- 1Department of Exercise Physiology, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 2Non-communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 3Department of Biostatistics and Epidemiology, School of Public Health, Babol University of Medical Sciences, Babol, Iran
- 4Social Determinants of Health Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 5Department of Health Education and Health Promotion, Faculty of Health, Isfahan University of Medical Sciences, Isfahan, Iran
- 6Cardiovascular Research Center, Shahid Rajaei Educational & Medical Center, Alborz University of Medical Sciences, Karaj, Iran
- 7Songhor Healthcare Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 8Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
Background: Exercise and physical activity can improve circulation through various mechanisms, such as the increment of nitric oxide (NO) production, by affecting vascular endothelial nitric oxide synthase, and reducing reactive oxygen species (ROS). Although, theoretically, this mechanism is well known, studies in living subjects have made controversial findings regarding the association of NO production and its metabolites [nitrate/nitrite (NOx)] with physical activity. Hence, this systematic review and meta-analysis was designed to gather all these studies and evaluate the effects of exercise training, and physical activity duration and length on the mean change of serum/plasma NO and NOx.
Method: We searched all available bibliographic electronic databases from inception through to May 2022 to include all randomized controlled trials (RCT) and quasi-experimental trials which assessed the effect of exercise and training on NO and NOx levels. Random-effects meta-analysis was used to pool the standardized mean difference (SMD) and 95% confidence interval (CI) of included RCT studies which assessed the effect of training. Stratified meta-analysis was performed according to the type of exercise (high-intensity interval training (HIIT), aerobic training (AT), the duration of exercise (≤8 and > 8 weeks), and length of exercise in each session ≥40 and 40 < minutes).
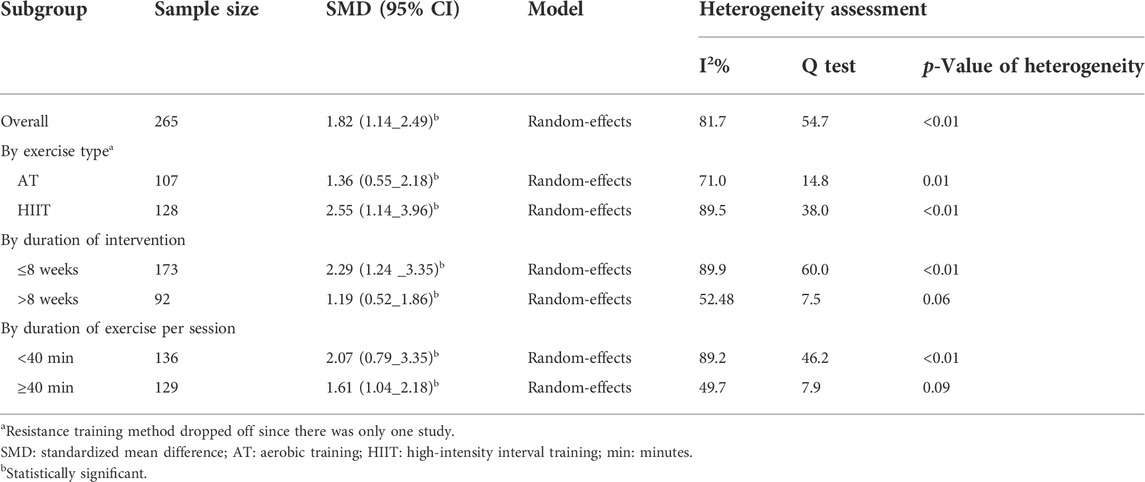
Results: Overall, 15 and 10 studies were included in the systematic review and meta-analysis, respectively. According to the random-effects meta-analysis, exercise significantly increased the mean change of NO and NOx compared to control (SMD: 1.82, 95%CI: 1.14 to 2.49. In the stratified meta-analysis, the mean change of NO and NOx in the intervention group was significantly higher than in the control group in the AT (SMD: 1.36, 95%CI: 0.55–2.18), HIIT (SMD: 2.55, 95%CI: 1.14–3.96), duration of ≤8 (SMD: 2.29, 95%CI: 1.24–3.35) and > 8 weeks (SMD: 1.19, 95%CI: 0.52–1.86), length of ≥40 (SMD: 1.61, 95%CI: 1.04–2.18), and 40 < minutes in each session (SMD: 2.07, 95%CI: 0.79–3.35).
Conclusion: The findings of this study indicate that, regardless of exercise duration, length, and type (AT or HIIT), exercise can significantly increase serum NO and NOx levels.
Background
Cardiovascular disease (CVD) is one of the major causes of disability and mortality in both developing and developed communities (Balakumar et al., 2016). The health of vascular endothelium tissue plays a major role in the incidence and control of cardiovascular diseases (Quyyumi, 1998). Vascular endothelium, a layer on the internal area of vessels, can produce various substances that control vascular constriction and relaxation (Förstermann et al., 2017). One of these substances is nitric oxide (NO)—later metabolized to nitrate/nitrite (NOx)—a cell-signaling molecule that is produced in endothelium through an enzymatic transformation of l-arginine by NO synthase (NOS) (Li et al., 2014). NOS presents in many cell types and tissues, including blood vessels, nerve cells, smooth muscles, myocytes, macrophages, and kidney endothelial cells (Di Pietro et al., 2017). The release of NO by relaxation of the vascular smooth muscles in the systemic and cerebral circulation plays a vital role in preventing CVD (Bondonno et al., 2016). The positive influences of physical activity and exercise on CVD control are well established (Bohn et al., 2015). Exercise-induced improvement of endothelial function can lead to a decrease in cardiovascular complications (Linke et al., 2006). Regular aerobic training (AT) and mild to moderate exercise are firmly prescribed for people who are not physically active, due to their beneficial impact on glycemic control and insulin sensitivity (Boulé et al., 2001). It has also been demonstrated that people with diabetes and CVD will benefit from such training and workouts as these have beneficial effects on lipid and glycemic control, postprandial glycemia and lipemia, fasting blood lipids, body mass, and blood pressure (Duncan, 2006; Harding et al., 2006).
