
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol., 14 December 2022
Sec. Vascular Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.995353
This article is part of the Research TopicVascular Involvement in Eye DiseasesView all 15 articles
Purpose: The aim of the study was to investigate microcirculation changes in the macula evaluated by optical coherence tomography angiography (OCTA)in patients receiving anatomical repair after surgery for rhegmatogenous retinal detachment (RRD).
Methods: A literature search was conducted in PubMed, EMBASE, Web of Science and the Cochrane Library. Studies including patients with macula-on or macula-off RRD and repaired successfully through primary surgery were selected. Foveal avascular zone (FAZ) area and macular vascular density (VD) in both the superficial capillary plexus (SCP) and deep capillary plexus (DCP) were analyzed using RevMan 5.4 software.
Results: Twelve studies including 430 RRD eyes and 430 control eyes were selected. In eyes with macula-on RRD, FAZ area, VD in the foveal SCP and DCP, and VD in the parafoveal SCP and DCP were not altered compared with control eyes, after the retina was reattached. In eyes with macula-off RRD that was repaired successfully through surgery, FAZ area in the DCP (0.13 mm2, 95% CI: 0.02 to 0.25, p = 0.02) remained enlarged compared with control eyes. Meanwhile, VD in the foveal DCP was also significantly reduced (−3.12%, 95% CI: −6.15 to −0.09%, p = 0.04), even though retinal reattachment was achieved by surgery in eyes with macula-off RRD.
Conclusion: In patients with macula-off rhegmatogenous retinal detachment, foveal avascular zone area in the deep capillary plexuses was enlarged and vascular density in the foveal deep capillary plexus was reduced, even after the retina was successfully reattached through a primary surgery.
Rhegmatogenous retinal detachment is a vision-threatening disease that affects visual activity in people of working age. Although it has been reported that the retina is reattached successfully after surgery in approximately 90% of patients (Shlomit et al., 2011), the recovery of visual function is limited, especially in patients with macula-off retinal detachment. The limitation of visual recovery has been demonstrated to be related with microstructural changes of the retina in macular region, such as the disruption of ellipsoid zone and reduction of photoreceptor outer segment length (Schocket et al., 2006; Shimoda et al., 2010; Park et al., 2018).
In recent years, optical coherence tomography angiography (OCTA) has been utilized to investigate detailed changes in microcirculation in the macular area and peripapillary area in various diseases, such as diabetic retinopathy, macular degeneration and glaucoma (Kuehlewein et al., 2015; Yarmohammadi et al., 2016; Greig et al., 2020; Zhang et al., 2020). In patients with diabetic retinopathy, an increased foveal avascular zone (FAZ) area, intraretinal microvascular abnormalities, and a decrease in vascular density (VD) in macular and peripapillary areas, as detected via OCTA, have been found correlated with progression of diabetic retinopathy and diabetic macular edema (Sun et al., 2019; Greig et al., 2020).
In patients with macula-off rhegmatogenous retinal detachment, FAZ area and VD in the macular area evaluated by OCTA have also been investigated. Several studies have reported that FAZ area and VD in the macular area remain different from those of healthy fellow eyes after the retina was successfully reattached through surgery (Woo et al., 2018; Agarwal et al., 2019; Tsen et al., 2019). Enlarged FAZ and decreased VD have also been showed to be associated with postoperative visual activity in eyes with successful anatomical repair (Woo et al., 2018; McKay et al., 2020; Ng et al., 2020). However, in other studies, VD in the macular area of eyes receiving surgery for retinal detachment has not changed significantly (Wang et al., 2019).
This study was to investigate and meta-analyze macular microcirculation changes assessed with OCTA after successful repair for rhegmatogenous retinal detachment with or without macular involvement.
This meta-analysis was conducted in accordance with the guidelines presented by the Meta-Analysis of Observational Studies statements (Stroup et al., 2000).
The databases PubMed, EMBASE, Web of Science and the Cochrane Library were searched using the terms “retinal detachment” and “Optical Coherence Tomography Angiography”, “OCT Angiography” or “OCTA” up to 07 July 2022. The detailed search query for PubMed was showed in Supplementary Table S1. Language was restricted to English.
Studies included should meet all of the following inclusion criteria: 1) the affected eyes had primary rhegmatogenous retinal detachment; 2) the contralateral healthy eyes or eyes of healthy people were used as controls; 3) the retina was successfully reattached after single surgery; and 4) optical coherence tomography angiography was applied to evaluate macular microcirculation. Exclusion criteria were as follows: 1) the affected eyes had any preexisting macular impairing disease, such as diabetic retinopathy, retinal vein occlusion, macular degeneration, uveitis and high myopia (axial length>26 mm); 2) the affected eyes had previous retinal surgery; 3) retinal detachment was complicated by macular hole, epi-macular membrane, submacular membrane/subfoveal fibrous band, macular scar or choroidal detachment; 4) retina was reattached after two or more surgeries; 5) internal limiting membrane was peeled in the surgery; 6) eyes with silicone oil tamponade were included and data of eyes with gas or air tamponade could not be extracted for analysis; 7) the affected eyes had any of postoperative complications including epi-macular membrane, macular edema, macular cyst and submacular fluid; 8) normal control eyes were not included; 9) children were included. The search results were reviewed by two investigators.
Data from each selected study were extracted by two investigators, including first author, year of publication, location, study design, number and mean age of patients, gender, time between initial symptoms and surgery, type of surgery, time of OCTA measurement, type of OCTA device, FAZ area, VD in the foveal area, and VD in the parafoveal area. If OCTA measurements were taken at different times after surgery, data containing sufficient information at the last time of follow up were collected. FAZ area was defined as the area inside the central border of the capillary network. VD was defined as the percentage of area occupied by vessels in a defined region. FAZ area and VD both in the superficial capillary plexus (SCP) and in the deep capillary plexus (DCP) were extracted. In studies in which the layer for measurement was not mentioned, the data were analyzed as in the SCP group in our study. Discrepancies were addressed through discussion.
