- 1Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Department of Health Management, Shengjing Hospital of China Medical University, Shenyang, China
- 3Department of Surgery, Shengjing Hospital of China Medical University, Shenyang, China
Background: Pernicious placenta previa (PPP) is one of the most dangerous complications in pregnancy after cesarean section, with high perinatal mortality. This study aimed to develop a nomogram to predict postpartum hemorrhage in patients with PPP.
Methods: A total of 246 patients with confirmed PPP at Shengjing Hospital of China Medical University from January 2018 to December 2021 were included. Patients were divided into to two cohorts depending on a postpartum blood loss of > 1000 ml (n = 146) or ≤ 1000 ml (n = 100). Lasso regression analysis was performed on the risk factors screened by univariate analysis to screen out the final risk factors affecting postpartum hemorrhage. Based on the final risk factors, a Nomogram prediction model with excellent performance was constructed using Logistic regression. A nomogram was constructed with further screening of the selected risk factors of postpartum hemorrhage in PPP. A second nomogram based only on the total ultrasonic risk score was constructed. Decision curve analysis (DCA) was used to evaluate the clinical efficacy of the nomograms.
Results: Older age, larger gestational age, larger neonatal birth weight, presence of gestational diabetes mellitus, larger amniotic fluid index, absence of gestational bleeding, and higher ultrasonic risk single score were selected to establish a nomogram for postpartum hemorrhage in PPP. The area under the curve of the nomogram constructed by Lasso regression analysis was higher than that of the ultrasonic total score alone (0.887 vs. 0.833). Additionally, DCA indicated better clinical efficacy in the former nomogram than in the later nomogram. Furthermore, internal verification of the nomogram constructed by Lasso regression analysis showed good agreement between predicted and actual values.
Conclusion: A nomogram for postpartum hemorrhage in PPP was developed and validated to assist clinicians in evaluating postpartum hemorrhage. This nomogram was more accurate than using the ultrasonic score alone.
Introduction
Placenta previa is a pregnancy complication in which the placenta (the organ that grows in the uterus to provide oxygen and nutrients to the baby) attaches low within the uterus, covering all or part of the cervix. It is a serious complication of pregnancy; one of the major complications is massive bleeding is massive bleeding during childbirth or the postpartum period, potentially resulting in hysterectomy, blood transfusion, and premature delivery (Oppenheimer, 2007). In 1993, Chattopadhyay et al. (1993) first proposed the concept of pernicious placenta previa (PPP), which refers to placenta previa associated with uterine scar tissue in a woman with a history of cesarean section or myomectomy, and is often accompanied by placenta accreta spectrum disorders (PAS). Clinically, because of a high incidence of PAS, PPP can easily cause fatal and refractory massive bleeding, and even threaten the life of the pregnant woman in serious cases (Zheng et al., 2021). Furthermore, excessive blood transfusion due to bleeding increases the risk of infection and transfusion reaction (Shafer et al., 1980). Additionally, the loss of reproductive opportunities after a hysterectomy in women of childbearing age has great negative impact on their physical and mental health and family life (Horng et al., 2021).
In China, because of “family planning” policies, the cesarean section rate rose rapidly in the 1980s. According to a global survey on maternal perinatal health conducted by the World Health Orga e incidence of caesarean section due to social factors was estimated as approximately 0.01%–2.10% globally and 11.6% in China (Souza et al., 2010). In 2018, Zhang reported that the cesarean section rate in most urban hospitals in China was as high as 40%, and was even 80% in some hospitals (WeiyuanZhang, 2018). Furthermore, due to a recent reform (within the last 5 years) in China’s family planning policies, the number of parturient women has increased, including those with uterine scars. Accordingly, there has been an increase in the incidence of cesarean section scar pregnancy, PAS, uterine rupture, placenta previa, and PPP. Chinese scholars have reported that the current incidence of PPP in China is 0.31%–0.89%, and approximately 53.3% of patients with PPP have accompanying PAS (Yunshan Chen, 2020).
PPP combined with severe intrapartum and postpartum bleeding threatens maternal and infant health, and is one of the great challenges faced by obstetricians in clinical work. In an effort to improve pregnancy outcomes, obstetricians are constantly seeking diagnostic methods that can predict the severity of PPP in order to appropriately intervene and prepare. At present, there are many reports on the prenatal diagnosis of PPP; however, methods to accurately judge and evaluate its severity are lacking. Ultrasound can diagnose about 80% of patients with PPP and is considered by obstetricians to be an ideal method for the prenatal diagnosis of PPP (Jauniaux et al., 2019). Because of its convenient operation, small impact on maternal and fetal health, and capacity for repeatable examinations, without special conditions, it is accepted by most pregnant women. By observing ultrasonic imaging characteristics of the placenta, including relationships between the placenta and cervical opening and, especially, between the placenta and cesarean section scar in the lower section of uterus, and selecting meaningful image features, it is possible to evaluate the type of PPP and whether it is accompanied by PAS (Berkley and Abuhamad, 2018). In addition, ultrasonic images for the diagnosis of placental implantation have the following characteristics: disappearance of the posterior placental space (Pasto et al., 1983), unclear uterine-placenta boundary (Tovbin et al., 2016), interruption or loss of a strong echo line at the uterine serosal laminum-bladder interface (Jauniaux et al., 2018), vortex protrusion (Comstock et al., 2004) and placental echo into the bladder (Wong et al., 2008; Calì et al., 2013), abundant blood flow at the base of the placenta (Finberg and Williams, 1992), and vascular bridge (Finberg and Williams, 1992).
