
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 16 January 2023
Sec. Invertebrate Physiology
Volume 14 - 2023 | https://doi.org/10.3389/fphys.2023.1088712
This article is part of the Research TopicThe Side Effects of Insecticides on Insects and the Adaptation Mechanisms of Insects to InsecticidesView all 15 articles
 Rahat Afza1†
Rahat Afza1† Ayesha Afzal2,3†
Ayesha Afzal2,3† Muhammad Asam Riaz1
Muhammad Asam Riaz1 Muhammad Zeeshan Majeed1
Muhammad Zeeshan Majeed1 Atif Idrees2,4*
Atif Idrees2,4* Ziyad Abdul Qadir5,6
Ziyad Abdul Qadir5,6 Muhammad Afzal1
Muhammad Afzal1 Babar Hassan7
Babar Hassan7 Jun Li2*
Jun Li2*Synthetic insecticides have been an inevitable part of plant protection throughout the world. Sublethal effects of these chemicals on beneficial insect species are one of the contemporary issues these days. Using the age-stage, two-sex life table model, this study evaluated the sublethal and transgenerational effects of six synthetic insecticides (imidacloprid, thiamethoxam, lambda-cyhalothrin, cypermethrin, chlorpyrifos and profenofos) commonly applied to winter vegetables, on the fitness and predation of the seven-spotted ladybeetle, Coccinella septempunctata, which is an efficient predator of aphids worldwide. According to results, all insecticides at their sublethal doses (LC30) significantly suppressed the emergence of adults, adult weight, fertility and fecundity of the parental generation compared to control treatment. The larval stage was prolonged and oviposition, fecundity and total longevity of the adult beetles were decreased in unexposed progeny whose parents were exposed to sublethal doses of all insecticides. Moreover, the biological parameters of adults, including the intrinsic rate of increase (r), finite rate of increase (λ) and net reproductive rate (R0) were significantly reduced when exposed to sublethal doses of insecticides. The predation rate of the F1 generation adults was also decreased after exposure to the sublethal doses of insecticides. However, chlorpyrifos, profenofos, lambda-cyhalothrin and cypermethrin exhibited more deleterious effects on the fitness and population parameters of beetles than imidacloprid and thiamethoxam.
The ladybeetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae) is a well-known predator of various species of agricultural insect pests worldwide including Pakistan (Hodek and Michaud 2013; Saljoqi and Ali 2013). It is a generalist predator often observed on arable crops, feeding on aphids, whiteflies, psyllids, mites, and scales that are destructive insect pests of several crops (Bianchi and Van der Werf 2003; Farooq and Tasawar 2008; Sarwar 2013; Afza et al., 2019; He et al., 2019; Jiang et al., 2019; Afza et al., 2021). This species is considered a useful biological control agent of brassica aphids because of its voracious appetite. It feeds on more than twelve aphid species and rapidly responds to aphid populations (Jiang et al., 2019).
The use of synthetic insecticides by farmers is the predominant practice to keep pest populations below economic injury level (Afza et al., 2019; Afza et al., 2021). Chemical insecticides are cost-effective, easy to use and adequate against target pests. However, their excessive usage may have various adverse effects on beneficial arthropods in agroecosystems. The predatory potential of C. septempunctata is negatively affected after feeding on contaminated prey and plant material during foraging. Both direct contact with spray droplets and residues of insecticides affect its predation (Desneux et al., 2007; Afza et al., 2019; Afza et al., 2021). The deleterious effects of insecticides on coccinellid beetles include acute toxicity and changes in physiological, biochemical and behavioural processes. A lethal dose may not represent the overall adverse effects of synthetic insecticides, as sublethal concentrations do also have effect on insect behaviour and physiology (Galvan et al., 2005; Desneux et al., 2007; Rahmani and Bandani 2016). Effects of sublethal doses on the physiology of insects range from immunological to biochemistry, neurophysiological, sex ratio, fecundity, longevity and weight. Behavioural effects include disturbed mobility, orientation, feeding behaviour and learning performance (Galvan et al., 2005; Desneux et al., 2007; Afza et al., 2021). Sublethal doses of synthetic insecticides also have deleterious effects on the functional response of predatory beetles and other biological control agents (He et al., 2012; Jiang et al., 2019).
Indigenous farmers in Pakistan use several insecticides to manage aphids and other pests on their crops. All these insecticides have different ranges of toxicity and adverse effects on C. septempunctata (Arshad et al., 2017; Afza et al., 2019; Afza et al., 2021). Although biological control is an integral part of integrated pest management (IPM) programs, chemical control is the primary and most effective method for combating pests. However, some of the synthetic insecticides are relatively safer to be used as components of IPM programs. Therefore, knowledge of the non-target effects of insecticides on the behavioural traits, growth and development of biological control agents, is essential for an effective incorporation of insecticides in successful pest management programs (Tillman and Mulrooney 2000; Youn et al., 2003; Galvan et al., 2005; Bozsik 2006; Stark et al., 2007; Tengfei et al., 2019).
Several studies have reported on the lethal and sublethal effects of pesticides on predatory coccinellid beetles, showing that pesticides are harmful to these natural enemies (Urbaneja et al., 2008; Cabral et al., 2011; He et al., 2012; He et al., 2019; Jiang et al., 2019). However, data on age-related life table analyses and demographic parameters of C. septempunctata to evaluate the effects of currently used insecticides is still lacking. No study has evaluated the sublethal impacts of formulated insecticides commonly used by farmers for the management of aphids in Pakistan. Hence, an overall risk assessment of the exposure of C. septempunctata to currently-used synthetic insecticides is necessary. Apropos of the context, this study was aimed to assess the sublethal effects of six commonly-used synesthetic insecticides at their lower concentrations (LC30), on the fecundity, development and demographic parameters of C. septempunctata based on the life table theory. Moreover, the effects of their sublethal doses on the functional response of C. septempunctata were also assessed. The results of the current study can be useful for guiding assessment of the compatibility between the C. septempunctata and different insecticides in future IPM strategies for aphids. They would also contribute to the conservation of C. septempunctata in the field.
C. septempunctata adults were collected from canola (Brassica napus L.) fields managed without application of insecticides, at a farm area of the University of Sargodha, Pakistan (32°4′ N; 72°40′ E). Adult beetles were identified using identification keys described by (Poorani 2002; Rafi et al., 2005; Saeed et al., 2016) and were sorted into fifty couples (male and female). Then each pair of male and female coccinellid beetles was released into glass bowl (7.0 × 2.5 cm), which were then covered with muslin cloth and placed in an incubator (at 25 ± 2°C and 60 ± 5% R.H.), to obtain eggs for single cohort progeny. Collected adults and newly-hatched larvae of beetles were offered twigs and branches of canola infested by aphids (Brevicoryne brassicae L.) in a bowl. Aphids collected from the canola field were provided after every 12 h. For this purpose, the canola crop was sown in the campus and no insecticide was sprayed on the crop. Adult beetles were also offered a continuous supply of 10% glucose solution via a soaked cotton wool plug (Sarwar and Saqib 2010; Afza et al., 2019; Afza et al., 2021).
A field survey was conducted in the brassica growing region of Punjab (Pakistan) for 2 years. One hundred farmers were asked about the insecticides sprayed for the control of aphids on brassica and other winter crops every year. Survey results revealed six insecticides which were commonly sprayed by the farmers to manage their crop pests. These included imidacloprid, thiamethoxam, lambda-cyhalothrin, cypermethrin, chlorpyrifos and profenofos. Hence, these six insecticides were selected for the current study. Trade names, type of formulations, percent active ingredients (a.i.) and sources of the tested insecticides are given in Supplementary Table S1.
The experiment for the determination of lethal and sublethal concentration values was conducted following Afza et al. (2021). Based on mortality results obtained from preliminary assays (0–90%), five to six concentrations of each insecticide were prepared by dissolving them in water. A sample of 25–30 fourth instar larvae of C. septempunctata were treated with the concentrations of each insecticide separately. The control involved treatment of larvae with water. The experiment was replicated five times. Larvae were placed on ice to reduce their mobility during the bioassays. Then, 1 μl of each insecticide at different concentration and water (for control) was applied separately on the mesonotum of fourth instar larvae using a micropipette.
Treated larvae were placed in Petri-plates (90 mm diameter) lined with moistened Whatman No. 1 filter paper discs. To prevent the larvae from escaping, the plates were covered with PVC film which had small holes for aeration and were placed in an incubator (at 25 ± 2°C and 60 ± 5% R.H). Aphids were provided ad-libitum daily. Larval mortality was recorded 24 h after treatment. All beetles used in this experiment were 12–15 weeks old. Probit analysis using the Polo Plus® software was performed to calculate the median lethal concentrations (LC50 and LC30) values for each insecticide. The toxicity of sublethal dose (LC30) was confirmed in a separate test against the fourth instar larval beetle.