Several pieces of research have demonstrated that AT can improve endothelium-dependent vasodilation by increasing NOS expression and phosphorylation (Linke et al., 2006; Gómez-Guzmán et al., 2012; Dyakova et al., 2015). Recent studies have also shown that exercise increases NO and, consequently, NOx production in the body (Shen et al., 1995). Another study on animals and humans has indicated that obesity reduces the bioavailability of NO (Lemaster et al., 2016). Even though several studies have examined the effect of different types of exercise training on NO and NOx production and, although theoretically, this mechanism is well known (Nosarev et al., 2014; Dyakova et al., 2015), the association between different modalities, lengths, and durations of physical activity, and NO/NOx production among studies in living subjects, are controversial (Nosarev et al., 2014). Moreover, no systematic review and meta-analysis has pooled the effect of exercise training on NO and NOx production. Therefore, this systematic review and meta-analytical study was designed to evaluate and pool the effect of different exercise durations, lengths, and modalities on the levels of serum/plasma NO and NOx.
Methods
Search strategy
A global systematic review of electronic databases was conducted to identify controlled clinical trials that have evaluated the effect of physical activity on circulating concentrations of serum/plasma NO and NOx. The search followed the guidelines of the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement. MEDLINE, Web of Sciences, EMBASE, Scopus, and the Cochrane Central Register of Controlled Trials (CENTRAL) electronic databases were searched up to May 2022 with search terms “NO” combined with exercise training and physical activity. The entire search details are available in Supplementary Appendix S1. In addition, reference lists of the relevant reviews and included studies were investigated to identify other potentially eligible articles. No language and publication date restrictions were used in the literature search. Two independent researchers (JH and ES) evaluated the retrieved articles by screening the title and abstract, then by a full-text evaluation. Disagreements regarding study selection were resolved through discussion.
Criteria for considering studies for this review
Studies were considered eligible for inclusion in this review if they met the following criteria (Balakumar et al., 2016): being a randomized controlled trial (RCT) with parallel design or quasi-experimental trial (before–after) (Quyyumi, 1998), an intervention study that applied physical activity as monotherapy or as adjunctive therapy provided that appropriate control group is included (Förstermann et al., 2017), reporting enough data on NO parameters at the beginning and that the end of the study in intervention and control groups (Li et al., 2014), had evaluated serum/plasma concentration of NOx. Exclusion criteria included (Balakumar et al., 2016): observational studies (cohort, case studies, case series, case–control, and cross-sectional) (Quyyumi, 1998), studies that did not report enough data on methodology or results (Förstermann et al., 2017), and studies that had evaluated exhaled or urinary excretion of NOx. Furthermore, the systematic review included all RCTs and quasi-experimental trials on NOx. However, only those studies with two parallel group designs as intervention and control groups—where the control group was sedentary without any exercise—were included in the meta-analysis. Moreover, if a study had assessed the effect of different exercise modes on NO and NOx in comparison with the control group, each comparison was considered a separate study in the meta-analysis.
Data extraction and quality assessment
The following data of eligible studies were extracted by the two reviewers (JH and ES) independently using a standard protocol. The extracted data included (Balakumar et al., 2016) the first author’s name (Quyyumi, 1998), publication date (Förstermann et al., 2017), study location (Li et al., 2014), number of participants in the case and control groups (Di Pietro et al., 2017), study design (Bondonno et al., 2016), information regarding randomization, blinding and drop-outs (Bohn et al., 2015), duration of intervention (Linke et al., 2006), means, and standard deviations of serum/plasma NO and NOx in treatment and control groups.
Eligible trials were methodologically and separately evaluated for their quality using the Cochrane risk of bias tool. This tool evaluates six parameters: selection bias (method for random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other sources of bias.
Ethical considerations
This current systematic review was approved by the ethical committee of the Alborz University of Medical Sciences. All primary articles included were cited in all reports and in the final manuscript.
Statistical analysis
Meta-analysis was conducted using Stata 17.0 software (Stata Corp, College Station, Texas). The result of each study was presented as a standardized mean difference (SMD) and 95% confidence interval (CI). Heterogeneity between the included studies was assessed using the Q-Cochrane test and was regarded as statistically significant at p < 0.1. The degree of heterogeneity was estimated using I2 statistics. In cases of significant heterogeneity between studies, the pooled SMD as a measure of effect size was estimated using the random-effects model (Der-Simonian and Laird method); otherwise, a fixed-effect model was used. Publication bias was scrutinized using Egger’s test. A p-value <0.10 was considered a statistically significant publication bias. If publication bias was significant, the trim and fill correction was used to impute missing studies and correct and adjust the pooled SMD. To determine each study’s effect on the overall pooled association of serum/plasma NO and NOx concentration with exercise, sensitivity (leave-one-out) analysis was also performed. Moreover, according to previous meta-analyses, a stratified meta-analysis was performed according to the type of exercise modes (high-intensity interval training (HIIT) and AT), length of exercise (≤8 weeks/> 8 weeks), and its duration (<40 min per session/≥40 min per session) (Wenger and Bell, 1986; Clark, 2016). All comparisons were two-tailed, with 95% CIs described if applicable.
