
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol., 26 September 2022
Sec. Exercise Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.987804
This article is part of the Research TopicEffects of recreational sports on improving body composition and physical fitnessView all 6 articles
Parts of this article's content have been modified or rectified in:
Erratum: Effects of different exercise types on visceral fat in young individuals with obesity aged 6–24 years old: A systematic review and meta-analysis
Introduction: The prevalence of pediatric obesity remains high all over the world. Various exercise interventions have been applied to decrease the visceral fat in young individuals with obesity. But the evidence remains controversial on the effect of the exercise on visceral fat. Moreover, it is unclear which type of the exercise is the most effective for young individuals with overweight or obesity to reduce visceral fat.
Objective: The objective of this review and meta-analysis is to evaluate and compare the effectiveness of different exercise interventions on visceral fat in young individuals with overweight or obesity.
Methods: Four databases consisting of PubMed, Web of Science, EBSCO, and Cochrane Library were searched prior to May 2022. Fifteen studies with a total of 30 data points involving 1,134 participants were included in this meta-analysis. And the interventions were limited to 4 exercise types [i.e., aerobic exercise (AE), resistance exercise (RE), aerobic exercise combined with resistance exercise (CE), and high-intensity interval training (HIIT)].
Data Synthesis: The results showed that AE (Standardized Mean Difference = −0.32; 95% CI = −0.50 to −0.13; p = 0.0007; I2 = 37%) and HIIT (SMD = −0.59; 95% CI = −0.87 to −0.31; p < 0.0001; I2 = 0%) had a significant reduction effect on visceral fat. And the effect of HIIT seemed better than AE. However, RE (SMD = −0.58; 95% CI = −1.34 to 0.17; p = 0.13; I2 = 76%) and CE (SMD = −0.21; 95% CI = −0.81 to 0.38; p = 0.48; I2 = 63%) had a non-significant effect on visceral fat decline. Additionally, compared with the control group, exercise interventions had a significant effect on reducing visceral fat in adolescents (SMD = −0.54; 95% CI = −0.82 to −0.26; p = 0.0001; I2 = 64%) and young adults (SMD = −0.42; 95% CI = −0.69 to −0.15; p = 0.003; I2 = 0%) rather than children (SMD = −0.15; 95% CI = −0.32 to 0.02; p = 0.08; I2 = 0%). And the gender-based subgroup analysis indicated that the effectiveness of the exercise on the reduction of visceral fat was more significant in males (SMD = −1.27; 95% CI = −1.67 to −0.87; p < 0.00001; I2 = 0%) than that in females (SMD = −0.31; 95% CI = −0.48 to −0.14; p = 0.0004; I2 = 0%).
Conclusion: This review and meta-analysis demonstrates that exercise interventions are efficient to decrease visceral fat in adolescents (12–18 years old) and young adults (18–24 years old). Among different exercise types, AE and HIIT are helpful for young individuals with overweight or obesity to reduce visceral fat and HIIT appears to be the most effective exercise intervention. In addition, the effect of exercise interventions on the consumption of visceral fat is more significant in males than that in females.
Systematic Review Registration: [http://www.crd.york.ac.uk/PROSPERO], identifier [CRD42022310878].
It is widely known that the prevalence of overweight and obesity trends towards the younger ages in recent years. In 2020, World Health Organization (WHO) announced that 38.9 million children under the age of 5 years old are overweight (WHO, 2021). Overweight and obese young individuals (e.g., children, adolescents, or young adults) are more prone to develop diabetes and cardiovascular diseases and to maintain obesity in older-aged adulthood (Wang and Lim, 2012; Al-Sulaiti et al., 2019; WHO, 2020). The sustained effect of obesity from childhood to adulthood makes a variety of diseases more apparent in the adult period, such as heart disease, stroke, diabetes, osteoarthritis, endometrial, breast, and colon (WHO, 2020). In addition to the threat to physical health, obesity can also impair young individuals’ mental soundness. It has been reported that overweight or obese adolescents have a higher prevalence of depression and anxiety symptoms than adolescents who are not obese (Wang et al., 2019). Those obese children or adolescents frequently fall into a vicious cycle between distress, damage and distortion of self-image, maintenance and deterioration of pain (Pervanidou and Chrousos, 2011).
Abnormal or excessive fat accumulation including subcutaneous fat and visceral fat can lead to obesity at a young stage, which is identified as an important risk factor for health in the full life cycle. Various studies have demonstrated that, compared to total body fat mass or subcutaneous fat, excessive visceral fat should be more blamed for several chronic metabolic diseases (e.g., insulin resistance and hypertension) (Fox et al., 2007; Gastaldelli et al., 2007; Suliga, 2009; Karlsson et al., 2019). Tadokoro et al. have demonstrated that boys aged 15–16 years old with more accumulated visceral fat were detected with a higher level of atherosclerosis, liver dysfunction, hyperlipidemia, and hyperinsulinemia (Tadokoro et al., 2010). It has been reported that visceral fat thickness is strongly correlated to the elastic properties of the abdominal aorta (Polat et al., 2008). Hence, the extra visceral fat may be a hidden danger for young people with visceral obesity to develop certain cardiovascular diseases. Besides that, the excessive accumulation of visceral fat can lead to the decreased immunity (de Heredia et al., 2012), the high occurrence of chronic obstructive pulmonary disease (Furutate et al., 2011), and even some common cancers such as colorectal cancer (Larsson and Wolk, 2007; Tsang et al., 2009; Kang et al., 2010) and esophageal cancer (Elliott and Reynolds, 2021). Therefore, it is necessary to pay more attention to excessive visceral fat accumulation rather than total body fat or subcutaneous fat and to find effective interventions to reduce visceral fat in young individuals with overweight or obesity.
Exercise interventions are the effective and cost-efficient strategies to optimize the body composition of individuals at a younger age (Stoner et al., 2016; Kim et al., 2020; Bogataj et al., 2021; Ouerghi et al., 2022). However, the evidence remains controversial on the effect of the exercise on visceral fat. For example, Tadokoro et al. have found that even in healthy high school students who do regular exercises, visceral obesity also occurs in 9.6% of participants (Tadokoro et al., 2010). In a randomized controlled trial (RCT) that examined the effect of the exercise on visceral fat, although subjects were instructed with exercise interventions in the experimental group, visceral fat did not decrease significantly in obese young individuals (Alberga et al., 2015).
