
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol. , 15 September 2022
Sec. Exercise Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.965366
This article is part of the Research Topic Current Advances in Exercise Immunology View all 10 articles
Pyroptosis plays a crucial role in a variety of human diseases, including atherosclerosis, obesity, diabetes, depression, and Alzheimer’s disease, which usually release pyroptosis-related cytokines due to inflammation. Many studies have demonstrated that aerobic exercise is a good option for decreasing the release of pyroptosis-related cytokines. However, the molecular mechanisms of aerobic exercise on pyroptosis-related diseases remain unknown. In this review, the effects of aerobic exercise on pyroptosis in endothelial cells, adipocytes and hippocampal cells, and their potential mechanisms are summarized. In endothelial cells, aerobic exercise could inhibit NOD-like receptor protein 3 (NLRP3) inflammasome-mediated pyroptosis by improving the endothelial function, while reducing vascular inflammation and oxidative stress. In adipocytes, aerobic exercise has been shown to inhibit pyroptosis by ameliorating inflammation and insulin resistance. Moreover, aerobic exercise could restrict pyroptosis by attenuating microglial activation, neuroinflammation, and amyloid-beta deposition in hippocampal cells. In summary, aerobic exercise alleviates the pyroptosis-related diseases by regulating the NLRP3 inflammation si0067naling.
Pyroptosis, a type of lytic programmed cell death caused by inflammasomes, is an important natural immune response in our body (Kovacs and Miao 2017). Pore formation in the plasma membrane, swelling and rupture of cells, massive leakage of cytoplasmic contents, and release of inflammatory factors are typical features of pyroptosis (Man et al., 2017). Pyroptosis is induced by the NOD-like receptor protein 3 (NLRP3) inflammasome, and triggered by Caspase-1 (Tang et al., 2020), which controls the N-terminal domain of gasdermin D (GSDMD) by assembling channels in the cell membrane and activates interleukin (IL)-1β and IL-18 (Schroder and Tschopp 2010) (Figure 1).
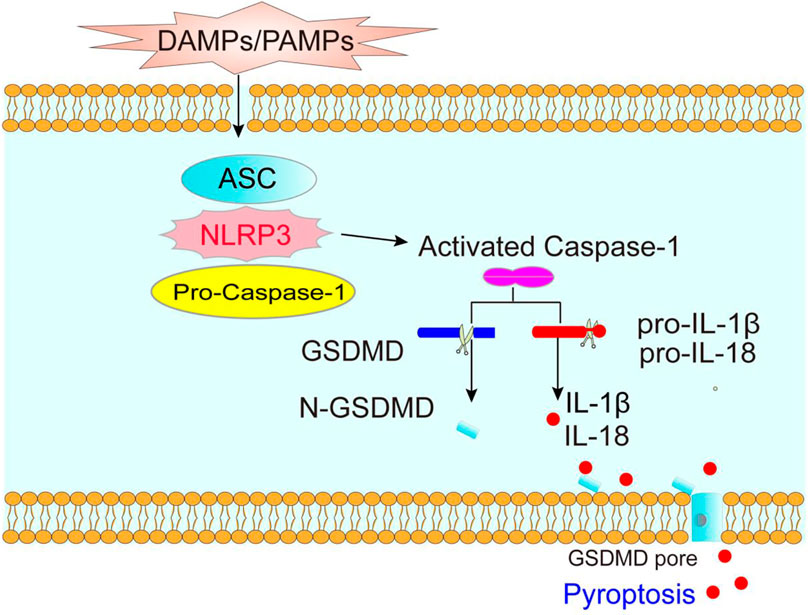
FIGURE 1. The molecular mechanism of pyroptosis. DAMPs (danger-associated molecular patterns) and PAMPs (pathogen-related molecular patterns) activate NLRP3 inflammasome, promotes Caspase-1 activation, which cleavages GSDMD and the precursor of IL-1β and IL-18, forming mature IL-1β and IL-18, thereby causing pyroptosis.
Pyroptosis occurs in multiple cell types (Shi et al., 2017), including endothelial cells, adipocytes and hippocampal cells. Many studies have suggested that pyroptosis takes an important role in the development of human diseases, including obesity (Mardare et al., 2016), diabetes (Vandanmagsar et al., 2011), atherosclerosis (Hong et al., 2021), Alzheimer’s disease (AD) (Liang et al., 2020), and depression (Liu et al., 2015). Aerobic exercise exhibits an obvious anti-inflammatory effect and is closely related to pyroptosis (Kar et al., 2019). As it is known, aerobic exercise could reduce chronic inflammation and effectively inhibit the expression of inflammatory factors, thereby increasing the release of anti-inflammatory cytokines. Previous studies have found that aerobic exercise could decrease the expression of NLRP3 inflammasome and markedly inhibit the activation of ASC, Caspase-1, IL-1β, and IL-18 (Kar et al., 2019; Lee et al., 2020). Although aerobic exercise can regulate cell pyroptosis, its specific effects on pyroptosis-related diseases and potential mechanisms still need further clarification.
The present review aimed to identify the relationship between aerobic exercise and NLRP3 inflammasome-mediated pyroptosis in endothelial cells, adipocytes, and hippocampal cells, and to investigate the potential mechanism of the effect of aerobic exercise on pyroptosis-related diseases.
Endothelial cell’s pyroptosis is among the major causes of cardiovascular diseases (Zhang L. et al., 2019). Aerobic exercise is an important strategy to control the endothelial cell’s pyroptosis, and inhibiting the development of cardiovascular diseases.
Endothelial cells are considered to be an important modulator in vascular homeostasis, regulated by various paracrine factors, and they play a critical role in maintaining normal vascular tension and blood flow and in inhibiting vascular inflammation and oxidative stress. Endothelial dysfunction is a classical symbol and predictor of cardiovascular diseases (Bai et al., 2020), and pyroptosis confers a decisive contribution to vascular endothelial dysfunction during the development of related diseases. Previous studies have suggested that endothelial cell’s pyroptosis was associated with cardiovascular diseases, including atherosclerosis (Zhang L. et al., 2019) and hypertension (Wu et al., 2022). Besides, the activation of NLRP3, ASC, Caspase-1, and GSDMD is increased significantly in atherosclerotic endothelial cells (Zhang et al., 2018). Furthermore, NLRP3 inflammasome, Caspase-1, and IL-1β trigger inflammation in the blood vessel wall, thereby leading to atherosclerosis (Karasawa and Takahashi 2017). Oxidized low-density lipoprotein (ox-LDL) and cholesterol crystals are abundant in atherosclerotic lesions (Zhang et al., 2015; Keping et al., 2020). Ox-LDL (Keping et al., 2020) and cholesterol crystals (Duewell et al., 2010; Zhang et al., 2015) could also promote NLRP3 inflammasome and Caspase-1 activation, leading to the release of IL-1β and IL-18 in immune cells. Especially, NLRP3 inflammasome promotes plaque formation and contributes to the development of atherosclerosis by affecting several targets, including signal transducer and activator of transcription (STAT), mitogen-activated protein kinases (MAPK), c-Jun N-terminal kinase (JNK), microRNA network, reactive oxygen species (ROS), and protein kinase R (PKR) (Hoseini et al., 2018). Thus, endothelial cell’s pyroptosis contributes to atherosclerosis formation and development by accelerating the release of inflammatory cytokines and increasing the vascular permeability (Zhaolin et al., 2019).
