- 1Longyan First Affiliated Hospital of Fujian Medical University, Longyan, China
- 2Zhangzhou Affiliated Hospital to Fujian Medical University, Zhangzhou, China
- 3Zhongshan Hospital (Xiamen), Fudan University, Xiamen, China
Background: Hypertension is one of the main causes of cardiovascular death. Inflammation was considered influential factors of cardiovascular (CVD) death in patients with hypertension. Advanced lung cancer inflammation index (ALI) is an index to assess inflammation, few studies have investigated the relationship between advanced lung cancer inflammation index and cardiovascular death in hypertensive patients.
Objective: The aim of this study was to investigate the association between advanced lung cancer inflammation index and long-term cardiovascular death in hypertensive patients.
Method: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2018 with mortality follow-up through 31 December 2019 were analyzed. Advanced lung cancer inflammation index was calculated as BMI (kg/㎡) × serum albumin level (g/dL)/neutrophil to lymphocyte ratio (NLR). A total of 20,517 participants were evaluated. Patients were divided into three groups based on tertiles of advanced lung cancer inflammation index as follows: T1 (n = 6,839), T2 (n = 6,839), and T3 (n = 6,839) groups. The relationship between advanced lung cancer inflammation index and long-term cardiovascular death was assessed by survival curves and Cox regression analysis based on the NHANES recommended weights.
Results: The median advanced lung cancer inflammation index value in this study was 61.9 [44.4, 84.6]. After full adjustment, the T2 group (hazard ratio [HR]: 0.59, 95% confidence interval [CI]: 0.50–0.69; p < 0.001) and T3 group (HR: 0.48, 95% CI: 0.39–0.58; p < 0.001) were found to have a significantly lower risk of cardiovascular death compared to the T1 group.
Conclusion: High levels of advanced lung cancer inflammation index were associated with reduced risk of cardiovascular death in hypertensive patients.
Introduction
Hypertension is a chronic disease that can be effectively intervened, and it is one of the primary causes of death from cardiovascular disease (CVD) (Shin et al., 2019). In fact, approximately half of all CVD deaths were related to hypertension (Kearney et al., 2005; Lim et al., 2012). Despite significant advancements in hypertension treatment and management, its impact on CVD mortality continues to rise worldwide. (Mills et al., 2016; Das, 2017).
Past research has revealed that inflammation plays a crucial role in the onset and progression of hypertension. Chronic inflammation can significantly elevate the risk of death from CVD. (Virdis et al., 2014; Boos et al., 2021). The majority of present-day studies that employ inflammatory markers to evaluate the prognosis of hypertension only utilize individual inflammatory markers (Engström et al., 2006; Cortez et al., 2016; Sun et al., 2017). Nevertheless, inflammation can result in decreased albumin and weight loss (Lennie, 1998; Sheinenzon et al., 2021). and relying on a single inflammatory marker may not provide sufficient precision to evaluate the prognosis of patients with hypertension.
The advanced lung cancer inflammation index, an index that combines body weight, albumin and neutrophil to lymphocyte ratio (NLR), was originally used to assess systemic inflammation levels in cancer patients (Jafri et al., 2013). In previous studies, ALI showed good efficacy in assessing inflammatory status in coronary artery disease and heart failure patients, and was associated with prognosis in these patients (Maeda et al., 2020; Fan et al., 2021). Given that hypertension was believed to have a connection with inflammation, we utilized the ALI to evaluate the inflammatory status among patients with hypertension, and investigated its correlation with death from CVD in hypertensive patients.
The purpose of this study was to investigate the relationship between ALI and the risk of CVD death in patients with hypertension and to provide some reference for the treatment and management of hypertensive patients.
Materials and methods
Study population
NHANES is a nationally representative cross-sectional survey recursively conducted in the United States by the National Center for Health Statistics. NHANES is based on a stratified multistage random sampling design. A retrospective analysis was performed using publicly available data from NHANES from 1999 to 2018.
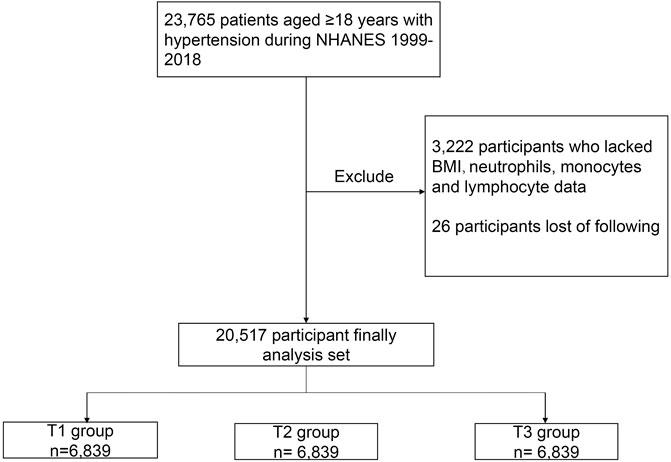
In NHANES 1999–2018, our analysis was limited to 23,765 participants aged 18 years and older with hypertension. Hypertension was defined as an affirmative answer by participants to the question “Have you ever been told by a doctor or other health professional that you have hypertension, also called high blood pressure?” In addition, participants with systolic blood pressure (SBP) ≥140 mmHg or/and diastolic blood pressure (DBP) ≥90 mmHg were defined as having hypertension. If the participants had their blood pressure measured more than one time, their average blood pressure was used to determine whether the patient had hypertension (Unger et al., 2020). In addition, participants who were receiving antihypertensive medications are also considered to have hypertension. Of these participants, 3,222 people who lacked body mass index (BMI), albumin, neutrophil, and lymphocyte data were excluded. In addition, 26 participants who were lost follow-up were excluded. Ultimately, a total of 20,517 participants were included in this cohort study (Figure 1).