Results
Literature search
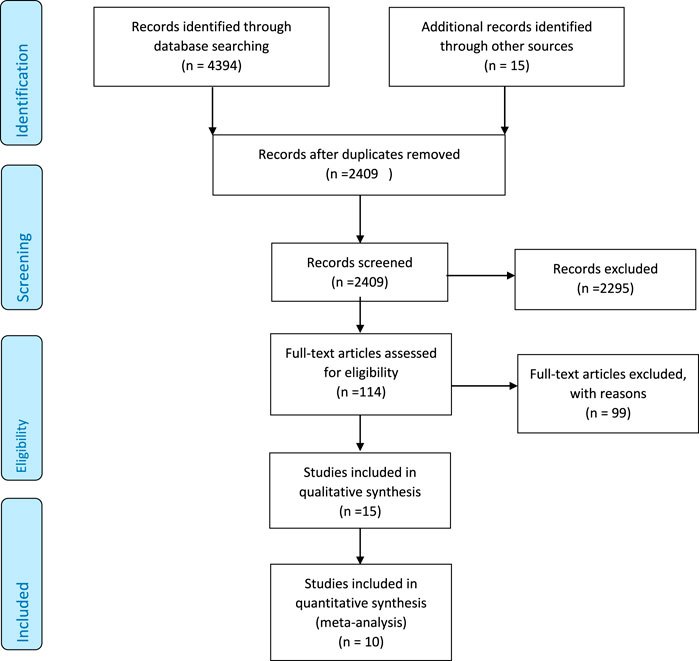
The primary database search identified 4394 relevant articles. There were 2409 unique articles after removing duplicates, and 2295 were further excluded due to irrelevant content. Of the 114 full-text screened articles, 104 did not qualify for inclusion criteria either because they lacked sufficient information on the outcomes of interest, inappropriate design, or did not respond to our email request for additional data. In the end, 15 and 10 studies were included in the qualitative synthesis and meta-analysis, respectively, with each study containing two different interventions and one control group (Figure 1).
Summary of study characteristics and main findings
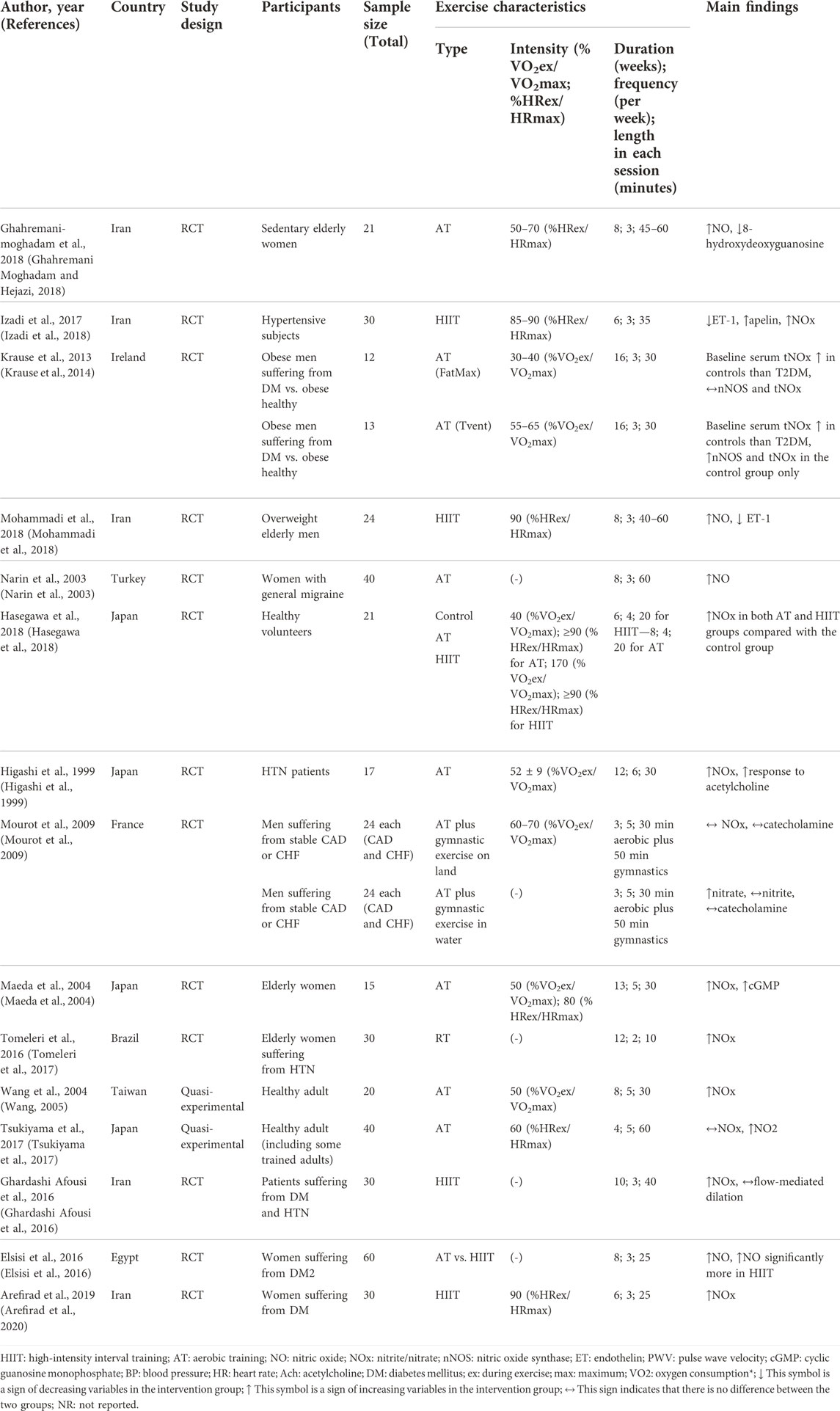
The main characteristics and findings of all 15 included studies (Higashi et al., 1999; Narin et al., 2003; Maeda et al., 2004; Wang, 2005; Mourot et al., 2009; Krause et al., 2014; Elsisi et al., 2016; Ghardashi Afousi et al., 2016; Tomeleri et al., 2017; Tsukiyama et al., 2017; Ghahremani Moghadam and Hejazi, 2018; Hasegawa et al., 2018; Izadi et al., 2018; Mohammadi et al., 2018; Arefirad et al., 2020) in the systematic review are presented in Table 1 comprising 527 participants. Ten studies were included in the meta-analysis, comprising 265 participants (Higashi et al., 1999; Narin et al., 2003; Maeda et al., 2004; Ghardashi Afousi et al., 2016; Tomeleri et al., 2017; Ghahremani Moghadam and Hejazi, 2018; Hasegawa et al., 2018; Izadi et al., 2018; Mohammadi et al., 2018; Arefirad et al., 2020). All studies were performed from 1999 to 2020, with sample sizes ranging from 12 to 60 healthy or unhealthy individuals. Intervention durations ranged from three to 16 weeks. The age of participants ranged from 20 to 70 years. Overall, 13 studies were RCTs and two were quasi-experimental. The majority of the studies were conducted in Iran (five), followed by Japan (four), and Ireland, Egypt, France, Turkey, Taiwan, and Brazil (one study each). Based on exercise training mode, there were five studies on AT, five on HIIT, and one on resistance training. Of these, three found a significant association between serum/plasma NOx levels and AT, and five found a significant association between serum/plasma NOx levels and HITT.
