
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 22 July 2022
Sec. Avian Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.934660
This article is part of the Research Topic Women in Avian Physiology: 2022 View all 13 articles
 R. Shanmugasundaram1*
R. Shanmugasundaram1* D. Adams2
D. Adams2 S. Ramirez3
S. Ramirez3 G. R. Murugesan3
G. R. Murugesan3 T. J. Applegate2
T. J. Applegate2 S. Cunningham1
S. Cunningham1 A. Pokoo-Aikins1
A. Pokoo-Aikins1 A. E. Glenn1
A. E. Glenn1Fumonisins (FB) and deoxynivalenol (DON) are mycotoxins which may predispose broiler chickens to necrotic enteritis (NE). The objective of this study was to identify the effects of subclinical doses of combined FB and DON on NE. A total of 480 day-old male broiler chicks were divided into four treatment groups; 1) control group (basal diet + Clostridium perfringens); 2) necrotic enteritis group (basal diet + Eimeria maxima + C. perfringens); 3) FB + DON group (basal diet + 3 mg/kg FB + 4 mg/kg DON + C. perfringens); and 4) FB + DON + NE group (basal diet + 3 mg/kg FB + 4 mg/kg DON + E. maxima + C. perfringens). Birds in NE and FB + DON + NE groups received 2.5 × 103 E. maxima on day 14. All birds were inoculated with C. perfringens on days 19, 20, and 21. On day 35, birds in the NE, FB + DON, and FB + DON + NE groups had 242, 84, and 339 g lower BWG and a 19-, 2-, and 22-point increase in FCR respectively, than in the control group. Subclinical doses of FB + DON increased (p < 0.05) the NE lesion scores compared to the control group on day 21. On day 21, birds in the NE, FB + DON, and FB + DON + NE groups had increased (p < 0.05) serum FITC-D, lower (p < 0.05) jejunal tight junction protein mRNA, and increased (p < 0.05) cecal tonsil IL-1 mRNA compared to control group. On day 21, birds in the NE group had decreased (p < 0.05) villi height to crypt depth ratio compared to the control group and the presence of FB + DON in NE-induced birds further decreased the villi height to crypt depth ratio. Birds in the NE, FB + DON, and FB + DON + NE groups had increased (p < 0.05) C. perfringens, lower (p < 0.05) Lactobacillus loads in the cecal content, and a lower (p < 0.05) CD8+: CD4+ cell ratio in the cecal tonsils compared to the control group. It can be concluded that subclinical doses of combined FB and DON predispose C. perfringens-inoculated birds to NE, and the presence of FB + DON in NE-induced birds exacerbated the severity of NE.
Corn is one of the major components of poultry feed, and up to 65% of finished poultry feed can be comprised of corn and corn byproducts (Alqaisi et al., 2017). Poultry diets are often contaminated with more than one mycotoxin. Fumonisins (FB) and deoxynivalenol (DON) are secondary mycotoxin metabolites produced by Fusarium verticillioides and Fusarium graminearum, respectively (Glenn, 2007). According to the 2021 survey by Biomin, FB and DON are the most prevalent mycotoxins in poultry feed samples in North America and were detected in 64% and 47% of poultry diets, respectively (Biomin, 2021). Recent surveys have identified that, on average, the amount of DON in corn and cereal grain was 808 μg/kg and 1,721 μg/kg, respectively; the amount of FB in corn was 2,405 μg/kg. Furthermore, DON and FB can co-occur in poultry feed ingredients, and 92% of feed samples analyzed in 2021 had more than one mycotoxin (Biomin, 2021). Though negative effects of FB have been reported when FB are present at 100 mg/kg in chicken feed, FB has been suggested to cause negative effects even at a lower dose when co-occurring with other mycotoxins such as aflatoxins, DON, and zearalenone in poultry (Ogbuewu, 2011). Co-occurrence of mycotoxins decreases the tolerance to individual mycotoxins and, therefore, the existence of multiple mycotoxins in poultry feed even at subclinical levels can be expected to exacerbate the pathology of individual mycotoxins in poultry.