Additionally, NLRP3-dependent pyroptosis mediates endothelial dysfunction, which provides an impetus for hypertension (Pasqua et al., 2018), cardiovascular complications of coronary heart disease, and atherosclerosis in endothelial cells. The study highlighted that pyroptosis is a significant mediator of vascular dysfunction and injury in hypertensive patients (De Miguel et al., 2021). The serum level of IL-1β was higher in patients with essential hypertension than in healthy persons (Zeng et al., 2019). Besides, the research shows that the downregulation of the expression of key components of the NLRP3 inflammasome can delay the development of hypertension (De Miguel et al., 2021). The study found that microcrystals, and high levels of extracellular ATP and ROS could activate the NLRP3 inflammasome in the hypertensive patients (Krishnan et al., 2014). Overall, the endothelial cell’s pyroptosis is closely associated with the development of cardiovascular diseases. Aerobic exercise is an ideal non-drug management to inhibit endothelial cell’s pyroptosis and takes an essential role in treating cardiovascular diseases.
Aerobic exercise is beneficial for maintaining the function of vascular endothelial cells (Kourek et al., 2021). Notably, aerobic exercise could significantly alleviate the endothelial dysfunction and reduce the risk of cardiovascular diseases (Neunhäuserer et al., 2021). The study also showed that aerobic exercise could increase the blood flow and laminar shear stress as well as reduce leukocyte adhesion (You et al., 2013), and risk of inflammation, thereby improving the antioxidant system of enzymes and immune responses. Many studies have found that endothelial cell’s pyroptosis can be inhibited by aerobic exercise (Lee et al., 2018; Lee et al., 2020). Lee et al. (2018) have proved that voluntary running could reduce the activation of NLRP3 inflammasome in the endothelial cells of the coronary arteries. Their findings further suggested that aerobic exercise improves the vascular function by inhibiting NLRP3 inflammasome signaling (Lee et al., 2020). Other studies also reported that treadmill exercise of >12 weeks could reduce the endothelial cell’s pyroptosis in arteriosclerosis (Hong et al., 2018; Hong et al., 2021) (as shown in Table 1).
Accumulating evidence has demonstrated that NLRP3 inflammasome plays a vital role in vascular inflammation (Wang L. et al., 2016). In endothelial cells, NLRP3 inflammasomes could be activated in response to multiple stimuli and are involved in vascular pathology (Lee et al., 2020).
Stimuli, including oxidative stress, mitochondrial dysfunction and lysosomal rupture have been demonstrated to activate the NLRP3 inflammasomes (Hoseini et al., 2018), which are important initiators in the development of vascular diseases. Moreover, ox-LDL and cholesterol crystals stimulate nuclear factor-κB (NF-κB) activation and TNF-α secretion (Steyers and Miller 2014). Then, the activated NF-κB further affects the NLRP3 signaling and contributes to the development of atherosclerosis (Hoseini et al., 2018). Studies have demonstrated that 12 weeks of treadmill exercise could down-regulate NF-κB protein expression and inhibit NF-κB-mediated aortic inflammation in participants (Wu et al., 2017).
Additionally, NLRP3 inflammasomes can be activated by the thioredoxin-interacting protein (TXNIP), which plays a crucial role in inflammatory response (Byon et al., 2015). The TXNIP/NLRP3 inflammasome signaling is closely associated with the development and progression of atherosclerosis (Hoseini et al., 2018). The activated NLRP3 inflammasome could increase the expression and release of the high-mobility histone box-1 (HMGB1) in endothelial cells (Lee et al., 2020), promoting endothelial hyperpermeability and leading endothelial dysfunction (Wang L. et al., 2016; Wang et al., 2016a). Several studies have demonstrated that aerobic exercise can significantly reduce vascular inflammation by inhibiting NLRP3 inflammasome, HMGB1, and its downstream effects (Goh and Behringer 2018; Kar et al., 2019; Lee et al., 2020).
Vascular elasticity is regulated by generating many potent vasoactive substances, including vasodilator nitric oxide (NO) and contractile factor endothelin-1 in endothelial cells (Haybar et al., 2019). NO is a vasomotor factor produced and released by vascular endothelial cells, which has an important protective effect on the vascular wall and endothelial function (Ferentinos et al., 2022). NO bioavailability refers to the production and utilization of NO in endothelial cells, which is closely related with endothelial dysfunction. The reduction of NO bioavailability reportedly resulted from oxidative stress and expression of inflammatory factors (Chen et al., 2018). Similarly, a previous study has found that NO inhibits NLRP3 activation, thereby preventing pyroptosis in endothelial cells (Jiang et al., 2020).
Aerobic exercise is a promising non-medical treatment for preventing early endothelial dysfunction and redox imbalance by increasing NO bioavailability and reducing chronic inflammation (Gao et al., 2021). Moreover, aerobic exercise can effectively increase the NO content and enhance the diastolic function of vascular endothelial cells (Gao et al., 2021). NO can further increase the blood flow in the body during aerobic exercise.
In summary, aerobic exercise could regulate NO production and bioavailability to improve the endothelial cell’s function. Firstly, aerobic exercise increased the NO bioavailability by enhancing phosphorylated eNOS expression and reversing aortic endothelial dysfunction. In the vascular endothelium, aerobic exercise improves the NO bioavailability by enhancing endothelial NO synthase (eNOS) expression and eNOS/NO signaling (Lee et al., 2018), decreasing oxidative stress and inflammatory pathways. Aerobic exercise can enhance the heart’s pumping function, increase the heart’s output, accelerate the blood flow and blood shear stress, thereby stimulating the NO synthesis by vascular endothelial cells (Inoue et al., 2020). Secondly, aerobic exercise could improve the NO production by aggrandizing adiponectin (APN) and AdipoR1 levels (Lee et al., 2020). Thirdly, aerobic exercise can elevate the expression of junction proteins zonula occludin-1 (ZO-1) and ZO-2 (these are associated with endothelial permeability and dysfunction (Wang L. et al., 2016)) in endothelial cells, thereby facilitating NO production (Lee et al., 2020). Lastly, aerobic exercise induces the activity of superoxide dismutase (SOD), which results in the decrease in ROS production and ultimately improves the generation of NO (Cao et al., 2020). Studies have indicated that aerobic exercise could improve the endothelial cell’s function by downregulating TXNIP/NLRP3 inflammasome signaling (Hong et al., 2021). Overall, aerobic exercise could inhibit endothelial cell’s pyroptosis by improving the vascular endothelial cell’s function.
Nicotinamide adenine dinucleotide-phosphate (NADPH) oxidases take a vital role in oxidative stress. NADPH oxidases could produce superoxides (O2−), which induce reactive free radicals (Gjevestad et al., 2015) and act as the main source of ROS in blood vessels. The ROS-dependent activation of NLRP3 inflammasome can induce endothelial impairment (Rovira-Llopis et al., 2018) and oxidative stress. Previous studies have shown that NADPH subunit p22phox decreased the expression of IL-1β (Liao et al., 2020). Aerobic exercise could inhibit superoxide production and NADPH oxidases activity in coronary arteries (Hong et al., 2021), thereby reducing ROS production and oxidative stress (Cunha et al., 2017). Treadmill exercise reportedly could suppress ROS production by reducing the activity of NADPH oxidases (Jeong et al., 2018). As shown in Figure 2, the potential mechanisms of aerobic exercise modulating endothelial cell’s pyroptosis are as follows: 1) reduces vascular inflammation by inhibiting the expression of NLRP3 inflammasome. 2) improves endothelial function by enhancing NO bioavailability, and 3) decreases oxidative stress by reducing the activity of NADPH oxidase and IL-1β.
