- 1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
- 2Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, Shanghai, China
- 3MOE Frontiers Center for Brain Science, Fudan University, Shanghai, China
- 4University Center UNIFATEB, Telêmaco Borba, Brazil
- 5Graduate Program in Chemical Engineering Federal Technological University of Paraná, Ponta Grossa, Brazil
- 6Physics Institute, University of São Paulo, Oscillation Control Group, São Paulo, Brazil
- 7School of Information Science and Technology, Fudan University, Shanghai, China
- 8Research Institute of Intelligent Complex Systems, Fudan University, Shanghai, China
- 9Potsdam Institute for Climate Impact Research, Potsdam, Germany
- 10Humboldt University, Berlin, Germany
Networks of identical coupled oscillators display a remarkable spatiotemporal pattern, the chimera state, where coherent oscillations coexist with incoherent ones. In this paper we show quantitatively in terms of basin stability that stable and breathing chimera states in the original two coupled networks typically have very small basins of attraction. In fact, the original system is dominated by periodic and quasi-periodic chimera states, in strong contrast to the model after reduction, which can not be uncovered by the Ott-Antonsen ansatz. Moreover, we demonstrate that the curve of the basin stability behaves bimodally after the system being subjected to even large perturbations. Finally, we investigate the emergence of chimera states in brain network, through inducing perturbations by stimulating brain regions. The emerged chimera states are quantified by Kuramoto order parameter and chimera index, and results show a weak and negative correlation between these two metrics.
1 Introduction
The Kuramoto model is known to exhibit various complex phenomena of collective synchronization, where nonidentical oscillators spontaneously lock in a common frequency, except those with very different natural frequency (Arenas et al., 2008). Identical oscillators, however, were expected to display simple collective behaviors until the discovery of a chimera state (Kuramoto and Battogtokh, 2002; Abrams and Strogatz, 2004). The chimera state is a spatiotemporal pattern where a network of coupled oscillators is split into coexisting subpopulations of synchronized and desynchronized oscillations (Kuramoto and Battogtokh, 2002; Abrams and Strogatz, 2004). It has been observed theoretically in networks of general types of oscillators (Abrams et al., 2008; Pikovsky and Rosenblum, 2008; Panaggio and Abrams, 2015), as well as in experiments including chemical systems (Hagerstrom et al., 2012; Tinsley et al., 2012; Nkomo et al., 2013) and mechanical oscillators (Martens et al., 2013). In biology, the chimera state is observed in Wilson-Cowan oscillators (WCOs), which obey a nonlinear mean-field model to describe the dynamics of brain network (Wilson and Cowan, 1972; Bansal et al., 2019).
Mathematical studies of chimera states have focused on a special class of density function based on the Ott-Antonsen ansatz and have analytically described stable and breathing chimera states (Abrams et al., 2008). Via the Watanabe-Strogatz theory, governing equations are reduced to low-dimensional systems with only three transformed parameters (Pikovsky and Rosenblum, 2008), which complements the results of chimera states (Abrams et al., 2008). This ansatz is very efficient for a network of Kuramoto oscillators. Note that chimera states are stable, persistent phenomena for N → ∞ (Omel’chenko, 2013) and are sensitive to perturbations with typically small basins of attraction. Therefore, it is crucial to investigate the stability of chimera states against perturbations. Basin stability indicates the likelihood that the system (or a group) will retain a desirable state after being subjected to even large perturbations and is calculated proportional to the volume of the basin of attraction of the desirable state (Menck et al., 2013).
In addition to the mathematical studies, chimera states in brain networks have also received great attentions to deepen the understanding of cognitive function (input, integration and output), from various perspectives. From dynamical perspectives, networked FitzHugh-Nagumo oscillators could induce chimera states for certain range of coupling strengths (Chouzouris et al., 2018). Adaptive couplings could also yield a self-organized state and induce chimera states (Huo et al., 2019). From structural perspectives, an empirical brain network is applied to explore how brain structures impact chimera states through numerical disruptions (Bansal et al., 2019). The two-layer brain network reproduces the phenomena of unihemispheric sleep with one hemisphere synchronized and the other desynchronized (Kang et al., 2019). This further explains the first-night effect in human sleep (Tamaki et al., 2016). These results could be further utilized to analyze the mechanism of brain functions, e.g., cognition and memory, and so on (Wang and Liu, 2020; Parastesh et al., 2021).
In this paper, we investigate the stability of chimera states of the coupled networks by using the coupling scheme of Ref. (Abrams et al., 2008), where two populations are fully connected but with different intra- and inter-coupling strengths, by means of basin stability. We first analyze the stability of the low-dimensional model after the phase reduction using the Ott-Antonsen ansatz and approximate basin stability of chimera states in terms of their attracting basins. In comparison to the model after reduction, we substantially perturb the original dynamics in the coupled networks. In this way, instead of stable or breathing chimera states, we quantitatively show that the original system is dominated by periodic or quasi-periodic chimera states. We also observe that after the system being subjected to even more and large perturbations, the curve of basin stability of the chimera states behaves bimodally. To investigate how chimera states in brain are influenced by stimulation of various brain regions, we integrate WCOs on brain networks and stimulate single region with three global coupling strengths. Results show the existing of three different states, i.e., the coherent, chimera, and metastable state (Yeo et al., 2011; Dimulescu et al., 2021), and suggest that the Kuramoto order parameter is weakly and negatively correlated with the chimera index. Besides, higher degree nodes have a more centralized and compact distribution of the ranked order parameter compared to lower ones.
2 Model
In this paper, we focus on chimera states in theoretical and applied aspects, with Kuramoto model by dimensional reduction and the coupled Wilson-Cowan oscillators on brain networks.