Method quality of the selected studies was evaluated according to the Newcastle-Ottawa Scale (NOS) (Stang, 2010). Scores ranging from 1 to 9 were applied to assess the selection criteria of subjects, comparability between controls and cases, and measurement values of each study. This procedure was completed by two reviewers and disagreements were discussed to achieve consensus. Publication bias was evaluated by Egger’s test and Begg’s test.
Data analysis was performed with Cochrane Collaboration’s Review Manager Software (RevMan 5.4, Cochrane Collaboration, Oxford, United Kingdom). Means and standard deviations of the area of foveal avascular zone and vessel density were calculated as continuous variables to obtain the weighted mean difference. Heterogeneity among the selected studies was tested by Chi-square test and Higgins I2 test. If the heterogeneity was not significant (p > 0.10, I2<50%), the fixed-effect analysis model was used. Otherwise, the random-effect model was applied. A p value < 0.05 was considered significant for all data analysis.
Twelve studies including 430 RRD eyes and 430 control eyes were selected in this study (Agarwal et al., 2019; Woo et al., 2018; McKay et al., 2020; Ng et al., 2020; Wang et al., 2019; Hong et al., 2020; Bonfiglio et al., 2020; Barca et al., 2020; Christou et al., 2021; Liu et al., 2021; D'Aloisio et al., 2022; Chatziralli et al., 2022), as indicated in Table 1. The search strategy for Pubmed is showed in Supplementary Table S1. The selection process is showed in Figure 1. As showed in Table 1, two studies selected macular-on RRD, six studies collected macula-off RRD, and the remaining four studies included both macula-on and macula-off RRD. In the study by D'Aloisio et al., part of the macula was detached before surgery in 6 RRD patients who received treatment within 24 h of onset (D'Aloisio et al., 2022). This group of eyes was considered as macular-on RRD for analysis in our study. Data were collected and analyzed separately in macula-on retinal detachment and macula-off retinal detachment. Patients with RRD received pars plana vitrectomy (PPV) with gas or air tamponade or scleral buckling (SB) in selected studies. One study was exclude since patients with RRD were treated with pneumatic retinopexy (Kaderli et al., 2021). Since silicone oil tamponade has been reported to be related to changes of macular microcirculation (Ma et al., 2020; Lee and Park, 2021), eyes with silicone oil tamponade were exclude in this study. The duration between symptoms and surgery was less than 14 days in most studies. However, in one study (Agarwal et al., 2019), the duration time was 1.76 ± 2.84 months (10 days–6 months), which was converted to the day unit in Table 1. The time that from surgery to OCTA measurement was at least 1 month in all studies, as showed in Table 1. As indicated in the selected studies, the foveal area was defined as a central circular region within a diameter of 1–1.5 mm, and the parafoveal area was defined as a circular annulus region between 1 and 2.5–3 mm in diameter. VD in the foveal area and the parafoveal area were analyzed separately. In the study of McKay et al. (McKay et al., 2020), VD was evaluated in a central area of 3 × 3 mm, which was not included for analysis in our study. The NOS score of each selected study is also showed in Table 1, and detailed information is showed in Supplementary Table S2.
Seven studies were included for analyzing FAZ area of macula-on RRD. As showed in Figure 2, FAZ area in the SCP and in the DCP of macula-on RRD was not different from that of control eyes after surgery (−0.01 mm2, 95% CI: −0.03 to 0.01, p = 0.40; 0.03 mm2, 95% CI: −0.04 to 0.09, p = 0.43). Nine studies collecting 289 eyes with RRD and 304 control eyes were selected for analyzing FAZ area in the SCP of macula-off RRD. FAZ area in the SCP was not changed after treatment (0.05 mm2, 95% CI: −0.01 to 0.10, p = 0.08). To calculate FAZ area in the DCP of macula-off RRD, five studies including 191 eyes in the RRD group and 206 eyes in the control group were collected. As indicated in Figure 3, FAZ area in the DCP was significantly increased even after the retina was reattached successfully through surgery in macula-off RRD eyes compared with that of control eyes (0.13 mm2, 95% CI: 0.02 to 0.25, p = 0.02).
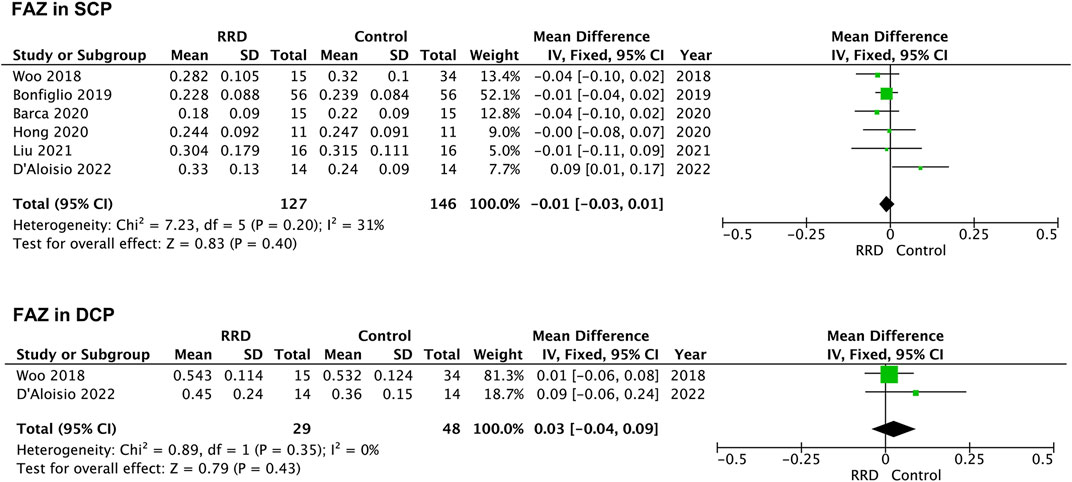
FIGURE 2. Forest plots of changes in FAZ area in eyes with Macula-on RRD. FAZ, foveal avascular zone; SCP, superficial capillary plexus; DCP, deep capillary plexus, RRD, rhegmatogenous retinal detachment.