Three groups of freshly laid (0–6 h old) 100 eggs were placed on moistened Whatman No. 1 filter paper discs lined in Petri-plates (90 mm diameter). After inspection every 6 h, newly-hatched larvae were transferred to new Petri-plates and were fed on mixed instars of canola aphids (Brevicoryne brassicae L). All Petri-plates were incubated in an environmental chamber at 25 ± 2°C, 60 ± 5% R.H. and 14:10 h (L: D) photoperiod. When larvae reached the fourth instar (0–12 h old), sublethal concentrations (LC30) of each insecticide was applied topically. The treatment method was similar as described above. Forty-five larvae (15 larvae per replicate) were treated in each treatment and the experiment was repeated thrice. For the control treatment, larvae were treated with water. Fourth instar larvae were chosen for this experiment because of their lower natural mortality, compared to that of the initial three instars. Larval mortality, percentage of pupae formed and adult emergence from pupae were recorded at every 12 h until the adult stage. Larvae found motionless were considered dead. Newly-emerged adults were weighed and fed with B. brassicae individuals and 10% glucose solution. Male and female beetles from the same treatment were paired the same or second day of emergence and were observed daily to record their survival rate, fecundity and egg hatchability, until all the individuals were dead. For the fecundity, fifteen couples were tested per insecticide treatment.
A total 45–50 eggs (0–6 h old) from three to four couples of the parental generation, from the same treatment, were separated and kept in Petri-plates. Eggs were checked at 12 h intervals and newly-emerged larvae were transferred to new Petri-plates and fed on aphids. Each larva was considered as one replicate and its developmental time was monitored until pupation. Newly-emerged adults were sexed (12–15 couples) and their fecundity, survival rate and developmental time were recorded until the death of all individuals of the F1 generation.
F1 adult beetles starved for 24 h, were confined on canola foliage in Petri-plates (150 mm diameter) lined with moistened Whatman No. 1 filter paper discs and were offered B. brassicae aphid individuals at five densities, i.e. 20, 80, 160, 320 and 640 aphid individuals, as a food source. These aphids were pre-exposed to sublethal doses (LC30) of the insecticides. Water was used as control treatment. Twelve replications were carried out for each aphid density treatment. Individual coccinellids were offered insecticide-treated aphids. The data on aphid consumption by each beetle was recorded. One replicate under each density served as no-predator control, to determine the mortality of aphids after treating with insecticides. Experiments were conducted under controlled condition at 25 ± 2°C, 60 ± 5% R.H, and 14:10 h (L: D) photoperiod.
The data obtained from the toxicity bioassays were corrected using Abbott’s formula before analysis. The LC50 and LC30 values were calculated for each insecticide by probit analysis using the Polo Plus® software (Finney 1971). The normality of the parental generation (F0) data was assessed using the Kolmogorov-Smirnov test in SPSS® version 21.0 (SPSS Inc, Chicago, IL, United States) and data were analysed using one-way ANOVA in GraphPad Prism version 7.0 (GraphPad Software Inc, San Diego, CA, United States). Treatment means were compared by Tukey’s honest significant difference (HSD) post hoc test. Life table data were analysed using age-stage, two-sex life table using Two Sex-MS Chart program (Chi 1988). Standard errors of the life table parameters were calculated via 100,000 bootstrap replicates to obtain stable S.E. estimates. Sigma Plot® version 12.0 (Systat Software, Inc, San Jose, CA, United States) was used to generate curves for all population parameters including age-stage specific survival rate (Sxj), age-stage life expectancy (exj), age-specific survival rate (lx), age-specific fecundity (mx), net maternity (lx*mx) and reproductive value (vxj). Moreover, a two-step approach recommended by Juliano (2001) was used to determine the type of functional response and its parameters. Further details are given in Supplementary Table S1 and Supplementary Table S2.
The LC50 values of the six insecticides against C. septempunctata larvae were 256.6, 191.3, 122.3, 92.2, 153.1 and 235.9 mg a. i./ml for imidacloprid, thiamethoxam, profenofos, chlorpyrifos, lambda-cyhalothrin and cypermethrin, respectively. The highest LC50 was recorded for imidacloprid, while the minimum LC50 was observed in chlorpyrifos. The organophosphate insecticides, profenofos and chlorpyrifos, exhibited comparatively lower LC50 values against the fourth instar larvae of C. septempunctata than that of the other insecticides. The LC30 values of these insecticides were determined as 158.7, 117.5, 75.5, 56.4, 95.9, and 110.4 mg a. i./ml for imidacloprid, thiamethoxam, profenofos, chlorpyrifos, lambda-cyhalothrin and cypermethrin, respectively.
The treatment of larvae with sublethal concentrations of the six insecticides had significant negative effects on the biological parameters of C. septempunctata compared to the control groups (Figure 1). Exposure to sublethal concentration of lambda-cyhalothrin, chlorpyrifos and profenofos, significantly increased the pupal duration of beetles (>7 days), compared to the other insecticides and control (water-treated) insects (<6.8 days) (F = 6.22; p < 0.0001) (Figure 1A). The adult emergence rate of insecticide-treated C. septempunctata was also significantly affected compared to the control insects (F = 58.84; p < 0.0001). The sublethal doses of lambda-cyhalothrin, chlorpyrifos and profenofos significantly reduced the emergence rate compared to insects treated to cypermethrin, imidacloprid and thiamethoxam. A higher adult emergence rate was recorded for control (water treated) insects (Figure 1B). Similarly, the weight of newly-emerged beetles treated with insecticides was reduced compared to control groups (F = 5.09; p = 0.001). A significantly higher decrease in weight of adult beetles was observed after treatment with chlorpyrifos, followed by lambda-cyhalothrin and profenofos. Thiamethoxam and cypermethrin caused similar effects on the weight of beetles (Figure 1C). The fecundity rate of females was also significantly suppressed after treatment with sublethal concentrations of the six insecticides, compared to control treatment (F = 83.01; p < 0.0001). The most significant reduction in fecundity occurred after treatment with lambda-cyhalothrin, chlorpyrifos and profenofos followed by cypermethrin. Thiamethoxam and imidacloprid caused similar effects on the fecundity of beetles. A higher fecundity was observed in beetles treated with water (872.4 ± 15.75 eggs) (Figure 1D). Egg hatchability (%) (F = 103.40; p < 0.0001) and survival (%) (F = 80.50; p < 0.0001) of C. septempunctata treated with sublethal doses of six insecticides was also significantly reduced compared to control groups. A sublethal treatment of chlorpyrifos and lambda-cyhalothrin, significantly reduced the egg viability and survival of beetles compared to the rest of insecticides. However, egg hatchability and survival of water-treated beetles were substantially higher compared to insecticides-treated beetles (Figure 1E, F).
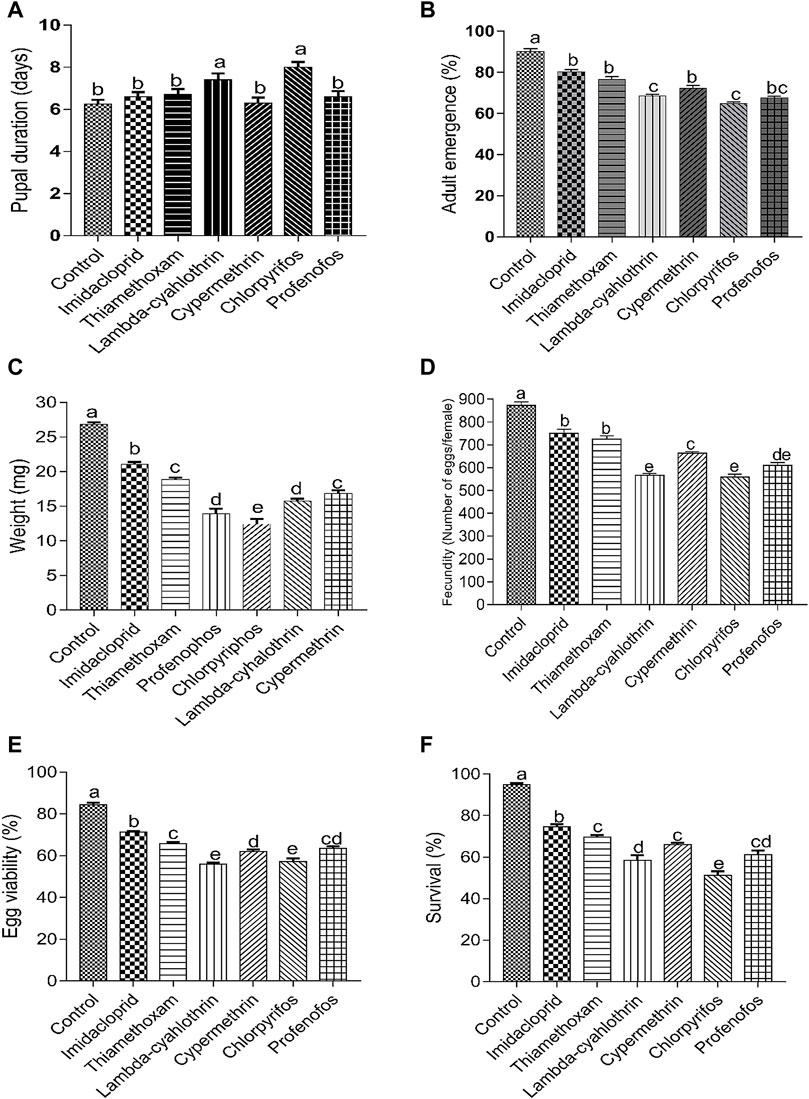
FIGURE 1. Effect of sublethal concentrations (LC30) of synthetic insecticides on the pupal duration (A), adult emergence (B), weight of newly-emerged adults (C), fecundity (eggs/female) (D) egg viability (E) and adult survival (F) of the F0 generation of Coccinella septempunctata. Larvae of Coccinella septempunctata were exposed to sublethal concentrations of imidacloprid, thiamethoxam, profenofos, chlorpyrifos, lambda-cyhalothrin and cypermethrin. Treatment means were compared by Tukey’s Honest Significant Difference (HSD) post hoc test. Means with the same letters are not significantly different at p ≤ 0.005.