The governing equations for Kuramoto model follow (Abrams et al., 2008)
where
The dynamics on brain behavior are modeled by Wilson-Cowan oscillators (WCOs), describing the evolution of excitatory and inhibitory activity in a coupled brain network (Wilson and Cowan, 1972; Bansal et al., 2019). In particular, we consider a brain network with N brain regions, the connection strength between brain regions i and j accounted for by Aij. At time t, we use Ei(t) and Ii(t) to denote the fraction of excitatory and inhibitory neurons activities, respectively, in the i-th brain region. The temporal dynamics of Ei(t) and Ii(t) are governed by
where
with the maximal values
In Eq. 2, the parameters c5 and c6 represent the excitatory and inhibitory global coupling strength between brain regions, respectively, with c6 = c5/4. The term Pi(t) determines the external stimulation to excitatory neurons activities. The parameter
To quantitatively analyze the degree of synchronization of the population σ, we consider the complex order parameter
as a macroscopic quantity, where
In what follows, we focus on the emergence of chimera states from the theoretical derivations and numerical investigation on an empirical brain network. The theoretical part provides the quantitatively basin stability of the chimera state, as well as comparing the difference of attracting basins of chimera states between the original system and the reductional system. The applied part provides the investigation of the impact of stimulating single region on the brain dynamics and how the induced chimera states are influenced by the stimulation of various regions.
3 Low-dimensional system
To analytically investigate the dynamics, stability and bifurcations of the system (1), it is convenient to explore a reduction of the phase model to a low-dimensional description of each population (Abrams et al., 2008) in terms of the ansatz imposed by Ott and Antonsen (Ott and Antonsen, 2008). Previous studies of chimera states focused on a special class of density function based on the Ott-Antonsen ansatz and have analytically described stable and breathing chimera states (Abrams et al., 2008). In this paper, we use a special Poisson kernel density function based on the Ott-Antonsen ansatz and obtain a reduced system of the original Kuramoto system. The reduced system is derived to provide the quantitatively basin stability of the chimera state, and to compare the difference of attracting basins of chimera states between the original systems and the reduced system.
Assuming that the density function fσ(θσ, t) follows a special Poisson kernel and expanding fσ(θσ, t) in a Fourier series in θσ, we have
where c.c. Stands for complex conjugate and |aσ(t)| ≤ 1 to avoid divergence. Substituting the Fourier expansion Eq. 5 of the density function into the order parameter Eq. 4 yields
where
In the limit Nσ → ∞, we can get a reduction of the governing Eq. 1. Recall that fσ satisfies the continuity equation
The amplitude equations can be rewritten in terms of polar coordinates rσ and ψσ according to Eq. 6 and obtain a two-dimensional system given by
where ψ = ψ1 − ψ2.
We have investigated the behavior of this low-dimensional system Eq. 7. The linear stability analysis of the system has been well performed (Abrams et al., 2008), but their attracting basins was not studied. In particular, the basins of attraction of chimera states have not attracted great attention (Panaggio and Abrams, 2015). Basins of attraction of Chimera states of a simple system of two populations have been investigated using perturbative analysis Martens et al. (2016). Following the similar notation of symbols (e.g., 1S2D) of the reference Martens et al. (2016), we analyze the stability diagram A and β of chimera states in low dimensional systems.
System Eq. 7 consists of the order parameter r1 of the first population, that of the second one, and the mean-phase difference ψ. With special initial conditions, one could observe remarkable phenomena (Abrams et al., 2008), where the first (second) population is synchronized with r1 = 1 (r2 = 1) and the second (first) is desynchronized with r2 < 1 (r1 < 1). For convenience, we denote these two kinds of chimera states by 1S2D and 1D2S, correspondingly. Figures 1A,C,E exhibit scatter plots of the basins of attraction of chimera states colored in red (blue), a set of initial conditions leading the low-dimensional system Eq. 7 to approaching 1S2D (1D2S). At each initial value of r1, r2 and ψ, we independently integrate Eq. 7 long enough, so that the distribution of the state of the oscillators becomes stationary. As predicted by the stability diagram of chimera states (Abrams et al., 2008), stable chimera corresponds to a point, breathing chimera show as a stable limit cycle. The colored region in Figures 1A,C,E indicates, respectively, the basin of attraction of stable, breathing and long-period breathing chimera states. The colored regions of Figures 1B,D,F show the basin of attraction especially with initial value r1 = 1 for 1S2D and r2 = 1 for 1D2S. Solid lines in red and blue are trajectories with random initial conditions within the red and blue basins, and they will approach 1S2D and 1D2S respectively. Here, we fix the phase shift β = 0.1 and set the coupling disparity A = 0.20 in Figures 1A,B, A = 0.28 in Figures 1C,D and A = 0.35 in Figures 1E,F.