The possible mechanisms, proposed by the majority of studies, are presented in Figure 2. According to this figure, exercise training increases the serum/plasma NOx levels either via the increment of eNOS or reduction of NO clearance.
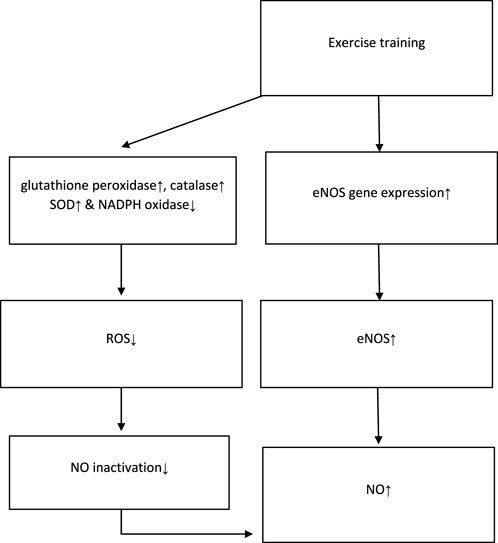
FIGURE 2. Possible mechanisms of NO increment proposed by studies. NO: nitric oxide; eNOS: endothelial nitric oxide synthase; ROS: reactive oxygen species; SOD: superoxide dismutase; NADPH: nicotinamide adenine dinucleotide phosphate. ↓ This symbol is a sign of decreasing variables in the intervention group. ↑ This symbol is a sign of increasing variables in the intervention group.
Risk of bias assessment
Supplementary Appendix S2 provides the methodological features of included trials in the meta-analysis. All assessed studies were considered as being at high risk of bias for allocation concealment and blinding participants and personnel because detailed blinding processes were not found in any of the included RCTs.
Serum/plasma NOx
The mean changes of serum/plasma NOx as the final metabolites of produced NO were assessed between the exercise and control groups in ten articles, in which one study performed two exercise training modes in comparison with the control groups (Hasegawa et al., 2018). The random effect meta-analysis model (Q = 54.69, p-value < 0.01, I2 = 81.72%) showed a significant association between exercise training and serum/plasma NOx with a pooled SMD of 1.82 (95%CI: 1.14–2.49) (Figure 3).
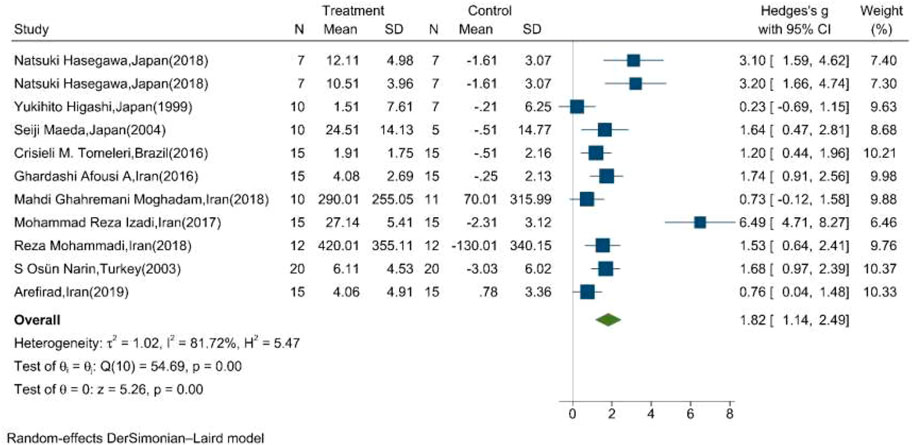
FIGURE 3. Forest plot for the effect of training on mean change of serum NOx concentration. (The vertical line represents null effect.)
Publication bias
The results of Egger’s test supported the existence of publication bias regarding the effect of exercise training on serum/plasma NOx levels (β: 7.31, SE: 1.46, p-value<0.001). Nonetheless, upon “trim and fill” correction, no missing studies were imputed and the results of the analysis did not change; therefore, publication bias did not substantially affect the results.
Stratified meta-analysis
Table 2 shows the results of the subgroup meta-analysis according to the type of exercise training mode, duration of exercise in terms of weeks, and length of each session. Random effect sub-group meta-analysis showed that the effects of HIIT and AT on the mean change of serum/plasma NOx concentration were statistically significant with severe heterogeneity between studies (Q = 38, p < 0.01, I2 = 89.5%) (pooled SMD: 2.55, 95%CI: 1.14–3.96) and (Q = 14.8, p = 0.01, I2 = 71%) (pooled SMD: 1.36, 95%CI: 0.55–2.18), respectively (Figure 4).