Results of the effect of sublethal doses of the insecticides on the developmental time of the F1 generation are presented in Table 1. All sublethal insecticidal treatments did not significantly affect the egg hatching and first instar larval period of the beetles (egg hatching duration: F = 4.55; p = 0.090; first instar duration: F = 12.33; p = 0.10). The larval duration of the second instar of all insecticide-treated beetles significantly lengthened, compared to control treatment (F = 47.15; p = 0.040). Here, lambda-cyhalothrin had the most significant effect on this duration compared to other insecticidal treatments. Similarly, the period of the third instar larvae was significantly increased after treatment with lambda-cyhalothrin, chlorpyrifos, and profenofos compared to other insecticides. Overall, the third instar larval period of insecticide-treated beetles was substantially longer compared to that of water-treated beetles (F = 43.65; p = 0.030). The fourth instar period of beetles treated with lambda-cyhalothrin and cypermethrin was significantly increased compared to thiamethoxam and imidacloprid (F = 25.19; p = 0.032). Moreover, the pupal duration of beetles treated with profenofos significantly lengthened compared to water-treated beetles. Nevertheless, the pupal period of beetles treated with the other insecticides did not differ significantly from control treatment and with profenofos (F = 51.23; p = 0.041). Adult duration and total longevity of insecticide-treated beetles were reduced considerably compared to water-treated beetles (Adult duration: F = 157.11; p = 0.002; overall longevity: F = 412.13; p = 0.001) (Table 1).
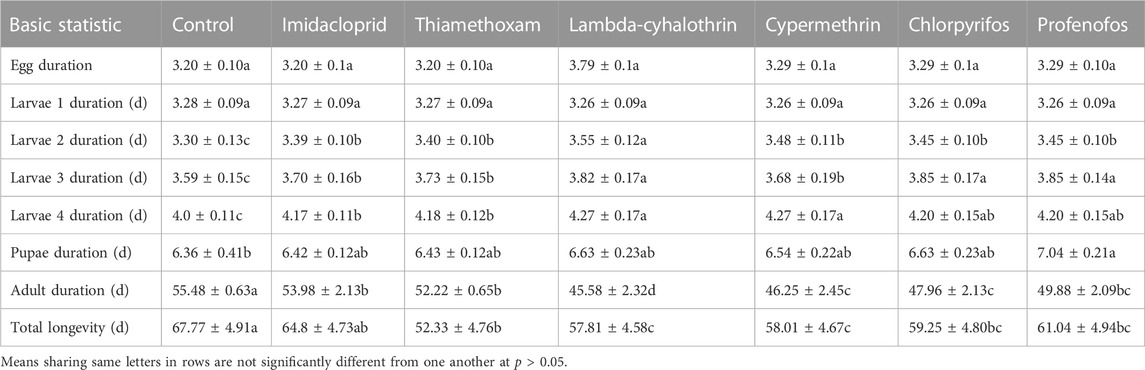
TABLE 1. Means (days ±SE) developmental time of the different life stages of Coccinella septempunctata F1 generation.
Results of the effects of the six insecticides at LC30, on the population parameters of the F1 generation of C. septempunctata are presented in Table 2. Sublethal doses of all six insecticides significantly reduced the intrinsic rate of increase (r) and finite rate (λ) of the beetles. However, the finite rate of beetles treated with thiamethoxam and imidacloprid was similar to that of water-treated beetles. Sublethal treatments of chlorpyrifos, profenofos, and lambda-cyhalothrin significantly decreased the intrinsic rate of increase compared to the other three insecticides (p < 0.05). All insecticides at sublethal doses (LC30) significantly reduced the net reproduction rate (R0) (F = 351.65; p < 0.0001) and increased the mean generation time (T) (F = 253.15; p = 0.002) compared to that of water-treated C. septempunctata. However, chlorpyrifos, profenofos, and lambda-cyhalothrin had significantly greater effects on R0 and T, compared to the other insecticides (Table 2). Adult pre-oviposition period (APOP) (F = 51.20; p = 0.001) and total pre-oviposition period (TPOP) (F = 98.27; p = 0.002) were significantly prolonged in beetles treated with the six insecticides compared to those of water-treated beetles. Chlorpyrifos, profenofos, and lambda-cyhalothrin had greater effects on TPOP and APOP compared to those of the other three insecticides. Female fecundity was also reduced in beetles treated with insecticides compared to that of the control (water-treated) beetles (F = 698.27; p < 0.0001) (Table 2). A higher reduction in eggs/female was recorded in beetles treated with sublethal doses of profenofos, chlorpyrifos, and lambda-cyhalothrin than that from the other insecticides. Oviposition durations of females were significantly higher in water-treated beetles, compared to insecticide-treated beetles (F = 173.27; p < 0.0001).
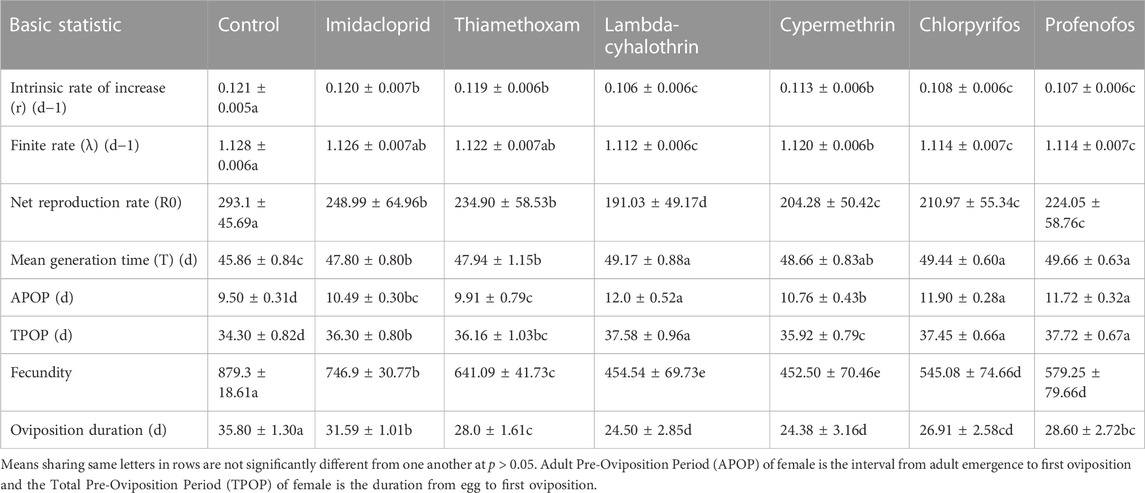
TABLE 2. Population parameters (means ± SE) of Coccinella septempunctata F1 generation adults in response to sublethal exposure (LC30) to six insecticides.