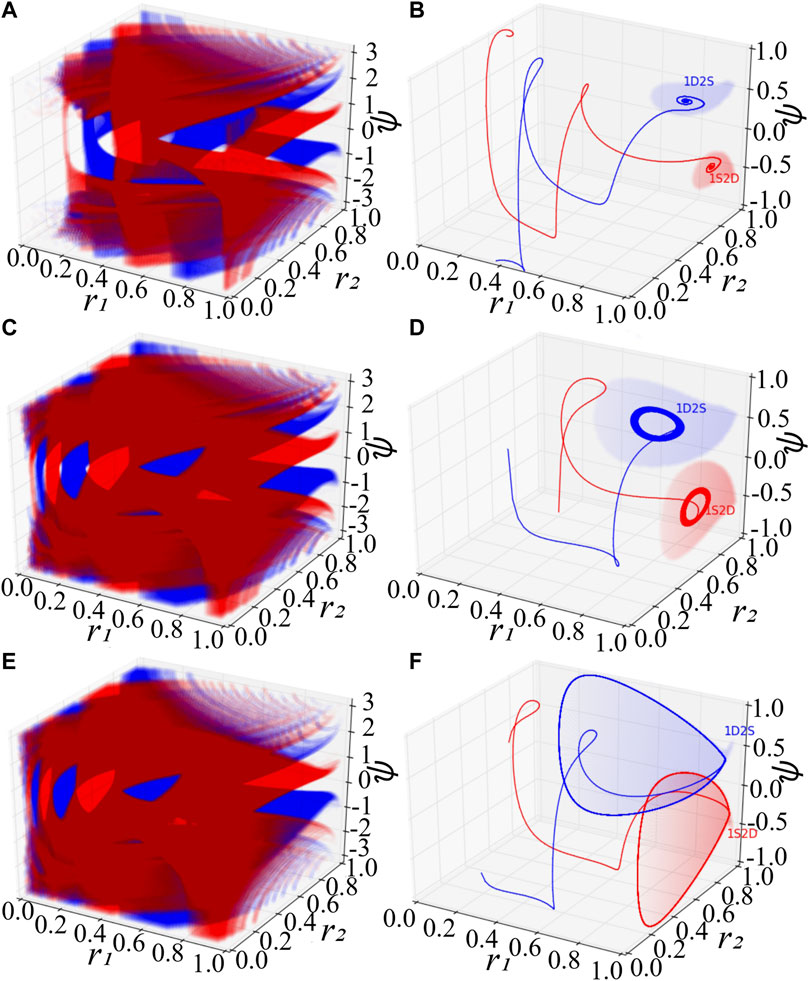
FIGURE 1. Basins of attraction of chimera states for the low-dimensional system Eq. 7. (A,B) The stable chimera states. (C,D) The breathing chimera states. (E,F) The long-period breathing chimera states. In (A), (C), and (E), the red and blue color denote respectively the basins of attraction of 1S2D and 1D2S. The notations 1S2D represent that the first population is synchronized and the second is desynchronized, and 1D2S denote that the first population is desynchronized and the second is synchronized. Mathematically, 1S2D with r1 = 1 and r2 < 1, and 1D2S with r1 < 1 and r2 = 1. In (B), (D) and (F), red and blue solid lines are trajectories with random initial conditions inside the red and blue attracting basins respectively. Red and blue areas in (B), (D) and (F) show the special attracting basins with the initial conditions r1 = 1 and r2 = 1 respectively. For the simulation, we set β = 0.1, A = 0.20 in (A,B), A = 0.28 in (C,D), and A = 0.35 in (E,F).
Figure 2 provides the basins of attraction of the stable chimera in (a), of the breathing chimera in (b), and of the long-period breathing chimera in (c), via perturbing the second population r2 and keeping the first population synchronized, i.e., r1 = 1. As predicted by the stability diagram of chimera states (Abrams et al., 2008), the red region in Figures 2A–C indicates, respectively, the basin of attraction of stable, breathing and long-period breathing chimera states with Max(r2) < 1. This system Eq. 7 always has a fixed point at r2 = 1 and ψ = 0, and its corresponding attracting basin is colored in white with Max(r2) = 1. Here, for simplicity, we use the maximum value of r2 denoted by Max(r2) to approximate the basins of attraction of the different states. Saddles colored in blue are always located at the basin boundary.
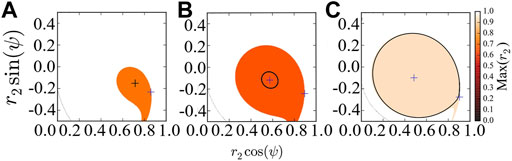
FIGURE 2. Basins of attraction of chimera states of the reduced system Eq. 7, which are colored with respect to the maximum value Max(r2). (A) The system converges to the stable chimera (denoted by black plus). (B) The system converges to the breathing chimera (denoted by black circle). (C) The system converges to the long-period breathing chimera (denoted by black circle). In (A–C), we regard r2 and ψ as polar coordinates, and the blue pluses indicate the location of fixed points. For the simulation, the initial values (r2, ψ) = (0.7, − 0.1) are inside the basin of attraction of chimera states, and we fix β = 0.1 and vary A = 0.20 (A), A = 0.28 (B) and A = 0.35 (C).
The linear stability diagram for chimera states was identified by a bifurcation analysis (Abrams et al., 2008), but it does not show how stable a chimera state is under large perturbations. Moreover, in realistic situations, a certain degree of perturbations are largely unavoidable and may drive the system from one desirable state to other unpredictable states. Therefore, it is crucial to investigate its stability against even large perturbations.
Menck et al. (2013) proposed the concept of basin stability
Numerically, we perturb the three parameters (r1, r2, ψ) M times independently inside the range of [0, 1] × [0, 1] × [ − π, π], count the number denoted by S of the system retaining back to chimera states, and then approximate the basin stability
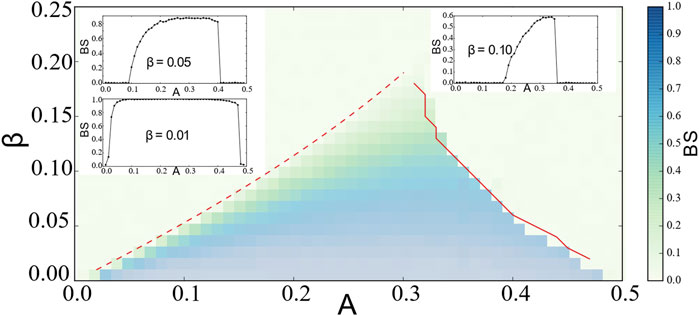
FIGURE 3. The projection of basin stability
4
The above results are achieved under the Ott-Antonsen ansatz by considering a restricted class of density functions following the form of a Poisson kernel. Next, we analyze the original dynamics (1) and investigate its basin stability, to compare with the three cases of Figure 1 as observed from the low-dimensional system Eq. 7. Initially, the system is in the state of stable chimera, breathing chimera or long-period breathing chimera as predicted by the low-dimensional solution. Numerically, we observe that the system after perturbations will probably converge to periodic chimera instead of stable chimera with A = 0.20, quasi-periodic chimera instead of breathing chimera with A = 0.28, quasi-periodic chimera instead of long-period breathing chimera with A = 0.35. With the variations of r1, r2 and ψ, trajectories of periodic chimera are stable and periodic with a closed curve in polar coordinates, and trajectories of quasi-periodic chimera are periodic while not stable with many closed curves. We show different realizations of periodic chimera in Figure 4A and quasi-periodic chimeras Figures 4B,C after perturbations with different parameter values. As shown in Figure 4, with periodic chimera suggests that the trajectories of r1, r2 and ψ are stable and periodic, and the trajectories are a closed curve in polar coordinates.