Calculation of ALI
ALI was calculated as BMI (kg/m2) × serum albumin level (g/dL)/neutrophil-to-lymphocyte ratio (NLR). Patients were divided into three groups based on the tertiles of ALI: T1 group (≤50.0), T2 group (>50.0 and ≤77.0), and T3 group (>77.0).
Primary outcome
The primary outcome was CVD death. Cause of death was categorized using the International Classification of Diseases 10th edition (ICD-10). Cardiovascular mortality was categorized using ICD-10 codes I00–I078. For participants in NHANES 1999–2018, mortality follow-up data was available through 31 December2019.
Definitions of variables of interest
Age, sex, race, smoking status and drink status were self-reported by participants. Participants who were currently taking calcium channel blockers (CCB), diuretics, beta blockers, and angiotensin converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARB) were considered to be taking antihypertensive drugs. Laboratory measurements, such as creatinine (Cr), triglyceride (TG), total cholesterol (TC), blood glucose (Glu), albumin, neutrophil counts, and lymphocyte counts, were collected using automated hematological analysis equipment. Detailed procedures for obtaining laboratory measurements were provided in a document on the website of the National Center for Health Statistics. The Healthy Eating Index (HEI-2015) was calculated based on the patient’s total nutrient intake on the first day. Diagnosis of comorbidities was based on an affirmative response to the question “Has a doctor or other health professional ever told you that you had chronic heart failure (CHF), chronic heart disease (CHD), diabetes mellitus (DM), stroke, or cancer?”
Statistical analyses
We used the NHANES recommended weights to calculate the weights for specific groups. Continuous variables were expressed as the mean ± standard deviation. Variables that do not conform to the normal distribution are represented by the median (25th percentile, 75th percentile). Categorical variables were presented as counts (percentages). Baseline characteristics between the three groups were compared using an analysis of variance (ANOVA) for continuous variables and an χ2 test for categorical variables.