Chinese scholars have proposed a quantitative table based on ultrasound imaging characteristics of the placenta and risk factors of PPP, assigning various scores (Chen et al., 2021). At present, this table is commonly used clinically, and the total score has been widely reported to predict the risk of placenta previa, severe postpartum hemorrhage, hysterectomy, and premature birth in patients with PPP (Chong et al., 2018; Liu et al., 2019; Jingyi Huang and Gu, 2020). However, obstetricians are beginning to pay attention to nomograms for perinatal prediction. The nomograph is a visualization of a complex statistical formula that is increasingly being used in medicine. Nomographs graphically describe a statistical prognostic model that generates the probability of a clinical event (such as cancer recurrence or death) in a particular individual using biological and clinical variables (such as tumor grade and patient age). In order to improve the accuracy of the preoperative assessment of PPP, this study combined reported clinical risk factors with ultrasound scores to construct a model to predict the risk of postpartum hemorrhage in PPP. We consider it more intuitive and concise to predict postpartum hemorrhage in PPP with the developed line diagram, which is convenient for clinical use, than with a quantitative table.
Materials and methods
Ethical approval
Ethical approval was obtained from the Ethics Committee of Shengjing Hospital of China Medical University (No. 2022PS132K), and the study conformed to the principles outlined in the Declaration of Helsinki (World Medical Association Declaration of Helsinki).
Study design
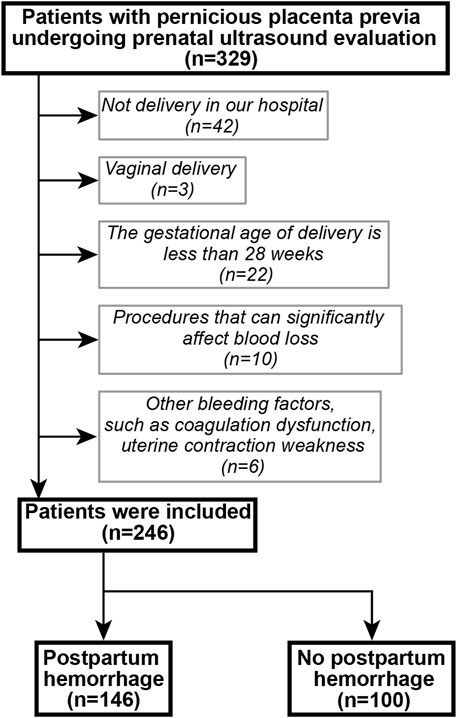
A retrospective cohort was established. Patients with suspected PPP who underwent ultrasonography and delivered by cesarean section between January 2018 and December 2019 at the Shengjing Hospital of China Medical University were included. Inclusion criteria were as follows: a history of cesarean section; singleton pregnancy; postoperatively confirmed PPP, with or without PAS; complete clinical data; and ultrasound scoring completed before delivery. The following exclusion criteria were applied: delivery at another hospital; vaginal delivery; gestational age at delivery was less than 28 weeks; underwent procedures that could significantly affect blood loss, such as balloon tamponade, brace sutures, surgical devascularization, radiological embolization and total hysterectomy; presence of a gynecological disease, uterine malformation, uterine fibroids, or adenomyosis; and presence of a disease affecting coagulation function. The patient selection flowchart is shown in Figure 1. PPP with or without PAS disorders were diagnosed using the intraoperative findings or postoperative pathology.
Patient information was collected through the Health Insurance System of the Shengjing Hospital of China Medical University, and included general information (age, ethnicity, times of previous cesarean sections\pregnancies\childbirth\induced abortions, and history of uterine cavity surgery); previous pregnancy information (history of placenta previa, previous operation times); prenatal examination results (placenta previa status and ultrasound examination results in the third trimester); clinical manifestations during pregnancy (prenatal vaginal bleeding); and amount of blood loss during cesarean section. Postpartum hemorrhage was defined as blood loss greater than 1000 ml during cesarean delivery.
Doppler ultrasound examination
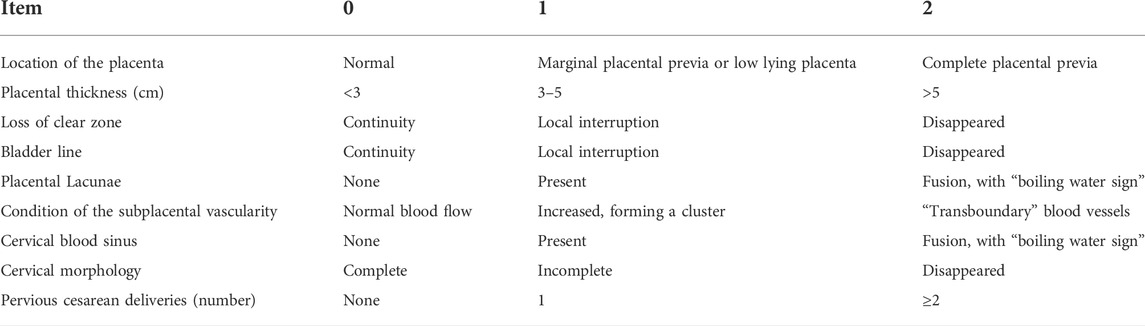
Ultrasound devices (Voluson E10; GE Medical Systems, Milan, Italy) with curved probes (1–5 MHz) and endovaginal transducers (5–9 MHz) were used. Before the examination, the patient, with a full bladder, was placed in a supine position. Patients were scanned for placental location and thickness, placental echo, placental margin, and uteral margin and thickness. When the placenta was located at the base of the anterior wall, the relationship between the continuity of the posterior wall and bladder wall was specifically scanned. Sonographic scores were computed using complete scan data, including location of the placenta, placental thickness, continuity of the clear space, bladder line, placental lacunae, condition of the subplacental vascularity, and cervical morphology, cervical sinus blood, as well as the number of pervious cesarean deliveries (Chen et al., 2021) (Table 1).
