
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol. , 30 September 2022
Sec. Computational Physiology and Medicine
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.952709
This article is part of the Research Topic Biomedical Image Segmentation and Analysis View all 9 articles
Cancer is one of the top causes of death globally. Recently, microarray gene expression data has been used to aid in cancer’s effective and early detection. The use of DNA microarray technology to uncover information from the expression levels of thousands of genes has enormous promise. The DNA microarray technique can determine the levels of thousands of genes simultaneously in a single experiment. The analysis of gene expression is critical in many disciplines of biological study to obtain the necessary information. This study analyses all the research studies focused on optimizing gene selection for cancer detection using artificial intelligence. One of the most challenging issues is figuring out how to extract meaningful information from massive databases. Deep Learning architectures have performed efficiently in numerous sectors and are used to diagnose many other chronic diseases and to assist physicians in making medical decisions. In this study, we have evaluated the results of different optimizers on a RNA sequence dataset. The Deep learning algorithm proposed in the study classifies five different forms of cancer, including kidney renal clear cell carcinoma (KIRC), Breast Invasive Carcinoma (BRCA), lung adenocarcinoma (LUAD), Prostate Adenocarcinoma (PRAD) and Colon Adenocarcinoma (COAD). The performance of different optimizers like Stochastic gradient descent (SGD), Root Mean Squared Propagation (RMSProp), Adaptive Gradient Optimizer (AdaGrad), and Adaptive Momentum (AdaM). The experimental results gathered on the dataset affirm that AdaGrad and Adam. Also, the performance analysis has been done using different learning rates and decay rates. This study discusses current advancements in deep learning-based gene expression data analysis using optimized feature selection methods.
Cancer is one of the deadliest diseases, and with its increasing prevalence, early identification and treatment are critical (Sung et al., 2021) (Schiff et al, 2007; Reid et al, 2011). Lung cancer cases have been surpassed by female breast cancer cases and are one of the most often detected forms of cancer. Figure 1 shows the cancer cases and deaths in 2020.
About two-third of cases are detected at initial stages (Fotouhi et al, 2019; Id et al., 2021, Kashyap et al, 2022). The classification and identification of gene expression using DNA microarray data is an effective tool for cancer diagnosis and prognosis for specific cancer subtypes. AI-based learning algorithms are vital tools and the most often used way to achieve significant features of gene expression data and play an essential part in gene categorization. This article will give a review of some of those strategies from the literature and information on the various datasets on which these techniques are applied and their associated benefits and drawbacks. The most classic variants of deep learning, such as Convolution Neural Networks, Artificial Neural Networks, and Autoencoders, have been established as essential tools for clinical oncology research and can be used to drive decision-making regarding disease diagnosis and therapy. As time passes, sickness in general, and cancer in particular, grow increasingly complex and challenging to identify, analyze, and treat. Cancer research is a prominent topic of study in the medical world.
The selected articles for analysis have been published in last 5-years. Most of the research articles explored in this study have been published in 2018 and 2019. The articles that have explored gene expression data for cancer diagnosis/survival/stage prediction have been included in this study. Figure 2 presents the year-wise distribution of articles.
The study contributes in a number of ways. Following are the significant contributions made by the study:
• This article reviews recent developments in deep learning-based feature selection techniques for gene expression data interpretation and offers an extensive review of Deep Learning architectures that have demonstrated success across a wide range of industries and are now used to help doctors identify various chronic conditions.
• In this work, we have compared the outcomes of several optimizers on a dataset of RNA sequences. The study’s deep learning system categorizes five types of cancer: colon cancer, lung adenocarcinoma, prostate cancer, invasive breast carcinoma, and kidney clear cell carcinoma (COAD).
• The efficiency of several optimizers, including adaptive gradient optimization (AdaGrad), stochastic gradient descent (SGD), root mean square propagation (RMSProp), as well as adaptive momentum (Adam). AdaGrad and Adam are more precise, according to the experimental findings discovered in the dataset. The performance of a variety of learning and decay rates was explored in the performance study.
This paper is organized in a way that boosts the comprehensibility of the article. Second section gives the description of the significance of gene-expression analysis in cancer research. Section 2 gives description of search strategy used to select the articles for this study. Further Section 3 presents an overview of deep learning approaches where conventional approaches are discussed. Section 4 illustrates the importance of deep learning techniques in Cancer Prediction. Further, Section 5 embraces the literature of recent studies that have explored the deep learning strategies for gene section or survival prediction from microarray gene expression datasets. The article is discussed and concluded in Section 6 and Section 7, respectively. This study reviews and presents a comparative analysis of the previous studies. This article aims to analyze the concepts underlying deep learning-based classification algorithms used in healthcare.