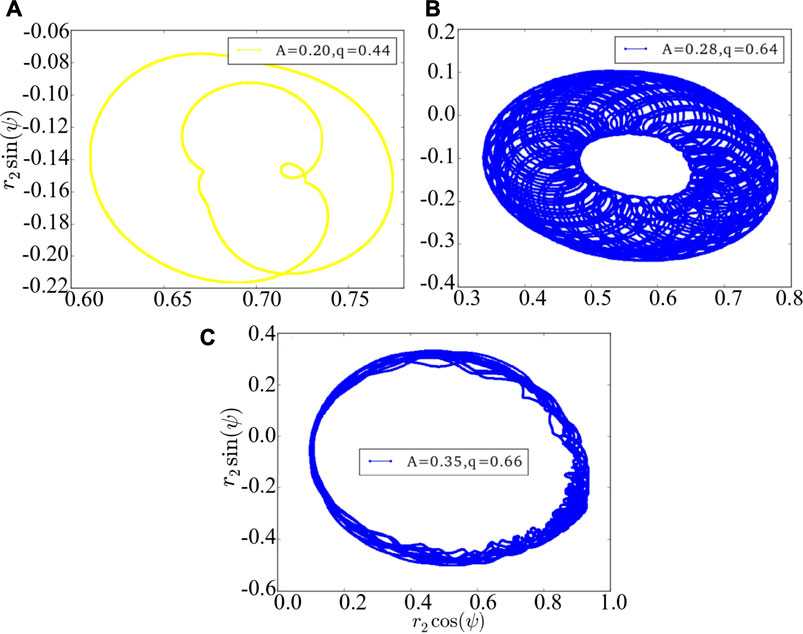
FIGURE 4. The phase portrait of the original dynamics (1). (A) is periodic chimera, and (B,C) are quasi-periodic chimeras. The realizations vary depending crucially on the initial conditions, perturbations and parameter values. Here we use arbitrary values of parameters A = 0.20 and q = 0.44 in (A), A = 0.28 and q = 0.64 in (B), and A = 0.35 and q = 0.66 in (C).
In comparison with the results of the reduced system, in what follows, we provide the results via perturbing especially the second population.
Chimera states require carefully selected initial conditions, and, therefore, it is interesting to quantify
with Mq = ∑SNS;q.
Numerically, we observe that the degree of phase coherence becomes periodic or quasi-periodic after even large perturbations. In Figure 4A, with the coupling disparity A = 0.20, the order parameter r1 of the first population or r2 of the second population starts oscillating and the stable chimera states become periodic. With A = 0.28 in Figure 4B or 0.35 in Figure 4C, r1 or r2 displays irregular periodicity and breathing chimeras become quasi-periodic. Therefore, phase portraits can not be depicted solely by the Ott-Antonsen ansatz, though this does work for the case of the low-dimensional system. Realizations of periodic and quasi-periodic chimera states of Eq. 7 vary and depend heavily on the initial conditions and parameter values.
In Figure 5, we plot the basin stability
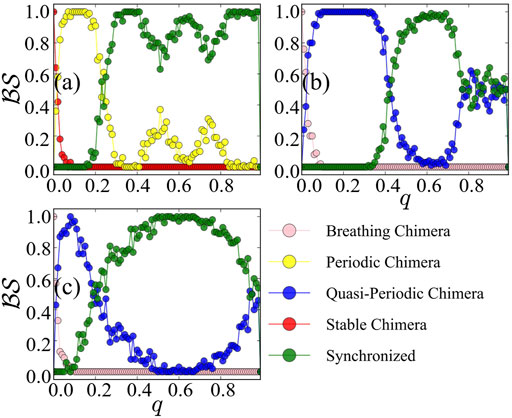
FIGURE 5. Basin stability
To compare with the solution of the low-dimensional model Eq. 7, we record values of polar coordinates r2 and ψ with the initial value r1 = 1 as basin of attraction of the corresponding stationary state with respect to A and then project basins of attractions of different states on the space regarding the polar coordinates as shown in Figure 6. We observe that dominant chimeras are periodic in Figure 6A rather than stable or quasi-periodic in Figures 6B,C instead of breathing chimera. The basin boundary between different states is not clearly separated, in contrast to that shown in Figure 1 of the reduced system Eq. 7. Moreover, the basins of attraction between different states are overlapped. Recall that the calculation of
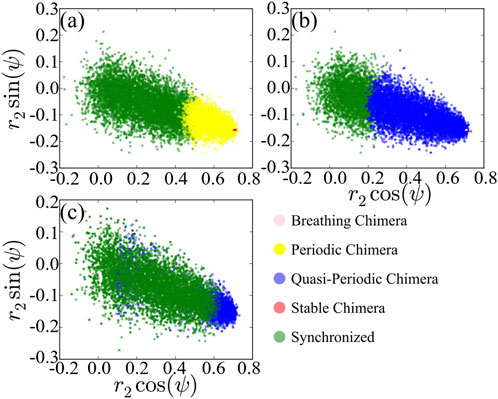
FIGURE 6. Projection of basins of attraction of different states of the original system, with A = 0.20 (A), A = 0.28 (B) and A = 0.35 (C). For comparing the results to Figure 1, we regard r2 and ψ as polar coordinates.