European Food Safety Authority (EFSA) and Food and Drug Administration (FDA) have set guidelines for maximal permissible levels of major mycotoxins in poultry feed. However, subclinical doses of FB (20 mg/kg diet) and DON (5 mg/kg diet), alone (Antonissen et al., 2014; Antonissen et al., 2015) or in combination (Grenier et al., 2016), cause metabolic and immunological disturbances that amplify the severity of necrotic enteritis (NE), coccidiosis, and increase the susceptibility to bacterial diseases in chickens. Mycotoxin interactions within the animal system are mainly additive, but depending on the endpoint assessment these interactions can also be synergistic or antagonistic (Grenier and Oswald, 2011).
Currently, NE is an economically important disease affecting the modern broiler industry. Subclinical NE affects broilers between 2–5-weeks of age and is characterized by intestinal mucosal damage, with no apparent clinical signs or mortality (Hofacre et al., 2018). Subclinical NE leads to decreased digestion and absorption of nutrients, reduced weight gain, and impaired feed conversion rate in poultry (Immerseel et al., 2004). Coccidiosis and feed contaminated with mycotoxins, particularly FB and DON (Antonissen et al., 2015), are considered to be the predisposing factors for NE. In addition, mycotoxins reduce the efficacy of coccidiosis vaccines and, therefore, contribute to NE incidence in chickens (Broom, 2017). Recent restrictions on the use of antibiotics and ionophores in broiler production led to an increase in the occurrence of NE by altering the composition and microbial balance in the gut microbiome (Smith, 2019). The causative organism for NE is Clostridium perfringens, a commensal bacterium in the gastrointestinal tract of healthy broilers. C. perfringens loads range up to 1 × 105 CFU/g of digesta in healthy chickens, while in chickens with clinical NE symptoms, C. perfringens loads increase to 1 × 106 to 1 × 108 CFU/g of digesta, along with associated toxins that include necrotic toxin enteritis B-like (NetB) (Timbermont et al., 2011; Mora et al., 2020).
In the past, FB below 50 mg/kg feed and DON at 5 mg/kg feed were considered not to cause negative effects in poultry (Dänicke et al., 2001; Filazi et al., 2017). However, recent studies have identified that a combined dose of 20 mg/kg FB and 1.5 mg/kg DON decreases the production performances, causes gut damage, and increases coccidiosis severity (Antonissen et al., 2014; Antonissen et al., 2015; Grenier et al., 2016), which can be expected to predispose the broilers to NE. Information regarding the role of chronic exposure of subclinical doses, even at doses much lower than previous studies, of mycotoxins is lacking. Continuous exposure to mycotoxins is expected to damage the gut wall and increase gut permeability to negatively affect the FDA recommendation on NE, gut health, and immune response in chickens. Therefore, the objective of this study was to evaluate the combined effects of FB (3 mg/kg diet) and DON (4 mg/kg diet) on gut health and immune parameters and evaluate the role of mycotoxins as a predisposing factor in inducing and increasing the severity of a NE in poultry.
A non-medicated corn–soybean meal-based mash diet was applied as a basal diet (Table 1). The feeding study was divided into two experimental phases: 1) d0–18, starter feed, and 2) d19–35, finisher feed. Two strains of Fusarium, F. graminearum strain PH-1 and F. verticillioides strain M3125 were cultured for DON and FB production, respectively (Altpeter and Posselt, 1994). In brief, Fusarium strains were cultured separately in carboxymethyl cellulose liquid media and shaken for 5 days (F. verticillioides) or 7 days (F. graminearum), and spores were collected. Fungal spores were added separately to rice media and incubated until mycotoxin content was analyzed. The homogenized rice cultures with FB and DON were mixed with a small portion of the basal diet and re-mixed with the appropriate amount of basal feed to create the experimental diets. The starter diet (d0–18) and the finisher diet were formulated to contain 3 mg/kg FB and 4 mg/kg DON, respectively. The final diets were analyzed by LC-MS-MS to determine the actual content of FB and DON and the content of other major mycotoxins (Romer Labs, Union, MO, United States). The mycotoxin content of the formulated experimental diet is provided in Table 2.