Statistical analysis
Analyses were performed in the R-Studio environment using R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). In order to identify the risk factors of postpartum hemorrhage, the odds ratio (OR) and 95% confidence interval (CI) were calculated for each clinical factor using univariate logistics regression analysis. Lasso regression analysis was performed on the risk factors screened by univariate analysis to screen out the final risk factors affecting postpartum hemorrhage. Logical Lasso regression modeling is a shrinkage approach that actively selects from a large and potentially multicollinear set of variables to produce a more relevant and interpretable set of predictive variables (Muthukrishnan and Rohini, 2016). We used tenfold cross validation to select the penalty term, λ. The built-in function in R produces two automatic λs, one of which minimizes the binomial bias, rendering the covariables included in the study more comprehensive. We also constructed a nomogram of postpartum hemorrhage based entirely on the total ultrasound risk score The prediction ability of the nomograms was evaluated by the area under the receiver operating characteristic (ROC) curve (AUC); AUCs closer to 1.0 are considered to have better recognition ability (Obuchowski and Bullen, 2018). The nomograms were internally verified by bootstrapping (1,000 resamplings) (Henderson, 2005). Decision curve analysis (DCA) (Vickers et al., 2019) was used to calculate the total benefit at each possible risk threshold and evaluate the clinical efficacy of the nomogram. Statistical significance was set as p < 0.05.
Results
Patient characteristics
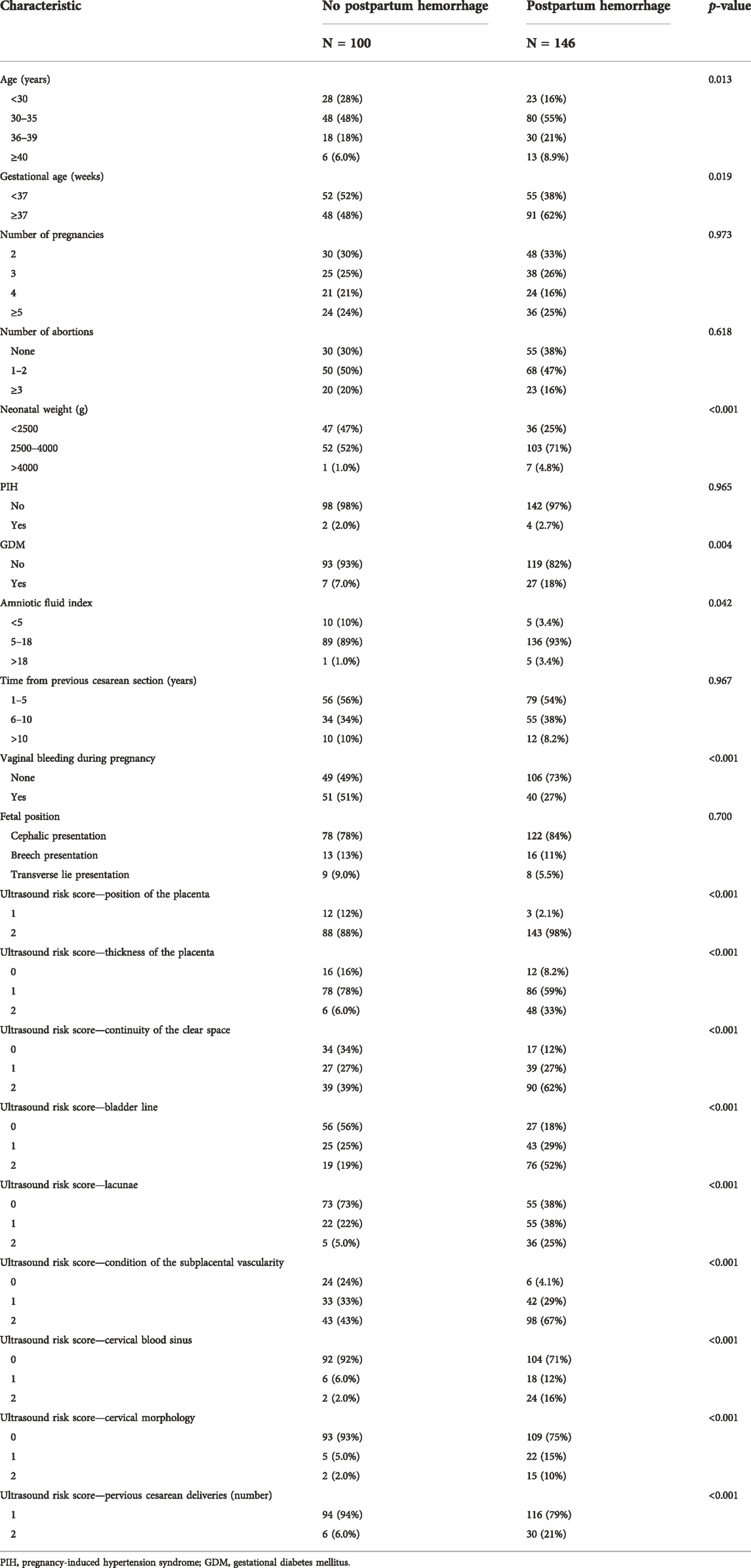
During the study period, 329 patients underwent ultrasound examination for suspected PPP. After applying the exclusion criteria, 246 patients remained and were included in the study. Of these, 146 patients experienced postpartum bleeding. Specific patient characteristics are shown in Table 2.
Selected risk factors
Results of the univariate logistic regression and Lasso regression analyses of postpartum hemorrhage in PPP are shown in Table 3; Figure 2. In the final Lasso analysis, older age, larger gestational age, larger neonatal birth weight, presence of gestational diabetes mellitus (GDM), larger amniotic fluid index, absence of gestational bleeding, and higher ultrasound risk single score were associated with a higher risk of postpartum hemorrhage.