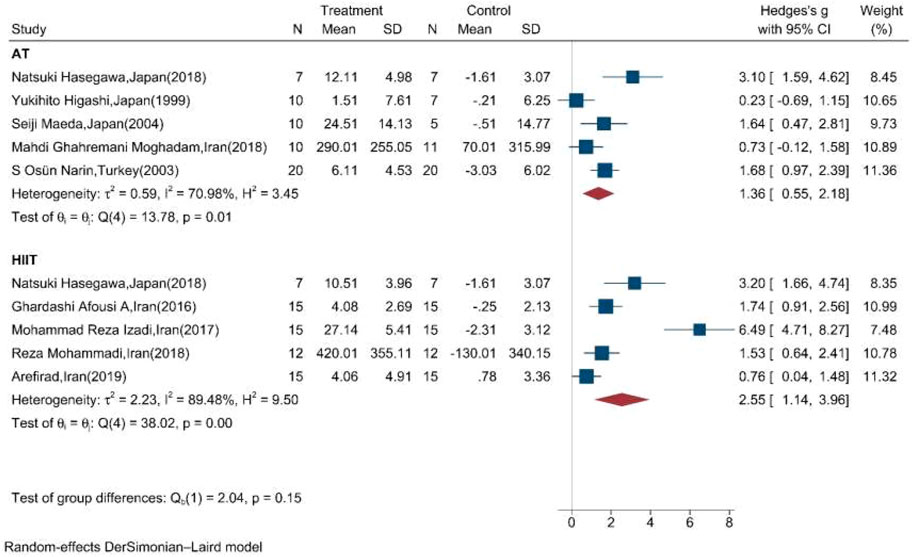
FIGURE 4. Forest plot for the effect of training on mean change of serum NOx concentration according to the type of training (AT: aerobic training; HIIT: high-intensity interval training. The vertical line represents null effect).
Subgroup meta-analysis according to the length of exercise training in each session (<40 min per session/≥40 min per session) showed that both types of length significantly increased the mean change of NOx in the intervention compared to the control group (Figure 5).
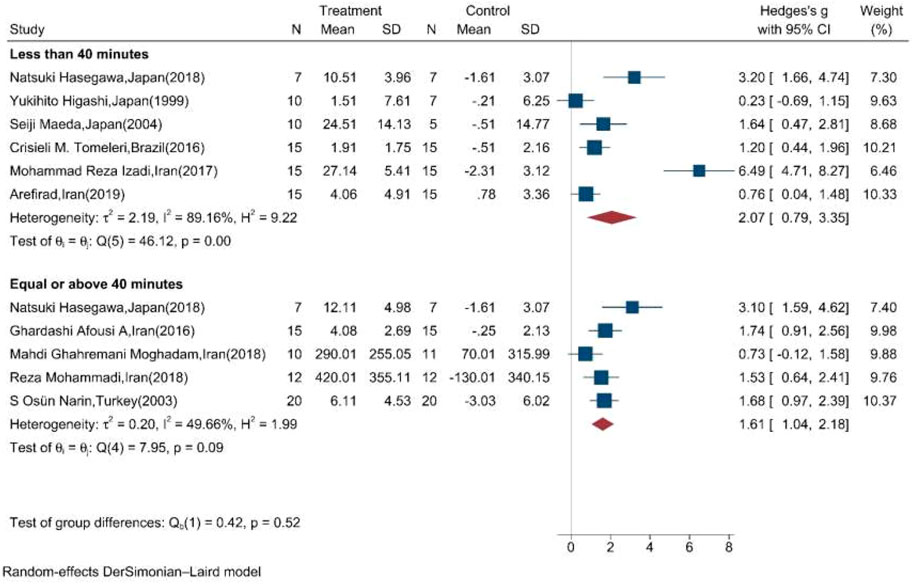
FIGURE 5. Forest plot for the effect of training on mean change of serum NOx according to the length of training in each session. (The vertical line represents null effect.)
Moreover, the random effect sub-group meta-analysis showed that the duration of exercise training equal, less, and above eight weeks significantly increased the mean concentration of NOx (Figure 6).
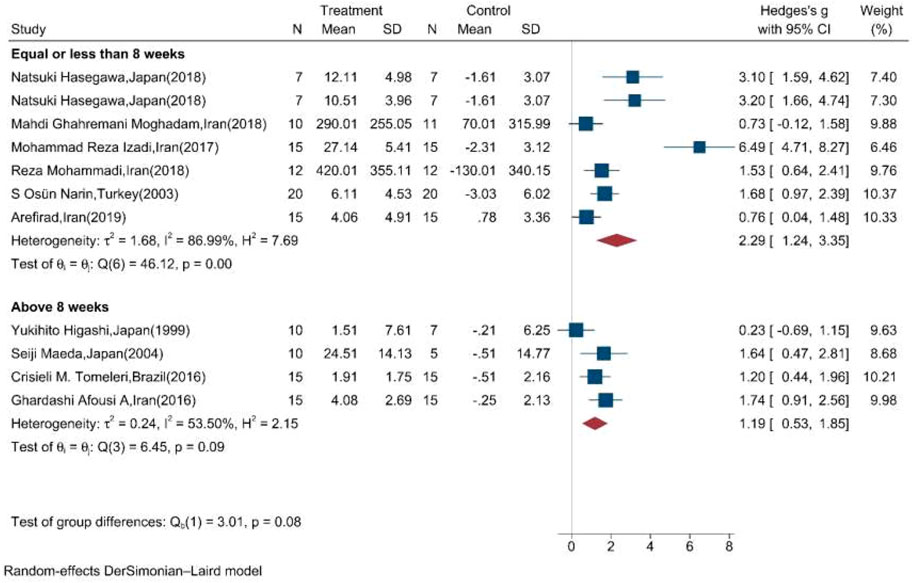
FIGURE 6. Forest plot for the effect of training on mean change of serum NOx concentration according to the duration of training initiation. (The vertical line represents null effect.)
Sensitivity analysis
Figure 7 shows the results of the “leave-one-out” analysis. Upon omitting each study, the overall pooled association of serum/plasma NOx levels and exercise remained significant, meaning that the significance of the association was not substantially affected by the results of each study.
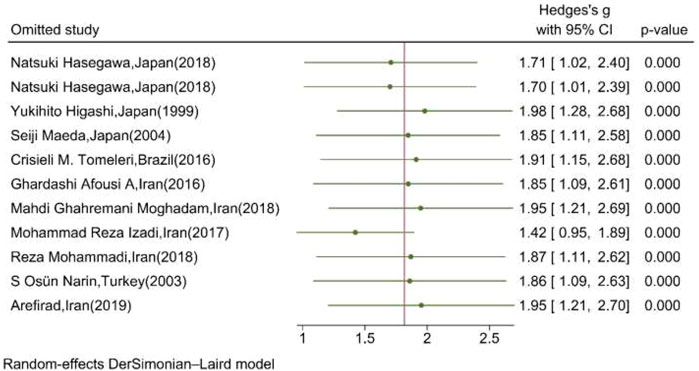
FIGURE 7. Leave-one-out analysis illustrating the overall pooled association of serum/plasma NOx levels upon omitting each study. (The vertical line represents the overall effect of training on the mean change of serum NOx.)