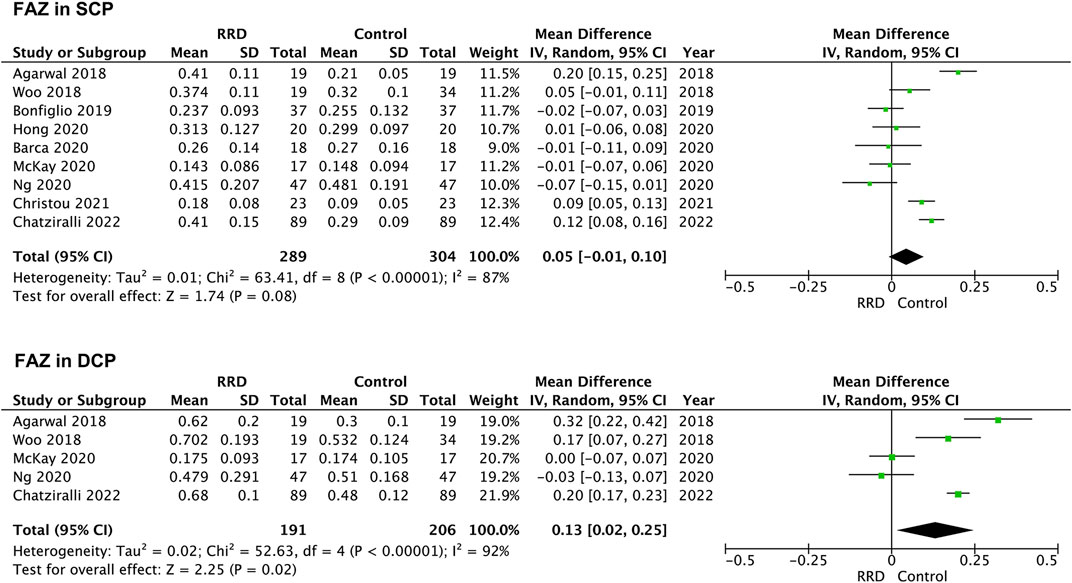
FIGURE 3. Forest plots of changes in FAZ area in eyes with Macula-off RRD. FAZ, foveal avascular zone; SCP, superficial capillary plexus; DCP, deep capillary plexus, RRD, rhegmatogenous retinal detachment.
In macula-on RRD, VD in the SCP and in the DCP of the foveal area was not changed compared with that of control eyes after surgery (1.04%, 95% CI: −0.80%–2.89%, p = 0.27; −0.30%, 95% CI: −1.67%–2.26%, p = 0.77, Figure 4). A total of 183 eyes with RRD and 183 control eyes in five studies were analyzed for VD changes in the foveal area of macula-off RRD. As demonstrated in Figure 5, no change in VD in the SCP was observed when compared with that of controls (−0.95%, 95% CI: −4.56 to 2.67%, p = 0.61). However, VD in the DCP of the foveal area was significantly reduced in macula-off RRD (−3.12%, 95% CI: −6.15% to −0.09%, p = 0.04), even though retinal detachment was repaired successfully after surgery.
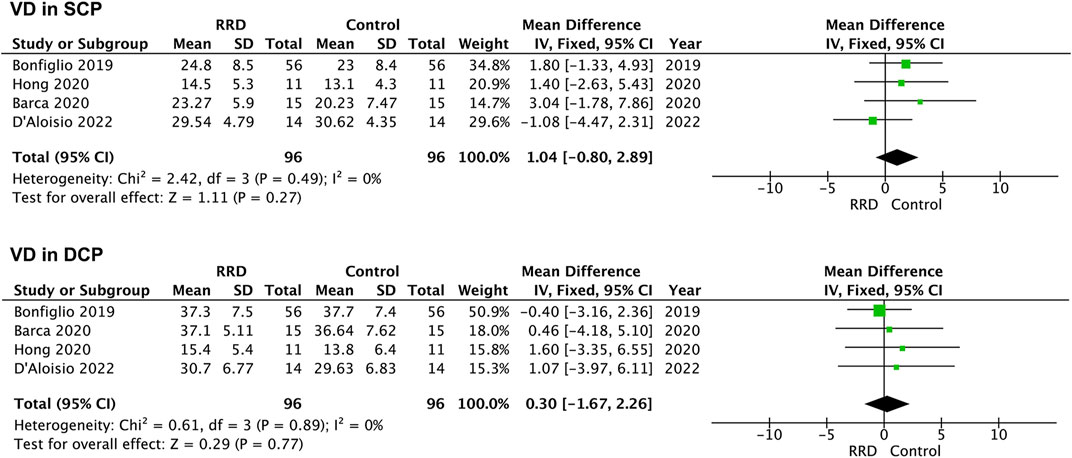
FIGURE 4. Forest plots of changes in foveal VD in eyes with Macula-on RRD. VD, vascular density, SCP, superficial capillary plexus; DCP, deep capillary plexus, RRD, rhegmatogenous retinal detachment.