The search strategy used in this paper is Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) strategy. All the research studies selected for this systematic review have been extracted from databases like PubMed, Web of Science, EBSCO, and EMBASE. All the research articles that have been published before 2016 are excluded from the analysis. The keywords used for extraction of articles include “Deep Learning”, “Artificial Intelligence”, “Cancer”, “Micro-array analysis”, “gene-expression”, and combination of these keywords. The research articles that have focused on the optimization of gene selection using deep learning techniques have been included in the study. Figure 3 shows the PRISMA strategy flowchart.
The Artificial intelligence is the idea of making innovative and intelligent machines. Machine learning is an artificial intelligence subset that aids in developing AI-driven applications. Deep learning is a subtype of machine learning that trains a model using large amounts of data and advanced methods. Figure 4 shows hierarchy of AI, Machine Learning, Deep Learning.
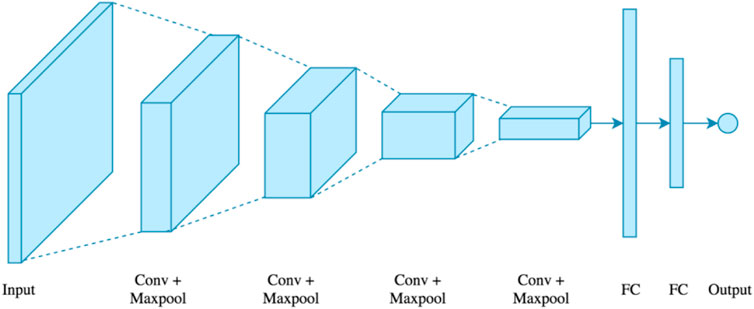
FIGURE 6. Convolutional neural network. • Long Short-Term Memory (LSTM) Network: Hochreiter and Schimdhuber collaborated to create the LSTM (Lecun et al, 2015), which is utilized in various applications. LSTMs were chosen by IBM primarily for voice recognition. The LSTM employs a memory unit known as a cell that may retain its value for an extended period and aids the device in remembering the most recent computed value. The memory unit, also known as a cell, comprises three gates that regulate the movement of data inside the unit. Figure 7 shows the logical structure of a LSTM model.
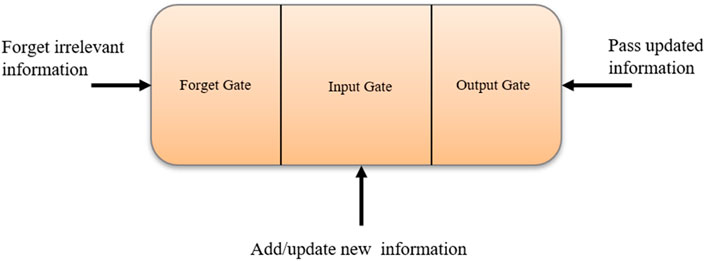
FIGURE 7. Long short-term memory.• The input port, also known as the gate, controls new data flow into the memory. • The forget gate forgets the irrelevant/unnecessary information. • The third port must regulate the information stored as output.
The significant differences between deep learning approaches and traditional learning are summarized in Table 1.
• Artificial Neural Networks: One of the most often used data modeling algorithms in medicine is neural networks. In the early 20th century, neural networks were developed (Daoud and Mayo, 2019). The primary goal of employing neural networks is to recognize patterns and conduct classification tasks. A human brain is used to represent the neural network system. The human brain is made up of millions of neurons that are all linked together. Figure 5 shows the representation of an artificial neural network.
Similarly, a neural network represents multiple neurons with a weight assigned to each link. These neurons act in parallel. During the learning stage, the network updates the weights for prediction of proper input to produce the output function (Gupta and Gupta, 2021b). Different optimization tasks are done by neural networks using different optimization techniques. Sigmoid optimization is mathematically given in Equation 1.
The mathematical working of Hyperbolic Tangent (Tanh) optimization technique is given in Equation 2.
The working of Rectilinear Unit (Relu) optimization technique is expressed in Eq. 3.
Because of its adaptive character, altering the weights aids in the minimization of error. In contrast to basic modeling methods, neural networks have the advantage of predicting non-linear relationships. In the study of medical data, neural networks play a significant role such as medication development. The use of a neural network to predict cardiac disease is possible.
• Convolutional Neural Network (CNN): CNN is a multi-layer neural network based on the visual brain of animals. LeCun et al. constructed the first CNN. CNN’s major application areas include image processing and character recognition (Akkus et al, 2017; Zahras, 2018). In terms of construction, the initial layer recognizes features, however the intermediate layer recombines features to produce high-level input characteristics, followed by classification. The collected characteristics will then be pooled, which reduces their dimensionality. Convolution and pooling are the following steps, which are then put into a fully connected multi-layer perceptron. The last layer, known as the output layer, recognizes the image’s characteristics using back-propagation techniques (Gupta and Gupta, 2021a). Because of its unique properties, such as local connection and shared weights, CNN increases the system’s accuracy and performance. It outperforms all other deep learning techniques. In comparison to other types of architecture, it is the most often utilized. Figure 6 shows a convolutional neural network.