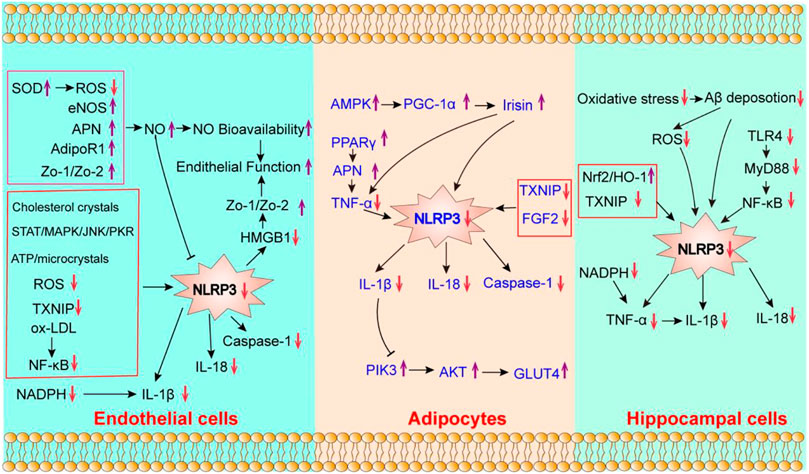
FIGURE 2. The potential effect of aerobic exercise on the pyroptosis of endothelial cells, adipocytes and hippocampal cells. “↓” indicates that its expression can be downregulated by aerobic exercise; “↑” indicates that it can be enhanced by aerobic exercise.
Adipocytes are closely related to metabolism, and aerobic exercise plays an important role in improving metabolic diseases by regulating adipocyte’s pyroptosis. Studies have shown that targeting the NLRP3 inflammasome would reduce diet-induced metabolic abnormalities in mice (Chiazza et al., 2016; Ding et al., 2019).
Adipose tissue is the largest endocrine organ of the human body capable of storing lipids, secreting a large amount of adipokines, and it takes an essential role in the metabolism of human nutrients (Carbone et al., 2019). Chronic inflammation and adipose tissue dysfunction usually occur in individuals or mice with diabetes or obesity (Šimják et al., 2018). Adipocyte’s pyroptosis is an important upstream event in metabolism-related diseases including obesity (Giordano et al., 2013) and diabetes (Vandanmagsar et al., 2011). The expressions of Caspase-1, NLRP3, and other related factors of adipocyte’s pyroptosis were abundantly present in obese patients and mice (Giordano et al., 2013), which are involved in systematic inflammation and glucose homeostasis of adipose tissues (Ding et al., 2019; Wu et al., 2020). Additionally, the elevated expressions of the NLRP3 inflammasome, IL-1β, and IL-18 in adipose tissues are directly associated with insulin resistance and severity of diabetes (Esser et al., 2013). Mitochondria are reportedly involved in regulating NLRP3 inflammasome activation in adipocytes (Zhang et al., 2021). Moreover, a previous study found that high-fat diet induced overactivation of NLRP3 inflammasome in mice, the protein expression of genes related to mitochondrial biogenesis decreased, suggesting that mitochondrial damage caused by glucose and lipid metabolism disorders may activate the NLRP3 inflammasomes (Zhang et al., 2021). Therefore, the adipocyte’s pyroptosis is mainly related to metabolic diseases. Further, aerobic exercise is an effective strategy to prevent metabolic diseases by limiting the adipocyte’s pyroptosis.
Lipids have important biological functions, in fact, fat is the energy provider in our body. The prominent roles of adipose tissue are to sequester fatty acids in times of energy excess and to release fatty acids via the process of lipolysis during times of high-energy demand, such as during an exercise. (Tsiloulis and Watt 2015). Several studies demonstrated that aerobic exercise could improve the function of adipocytes (Stanford et al., 2015), alter the expression of adipokines (Stinkens et al., 2018), and decrease adipocyte’s inflammation. Aerobic exercise training has been reported to inhibit the expression of pro-inflammatory factors in adipocytes, promotes the balance of the oxidative and antioxidant systems, and improves the inflammatory state. Researches have also demonstrated that 10 weeks of aerobic exercise ameliorates HFD-induced complications through the reduction of NLRP3, IL-18, TNF-α, TLR4 and IL-1β activation in adipocytes (Mardare et al., 2016). Therefore, aerobic exercise training is an effective strategy to reduce the expression of pyroptosis-related factors in adipocytes. As shown in Table 1, previous studies have shown that treadmill exercise training for >8 weeks can decrease the release of pyroptosis-associated factors in the adipocytes of obese or HFD rats (Mardare et al., 2016; ZhuGe et al., 2020). Nevertheless, the molecular mechanism of the effect of aerobic exercise on adipocyte’s pyroptosis remains unclear.
Inflammation in adipocytes plays a vital role in metabolic diseases, as it increases the expression of NLRP3 and its related inflammatory factors (Vandanmagsar et al., 2011). TXNIP (Wu et al., 2020) and FGF2 (ZhuGe et al., 2020) can exacerbate the inflammatory response in adipocytes by activating NLRP3 inflammasomes and Caspase-1. A previous study has shown that 8 weeks of treadmill training effectively inhibited the NLRP3 expression and reduced the FGF2 levels in adipose tissues (ZhuGe et al., 2020). Moreover, it has been reported that TNF-α is responsible for regulating the transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies (McGeough et al., 2017). Similar studies confirmed that the expression of NLRP3 was positively correlated with the release of TNF-α in adipose tissues (Bauernfeind et al., 2016). The increase in peroxisome proliferator-activated receptor-γ (PPARγ) levels raises the expression of APN as well as inhibits TNF-α release (Xia 2015). As it is known, PPARγ is responsible for regulating adipocyte differentiation (Ahmadian et al., 2013). In adipose tissues, the expression levels of PPARγ and APN could be increased significantly after aerobic exercise, while that of TNF-α was decreased (Xia 2015). Moreover, after 10 weeks of treadmill training, the significantly decreased expressions of NLRP3, TNF-α, and IL-1β were observed in adipose tissues (Mardare et al., 2016). The above mentioned results suggest that aerobic exercise training may inhibit adipocyte’s pyroptosis by reducing adipocyte inflammation.
Irisin, also known as fibronectin domain-containing protein 5 (FNDC5), is an exercise-inducing factor; it is not only a muscle factor but also an adipocytokine. A previous study has shown that irisin is a promising therapeutic agent that inhibits NLRP3-mediated pyroptosis of cardiomyocytes (Yue et al., 2021). AMP-activated protein kinase (AMPK) is essential for maintaining peroxisome proliferator-activated receptor-coactivator-1α (PGC-1α) (Gholamnezhad et al., 2020) and irisin (Lally et al., 2015) expressions. Irisin could inhibit the ROS/NLRP3 inflammatory signaling (Peng et al., 2017), TNF-α (Clark and Vissel 2019), and pyroptosis (Yue et al., 2021). Aerobic exercise has been demonstrated to activate AMPK and PGC1-α (Lally et al., 2015), increasing irisin expression in adipose tissues (Sanchez-Delgado et al., 2015) and inhibiting the NLRP3 related signaling.
Phosphatidylinositol 3-hydroxy kinase (PI3K)/protein kinase B (AKT) signaling has been regarded as a key signaling pathway in glucose homeostasis, lipid metabolism and insulin resistance (Abeyrathna and Su 2015). The activation of the NLRP3 inflammasome could enhance the expression of IL-1β, IL-18, and interferonγ (IFNγ), while it inhibits IRS-1/PI3K/AKT signaling (Sun et al., 2017) (Vandanmagsar et al., 2011), thereby leading to insulin resistance. Vandanmagsar et al. (2011) proved that aerobic exercise ameliorated insulin resistance in the adipose tissues of T2DM patients by inhibiting the expression of NLRP3 and IL-1β. Another study found that the decreased expression of IL-1β and NLRP3 was positively associated with decreased blood glucose levels and improved insulin resistance index (Vandanmagsar et al., 2011). Moreover, aerobic exercise could enhance the expression of PI3K and AKT, and sequentially activate the PI3K/AKT/glucose transporter 4 (GLUT4) signaling pathway in adipose tissues, thereby improving insulin sensitivity (Yi et al., 2020). Taken together, ameliorating adipocyte’s inflammation and insulin resistance are the potential molecular mechanisms of the effects of aerobic exercise on adipocyte’s pyroptosis (Figure 2).