To evaluate the association between ALI and long-term CVD death, we used Kaplan-Meier and Cox regression analyses. Both estimates and probabilities were based on weights recommended by NHANES. Model 1 was a crude model unadjusted for potential confounders. Model 2 was adjusted for age and sex. Model 3 was fully adjusted for potential confounders. Furthermore, we explored the relationship between ALI and CVD mortality in different subgroups including age, sex, BMI, antihypertensive drug and DM. Restricted regression cubic splines were used to explore the potential non-linear relationship between ALI and CVD death in hypertensive patients. COX regression analysis was performed on the variables required for ALI calculation.
All data analyses were performed by using the Survey package in R software (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value <0.05 indicated significance for all analyses.
Results
Participant characteristics
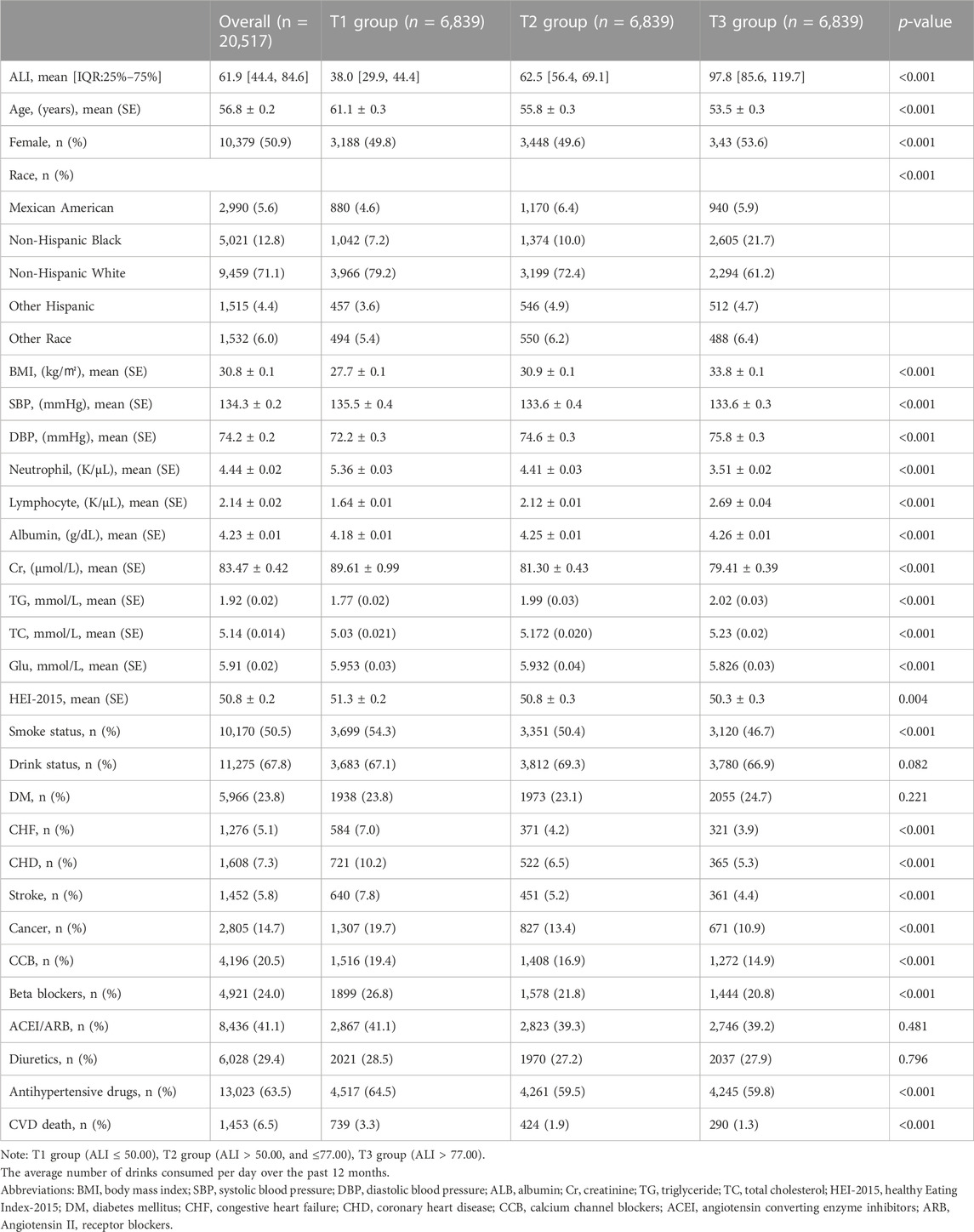
Among all 20,517 participants eligible for the study, the average age was 54.8 ± 0.2 years. The distribution of ALI is shown in Supplementary Figure S1. Approximately half (n = 10,379, 50.9%) were female. Patients were divided into three groups based on the tertiles of ALI: T1 group (n = 6,839), T2 group (n = 6,839), and T3 group (n = 6,839). The median ALI of the T2 [62.5 (IQR:56.4–69.1)] and T3 [97.8 (IQR:85.6–119.7)] groups was found to be higher than that of the T1 group [38.0 (IQR:29.9–44.4)]. Participants in the group with higher ALI were younger (T1 group: 61.1 ± 0.3 vs. T2 group: 55.8 ± 0.3 vs. T3 group: 53.5 ± 0.3 years) and had higher BMI (T1 group: 27.7 ± 0.1 vs. T2 group: 30.9 ± 0.1 vs. T3 group: 33.8 ± 0.1 kg/m2) and were more likely to be female (T1 group: 49.8% vs. T2 group: 49.6% vs. T3 group: 53.6%). In the group with higher ALI, participants have lower Cr (T1 group: 89.61 ± 0.99 vs. T2 group: 81.30 ± 0.43 vs. T3 group: 79.41 ± 0.39 μmol/L) and Healthy Eating Index–2015 (HEI-2015) (T1 group: 51.3 ± 0.2 vs. T2 group: 50.8 ± 0.3 vs. T3 group: 50.3 ± 0.3). With increased ALI, the proportion of smokers gradually deceased (T1 group: 54.3% vs. T2 group: 50.4% vs. T3 group: 46.7%) and was less likely to be combined with stroke (T1 group: 7.8% vs. T2 group: 5.2% vs. T3 group: 4.4%), CHD (T1 group: 10.2% vs. T2 group: 6.5% vs. T3 group: 5.3%), CHF (T1 group: 7.0% vs. T2 group: 4.2% vs. T3 group: 3.9%) and cancer (T1 group: 19.7% vs. T2 group: 13.4% vs. T3 group: 10.9%). There was no statistical difference in DM among the three groups. In addition, the group with higher ALI levels had lower rates of CCB (T1 group: 19.4% vs. T2 group: 16.9% vs. T3 group: 14.9%) and β-block use (T1 group: 26.8% vs. T2 group: 21.8% vs. T3 group: 20.8%). There was no statistical difference in the number of people using ACEI/ARB and diuretics. More data on the baseline characteristics of study population are detailed in Table 1.