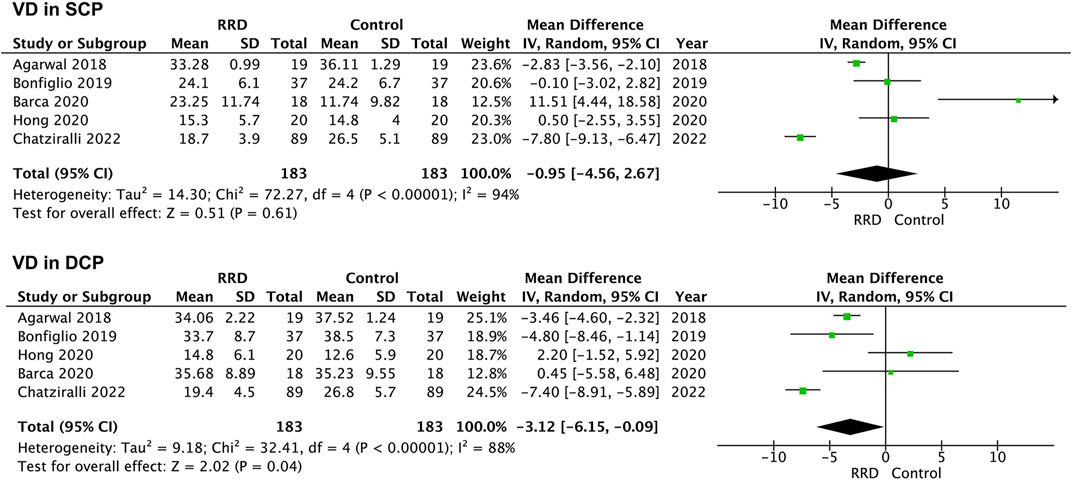
FIGURE 5. Forest plots of changes in foveal VD in eyes with Macula-off RRD. VD, vascular density, SCP, superficial capillary plexus; DCP, deep capillary plexus, RRD, rhegmatogenous retinal detachment.
VD in the parafoveal area was also analyzed in patients with RRD. No difference was detected in VD in the SCP or the DCP between eyes with macula-on RRD and control eyes (−0.76%, 95% CI: −1.84 to 0.31%, p = 0.16; −1.01%, 95% CI: −2.51 to −0.48%, p = 0.18, Figure 6). In patients with macula-off RRD, 225 eyes in the RRD group and 225 eyes in the control group from six studies were summarized for calculation. As showed in Figure 7 VD in the SCP and the DCP was not changed after retina was reattached (−2.00%, 95% CI: −5.99 to 2.00%, p = 0.33; −2.54%, 95% CI: −6.58% to 1.49%, p = 0.22).
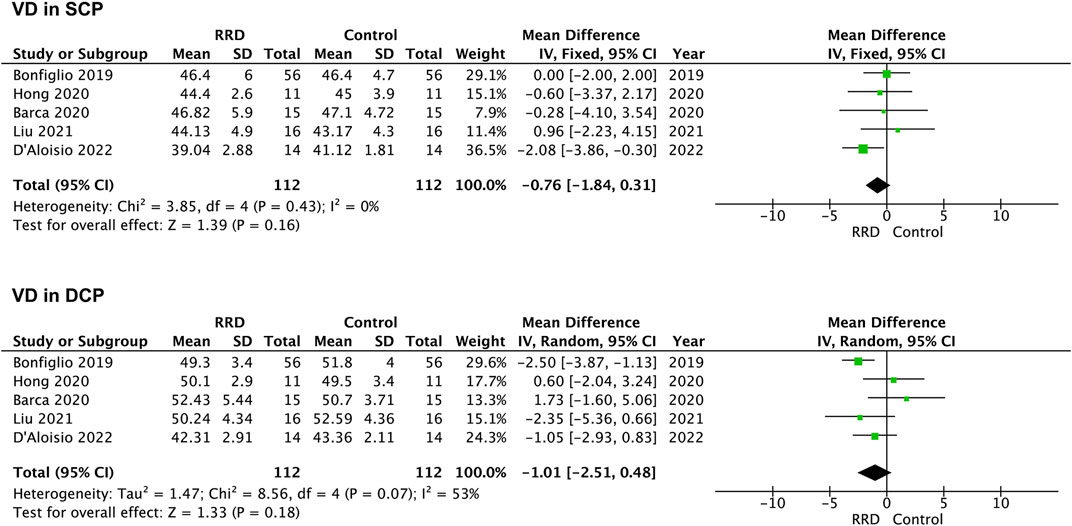
FIGURE 6. Forest plots of changes in parafoveal VD in eyes with Macula-on RRD. VD, vascular density, SCP, superficial capillary plexus; DCP, deep capillary plexus, RRD, rhegmatogenous retinal detachment.