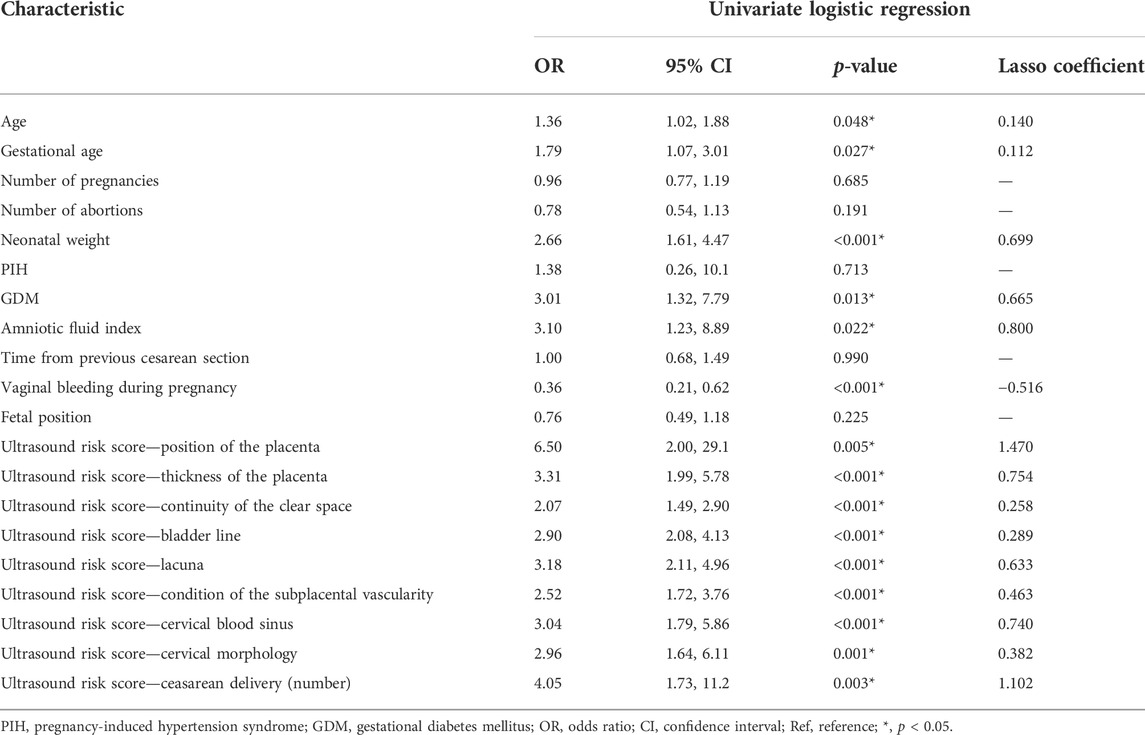
TABLE 3. Univariate logistic and Lasso regression analyses of postpartum hemorrhage in pernicious placenta previa.
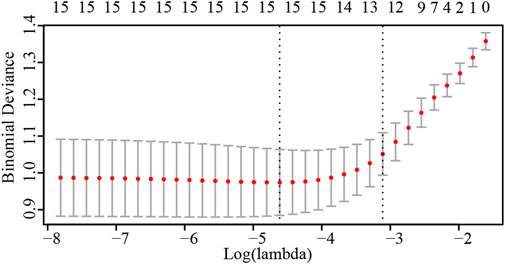
FIGURE 2. Number of risk factors determined by cross-validation of penalty terms in Lasso regression analysis.
Nomogram construction
The nomogram of postpartum hemorrhage based on variables from the Lasso regression analysis, including age, gestational age, neonatal birth weight, GDM, amniotic fluid index, gestational bleeding, and ultrasound risk single score is shown in Figure 3. The nomogram of postpartum hemorrhage based entirely on the ultrasonic risk total score is shown in Supplementary Material S1.
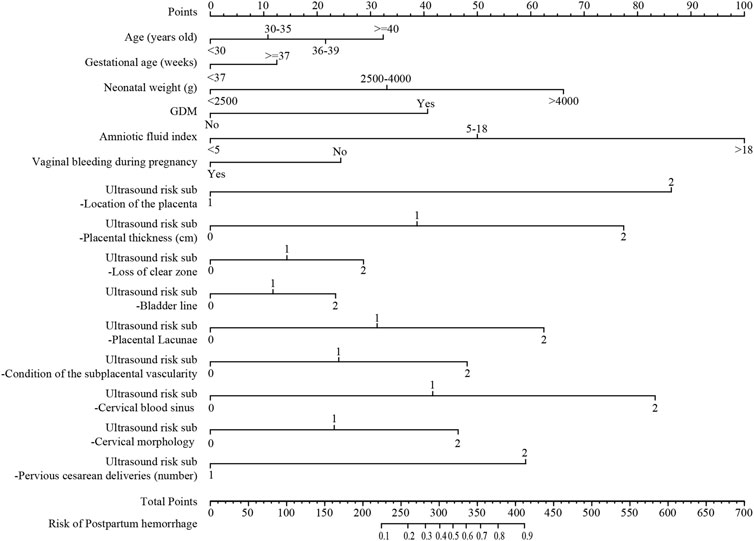
FIGURE 3. The nomogram of postpartum hemorrhage based on the Lasso regression analysis. GDM, gestational diabetes mellitus.