The cell’s weight can be utilized as a regulating factor. There is a requirement for a training approach known as Backpropagation through time (BPTT) that improves weight. For optimization, the technique requires network output error.
Deep learning has been widely utilized to improve prognosis (Huang et al., 2020). Gene expression profiles, which describe the molecular state, offer enormous promise as a medical diagnostic tool. However, current training data sets have a minimal sample size for classification compared to the number of genes involved, and these training data constraints challenge specific classification techniques. One of the most important new clinical applications of microarray data is abnormality detection. Because of the high dimensionality, gene selection is a crucial step in enhancing the classification performance of expression data. As a result, better approaches for selecting functional genes for cancer prediction and detection are required. Microarray studies yield a massive quantity of gene-expression information from a single sample. The quantity of gene-expressions (features) to cases (samples) ratio is highly skewed, resulting in the well-known curse-of-dimensionality issue. In a single experiment, microarray technology generates hundreds of gene expressions. However, comparing the quantity of characteristics, the quantity of samples/patients is significantly lower (up to a few hundred) (several thousand). The limited number of samples (training data) provided is insufficient to create an efficient model from the given data. This is referred to as data scarcity.
Processing microarray gene expression data is a diverse field of computer science that includes graph analysis, machine learning, clustering, and classification. Microarray technology allows for the measurement of thousands of gene expressions in a single experiment. Gene expression levels aid in identifying linked genes and disease development, which aids in the early detection and prognosis of many forms of cancer.
Using microarray gene expression patterns (Dwivedi, 2016), develop a framework of supervised machine learning approaches for discriminating acute lymphoblastic leukemia from acute myeloid leukemia. This classification was accomplished using an artificial neural network (ANN) (Tumuluru and Ravi, 2017). Using microarray gene expression patterns develop a framework of supervised machine learning approaches for discriminating acute lymphoblastic leukemia from acute myeloid leukemia. This classification was accomplished using an artificial neural network (ANN). In 2020, prostate cancer (Surbhi Gupta, 2021) was predicted using Multi-layer perceptrons and explored multiple data balancing techniques. Another recent study in 2021 (Gupta and Gupta, 2021b) predicted mesothelioma with 96% accuracy using ANN (Tumuluru and Ravi, 2017). presented an approach for cancer categorization based on gene-expression data. The logarithmic transformation pre-processed the gene expression data to reduce the classification’s complexity, while the Bhattacharya distance identified the most informative genes. The weight update in Deep Belief Neural Networks has estimated the average error using GOA and Gradient Descent.
The experimentation with colon and leukemia data demonstrates the proposed cancer classification’s efficacy. The accuracy rate of the proposed classification approach employing gene expression data is 0.9534, and 0.9666 detection rate.
Despite decades of research, clinical diagnosis of cancer and the identification of tumor-specific markers remain unknown (Danaee et al., 2017). offered a deep learning technique for cancer detection and identifying critical genes for breast cancer diagnosis using autoencoders. The error rates are computed using log loss function given in Equation 4.
In the above equation,
A retrospective study (Lin et al., 2018) investigated the use of Deep Learning (DL) to predict acute myeloid leukemia (AML) prognosis. This study used 94 AML cases from the TCGA database. Age, ten common cytogenetic mutations, and the 23 most common mutations have been used as input data. Also, the results suggested feasible applications of deep learning (DL) in the prognostic prediction utilizing next-generation sequencing (NGS) data as proof-of-concept research.
Research work (Parvathavardhini and Manju, 2020) proposed a Neuro-Fuzzy approach for interpreting gene-expression data from microarray experiments. The analysis enabled the detection and classification of cancer, hence facilitating treatment selection and development. The proposed strategy was evaluated against three publicly available datasets of cancer gene expression. Also (Sevakula et al, 2018), proposed a cancer-verification transfer learning process in combination with autoencoders. The cross entropy function is used for optimizing the neural models. The cross entropy (
The term
(Xu et al., 2019b) employed numerous computational methods for classifying cancer subtypes have been presented. However, the majority of them create the model only using gene expression data. 2019 (Huynh et al, 2019). proposed a new support vector machine (SVM) classification model for gene expression based on features collected from a deep convolutional neural network (DCNN). The Equation 6 illustrates the working of CNN.