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis that has assessed and pooled the effect of exercise training on serum/plasma NO and NOx levels. In our meta-analysis, exercise significantly increases serum/plasma NOx levels. These results accord with those of Olszanecka-Glinianowicz et al. (2008), Bechara et al. (2008), and Chikara Goto et al. (2003).
In the studies included, some mechanisms have been suggested to explain the association between exercise and serum/plasma NO and NOx levels. Previous studies have shown that exercise increases eNOS gene expression in the lungs, increasing nitric acid production from arginine (Miyauchi et al., 2003). It is also established that aerobic exercise training increases eNOS genes, phosphorylation, and NO levels in the aorta (Franklin et al., 2020).
As previously mentioned (Figure 2), most studies propose that physical activity stimulates the eNOS which lies near the sarcolemma and dystrophin complex within the muscle fibers, thus increasing NO production (Tidball and Wehling-Henricks, 2014). Furthermore, another significant mechanism discussed by studies was that reduced NO bioavailability resulted from its direct quenching by superoxide, even without altering NOS activity (Paolocci et al., 2001). In this regard, it has been demonstrated that regular exercise improves endothelial function by increasing vascular endothelial growth factor-induced angiogenesis and reduces NO deactivation with the reinforcement of components of the antioxidant defense system functions, such as glutathione peroxidase, catalase, and superoxide dismutase (SOD). It decreases nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-derived production of reactive oxygen species (ROS) and angiotensin receptor I and II expression, leading to elevated NO bioavailability and vasodilation (Hollander et al., 1999; Linke et al., 2006). These studies suggest that training and exercise increase NO generation and reduce NO deactivation, thus increasing serum/plasma NO levels (Powers et al., 2016).
The stratified meta-analysis of our findings showed that the effect of exercise on NOx on both AT and HIIT, duration of exercise ≤8 and >8 weeks, and duration of exercise ≥40 and <40 min in each session were statistically significant, thus promoting NO production. Regular exercise can improve body composition and cardiovascular efficiency (Gibson et al., 2018). NO also plays an essential role in the control of CVD by improving endothelial function and vasodilation, glucose homeostasis, and insulin resistance (Lundberg et al., 2015). However, the mechanism associated with training-induced eNOS activation is still unclear; however, mechanical pressures such as shear stress, known to elevate during AT, have been recognized as a major stimulus for eNOS activation (Boo et al., 2002; Tang et al., 2006). Therefore, the intensity of exercise in studies can vary, which may lead our study to differ from the outcomes of previous studies. Moreover, further research is needed to elucidate the actual effect of physical activity on NO levels in those with comorbid disease.
Limitations and strength
This systematic review and meta-analysis faced some limitations. First, the included studies were heterogeneous in regard to training mode, duration of intervention, and length. Second, the heterogeneity between studies in terms of the study population (healthy, subjects with metabolic disorders, and subjects with hypertension) in this review limited our findings. Moreover, the variation in control groups in included studies made it difficult to precisely conclude that the pooled estimated effect is attributed to the training effect. In addition, although the sample size was small in most of the included studies and, in the meta-analysis, the sample and the effect size were pooled, the pooled sample size was not large enough, particularly in the subgroup analysis (according to training mode, prolonged/shortened training time in each session, and duration of exercise training) to robustly justify the findings. Nonetheless, the present systematic review and meta-analysis were based on a comprehensive literature search; it is thus unlikely that much related research was overlooked.
Conclusion
The findings of this study indicate that, regardless of duration, length, and type (AT or HIIT), exercise can significantly increase serum NOx levels. Further randomized trials in different study populations (healthy/unhealthy) with larger sample sizes, homogenous duration, and similar training are needed to approve the findings of this systematic and meta-analytical review.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
TA, MS, and MQ designed the study. ES and JH searched the databases screened and extracted the data. NM and MQ analyzed the data and prepared the results. FR, SM, and NM wrote the manuscript. SY, NM, and FE drafted the final manuscript. All other authors read and approved the final manuscript.
Funding
This study was supported by Alborz University of Medical Sciences, Karaj, Iran.
Acknowledgments
We appreciate Rajaee Hospital Clinical Development Research Unit for their administrative assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.953912/full#supplementary-material
References
Arefirad T., Shakeri N., Ebrahim K., Nasli-Esfahani E. (2020). Effects of interval training on cardio metabolic risk factors and nitric oxide in type 2 diabetes patients: A randomized controlled trial. J. Diabetes Metab. Disord. 19, 669–674. doi:10.1007/s40200-019-00486-z
Balakumar P., Maung U. K., Jagadeesh G. (2016). Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 113 600–609. doi:10.1016/j.phrs.2016.09.040
Bechara L. R. G., Tanaka L. Y., dos Santos A. M., JORDãO C. P., de Sousa L. G. O., Bartholomeu T., et al. (2008). A single bout of moderate-intensity exercise increases vascular NO bioavailability and attenuates adrenergic receptor-dependent and-independent vasoconstrictor response in rat aorta. J. Smooth Muscle Res. 44 (3+ 4), 101–111. doi:10.1540/jsmr.44.101
Bohn B., Herbst A., Pfeifer M., Krakow D., Zimny S., Kopp F., et al. (2015). Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: A cross-sectional multicenter study of 18, 028 patients. Diabetes Care 38 (8), 1536–1543. doi:10.2337/dc15-0030
Bondonno C. P., Croft K. D., Hodgson J. M. (2016). Dietary nitrate, nitric oxide, and cardiovascular health. Crit. Rev. Food Sci. Nutr. 56 (12), 2036–2052. doi:10.1080/10408398.2013.811212
Boo Y. C., Sorescu G., Boyd N., Shiojima I., Walsh K., Du J., et al. (2002). Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms role of protein kinase A. J. Biol. Chem. 277 (5), 3388–3396. doi:10.1074/jbc.M108789200
Boulé N. G., Haddad E., Kenny G. P., Wells G. A., Sigal R. J. (2001). Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. Jama 286 (10), 1218–1227. doi:10.1001/jama.286.10.1218
Chikara Goto R., Higashi Y., Kimura M., Noma K., Hara K., Nakagawa K., et al. (2003). Effect of different intensities of exercise on endothelium-dependent vasodilation in humans.