This 35-day feeding trial was conducted with 480 day-old male Ross × Ross 708 strain broiler chicks (Aviagen, Blairsville, GA, United States). The animal care practices and use procedures were followed under the Guide for the Care and Use of Agricultural Animals in Research and Teaching (McGlone, 2010). All animal protocols were approved by the Institutional Animal Care and Use Committee at the Southern Poultry Research Group, Athens, GA. The birds were raised under the supervision of a licensed poultry veterinarian. All birds were euthanized by methods approved by the American Veterinary Medical Association (AVMA). Day-old broiler chicks were raised in 1.5 m × 1.5 m floor pens (stocking density of 15 birds/m2) on new shavings/litter following standard industry practice in North America and raised under ambient humidity. Chickens were weighed individually and randomly distributed into either one of the four treatment groups. The experimental treatment groups were 1) control group (basal diet + C. perfringens challenge), 2) NE group (basal diet + E. maxima + C. perfringens), 3) FB + DON group (basal diet + 3 mg/kg FB +4 mg/kg DON + C. perfringens), and 4) FB + DON + NE group (basal diet + 3 mg/kg FB + 4 mg/kg DON + E. maxima + C. perfringens). Each treatment was replicated in 8 pens with 15 birds/pen in a completely randomized design. Chicks had ad libitum access to the feed and water throughout the experimental period. The mortality of the birds was recorded daily. The birds were housed in floor pens equipped with nipple-type waterers and thermostatically controlled heaters.
On day 14, 2.5 × 103 Eimeria maxima sporulated oocysts/bird were mixed in the feed of NE and FB + DON + NE groups. On days 19, 20, and 21, birds in all treatment groups were challenged with 1 × 108 CFU/bird C. perfringens (strain #6) through the feed to target 3%–5% NE mortality as described earlier (Hofacre et al., 1998). Before the C. perfringens challenge, feed and water were withdrawn for 4 h and 2 h, respectively. Three birds from each pen were randomly sacrificed and examined for the NE lesion score on day 21. Lesion scoring was based on a 0 to 3 scale as described earlier (Hofacre et al., 1998), wherein 0 is normal, 1 is a slight mucus covering the small intestine, 2 is a necrotic small intestinal mucosa, and 3 is a sloughed cells and blood in the small intestinal mucosa and contents. Bodyweight and feed intake were measured at 0, 7, 14, 21, 27, and 35 days of age. Average feed intake and body weight gain (BWG) were corrected for mortality for calculating the feed conversion ratio (FCR) for each pen.
Gut permeability was measured using the FITC-D assay as described earlier (Kuttappan et al., 2015). On days 21, 28, and 35, one bird/pen (n = 8) was orally gavaged with 1 ml of fluorescein isothiocyanate dextran (FITC-D, MW 4000; Sigma-Aldrich, United States) 2.2 mg/bird. 2 h later, the birds were euthanized, and blood was collected by cardiac puncture. Blood samples were centrifuged at 450 × g for 10 min to separate the serum from red blood cells. The serum was diluted in PBS with pH 7.4 at a 1:1 ratio. The serum FITC-D concentration was determined based on a standard curve. A standard curve with 0, 0.2, 0.4, 0.6, 0.8, 1.0, and 2 μg/ml FITC-D was drawn using Gen5 software on the same plate as the samples. The samples and standards were measured at an excitation wavelength of 485 nm and emission wavelength of 528 nm (Synergy HT, multi-mode microplate reader, BioTek Instruments, Inc., VT).
On days 21, 28, and 35, post-challenge, the effect of FB and DON on the spleen and cecal tonsil CD4+ and CD8+ cell percentages were determined by flow cytometry as described previously (Shanmugasundaram et al., 2015). In brief, single-cell suspensions from the spleen and cecal tonsils were enriched for mononuclear cells by density centrifugation over Histopaque (1.077 g/ml, Sigma-Aldrich, St. Louis, MO) for 15 min at 400 g. The cells were incubated with a 1:250 dilution of fluorescent-isothiocyanate conjugated mouse anti-chicken CD4+ (Southern Biotech, Birmingham, AL), 1:450 dilution of phycoerythrin-conjugated mouse anti-chicken CD8+ (Southern Biotech, Birmingham, AL), and 1:200 dilution of unlabeled mouse IgG for 15 min. The unbound antibodies were removed by centrifugation, the percentages of CD4+ and CD8+ cells were analyzed using a flow cytometer (Guava EasyCyte, Millipore, MA), and CD8+: CD4+ ratio was calculated.
On days 21, 28, and 35, 1 bird per pen (n = 8) was euthanized by cervical dislocation. A portion of distal-jejunum and proximal ileum (1 cm proximal and 1 cm distal to the Meckel’s diverticulum) and cecal tonsils were collected in cryovials containing RNAlater® (Ambion Inc., Austin, TX, United States) and stored at −70°C until further analysis. The jejunum was analyzed for claudin-1, claudin-2, and zona-occluden-1 tight junction protein mRNAs, and cecal tonsils were analyzed for pro-and anti-inflammatory cytokines IL-1β, IL-10, LITAF, and IFN-γ mRNA expression, as described previously (Shanmugasundaram and Selvaraj, 2012).