Nomogram performance
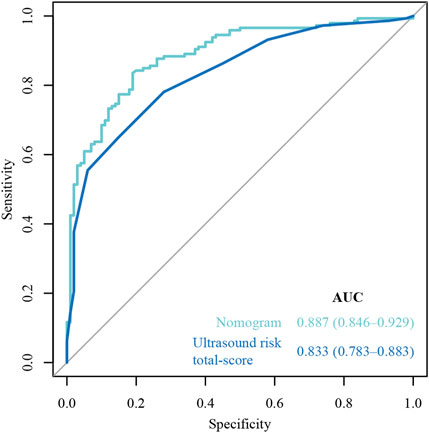
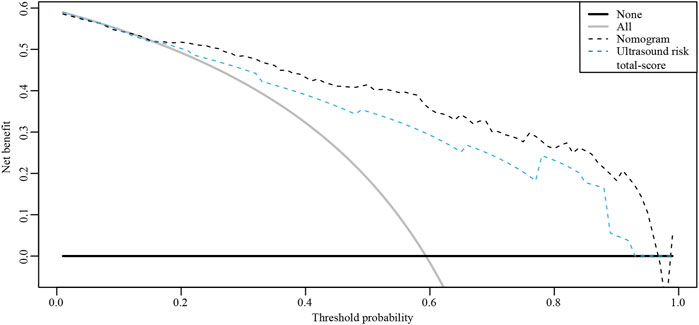
The ROC curves of the nomograms of postpartum hemorrhage are shown in Figure 4. The AUC of the nomogram constructed by Lasso regression analysis was higher than that of the nomogram constructed by the ultrasonic risk total score alone, suggesting that the former nomogram has a better ability to predict postpartum hemorrhage than the latter nomogram. In addition, DCA indicated that the former nomogram has better clinical efficacy than the latter nomogram (Figure 5). Internal validation of the nomogram based on Lasso regression analysis showed a calibration curve close to 45 degrees (Figure 6), indicating good agreement between predicted and actual values.
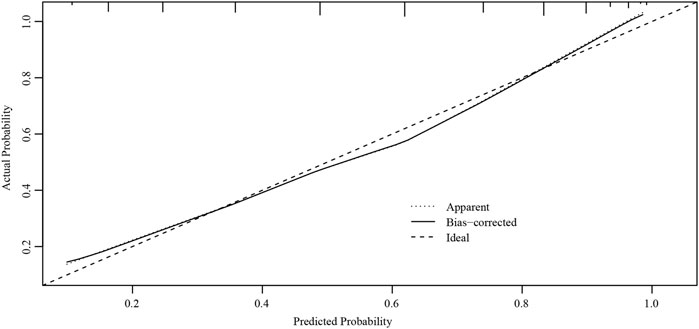
FIGURE 6. Internal verification plots of the nomogram calibration curves by bootstrapping with 1000 resamples.
Discussion
In 2016, the Chinese scholar, Yang, and colleagues reported that the average incidence of PPP at Peking University Third Hospital was 2.08/1000, which increased each year from 0.9/1000 in 2008 to 3.08/1000 in 2014. In addition, 53.3% of patients had placenta implantation, and 90% of patients suffered bleeding of more than 3,000 ml during surgery (Lin Yu et al., 2016). At present, research on a prenatal diagnostic method for PPP is an important and active field in obstetrics worldwide. However, due to a lack of objective indicators for an accurate preoperative judgment and evaluation of the degree of risk in PPP, preoperative preparation is often inadequate or excessive, resulting in unnecessary hysterectomy or a waste of medical resources.
The main cause of PPP postpartum hemorrhage is placental implantation, which is collectively referred to in the 2018 FIGO guidelines as placenta accreta spectrum (PAS) (Hecht et al., 2020). Numerous studies on the ultrasonic diagnosis of PAS, have reported diagnostic sensitivities ranging from 50% to 87% (Jauniaux et al., 2018; D’Antonio et al., 2013; Jauniaux and Bhide, 2017). Therefore, regardless of the imaging method, the prenatal diagnosis of PPP with/without PAS is relatively subjective, and its accuracy depends largely on the experience of the operator. A study published by Dimitrova and colleagues suggested that the diagnostic accuracy of PPP was higher in ultrasound operators who received standard training than in ultrasound operators who only received basic obstetrics training (Dimitrova et al., 2019). Naturally, in addition to subjective factors, some objective factors, such as subcutaneous fat and poor patient compatibility, contribute to the occurrence of missed diagnoses and the misdiagnosis of ultrasound results. A meta-analysis conducted by Xu and colleagues showed that magnetic resonance imaging was more effective than ultrasound in the prenatal diagnosis of PPP (Zeng et al., 2018).
Zhao and colleagues published an ultrasonic scoring system for PAS in 2018, which can not only predict the type of PAS, but also predict the risk of intraoperative bleeding and hysterectomy (Chong et al., 2018). Higher PAS scores indicate higher risk of intraoperative hemorrhage and hysterectomy. By drawing an ROC curve, the cut-off values of PAS were determined by a score of 5 for placenta increta (PI) and 10 for placenta percreta (PP). The scale is easy to operate and understand by using typical signs of placenta implantation as the prediction standard, combined with the high-risk factor of a previous cesarean section, to evaluate postpartum hemorrhage in PPP with specific scores. Use of this scale can effectively avoid the influence of subjective factors and has been promoted and recognized in China’s clinical practice (Lingyan Zhu et al., 2018; Hui Cen et al., 2019; Zhu and Xie, 2019). Building upon this scale, the present study combined ultrasonic data with clinical characteristics; all factors were fitted and quantified to establish a prediction model. Its accuracy was significantly higher than that of the ultrasonic risk total score, and it had better application value.
Calì and colleagues (Calì et al., 2013) reported that hypervascularity of the entire uterine serosa-bladder wall interface showed a sensitivity, specificity, negative predictive value, and positive predictive value of 90%, 100%, 100%, and 97%, respectively, in 17 PP cases. Furthermore, irregular intraplacental vascular formation and curved vessels affecting the entire width of the placenta showed high specificity (100%) and positive predictive value (100%). In a retrospective analysis of ultrasound imaging data from 232 pregnant women with high-risk factors for PAS, Chalubinski et al. (2013) predicted the implant type based on specific recognized ultrasound signs for PAS diagnosis, with clinical outcomes and pathological returns as the final evaluation criteria; this method did not misdiagnose PI or PP as normal placenta or PA, and the accuracy of screening reached 100%. Han et al (Penghui Han et al., 2016) reported that the degree of abnormal thickening of blood vessels in the placenta and the size of the blood sinus may be related to the range and depth of PA. Furthermore, the report by Yanmei Liu et al. (2017) suggested that the risk of hysterectomy during cesarean section was very high when the ultrasound examination found extensive lacunae in the placenta, focal or extensive lacunae in the placenta with visible blood flow, and abundant blood flow at the serum-bladder junction.