In addition, it is widely reported that various exercise types are beneficial for young individuals with overweight or obesity to decrease visceral fat. Specifically, some researchers have suggested that aerobic training can effectively reduce visceral fat mass in obese adolescents (Gutin et al., 2002; Foschini et al., 2010; Alberga et al., 2013). A case in point showed that a 12-week aerobic exercise (AE) without calorie restriction (30 min/session, 4 sessions/week) was effective for the decline of visceral adipose tissue (VAT) (van der Heijden et al., 2010). While, many studies have also confirmed the role of resistance training in the decrease of visceral fat (Lee et al., 2012; Shultz et al., 2014; Lee et al., 2020). For example, a non-contact boxing-oriented intervention followed by the progressive resistance exercise (RE) has been regarded as an active exercise program to help obese young people decrease their visceral fat thickness (Shultz et al., 2014). Several studies have illustrated that aerobic combined with resistance exercise (CE) has a more obvious effect on the decline of visceral fat in young people with obesity than the isolated AE or the isolated RE (Damaso et al., 2014; Jung et al., 2019). High-intensity interval training (HIIT), as a novel exercise training pattern, has also been proposed to burn visceral fat and optimize body composition in obese individuals (Maillard et al., 2018; Mendelson et al., 2022). It has been revealed that HIIT can produce a variety of health benefits in school-aged individuals with obesity, such as reducing total body mass and visceral fat (Zhang et al., 2017), improving cardiovascular fitness (Costigan et al., 2015; Kong et al., 2016), increasing muscle strength and peak oxygen uptake (Abarzua et al., 2019). Although different types of exercise interventions have been suggested conducive to the reduction of visceral fat in obese or overweight young individuals. It is still unclear which type of the exercise is the most effective intervention for decreasing visceral fat. Considering that obesity at a young age has a lasting harmful effect on adulthood, it is necessary to examine studies related to obese or overweight young individuals to compare the effectiveness of different types of exercise interventions on visceral fat.
The purpose of our study is to evaluate and compare the effect of exercise interventions (AE, RE, CE, and HIIT) on visceral fat in overweight or obese young individuals through a meta-analysis from a more comprehensive and systematic perspective.
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was registered on the international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO), registration number: CRD42022310878. No similar systematic review protocol exists.
Four databases, namely, PubMed (1988–2022), Web of Science (2003–2022), EBSCO (1997–2022), and Cochrane Library (1991–2022) were searched using the following keywords: [“exercise” OR “training” OR “aerobic” OR “resistance” OR “aerobic plus resistance” OR “combined training” OR “HIIT” OR “sprint interval training (SIT)” OR “repeated sprint interval training (RST)”] AND (“body composition” OR “visceral fat” OR “VAT” OR “visceral adipose” OR “abdominal fat” OR “abdominal adipose”) AND (“obesity” OR “obese” OR “overweight”) AND (“adolescent” OR “youth” OR “children” OR “teenagers” OR “young”). Two authors (R.W. and X.Z.) screened and extracted relevant studies independently through reviewing all titles, abstracts, and full-text articles. Any disagreement was resolved by discussion until consensus was reached or by consulting a third arbitrator (X.H.).
According to the PICOS principle (patient/population, intervention, comparison/control, outcome and study design), trials were eligible for inclusion if they met the criteria as follows: 1) participants were defined as overweight [body mass index (BMI) ≥ 85th percentile or BMI ≥25 kg/m2] or obese (BMI ≥95th percentile or BMI ≥30 kg/m2) young individuals aged 6–24 years old, participants with body fat percentage (BFP)≥30% for females and≥25% for males, or participants whose triceps skinfold thickness (TSF) was greater than the 85th percentile; 2) the exercise interventions were limited to AE, RE, CE and HIIT; 3) no exercise intervention was applied in the control group in the same duration as the experimental group; 4) the outcomes of the trial included the common indices reflecting visceral fat: visceral fat mass, VAT, the thickness of abdominal visceral fat, abdominal visceral fat area, the prevalence of visceral obesity or abdominal adipose; 5) only the trials designed as RCTs were covered; and 6) the selected articles were peer-reviewed publications written in English.
Trials were excluded when they met any of the following exclusion criteria: 1) reviews, abstracts, case reports, observational studies, non-peer-reviewed articles, such as dissertations or conference posters; 2) participants have been diagnosed with mental disorders or eating disorders; 3) participants were pregnant women or individuals with orthopedic/neurological disorders that limited the exercise ability; 4) the specific types of exercise interventions couldn’t be identified; 5) the experimental group was intervened with the exercise and drugs; 6) the control group received any drug treatment; 7) incomplete data or inadequate descriptions of research methods in the study.
Two independent authors used the Cochrane Collaboration tool to evaluate the quality of the included studies according to the seven domain biases as follows: 1) Random sequence generation (selection bias); 2) allocation concealment (selection bias); 3) blinding of participants and personnel (performance bias); 4) blinding of outcome assessment (detection bias); 5) incomplete outcome data (attrition bias); 6) selective reporting (reporting bias); and 7) other bias (Higgins et al., 2011). Three grades of high, low, or unclear bias were labeled for each included study. Any disagreement was resolved by discussion until consensus was reached or by consulting a third arbitrator (X.H.).
Two authors independently extracted the relevant data from each included study as the following: author(s), publication year, country/region, subjects’ characteristics (e.g., age, BMI, gender), sample size, intervention (e.g., intensity, frequency, duration, exercise type), reported outcomes (e.g., VAT, the thickness of abdominal visceral fat, and the abdominal visceral fat area).
The authors used the Review Manager software (Review Manager 5.3; The Nordic Cochrane Center, The Cochrane Collaboration) to perform this meta-analysis. The outcomes in the meta-analysis included visceral fat mass (g, kg, L, cm3), the thickness of abdominal visceral fat (cm, defined as the distance between the inner wall of the abdominal cavity and the anterior wall of the aorta), or visceral fat area (quantified in cm2 at the level between the 4th and 5th lumbar vertebra). In accordance with the Cochrane Handbook for Systematic Reviews (Higgins and Green, 2008), either post-intervention values (Mean post−intervention ± SD post−intervention) of the outcome or changes from baseline (Mean of changes ± SD of changes) could be used to calculate the summary statistic value.
If studies reported SE instead of SD, we would calculate SD using the formula: SD = SE × √N (Higgins et al., 2011). If studies only presented CI, the SD would be calculated by the formula “√N × (lupper-llower)/c”, in which lupper and llower, respectively, represented the upper and lower limits of the CI. And c was a constant which depended on the CI and the sample size (Huedo-Medina et al., 2006). If articles presented the outcomes using figures, the GetData Graph Digitizer 2.26 would be used to extract the relevant data. If the mean and SD were not presented in the articles, we would contact the authors by email and if the authors didn’t reply, the articles would be excluded.