ALI and CVD mortality
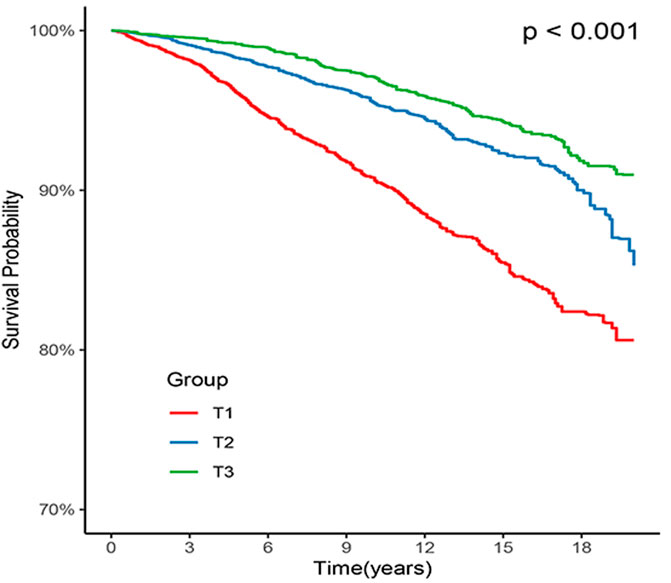
Out of all the participants, 1,453 (6.5%) individuals died due to CVD, with 739 (3.3%) in the T1 group, 424 (1.9%) in the T2 group, and 290 (1.3%) in the T3 group. Kaplan-Meier survival analysis curves revealed that the groups with higher ALI had lower mortality rates from CVD (P-log rank <0.001, Figure 2). The results of univariate Cox proportional hazard analysis showed that the risk of death from CVD decreased by 16% for each 10 unit increase in ALI (95% confidence interval (CI): 0.81–0.87; p < 0.001). In comparison to the T1 group, both the T2 (hazard ratio (HR): 0.47, 95% CI: 0.41–0.53; p < 0.001) and T3 (HR: 0.32, 95% CI: 0.27–0.38; p < 0.001) groups had a lower risk of death from CVD. After adjusting for potential confounders including age, sex, ethnicity, smoking, drinking, Cr, TG, TC, Glu, CHF, CHD, DM, stroke, antihypertensive drugs, cancer, HEI-2015, DBP, and SBP, the risk of CVD death decreased by 11% (95% CI: 0.87–0.92; p < 0.001) for each 10 unit increase in ALI. The T2 (HR: 0.59, 95% CI: 0.50–0.69; p < 0.001) and T3 (HR: 0.48, 95% CI: 0.39–0.58; p < 0.001) groups had a lower risk of CVD death compared to the T1 group (Table 2).
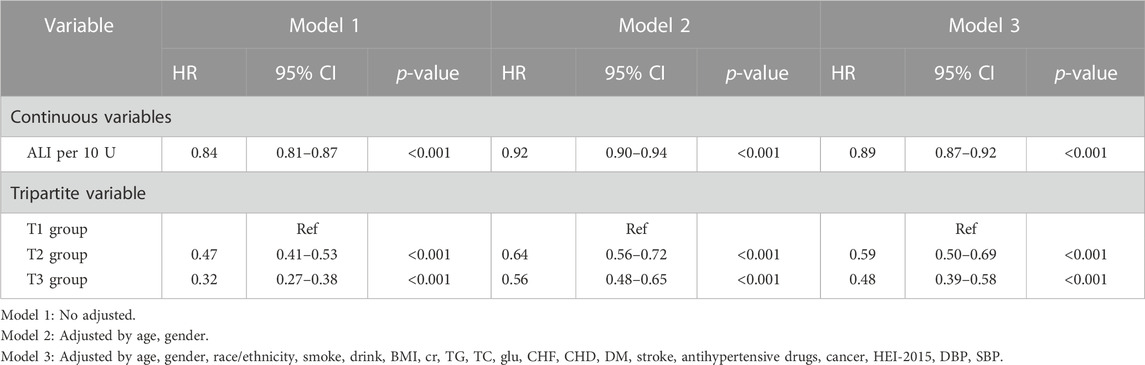
TABLE 2. Associations between ALI and cardiovascular mortality in NHANES 1999–2018 followed through 2019.
Restricted regression cubic spline
The results of the restricted RCS analysis indicated no non-linear relationship between ALI and CVD death in hypertensive patients, and low levels of ALI were associated with an increased risk of CVD death in this population. Stratification by BMI did not significantly alter the results. (Figure 3).
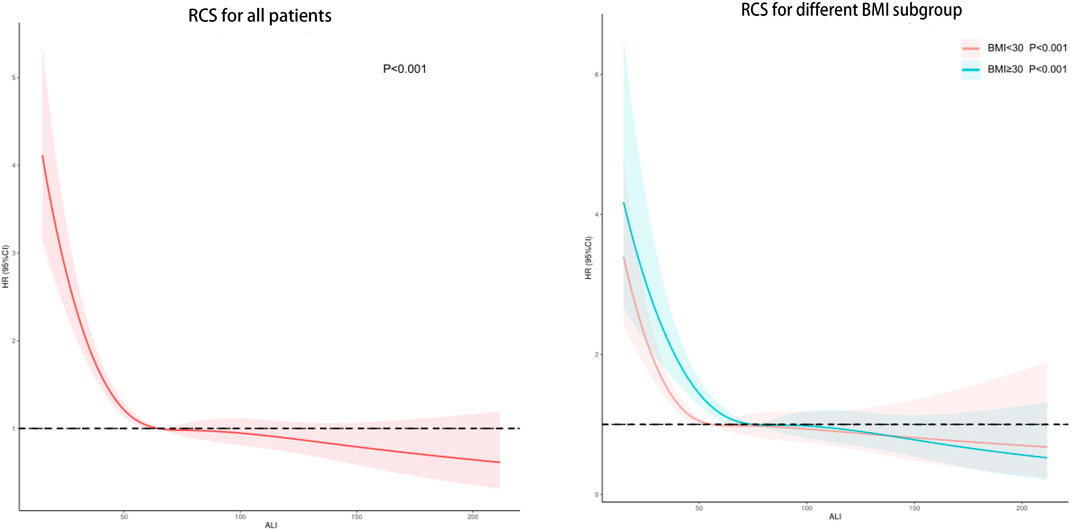
FIGURE 3. Potential nonlinear relationship between ALI and cardiovascular death in hypertensive patients (weighted).