Total RNA was extracted from all experimental groups using the TRI reagent (Molecular Research Center, Cincinnati, OH) following the manufacturer’s instructions. RNA concentration and purity were determined using an Epoch spectrophotometer (BioTek, Winooski, VT, United States), using the 260/280 and 260/230 ratios. 2 mg RNA was reverse transcribed into cDNA and analyzed for IL-1β, IL-10, LITAF, IFN-γ, claudin-1, claudin-2, and zona-occluden-1 by real-time PCR (CFX96 Touch Real-Time System, BioRad, Hercules, CA) using SYBR Green. Primer sequences and annealing temperature are provided in Table 3. Each well contained 10 µl SYBR Green PCR master mix, 7 µl RNAse-free water using C1000 TouchTM Thermal cycler (BioRad, Hercules, CA), 2 µl (∼600 ng/μl) cDNA, 0.5 µl forward primer (5 µM), and 0.5 µl reverse primer (5 µM). To perform real-time PCR, the following settings were used for all genes: an initial denaturation of 95°C for 10 min (1 cycle); followed by 95°C for 15 s; and 60°C for 45 s (40 cycles). The melting profile was determined by heating samples at 65°C for 30 s and then increasing the temperature at a linear rate of 10°C/s to 95°C while continuously monitoring fluorescence. Housekeeping genes of β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and ribosomal protein S13 (RPS13) were selected, and the stability was analyzed using Normfinder software (Department of Molecular Medicine, Aarhus University Hospital, Denmark) as described previously (Shanmugasundaram et al., 2018). The RPS13 gene was selected for data normalization because it was the most stable expression among the set of housekeeping genes analyzed for normalization. The cecal tonsil IL-1β, IL-10, LITAF, and IFN-γ, the jejunal claudin-1, claudin-4, and zona-occluden-1 mRNAs were normalized with RPS13. The 2ˉ∆∆Ct method, as previously described (Livak and Schmittgen, 2001), where Ct is the threshold cycle, was used to calculate the mRNA fold change. The fold change was calculated as 2(Ct Sample – housekeeping)/2 (Ct Reference – housekeeping). The reference group was the control group.
On days 21, 28, and 35, cecal content from one bird/pen (n = 8) was collected and stored at −20°C until further use. The DNA from the cecal microflora DNA was extracted as described earlier (Amit-Romach et al., 2004; Shanmugasundaram et al., 2019a). The DNA pellet was resuspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and stored at −20°C until further use. The final concentration of the isolated DNA was determined using an Epoch spectrophotometer (BioTek, Winooski, VT, United States). The DNA samples were diluted to a final concentration of 100 ng/μl. The primers for Lactobacillus, Bifidobacterium, and C. Perfringens were adapted from an earlier publication (Amit-Romach et al., 2004). The Ct values were converted into CFU/g using a standard curve as described previously (Shanmugasundaram et al., 2019b). The PCR efficiency and the slope and intercept of the standard curve were determined by the CFX software (Bio-Rad, Hercules, CA). The PCR efficiency of the C. perfringens, Lactobacillus, and Bifidobacteria standard curve analysis was 98%, 99%, and 99%, respectively.
On 21, 28, and 35 days, jejunal and ileal samples were collected from one bird/pen (n = 8) from each replication post-challenge. Approximately 4 cm of jejunal and ileal samples were cut proximal and distal to the Meckel’s diverticulum and stored in buffered formalin. The jejunal and ileal samples were processed at room temperature in a graded series of alcohols (15 min in 50% ethanol, 15 min in 70% ethanol, 15 min in 96% ethanol, and 30 min in 100% ethanol with one change at 15 min), cleared in Pro-par (Anatech, Battle Creek, MI) for 45 min with 2 changes at 15 and 30 min, and infiltrated with paraffin at 60°C overnight with one change at 15 min using a tissue processor (Sakura Finetek USA, Inc., Torrance, CA, United States). Paraffin blocks were cut into 5-μm cross-sections and mounted on super frost slides (Thermo Fisher Scientific, Waltham, MA, United States). Slides were then stained with hematoxylin and eosin. Cross-sections were viewed using the cellSens Imaging software (Olympus America, Central Valley, PA) to measure villi length and crypt depth. Ten intact lamina propria villi and crypts per section and 5 sections per sample were analyzed as described earlier (Shanmugasundaram et al., 2020). The tip of the villus to the villus–crypt junction was measured as villus height. The crypt depth was defined by the depth of the invagination between adjacent villi. All the samples in a time point were collected from the same bird, except for the gut permeability analysis for which a second bird was used.