Here a
Nonetheless, it is characterized by highly high-dimensional data, which results in an over-fitting problem for the classifying model (Lin et al, 2018). purposed a novel way for incorporating deep learning into an ensemble approach that included numerous machine learning models. First, the study provided valuable gene data to five distinct categorization models using differential gene expression analysis. Then outputs of the five classifiers are then combined using a deep learning algorithm.
Significant bioinformatics research (Shon et al, 2021) has been undertaken in cancer research, and bioinformatics methodologies may aid in developing methods and models for early prediction of stomach cancer. This study aimed to build a CNN algorithm to analyze TCGA data. This study merged RNA-seq, and clinical data looked for and assessed potential genes employing the CNN model. In addition, this study performed learning and evaluated the status of cancer patients. The proposed model acquired an accuracy of 95.96 percent and a critical status accuracy of 50.51 percent. Despite overfitting due to the small sample size, reasonably accurate results for the sample type were achieved. This method can be used to forecast the diagnosis of stomach cancer, which comes in various forms and has a variety of underlying causes.
(Gupta and manoj, 2021) discovered that group algorithms for chronic disease diagnosis could be more effective than baseline algorithms. Additionally, it outlines many impediments to furthering the use of machine learning classification to detect illness. The proposed strategy achieved 98.5, 99, and 100% accuracy in this study. The disease datasets used in the study includes Diabetes, Cardiovascular Disease, and Breast Cancer. The algorithms used for the disease prediction are Group Algorithms, Stacked, and Neural Network.
(Abdollahi et al, 2021) proposed a novel strategy for reducing the number of features by utilizing an autoencoder. Each gene’s weight is determined as a consequence of our autoencoder model. The weights indicate the magnitude of each gene’s effect on survival probability. Our approach enhances survival analysis by speeding up the procedure, increasing prediction accuracy, and decreasing the calculated survival probability’s error rate. The error rates are computed using root mean squared error (RMSE). The mathematical formula of RMSE is given in Equation 7) where A and O represent actual and observed values respectively.
Multiple studies aimed to investigate cancer prediction models. Table 2 presents the research analysis table.
This section holds the simulation results achieved using ANN model along with multiple optimizers like Stochastic gradient descent (SGD), Root Mean Squared Propagation (RMSProp), Adaptive Gradient Optimizer (AdaGrad), and Adaptive Momentum (AdaM). Also, the performance analysis has been done using different learning rates and decay rates.
TCGA dataset is available at https://archive.ics.uci.edu/ml/datasets/gene + expression + cancer + RNA-Seq. This dataset comprises data on five different forms of cancer, including kidney renal clear cell carcinoma (KIRC), Breast Invasive Carcinoma (BRCA), lung adenocarcinoma (LUAD), Prostate Adenocarcinoma (PRAD) and Colon Adenocarcinoma (COAD). The dataset consists of 20,531 attributes of 801 patients.
The performance of multiple optimizers is analyzed and shown in Figure 8. From Figure 8, it is clear that both “Adam” and “Adagrad” performed the best on training and testing data.
The ANN model using SGD and rmsprop optimizer attained 35.3% on training data and 43.8% on test data. Both the Adam approaches performed well. Hence, we considered analyzing the performance of different parameters like learning rates and decay rates.
The performance of ADAM optimizer using different learning rates is analyzed and shown in Figure 9.
From the figure, it is clear that learning rate (’0.01’, ‘0.001’, ‘0.0001’, ‘1e−05) performed the best on training and testing data. The ANN models performed worst (35% on train and 43.8% on test set) with slowest (lrate = “1.0”, “0.1”).
The technique of learning rate decay (lrDecay) is used to train current neural networks. It begins with a high rate of learning and then decays several times. It has been demonstrated empirically to aid in both optimization and generalization. The performance of ADAM optimizer using different decay rates is investigated and revealed in Figure 10.
From the figure, it is clear that decay rate (“0.1”, “0.001”) performed the best on training and testing data. The ANN models performed worst (35.3% on train and 43.8% on test set) and (63.5% on train and 68.7% on test set) with decay rates “0.01” and “0.0001” respectively.
Several strategies for gene selection in cancer categorization have been proposed in prior studies. The advent of deep learning has profoundly affected a wide variety of machine learning applications and research. Few of such studies (Gupta and Gupta, 2021a), (Gupta and Gupta, 2021b), (Gupta and Gupta, 2021c) are described in this section. The work flow used for classification of cancer data is shown in Figure 11.