Clark J. E. (2016). The impact of duration on effectiveness of exercise, the implication for periodization of training and goal setting for individuals who are overfat, a meta-analysis. Biol. Sport 33 (4), 309–333. doi:10.5604/20831862.1212974
Di Pietro N., Marcovecchio M. L., Di Silvestre S., de Giorgis T., Cordone V. G. P., Lanuti P., et al. (2017). Plasma from pre-pubertal obese children impairs insulin stimulated Nitric Oxide (NO) bioavailability in endothelial cells: Role of ER stress. Mol. Cell. Endocrinol. 443, 52–62. doi:10.1016/j.mce.2017.01.001
Duncan G. E. (2006). Exercise, fitness, and cardiovascular disease risk in type 2 diabetes and the metabolic syndrome. Curr. Diab. Rep. 6 (1), 29–35. doi:10.1007/s11892-006-0048-1
Dyakova E. Y., Kapilevich L. V., Shylko V. G., Popov S. V., Anfinogenova Y. (2015). Physical exercise associated with NO production: Signaling pathways and significance in health and disease. Front. Cell Dev. Biol. 3, 19. doi:10.3389/fcell.2015.00019
Elsisi H. F., Albady G. M., Mohammed M. A., Rahmy A. F. (2016). Insulin resistance and nitric oxide response to low volume high intensity interval exercise versus continuous moderate intensity aerobic exercise in type 2 diabetes mellitus. Int. J. Ther. Rehabilitation Res. 5 (1), 15. doi:10.5455/ijtrr.000000112
Förstermann U., Xia N., Li H. (2017). Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120 (4), 713–735. doi:10.1161/CIRCRESAHA.116.309326
Franklin B. A., Thompson P. D., Al-Zaiti S. S., Albert C. M., Hivert M-F., Levine B. D., et al. (2020). Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: Placing the risks into perspective–an update: A scientific statement from the American heart association. Circulation 141 (13), e705–e736. doi:10.1161/CIR.0000000000000749
Ghahremani Moghadam M., Hejazi K. (2018). Effects of eight weeks of aerobic exercise on markers of oxidative stress in elderly women. mljgoums. 12 (3), 17–23. doi:10.29252/mlj.12.3.17
Ghardashi Afousi A., Gaeini A., Gholami Borujeni B. (2016). The effect of aerobic interval training on endothelial vasculature function in type 2 diabetes patient. Iran. J. Rehabilitation Res. 2 (3), 27–39.
Gibson A. L., Wagner D., Heyward V. (2018). Advanced fitness assessment and exercise prescription, 8E. Human kinetics.
Gómez-Guzmán M., Jiménez R., Sánchez M., Zarzuelo M. J., Galindo P., Quintela A. M., et al. (2012). Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic. Biol. Med. 52 (1), 70–79. doi:10.1016/j.freeradbiomed.2011.09.015
Harding A-H., Griffin S. J., Wareham N. J. (2006). Population impact of strategies for identifying groups at high risk of type 2 diabetes. Prev. Med. 42 (5), 364–368. doi:10.1016/j.ypmed.2006.01.013
Hasegawa N., Fujie S., Horii N., Miyamoto-Mikami E., Tsuji K., Uchida M., et al. (2018). Effects of different exercise modes on arterial stiffness and nitric oxide synthesis. Med. Sci. Sports Exerc. 50 (6), 1177–1185. doi:10.1249/MSS.0000000000001567
Higashi Y., Sasaki S., Kurisu S., Yoshimizu A., Sasaki N., Matsuura H., et al. (1999). Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: Role of endothelium-derived nitric oxide. Circulation 100 (11), 1194–1202. doi:10.1161/01.cir.100.11.1194
Hollander J., Fiebig R., Gore M., Bejma J., Ookawara T., Ohno H., et al. (1999). Superoxide dismutase gene expression in skeletal muscle: Fiber-specific adaptation to endurance training. Am. J. Physiol. 277 (3), R856–R862. doi:10.1152/ajpregu.1999.277.3.R856
Izadi M. R., Ghardashi Afousi A., Asvadi Fard M., Babaee Bigi M. A. (2018). High-intensity interval training lowers blood pressure and improves apelin and NOx plasma levels in older treated hypertensive individuals. J. Physiol. Biochem. 74 (1), 47–55. doi:10.1007/s13105-017-0602-0
Krause M., Rodrigues-Krause J., O’Hagan C., Medlow P., Davison G., Susta D., et al. (2014). The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: Implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur. J. Appl. Physiol. 114 (2), 251–260. doi:10.1007/s00421-013-2769-6
Lemaster K., DeVallance E., Branyan K., Skinner R., Brooks S., Asano S., et al. (2016). Aerobic exercise improves nitric oxide bioavailability and endothelium-dependent vasorelaxation in aortic rings of obese Zucker rats. FASEB J. 30 1240–2828.