The age-stage specific survival rate (sxj) curves showed the variation in different developmental stages of the coccinellids. There were distinct overlaps between the control and insecticidal treatments (Figure 2). The survival rate of adult males and females was decreased after treatment with lambda-cyhalothrin and chlorpyrifos, compared to the control and that of the other insecticidal treatments. The larval duration after treatments with lambda-cyhalothrin, profenofos, and chlorpyrifos was significantly reduced compared to that of control and other insecticidal treatments. The exj was significantly reduced in beetles treated with sublethal doses of all insecticides except imidacloprid, compared to that of the control treatment (Figure 3). The lx, mx, and lxmx are presented in Figure 4. Age-stage specific survival rate was significantly reduced in beetles treated with chlorpyrifos and lambda-cyhalothrin compared to that of beetles treated with water and other insecticides. The maximal survival time for control treatment was 82 days, which was higher than that under insecticidal treatments. Similarly, compared to control treatment, the vxj of C. septempunctata was significantly reduced after treatment with sublethal doses of all insecticides except for imidacloprid and chlorpyrifos (Figure 5). A higher reduction in vxj was observed in beetles treated with a sublethal dose of lambda-cyhalothrin followed by thiamethoxam compared to that from the other insecticides.
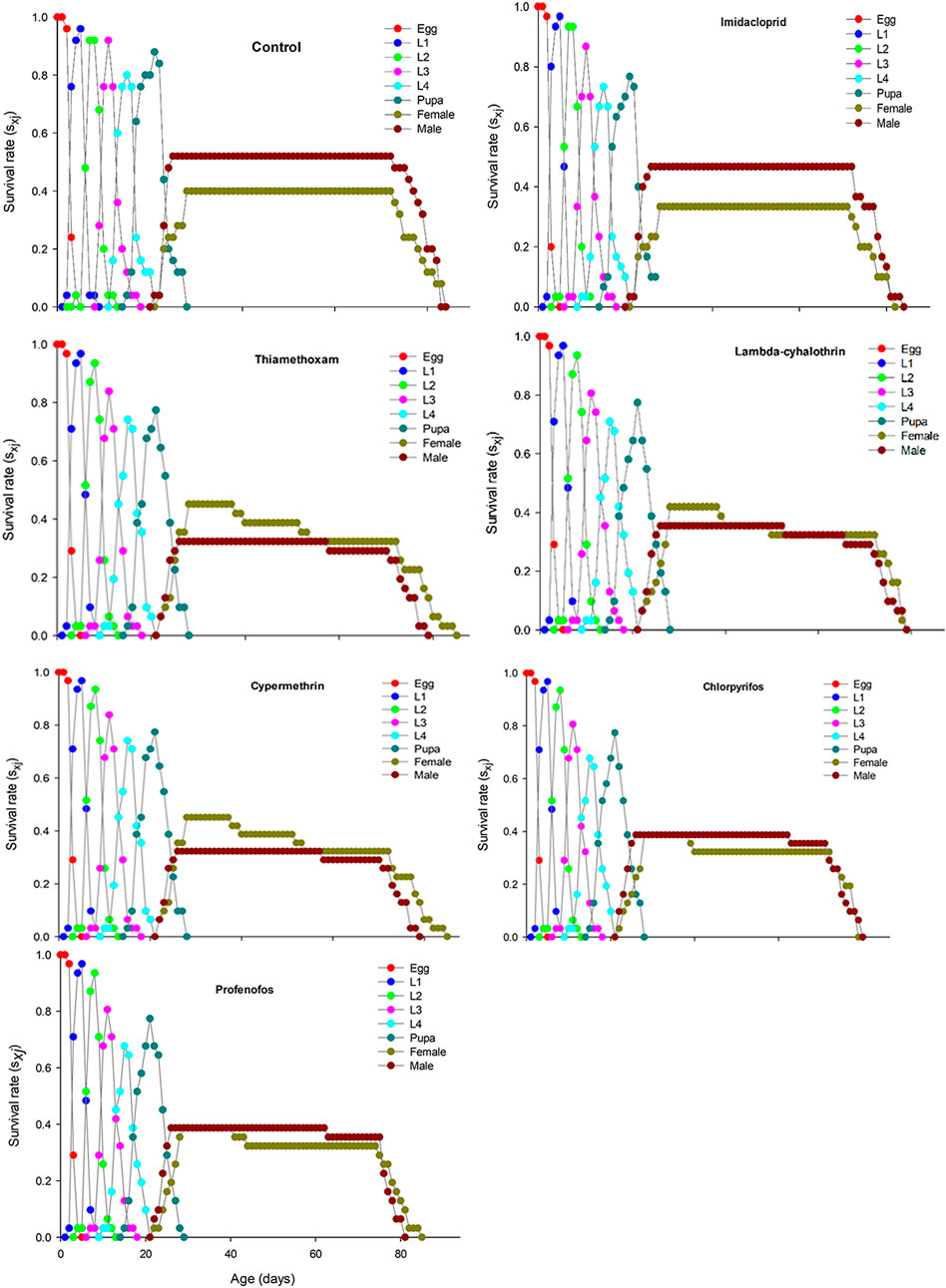
FIGURE 2. Age-stage specific survival (sxj) of Coccinella septempunctata (F1) treated with water (control) and sublethal concentrations (LC30) of synthetic insecticides.
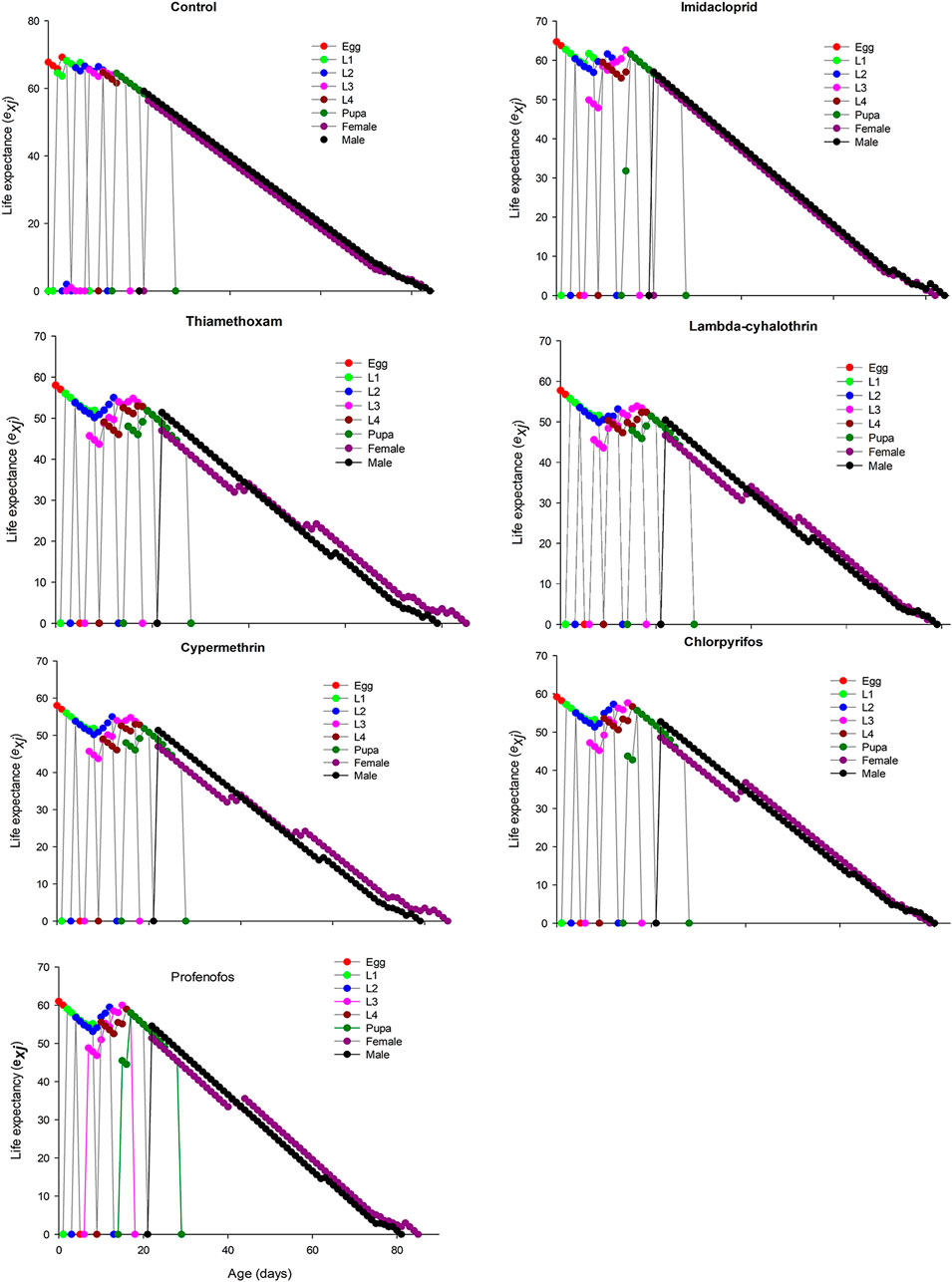
FIGURE 3. Age-stage specific life expectancy (exj) of Coccinella septempunctata (F1) treated with water (control) and sublethal concentration (LC30) of synthetic insecticides.