A one-way ANOVA (JMP Pro 15 software, Cary, NC) was used to examine the effects of the subclinical dose of FB + DON on dependent variables, with the pen being considered as the experimental unit. When the main effects were significant (p < 0.05), differences between means were analyzed by Tukey’s least-square means comparison. Values reported are least-squares means ± SEM. The lesion scores were analyzed by a non-parametric test, and a Wilcoxon/Kruskal–Wallis rank-sum test was used to separate the means. The heatmap was rendered with JMP’s plotting library (Šefcová et al., 2020).
There were significant (p < 0.05) treatment effects on body weight gain on days 14, 21, 28, and 35 (Figure 1A). On day 14, birds in the FB + DON had lower BWG compared to the birds in the control group. On day 35, birds in the NE and FB + DON + NE groups had 242 g (p < 0.05) and 339 g (p < 0.05) lower BWG than the birds in the control group, respectively.
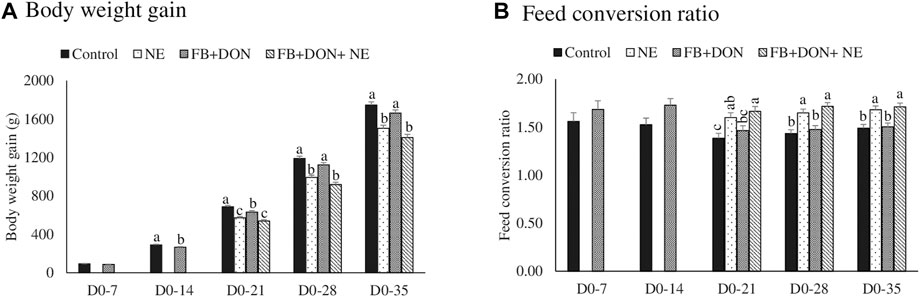
FIGURE 1. Effect of subclinical dose of FB + DON and E. maxima/C. perfringens challenge on production performances. Day-old chicks were distributed into four treatment groups: control, necrotic enteritis (NE), fumonisin + deoxynivalenol (FB + DON), and FB + DON + NE groups. Birds in the NE and FB + DON + NE groups received 2.5 × 103 Eimeria maxima oocyst per bird on day 14. All birds received 1 × 108 CFU/bird of Clostridium perfringens on days 19, 20, and 21. Body weight and feed consumption was measured on days 0, 7, 14, 21, 28, and 35 of age to calculate body weight gain (Panel 1A) and feed consumption ratio (Panel 1B). Mortality-corrected body weight gain and feed conversion ratio are presented. Bars (+SEM) without a common superscript differ significantly (p < 0.05). n = 8 pens of 15 birds/pen.
There were significant (p < 0.05) treatment effects on the FCR on days 21, 28, and 35 (Figure 1B). On day 14, birds in the FB + DON group had 21 points (p = 0.05) increase in FCR compared to the birds in the control group. On day 35, birds in the NE and FB + DON + NE groups had 19 points and 22 points significant increase in FCR than those in the control group.
There were significant (p < 0.05) treatment effects on the NE lesion score on day 21 (Table 4). Birds in the control group had the lowest Wilcoxon/Kruskal–Wallis score means for lesion scores. In birds with induced NE, 4.2% (1 out of 24) had a NE lesion score of 0, 58.3% (14 out of 24) had a NE lesion score of 1, 37.5% (9 out of 24) had a NE lesion score of 2, and 0% had a NE lesion score of 3. In birds exposed to FB + DON and induced with NE, 0% had a NE lesion score of 0 , 37.5% (9 out of 24) had a NE lesion score of 1, 62.5% (15 out of 24) had a NE lesion score of 2, and 0% had a NE lesion score of 3. Birds in the NE group had higher (p < 0.05) Wilcoxon/Kruskal–Wallis Score Means for lesion scores than scores observed in the control group. Subclinical dose of FB + DON increased (p < 0.05) the Wilcoxon/Kruskal–Wallis Score Means for lesion scores compared with the control group on day 21. The presence of FB + DON in NE-challenged birds increased (p < 0.05) the Wilcoxon/Kruskal–Wallis Score Means for lesion scores compared to the NE group.