The I2 index was used to assess the heterogeneity among studies. Low, moderate, high and very high heterogeneity were identified when I2 ≤ 25%, I2 ≤ 50% and >25%, I2 ≤ 75% and >50%, I2 > 75% respectively (Huedo-Medina et al., 2006). If the heterogeneity was low or moderate, a fixed-effect model would be used. While, if the heterogeneity was high or very high, a random-effect model would be applied (Hou et al., 2021). Considering that, in this meta-analysis, the units (e.g., g, kg, L, cm, cm2, cm³) of outcomes reflecting visceral fat were different. We performed the Standardized Mean Difference (SMD) to analyze the compositive effects. The subgroup analysis was used to analyze the effectiveness of different exercise types (AE, RE, CE, and HIIT) on visceral fat in young individuals with overweight or obesity. And the other two subgroup analyses based on participants’ age and gender were also performed. When I2 > 50%, possible publication bias was assessed by examining the asymmetry of funnel plots or using Egger’s test (Egger et al., 1997). The level of significance was set at p < 0.05.
The flowchart in Figure 1 presents the search procedure. Based on our preliminary search of four databases, a total of 2,144 records were identified. 1772 records of them remained after excluding duplicates. 28 potentially eligible articles remained after screening titles and abstracts. 15 articles met the inclusion after reviewing the full-text articles. The 15 articles were pooled in this meta-analysis and a total of 30 data points (involving 1,134 participants) were included.
Table 1 shows the basic characteristics of the included articles. The subjects in all studies were children aged 6–12 years old, adolescents aged 12–18 years old, and young adults aged 18–24 years old. Among these studies, the subjects in three articles were children (Barbeau et al., 2007; Saelens et al., 2011; Davis et al., 2012). The subjects in eight articles were adolescents (Mitchell et al., 2002; Davis et al., 2011; Lee et al., 2012; Lee et al., 2013; Alberga et al., 2015; Monteiro et al., 2015; Staiano et al., 2017; Cao et al., 2022). The subjects in three articles were young adults (Zhang et al., 2017; Tong et al., 2018; Zhang et al., 2021). And one article included both children and adolescents (Dias et al., 2018). For the gender of the recruited subjects, seven articles only recruited females. Two articles only recruited males. And six articles recruited both males and females as subjects.
Of these 15 articles, thirteen (87%) involved the isolated AE intervention, three (20%) involved the isolated RE intervention, three (20%) involved the CE intervention and five (33%) involved the HIIT intervention. For studies related to AE, nine (69%) studies designed running or walking as an AE intervention. Six (46%) studies designed cycling as an AE intervention. Only one (8%) study conducted dancing as an AE intervention and the other three (23%) studies designed several ball games like basketball, softball, badminton, and kickball as AE interventions. For studies related to RE, all studies (n = 3, 100%) performed the whole-body muscle strength training (e.g., leg press, leg extension, leg flexion, chest press, latissimus pulldown, seated row, bicep curl, and triceps extension) with weight machines as RE interventions. For the studies related to CE, all of them (n = 3, 100%) combined the AE and RE modalities mentioned above. Of the five studies involving HIIT, three conducted the cycling exercise as an intervention. And one study designed walking or running on a treadmill as well as cycling on a stationary bike as interventions. One study designed running on an outdoor track as an intervention.
According to the instruction developed by Higgins (Higgins et al., 2011), we evaluated the quality of included articles. The bias of included studies mainly came from the random sequence generation (selection bias), the blinding of participants and personnel (performance bias), and the blinding of outcome assessment (detection bias) (shown in Figure 2).
A total of 28 data points from fifteen studies presented the influence of exercise interventions on visceral fat in children, adolescents, and young adults with overweight or obesity. As shown in Figure 3, there was a significant difference between the exercise intervention and control groups based on a random-effect model (SMD = −0.39; 95% CI = −0.54 to −0.24; p < 0.00001; I2 = 42%), which indicated that exercise interventions had a significant effect on reducing visceral fat in obese or overweight young individuals. To compare the effectiveness of different exercise interventions on the decline of visceral fat, we made a subgroup analysis based on the exercise types (i.e., AE, RE, CE, HIIT). Fourteen data points from thirteen studies demonstrated the effectiveness of AE on visceral fat in young individuals. As shown in Figure 3, there was a significant difference between the AE and control groups (SMD = −0.32; 95% CI = −0.50 to −0.13; p = 0.0007; I2 = 37%). It indicated that AE was an effective intervention for young individuals to decrease visceral fat. For RE, three data points from three studies were involved in this meta-analysis. No significant difference was found between the RE and control groups (SMD = −0.58; 95% CI = −1.34 to 0.17; p = 0.13; I2 = 76%). It implied that RE had a non-significant effect on the decline of the visceral fat in obese or overweight young people. For CE, three data points from three studies reported the effectiveness of the combination of AE and RE interventions on obese or overweight subjects’ visceral fat. As presented in Figure 3, no significant difference was found between the CE and control groups (SMD = −0.21; 95% CI = −0.81 to 0.38; p = 0.48; I2 = 63%). It suggested that CE couldn’t help young individuals decrease visceral fat. For HIIT, eight data points from five studies were used to report the effectiveness of HIIT on the visceral fat of young individuals with obesity. As shown in Figure 3, there was a significant difference between the HIIT and control groups (SMD = −0.59; 95% CI = −0.87 to −0.31; p < 0.0001; I2 = 0%), which implied that HIIT was significantly effective for obese or overweight young individuals to reduce visceral fat. Based on the effect size in AE (SMD = −0.32) and HIIT (SMD = −0.59), the effect of HIIT on reducing the visceral fat appeared to be more obvious than that of AE.
As shown in Figure 4, to compare the effects of exercise interventions on visceral fat consumption in different age groups, we made a subgroup analysis based on the subjects’ age (i.e., children aged 6–12 years old, adolescents aged 12–18 years old, young adults aged 18–24 years old). Four data points from three studies evaluated the effectiveness of exercise interventions on visceral fat in overweight or obese children aged 6–12 years old. For children (6–12 years old) group, no significant difference between the exercise and control groups (SMD = −0.15; 95% CI = −0.32 to 0.02; p = 0.08; I2 = 0%) was found, which proposed that exercise interventions couldn’t help obese children burn the visceral fat. For adolescents aged 12–18 years old, fourteen data points from eight studies were pooled in our meta-analysis. As shown in Figure 4, for adolescents (12–18 years old) group, there was a significant difference between experimental and control groups based on a random-effect model (SMD = −0.54; 95% CI = −0.82 to −0.26; p = 0.0001; I2 = 64%). It indicated that exercise interventions were conducive to decreasing visceral fat in adolescents with obesity or overweight. For young adults aged 18–24 years old, eight data points from three studies reported the effectiveness of exercise interventions on visceral fat consumption. Figure 4 showed that there was a significant difference between experimental and control groups (SMD = −0.42; 95% CI = −0.69 to −0.15; p = 0.003; I2 = 0%), which implied that the exercise could significantly decrease young adults’ visceral fat.