Initially, the exploration of data is done and termed as “exploratory data analysis”. Further, data preprocessing steps are used like cleaning data, reducing dimension (feature reduction), normalizing the data. Further the next stage splits the preprocessed data into sets. The deep learning classification algorithm is trained on the training set for classification of data. The trained classification model is further evaluated on the test set. The evaluation of the data can express the accurateness of the model. The number of cancer cases is rapidly increasing. It is difficult to diagnose because the illness is frequently asymptomatic in its early stages. Early detection can increase the odds of a patient’s recovery and cure. Cancer is notoriously difficult to diagnose in its early stages and is prone to recurrence after treatment. Cancer classification is a crucial topic. One of the most effective methods for cancer classification is gene selection (Gupta and Gupta, 2021d). The task of choosing a set of genes that enhances classification accuracy is NP-Hard. Furthermore, making accurate and specific cancer diagnostic forecasts is quite tricky. Because of the nonspecific symptoms and imprecise scans, certain tumors are more challenging to diagnose in their early stages. As a result, improving the prediction model in diagnostic cancer research is vital. Furthermore, most cancer research articles have increased dramatically, particularly those that use deep learning methodologies (Shimizu and Nakayama, 2020). Again, the present research shows that traditional analysis techniques (Akkus et al., 2017; Ronoud and Asadi, 2019; Chaunzwa et al., 2021) aid in improving the prediction accurateness and is frequently applied in healthcare sector. Its success is since it enables the discovery of highly complicated non-linear correlations between characteristics; and the extraction of information from unlabeled data unrelated to the situation at hand. Statistical studies demonstrate that deep learning models outperform numerous widely used cancer categorization algorithms.
Several academics have investigated automated learning methodologies; however, these approaches still have several flaws that make cancer classification difficult. Specific machine learning algorithms have been found incapable of exploiting unstructured data in cancer classification. CNNs are particularly appropriate for analyzing a wide range of unstructured data. This capability enabled deep learning algorithms to take an active role in the early diagnosis of cancer through data classification. Deep learning approaches have achieved high accuracy and other statistical characteristics. Deep Learning has succeeded in various domains, including image, video, audio, and text processing. Deep Learning faces a unique problem in gene expression analysis for various cancer detection and prediction tasks to define appropriate biomarkers for different cancer subtypes. Despite several research studies on multimodal treatment approaches, survival times remain short. The gathering of significant genes that can increase accuracy can provide adequate guidance in early cancer detection. Cancer can be classified into several subgroups. However, it is a complex task because of the vast number of genes and the comparatively few experiments in gene expression data (Kumar et al, 2021). Cancer identification from microarray gene expression data presents a significant difficulty due to the small sample size, high dimensionality, and complexity of the data (Dargan et al, 2020). There is a need for rapid and computationally efficient methods to address such issues. This study briefly explores the research studies that employed deep learning architectures that selected the most relevant genes for cancer prediction using gene expression data. Although Deep Learning has had success in various domains, it has yet to be thoroughly explored in genomics, notably in genomic cancer.
Cancer has become one of the top causes of death worldwide in recent years. As a result, increasing research is being done to determine the most effective diagnosing and treating cancer. However, cancer treatment faces numerous obstacles, as possible causes of cancer include genetic problems or epigenetic modifications in the cells. RNA sequencing is a substantial approach for assessing gene expression in model organisms and can provide information for bio-molecular cancer diagnosis. Microarray gene expression profiles can be used to classify tumors efficiently and effectively. Predicting various tumors is a significant problem, and offering accurate predictions would be highly beneficial in delivering better therapy to patients. The advent of deep learning approaches is critical for improving patient monitoring, as it can aid clinicians in making decisions regarding deadly diseases. Furthermore, Gene expression data are utilized to develop a classification model that will help cancer treatment. Classification of cancer subtypes is critical for effective diagnosis and individualized cancer treatment. The article concludes that the recent advances in high-throughput sequencing technology have resulted in the quick generation of multi-omics data from the same cancer sample. Thus, deep learning-based molecular illness classification holds considerable promise in the realm of genomics, particularly concerning gene microarray data.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdollahi J., Nouri-Moghaddam B., Ghazanfari M. (2021). Deep Neural Network Based Ensemble learning Algorithms for the healthcare system diagnosis of chronic diseases. ArXiv Preprint Available at: https://ArXiv/org/abs.2103.08182.
Ahn T., Lee C. (2018). Deep learning-based identification of cancer or normal tissue using gene expression data.In Proceeding IEEE International Conference on Bioinformatics and Biomedicine (BIBM). Madrid Spain. 03-06 December 2018. IEEE, 1748–1752. doi:10.1109/BIBM.2018.8621108
Akkus Z., Galimzianova A., Hoogi A., Rubin D. L., Erickson B. J. (2017). Deep learning for brain MRI segmentation : State of the art and future directions. J. Digit. Imaging. 30 (4), 449–459.