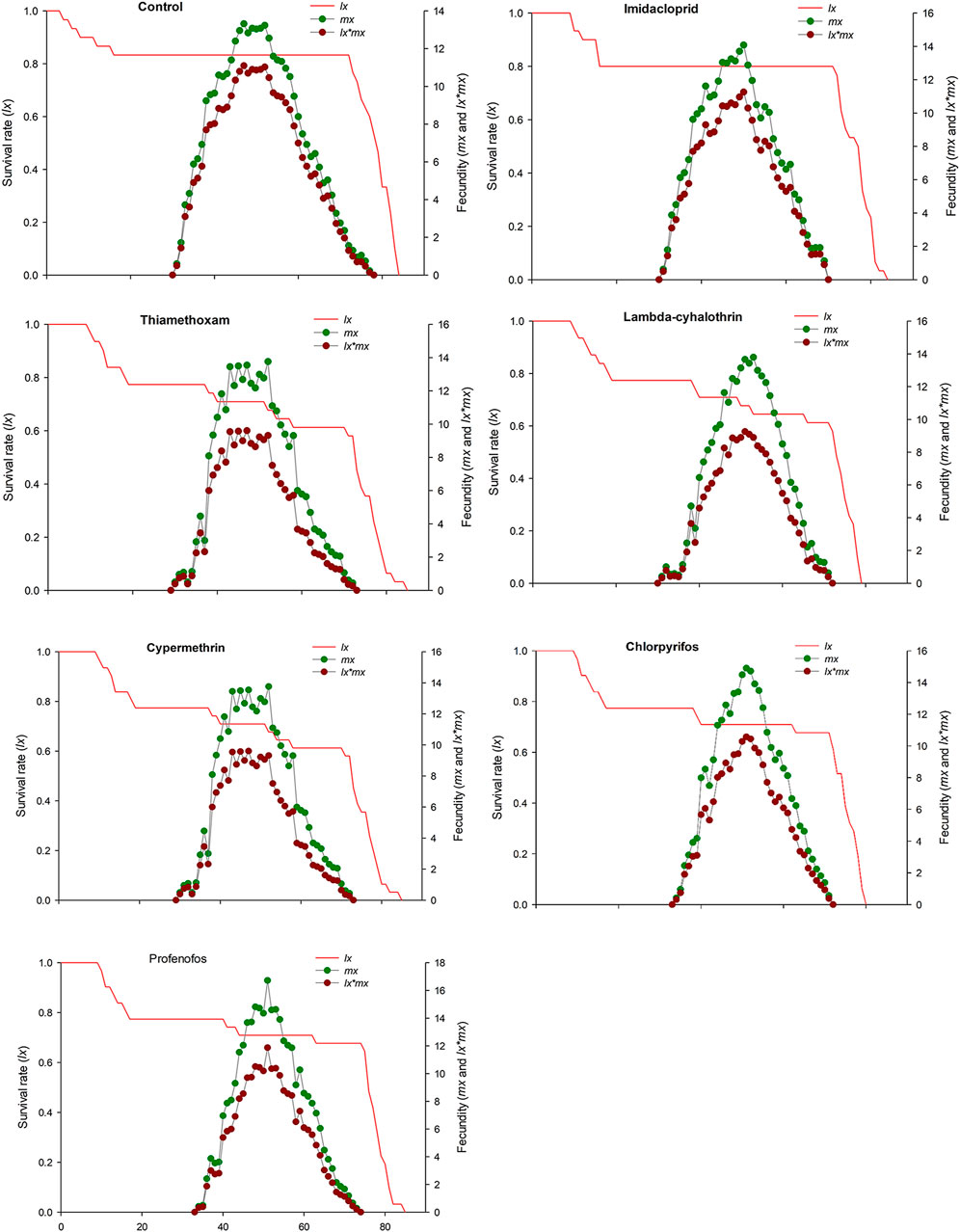
FIGURE 4. Age-stage specific survival rate (lx), age-specific fecundity (mx) and net maternity (lx × mx) of C. septempunctata (F1) treated with water (control) and sublethal concentration (LC30) of synthetic insecticides.
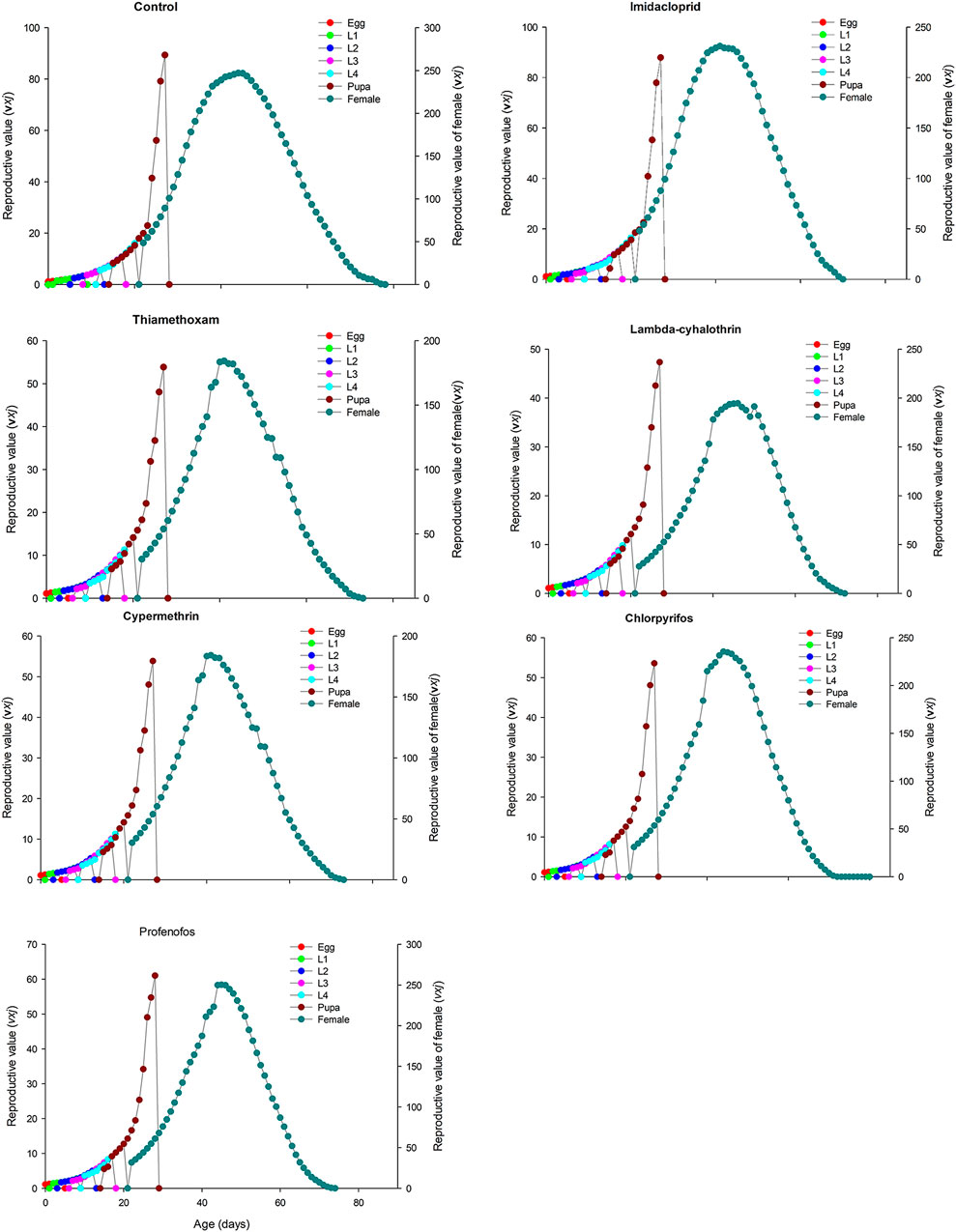
FIGURE 5. Age-stage specific reproductive value (vxj) of Coccinella septempunctata (F1) treated with water (control) and sublethal concentration (LC30) of synthetic insecticides.