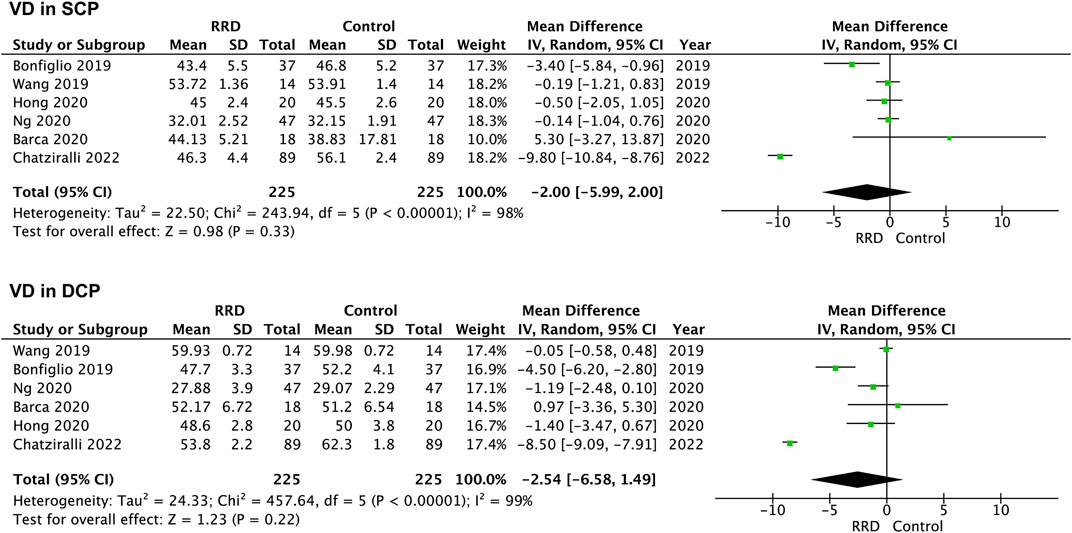
FIGURE 7. Forest plots of changes in parafoveal VD in eyes with Macula-off RRD. VD, vascular density, SCP, superficial capillary plexus; DCP, deep capillary plexus, RRD, rhegmatogenous retinal detachment.
VD of the choriocapillaris was evaluated in four studies. Three studies were selected for meta-analysis, because VD of the choriocapillaris was measured in an area of 8 mm × 8 mm while the measurement was performed within an area of 3 mm × 3 mm in other studies. VD of the choriocpillaris in eyes with macula-off RRD was analyzed. As showed in Figure 8, VD of the choriocpillaris was not altered after retinal reattachment (−0.73%, 95% CI: −1.64% to 0.17%, p = 0.11).
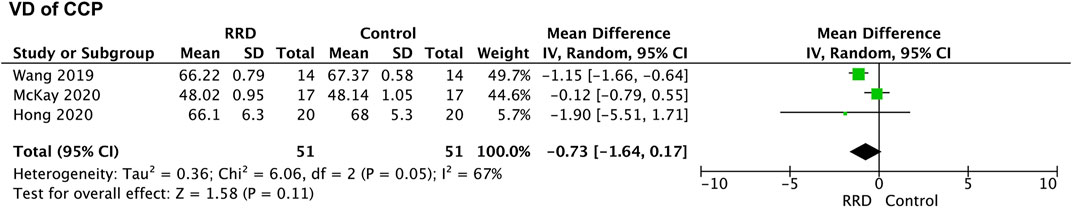
FIGURE 8. Forest plots of changes in VD of the choriocpillaris in eyes with macula-off RRD. VD, vascular density; RRD, rhegmatogenous retinal detachment.
Publication bias was assessed by Egger’s test and Begg’s test. No publication bias was revealed in FAZ area, VD in the foveal and the parafoveal areas of eyes with macular-off RRD. However, publication bias was found in VD in the DCP of the foveal area in eyes with macular-on RRD by Egger’s test (p = 0.04) but not by Begg’s test. No significant publication bias was observed in other measurements in eyes with macular-on RRD. Funnel plots of studies are showed in Supplementary Figure S1.
This study indicated that FAZ area in the DCP of eyes with macula-off RRD remained enlarged when the retina was reattached successfully after a single surgery, as compared with control eyes. Meanwhile, VD in the DCP of the foveal area in eyes with macula-off RRD also remained diminished after anatomical repair.
It has been reported that blood flow in the macular area was not different between eyes that received SB and those that received PPV and gas tamponade for retinal detachment (Sato et al., 2007). Other studies indicated that macular microcirculation was changed after silicone oil tamponade, including narrowing of arterioles and reduced blood flow (Agnieszka et al., 2011; Roohipoor et al., 2020). Accordingly, patients who received SB or PPV with gas tamponade, but not silicone oil tamponade, were selected in this study.
It has been found that capillaries in the DCP are more vulnerable to retinal and systemic diseases, such as retinal vein occlusion, diabetic macular edema, hypertension and systemic lupus erythematosus (Adhi et al., 2016; Lee et al., 2016; Chua et al., 2019; Arfeen et al., 2020). The mechanism is not clear. In the SCP of normal eyes, capillaries were located between arterioles and venules, forming an interconnected plexus. In the DCP, capillaries were arranged as polygonal units and were speculated to drain into the superficial venules (Bonnin et al., 2015). Moreover, as a single monolayer of the capillary plexus, VD in the DCP has been found to be lower than that in SCP (Lavia et al., 2019). The difference in structural pattern may be associated with unbalanced vulnerability to ischemia between the SCP and the DCP.
Decreased visual activity has been reported to be related to capillary loss in the DCP in type 1 diabetes without macular edema (Dupas et al., 2018). Furthermore, nonperfusion in the DCP has been proved to be correlated with photoreceptor disruption, which suggests that blood flow in the DCP is critical to the survival and function of photoreceptors (Scarinci et al., 2016).
In patients with macula-off retinal detachment, Woo et al. have reported that FAZ area in the SCP and the DCP are negatively associated with postoperative best-corrected visual acuity (BCVA) (Woo et al., 2018). In the study by Bonfiglio et al., BCVA has been proved to be related not only to FAZ area but also to VD in the foveal SCP and the parafoveal DCP (Bonfiglio et al., 2020). In McKay’s investigation, VD in the DCP, but not VD in the SCP and FAZ area, was found to be changed and correlated with BCVA (McKay et al., 2020). Ng et al. observed that a smaller FAZ area in the DCP was associated with better postoperative BCVA (Ng et al., 2020).