Hippocampal cell’s pyroptosis is closely related to the development of neurodegenerative diseases (Han et al., 2020; Li et al., 2021), and aerobic exercise is an ideal regimen to inhibit pyroptosis of hippocampal cells, which is beneficial for patients with neurodegenerative diseases.
The hippocampal cells take a vital role in storing information associated with memory. Pyroptosis of hippocampal cells is closely associated with AD’s pathogenesis (Han et al., 2020), depression (Li et al., 2021), and so on. Neuroinflammation mediated by hippocampal cells and microglia take a crucial role in AD, primarily owning to amyloid-beta (Aβ) deposition and pyroptosis. The inhibition of NLRP3 in AD mice reduced Caspase-1 expression and Aβ deposition, and improved the cognitive function (Dempsey et al., 2017). Moreover, the activation of IL-1β and GSDMD will induce neuronal pyroptosis, and plays a significant role in the pathogenesis of AD (White et al., 2017; Han et al., 2020).
Current evidence has demonstrated that the NLRP3-mediated pyroptosis was a key modulator in the development of depression (Li et al., 2021). Especially, the NLRP3 inflammasome promotes hippocampal neurons and depression-like behavior in the hippocampus in depressed rats (Herman and Pasinetti 2018; Yang et al., 2020).
In fact, the downstream cytokines of NLRP3, including IL-1β and TNF-α, were increased in the cerebral spinal fluid and serum of patients with depression (Herman and Pasinetti 2018). In brief, the hippocampal cell’s pyroptosis is closely related to AD and depression. Aerobic exercise is an important way to suppress hippocampal cell’s pyroptosis in patients with neurodegenerative diseases.
Emerging evidence indicates that aerobic exercise can improve the function of hippocampal cells (Zhang X. et al., 2019). The possible mechanism is that aerobic exercise effectively reduces Aβ deposition by regulating neuroinflammation and oxidative stress (Zhang X. et al., 2019). Some studies have proved that aerobic exercise can inhibit hippocampal cell’s pyroptosis. As shown in Table 1, the studies indicated that aerobic exercise could inhibit NLRP3 inflammasome-related inflammatory cytokines, including Toll-like receptor 4 (TLR4), NF-κB, TXNIP, IL-1β, and IL-18. As mentioned above, these studies have suggested that aerobic exercise can reduce the expression of pyroptosis-related factors in the hippocampal cells. Aerobic exercise could inhibit the TLR4/NF-κB/NLRP3 signaling pathway in the dentate gyrus region of the hippocampus of post-stroke depression models (Li et al., 2020), which could prevent the activation of TXNIP and NLRP3 inflammasome pathways in AD rats (Rosa et al., 2021), and ameliorate depression-like behaviors by decreasing NLRP3, IL-1β, and IL-18 expressions in the hippocampal tissues (Wang et al., 2016c). These studies suggested that aerobic exercise could reduce hippocampal cell’s pyroptosis.
Microglia are the major source of inflammatory cytokines in the central nervous system (Habib and Beyer 2015) and coordinate the brain’s inflammatory response (Andoh and Koyama 2020). Studies have demonstrated that TLR4 could activate microglia, which transmit downstream inflammatory signals through the adaptor protein MyD88 (Kang et al., 2016), then activate NF-κB and NLRP3 inflammasome. NLRP3 inflammasome has been demonstrated to activate the microglia (Freeman et al., 2017), and NLRP3 protein was preferentially expressed in the microglia (Xia et al., 2021). The NLRP3 complex secretes IL-1β and IL-18, leading to pro-inflammatory response and pyroptosis (Zhou et al., 2011).
Numerous studies have found that aerobic exercise upregulated the expression of anti-inflammatory cytokines, thereby inhibiting the activation of microglia (Andoh and Koyama 2020) and expression of the NLRP3 inflammasome (Wang et al., 2016b). Aerobic exercise can inhibit microglial activation by decreasing the levels of IL-1β and TNF-α (Zhang X. et al., 2019), and regulating TLR signaling pathways (Mee-Inta et al., 2019). Long-term treadmill running could also reduce the expression of IL-1β and IL-18, inhibiting microglial activation caused by the activation of NLRP3 inflammasome in the hippocampal tissues (Wang et al., 2016b). Therefore, aerobic exercise can inhibit hippocampal cell’s pyroptosis by reducing microglial activation.
Neuroinflammation is an immune response mediated by cytokines released from the microglia, which is related to increased expression of inflammatory cytokines, including NLRP3, IL-1β, and IL-18, in the hippocampal cells. Aerobic exercise has been shown to relieve neuroinflammation and protect neurons by decreasing the expression of NLRP3, IL-1β, and IL-18 (Wang et al., 2016b; Rosa et al., 2021). Wang et al. (2019) have shown that 4 weeks of treadmill exercise training inhibited neuroinflammation and played a neuroprotective role by suppressing the NF-κB/NLRP3 signaling pathway. The potential mechanism for aerobic exercise inhibits the expression of hippocampal NLRP3 inflammasome by reducing the TXNIP levels in the hippocampal dissection (Rosa et al., 2021). Moreover, TXNIP mediates the activation of NLRP3-related inflammatory signaling pathways through oxidative stress (Italiani et al., 2018). Moreover, aerobic exercise activates the Nrf2/HO-1 pathways, although it suppresses the NLRP3/IL-1β pathway (Cai et al., 2016), thereby inhibiting hippocampal cell’s pyroptosis.
Multiple studies have demonstrated that aerobic exercise could inhibit upstream signaling of hippocampal cell’s pyroptosis. Specifically, Qu et al. have demonstrated that 8 weeks of aerobic exercise training inhibited the TLR4/myeloid differentiation 88 (MyD88)/NF-κB signaling pathway in the hippocampal tissue (Qu et al., 2020). Li et al. (2020) found that 28 days of running training inhibited the TLR4/NF-κB/NLRP3 inflammatory signaling pathway, which mediates the hippocampal neurons’ protective effect in post-stroke depressed mice. Qu et al. (2019) identified that 8 weeks of moderate-intensity treadmill exercise significantly reduced the expression of TLR4 in the hippocampal tissue of mice, and activated the TLR4/miR-223/NLRP3 pathway axis, thereby improving the hippocampal function and promoting the repair of the damaged hippocampal tissue. Furthermore, Li et al. (2019) proved that 4 weeks of treadmill exercise could modulate the NF-κB/NLRP3/IL-1β signaling pathways in the hippocampal proteins. Moreover, long-term running wheel exercise training inhibited the expression of NADPH oxidase, and release of TNF-α and IL-1β, and induced the antioxidant and protective effects of microglia on nerves (Simioni et al., 2018). In other words, aerobic exercise could inhibit hippocampal cell’s pyroptosis by reducing neuroinflammation.
Aβ deposition is neurotoxic and can destroy the neurons, resulting in abnormal autophagy, blocking the clearance of Aβ, and affecting the cognitive function of neurodegenerative diseases. Aβ deposition promotes ROS production oxidative stress (Matěj et al., 2015) and activates the NLRP3 inflammasome in microglial cells in vitro and in vivo (Luciunaite et al., 2020). Aerobic exercise reduces microglia-mediated neuroinflammation, oxidative stress and Aβ deposition by inhibiting NLRP3 expression in the microglia (Zhang X. et al., 2019; Liang et al., 2020; Nakanishi et al., 2021). Together, these studies suggested that aerobic exercise could inhibit hippocampal cell’s pyroptosis by decreasing Aβ deposition.