In addition, many researchers have reported on the risk factors of PPP accompanied by PAS, but controversy still exists. The reported risk factors mainly comprised old age, history of multiple uterine surgeries, history of multiple abortions, intrauterine membrane inflammation, and maternal lifestyle habits such as smoking (Eshkoli et al., 2013). Furthermore, medicines used during pregnancy may lead to PA by affecting the secretion of some factors in the placenta, thus precipitating trophoblast cell invasion and abnormal angiogenesis of the placenta. In addition, medicines such as heparin and aspirin may affect blood circulation and cytokine secretion throughout the body, which may lead to changes in maternal endometrial hormone levels, which in turn affect endometrial decidualization, leading to PAS (Niringiyumukiza et al., 2018). Additionally, the study by Bowman et al. (2014) showed that the number of previous cesarean sections was a risk factor affecting the degree of PPP. In the present study, postpartum hemorrhage in PPP was associated with neonatal weight, GDM, and vaginal bleeding during pregnancy.
At present, there are few reports on the relationship between vaginal bleeding in early pregnancy and PAS/PPP. Multivariate logistic regression analysis in Chou et al. (2000) identified a history of vaginal bleeding in early pregnancy as an independent risk factor for PAS (p < 0.05), which significantly increased the risk of placental implantation approximately fivefold (OR = 5.336, 95% CI 1.874–15.197). Wu and Yan, 2015 reported that slight vaginal bleeding in early pregnancy was related to embryo implantation, but long-term and severe bleeding could cause pathological damage to the decidua and placenta, thus reducing the physiological function of the placenta and ultimately leading to pregnancy complications such as PAS. There are also studies that patients with PI and PP, but not easy to cause vaginal bleeding (Bhide et al., 2019). In the present study, vaginal bleeding during pregnancy was an independent risk factor for postpartum hemorrhage in PPP, and patients with PPP with vaginal bleeding during pregnancy had a lower rate of postpartum bleeding, which is consistent with the results of Bhide et al. (2019).
Additionally, GDM is an independent risk factor for postpartum hemorrhage in PPP. There are numerous studies on angiogenesis and vascular remodeling associated with the placenta in GDM. The most common placental changes associated with GDM are chorangiosis (Stanek, 2016) and placental venous immaturity (Ogino and Redline, 2000; Rossi et al., 2012; Soma et al., 2013), and GDM is associated with accelerated microangiopathy. The number of villous capillaries is increased, especially in the center of the villi, in the placenta in GDM. Daskalakis et al. (2008) investigated 40 placentae associated with GDM and found that 40% of placentae showed chorangiosis. The proliferation of capillaries and pathophysiological changes in the placenta may increase the risk of postpartum hemorrhage in PPP.
Due to the establishment of inclusion and exclusion criteria and the limited number of PPP patients, the study results have some limitations. The ultrasound-based nomogram was designed to predict the risk of postpartum bleeding and the risk of adverse outcomes in PPP, should be verified in prospective cohort in the future. In addition, the prediction efficiency of different ultrasonic signs for PPP combined with PAS is different. If multicenter studies are included in which different intraoperative treatments are used, the current predictions might have changed considerably.
Conclusion
Our ultrasound-based nomogram can be used to predict postpartum hemorrhage and the risk of adverse outcomes in PPP. Our nomogram can aid clinicians in accurately assessing the severity of PPP before surgery, to determine the best time to terminate pregnancy according to clinical characteristics and ultrasound results, and fully prepare their personnel and supplies before surgery. The planned organization of a multiple disciplinary team with member from obstetrics, neonatology, anesthesiology, the intensive care unit, interventional radiology, and the blood bank can improve the outcomes of PPP as much as possible, while ensuring the safety of patients, taking into account fetal maturity and post-birth survival.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shengjing Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ provided research ideas and contributed to the drafting of the manuscript. XW and MZ extracted the data and performed the statistical analysis. ZS performed the statistical analysis and wrote the manuscript. XC and DZ conceptualized the research and conducted quality control. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by internal funding from Shengjing Hospital, China Medical University (SJ-M0133), 345 Talent Project of Shengjing Hospital of China Medical University (No. M0946), and Medical Education Research Project of Liaoning Province (No. 2022-N005-03).
Acknowledgments
We would like to express our gratitude to all those who helped us during the writing of this manuscript. We thank to all of the peer reviewers for their opinions and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.982080/full#supplementary-material
Abbreviations
AUC, area under the receiver operating characteristic curve; CI, confidence interval; DCA, decision curve analysis; GDM, gestational diabetes mellitus; OR, odds ratio; PA, placenta accrete; PAS, placenta accreta spectrum; PI, placenta increta; PP, placenta percreta; PPP, pernicious placenta previa; ROC, receiver operating characteristic.