Subgroup analysis
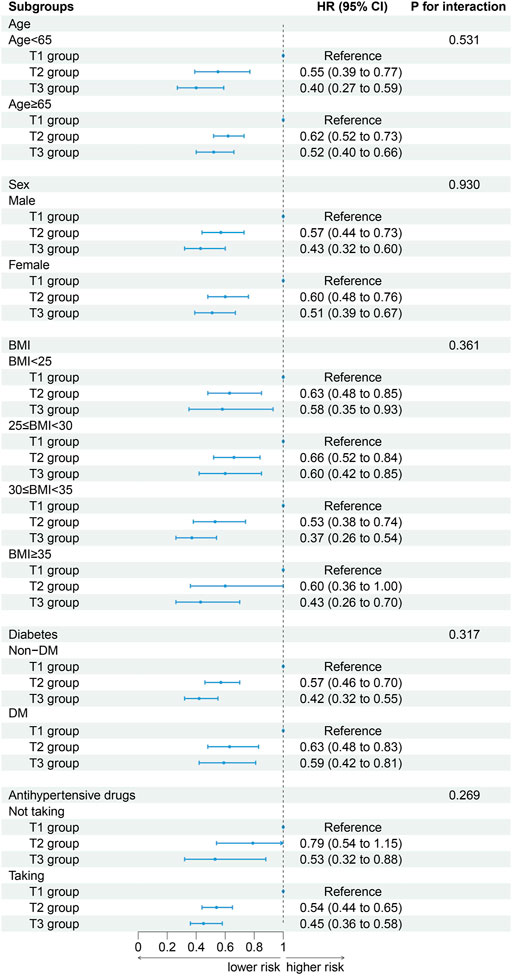
When participants were stratified by age (P for interaction = 0.531), sex (P for interaction = 0.930), BMI (P for interaction = 0.361), DM (P for interaction = 0.317) and antihypertensive drugs (P for interaction = 0.269) the association between ALI and CVD mortality did not change. With the increased of ALI, the risk of CVD death decreased. The results of a stratified analysis by drug were consistent with the main effect (Figure 4).
Supplementary analysis
Among the items required for ALI calculations, alb and NLR were associated with the risk of CVD death in hypertensive patients after full adjustment for confounding variables (Supplementary Table S1). After grouping by BMI quintile, groups Q2, Q3, and Q4 had a lower risk of CVD death compared to group Q1. The Q5 group was not statistically significant (Supplementary Table S2). In addition, the results of a subgroup analysis of ALI showed that elevated ALI was associated with a reduced risk of CVD death in hypertensive patients in the subgroup with ALI≤60. p values were not statistically significant in the subgroup with ALI>60, despite a downward trend in CVD death risk (Supplementary Table S3).
Discussion
This cohort study conducted in the United States utilized ALI to evaluate the inflammatory status of hypertensive patients. The findings indicated that patients with higher ALI had a decreased risk of CVD death, even after controlling for various confounding factors. This association remained consistent across different age groups, genders, BMI categories, and DM status. These results suggest that lower levels of inflammation were associated with a lower risk of CVD death in individuals with hypertension.
Hypertension was considered to be disease associated with inflammation (Steven et al., 2019). The chronic, low-grade inflammatory state in hypertensive patients can contribute to vascular remodeling, leading to vascular fibrosis and an increased risk of CVD-related mortality. (Intengan and Schiffrin, 2001). In a prospective study, hypertension patients with higher levels of inflammation had a 2-fold increased risk of all-cause death and a 1.8-fold increased risk of CVD (Cortez et al., 2016). The findings of these studies all suggest that inflammation was detrimental prognostic factors in patients with hypertension, which was in line with our results. However, it was worth noting that prior research has typically relied on a single inflammatory marker to evaluate the prognosis of hypertensive patients (Engström et al., 2006; Cortez et al., 2016; Sun et al., 2017). As mentioned in the introduction, inflammation can accelerate protein breakdown, resulting in a reduction in albumin levels. Moreover, inflammation can also induce insulin resistance, diminish appetite, and hinder the absorption of nutrients, ultimately leading to weight loss (Yoon et al., 2016; Guescini et al., 2017; Merker et al., 2020; Aldhwayan et al., 2022; Dou et al., 2022). Hence, relying solely on a single inflammatory marker to evaluate the mortality risk in patients with hypertension may not provide sufficiently accurate results.
ALI was calculated as BMI (kg/m2) × serum albumin level (g/dL)/NLR. Due to the fact that inflammation often results in hypoproteinemia and decreased BMI, previous studies have combined these two parameters with inflammatory markers to comprehensively evaluate the systemic levels of inflammation in cancer patients. It has been reported that lung cancer patients with higher ALI have a reduced risk of death (Jafri et al., 2013). In non-cancer populations, the effectiveness of ALI has also been demonstrated. Several studies have shown that low ALI is associated with increased risk of coronary artery calcification, readmissions and death in patients with heart failure (Maeda et al., 2020; Fan et al., 2021; Yuan et al., 2022). However, few studies have utilized ALI to evaluate the risk of CVD death in patients with hypertension. Our findings demonstrate that higher ALI levels are associated with a reduced risk of CVD death in patients with hypertension. Moreover, the results remained consistent when stratified by age, sex, BMI, antihypertensive drug use, and DM.