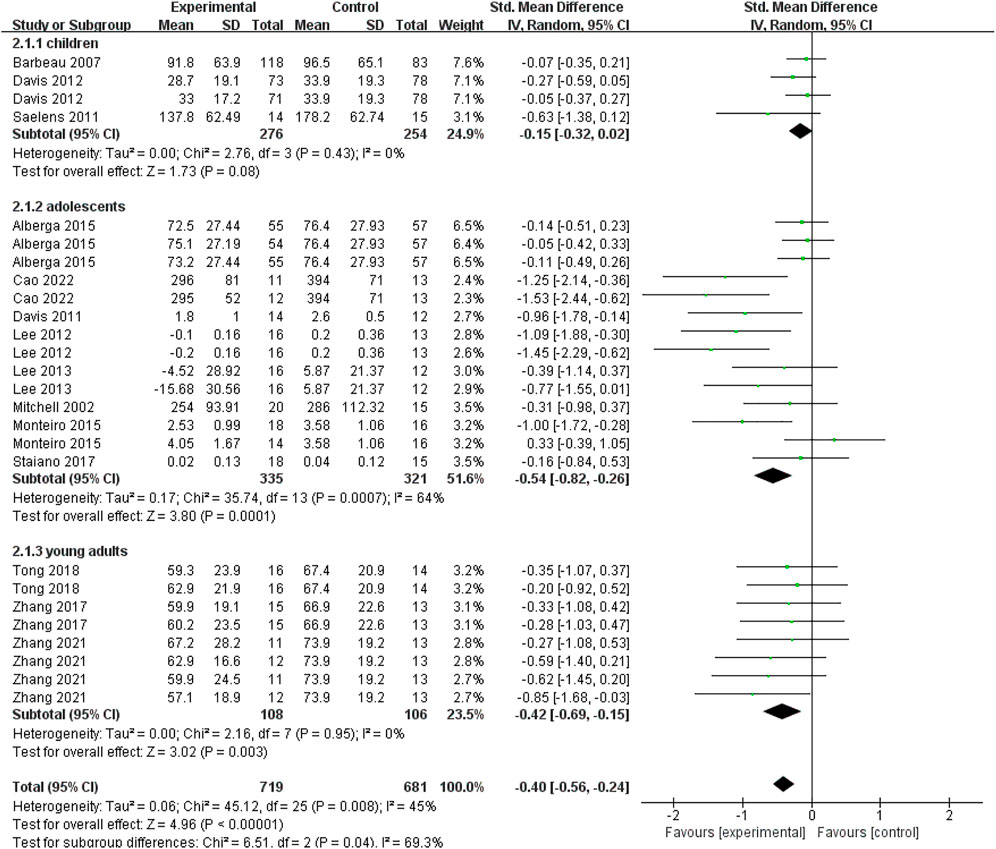
FIGURE 4. Subgroup analysis of age related to the effect of exercise interventions on visceral fat in young individuals with obesity or overweight.
A total of nineteen data points from ten studies reported the specific genders of subjects. To distinguish the gender difference in the effect of exercise interventions on visceral fat in young individuals, we conducted a subgroup analysis based on the genders of young participants. As shown in Figure 5, for males, there was a significant difference between experimental and control groups based on a fixed-effect model (SMD = −1.27; 95% CI = −1.67 to −0.87; p < 0.00001; I2 = 0%). For females, there was also a significant difference between experimental and control groups based on a fixed-effect model (SMD = −0.31; 95% CI = −0.48 to −0.14; p = 0.0004; I2 = 0%). It indicated that exercise interventions could significantly decrease the visceral fat in both young males and females with overweight or obesity. Based on the effect size in different genders, the decreased effect of exercise interventions on visceral fat was more obvious in male subjects (SMD = −1.27) than that in female subjects (SMD = −0.31).
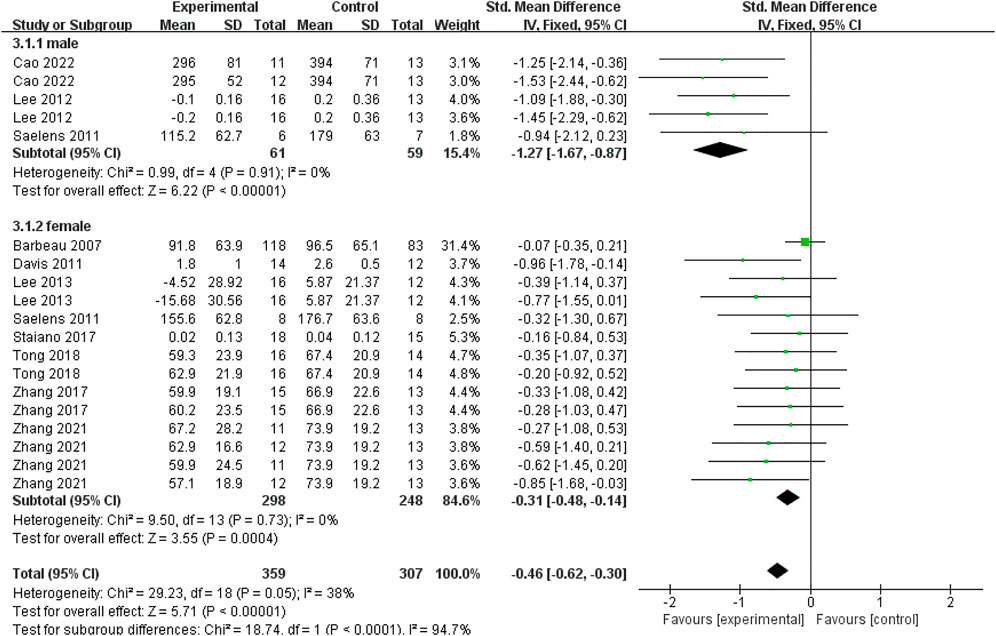
FIGURE 5. Subgroup analysis of gender related to the effect of exercise interventions on visceral fat in young individuals with obesity or overweight.
In this meta-analysis, the evidence from 15 studies regarding the effects of AE, RE, CE, and HIIT was summarized. This synthesis has suggested that the exercise is highly associated with a reduction in visceral fat of obese or overweight young individuals aged 6–24 years old. Among the exercise interventions included in our study, the effectiveness of AE and HIIT is significantly positive for reducing the visceral fat in young people with obesity. And the effect of HIIT seems better than AE. However, the effect of RE and CE on visceral fat was not statistically significant. For different age groups, the effect of the exercise on visceral fat consumption was significant in adolescents (12–18 years old) and young adults (18–24 years old) with overweight or obesity, but not in children (6–12 years old). In addition, the results derived from the gender-based subgroup analysis illustrated that exercise interventions could decrease visceral fat in both males and females. And the effect of the exercise was more significant in males than that in females.