In summary, the review highlighted the close association between aerobic exercise and pyroptosis-related diseases, suggesting that aerobic exercise can alleviate the pyroptosis by regulating the NLRP3 inflammation signaling. Aerobic exercise inhibits endothelial cell’s pyroptosis by improving the endothelial function, while reducing vascular inflammation and oxidative stress. Moreover, aerobic exercise affects adipocyte’s pyroptosis by ameliorating adipocyte inflammation and insulin resistance. The potential mechanism of the effects of aerobic exercise on hippocampal cell’s pyroptosis is the reduction of microglial activation, neuroinflammation and Aβ deposition.
Different patterns of exercise have varying effects on cell pyroptosis. For example, Khakroo Abkenar et al. (2019) and Comassi et al. (2018) have found that one-time acute and acute high-intensity exercises can promote the activation of pyroptosis-associated protein, which are related to exercise intensity. However, aerobic exercise, resistance training and chronic high-intensity intermittent exercise can inhibit the activation of pyroptosis. Thus, further studies are needed to define the optimal effects of different patterns of exercise on specific cell’s pyroptosis and their molecular mechanism. Additionally, at present, animal experiments to investigate the effect of exercise on cell pyroptosis are more frequently performed, as compared to human experiments, which are scarce and more likely involved a small sample size. More in-depth research on the human body can provide a more scientific basis on the efficacy of exercise in regulating cell pyroptosis and promoting health. Therefore, more methodological, high-quality, and large-sized human studies are needed to determine the ideal patterns of exercise.
SH and XWW designed the study. SH and XXW drafted the manuscript. SH and XXW drew the figures and filled the table. XWW and XHL revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Philosophy and Social Science Research Project of the Hubei Education Department (21Q050), the Central Government guides local funds for scientific and Technological Development (XZ202201YD0024C), Key R & D Program of Hubei Province (2021BGD010), Hubei Province Scientific and Technological Research Project (D20201306), Hubei Province Key Project of Research and Development Plan (to XWW), Hubei Province Health Research Project (WJ 2019-01), Hubei Medical Youth Tip-Top Talent (to XWW), Leading Talent Program of Yangtze Talent Project (to XWW) and the College Students Innovative Entrepreneurial Training Program in Yangtze University (Yz2020334, Yz2021296).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abeyrathna P., Su Y. (2015). The critical role of Akt in cardiovascular function. Vasc. Pharmacol. 74, 38–48. doi:10.1016/j.vph.2015.05.008
Ahmadian M., Suh J. M., Hah N., Liddle C., Atkins A. R., Downes M., et al. (2013). PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 19 (5), 557–566. doi:10.1038/nm.3159
Bai B., Yang Y., Wang Q., Li M., Tian C., Liu Y., et al. (2020). NLRP3 inflammasome in endothelial dysfunction. Cell. Death Dis. 11 (9), 776. doi:10.1038/s41419-020-02985-x
Bai Y., Feng Y., Jiang B., Yang Y., Pei Z., Yang Q., et al. (2021). The role of exercise in reducing hyperlipidemia-induced neuronal damage in apolipoprotein E-deficient mice. BioMed Res. Int. 2021, 1–9. doi:10.1155/2021/5512518
Bauernfeind F., Niepmann S., Knolle P. A., Hornung V. (2016). Aging-associated TNF production primes inflammasome activation and NLRP3-related metabolic disturbances. J. I. 197 (7), 2900–2908. doi:10.4049/jimmunol.1501336
Byon C. H., Han T., Wu J., Hui S. T. (2015). Txnip ablation reduces vascular smooth muscle cell inflammation and ameliorates atherosclerosis in apolipoprotein E knockout mice. Atherosclerosis 241 (2), 313–321. doi:10.1016/j.atherosclerosis.2015.05.020
Cai M., Wang H., Li J. J., Zhang Y. L., Xin L., Li F., et al. (2016). The signaling mechanisms of hippocampal endoplasmic reticulum stress affecting neuronal plasticity-related protein levels in high fat diet-induced obese rats and the regulation of aerobic exercise. Brain, Behav. Immun. 57, 347–359. doi:10.1016/j.bbi.2016.05.010
Cao P., Ito O., Ito D., Rong R., Zheng Y., Kohzuki M. (2020). Combination of exercise training and SOD mimetic tempol enhances upregulation of nitric oxide synthase in the kidney of spontaneously hypertensive rats. Int. J. Hypertens. 2020, 1–10. doi:10.1155/2020/2142740
Carbone S., Del Buono M. G., Ozemek C., Lavie C. J. (2019). Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 62 (4), 327–333. doi:10.1016/j.pcad.2019.08.004
Chen J. Y., Ye Z. X., Wang X. F., Chang J., Yang M. W., Zhong H. H., et al. (2018). Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 97, 423–428. doi:10.1016/j.biopha.2017.10.122
Chiazza F., Couturier-Maillard A., Benetti E., Mastrocola R., Nigro D., Cutrin J. C., et al. (2015). Targeting the NLRP3 inflammasome to reduce diet-induced metabolic abnormalities in mice. Mol. Med. 21 (1), 1025–1037. doi:10.2119/molmed.2015.00104
Clark I. A., Vissel B. (2019). Neurodegenerative disease treatments by direct TNF reduction, SB623 cells, maraviroc and irisin and MCC950, from an inflammatory perspective - a Commentary. Expert Rev. Neurother. 19 (6), 535–543. doi:10.1080/14737175.2019.1618710
Comassi M., Santini E., Rossi C., Vitolo E., Seghieri M., Tocchini L., et al. (2018). The level of physical training modulates cytokine levels through P2X7 receptor in healthy subjects. Eur. J. Clin. Invest. 48 (2), e12880. doi:10.1111/eci.12880
Cunha T. F., Bechara L. R., Bacurau A. V., Jannig P. R., Voltarelli V. A., Dourado P. M., et al. (2017). Exercise training decreases NADPH oxidase activity and restores skeletal muscle mass in heart failure rats. J. Appl. Physiology 122 (4), 817–827. doi:10.1152/japplphysiol.00182.2016
De Miguel C., Pelegrín P., Baroja-Mazo A., Cuevas S. (2021). Emerging role of the inflammasome and pyroptosis in hypertension. Ijms 22 (3), 1064. doi:10.3390/ijms22031064
Dempsey C., Rubio Araiz A., Bryson K. J., Finucane O., Larkin C., Mills E. L., et al. (2017). Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain, Behav. Immun. 61, 306–316. doi:10.1016/j.bbi.2016.12.014
Ding S., Xu S., Ma Y., Liu G., Jang H., Fang J. (2019). Modulatory mechanisms of the NLRP3 inflammasomes in diabetes. Biomolecules 9 (12), 850. doi:10.3390/biom9120850
Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., et al. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464 (7293), 1357–1361. doi:10.1038/nature08938
Esser N., L’homme L., De Roover A., Kohnen L., Scheen A. J., Moutschen M., et al. (2013). Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia 56 (11), 2487–2497. doi:10.1007/s00125-013-3023-9