References
Berkley E. M., Abuhamad A. (2018). Imaging of placenta accreta spectrum. Clin. Obstet. Gynecol. 61 (4), 755–765. doi:10.1097/grf.0000000000000407
Bhide A., Laoreti A., Kaelin Agten A., Papageorghiou A., Khalil A., Uprichard J., et al. (2019). Lower uterine segment placental thickness in women with abnormally invasive placenta. Acta Obstet. Gynecol. Scand. 98 (1), 95–100. doi:10.1111/aogs.13422
Bowman Z. S., Eller A. G., Bardsley T. R., Greene T., Varner M. W., Silver R. M. (2014). Risk factors for placenta accreta: A large prospective cohort. Am. J. Perinatol. 31 (9), 799–804. doi:10.1055/s-0033-1361833
Calì G., Giambanco L., Puccio G., Forlani F. (2013). Morbidly adherent placenta: Evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta. Ultrasound Obstet. Gynecol. 41 (4), 406–412. doi:10.1002/uog.12385
Chalubinski K. M., Pils S., Klein K., Seemann R., Speiser P., Langer M., et al. (2013). Prenatal sonography can predict degree of placental invasion. Ultrasound Obstet. Gynecol. 42 (5), 518–524. doi:10.1002/uog.12451
Chattopadhyay S. K., Kharif H., Sherbeeni M. M. (1993). Placenta praevia and accreta after previous caesarean section. Eur. J. Obstet. Gynecol. Reprod. Biol. 52 (3), 151–156. doi:10.1016/0028-2243(93)90064-j
Chen L., Shi H. F., Jiang H., Shi X. M., Wang Y. Y., Zhang A. Q., et al. (2021). Correlation of an ultrasonic scoring system and intraoperative blood loss in placenta accreta spectrum disorders: A retrospective cohort study. Biomed. Environ. Sci. 34 (2), 163–169. doi:10.3967/bes2021.022
Chong Y., Zhang A., Wang Y., Chen Y., Zhao Y. (2018). An ultrasonic scoring system to predict the prognosis of placenta accreta: A prospective cohort study. Medicine 97 (35), e12111. doi:10.1097/md.0000000000012111
Chou M. M., Ho E. S., Lee Y. H. (2000). Prenatal diagnosis of placenta previa accreta by transabdominal color Doppler ultrasound. Ultrasound Obstet. Gynecol. 15 (1), 28–35. doi:10.1046/j.1469-0705.2000.00018
Comstock C. H., Love J. J., Bronsteen R. A., Lee W., Vettraino I. M., Huang R. R., et al. (2004). Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am. J. Obstet. Gynecol. 190 (4), 1135–1140. doi:10.1016/j.ajog.2003.11.024
D'Antonio F., Iacovella C., Bhide A. (2013). Prenatal identification of invasive placentation using ultrasound: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 42 (5), 509–517. doi:10.1002/uog.13194
Daskalakis G., Marinopoulos S., Krielesi V., Papapanagiotou A., Papantoniou N., Mesogitis S., et al. (2008). Placental pathology in women with gestational diabetes. Acta Obstet. Gynecol. Scand. 87 (4), 403–407. doi:10.1080/00016340801908783
Dimitrova I., Jauniaux E., Zosmer N., De Stefani L. B., Andrade W., Bourmpaki E., et al. (2019). Development of a training program for the ultrasound screening of placenta accreta spectrum disorders. Int. J. Gynaecol. Obstet. 147 (1), 73–77. doi:10.1002/ijgo.12900
Eshkoli T., Weintraub A. Y., Sergienko R., Sheiner E. (2013). Placenta accreta: Risk factors, perinatal outcomes, and consequences for subsequent births. Am. J. Obstet. Gynecol. 208 (3), e1–e17. doi:10.1016/j.ajog.2012.12.037
Finberg H. J., Williams J. W. (1992). Placenta accreta: Prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J. Ultrasound Med. 11 (7), 333–343. doi:10.7863/jum.1992.11.7.333
Hecht J. L., Baergen R., Ernst L. M., Katzman P. J., Jacques S. M., Jauniaux E., et al. (2020). Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: Recommendations from an expert panel. Mod. Pathol. 33 (12), 2382–2396. doi:10.1038/s41379-020-0569-1
Henderson A. R. (2005). The bootstrap: A technique for data-driven statistics. Using computer-intensive analyses to explore experimental data. Clin. Chim. Acta. 359 (1-2), 1–26. doi:10.1016/j.cccn.2005.04.002
Horng H. C., Lai M. J., Chang W. H., Wang P. H. (2021). Placenta accreta spectrum (PAS) and peripartum hysterectomy. Taiwan. J. Obstet. Gynecol. 60 (3), 395–396. doi:10.1016/j.tjog.2021.03.001
Hui Cen L. S., Chen X., Zhong L. (2019). Application of placenta accreta score by ultrasound for evaluating patients with dangerous placenta previa. Chin. J. Fam. Plan. 27 (9), 1170–1173.
Jauniaux E., Bhide A. (2017). Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 217 (1), 27–36. doi:10.1016/j.ajog.2017.02.050
Jauniaux E., Collins S., Burton G. J. (2018). Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am. J. Obstet. Gynecol. 218 (1), 75–87. doi:10.1016/j.ajog.2017.05.067
Jauniaux E., Hussein A. M., Fox K. A., Collins S. L. (2019). New evidence-based diagnostic and management strategies for placenta accreta spectrum disorders. Best. Pract. Res. Clin. Obstet. Gynaecol. 61, 75–88. doi:10.1016/j.bpobgyn.2019.04.006
Jingyi Huang Z. L., Gu D. (2020). Ultrasound scoring for prediction of dangerous placenta previa combined with placenta accreta and analysis of adverse pregnancy outcomes. Chin. J. Med. Phys. 37 (9), 1160–1163.
Lin Yu K. H., Yang H., Yang H. X. (2016). A retrospective analysis on the pernicious placenta previa from 2008 to 2014. Zhonghua Fu Chan Ke Za Zhi 51 (103), 169–173. doi:10.3760/cma.j.issn.0529-567X.2016.03.002
Lingyan Zhu Z. N., Jin Q., Li L., Ma X. (2018). The value of prenatal diagnosis of ultrasonographic scoring system in placenta previa with placenta accrete. J. Kunming Med. Univ. 39 (4), 113–116.
Liu X. D., Liu X., Zhang Y., He X. (2019). Development of prenatal ultrasound diagnosis and risk prediction for pernicious previa with placental implantation. Chin. J. Fam. Plan. 27 (4), 539–543.
Muthukrishnan R., Rohini R. (2016). Lasso: A feature selection technique in predictive modeling for machine learning. 2016 IEEE International Conference on Advances in Computer Applications (ICACA). 18–20 p.