The results demonstrated that AE and HIIT had a significant effect on the reduction of visceral fat in young individuals with obesity. For AE, it has been suggested that AE can increase the secretion of catecholamines, which are regarded as the lipolytic hormone, by inducing sympathetic nervousness tension (Arner, 1999; Monda et al., 2019). Some studies found that the lipolytic effect of catecholamines on VAT was even more pronounced than the abdominal subcutaneous adipose (Ostman et al., 1979; Marin et al., 1992). This may partly explain that, in our meta-analysis, AE is helpful for young individuals to lose visceral fat. Another effective exercise intervention identified in this meta-analysis was HIIT. On the one hand, it has been shown that HIIT also can stimulate visceral adipose lipolysis by facilitating catecholamine secretion (Lonnqvist et al., 1995; Trapp et al., 2007). On the other hand, HIIT can suppress appetite, which may decrease the energy intake and reduce visceral fat accumulation (Boutcher, 2011; Panissa et al., 2019). Moreover, we found that the effect of HIIT seemed even better than that of AE. This may be partly explained by that the higher intensity of HIIT rather than the moderate intensity of AE has been illustrated to be related to the release of more hormones (e.g., catecholamine, growth hormone) (Pritzlaff et al., 2000; Trapp et al., 2007), which can accelerate adiposity oxidation and decrease visceral fat (Stanley and Grinspoon, 2015). However, an original study found a similar effect of 6-week moderate-intensity continuous training and HIIT on visceral fat size when the energy expenditure of exercise bout was equal (Gerosa-Neto et al., 2019). The same result wasn’t obtained in this review, because our meta-analysis could not uniformly control the energy of all the included studies.
This meta-analysis indicated that RE and CE had non-effective influence on decreasing visceral fat for young individuals with obesity. For RE, the non-significant effect of the exercise may be due to the different proportions of the energy supply system and different energy substrates (Moghetti et al., 2016). It is known that the energy of the RE is mainly supplied by the anaerobic energy system. Consequently, the adenosine triphosphate (ATP), creatine phosphate (CP), and muscle glycogen rather than the fat are mainly consumed to provide the energy during the resistance training. For CE, it is widely documented that the long-term and low-intensity AE is easier to mobilize fat for the energy supply (Turcotte et al., 1992; Kiens et al., 1993; Ranallo and Rhodes, 1998). However, when the AE is combined with the RE, the decrease in the proportion of AE during the exercise period may illustrate the reason why CE failed to burn visceral fat in young individuals with overweight or obesity. Additionally, the small sample size (n = 83, 7%) might cause the bias.
Our meta-analysis revealed that exercise interventions could effectively decrease the visceral fat in adolescents aged 12–18 years old and young adults aged 18–24 years old, but not in 6–12-year-old children. For adolescents and young adults, the significant effect of the visceral fat decline could be interpreted as that they may have a better understanding of the technical movements during the exercise and the level of the exercise completion is higher. For children, a study suggested that children including obese or overweight children had less visceral fat in the abdomen area (Staiano et al., 2013). Hence, the exercise may have a more obvious effect on decreasing subcutaneous fat rather than visceral fat in low-aged obese children.
For subjects in different gender groups, we found that exercise interventions were more helpful to reduce visceral fat in young males than that in young females with obesity or overweight. This could be explained by the gender differences in hormone levels. Various studies have indicated that the estrogen can promote the proliferation of adipocyte precursors to produce new mature fat cells in the visceral region (Tchoukalova et al., 2010; Gavin and Bessesen, 2020). Conversely, during puberty, boys can secrete more androgens that stimulate lipolysis than girls (Blouin et al., 2008). Therefore, the dominance of androgens in boys has more tendency for the visceral fat removal. Additionally, we speculated that males were more compliant and interested in the exercise than females. So, females might not well finish the exercise in experiments due to a lack of interest in the exercise. On the other hand, males might spend more time exercising in their daily lives out of experiments. In contrast, females often conducted sedentary behavior. Win et al. have also suggested that females exercised less regularly than males (prevalence rate = 0.63, 95% CI = 0.51–0.76) (Win et al., 2015).
Although this study demonstrated the outstanding effects of AE and HIIT on visceral fat in young individuals with obesity, there are some limitations. First, it is worth mentioning that the outcomes of this meta-analysis are restricted to the effects of various exercise types on the visceral fat of young people with obesity. Hence, the findings of our study do not apply to other benefits of exercise interventions for obese or overweight young individuals, such as the improved cardiorespiratory fitness, the increased muscle mass. The exercise interventions that have no apparent effects on reducing visceral fat in young individuals with obesity may have other health benefits. Second, except for age and gender, the effect of exercise interventions on visceral fat may vary from the degree of overweight (i.e., overweight or obesity) of young individuals. But several covered studies in our meta-analysis provided a mixed sample including overweight and obese individuals. This prevented us from extracting the independent data points from the mixed BMI or percentage of body fat group. Third, our study focused on comparing the experimental group (exercise) with the control group (no exercise). Thus, this meta-analysis did not consider the effect of diet on visceral fat. Future meta-analyses can investigate the interaction effect of the diet and the exercise on visceral fat. Last, the limited number of included articles (n = 15) may result in a certain degree of influence on the effect. Although this review and meta-analysis is systematic and rigorous, we did not include unpublished articles. This may affect the comprehensiveness of this study.