The functional response of F1 coccinellid adults to B. brassicae aphids fitted o the Holling’s type II curves (Figure 6). Logistic regression analysis coefficients of the proportion of sublethal (LC30) dose-treated aphids devoured by adult coccinellids are presented in Supplementary Table S2. Values of the parameters α, Th and T/Th derived from all treatments are presented in Table 3. The instantaneous attacking rate (α) derived from control treatment remained significantly higher (0.075) compared to that from insecticidal treatments. Significantly minimum attack rate was observed in beetles which fed on aphids treated with lambda-cyhalothrin (0.001), followed by chlorpyrifos (0.011) and thiamethoxam (0.010). The maximum handling time (Th) (0.069) was recorded for beetles which fed on aphids treated with chlorpyrifos, followed by lambda-cyhalothrin (0.051) and profenofos (0.050). The handling time of prey by beetles treated with imidacloprid, thiamethoxam and cypermethrin did not differ significantly. The minimum handling (0.017) time was recorded under control treatment.
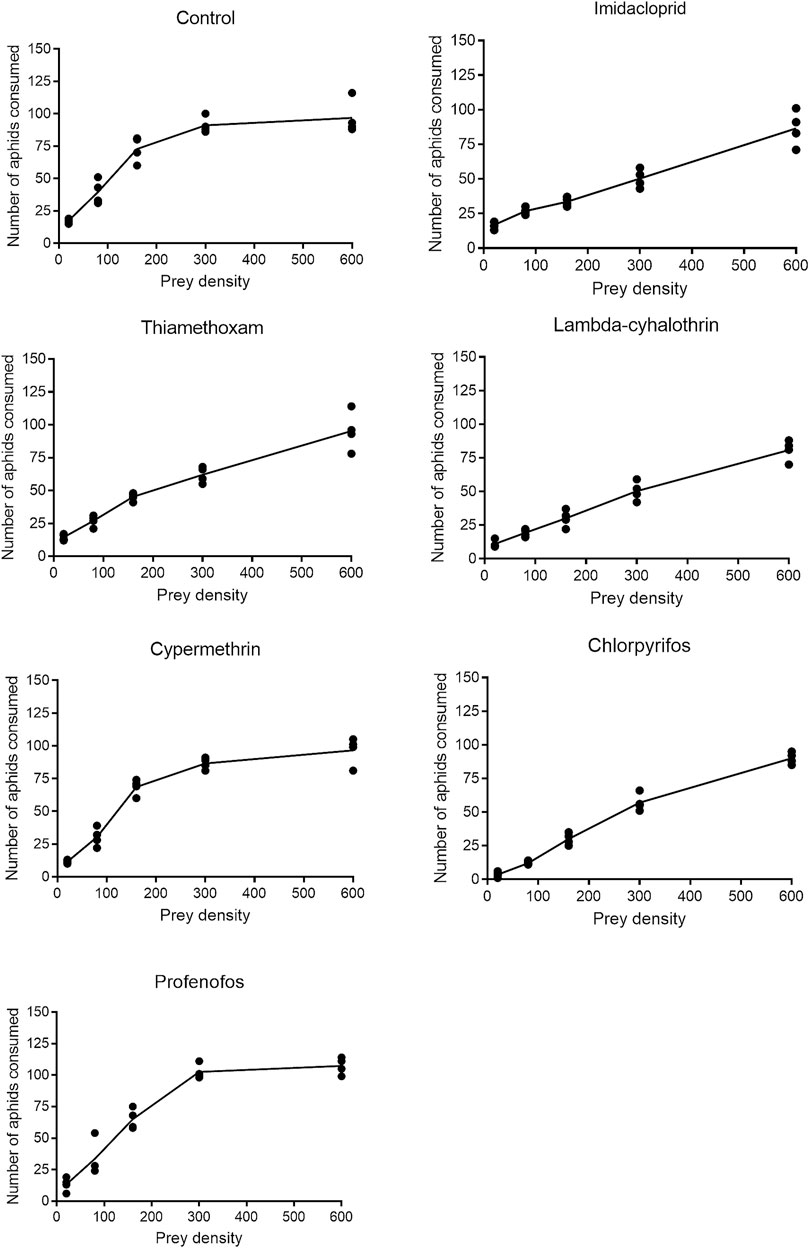
FIGURE 6. Functional responses of Coccinella septempunctata adults exposed to sublethal doses of synthetic insecticides and control.
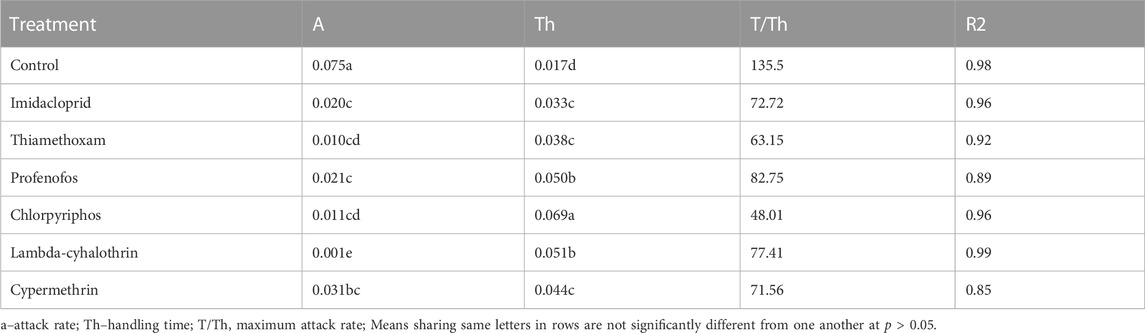
TABLE 3. Parameters for functional response model for adult Coccinella septempunctata exposed to sublethal concentrations (LC30) of six insecticides.
Insecticides are mainly used as a foliar spray on canola and other winter crops. Therefore, predators of insect pests, including coccinellid beetles are likely to be exposed to insecticides while foraging on the treated plants and pests. Exposure to pesticides may cause various adverse effects on the life-history traits and feeding behaviour of coccinellid beetles. In the current study, the sublethal effect of six insecticides belonging to three chemical classes was presented with experimental evidences. We found that the sublethal doses (LC30) of the six insecticides adversely affected the biological parameters of C. septempunctata (F0 generation). Lambda-cyhalothrin and chlorpyrifos increased the pupal duration compared to that from the other insecticides. All the tested insecticides reduced the adult emergence, fecundity rates, weight of adults, and survival compared to the control treatment. These results are similar to those from other studies which showed that most of the tested insecticides impaired the reproduction and survival of C. septempunctata and other predators (Yu et al., 2014; Skouras et al., 2017; Kang et al., 2018; Jiang et al., 2019; Noelia et al., 2022).
Transgenerational impacts of the six insecticides on the predatory beetle, C. septempunctata, were studied by age-stage, two-sex life table model analyses. Results showed that the second, third, fourth instar and pupation stages of C. septempunctata in the F1 generation were prolonged when their parental generations (F0) were exposed to sublethal doses of all insecticides. This prolongation in the larval and pupal development may be due to physiological effects such as reduced food assimilation or stunted development at the cost of detoxification mechanism (Skouras et al., 2017). However, treatment with a sublethal dose of lambda-cyhalothrin had higher significant effects on the second instar than the other insecticides. Similarly, the sublethal dose of lambda-cyhalothrin, chlorpyrifos and profenofos prolonged the third instar duration compared to the other three insecticides. All insecticides reduced the adult duration compared to the control treatment, and beetles treated with a sublethal dose of lambda-cyhalothrin had the least adult duration. The total longevity of beetles treated with the sublethal dose was significantly reduced compared to that under control treatment. The differences between the sublethal effects of insecticides on insect depend on the variations in the structure and efficacy of insecticides (Alkassab and Kirchner 2016; Skouras et al., 2017; Dai et al., 2019; Tan et al., 2021).
The sublethal doses of all insecticides adversely influenced the reproductive performance of the F1 generation. Reductions in the fecundity of females exposed to insecticides may result from both physiological and behavioural effects (Desneux et al., 2007; Wu et al., 2022). The duration of the F1 generation’s pre-oviposition period (APOP and TPOP) and the oviposition period changed significantly in beetles treated with lambda-cyhalothrin, chlorpyrifos, and profenofos (Desneux et al., 2004; De Castro et al., 2013). Moreover, there was a significant reduction in the number of offspring (F2) from parent individuals exposed to sublethal doses of all insecticides (Fernandes et al., 2010). These insecticides might have disrupted the very precise coordination of the nervous and hormonal systems in the insects, resulting in a breakdown in behavioural and physiological events related to oviposition (Desneux et al., 2007).
Population parameters such as intrinsic (r) and finite (λ) rates of increase, net reproductive rate (R0) and mean generation time (T) can be useful to better understand the population dynamics of insects (Liu et al., 2017; Zheng et al., 2017; Negi et al., 2018; Jiang et al., 2019). Results showed that the treatment of F0 with sublethal doses of all insecticides significantly reduced the r, λ, and R0 parameters of the F1 generation.