Ferentinos P., Tsakirides C., Swainson M., Davison A., Martyn-St James M., Ispoglou T. (2022). The impact of different forms of exercise on circulating endothelial progenitor cells in cardiovascular and metabolic disease. Eur. J. Appl. Physiol. 122 (4), 815–860. doi:10.1007/s00421-021-04876-1
Freeman L., Guo H., David C. N., Brickey W. J., Jha S., Ting J. P. (2017). NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 214 (5), 1351–1370. doi:10.1084/jem.20150237
Gao J., Pan X., Li G., Chatterjee E., Xiao J. (2021). Physical exercise protects against endothelial dysfunction in cardiovascular and metabolic diseases. J. Cardiovasc. Trans. Res. 15 (3), 604–620. doi:10.1007/s12265-021-10171-3
Gholamnezhad Z., Mégarbane B., Rezaee R. (2020). Molecular mechanisms mediating adaptation to exercise. Adv. Exp. Med. Biol. 1228, 45–61. doi:10.1007/978-981-15-1792-1_3
Giordano A., Murano I., Mondini E., Perugini J., Smorlesi A., Severi I., et al. (2013). Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 54 (9), 2423–2436. doi:10.1194/jlr.M038638
Gjevestad G. O., Holven K. B., Ulven S. M. (2015). Effects of exercise on gene expression of inflammatory markers in human peripheral blood cells: A systematic review. Curr. Cardiovasc Risk Rep. 9 (7), 34. doi:10.1007/s12170-015-0463-4
Goh J., Behringer M. (2018). Exercise alarms the immune system: A HMGB1 perspective. Cytokine 110, 222–225. doi:10.1016/j.cyto.2018.06.031
Habib P., Beyer C. (2015). Regulation of brain microglia by female gonadal steroids. J. Steroid Biochem. Mol. Biol. 146, 3–14. doi:10.1016/j.jsbmb.2014.02.018
Han C., Yang Y., Guan Q., Zhang X., Shen H., Sheng Y., et al. (2020). New mechanism of nerve injury in alzheimer's disease: β‐amyloid‐induced neuronal pyroptosis. J. Cell. Mol. Med. 24 (14), 8078–8090. doi:10.1111/jcmm.15439
Haybar H., Shahrabi S., Rezaeeyan H., Shirzad R., Saki N. (2019). Endothelial cells: From dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovasc Toxicol. 19 (1), 13–22. doi:10.1007/s12012-018-9493-8
Herman F. J., Pasinetti G. M. (2018). Principles of inflammasome priming and inhibition: Implications for psychiatric disorders. Brain, Behav. Immun. 73, 66–84. doi:10.1016/j.bbi.2018.06.010
Hong J., Kim K., Park E., Lee J., Markofski M. M., Marrelli S. P., et al. (2018). Exercise ameliorates endoplasmic reticulum stress-mediated vascular dysfunction in mesenteric arteries in atherosclerosis. Sci. Rep. 8 (1), 7938. doi:10.1038/s41598-018-26188-9
Hong J., Park E., Lee J., Lee Y., Rooney B. V., Park Y. (2021). Exercise training mitigates ER stress and UCP2 deficiency-associated coronary vascular dysfunction in atherosclerosis. Sci. Rep. 11 (1), 15449. doi:10.1038/s41598-021-94944-5
Hoseini Z., Sepahvand F., Rashidi B., Sahebkar A., Masoudifar A., Mirzaei H. (2018). NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell. Physiol. 233 (3), 2116–2132. doi:10.1002/jcp.25930
Inoue K., Fujie S., Hasegawa N., Horii N., Uchida M., Iemitsu K., et al. (2020). Aerobic exercise training-induced irisin secretion is associated with the reduction of arterial stiffness via nitric oxide production in adults with obesity. Appl. Physiol. Nutr. Metab. 45 (7), 715–722. doi:10.1139/apnm-2019-0602
Italiani P., Puxeddu I., Napoletano S., Scala E., Melillo D., Manocchio S., et al. (2018). Circulating levels of IL-1 family cytokines and receptors in alzheimer's disease: New markers of disease progression? J. Neuroinflammation 15 (1), 342. doi:10.1186/s12974-018-1376-1
Jeong J. H., Koo J. H., Cho J. Y., Kang E. B. (2018). Neuroprotective effect of treadmill exercise against blunted brain insulin signaling, NADPH oxidase, and Tau hyperphosphorylation in rats fed a high-fat diet. Brain Res. Bull. 142, 374–383. doi:10.1016/j.brainresbull.2018.08.001
Jiang M., Wang H., Liu Z., Lin L., Wang L., Xie M., et al. (2020). Endoplasmic reticulum stress‐dependent activation of iNOS/NO‐NF‐κB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity. FASEB J. 34 (8), 10835–10849. doi:10.1096/fj.202000734R
Kang E. B., Koo J. H., Jang Y. C., Yang C. H., Lee Y., Cosio-Lima L. M., et al. (2016). Neuroprotective effects of endurance exercise against high-fat diet-induced hippocampal neuroinflammation. J. Neuroendocrinol. 28 (5). doi:10.1111/jne.12385
Kar S., Shahshahan H. R., Hackfort B. T., Yadav S. K., Yadav R., Kambis T. N., et al. (2019). Exercise training promotes cardiac hydrogen sulfide biosynthesis and mitigates pyroptosis to prevent high-fat diet-induced diabetic cardiomyopathy. Antioxidants 8 (12), 638. doi:10.3390/antiox8120638
Karasawa T., Takahashi M. (2017). Role of NLRP3 inflammasomes in atherosclerosis. Jat 24 (5), 443–451. doi:10.5551/jat.RV17001
Keping Y., Yunfeng S., Pengzhuo X., Liang L., Chenhong X., Jinghua M. (2020). Sestrin1 inhibits oxidized low‐density lipoprotein‐induced activation of NLRP3 inflammasome in macrophages in a murine atherosclerosis model. Eur. J. Immunol. 50 (8), 1154–1166. doi:10.1002/eji.201948427
Khakroo Abkenar I., Rahmani-nia F., Lombardi G. (2019). The effects of acute and chronic aerobic activity on the signaling pathway of the inflammasome NLRP3 complex in young men. Medicina 55 (4), 105. doi:10.3390/medicina55040105
Kourek C., Alshamari M., Mitsiou G., Psarra K., Delis D., Linardatou V., et al. (2021). The acute and long-term effects of a cardiac rehabilitation program on endothelial progenitor cells in chronic heart failure patients: Comparing two different exercise training protocols. IJC Heart & Vasc. 32, 100702. doi:10.1016/j.ijcha.2020.100702
Kovacs S. B., Miao E. A. (2017). Gasdermins: Effectors of pyroptosis. Trends Cell. Biol. 27 (9), 673–684. doi:10.1016/j.tcb.2017.05.005
Koyama M., Andoh R. (2020). Exercise, microglia, and beyond - workout to communicate with microglia. Neural Regen. Res. 15 (11), 2029–2030. doi:10.4103/1673-5374.282241
Krishnan S. M., Sobey C. G., Latz E., Mansell A., Drummond G. R. (2014). IL ‐1β and IL ‐18: Inflammatory markers or mediators of hypertension? Br. J. Pharmacol. 171 (24), 5589–5602. doi:10.1111/bph.12876
Lally J. S., Ford R. J., Johar J., Crane J. D., Kemp B. E., Steinberg G. R. (2015). Skeletal muscle AMPK is essential for the maintenance of FNDC5 expression. Physiol. Rep. 3 (5), e12343. doi:10.14814/phy2.12343
Lee J., Hong J., Umetani M., Lavoy E. C., Kim J. H., Park Y. (2020). Vascular protection by exercise in obesity: Inflammasome-associated mechanisms. Med. Sci. Sports Exerc 52 (12), 2538–2545. doi:10.1249/MSS.0000000000002419
Lee J., Lee Y., LaVoy E. C., Umetani M., Hong J., Park Y. (2018). Physical activity protects NLRP3 inflammasome-associated coronary vascular dysfunction in obese mice. Physiol. Rep. 6 (12), e13738. doi:10.14814/phy2.13738