As high BMI is a well-established risk factor for CVD mortality, it is crucial to account for this factor when assessing the link between ALI levels and CVD mortality in hypertensive patients (Li et al., 2020). Therefore, we adjusted for BMI and utilized RCS to examine the possible non-linear relationship between ALI and hypertension. After controlling for BMI, we found that higher levels of ALI remained associated with a lower risk of CVD mortality in hypertensive patients. While elevated ALI levels may correspond to higher BMI levels, our RCS analysis did not reveal any U-shaped relationship. Although the regression analysis indicated that an elevated BMI was linked to a higher risk of CVD mortality when treated as a continuous variable, this association was not significant (p = 0.091). On the other hand, our findings revealed that hypertensive patients with a BMI ranging from 24.9 to 35.5 kg/㎡ had a lower risk of CVD mortality compared to those with a BMI ≤24.9 kg/㎡. Consequently, the results of the regression analysis of BMI as a continuous variable may have been influenced by the severely obese population (BMI >35.5 kg/㎡), leading to inconsistent findings. Similar results have been reported in previous studies. For instance, a retrospective study showed that low BMI was linked to an increased 3-year risk of all-cause mortality in hypertensive patients, while obesity was related to a reduced risk of all-cause mortality (Kim et al., 2022). In Zhu et al. (2022) ‘s study, being underweight was associated with higher mortality in people with high blood pressure, while being overweight was associated with lower mortality. In addition, the study of Zhou et al. (2021) also confirmed that low BMI was an independent risk factor for death in patients with hypertension, while high BMI was not Obese individuals may have greater metabolic reserves to cope with inflammation and metabolic demands (Doehner et al., 2010). In addition, systemic vascular resistance and plasma renin activity were lower in hypertensive patients with higher BMI compared with those with lower BMI, which can improve the prognosis of hypertensive patients (Lavie et al., 2007). Therefore, although high BMI is a recognized risk factor for CVD death, ALI can still be used as a biomarker for prognosis in hypertensive patients with BMI≤35.5kg/㎡.
In the supplementary analysis, it was found that increased levels of ALI were associated with a decreased risk of CVD mortality only in the subgroup with ALI ≤60, which is in line with the RCS findings. This could be attributed to the fact that high ALI may be linked to high BMI, as previously mentioned. The accuracy of ALI may be significantly impacted when BMI >35.5 kg/㎡, indicating that ALI might not be suitable for individuals with severe obesity.
Previous studies have suggested that low ALI is associated with a higher risk of death and may require early intervention (Jafri et al., 2013; Maeda et al., 2020). increasing the intake of nutraceuticals and fruits has been shown to be associated with lower levels of inflammation (Scicchitano et al., 2014; Maaliki et al., 2019; Zuraini et al., 2021). As this was a retrospective cohort study, we were unable to confirm whether the use of nutritional supplements and increased fruit intake could improve the risk of CVD death in hypertensive patients. Therefore, these are only hypotheses for the treatment and management of hypertension, and more clinical trials are needed to confirm their effectiveness.
This cohort study, which included 20,517 individuals, has a large sample size that lends reliability to our findings. Our results indicate that hypertensive patients with high ALI levels have a lower risk of CVD death. As a simple and easily calculated index, ALI may provide a more comprehensive assessment of the risk of CVD death in hypertensive patients than a single inflammatory parameter. Early intervention in hypertensive patients with low ALI levels may potentially have a positive effect in reducing the risk of CVD death. However, further experimental studies are needed to confirm this hypothesis.
Limitations
There are some limitations in our study. First, the diagnosis of hypertension and comorbidities was mostly based on self-reported information, which may have introduced recall bias. Second, the use of a single blood draw may not have provided a complete picture of a patient’s physical state, which could change over long-term follow-up. Finally, our study design as a cohort study means that the results should be interpreted as correlational rather than causal, given the possibility of unmeasured confounding factors (Reddy et al., 2018). Therefore, further clinical trials are necessary to confirm our findings.
Conclusion
ALI was an effective indicator of systemic inflammation in hypertensive patients. High levels of ALI were associated with a reduced risk of CVD death in hypertensive patients.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
LC designed the research and is the guarantor of this work. LC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JT performed the analyses and wrote the first draft of the paper. BW, JX, JD, SL, JL, YY, PY, JZ, KC, SD, and LC revised the manuscript. All authors read and approved the final manuscript and its’ submission.
Funding
This research was funded and supported by the Longyan City Science and Technology Plan Project (2015LY33), and the Startup Fund for Scientific Research by Fujian Medical University (2019QH1205).