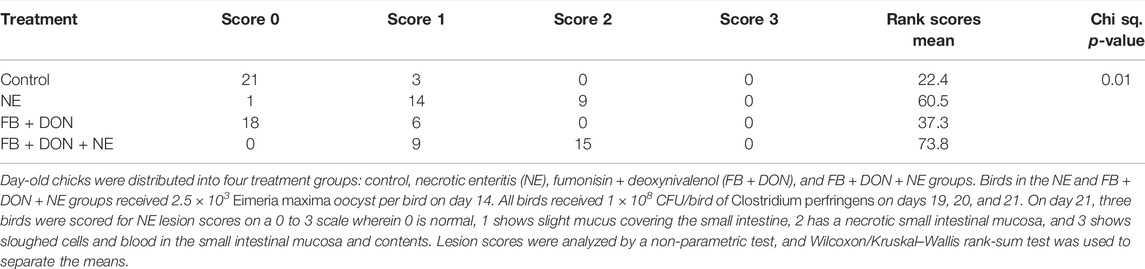
TABLE 4. Effect of subclinical dose of FB + DON and E. maxima/C. perfringens challenge on necrotic enteritis lesion score at 21 days of age.
There were significant (p < 0.05) treatment effects on the serum FITC-D concentration on days 21 and 28 (Figure 2). On day 21, birds in the NE, FB + DON, and FB + DON + NE groups had a 150% (p < 0.05), 51% (p > 0.05), and 293% (p < 0.05) increase in serum FITC-D compared to the birds in the control group. Similar trends were observed on day 28. The presence of FB + DON in NE-challenged birds increased (p < 0.05) the serum FITC-D concentration further by 57%, compared with NE group on day 21.
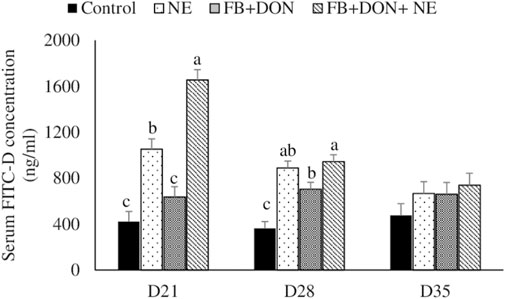
FIGURE 2. Effect of subclinical dose of FB + DON and E. maxima/C. perfringens challenge on gut permeability to FITC-dextran. Day-old chicks were distributed into four treatment groups: control, necrotic enteritis (NE), fumonisin + deoxynivalenol (FB + DON), and FB + DON + NE groups. Birds in the NE and FB + DON + NE groups received 2.5 × 103 Eimeria maxima oocyst per bird on day 14. All birds received 1 × 108 CFU/bird of Clostridium perfringens on days 19, 20, and 21. One bird/pen was orally gavaged with 2.2 mg/bird of 4,000 MW fluorescein isothiocyanate dextran (FITC-D), and blood was collected 2 h later. Serum FITC-D concentration was determined in a microplate reader. Bars (+SEM) without a common superscript differ significantly (p < 0.05). n = 8 (8 pens of 15 birds/pen).
There were significant (p < 0.05) treatment effects on the jejunal mRNA expression on days 21, 28, and 35 (Figure 3). On day 21, birds in the NE, FB + DON, and FB + DON + NE groups had lower claudin-1, claudin-2, and zona occludens-1 mRNA expression compared to the birds in the control group. On days 28 and 35, birds in the NE group had similar claudin-1 and zona occludens-1 mRNA expression when compared with the control group, but birds in the FB + DON and FB + DON + NE groups still had downregulated claudin-1 and zona occludens-1 mRNA compared to the control group. Similar trends were observed on days 28 and 35.