However, the sublethal doses of lambda-cyhalothrin, chlorpyrifos, and profenofos significantly reduced r and λ than sublethal doses of imidacloprid and thiamethoxam. In general, the fluctuations in demographic features demonstrated that sublethal concentrations pyrethroid and organophosphate insecticides had the greatest effects on the reproduction and survival of C. septempunctata transgenerationally than the two neonicotinoids.
Functional response is generally determined to assess the feeding efficiency of predatory species to agricultural pests (Juliano 2001; Xue et al., 2009; He et al., 2012; Sarkar et al., 2022). Sublethal exposure to all insecticides impaired the functional responses and prolonged the handling time of aphid by the predator beetle. This agrees with studies of He et al. (2012) and Yao et al. (2015) which showed that thiamethoxam and imidacloprid prolonged the handling time of Bemisia tabaci eggs by the predatory coccinellid beetle, Serangium japonicum at sublethal doses. However, a maximum handling time was observed when beetles were offered aphids treated with chlorpyrifos, followed by profenofos (GholamzadehChitgar et al., 2014).
Overall, sublethal doses of all the tested insecticides impaired the fitness and predation efficacy of C. septempunctata individuals up to two successive generations. However, the effects of the pyrethroids and organophosphates were greater, compared to neonicotinoids. This agrees with previous studies (Ahmad et al., 2011). Organophosphates and pyrethroids have low selectivity to insect predators and parasitoids than neonicotinoids (Galvan et al., 2002; Fernandes et al., 2010). This greater negative effects of the two insecticide groups, may be associated with their pro-insecticide activities. Moreover, organophosphates and pyrethroids have lipophilic characteristic and can be adsorbed in the beetle cuticle very easily when topically applied (GholamzadehChitgar et al., 2014; Costa 2015). This may be responsible for thegreater negative effects they have on insects.
Our laboratory experiments demonstrated that all the tested insecticides had adverse effects on the fitness and predation efficacy of C. septempunctata individuals. However, among these, the organophosphates and pyrethroids had greater negative effects on the population parameters of beetles compared to the neonicotinoids. Therefore, these neonicotinoids have some potential which can enable their integration into IPM programs. Additionally, we also evaluated the demographic and transgenerational effects mediated by exposure to sublethal doses of these insecticides. Future work will focus on the mechanisms underlying these adverse effects of the sublethal doses of the insecticides on C. septempunctata.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
All authors listed have made considerable direct or indirect and intellectual contribution to the work and have read and agreed to the manuscript.
This research work was financially supported by the GDAS Special Project of Science and Technology Development (No. 2020GDASYL-20200301003), GDAS Action Capital Project to build a comprehensive industrial technology innovation Center (No. 2022GDASZH-2022010106). Higher Education Commission of Pakistan (HEC) (Project No. NRPU-11326).
The authors are thankful to Hsin Chi (Laboratory of Theoretical and Applied Ecology, Department of Entomology, National Chung Hsing University, Taiwan) for providing the age-stage, two-sex life-table program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1088712/full#supplementary-material
Afza R., Afzal M., Majeed M. Z., Riaz M. A. (2019). Effect of intra-guild predation and sub lethal concentrations of insecticides on the predation of coccinellids. Pak. J. Zool. 51, 611–617. doi:10.17582/journal.pjz/2019.51.2.611.617
Afza R., Riaz M. A., Afzal M., Majeed M. Z. (2021). Adverse effect of sublethal concentrations of insecticides on the biological parameters and functional response of predatory beetle Coccinella septempunctata (Coleoptera: Coccinellidae) of Brassica aphid. Sarh. J. Agric. 37, 226–234. doi:10.17582/journal.sja/2021/37.1.226.234
Ahmad M., Rafiq M., Arif M. I., Sayyed A. H. (2011). Toxicity of some commonly used insecticides against Coccinella undecimpunctata (Coleoptera: Coccinellidae). Pak. J. Zool. 43, 1161–1165.
Alkassab A. T., Kirchner W. H. (2016). Impacts of chronic sublethal exposure to clothianidin on winter honeybees. Ecotoxico. L. 25, 1000–1010. doi:10.1007/s10646-016-1657-3
Arshad M., Khan H. A. A., Hafeez F., Sherazi R., Iqbal N. (2017). Predatory potential of Coccinella septempunctata L. Against four aphid species. Pak. J. Zool. 49, 623–627. doi:10.17582/journal.pjz/2017.49.2.623.627
Bianchi F. J., Van Der Werf W. (2003). The effect of the area and configuration of hibernation sites on the control of aphids by Coccinella septempunctata (Coleoptera: Coccinellidae) in agricultural landscapes: A simulation study. Environ. Entomol. 32, 1290–1304. doi:10.1603/0046-225X-32.6.1290
Bozsik A. (2006). Susceptibility of adult Coccinella septempunctata (Coleoptera: Coccinellidae) to insecticides with different modes of action. Pest. Manage. Sci. 62, 651–654. doi:10.1002/ps.1221
Cabral S., Soares A. O., Garcia P. (2011). Voracity of Coccinella undecimpunctata: Effects of insecticides when foraging in a prey/plant system. J. Pest. Sci. 84, 373–379. doi:10.1007/s10340-011-0373-2
Chi H. (1988). Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17, 26–34. doi:10.1093/ee/17.1.26
Costa L. G. (2015). “The neurotoxicity of organochlorine and pyrethroid pesticides,” in Handbook of clinical neurology. Editors L. Marcello, and L. B. Margit (Amsterdam, Netherlands: Elsevier), 135–148. doi:10.1016/B978-0-444-62627-1.00009-3
Dai P., Jack C. J., Mortensen A. N., Bustamante T. A., Bloomquist J. R., Ellis J. D. (2019). Chronic toxicity of clothianidin, imidacloprid, chlorpyrifos, and dimethoate to Apis mellifera L. Larvae reared in vitro. Pest. Manage. Sci. 75, 29–36. doi:10.1002/ps.5124
De Castro A., Corrêa A., Legaspi J., Guedes R., Serrão J., Zanuncio J. (2013). Survival and behavior of the insecticide-exposed predators podisus nigrispinus and supputius cincticeps (heteroptera: Pentatomidae). Chemosphere 93, 1043–1050. doi:10.1016/j.chemosphere.2013.05.075
Desneux N., Decourtye A., Delpuech J. M. (2007). The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106. doi:10.1146/annurev.ento.52.110405.091440
Desneux N., Pham-Delègue M. H., Kaiser L. (2004). Effects of sub-lethal and lethal doses of lambda-cyhalothrin on oviposition experience and host-searching behaviour of a parasitic wasp. Aphidius Ervi. Pest. Manage. Sci. 60, 381–389. doi:10.1002/ps.822
Farooq A., Tasawar Z. (2008). Evaluation of integrated management of aphid pests, brevicoryne brassicae and lipaphis erysimi on canola crop in southern Punjab, Pakistan. Pak. J. Zool. 40, 13–17.
Fernandes F. L., Bacci L., Fernandes M. S. (2010). Impact and selectivity of insecticides to predators and parasitoids. Entomo. Bras. 3, 1–10. doi:10.12741/ebrasilis.v3i1.52
Galvan T., Koch R. L., Hutchison W. D. (2005). Effects of spinosad and indoxacarb on survival, development, and reproduction of the multicolored asian lady beetle (Coleoptera: Coccinellidae). Biol. Control. 34, 108–114. doi:10.1016/j.biocontrol.2005.04.005
Galvan T. L., Picanço M. C., Bacci L., Pereira E. J. G., Crespo A. L. B. (2002). Seletividade de oito inseticidas a predadores de lagartas em citros. Pesqui. Agropecu. Bras. 37, 117–122. doi:10.1590/S0100-204X2002000200001
Gholamzadehchitgar M., Hajizadeh J., Ghadamyari M., Karimi-Malati A., Hoda H. (2014). Sublethal effects of diazinon, fenitrothion and chlorpyrifos on the functional response of predatory bug, andrallus spinidens fabricius (Hemiptera: Pentatomidae) in the laboratory conditions. J. King. Saud. Univ-Sci. 26, 113–118. doi:10.1016/j.jksus.2013.09.001
He F., Sun S., Tan H., Sun X., Shang D., Yao C., et al. (2019). Compatibility of chlorantraniliprole with the generalist predator Coccinella septempunctata L. (Coleoptera: Coccinellidae) based toxicity, life-cycle development and population parameters in laboratory microcosms. Chemosphere 225, 182–190. doi:10.1016/j.chemosphere.2019.03.025
He Y., Zhao J., Zheng Y., Desneux N., Wu K. (2012). Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicol 21, 1291–1300. doi:10.1007/s10646-012-0883-6
Hodek I., Michaud J. (2013). Why is Coccinella septempunctata so successful? (a point-of-view). Eur. J. Entomol. 105, 1–12. doi:10.14411/eje.2008.001
Jiang J., Zhang Z., Yu X., Yu C., Liu F., Mu W. (2019). Sublethal and transgenerational effects of thiamethoxam on the demographic fitness and predation performance of the seven-spot ladybeetle Coccinella septempunctata L.(Coleoptera: Coccinellidae). Chemosphere 216, 168–178. doi:10.1016/j.chemosphere.2018.10.126
Juliano S. A. (2001). Nonlinear curve fitting: Predation and functional response curves. Des. Anal. Ecol. Exp. 2, 178–196.