For a comparison between the reduced system and the original system, in terms of basin stability, the dominated states in the reduced system are stable and are breathing chimera states, with small basins of attraction. However, the original system is dominated by periodic and quasi-periodic chimera states, in contrast to the model after reduction. The original system being subjected to even more and large perturbations, the curve of basin stability of the chimera states behaves bimodally. Therefore, the low-dimensional system under the Ott-Antonsen ansatz cannot capture the behavior of the basins.
5 Chimera states on brain networks
Up to now, we have investigated the basins of attraction of chimera states in original and reduced Kuramoto networks. In this section, we focus the influence of stimulating regions on the chimera states on brain networks.
Firstly, we use the diffusion imaging data to generate brain networks. The Diffusion imaging data are available from the Human Connectome Project (HCP), WU-Minn Consortium (https://www.humanconnectome.org). HCP recruits subjects in the age range of 22–35 years, and subjects are scanned on a customized Siemens 3 T “Connectome Skyra” at Washington University, using a standard 32-channel Siemens receive head coil and a “body” transmission coil (Van Essen et al., 2013). For the simplification, we take the diffusion pre-processed data of 30 individual participant scans, randomly selected, and use DSI Studio (http://dsi-studio.labsolver.org/) to perform whole-brain fiber tractography between brain regions. To obtain the fiber connectivity matrix of each participant, the fiber threshold is set by 0.001, as the default value of DSI Studio, to filter out a small number of connecting tracks. In this case, the fiber connectivity between brain regions smaller than the threshold will be ignored in the connectivity matrix (Yeh, 2017). We use the automated anatomical atlas (AAL2), with 94 cortical brain regions (Rolls et al., 2015), and further obtain the fiber connectivity matrices for these participants, accounting for fiber numbers between brain regions. The connectivity matrix and graph theoretical analysis are conducted by using DSI Studio (http://dsi-studio.labsolver.org). To minimize the impact of bias in the tractography parameter scheme on connectivity matrix generation, we use the averaged fiber connectivity matrix, across 30 subjects, to simulate the brain networks.
Based on the generated networks, we use the WCOs Eq. 2, employ the stochastic Euler-Maruyama method with time step size 0.001 s, and set the initial conditions Ei(0) = 0.1, Ii(0) = 0.1 with i = 1, …, N. The connection strength Aij in Eq. 2 is obtained by normalizing the averaged fiber connectivity matrix, i.e., Aij = nij/ns, where nij is the fiber count between regions i and j, and ns is the sum of fiber count in the whole brain. The dynamical perturbation of the stimulated region Pi = 1.15 is utilized as a single regional stimulation. After stimulation, signals propagate through the network connectivity from the stimulated regions and others, (Bansal et al., 2019).
In what follows, we focus on the investigation of the impact on brain dynamics (stimulated region) and the quantification of the induced chimera states from different regions. To characterize the emerged states in brain dynamics, we focus on synchronized patterns on brain systems, where regions are divided into different functional regions with similar cognitive processes. For this network, each region is assigned to one of eight cognitive systems (Yeo et al., 2011), including somatomotor (Som), default mode network (DMF), control (Con), dorsal attention (DA), limbic (Lim), visual (Vis), ventral attention (VA), other (Oth, subcortical regions could not be assigned to any system) (Dimulescu et al., 2021). Figure 7 exhibits the assignment of 94 AAL2 brain regions within eight cognitive systems. Figures 7A–C shows the three dimensional distribution of 94 AAL2 brain regions within brain, with same color nodes assigned to one cognitive system. Figure 7D is the spatial mappings of 8 cognitive systems.
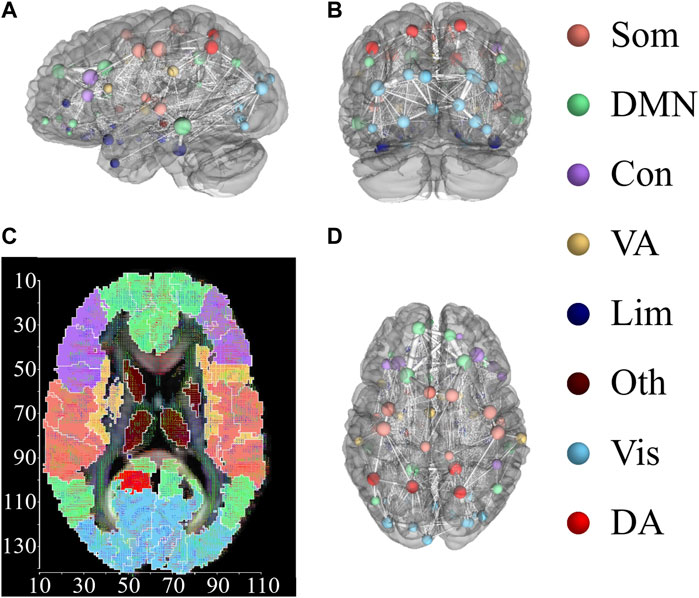
FIGURE 7. Distribution of 94 AAL2 brain regions within 8 cognitive systems. (A,B) and (D) show 3-dimensional spatial distribution of the 94 brain regions in 3 directional views, with left view (A), back view (B), and top view (D). Brain regions with the same color belong to the same cognitive systems. The connections between brain regions are displayed, with the thickness representing the connection strength. (C) Spatial mappings show the distribution of 8 cognitive systems. The color of map is connected to the cognitive system shown in the right column. This figure is drawn by using DSI Studio.