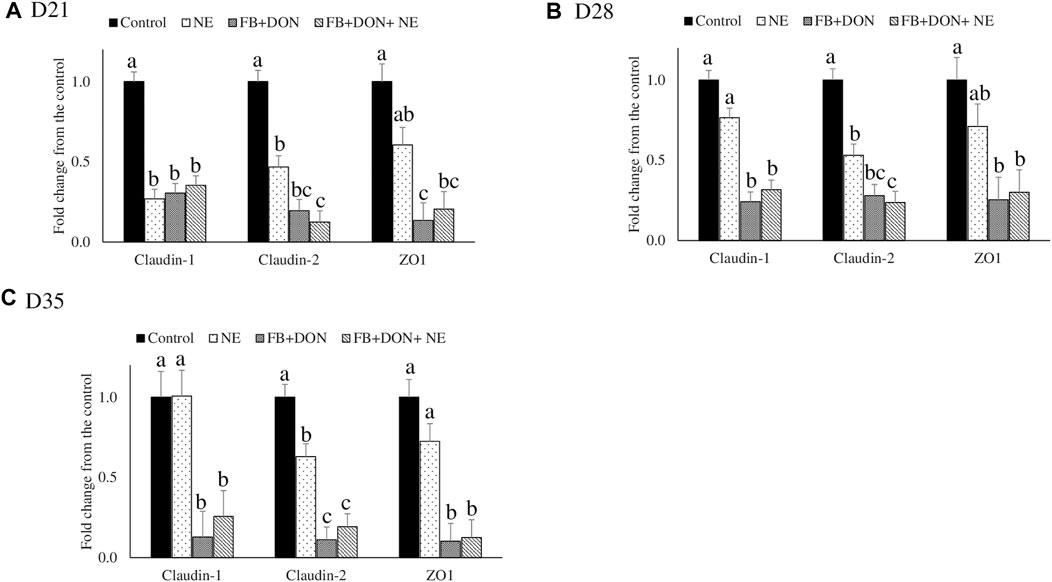
FIGURE 3. Effect of subclinical dose of FB + DON and E. maxima/C. perfringens challenge on jejunal tight junction protein mRNA expression. Day-old chicks were distributed into four treatment groups: control, necrotic enteritis (NE), fumonisin + deoxynivalenol (FB + DON), and FB + DON + NE groups. Birds in the NE and FB + DON + NE groups received 2.5 × 103 Eimeria maxima oocyst per bird on day 14. All birds received 1 × 108 CFU/bird of Clostridium perfringens on days 19, 20, and 21. Tight junction protein mRNA content was analyzed after correcting for the housekeeping gene RPS13 mRNA content and normalizing to the mRNA content of the control group at D21 (A), D28 (B) and D35 (C), so all bars represent fold change compared to the control group. Bars (+SEM) without a common superscript differ significantly (p < 0.05). n = 8 (8 pens of 15 birds/pen).
There were significant (p < 0.05) treatment effects on the cecal tonsil IL-1β, IL-10, LITAF, and IFN-γ jejunal mRNA expression on day 21 (Figure 4). On day 21, birds in the NE, FB + DON, and FB + DON + NE groups had an approximately 4-fold increase in IL-1β mRNA compared to the birds in the control group. Similar trends were observed on days 28 and 35.
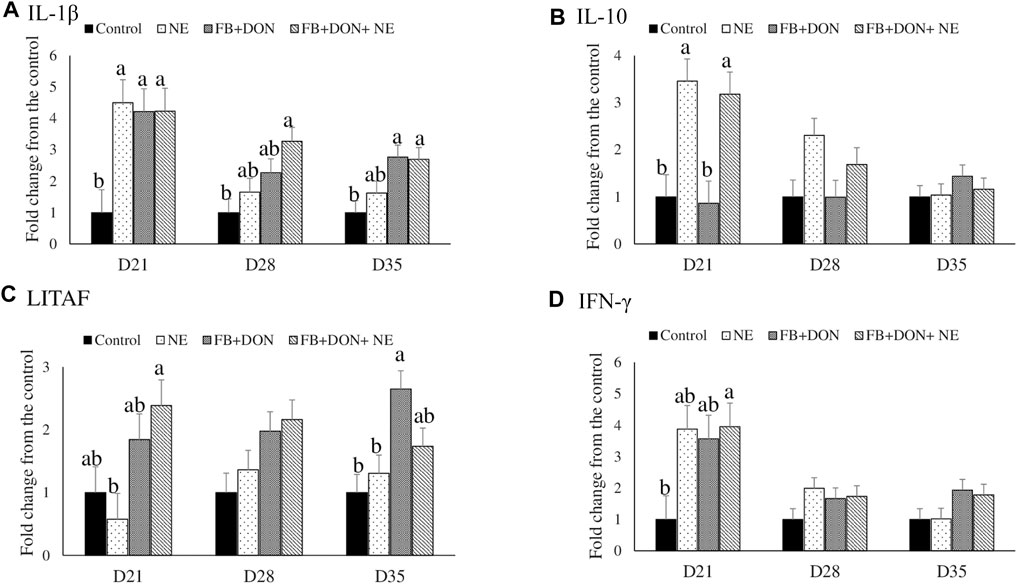
FIGURE 4. Effect of subclinical dose of FB + DON and E. maxima/C. perfringens challenge on cecal tonsil cytokine mRNA expression. Day-old chicks were distributed into four treatment groups: control, necrotic enteritis (NE), fumonisin + deoxynivalenol (FB + DON), and FB + DON + NE groups. Birds in the NE and FB + DON + NE groups received 2.5 × 103 Eimeria maxima oocyst per bird on day 14. All birds received 1 × 108 CFU/bird of Clostridium perfringens on days 19, 20, and 21. IL-1β, IL-10, LITAF, and IFN-γ mRNA content was analyzed after correcting for the housekeeping gene RPS13 mRNA content and normalizing to the mRNA content of the control group, so all bars represent fold change compared to the control group. Bars (+SEM) without a common superscript differ significantly (p < 0.05). n = 8 (8 pens of 15 birds/pen).