Niringiyumukiza J. D., Cai H., Xiang W. (2018). Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 16 (1), 43. doi:10.1186/s12958-018-0359-5
Obuchowski N. A., Bullen J. A. (2018). Receiver operating characteristic (ROC) curves: Review of methods with applications in diagnostic medicine. Phys. Med. Biol. 63, 07TR01. doi:10.1088/1361-6560/aab4b1
Ogino S., Redline R. W. (2000). Villous capillary lesions of the placenta: Distinctions between chorangioma, chorangiomatosis, and chorangiosis. Hum. Pathol. 31 (8), 945–954. doi:10.1053/hupa.2000.9036
Oppenheimer L. (2007). Diagnosis and management of placenta previa. J. obstetrics Gynaecol. Can. JOGC = J. d'obstetrique Gynecol. du Can. JOGC 29 (3), 261–266. doi:10.1016/s1701-2163(16)32401-x
Pasto M. E., Kurtz A. B., Rifkin M. D., Cole-Beuglet C., Wapner R. J., Goldberg B. B. (1983). Ultrasonographic findings in placenta increta. J. Ultrasound Med. 2 (4), 155–159. doi:10.7863/jum.1983.2.4.155
Penghui Han K. J., Guo Q., Ouyang C., Lei Q., Ou J. (2016). The value of ultrasonography and MRI in the diagnosis of placenta accreta. J. China Clin. Med. Imaging 27 (03), 194–197.
Rossi R., Scillitani G., Vimercati A., Fiore M. G., Mastrodonato M., Resta L. (2012). Diabetic placenta: Ultrastructure and morphometry of the term villi. Anal. Quant. Cytopathol. Histpathol. 34 (5), 239–247.
Soma H., Murai N., Tanaka K., Oguro T., Kokuba H., Fujita K., et al. (2013). Angiogenesis in villous chorangiosis observed by ultrastructural studies. Med. Mol. Morphol. 46 (2), 77–85. doi:10.1007/s00795-013-0010-7
Souza J. P., Gülmezoglu A., Lumbiganon P., Laopaiboon M., Carroli G., Fawole B., et al. (2010). Caesarean section without medical indications is associated with an increased risk of adverse short-term maternal outcomes: The 2004-2008 WHO global survey on maternal and perinatal health. BMC Med. 8, 71. doi:10.1186/1741-7015-8-71
Stanek J. (2016). Chorangiosis of chorionic villi: What does it really mean? Arch. Pathol. Lab. Med. 140 (6), 588–593. doi:10.5858/arpa.2015-0160-OA
Tovbin J., Melcer Y., Shor S., Pekar-Zlotin M., Mendlovic S., Svirsky R., et al. (2016). Prediction of morbidly adherent placenta using a scoring system. Ultrasound Obstet. Gynecol. 48 (4), 504–510. doi:10.1002/uog.15813
Vickers A. J., van Calster B., Steyerberg E. W. (2019). A simple, step-by-step guide to interpreting decision curve analysis. Diagn. Progn. Res. 3, 18. doi:10.1186/s41512-019-0064-7
WeiyuanZhang L. H. (2018). Insight of the big data survey of cesarean section situation in mainland China. Chin. J. Pract. Gynecol. Obstetrics 34 (1), 38–40. doi:10.19538/j.fk2018010110
Wong H. S., Cheung Y. K., Zuccollo J., Tait J., Pringle K. C. (2008). Evaluation of sonographic diagnostic criteria for placenta accreta. J. Clin. Ultrasound 36 (9), 551–559. doi:10.1002/jcu.20524
Wu X., Yan J. (2015). Relationship between abnormality of maternal-fetal interface and placenta implantation Progress in Obstetrics and Gynecology. 24(10), 792–795.
Yanmei Liu H. L., Zuo C., Dong D, Zhang C., Hu Y. (2017). Value of ultrasonic image features in prenatal diagnosis of placenta implantation and prediction of postpartum hysterectomy. Shaanxi Med. J. 46 (04), 521–522.
Yunshan Chen H. L. (2020). Changes of indications for cesarean section. Chin. J. Obstetric Emerg. Electron. Ed. 9 (2), 65–68.
Zeng C., Yang M., Ding Y., Duan S., Zhou Y. (2018). Placenta accreta spectrum disorder trends in the context of the universal two-child policy in China and the risk of hysterectomy. Int. J. Gynaecol. Obstet. 140 (3), 312–318. doi:10.1002/ijgo.12418
Zheng W., Zhang H., Ma J., Dou R., Zhao X., Yan J., et al. (2021). Validation of a scoring system for prediction of obstetric complications in placenta accreta spectrum disorders, J. Maternal-Fetal Neonatal Med., Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 1–7. doi:10.1080/14767058.2020.1847077
Keywords: pernicious placenta previa, postpartum hemorrhage, nomogram, univariate logistic regression, LASSO regression, decision curve analysis, area under the curve
Citation: Zhou Y, Song Z, Wang X, Zhang M, Chen X and Zhang D (2022) Ultrasound-based nomogram for postpartum hemorrhage prediction in pernicious placenta previa. Front. Physiol. 13:982080. doi: 10.3389/fphys.2022.982080
Received: 30 June 2022; Accepted: 26 July 2022;
Published: 22 August 2022.
Edited by:
Xun Jia, Johns Hopkins Medicine, United StatesReviewed by:
Dazhi Fan, Foshan Women and Children Hospital, ChinaMengjun Dai, Shanghai Jiao Tong University, China
Xiaodong Wang, Sichuan University, China
Copyright © 2022 Zhou, Song, Wang, Zhang, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Zhang, emhhbmdkZEBzai1ob3NwaXRhbC5vcmc=
 Yangzi Zhou
Yangzi Zhou Zixuan Song
Zixuan Song Xiaoxue Wang2
Xiaoxue Wang2 Dandan Zhang
Dandan Zhang