To quantify the degree of synchronization between brain regions, we use the order parameter based on a single regional stimulation. For the N coupling WCOs, the phase of the i-th node follows
The order parameter averaged across a long period of time T indicates the global synchrony, i.e.,
where σ represents all regions, i.e., Nσ = N. For instance, if all regions move coherently and act like a giant component, rN ≈ 1, otherwise, rN ≈ 0 (Strogatz, 2000). In numerical simulations, we set T = 1 s to estimate the averaged order parameter.
Additionally, we use the order parameter to investigate the synchronized activities between pairs of cognitive systems based on a regional stimulation. In particular, for each pair of systems ξi and ξj with
where Φ(t) is the averaged phase of oscillators within cognitive systems ξi and ξj. The cognitive system-level order parameter is calculated by averaging
for each pair of cognitive systems, i.e., i, j = 1, 2, …, 8. For a given regional stimulation, the system Eq. 2 may exhibit different final states based on different coupling strengths c5. To identify the synchronization patterns, we define a synchronization threshold rTh, such that two cognitive systems ξi and ξj are considered to be synchronized if
To explore how a single region drives the brain dynamics with different underlying couplings, based on varying the coupling strength c5, we simulate the dynamics Eq. 2 and obtain three different synchronization patterns, including (i) the coherent state, (ii) the chimera state with coexisting synchronized and desynchronized subpopulations, and (iii) the metastable state with the absence of any large-scale stable synchronized oscillations (Bansal et al., 2019). Figure 8 shows the corresponding numerical results, with (a) the coherent state with the order parameter
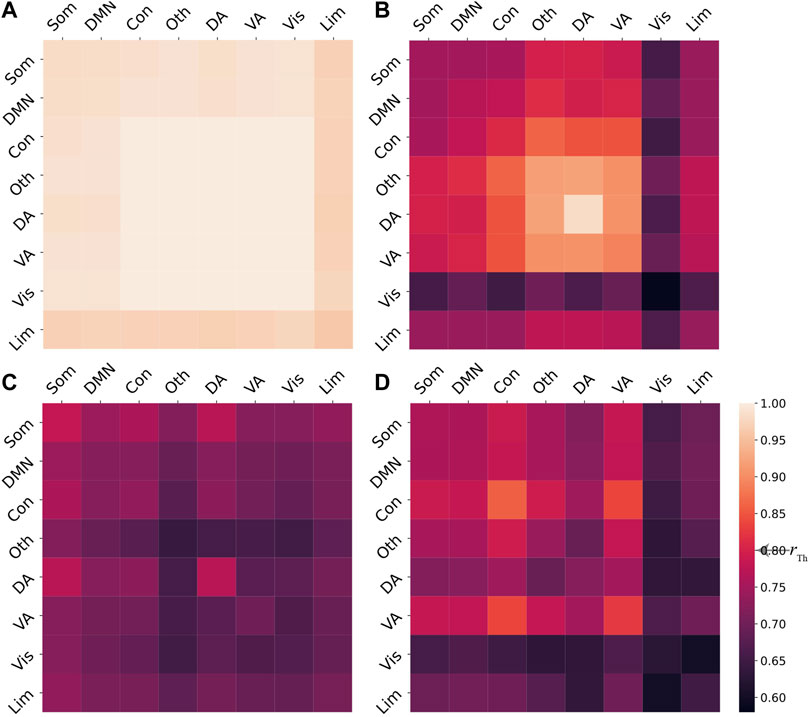
FIGURE 8. (A) Coherent state generated with the coupling strength c5 = 1000. (B) Chimera state with the coupling strength c5 = 330. (C) Metastable state with the coupling strength c5 = 200. In (A–C), we stimulate the 1-st brain region by applying a constant external input, i.e., Pi = 1.15 if i = 1 and Pi = 0 otherwise. (D) Chimera state of the brain dynamics without stimulation for c5 = 330. The order parameter
We further focus on how stimulation on brain regions impacts the induced chimera states of the model Eq. 2. Given the coupling strength c5 = 330, we stimulate the i-th brain region and integrate Eq. 2 10 times for each region i, with i ranging from 1 to N. For each simulation, the system will converge to either coherent, chimera, or metastable states. To identify the final state, we calculate the system-level order parameter matrices with elements
We stimulate each region i and integrate Eq. 2 10 times for each i, with N = 94. For the identified chimera state based on P, we calculate the index of the stimulated region is and the corresponding number of simulation
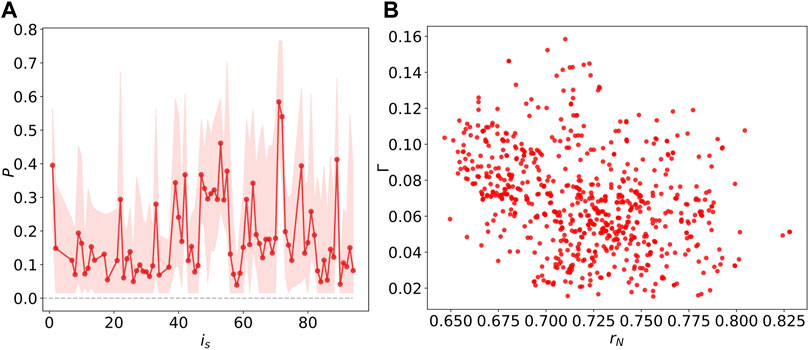
FIGURE 9. (A) The probability P of
As described in (Shanahan, 2010; Bansal et al., 2019), an ideal chimera state occurs with half of the population synchronized and the others desynchronized. After identifying the chimera states with different stimulated node is and the corresponding number of simulation
where
The chimera index Γ is averaged over the long time T, representing the averaged diversities in the order parameters within the M cognitive systems (Bansal et al., 2019). The normalization factor Γmax = 5/36 depicts the maximal variations of the order parameter corresponding to an ideal chimera state (Shanahan, 2010; Bansal et al., 2019). The instantaneous quantity
To illustrate further how the chimera states are constrained by the underlying network structure, we investigate the Kuramoto order parameter and the chimera index, as a function of the degree
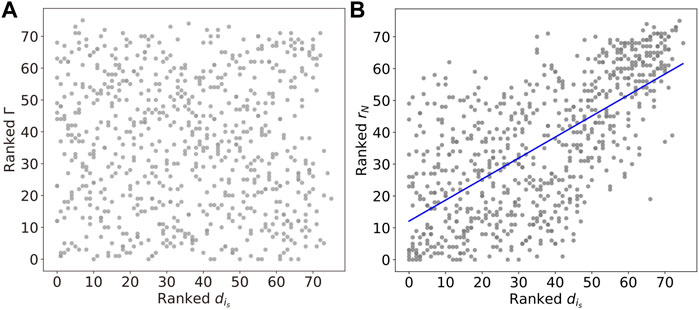
FIGURE 10. (A) The linear relations between the Kuramoto order parameter rN and degree
6 Conclusion
In the paper, we have firstly investigated the basin of attraction of stable and breathing chimera states of the solvable model (Abrams et al., 2008) with and without the Ott-Antonsen ansatz, and we have implemented basin stability on chimera states and quantified their stability after even large perturbations. Quantitatively, we have shown that periodic and quasi-periodic chimera states of networked oscillators, instead of stable and breathing chimera states in the reduced system, dominate the desynchronized states of the full system. Interestingly, we have observed that the curve of basin stability of chimera states becomes bimodal. The same process could be widely implemented on where chimera states are observed. It would also be worth looking at experiments for future work.