Li H., Horke S., Forstermann U. (2014). Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237 (1), 208–219. doi:10.1016/j.atherosclerosis.2014.09.001
Linke A., Erbs S., Hambrecht R. (2006). Exercise and the coronary circulation—alterations and adaptations in coronary artery disease. Prog. Cardiovasc. Dis. 48 (4), 270–284. doi:10.1016/j.pcad.2005.10.001
Lundberg J. O., Gladwin M. T., Weitzberg E. (2015). Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 14 (9), 623–641. doi:10.1038/nrd4623
Maeda S., Tanabe T., Otsuki T., Sugawara J., Iemitsu M., Miyauchi T., et al. (2004). Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens. Res. 27 (12), 947–953. doi:10.1291/hypres.27.947
Miyauchi T., Maeda S., Iemitsu M., Kobayashi T., Kumagai Y., Yamaguchi I., et al. (2003). Exercise causes a tissue-specific change of NO production in the kidney and lung. J. Appl. Physiol. 94 (1), 60–68. doi:10.1152/japplphysiol.00269.2002
Mohammadi R., Fathei M., Hejazi K. (2018). Effect of eight-weeks aerobic training on serum levels of nitric oxide and endothelin-1 in overweight elderly men. Salmand 13 (1), 74–85. doi:10.21859/sija.13.1.74
Mourot L., Teffaha D., Bouhaddi M., Ounissi F., Vernochet P., Dugue B., et al. (2009). Training-induced increase in nitric oxide metabolites in chronic heart failure and coronary artery disease: An extra benefit of water-based exercises? Eur. J. Cardiovasc. Prev. Rehabil. 16 (2), 215–221. doi:10.1097/HJR.0b013e3283292fcf
Narin S. O., Pinar L., Erbas D., Ozturk V., Idiman F. (2003). The effects of exercise and exercise-related changes in blood nitric oxide level on migraine headache. Clin. Rehabil. 17 (6), 624–630. doi:10.1191/0269215503cr657oa
Nosarev A. V., Smagliy L. V., Anfinogenova Y., Popov S. V., Kapilevich L. V. (2014). Exercise and NO production: Relevance and implications in the cardiopulmonary system. Front. Cell Dev. Biol. 2, 73. doi:10.3389/fcell.2014.00073
Olszanecka-Glinianowicz M., Zahorska-Markiewicz B., Plewa M., Janowska J. (2008). The effect of short-term exercise on nitric oxide (no) serum concentrations in overweight and obese women. Biol. Sport 25 (2), 125.
Paolocci N., Biondi R., Bettini M., Lee C-I., Berlowitz C. O., Rossi R., et al. (2001). Oxygen radical-mediated reduction in basal and agonist-evoked NO release in isolated rat heart. J. Mol. Cell. Cardiol. 33 (4), 671–679. doi:10.1006/jmcc.2000.1334
Powers S. K., Radak Z., Ji L. L. (2016). Exercise-induced oxidative stress: Past, present and future. J. Physiol. 594 (18), 5081–5092. doi:10.1113/JP270646
Quyyumi A. A. (1998). Endothelial function in health and disease: New insights into the Genesis of cardiovascular disease. Am. J. Med. 105 (1), 32S–9S. doi:10.1016/s0002-9343(98)00209-5
Shen W., Zhang X., Zhao G., Wolin M. S., Sessa W., Hintze T. H. (1995). Nitric oxide production and NO synthase gene expression contribute to vascular regulation during exercise. Med. Sci. Sports Exerc. 27 (8), 1125–1134. doi:10.1249/00005768-199508000-00005
Tang B. T., Cheng C. P., Draney M. T., Wilson N. M., Tsao P. S., Herfkens R. J., et al. (2006). Abdominal aortic hemodynamics in young healthy adults at rest and during lower limb exercise: Quantification using image-based computer modeling. Am. J. Physiol. Heart Circ. Physiol. 291 (2), H668–H676. doi:10.1152/ajpheart.01301.2005
Tidball J. G., Wehling-Henricks M. (2014). Nitric oxide synthase deficiency and the pathophysiology of muscular dystrophy. J. Physiol. 592 (21), 4627–4638. doi:10.1113/jphysiol.2014.274878
Tomeleri C. M., Marcori A. J., Ribeiro A. S., Gerage A. M., de Souza Padilha C., Schiavoni D., et al. (2017). Chronic blood pressure reductions and increments in plasma nitric oxide bioavailability. Int. J. Sports Med. 38 (04), 290–299. doi:10.1055/s-0042-121896
Tsukiyama Y., Ito T., Nagaoka K., Eguchi E., Ogino K. (2017). Effects of exercise training on nitric oxide, blood pressure, and antioxidant enzymes. J. Clin. Biochem. Nutr. 60, 180–186. doi:10.3164/jcbn.16-108
Wang J-S. (2005). Effects of exercise training and detraining on cutaneous microvascular function in man: The regulatory role of endothelium-dependent dilation in skin vasculature. Eur. J. Appl. Physiol. 93 (4), 429–434. doi:10.1007/s00421-004-1176-4
Keywords: exercise, training, aerobic, nitric oxide, NO
Citation: Arefirad T, Seif E, Sepidarkish M, Mohammadian Khonsari N, Mousavifar SA, Yazdani S, Rahimi F, Einollahi F, Heshmati J and Qorbani M (2022) Effect of exercise training on nitric oxide and nitrate/nitrite (NOx) production: A systematic review and meta-analysis. Front. Physiol. 13:953912. doi: 10.3389/fphys.2022.953912
Received: 26 May 2022; Accepted: 06 September 2022;
Published: 04 October 2022.
Edited by:
Cain Craig Truman Clark, Coventry University, United KingdomReviewed by:
Nazareno Paolocci, Johns Hopkins University, United StatesShin-Da Lee, China Medical University, Taiwan
Ryoma Michishita, Fukuoka University, Japan
Sandra Lia Amaral, São Paulo State University, Brazil
Copyright © 2022 Arefirad, Seif, Sepidarkish, Mohammadian Khonsari, Mousavifar, Yazdani, Rahimi, Einollahi, Heshmati and Qorbani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javad Heshmati, amF2YWQuaGVzaG1hdGlAZ21haWwuY29t; Mostafa Qorbani, bXFvcmJhbmkxMzc5QHlhaG9vLmNvbQ==
 Tahereh Arefirad1
Tahereh Arefirad1 Ehsan Seif
Ehsan Seif Nami Mohammadian Khonsari
Nami Mohammadian Khonsari Fatemeh Rahimi
Fatemeh Rahimi Faezeh Einollahi
Faezeh Einollahi Javad Heshmati
Javad Heshmati Mostafa Qorbani
Mostafa Qorbani