This review and meta-analysis indicates that exercise interventions are effective in reducing visceral fat in young people with overweight and obesity, especially in adolescents (12–18 years old) and young adults (18–24 years old). Among different exercise types, AE and HIIT have a significant effect on decreasing visceral fat and HIIT appears to be the most effective exercise type. The effectiveness of exercise interventions on the decline of visceral fat is more significant in boys than that in girls.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conceptualization, XH and HR; methodology, RW and XZ; formal analysis, RW; investigation, HZ and YY; resources, YC; writing—original draft preparation, RW; writing—review and editing, XH and RW; visualization, RW; supervision, XH. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This study was funded by The Ministry of education of Humanities and Social Science project (grant number: 22YJC890008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abarzua V. J., Viloff C. W., Bahamondes V. J., Olivera P. Y., Poblete-Aro C., Herrera-Valenzuela T., et al. (2019). High intensity interval training in teenagers. Rev. Med. Chil. 147 (2), 221–230. doi:10.4067/s0034-98872019000200221
Al-Sulaiti H., Diboun I., Agha M. V., Mohamed F. F. S., Atkin S., Domling A. S., et al. (2019). Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J. Transl. Med. 17 (1), 348. doi:10.1186/s12967-019-2096-8
Alberga A. S., Frappier A., Sigal R. J., Prud'homme D., Kenny G. P. (2013). A review of randomized controlled trials of aerobic exercise training on fitness and cardiometabolic risk factors in obese adolescents. Phys. Sportsmed. 41 (2), 44–57. doi:10.3810/psm.2013.05.2014
Alberga A. S., Prud'homme D., Kenny G. P., Goldfield G. S., Hadjiyannakis S., Gougeon R., et al. (2015). Effects of aerobic and resistance training on abdominal fat, apolipoproteins and high-sensitivity C-reactive protein in adolescents with obesity: The HEARTY randomized clinical trial. Int. J. Obes. 39 (10), 1494–1500. doi:10.1038/ijo.2015.133
Arner P. (1999). Catecholamine-induced lipolysis in obesity. Int. J. Obes. Relat. Metab. Disord. 23 (1), 10–13. doi:10.1038/sj.ijo.0800789
Barbeau P., Johnson M. H., Howe C. A., Allison J., Davis C. L., Gutin B., et al. (2007). Ten months of exercise improves general and visceral adiposity, bone, and fitness in black girls. Obes. (Silver Spring) 15 (8), 2077–2085. doi:10.1038/oby.2007.247
Blouin K., Boivin A., Tchernof A. (2008). Androgens and body fat distribution. J. Steroid Biochem. Mol. Biol. 108 (3-5), 272–280. doi:10.1016/j.jsbmb.2007.09.001
Bogataj S., Trajkovic N., Cadenas-Sanchez C., Sember V. (2021). Effects of school-based exercise and Nutrition intervention on body composition and physical fitness in overweight Adolescent girls. Nutrients 13 (1), 238. doi:10.3390/nu13010238
Boutcher S. H. (2011). High-intensity intermittent exercise and fat loss. J. Obes. 2011, 868305. doi:10.1155/2011/868305
Cao M., Tang Y., Li S., Zou Y. (2022). Effects of school-based high-intensity interval training on body composition, cardiorespiratory fitness and cardiometabolic markers in adolescent boys with obesity: A randomized controlled trial. BMC Pediatr. 22 (1), 112. doi:10.1186/s12887-021-03079-z
Costigan S. A., Eather N., Plotnikoff R. C., Taaffe D. R., Lubans D. R. (2015). High-intensity interval training for improving health-related fitness in adolescents: A systematic review and meta-analysis. Br. J. Sports Med. 49 (19), 1253–1261. doi:10.1136/bjsports-2014-094490
Damaso A. R., da Silveira Campos R. M., Caranti D. A., de Piano A., Fisberg M., Foschini D., et al. (2014). Aerobic plus resistance training was more effective in improving the visceral adiposity, metabolic profile and inflammatory markers than aerobic training in obese adolescents. J. Sports Sci. 32 (15), 1435–1445. doi:10.1080/02640414.2014.900692
Davis C. L., Pollock N. K., Waller J. L., Allison J. D., Dennis B. A., Bassali R., et al. (2012). Exercise dose and diabetes risk in overweight and obese children: A randomized controlled trial. JAMA 308 (11), 1103–1112. doi:10.1001/2012.jama.10762
Davis J. N., Gyllenhammer L. E., Vanni A. A., Meija M., Tung A., Schroeder E. T., et al. (2011). Startup circuit training program reduces metabolic risk in Latino adolescents. Med. Sci. Sports Exerc. 43 (11), 2195–2203. doi:10.1249/MSS.0b013e31821f5d4e
de Heredia F. P., Gomez-Martinez S., Marcos A. (2012). Obesity, inflammation and the immune system. Proc. Nutr. Soc. 71 (2), 332–338. doi:10.1017/S0029665112000092
Dias K. A., Ingul C. B., Tjonna A. E., Keating S. E., Gomersall S. R., Follestad T., et al. (2018). Effect of high-intensity interval training on fitness, fat mass and cardiometabolic Biomarkers in children with obesity: A randomised controlled trial. Sports Med. 48 (3), 733–746. doi:10.1007/s40279-017-0777-0
Egger M., Davey Smith G., Schneider M., Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Elliott J. A., Reynolds J. V. (2021). Visceral obesity, metabolic syndrome, and esophageal Adenocarcinoma. Front. Oncol. 11, 627270. doi:10.3389/fonc.2021.627270
Foschini D., Araujo R. C., Bacurau R. F., De Piano A., De Almeida S. S., Carnier J., et al. (2010). Treatment of obese adolescents: The influence of periodization models and ACE genotype. Obes. (Silver Spring) 18 (4), 766–772. doi:10.1038/oby.2009.247
Fox C. S., Massaro J. M., Hoffmann U., Pou K. M., Maurovich-Horvat P., Liu C. Y., et al. (2007). Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham heart study. Circulation 116 (1), 39–48. doi:10.1161/CIRCULATIONAHA.106.675355
Furutate R., Ishii T., Wakabayashi R., Motegi T., Yamada K., Gemma A., et al. (2011). Excessive visceral fat accumulation in advanced chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 6, 423–430. doi:10.2147/COPD.S22885
Gastaldelli A., Cusi K., Pettiti M., Hardies J., Miyazaki Y., Berria R., et al. (2007). Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 133 (2), 496–506. doi:10.1053/j.gastro.2007.04.068
Gavin K. M., Bessesen D. H. (2020). Sex differences in adipose tissue function. Endocrinol. Metab. Clin. North Am. 49 (2), 215–228. doi:10.1016/j.ecl.2020.02.008
Gerosa-Neto J., Panissa V. L. G., Monteiro P. A., Inoue D. S., Ribeiro J. P. J., Figueiredo C., et al. (2019). High- or moderate-intensity training promotes change in cardiorespiratory fitness, but not visceral fat, in obese men: A randomised trial of equal energy expenditure exercise. Respir. Physiol. Neurobiol. 266, 150–155. doi:10.1016/j.resp.2019.05.009
Gutin B., Barbeau P., Owens S., Lemmon C. R., Bauman M., Allison J., et al. (2002). Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. Am. J. Clin. Nutr. 75 (5), 818–826. doi:10.1093/ajcn/75.5.818