The pathogenetic mechanism is not clear. Deep capillaries are located in the inner nuclear layer. The avascular outer retinal layers are supplied by diffusion of oxygen and nourishment from the choriocapillaris (Tasman and Jaeger, 1991). However, it has also been found that 10%–15% of oxygen supplied to photoreceptors comes from retinal circulation (Birol et al., 2007), which indicates capillaries in the DCP may play an important role. In addition, capillaries in the DCP have also been considered important to provide oxygen and nutrition to synapses formed between photoreceptors and bipolar cells (Provis, 2001; Masland, 2012), which are therefore important for visual signal transmission. Usui et al. have speculated that vasculature, glia and neurons within retinal neurovascular units are highly interdependent and that their interactions are vital to maintain normal metabolism. Loss of either or both retinal interneurons and vasculature may lead to photoreceptor dysfunction (Usui et al., 2015). Inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and monocyte chemotactic protein-1 (MCP-1), may take part in the process (Nakazawa et al., 2006; Nakazawa et al., 2011).
Some limitations should be mentioned in this meta-analysis. First, the sample size of most selected studies was small. Second, the duration between symptoms and surgery, type of surgery, time and device of OCTA examination were different among the included studies, which contributed to the heterogeneity. The consistency of measurements among different OCTA devices has been investigated. It has been reported that FAZ area and VD in the SCP show good repeatability, while VD in the DCP dose not (Anegondi et al., 2018). In another study, FAZ area measurements were consistent across different devices, while differences in VD measurements were observed (Lu et al., 2021). Subgroup analysis could be performed when more studies are collected. Third, variations in software, layer segmentation and area selection during OCTA measurement also added to the heterogeneity. The SCP and the DCP were segmented automatically in most studies. The SCP was located from 3 μm under the internal limiting membrane (ILM) to 15 μm under the inner plexiform layer (IPL), and the DCP extended from 15 to 70 μm below the IPL. In the study by Liu et al., however, SCP was located between the ILM and 10 μm above the IPL and the inner nuclear layer (IPL-INL) junction, while the DCP was located between 10 μm above the IPL-INL junction and 10 μm below the outer plexiform layer and outer nuclear layer junction. Finally, the correlation between visual activity and FAZ or VD were not analyzed because of insufficient data in the selected studies.
In conclusion, in eyes with macula-off rhegmatogenous retinal detachment, FAZ area in the DCP was enlarged and VD in foveal DCP was reduced as compared with normal control eyes, even after successful anatomical repair through primary surgery. Multicenter studies with larger sample sizes are needed to evaluate changes in macular microcirculation in rhegmatogenous retinal detachment and its association with vision recovery after surgery.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
TL and XC conceived and designed the study. XC and WL searched literature. RL and XC extracted data and performed data analysis. XJ and TL conducted the quality assessment. XC drafted the manuscript. TL and YZ reviewed and revised the manuscript. All authors have read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.995353/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Funnel plots of studies. (A) , FAZ area in SCP of eyes with Macula-on RRD; (B), FAZ area in DCP of eyes with Macula-on RRD; (C), FAZ area in SCP of eyes with Macula-off RRD; (D), FAZ area in DCP of eyes with Macula-off RRD; (E), Foveal VD in SCP of eyes with Macula-on RRD; (F), Foveal VD in DCP of eyes with Macula-on RRD; (G), Foveal VD in SCP of eyes with Macula-off RRD; (H), Foveal VD in DCP of eyes with Macula-off RRD; (I), Parafoveal VD in SCP of eyes with Macula-on RRD; (J), Parafoveal VD in DCP of eyes with Macula-on RRD; (K), Parafoveal VD in SCP of eyes with Macula-off RRD; (L), Parafoveal VD in DCP of eyes with Macula-off RRD. FAZ, foveal avascular zone; SCP, superficial capillary plexus; DCP, deep capillary plexus, RRD, rhegmatogenous retinal detachment; VD, vascular density.
Adhi M., Filho M. A. B., Louzada R. N., Kuehlewein L., Carlo T. E. D., Baumal C. R., et al. (2016). Retinal capillary network and foveal avascular zone in eyes with vein occlusion and fellow eyes analyzed with optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci. 57, OCT486–94. doi:10.1167/iovs.15-18907
Agarwal A., Aggarwal K., Akella M., Agrawal R., Khandelwal N., Bansal R., et al. (2019). Fractal dimension and optical coherence tomography angiography features of the central macula after repair of rhegmatogenous retinal detachments. Retina 39 (11), 2167–2177. doi:10.1097/IAE.0000000000002276
Agnieszka K-T., Joanna K., Bozena R-D. (2011). Macular microcirculation blood flow after pars plana vitrectomy with silicone oil tamponade. Klin. Ocz. 113, 146–148.