Alomari O. A., Khader A. T., Al-Betar M. A., Awadallah M. A. (2018). A novel gene selection method using modified MRMR and hybrid bat-inspired algorithm with β-hill climbing. Appl. Intell. (Dordr). 48 (11), 4429–4447. doi:10.1007/s10489-018-1207-1
Aziz R., Verma C. K., Srivastava N. (2017). A novel approach for dimension reduction of microarray. Comput. Biol. Chem. 71, 161–169. doi:10.1016/j.compbiolchem.2017.10.009
Basavegowda H. S., Dagnew G. (2020). Deep learning approach for microarray cancer data classification. CAAI Trans. Intell. Technol. 5, 22–33. doi:10.1049/trit.2019.0028
Chaunzwa T. L., Hosny A., Xu Y., Shafer A., Diao N., Lanuti M., et al. (2021). Deep learning classification of lung cancer histology using CT images. Sci. Rep. 1, 5471. doi:10.1038/s41598-021-84630-x
Chen X., Xie J., Yuan Q. (2018). A method to facilitate cancer detection and type classification from gene expression data using a deep autoencoder and neural network. Mach. Learn. Available at: https://arXiv/org/abs1812.08674.
Ching T., Zhu X., Garmire L. X. (2018). Cox-nnet : An artificial neural network method for prognosis prediction of high-throughput omics data. PLoS Comput. Biol. 14, e1006076–18. doi:10.1371/journal.pcbi.1006076
Cho H., Lee S., Ji Y. G., Hyeon D., Id L. (2018). Association of specific gene mutations derived from machine learning with survival in lung adenocarcinoma. PLoS One 13, e0207204. doi:10.1371/journal.pone.0207204
Danaee P., Ghaeini R., Hendrix D. A. (2017). A deep learning approach for cancer detection and relevant gene identification. Pac. Symp. Biocomput. 22, 219–229. doi:10.1142/9789813207813_0022
Daoud M., Mayo M. (2019). A survey of neural network-based cancer prediction models from microarray data. Artif. Intell. Med. 97, 204–214. doi:10.1016/j.artmed.2019.01.006
Dargan S., Kumar M., Rohit M., Gulshan A. (2020). A survey of deep learning and its applications : A new paradigm to machine learning. Arch. Comput. Methods Eng. 27 (4), 1071–1092. doi:10.1007/s11831-019-09344-w
Dwivedi A. K. (2016). Artificial neural network model for effective cancer classification using microarray gene expression data. Neural comput. Appl. 29, 1545–1554. doi:10.1007/s00521-016-2701-1
Extraction S. F. (2017). “Prognosis prediction of human breast cancer by integrating deep neural network and support vector machine supervised feature extraction and classification for breast cancer prognosis prediction,” in Proceeding International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Shanghai China, 14-16 October 2017 (IEEE). doi:10.1109/CISP-BMEI.2017.8301908
Fotouhi S., Asadi S., Kattan M. W. (2019). A comprehensive data level analysis for cancer diagnosis on imbalanced data. J. Biomed. Inf. 90, 103089. doi:10.1016/j.jbi.2018.12.003
Gao F., Wang W., Tan M., Zhu L., Zhang Y., Fessler E., et al. (2019). DeepCC : A novel deep learning-based framework for cancer molecular subtype classification. Oncogenesis 8, 44. doi:10.1038/s41389-019-0157-8
García-díaz P., Sánchez-berriel I., Martínez- J. A., Diez-pascual A. M. (2019). Unsupervised feature selection algorithm for multiclass cancer classification of gene expression RNA-Seq data. Genomics 112, 1196. doi:10.1016/j.ygeno.2019.11.004
Guia J. M. De. (2019). “DeepGx : Deep learning using gene expression for cancer classification,” in Proceeding IEEE/ACM International Conference on Advances in Social Networks Analysis and Mining (ASONAM), Vancouver BC Canada, 27-30 August 2019 (IEEE), 913–920. doi:10.1145/3341161.3343516
Guo Y., Liu S., Li Z., Shang X. (2018). BCDForest : A boosting cascade deep forest model towards the classification of cancer subtypes based on gene expression data. BMC Bioinforma. 19 (5), 118–213. doi:10.1186/s12859-018-2095-4