Higgins J. P., Altman D. G., Gotzsche P. C., Juni P., Moher D., Oxman A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins J. P. T., Green S. (2008). Cochrane Handbook for systematic reviews of interventions: Cochrane Book Series. Chichester, West Sussex: The Cochrane Collaboration. doi:10.1002/9780470712184
Hou X., Liu J., Weng K., Griffin L., Rice L. A., Jan Y. K. (2021). Effects of various physical interventions on reducing Neuromuscular Fatigue assessed by Electromyography: A systematic review and meta-analysis. Front. Bioeng. Biotechnol. 9, 659138. doi:10.3389/fbioe.2021.659138
Huedo-Medina T. B., Sanchez-Meca J., Marin-Martinez F., Botella J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 11 (2), 193–206. doi:10.1037/1082-989X.11.2.193
Jung H. C., Jeon S., Lee N. H., Kim K., Kang M., Lee S. (2019). Effects of exercise intervention on visceral fat in obese children and adolescents. J. Sports Med. Phys. Fit. 59 (6), 1045–1057. doi:10.23736/S0022-4707.18.08935-1
Kang H. W., Kim D., Kim H. J., Kim C. H., Kim Y. S., Park M. J., et al. (2010). Visceral obesity and insulin resistance as risk factors for colorectal adenoma: A cross-sectional, case-control study. Am. J. Gastroenterol. 105 (1), 178–187. doi:10.1038/ajg.2009.541
Karlsson T., Rask-Andersen M., Pan G., Hoglund J., Wadelius C., Ek W. E., et al. (2019). Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat. Med. 25 (9), 1390–1395. doi:10.1038/s41591-019-0563-7
Kiens B., Essen-Gustavsson B., Christensen N. J., Saltin B. (1993). Skeletal muscle substrate utilization during submaximal exercise in man: Effect of endurance training. J. Physiol. 469, 459–478. doi:10.1113/jphysiol.1993.sp019823
Kim J., Son W. M., Headid R. J., Pekas E. J., Noble J. M., Park S. Y. (2020). The effects of a 12-week jump rope exercise program on body composition, insulin sensitivity, and academic self-efficacy in obese adolescent girls. J. Pediatr. Endocrinol. Metab. 33 (1), 129–137. doi:10.1515/jpem-2019-0327
Kong Z., Sun S., Liu M., Shi Q. (2016). Short-term high-intensity interval training on body composition and blood Glucose in overweight and obese young women. J. Diabetes Res. 2016, 4073618. doi:10.1155/2016/4073618
Larsson S. C., Wolk A. (2007). Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 86 (3), 556–565. doi:10.1093/ajcn/86.3.556
Lee S., Bacha F., Hannon T., Kuk J. L., Boesch C., Arslanian S. (2012). Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: A randomized, controlled trial. Diabetes 61 (11), 2787–2795. doi:10.2337/db12-0214
Lee S., Deldin A. R., White D., Kim Y., Libman I., Rivera-Vega M., et al. (2013). Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: A randomized controlled trial. Am. J. Physiol. Endocrinol. Metab. 305 (10), E1222–E1229. doi:10.1152/ajpendo.00285.2013
Lee S., Libman I., Hughan K. S., Kuk J. L., Barinas-Mitchell E., Chung H., et al. (2020). Effects of exercise modality on body composition and cardiovascular disease risk factors in adolescents with obesity: A randomized clinical trial. Appl. Physiol. Nutr. Metab. 45 (12), 1377–1386. doi:10.1139/apnm-2019-0993
Lonnqvist F., Thome A., Nilsell K., Hoffstedt J., Arner P. (1995). A pathogenic role of visceral fat beta 3-adrenoceptors in obesity. J. Clin. Invest. 95 (3), 1109–1116. doi:10.1172/JCI117758
Maillard F., Pereira B., Boisseau N. (2018). Effect of high-intensity interval training on total, abdominal and visceral fat mass: A meta-analysis. Sports Med. 48 (2), 269–288. doi:10.1007/s40279-017-0807-y
Marin P., Andersson B., Ottosson M., Olbe L., Chowdhury B., Kvist H., et al. (1992). The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism. 41 (11), 1242–1248. doi:10.1016/0026-0495(92)90016-4
Mendelson M., Chacaroun S., Baillieul S., Doutreleau S., Guinot M., Wuyam B., et al. (2022). Effects of high intensity interval training on sustained reduction in cardiometabolic risk associated with overweight/obesity. A randomized trial. J. Exerc. Sci. Fit. 20 (2), 172–181. doi:10.1016/j.jesf.2022.03.001
Mitchell B. M., Gutin B., Kapuku G., Barbeau P., Humphries M. C., Owens S., et al. (2002). Left ventricular structure and function in obese adolescents: Relations to cardiovascular fitness, percent body fat, and visceral adiposity, and effects of physical training. Pediatrics 109 (5), E73–E73. doi:10.1542/peds.109.5.e73
Moghetti P., Bacchi E., Brangani C., Dona S., Negri C. (2016). Metabolic effects of exercise. Front. Horm. Res. 47, 44–57. doi:10.1159/000445156
Monda V., Villano I., Messina A., Valenzano A., Salerno M., Santo Signorelli S., et al. (2019). Aerobic exercise and Orexin A: Role of sympathetic activity and redox system. J. Biol. Regul. Homeost. Agents 33 (2), 587–592.
Monteiro P. A., Chen K. Y., Lira F. S., Saraiva B. T., Antunes B. M., Campos E. Z., et al. (2015). Concurrent and aerobic exercise training promote similar benefits in body composition and metabolic profiles in obese adolescents. Lipids Health Dis. 14, 153. doi:10.1186/s12944-015-0152-9
Ostman J., Arner P., Engfeldt P., Kager L. (1979). Regional differences in the control of lipolysis in human adipose tissue. Metabolism. 28 (12), 1198–1205. doi:10.1016/0026-0495(79)90131-8
Ouerghi N., Fradj M. K. B., Duclos M., Bouassida A., Feki M., Weiss K., et al. (2022). Effects of high-intensity interval training on selected Adipokines and cardiometabolic risk markers in normal-weight and overweight/obese young males-A Pre-post test trial. Biol. (Basel) 11 (6), 853. doi:10.3390/biology11060853
Panissa V. L. G., Julio U. F., St-Pierre D. H., Tavares da Silva Gomes A., Caldeira R. S., Lira F. S., et al. (2019). Timing of high-intensity intermittent exercise affects ad libitum energy intake in overweight inactive men. Appetite 143, 104443. doi:10.1016/j.appet.2019.104443
Pervanidou P., Chrousos G. P. (2011). Stress and obesity/metabolic syndrome in childhood and adolescence. Int. J. Pediatr. Obes. 6 (1), 21–28. doi:10.3109/17477166.2011.615996
Polat T. B., Urganci N., Caliskan K. C., Akyildiz B. (2008). Correlation of abdominal fat accumulation and stiffness of the abdominal aorta in obese children. J. Pediatr. Endocrinol. Metab. 21 (11), 1031–1040. doi:10.1515/jpem.2008.21.11.1031
Pritzlaff C. J., Wideman L., Blumer J., Jensen M., Abbott R. D., Gaesser G. A., et al. (2000). Catecholamine release, growth hormone secretion, and energy expenditure during exercise vs. recovery in men. J. Appl. Physiol. 89 (3), 937–946. doi:10.1152/jappl.2000.89.3.937
Ranallo R. F., Rhodes E. C. (1998). Lipid metabolism during exercise. Sports Med. 26 (1), 29–42. doi:10.2165/00007256-199826010-00003
Saelens B. E., Grow H. M., Stark L. J., Seeley R. J., Roehrig H. (2011). Efficacy of increasing physical activity to reduce children's visceral fat: A pilot randomized controlled trial. Int. J. Pediatr. Obes. 6 (2), 102–112. doi:10.3109/17477166.2010.482157
Shultz S. P., Stoner L., Lambrick D. M., Lane A. M. (2014). A boxing-oriented exercise intervention for obese adolescent males: Findings from a pilot study. J. Sports Sci. Med. 13 (4), 751–757.