Anegondi N., Kshirsagar A., Mochi T. B., Roy A. S. (2018). Quantitative comparison of retinal vascular features in optical coherence tomography angiography images from three different devices. Ophthalmic Surg. Lasers Imaging Retina 49 (7), 488–496. doi:10.3928/23258160-20180628-04
Arfeen S. A., Bahgat N., Adel N., Eissa M., Khafagy M. M. (2020). Assessment of superficial and deep retinal vessel density in systemic lupus erythematosus patients using optical coherence tomography angiography. Graefes Arch. Clin. Exp. Ophthalmol. 258, 1261–1268. doi:10.1007/s00417-020-04626-7
Barca F., Bacherini D., Dragotto F., Tartaro R., Lenzetti C., Finocchio L., et al. (2020). OCT angiography findings in macula-on and macula-off rhegmatogenous retinal detachment: A prospective study. J. Clin. Med. 9 (12), 3982. doi:10.3390/jcm9123982
Birol G., Wang S., Budzynski E., Wangsa-Wirawan N. D., Linsenmeier R. A. (2007). Oxygen distribution and consumption in the macaque retina. Am. J. Physiol. Heart Circ. Physiol. 293, H1696–H1704. doi:10.1152/ajpheart.00221.2007
Bonfiglio V., Ortisi E., Scollo D., Reibaldi M., Russo A., Pizzo A., et al. (2020). Vascular changes after vitrectomy for rhegmatogenous retinal detachment: Optical coherence tomography angiography study. Acta Ophthalmol. 98 (5), E563–E9. doi:10.1111/aos.14315
Bonnin S., Mané V., Couturier A., Julien M., Paques M., Tadayoni R., et al. (2015). New insight into the macular deep vascular plexus imaged by optical coherence tomography angiography. Retina 35, 2347–2352. doi:10.1097/IAE.0000000000000839
Chatziralli I., Theodossiadis G., Chatzirallis A., Dimitriou E., Parikakis E., Theodossiadis P. (2022). Evolution of macular microvasculature and retinal layers alterations in patients with macula off retinal detachment after vitrectomy. Eur. J. Ophthalmol. 32 (1), 520–526. doi:10.1177/1120672121992984
Christou E. E., Kalogeropoulos C., Georgalas I., Stavrakas P., Christodoulou E., Batsos G., et al. (2021). Assessment of anatomical and functional macular changes with optical coherence tomography angiography after macula-off rhegmatogenous retinal detachment repair. Semin. Ophthalmol. 36 (3), 119–127. doi:10.1080/08820538.2021.1889618
Chua J., Chin C. W. L., Hong J., Chee M. L., Le T-T., Ting D. S. W., et al. (2019). Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J. Hypertens. 37, 572–580. doi:10.1097/HJH.0000000000001916
D'Aloisio R., Gironi M., Verdina T., Vivarelli C., Leonelli R., Mariotti C., et al. (2022). Early structural and vascular changes after within-24 hours vitrectomy for recent onset rhegmatogenous retinal detachment treatment: A pilot study comparing bisected macula and not bisected macula. J. Clin. Med. 11 (12), 3498. doi:10.3390/jcm11123498
Dupas B., Minvielle W., Bonnin S., Couturier A., Erginay A., Massin P., et al. (2018). Association between vessel density and visual acuity in patients with diabetic retinopathy and poorly controlled type 1 diabetes. JAMA Ophthalmol. 136, 721–728. doi:10.1001/jamaophthalmol.2018.1319
Greig E. C., Brigell M., Cao F., Levine E. S., Peters K., Moult E. M., et al. (2020). Macular and peripapillary optical coherence tomography angiography metrics predict progression in diabetic retinopathy: A sub-analysis of TIME-2B study data. Am. J. Ophthalmol. 219, 66–76. doi:10.1016/j.ajo.2020.06.009
Hong E. H., Cho H., Kim D. R., Kang M. H., Shin Y. U., Seong M. (2020). Changes in retinal vessel and retinal layer thickness after vitrectomy in retinal detachment via swept-source OCT angiography. Invest. Ophthalmol. Vis. Sci. 61 (2), 35. doi:10.1167/iovs.61.2.35
Kaderli S. T., Karalezli A., Sul S. (2021). Microvascular retinal alterations in rhegmatogenous retinal detachment after pneumatic retinopexy. Acta Ophthalmol. 99 (4), 383–389. doi:10.1111/aos.14624
Kuehlewein L., Bansal M., Lenis T. L., Iafe N. A., Sadda S. R., Filho M. A. B., et al. (2015). Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Am. J. Ophthalmol. 160, 739–748. doi:10.1016/j.ajo.2015.06.030
Lavia C., Bonnin S., Maule M., Erginay A., Tadayoni R., Gaudric A. (2019). Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina 39, 247–258. doi:10.1097/IAE.0000000000002413
Lee J., Moon B. G., Cho A. R., Yoon Y. H. (2016). Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology 123, 2368–2375. doi:10.1016/j.ophtha.2016.07.010
Lee J. H., Park Y. G. (2021). Microvascular changes on optical coherence tomography angiography after rhegmatogenous retinal detachment vitrectomy with silicone tamponade. PLoS One 16 (3), e0248433. doi:10.1371/journal.pone.0248433
Liu Y., Lei B., Jiang R., Huang X., Zhou M., Xu G. (2021). Changes of macular vessel density and thickness in gas and silicone oil tamponades after vitrectomy for macula-on rhegmatogenous retinal detachment. BMC Ophthalmol. 21 (1), 392. doi:10.1186/s12886-021-02160-6
Lu Y., Wang J. C., Cui Y., Zhu Y., Zeng R., Lu E. S., et al. (2021). A quantitative comparison of four optical coherence tomography angiography devices in healthy eyes. Graefes Arch. Clin. Exp. Ophthalmol. 259 (6), 1493–1501. doi:10.1007/s00417-020-04945-9
Ma Y., Zhu X. Q., Peng X. Y. (2020). Macular perfusion changes and ganglion cell complex loss in patients with silicone oil-related visual loss. Biomed. Environ. Sci. 33 (3), 151–157. doi:10.3967/bes2020.021
Masland R. H. (2012). The neuronal organization of the retina. Neuron 76, 266–280. doi:10.1016/j.neuron.2012.10.002
McKay K. M., Vingopoulos F., Wang J. C., Papakostas T. D., Silverman R. F., Marmalidou A., et al. (2020). Retinal microvasculature changes after repair of macula-off retinal detachment assessed with optical coherence tomography angiography. Clin. Ophthalmol. 14, 1759–1767. doi:10.2147/OPTH.S214623
Nakazawa T., Kayama M., Ryu M., Kunikata H., Watanabe R., Yasuda M., et al. (2011). Tumor necrosis factor-alpha mediates photoreceptor death in a rodent model of retinal detachment. Invest. Ophthalmol. Vis. Sci. 52, 1384–1391. doi:10.1167/iovs.10-6509
Nakazawa T., Matsubara A., Noda K., Hisatomi T., She H., Skondra D., et al. (2006). Characterization of cytokine responses to retinal detachment in rats. Mol. Vis. 12, 867–878.