Li C., Xu X., Wang Z., Wang Y., Luo L., Cheng J., et al. (2020). Exercise ameliorates post-stroke depression by inhibiting PTEN elevation-mediated upregulation of TLR4/NF-κB/NLRP3 signaling in mice. Brain Res. 1736, 146777. doi:10.1016/j.brainres.2020.146777
Li J., Liu Y., Liu B., Li F., Hu J., Wang Q., et al. (2019). Mechanisms of aerobic exercise upregulating the expression of hippocampal synaptic plasticity-associated proteins in diabetic rats. Neural Plast. 2019, 1–12. doi:10.1155/2019/7920540
Li Y., Song W., Tong Y., Zhang X., Zhao J., Gao X., et al. (2021). Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflammation 18 (1), 1. doi:10.1186/s12974-020-02040-8
Liang F., Huang T., Li B., Zhao Y., Zhang X., Xu B. (2020). High-intensity interval training and moderate-intensity continuous training alleviate β-amyloid deposition by inhibiting NLRP3 inflammasome activation in APPswe/PS1dE9 mice. Neuroreport 31 (5), 425–432. doi:10.1097/WNR.0000000000001429
Liao C. R., Wang S. N., Zhu S. Y., Wang Y. Q., Li Z. Z., Liu Z. Y., et al. (2020). Advanced oxidation protein products increase TNF-α and IL-1β expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 28, 101306. doi:10.1016/j.redox.2019.101306
Liu W., Wang H., Wang Y., Li H., Ji L. (2015). Metabolic factors-triggered inflammatory response drives antidepressant effects of exercise in CUMS rats. Psychiatry Res. 228 (3), 257–264. doi:10.1016/j.psychres.2015.05.102
Lučiūnaitė A., McManus R. M., Jankunec M., Rácz I., Dansokho C., Dalgėdienė I., et al. (2020). Soluble Aβ oligomers and protofibrils induce NLRP3 inflammasome activation in microglia. J. Neurochem. 155 (6), 650–661. doi:10.1111/jnc.14945
Man S. M., Karki R., Kanneganti T.-D. (2017). Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277 (1), 61–75. doi:10.1111/imr.12534
Mardare C., Krüger K., Liebisch G., Seimetz M., Couturier A., Ringseis R., et al. (2016). Endurance and resistance training affect high fat diet-induced increase of ceramides, inflammasome expression, and systemic inflammation in mice. J. Diabetes Res. 2016, 1–13. doi:10.1155/2016/4536470
Matej R., Rohan Z., Holada K., Olejar T. (2015). The contribution of proteinase-activated receptors to intracellular signaling, transcellular transport and autophagy in Alzheimer´s disease. Car 12 (1), 2–12. doi:10.2174/1567205012666141218123202
McGeough M. D., Wree A., Inzaugarat M. E., Haimovich A., Johnson C. D., Peña C. A., et al. (2017). TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J. Clin. Invest. 127 (12), 4488–4497. doi:10.1172/JCI90699
Mee-inta O., Zhao Z. W., Kuo Y. M. (2019). Physical exercise inhibits inflammation and microglial activation. Cells 8 (7), 691. doi:10.3390/cells8070691
Nakanishi K., Sakakima H., Norimatsu K., Otsuka S., Takada S., Tani A., et al. (2021). Effect of low-intensity motor balance and coordination exercise on cognitive functions, hippocampal Aβ deposition, neuronal loss, neuroinflammation, and oxidative stress in a mouse model of Alzheimer's disease. Exp. Neurol. 337, 113590. doi:10.1016/j.expneurol.2020.113590
Neunhäuserer D., Patti A., Niederseer D., Kaiser B., Cadamuro J., Lamprecht B., et al. (2021). Systemic inflammation, vascular function, and endothelial progenitor cells after an exercise training intervention in COPD. Am. J. Med. 134 (3), e171–e180. doi:10.1016/j.amjmed.2020.07.004
Pasqua T., Pagliaro P., Rocca C., Angelone T., Penna C. (2018). Role of NLRP-3 inflammasome in hypertension: A potential therapeutic target. Cpb 19 (9), 708–714. doi:10.2174/1389201019666180808162011
Peng J., Deng X., Huang W., Yu J. H., Wang J. X., Wang J. P., et al. (2017). Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol. Immunol. 91, 185–194. doi:10.1016/j.molimm.2017.09.014
Qu H., Liu R., Chen J., Zheng L., Chen R. (2020). Aerobic exercise inhibits CUMS-depressed mice hippocampal inflammatory response via activating hippocampal miR-223/TLR4/MyD88-NF-κB pathway. Ijerph 17 (8), 2676. doi:10.3390/ijerph17082676
Qu H. L., Xie J., Chen J. Q., Liu R. L., Tang C. F., Chen Y. L., et al. (2019). Aerobic training inhibits hippocampal inflammation by activating the Hippocampus TLR4/miR223/NLRP3 signaling pathway Axis in mice with CUMS depression. CHINA SPORT Sci. 39 (2), 40–50. doi:10.16469/j.css.201902005
Rosa J. M., Camargo A., Wolin I. A. V., Kaster M. P., Rodrigues A. L. S. (2021). Physical exercise prevents amyloid β1−40-induced disturbances in NLRP3 inflammasome pathway in the hippocampus of mice. Metab. Brain Dis. 36 (2), 351–359. doi:10.1007/s11011-020-00646-8
Rovira-Llopis S., Apostolova N., Bañuls C., Muntané J., Rocha M., Victor V. M. (2018). Mitochondria, the NLRP3 inflammasome, and sirtuins in type 2 diabetes: New therapeutic TargetsReviewing editors:markus bachschmid, dylan burger, vittorio calabrese, amadou camara, lukas kubala, giuseppe poli, and chandan K. Sen. Antioxidants Redox Signal. 29 (8), 749–791. doi:10.1089/ars.2017.7313
Sanchez-Delgado G., Martinez-Tellez B., Olza J., Aguilera C. M., Gil A., Ruiz J. R. (2015). Role of exercise in the activation of Brown adipose tissue. Ann. Nutr. Metab. 67 (1), 21–32. doi:10.1159/000437173
Schroder K., Tschopp J. (2010). The inflammasomes. Cell. 140 (6), 821–832. doi:10.1016/j.cell.2010.01.040
Shi J., Gao W., Shao F. (2017). Pyroptosis: Gasdermin-Mediated programmed necrotic cell death. Trends Biochem. Sci. 42 (4), 245–254. doi:10.1016/j.tibs.2016.10.004
Simioni C., Zauli G., Martelli A. M., Vitale M., Sacchetti G., Gonelli A., et al. (2018). Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9 (24), 17181–17198. doi:10.18632/oncotarget.24729
Šimják P., Cinkajzlová A., Anderlová K., Pařízek A., Mráz M., Kršek M., et al. (2018). The role of obesity and adipose tissue dysfunction in gestational diabetes mellitus. J. Endocrinol. 238 (2), R63–r77. doi:10.1530/joe-18-0032
Stanford K. I., Middelbeek R. J., Townsend K. L., Lee M. Y., Takahashi H., So K., et al. (2015). A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64 (6), 2002–2014. doi:10.2337/db14-0704
Steyers C. M., Miller F. J. (2014). Endothelial dysfunction in chronic inflammatory diseases. Ijms 15 (7), 11324–11349. doi:10.3390/ijms150711324
Stinkens R., Brouwers B., Jocken J. W., Blaak E. E., Teunissen-Beekman K. F., Hesselink M. K., et al. (2018). Exercise training-induced effects on the abdominal subcutaneous adipose tissue phenotype in humans with obesity. J. Appl. Physiology 125 (5), 1585–1593. doi:10.1152/japplphysiol.00496.2018
Sun X., Hao H., Han Q., Song X., Liu J., Dong L., et al. (2017). Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell. Res. Ther. 8 (1), 241. doi:10.1186/s13287-017-0668-1