Gupta G., Manoj G. (2021). “Deep learning for brain tumor segmentation using magnetic resonance images,” in Proceeding IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB), Melbourne, Australia, 13-15 October 2021 (IEEE), 1–6. doi:10.1109/CIBCB49929.2021.9562890
Gupta S. (2021). Computational prediction of cervical cancer diagnosis using ensemble-based classification algorithm. doi:10.1093/comjnl/bxaa198
Gupta S., Gupta M. (2021c). “Deep learning for brain tumor segmentation using magnetic resonance images,” in ProceedingIEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology, Melbourne, Australia, 13-15 October 2021 (IEEE). doi:10.1109/CIBCB49929.2021.9562890
Gupta S., Gupta M. K. (2021d). A comparative analysis of deep learning approaches for predicting breast cancer survivability. Archives Comput. Methods Eng., 1
Gupta S., Gupta M. K. (2021a). A comprehensive data‐level investigation of cancer diagnosis on imbalanced data. Comput. Intell. 38, 156–186. doi:10.1111/coin.12452
Gupta S., Gupta M. K. (2021b). Computational model for prediction of malignant mesothelioma diagnosis. Comput. J. doi:10.1093/comjnl/bxab146
He B., Luo H., Zhou Z., Wang B., Liang Y., Lang J., et al. (2020). A neural network framework for predicting the tissue-of-origin of 15 common cancer types based on RNA-seq data. Front. Bioeng. Biotechnol. 8 (8), 737–811. doi:10.3389/fbioe.2020.00737
Huang Z., Johnson T. S., Han Z., Helm B., Cao S., Zhang C., et al. (2020). Deep learning-based cancer survival prognosis from RNA-seq data : Approaches and evaluations. BMC Med. Genomics 13 (5), 41–12. doi:10.1186/s12920-020-0686-1
Huynh P., Nguyen V., Do T. (2019). Novel hybrid DCNN–SVM model for classifying RNA-sequencing gene expression data. J. Inf. Telecommun. 3, 533–547. doi:10.1080/24751839.2019.1660845
Id J. L., Zhou Z., Dong J., Fu Y., Li Y., Luan Z., et al. (2021). Predicting breast cancer 5-year survival using machine learning: A systematic review. PLoS One 16, e0250370–23. doi:10.1371/journal.pone.0250370
Jerez M., Franco L., Veredas F. J., Lo G. (2020). Transfer learning with convolutional neural networks for cancer survival prediction using gene-expression data. Plos One 15, e0230536–24. doi:10.1371/journal.pone.0230536
Joshi P., Park T. (2019). “Cancer subtype classification based on superlayered neural network,” in Proceeding IEEE International Conference on Bioinformatics and Biomedicine, San Diego, CA, USA, 18-21 November 2019 (IEEE), 1988–1992. doi:10.1109/BIBM47256.2019.8983343
Kashyap D., Pal D., Sharma R., Garg V. K., Goel N., Koundal D., et al. (2022). Global increase in breast cancer incidence: Risk Factors and preventive Measures. Biomed. Res. Int. 2022, 9605439. doi:10.1155/2022/9605439
Kim B., Yu K., Lee P. C. W. (2020). Cancer classification of single-cell gene expression data by neural network. Bioinformatics 36, 1360–1366. doi:10.1093/bioinformatics/btz772
Kong Y., Yu T. (2018). A deep neural network model using random forest to extract feature representation for gene expression data classification. Sci. Rep. 8 (1), 16477. doi:10.1038/s41598-018-34833-6
Kumar Y., Gupta S., Singla R., Chen Y. (2021). A systematic review of artificial intelligence techniques in cancer prediction and diagnosis. Arch. Comput. Methods Eng. 29 (4), 2043–2070. doi:10.1007/s11831-021-09648-w
Lecun Y., Bengio Y., Hinton G. (2015). Deep learning. Nature 521 (7553), 436–444. doi:10.1038/nature14539
Lin M., Jaitly V., Wang I., Hu Z., Chen L., Wahed M., et al. (2018). Application of deep learning on predicting prognosis of acute myeloid leukemia with cytogenetics age and mutations. Mach. Learn. Available at: https://arXiv/org/abs1810.13247.