Additionally, we have also employed a biologically motivated, networked model WCOs, to investigate how the induced chimera states are influenced by the stimulation of various regions. Stimulating single region with different coupling strength could potentially force the system to three states, consisting of the coherent, chimera, and metastable state. For the chimera behavior on brain networked model, the chimera state without stimulation exhibits low synchronization between cognitive systems. Besides, the variations of Kuramoto order parameter suggest that higher-degree nodes could induce higher synchronization influences compared to the lower ones.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
QL and PJ designed the research and contributed to the modelling. KL, DH, PJ, and JK contributed to the discussion and writing the paper.
Funding
DH acknowledges the support from the National Key R&D Program of China under Grant (No. 2018YFB2101302). PJ acknowledges support from the National Natural Science Foundation of China (No. 62076071) and Huawei. We acknowledge the support from the National Natural Science Foundation of China under Contracts (No.12147101), National Natural Science Foundation of China (11875133, 11075057, and 62076071), the Strategic Priority Research Program of Chinese Academy of Science (Grant No. XDB34030000), STCSM (Grant No. 22JC1402500), National Science and Technology Innovation 2030 Major Program (2021ZD0204500, 2021ZD0204504), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), and Russian Ministry of Science and Education (Agreement No. 075-15-2020-808).
Acknowledgments
The first part of the manuscript was initially finished in 2014, and we really appreciated thoughtful discussion with Kenneth Showalter. PJ also appreciated the nice discussion with Erik A. Martens and Daniel M. Abrams on the first part of the manuscript in the workshop of “Collective dynamics in coupled oscillator systems”, organized by Weierstrass Institute, Berlin. Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrams D. M., Mirollo R., Strogatz S. H., Wiley D. A. (2008). Solvable model for chimera states of coupled oscillators. Phys. Rev. Lett. 101, 084103. doi:10.1103/PhysRevLett.101.084103
Abrams D. M., Strogatz S. H. (2004). Chimera states for coupled oscillators. Phys. Rev. Lett. 93, 174102. doi:10.1103/PhysRevLett.93.174102
Arenas A., Díaz-Guilera A., Kurths J., Moreno Y., Zhou C. (2008). Synchronization in complex networks. Phys. Rep. 469, 93–153. doi:10.1016/j.physrep.2008.09.002
Bansal K., Garcia J. O., Tompson S. H., Verstynen T., Vettel J. M., Muldoon S. F. (2019). Cognitive chimera states in human brain networks. Sci. Adv. 5, eaau8535. doi:10.1126/sciadv.aau8535
Bansal K., Medaglia J. D., Bassett D. S., Vettel J. M., Muldoon S. F. (2018). Data-driven brain network models differentiate variability across language tasks. PLoS Comput. Biol. 14, e1006487. doi:10.1371/journal.pcbi.1006487
Chouzouris T., Omelchenko I., Zakharova A., Hlinka J., Jiruska P., Schöll E. (2018). Chimera states in brain networks: Empirical neural vs. modular fractal connectivity. Chaos 28, 045112. doi:10.1063/1.5009812
Dimulescu C., Gareayaghi S., Kamp F., Fromm S., Obermayer K., Metzner C. (2021). Structural differences between healthy subjects and patients with schizophrenia or schizoaffective disorder: A graph and control theoretical perspective. Front. Psychiatry 12, 991. doi:10.3389/fpsyt.2021.669783
Hagerstrom A. M., Murphy T. E., Roy R., Hövel P., Omelchenko I., Schöll E. (2012). Experimental observation of chimeras in coupled-map lattices. Nat. Phys. 8, 658–661. doi:10.1038/nphys2372
Hizanidis J., Kouvaris N. E., Zamora-López G., Díaz-Guilera A., Antonopoulos C. G. (2016). Corrigendum: Chimera-like states in modular neural networks. Sci. Rep. 6, 1–11. doi:10.1038/srep22314
Huo S., Tian C., Kang L., Liu Z. (2019). Chimera states of neuron networks with adaptive coupling. Nonlinear Dyn. 96, 75–86. doi:10.1007/s11071-019-04774-4
Kang L., Tian C., Huo S., Liu Z. (2019). A two-layered brain network model and its chimera state. Sci. Rep. 9, 1–12. doi:10.1038/s41598-019-50969-5
Kuramoto Y., Battogtokh D. (2002). Coexistence of coherence and incoherence in nonlocally coupled phase oscillators. Nonlinear Phenom. Complex Syst. 5, 380–385.