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2023.1074672/full#supplementary-material
References
Aldhwayan, M. M., Al-Najim, W., Ruban, A., Glaysher, M. A., Johnson, B., Chhina, N., et al. (2022). Does bypass of the proximal small intestine impact food intake, preference, and taste function in humans? An experimental medicine study using the duodenal-jejunal bypass liner. Nutrients 14 (10), 2141. doi:10.3390/nu14102141
Boos, C. J., Toon, L-T., and Almahdi, H. (2021). The relationship between ambulatory arterial stiffness, inflammation, blood pressure dipping and cardiovascular outcomes. BMC Cardiovasc Disord. 21 (1), 139. doi:10.1186/s12872-021-01946-2
Cortez, A. F., Muxfeldt, E. S., Cardoso, C. R. L., and Salles, G. F. (2016). Prognostic value of C-reactive protein in resistant hypertension. Am. J. Hypertens. 29 (8), 992–1000. doi:10.1093/ajh/hpw011
Das, U. N. (2017). Is there a role for bioactive lipids in the pathobiology of diabetes mellitus? Front. Endocrinol. (Lausanne) 8, 182. doi:10.3389/fendo.2017.00182
Doehner, W., Clark, A., and Anker, S. D. (2010). The obesity paradox: Weighing the benefit. Eur. Heart J. 31 (2), 146–148. doi:10.1093/eurheartj/ehp339
Dou, L., Shi, M., Song, J., Niu, X., Niu, J., Wei, S., et al. (2022). The prognostic significance of C-reactive protein to albumin ratio in newly diagnosed acute myeloid leukaemia patients. Cancer Manag. Res. 14, 303–316. doi:10.2147/CMAR.S343580
Engström, G., Hedblad, B., Janzon, L., and Lindgärde, F. (2006). Fatality of acute coronary events in relation to hypertension and low-grade inflammation: A population-based cohort study. J. Hum. Hypertens. 20 (8), 581–586. doi:10.1038/sj.jhh.1002037
Fan, W., Zhang, Y., Liu, Y., Ding, Z., Si, Y., Shi, F., et al. (2021). Nomograms based on the advanced lung cancer inflammation index for the prediction of coronary artery disease and calcification. Clin. Appl. Thromb. Hemost. 27, 10760296211060455. doi:10.1177/10760296211060455
Guescini, M., Tiano, L., Genova, M. L., Polidori, E., Silvestri, S., Orlando, P., et al. (2017). The combination of physical exercise with muscle-directed antioxidants to counteract sarcopenia: A biomedical rationale for pleiotropic treatment with creatine and coenzyme Q10. Oxid. Med. Cell Longev. 2017, 7083049. doi:10.1155/2017/7083049
Intengan, H. D., and Schiffrin, E. L. (2001). Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension 38, 581–587. doi:10.1161/hy09t1.096249
Jafri, S. H., Shi, R., and Mills, G. (2013). Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 13, 158. doi:10.1186/1471-2407-13-158
Kearney, P. M., Whelton, M., Reynolds, K., Muntner, P., Whelton, P. K., and He, J. (2005). Global burden of hypertension: Analysis of worldwide data. Lancet 365 (9455), 217–223. doi:10.1016/S0140-6736(05)17741-1
Kim, H-J., Kim, B. S., Lee, J. H., and Shin, J-H. (2022). Impact of underweight on 3-year all-cause mortality in patients with acute severe hypertension: A retrospective cohort study. Sci. Rep. 12 (1), 4798. doi:10.1038/s41598-022-08892-9
Lavie, C. J., Milani, R. V., and Ventura, H. O. (2007). Obesity, heart disease, and favorable prognosis-truth or paradox? Am. J. Med. 120 (10), 825–826. doi:10.1016/j.amjmed.2007.06.023
Lennie, T. A. (1998). Relationship of body energy status to inflammation-induced anorexia and weight loss. Physiol. Behav. 64 (4), 475–481. doi:10.1016/s0031-9384(98)00103-6
Li, M-H., Hu, L-H., Xiong, Y-R., Yu, Y., Zhou, W., Wang, T., et al. (2020). Association between body mass index and the risk of bleeding in elderly patients with non-valvular atrial fibrillation taking dabigatran: A cohort study. J. Geriatr. Cardiol. 17 (4), 193–201. doi:10.11909/j.issn.1671-5411.2020.04.008
Lim, S. S., Vos, T., Flaxman, A. D., Danaei, G., Shibuya, K., Adair-Rohani, H., et al. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet 380 (9859), 2224–2260. doi:10.1016/S0140-6736(12)61766-8
Maaliki, D., Shaito, A. A., Pintus, G., El-Yazbi, A., and Eid, A. H. (2019). Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 45, 57–65. doi:10.1016/j.coph.2019.04.014
Maeda, D., Kanzaki, Y., Sakane, K., Ito, T., Sohmiya, K., and Hoshiga, M. (2020). Prognostic impact of a novel index of nutrition and inflammation for patients with acute decompensated heart failure. Heart Vessels 35 (9), 1201–1208. doi:10.1007/s00380-020-01590-4
Merker, M., Felder, M., Gueissaz, L., Bolliger, R., Tribolet, P., Kägi-Braun, N., et al. (2020). Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: A secondary analysis of a randomized clinical trial. JAMA Netw. Open 3 (3), e200663. doi:10.1001/jamanetworkopen.2020.0663