Ng H., La Heij E. C., Andrinopoulou E. R., van Meurs J. C., Vermeer K. A. (2020). Smaller foveal avascular zone in deep capillary plexus is associated with better visual acuity in patients after macula-off retinal detachment surgery. Transl. Vis. Sci. Technol. 9 (10), 25–12. doi:10.1167/tvst.9.10.25
Park D. H., Choi K. S., Sun H. J., Lee S. J. (2018). Factors associated with visual outcome after macula-off rhegmatogenous retinal detachment surgery. Retina 38, 137–147. doi:10.1097/IAE.0000000000001512
Provis J. M. (2001). Development of the primate retinal vasculature. Prog. Retin. Eye Res. 20, 799–821. doi:10.1016/s1350-9462(01)00012-x
Roohipoor R., Tayebi F., Riazi-Esfahani H., Khodabandeh A., Karkhaneh R., Davoudi S., et al. (2020). Optical coherence tomography angiography changes in macula-off rhegmatogenous retinal detachments repaired with silicone oil. Int. Ophthalmol. 40, 3295–3302. doi:10.1007/s10792-020-01516-z
Sato E. A., Shinoda K., Kimura I., Ohtake Y., Inoue M. (2007). Microcirculation in eyes after rhegmatogenous retinal detachment surgery. Curr. Eye Res. 32, 773–779. doi:10.1080/02713680701531108
Scarinci F., Nesper P. L., Fawzi A. A. (2016). Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am. J. Ophthalmol. 168, 129–138. doi:10.1016/j.ajo.2016.05.002
Schocket L. S., Witkin A. J., Fujimoto J. G., Ko T. H., Schuman J. S., Rogers A. H., et al. (2006). Ultrahigh-resolution optical coherence tomography in patients with decreased visual acuity after retinal detachment repair. Ophthalmology 113, 666–672. doi:10.1016/j.ophtha.2006.01.003
Shimoda Y., Sano M., Hashimoto H., Yokota Y., Kishi S. (2010). Restoration of photoreceptor outer segment after vitrectomy for retinal detachment. Am. J. Ophthalmol. 149, 284–290. doi:10.1016/j.ajo.2009.08.025
Shlomit S., Mark P. S., Charles C. B., Henry J. K. (2011). Primary retinal detachment repair: Comparison of 1-year outcomes of four surgical techniques. Retina 31, 1500–1504. doi:10.1097/IAE.0b013e31820d3f55
Stang A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Stroup D. F., Berlin J. A., Morton S. C., Olkin I., Williamson G. D., Rennie D., et al. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-Analysis of observational studies in epidemiology (MOOSE) group. JAMA 283 (25), 2008–2012. doi:10.1001/jama.283.15.2008
Sun Z., Tang F., Wong R., Lok J., Szeto S. K. H., Chan J. C. K., et al. (2019). OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: A prospective study. Ophthalmology 126, 1675–1684. doi:10.1016/j.ophtha.2019.06.016
Tasman W., Jaeger J. (1991). Duane’s foudations of clinical ophthalmology. New York: Lippincott Williams & Wilkins.
Tsen C-L., Sheu S-J., Chen S-C., Wu T-T. (2019). Imaging analysis with optical coherence tomography angiography after primary repair of macula-off rhegmatogenous retinal detachment. Graefes Archive Clin. Exp. Ophthalmol. 257 (9), 1847–1855. doi:10.1007/s00417-019-04381-4
Usui Y., Westenskow P. D., Kurihara T., Aguilar E., Sakimoto S., Paris L. P., et al. (2015). Neurovascular crosstalk between interneurons and capillaries is required for vision. J. Clin. Invest. 125, 2335–2346. doi:10.1172/JCI80297
Wang H., Xu X., Sun X., Ma Y., Sun T. (2019). Macular perfusion changes assessed with optical coherence tomography angiography after vitrectomy for rhegmatogenous retinal detachment. Graefes Archive Clin. Exp. Ophthalmol. 257 (4), 733–740. doi:10.1007/s00417-019-04273-7
Woo J. M., Yoon Y. S., Woo J. E., Min J. K. (2018). Foveal avascular zone area changes analyzed using OCT angiography after successful rhegmatogenous retinal detachment repair. Curr. Eye Res. 43 (5), 674–678. doi:10.1080/02713683.2018.1437922
Yarmohammadi A., Zangwill L. M., Diniz-Filho A., Suh M. H., Manalastas P. I., Fatehee N., et al. (2016). Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest. Ophthalmol. Vis. Sci. 57, OCT451–9. doi:10.1167/iovs.15-18944
Keywords: rhegmatogenous retinal detachment, macular microcirculation, foveal avascular zone, optical coherence tomography angiography, meta analysis
Citation: Chen X, Li W, Jin X, Zhang Y, Li R and Liu T (2022) Macular microcirculation changes after repair of rhegmatogenous retinal detachment assessed with optical coherence tomography angiography: A systematic review and meta-analysis. Front. Physiol. 13:995353. doi: 10.3389/fphys.2022.995353
Received: 15 July 2022; Accepted: 24 November 2022;
Published: 14 December 2022.
Edited by:
Leopold Schmetterer, Medical University of Vienna, AustriaReviewed by:
Mehmet Cem Sabaner, Kutahya Evliya Celebi Training and Research Hospital, TurkeyCopyright © 2022 Chen, Li, Jin, Zhang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiecheng Liu, bHRjMzAxQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.