Kang Z. W., Liu F. H., Pang R. P., Tian H. G., Liu T. X. (2018). Effect of sublethal doses of imidacloprid on the biological performance of aphid endoparasitoid aphidius gifuensis (hymenoptera: Aphidiidae) and influence on its related gene expression. Front. Physiol. 9, 1729. doi:10.3389/fphys.2018.01729
Liu J., Huang W., Chi H., Wang C., Hua H., Wu G. (2017). Effects of elevated CO2 on the fitness and potential population damage of helicoverpa armigera based on two-sex life table. Sci. Rep. 7, 1119–1213. doi:10.1038/s41598-017-01257-7
Negi S., Sharma P. L., Sharma K. C., Verma S. C. (2018). Effect of host plants on developmental and population parameters of invasive leafminer, tuta absoluta (meyrick) (Lepidoptera: Gelechiidae). Phytoparasitica 46, 213–221. doi:10.1007/s12600-018-0661-y
Noelia F. M., Scorsetti A. C., Minardi G., Schneider M. I. (2022). Toxicity assessment of two IGR insecticides on eggs and larvae of the ladybird Eriopis connexa (Coleoptera: Coccinellidae). Pest Manage.Sci. doi:10.1002/ps.7293
Poorani J. (2002). An annotated checklist of the Coccinellidae (Coleoptera) (excluding epilachninae) of the Indian subregion. Orient. Insects. 36, 307–383. doi:10.1080/00305316.2002.10417335
Rafi M., Irshad M., Inayatullah M. (2005). National insect museum and insect pest informatics, IPM programme. Islamabad, Pakistan: Rohani Art Press.Predatory Ladybird Beetles of Pakistan.
Rahmani S., Bandani A. R. (2016). Pirimicarb, an aphid selective insecticide, adversely affects demographic parameters of the aphid predator Hippodamia variegata (goeze)(Coleoptera: Coccinellidae). J. Plant. Prot. Res. 56, 353–363. doi:10.1515/jppr-2016-0048
Saeed K., Khattak M. N. K., Khan F., Naz F., Akhtar N. (2016). Morphological characteristics of ladybird beetles (Coccinellidae: Coleoptera) of district buner, khyber pakhtunkhwa, Pakistan. Pak. J. Zool. 48, 1367–1372. doi:10.4172/2161-0983.1000187
Saljoqi A. U. R., Ali S. (2013). Population dynamics of Aphis gossypii (glover) and its associated natural enemies in different okra varieties. Pak. J. Zool. 45, 1197–1205.
Sarkar S. C., Milroy S. P., Xu W. (2022). Potential of variegated lady beetle Hippodamia variegata in management of invasive tomato potato psyllid Bactericera cockerelli. Pest Manage.Sci. doi:10.1002/ps.7247
Sarwar M., Saqib S. M. (2010). Rearing of predatory seven spotted ladybird beetle Coccinella septempunctata L.(Coleoptera: Coccinellidae) on natural and artificial diets under laboratory conditions. Pak. J. Zool. 42, 47–51.
Sarwar M. (2013). Studies on incidence of insect pests (aphids) and their natural enemies in canola Brassica napus L.(Brassicaceae) crop ecosystem. Int. J. Sci. Res. Environ. Sci. 1, 78–84. doi:10.12983/ijsres-2013-p078-084
Skouras P. J., Stathas G. J., Voudouris C. C., Darras A. I., Tsitsipis J. A., Margaritopoulos J. T. (2017). Effect of synthetic insecticides on the larvae of Coccinella septempunctata from Greek populations. Phytoparasitica 45, 165–173. doi:10.1007/s12600-017-0577-y
Stark J. D., Vargas R., Banks J. E. (2007). Incorporating ecologically relevant measures of pesticide effect for estimating the compatibility of pesticides and biocontrol agents. J. Econ. Entomol. 100, 1027–1032. doi:10.1603/0022-0493(2007)100[1027:iermop]2.0.co;2
Tan Y., Jia B., Foster S. P., Homem R. A., Williamson M. S., Han H. B., et al. (2021). Sublethal and transgenerational effects of lambda-cyhalothrin on the mirid bugs Lygus pratensis Linnaeus and Polymerus cognatus Fieber. Crop Prot. 139, 105354. doi:10.1016/j.cropro.2020.105354
Tengfei L., Yao W., Lixia Z., Yongyu X., Zhengqun Z., Wei M. (2019). Sublethal effects of four insecticides on the seven-spotted lady beetle (Coleoptera: Coccinellidae). J. Econ. Entomol. 112, 2177–2185. doi:10.1093/jee/toz146
Tillman P., Mulrooney J. (2000). Effect of selected insecticides on the natural enemies coleomegilla maculata and Hippodamia convergens (Coleoptera: Coccinellidae), geocoris punctipes (Hemiptera: Lygaeidae), and bracon mellitor, cardiochiles nigriceps, and cotesia marginiventris (hymenoptera: Braconidae) in cotton. J. Econ. Entomol. 93, 1638–1643. doi:10.1603/0022-0493-93.6.1638
Urbaneja A., Pascual-Ruiz S., Pina T., Abad-Moyano R., Vanaclocha P., Montón H., et al. (2008). Efficacy of five selected Acaricides against tetranychus urticae (Acari: Tetranychidae) and their side effects on relevant natural enemies occurring in citrus orchards. Pest. Manage. Sci. 64, 834–842. doi:10.1002/ps.1572
Wu H. M., Feng H. L., Wang G. D., Zhang L. L., Zulu L., Liu Y. H., et al. (2022). Sublethal effects of three insecticides on development and reproduction of spodoptera frugiperda (Lepidoptera: Noctuidae). Agronomy 12 (6), 1334. doi:10.3390/agronomy12061334
Xue Y., Bahlai C. A., Frewin A., Sears M., Schaafsma A., Hallett R. H. (2009). Predation by Coccinella septempunctata and Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Homoptera: Aphididae). Environ. Entomol. 38, 708–714. doi:10.1603/022.038.0322
Yao F. L., Zheng Y., Zhao J. W., Desneux N., He Y. X., Weng Q. Y. (2015). Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 128, 49–55. doi:10.1016/j.chemosphere.2015.01.010
Youn Y. N., Seo M., Shin J., Jang C., Yu Y. (2003). Toxicity of greenhouse pesticides to multicolored asian lady beetles, Harmonia axyridis (Coleoptera: Coccinellidae). Biol. L. Control. 28, 164–170. doi:10.1016/S1049-9644(03)00098-7
Yu C., Lin R., Fu M., Zhou Y., Zong F., Jiang H., et al. (2014). Impact of imidacloprid on life-cycle development of Coccinella septempunctata in laboratory microcosms. Ecotoxicol. Environ. Safe. 110, 168–173. doi:10.1016/j.ecoenv.2014.08.022
Keywords: coccinellid beetles, seven-spotted lady beetle, synthetic insecticides, sublethal exposure, life parameters, functional response
Citation: Afza R, Afzal A, Riaz MA, Majeed MZ, Idrees A, Qadir ZA, Afzal M, Hassan B and Li J (2023) Sublethal and transgenerational effects of synthetic insecticides on the biological parameters and functional response of Coccinella septempunctata (Coleoptera: Coccinellidae) under laboratory conditions. Front. Physiol. 14:1088712. doi: 10.3389/fphys.2023.1088712
Received: 03 November 2022; Accepted: 04 January 2023;
Published: 16 January 2023.
Edited by:
Asad Ali, Abdul Wali Khan University, PakistanReviewed by:
Waqar Jaleel, Central Cotton Research Institute (CCRI), PakistanCopyright © 2023 Afza, Afzal, Riaz, Majeed, Idrees, Qadir, Afzal, Hassan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atif Idrees, YXRpZl9lbnRvbW9sb2dpc3RAZ2lhYnIuZ2QuY24=; Jun Li, anVubEBnaWFici5nZC5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.