Kuramoto Y. (1975). “Self-entrainment of a population of coupled non-linear oscillators,” in International symposium on mathematical problems in theoretical physics (Springer), 420–422.
Martens E. A., Panaggio M. J., Abrams D. M. (2016). Basins of attraction for chimera states. New J. Phys. 18, 022002. doi:10.1088/1367-2630/18/2/022002
Martens E. A., Thutupalli S., Fourrière A., Hallatschek O. (2013). Chimera states in mechanical oscillator networks. Proc. Natl. Acad. Sci. U. S. A. 110, 10563–10567. doi:10.1073/pnas.1302880110
Menck P. J., Heitzig J., Marwan N., Kurths J. (2013). How basin stability complements the linear-stability paradigm. Nat. Phys. 9, 89–92. doi:10.1038/nphys2516
Muldoon S. F., Pasqualetti F., Gu S., Cieslak M., Grafton S. T., Vettel J. M., et al. (2016). Stimulation-based control of dynamic brain networks. PLoS Comput. Biol. 12, e1005076. doi:10.1371/journal.pcbi.1005076
Nkomo S., Tinsley M. R., Showalter K. (2013). Chimera states in populations of nonlocally coupled chemical oscillators. Phys. Rev. Lett. 110, 244102. doi:10.1103/PhysRevLett.110.244102
Omel’chenko O. (2013). Coherence–incoherence patterns in a ring of non-locally coupled phase oscillators. Nonlinearity 26, 2469–2498. doi:10.1088/0951-7715/26/9/2469
Ott E., Antonsen T. M. (2008). Low dimensional behavior of large systems of globally coupled oscillators. Chaos 18, 037113. doi:10.1063/1.2930766
Panaggio M. J., Abrams D. M. (2015). Chimera states: Coexistence of coherence and incoherence in networks of coupled oscillators. Nonlinearity 28, R67–R87. doi:10.1088/0951-7715/28/3/r67
Parastesh F., Jafari S., Azarnoush H., Shahriari Z., Wang Z., Boccaletti S., et al. (2021). Chimeras. Phys. Rep. 898, 1–114. doi:10.1016/j.physrep.2020.10.003
Pikovsky A., Rosenblum M. (2008). Partially integrable dynamics of hierarchical populations of coupled oscillators. Phys. Rev. Lett. 101, 264103. doi:10.1103/PhysRevLett.101.264103
Rolls E. T., Joliot M., Tzourio-Mazoyer N. (2015). Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 122, 1–5. doi:10.1016/j.neuroimage.2015.07.075
Shanahan M. (2010). Metastable chimera states in community-structured oscillator networks. Chaos 20, 013108. doi:10.1063/1.3305451
Strogatz S. (2000). From kuramoto to crawford: Exploring the onset of synchronization in populations of coupled oscillators. Phys. D. Nonlinear Phenom. 143, 1–20. doi:10.1016/s0167-2789(00)00094-4
Tamaki M., Bang J. W., Watanabe T., Sasaki Y. (2016). Night watch in one brain hemisphere during sleep associated with the first-night effect in humans. Curr. Biol. 26, 1190–1194. doi:10.1016/j.cub.2016.02.063
Tinsley M. R., Nkomo S., Showalter K. (2012). Chimera and phase-cluster states in populations of coupled chemical oscillators. Nat. Phys. 8, 662–665. doi:10.1038/nphys2371
Van Essen D. C., Smith S. M., Barch D. M., Behrens T. E., Yacoub E., Ugurbil K., et al. (2013). The Wu-minn human connectome project: An overview. Neuroimage 80, 62–79. doi:10.1016/j.neuroimage.2013.05.041
Wang Z., Liu Z. (2020). A brief review of chimera state in empirical brain networks. Front. Physiol. 11, 724. doi:10.3389/fphys.2020.00724
Wilson H. R., Cowan J. D. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophys. J. 12, 1–24. doi:10.1016/S0006-3495(72)86068-5
Yeh F. (2017). Diffusion mri reconstruction in dsi studio. Adv. Biomed. MRI Lab. Natl. Taiwan Univ. Hosp. Available: http://dsi-studio. labsolver. org/Manual/Reconstruction# TOC-Q-Space-Diffeomorphic-Reconstruction-QSDR.
Keywords: complex networks, synchronization patterns, basin stability, chimera states, brain network
Citation: Li Q, Larosz KC, Han D, Ji P and Kurths J (2022) Basins of attraction of chimera states on networks. Front. Physiol. 13:959431. doi: 10.3389/fphys.2022.959431
Received: 01 June 2022; Accepted: 02 August 2022;
Published: 08 September 2022.
Edited by:
Marcus A. Aguiar, State University of Campinas, BrazilReviewed by:
K. Sathiyadevi, Chennai Institute of Technology, IndiaBidesh Bera, Ben-Gurion University of the Negev, Israel
Copyright © 2022 Li, Larosz, Han, Ji and Kurths. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dingding Han, ZGRoYW5AZnVkYW4uZWR1LmNu; Peng Ji, cGVuZ2ppQGZ1ZGFuLmVkdS5jbg==
 Qiang Li
Qiang Li Kelly C. Larosz4,5,6
Kelly C. Larosz4,5,6 Peng Ji
Peng Ji