Tang R., Xu J., Zhang B., Liu J., Liang C., Hua J., et al. (2020). Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 13 (1), 110. doi:10.1186/s13045-020-00946-7
Tsiloulis T., Watt M. J. (2015). Exercise and the regulation of adipose tissue metabolism. Prog. Mol. Biol. Transl. Sci. 135, 175–201. doi:10.1016/bs.pmbts.2015.06.016
Vandanmagsar B., Youm Y. H., Ravussin A., Galgani J. E., Stadler K., Mynatt R. L., et al. (2011). The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17 (2), 179–188. doi:10.1038/nm.2279
Wang L., Chen Y., Li X., Zhang Y., Gulbins E., Zhang Y. (2016a). Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget 7 (45), 73229–73241. doi:10.18632/oncotarget.12302
Wang Q., Hu J., Liu Y., Li J., Liu B., Li M., et al. (2019). Aerobic exercise improves synaptic-related proteins of diabetic rats by inhibiting FOXO1/NF-κB/NLRP3 inflammatory signaling pathway and ameliorating PI3K/akt insulin signaling pathway. J. Mol. Neurosci. 69 (1), 28–38. doi:10.1007/s12031-019-01302-2
Wang W., Lv Z., Gao J., Liu M., Wang Y., Tang C., et al. (2021). Treadmill exercise alleviates neuronal damage by suppressing NLRP3 inflammasome and microglial activation in the MPTP mouse model of Parkinson's disease. Brain Res. Bull. 174, 349–358. doi:10.1016/j.brainresbull.2021.06.024
Wang Y., Xu Y., Sheng H., Ni X., Lu J. (2016b). Exercise amelioration of depression-like behavior in OVX mice is associated with suppression of NLRP3 inflammasome activation in hippocampus. Behav. Brain Res. 307, 18–24. doi:10.1016/j.bbr.2016.03.044
Wang Y., Zhong J., Zhang X., Liu Z., Yang Y., Gong Q., et al. (2016c). The role of HMGB1 in the pathogenesis of type 2 diabetes. J. Diabetes Res. 2016, 1–11. doi:10.1155/2016/2543268
White C. S., Lawrence C. B., Brough D., Rivers-Auty J. (2017). Inflammasomes as therapeutic targets for Alzheimer's disease. Brain Pathol. 27 (2), 223–234. doi:10.1111/bpa.12478
Wu K. K., Cheung S. W., Cheng K. K. (2020). NLRP3 inflammasome activation in adipose tissues and its implications on metabolic diseases. Ijms 21 (11), 4184. doi:10.3390/ijms21114184
Wu W., Wang H., Jiao G., Yue J., Wang G. (2017). Aerobic exercise suppresses atherosclerosis through adiponectin-nuclear transcription factor κB pathway in apolipoprotein E-deficient mice. Am. J. Med. Sci. 353 (3), 275–281. doi:10.1016/j.amjms.2016.11.002
Wu Y., Pan B., Zhang Z., Li X., Leng Y., Ji Y., et al. (2022). Caspase-4/11-Mediated pulmonary artery endothelial cell pyroptosis contributes to pulmonary arterial hypertension. Hypertension 79 (3), 536–548. doi:10.1161/hypertensionaha.121.17868
Xia D. Y., Yuan J. L., Jiang X. C., Qi M., Lai N. S., Wu L. Y., et al. (2021). SIRT1 promotes M2 microglia polarization via reducing ROS-mediated NLRP3 inflammasome signaling after subarachnoid hemorrhage. Front. Immunol. 12, 770744. doi:10.3389/fimmu.2021.770744
Xia S. Y. (2015). Effect of treadmill exercise of different intensity on the pparγ/adiponectin/TNF-α mRNA in adipose tissue of rats fed with high fat diet. J. Chengdu Sport Univ. 41, 98–102. doi:10.15942/j.jcsu.2015.03.19
Yang F., Zhu W., Cai X., Zhang W., Yu Z., Li X., et al. (2020). Minocycline alleviates NLRP3 inflammasome-dependent pyroptosis in monosodium glutamate-induced depressive rats. Biochem. Biophysical Res. Commun. 526 (3), 553–559. doi:10.1016/j.bbrc.2020.02.149
Yi X. J., Sun Y. X., Yao T. T., Li J., Gao C., Liu L., et al. (2020). Effects of acute and chronic exercise on fat PI3K/AKT/GLUT4 signal pathway in type 2 diabetic rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 36, 12–16. doi:10.12047/j.cjap.5802.2020.003
You T., Arsenis N. C., Disanzo B. L., LaMonte M. J. (2013). Effects of exercise training on chronic inflammation in obesity. Sports Med. 43 (4), 243–256. doi:10.1007/s40279-013-0023-3
Yue R., Zheng Z., Luo Y., Wang X., Lv M., Qin D., et al. (2021). NLRP3-mediated pyroptosis aggravates pressure overload-induced cardiac hypertrophy, fibrosis, and dysfunction in mice: Cardioprotective role of irisin. Cell. Death Discov. 7 (1), 50. doi:10.1038/s41420-021-00434-y
Zaidi H., Byrkjeland R., Njerve I. U., Åkra S., Solheim S., Arnesen H., et al. (2019). Effects of exercise training on inflammasome-related mediators and their associations to glucometabolic variables in patients with combined coronary artery disease and type 2 diabetes mellitus: Sub-study of a randomized control trial. Diabetes Vasc. Dis. Res. 16 (4), 360–368. doi:10.1177/1479164119836922
Zeng C., Wang R., Tan H. (2019). Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int. J. Biol. Sci. 15 (7), 1345–1357. doi:10.7150/ijbs.33568
Zhang L., Yuan M., Zhang L., Wu B., Sun X. (2019a). Adiponectin alleviates NLRP3-inflammasome-mediated pyroptosis of aortic endothelial cells by inhibiting FoxO4 in arteriosclerosis. Biochem. Biophysical Res. Commun. 514 (1), 266–272. doi:10.1016/j.bbrc.2019.04.143
Zhang T., Ding S., Wang R. (2021). Research progress of mitochondrial mechanism in NLRP3 inflammasome activation and exercise regulation of NLRP3 inflammasome. Ijms 22 (19), 10866. doi:10.3390/ijms221910866
Zhang X., He Q., Huang T., Zhao N., Liang F., Xu B., et al. (2019b). Treadmill exercise decreases Aβ deposition and counteracts cognitive decline in APP/PS1 mice, possibly via hippocampal microglia modifications. Front. Aging Neurosci. 11, 78. doi:10.3389/fnagi.2019.00078
Zhang Y., Li X., Pitzer A. L., Chen Y., Wang L., Li P. L. (2015). Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: Beyond inflammation. Antioxidants Redox Signal. 22 (13), 1084–1096. doi:10.1089/ars.2014.5978
Zhang Y., Liu X., Bai X., Lin Y., Li Z., Fu J., et al. (2018). Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J. Pineal Res. 64 (2), e12449. doi:10.1111/jpi.12449
Zhaolin Z., Jiaojiao C., Peng W., Yami L., Tingting Z., Jun T., et al. (2019). OxLDL induces vascular endothelial cell pyroptosis through miR‐125a‐5p/TET2 pathway. J. Cell. Physiology 234 (5), 7475–7491. doi:10.1002/jcp.27509
Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469 (7329), 221–225. doi:10.1038/nature09663
Keywords: aerobic exercise, pyroptosis, pyroptosis-related diseases, mechanisms, NLRP3 inflammasome
Citation: Hu S, Wan X, Li X and Wang X (2022) Aerobic exercise alleviates pyroptosis-related diseases by regulating NLRP3 inflammasome. Front. Physiol. 13:965366. doi: 10.3389/fphys.2022.965366
Received: 09 June 2022; Accepted: 25 July 2022;
Published: 15 September 2022.
Edited by:
Erik D. Hanson, University of North Carolina at Chapel Hill, United StatesReviewed by:
Marialuisa Perrotta, Sapienza University of Rome, ItalyCopyright © 2022 Hu, Wan, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianwang Wang, NTAwODUxQHlhbmd0emV1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.