Staiano A. E., Broyles S. T., Gupta A. K., Katzmarzyk P. T. (2013). Ethnic and sex differences in visceral, subcutaneous, and total body fat in children and adolescents. Obes. (Silver Spring) 21 (6), 1251–1255. doi:10.1002/oby.20210
Staiano A. E., Marker A. M., Beyl R. A., Hsia D. S., Katzmarzyk P. T., Newton R. L. (2017). A randomized controlled trial of dance exergaming for exercise training in overweight and obese adolescent girls. Pediatr. Obes. 12 (2), 120–128. doi:10.1111/ijpo.12117
Stanley T. L., Grinspoon S. K. (2015). Effects of growth hormone-releasing hormone on visceral fat, metabolic, and cardiovascular indices in human studies. Growth Horm. IGF Res. 25 (2), 59–65. doi:10.1016/j.ghir.2014.12.005
Stoner L., Rowlands D., Morrison A., Credeur D., Hamlin M., Gaffney K., et al. (2016). Efficacy of exercise intervention for weight loss in overweight and obese adolescents: Meta-analysis and implications. Sports Med. 46 (11), 1737–1751. doi:10.1007/s40279-016-0537-6
Suliga E. (2009). Visceral adipose tissue in children and adolescents: A review. Nutr. Res. Rev. 22 (2), 137–147. doi:10.1017/S0954422409990096
Tadokoro N., Shinomiya M., Yoshinaga M., Takahashi H., Matsuoka K., Miyashita Y., et al. (2010). Visceral fat accumulation in Japanese high school students and related atherosclerotic risk factors. J. Atheroscler. Thromb. 17 (6), 546–557. doi:10.5551/jat.3251
Tchoukalova Y. D., Koutsari C., Votruba S. B., Tchkonia T., Giorgadze N., Thomou T., et al. (2010). Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obes. (Silver Spring) 18 (10), 1875–1880. doi:10.1038/oby.2010.56
Tong T. K., Zhang H., Shi H., Liu Y., Ai J., Nie J., et al. (2018). Comparing time efficiency of sprint vs. High-intensity interval training in reducing abdominal visceral fat in obese young women: A randomized, controlled trial. Front. Physiol. 9, 1048. doi:10.3389/fphys.2018.01048
Trapp E. G., Chisholm D. J., Boutcher S. H. (2007). Metabolic response of trained and untrained women during high-intensity intermittent cycle exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293 (6), R2370–R2375. doi:10.1152/ajpregu.00780.2006
Tsang T. W., Briody J., Kohn M., Chow C. M., Singh M. F. (2009). Abdominal fat assessment in adolescents using dual-energy X-ray absorptiometry. J. Pediatr. Endocrinol. Metab. 22 (9), 781–794. doi:10.1515/jpem.2009.22.9.781
Turcotte L. P., Richter E. A., Kiens B. (1992). Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am. J. Physiol. 262 (1), E791–E799. doi:10.1152/ajpendo.1992.262.6.E791
van der Heijden G. J., Wang Z. J., Chu Z. D., Sauer P. J., Haymond M. W., Rodriguez L. M., et al. (2010). A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obes. (Silver Spring) 18 (2), 384–390. doi:10.1038/oby.2009.274
Wang S., Sun Q., Zhai L., Bai Y., Wei W., Jia L. (2019). The prevalence of depression and anxiety symptoms among overweight/obese and non-overweight/non-obese children/adolescents in China: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 16 (3), E340. doi:10.3390/ijerph16030340
Wang Y., Lim H. (2012). The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int. Rev. Psychiatry 24 (3), 176–188. doi:10.3109/09540261.2012.688195
WHO (2020). Noncommunicable diseases: Childhood overweight and obesity. Available at: https://www.who.int/news-room/questions-and-answers/item/noncommunicable-diseases-childhood-overweight-and-obesity.
WHO (2021). The UNICEF/WHO/WB Joint Child Malnutrition Estimates (JME) group released new data for 2021. Available at: https://www.who.int/news/item/06-05-2021-the-unicef-who-wb-joint-child-malnutrition-estimates-group-released-new-data-for-2021 (Accessed January 18, 2022).
Win A. M., Yen L. W., Tan K. H., Lim R. B., Chia K. S., Mueller-Riemenschneider F. (2015). Patterns of physical activity and sedentary behavior in a representative sample of a multi-ethnic south-East Asian population: A cross-sectional study. BMC Public Health 15, 318. doi:10.1186/s12889-015-1668-7
Zhang H., Tong T. K., Kong Z., Shi Q., Liu Y., Nie J. (2021). Exercise training-induced visceral fat loss in obese women: The role of training intensity and modality. Scand. J. Med. Sci. Sports 31 (1), 30–43. doi:10.1111/sms.13803
Keywords: exercise, visceral fat (VFA), young individuals, adolescents, obesity, overweight, meta-analysis
Citation: Wang R, Zhang X, Ren H, Zhou H, Yuan Y, Chai Y and Hou X (2022) Effects of different exercise types on visceral fat in young individuals with obesity aged 6–24 years old: A systematic review and meta-analysis. Front. Physiol. 13:987804. doi: 10.3389/fphys.2022.987804
Received: 06 July 2022; Accepted: 29 August 2022;
Published: 26 September 2022.
Edited by:
Ana Filipa Silva, Research Centre in Sports Sciences, Health Sciences and Human Development (CIDESD), PortugalReviewed by:
Zhaowei Kong, University of Macau, Macao SAR, ChinaCopyright © 2022 Wang, Zhang, Ren, Zhou, Yuan, Chai and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Hou, aG91eGlhbzAzMjdAYnN1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.