Mills, K. T., Bundy, J. D., Kelly, T. N., Reed, J. E., Kearney, P. M., Reynolds, K., et al. (2016). Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation 134 (6), 441–450. doi:10.1161/CIRCULATIONAHA.115.018912
Reddy, S. M., Barcenas, C. H., Sinha, A. K., Hsu, L., Moulder, S. L., Tripathy, D., et al. (2018). Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. Br. J. Cancer 118 (1), 17–23. doi:10.1038/bjc.2017.379
Scicchitano, P., Cameli, M., Maiello, M., Modesti, P. A., Muiesan, M. L., Novo, S., et al. (2014). Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J. Funct. Foods 6, 11–32. doi:10.1016/j.jff.2013.12.006
Sheinenzon, A., Shehadeh, M., Michelis, R., Shaoul, E., and Ronen, O. (2021). Serum albumin levels and inflammation. Int. J. Biol. Macromol. 184, 857–862. doi:10.1016/j.ijbiomac.2021.06.140
Shin, J., Lee, H. Y., Chung, W. J., Youn, H. J., Cho, E. J., Sung, K. C., et al. (2019). The association of smoothness index of central blood pressure with ambulatory carotid femoral pulse wave velocity after 20-week treatment with losartan in combination with amlodipine versus hydrochlorothiazide. J. Hypertens. 37 (12), 2490–2497. doi:10.1097/HJH.0000000000002202
Steven, S., Frenis, K., Oelze, M., Kalinovic, S., Kuntic, M., Bayo Jimenez, M. T., et al. (2019). Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell Longev. 2019, 7092151. doi:10.1155/2019/7092151
Sun, X., Luo, L., Zhao, X., Ye, P., and Du, R. (2017). The neutrophil-to-lymphocyte ratio on admission is a good predictor for all-cause mortality in hypertensive patients over 80 years of age. BMC Cardiovasc Disord. 17 (1), 167. doi:10.1186/s12872-017-0595-1
Unger, T., Borghi, C., Charchar, F., Khan, N. A., Poulter, N. R., Prabhakaran, D., et al. (2020). 2020 international society of hypertension global hypertension practice guidelines. Hypertension 75, 1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
Virdis, A., Dell'Agnello, U., and Taddei, S. (2014). Impact of inflammation on vascular disease in hypertension. Maturitas 78 (3), 179–183. doi:10.1016/j.maturitas.2014.04.012
Yoon, C-Y., Park, J. T., Kee, Y. K., Han, S. G., Han, I. M., Kwon, Y. E., et al. (2016). Low mitochondrial DNA copy number is associated with adverse clinical outcomes in peritoneal dialysis patients. Med. Baltim. 95 (7), e2717. doi:10.1097/MD.0000000000002717
Yuan, X., Huang, B., Wang, R., Tie, H., and Luo, S. (2022). The prognostic value of advanced lung cancer inflammation index (ALI) in elderly patients with heart failure. Front. Cardiovasc Med. 9, 934551. doi:10.3389/fcvm.2022.934551
Zhou, Q., Liu, X., Zhao, Y., Qin, P., Ren, Y., Liu, D., et al. (2021). BMI and risk of all-cause mortality in normotensive and hypertensive adults: The rural Chinese cohort study. Public Health Nutr. 24 (17), 5805–5814. doi:10.1017/S1368980021001592
Zhu, J., Liu, X., Zhang, J., Li, J., Chen, L., Huang, C., et al. (2022). Time-varying association between body mass index and all-cause mortality in patients with hypertension. Int. J. Obes. (Lond) 46 (2), 316–324. doi:10.1038/s41366-021-00994-0
Zuraini, N. Z. A., Sekar, M., Wu, Y. S., Gan, S. H., Bonam, S. R., Mat Rani, N. N. I., et al. (2021). Promising nutritional fruits against cardiovascular diseases: An overview of experimental evidence and understanding their mechanisms of action. Vasc. Health Risk Manag. 17, 739–769. doi:10.2147/VHRM.S328096
Keywords: hypertension, inflammation, cardiovascular death, advanced lung cancer inflammation index, NHANES
Citation: Tu J, Wu B, Xiu J, Deng J, Lin S, Lu J, Yan Y, Yu P, Zhu J, Chen K, Ding S and Chen L (2023) Advanced lung cancer inflammation index is associated with long-term cardiovascular death in hypertensive patients: national health and nutrition examination study, 1999–2018. Front. Physiol. 14:1074672. doi: 10.3389/fphys.2023.1074672
Received: 19 October 2022; Accepted: 17 April 2023;
Published: 03 May 2023.
Edited by:
Sebhat Erqou, Brown University, United StatesReviewed by:
Pietro Scicchitano, ASLBari—Azienda Sanitaria Localedella provincia di Bari (ASL BA), ItalyHanping Shi, Capital Medical University, China
Copyright © 2023 Tu, Wu, Xiu, Deng, Lin, Lu, Yan, Yu, Zhu, Chen, Ding and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaihong Chen, Y2hlbmthaWhvbmcxOTY0QDE2My5jb20=; Shan Ding, MTU5OTUyNDM2MUBxcS5jb20=; Liling Chen, Y2hlbmxpbGluZzE5NzkwMjA2QDE2My5jb20=
†These authors have contributed equally to this work
 Jiabin Tu
Jiabin Tu Bo Wu
Bo Wu Jiaming Xiu
Jiaming Xiu Jiayi Deng
Jiayi Deng Shuqiong Lin2
Shuqiong Lin2 Jin Lu
Jin Lu Yanfang Yan
Yanfang Yan Pei Yu
Pei Yu Shan Ding
Shan Ding Liling Chen
Liling Chen