Motieghader H., Najafi A., Sadeghi B., Masoudi-nejad A. (2017). A hybrid gene selection algorithm for microarray cancer classification using genetic algorithm and learning automata. Inf. Med. Unlocked 9, 246–254. doi:10.1016/j.imu.2017.10.004
Panda M. (2017). Elephant search optimization combined with deep neural network for microarray data analysis. J. King Saud Univ. - Comput. Inf. Sci. 32, 940–948. doi:10.1016/j.jksuci.2017.12.002
Parvathavardhini S., Manju S. (2020). Cancer gene detection using Neuro fuzzy classification algorithm. Int. J. Sci. Res. Comput. Sci. Eng. Inf. Technol. 3 (3), 2456
Reid A., Klerk N. De, Musk A. W. B. (2011). Does exposure to asbestos cause ovarian cancer ? A systematic literature review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 20, 1287–1295. doi:10.1158/1055-9965.EPI-10-1302
Ronoud S., Asadi S. (2019). An evolutionary deep belief network extreme learning-based for breast cancer diagnosis. Soft Comput. 23. 13139–13159. doi:10.1007/s00500-019-03856-0
Salman I., Ucan O., Bayat O., Shaker K. (2018). Impact of metaheuristic iteration on artificial neural network structure in medical data. Process. (Basel). 6, 57. doi:10.3390/pr6050057
Schiff M., Castle P. E., Jeronimo J., Rodriguez A. C., Wacholder S. (2007). Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 16, 1–17. doi:10.1128/CMR.16.1.1-17.2003
Sevakula R. K., Singh V., Member S., Kumar C., Cui Y. (2018). Transfer learning for molecular cancer classification using deep neural networks. IEEE/ACM Trans. Comput. Biol. Bioinform. 5963, 2089–2100. doi:10.1109/TCBB.2018.2822803
Shimizu H., Nakayama K. I. (2020). Artificial intelligence in oncology. Cancer Sci. 111 (5), 1452–1460. doi:10.1111/cas.14377
Shon H. S., Yi Y., Kim K. O., Cha E., Kim K. (2021). Classification of stomach cancer gene expression data using CNN algorithm of deep learning. J. Biomed. Transl. Res. 20 (1), 15–20. doi:10.12729/jbtr.2019.20.1.015
Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer statistics 2020 : GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Surbhi Gupta M. G. (2021). “Prostate cancer prognosis using multi-layer perceptron and class balancing techniques,” in Proceeding 2021 Thirteenth International Conference on Contemporary Computing (IC3-2021) (IC3 ’21), August 05-07, 2021 (New York NY USA: ACM), 1.
Torkey H., Atlam M., El-fishawy N., Salem H. (2021). A novel deep autoencoder based survival analysis approach for microarray dataset. Peer Comput. Sci. 1, e492. doi:10.7717/peerj-cs.492
Tumuluru P., Ravi B. (2017). “Goa-Based DBN : Grasshopper optimization algorithm-based deep belief neural networks for cancer classification Goa-based DBN : Grasshopper optimization algorithm-based deep belief neural networks for cancer classification,” in Proceeding International Journal of Applied Engineering Research, 14218–14231.
Urda D., Moreno F. (2017). “Deep learning to analyze RNA-seq gene expression data,” in International work-conference on artificial neural networks, 50–59. Springer: Cham. doi:10.1007/978-3-319-59147-6
Wessels F., Schmitt M., Krieghoff-henning E., Jutzi T., Worst T. S., Waldbillig F., et al. (2021). Deep learning approach to predict lymph node metastasis directly from primary tumor histology in prostate cancer. BJU Int. 128, 352. doi:10.1111/bju.15386
Xiao Y., Wu J., Lin Z., Zhao X. (2018a). A deep learning-based multi-model ensemble method for cancer prediction. Comput. Methods Programs Biomed. 153, 1–9. doi:10.1016/j.cmpb.2017.09.005
Xiao Y., Wu J., Lin Z., Zhao X. (2018b). A semi-supervised deep learning method based on stacked sparse auto-encoder for cancer prediction using RNA-seq data. Comput. Methods Programs Biomed. 166, 99–105. doi:10.1016/j.cmpb.2018.10.004
Xu J., Wu P., Chen Y., Meng Q., Dawood H., Dawood H. (2019a). A hierarchical integration deep flexible neural forest framework for cancer subtype classification by integrating multi-omics data. BMC Bioinforma. 20, 527. doi:10.1186/s12859-019-3116-7
Xu J., Wu P., Chen Y., Meng Q., Dawood H., Khan M. M. (2019b). A novel deep flexible neural forest model for classification of cancer subtypes based on gene expression data. IEEE Access 7, 22086–22095. doi:10.1109/ACCESS.2019.2898723
Yuan Y., Shi Y., Li C., Kim J., Cai W., Han Z., et al. (2016). DeepGene : An advanced cancer type classifier based on deep learning and somatic point mutations. BMC Bioinforma. 17 (17), 476. doi:10.1186/s12859-016-1334-9
Keywords: artificial intelligence, cancer, deep learning, gene expression, Rna-sequences
Citation: Gupta S, Gupta MK, Shabaz M and Sharma A (2022) Deep learning techniques for cancer classification using microarray gene expression data. Front. Physiol. 13:952709. doi: 10.3389/fphys.2022.952709
Received: 25 May 2022; Accepted: 01 September 2022;
Published: 30 September 2022.
Edited by:
Naveen Aggarwal, Panjab University, IndiaReviewed by:
Rajesh Kumar Garg, National Institute of Technology, Hamirpur, IndiaCopyright © 2022 Gupta, Gupta, Shabaz and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Shabaz, YmhhdHNhYjRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.