On day 21, birds in the NE and FB + DON + NE groups had an approximately 3-fold increase in IL-10 mRNA compared to the birds in the control group.
On day 21, birds in the FB + DON + NE group had higher LITAF mRNA compared to the birds in the NE group. On day 35, birds in the FB + DON group had higher LITAF mRNA compared to the birds in the control group.
On day 21, birds in the FB + DON + NE group had an approximately 4-fold increase in IFN-γ mRNA compared to the birds in the control group.
On day 21, birds in the FB + DON group had a lower CD8+: CD4+ ratio in the cecal tonsils compared to the birds in the control group (Figure 5). On day 35, birds in the FB + DON and FB + DON + NE groups had a lower CD8+: CD4+ ratio in the cecal tonsils compared to the birds in the control group
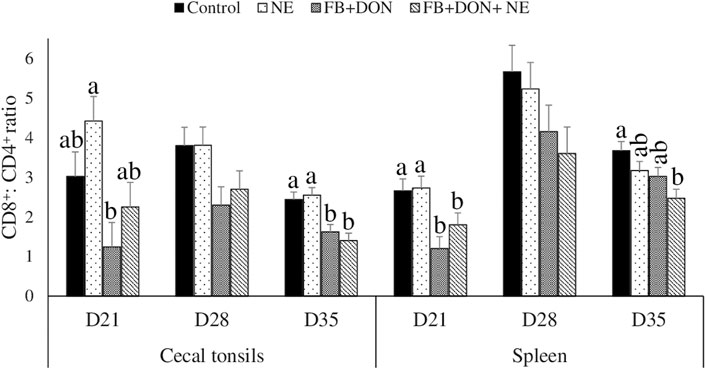
FIGURE 5. Effect of subclinical dose of FB + DON and E. maxima/C. perfringens challenge on the spleen and cecal tonsil CD8+: CD4+ ratio. Day-old chicks were distributed into four treatment groups: control, necrotic enteritis (NE), fumonisin + deoxynivalenol (FB + DON), and FB + DON + NE groups. Birds in the NE and FB + DON + NE groups received 2.5 × 103 Eimeria maxima oocyst per bird on day 14. All birds received 1 × 108 CFU/bird of Clostridium perfringens on days 19, 20, and 21. CD4+ and CD8+ cells were identified using fluorescent-linked anti-chicken CD4 and CD8 in a flow cytometer. Bars (+SEM) without a common superscript differ significantly (p < 0.05). n = 8 (8 pens of 15 birds/pen).
On day 21, birds in the FB + DON and FB + DON + NE groups had a lower CD8+:CD4+ ratio in the spleen compared to that in the birds in the control group. On day 35, birds in the FB + DON + NE group had a lower CD8+:CD4+ ratio in the spleen compared to the birds in the control group.
On day 21, birds in the NE group had a 24% decrease (p > 0.05) in villi height to crypt depth ratio compared to the birds in the control group and the presence of FB + DON in NE-induced birds further decreased the villi height to crypt depth ratio by 8.4% when compared with NE group (Figure 6). Similar results were observed in the ileum on day 21.
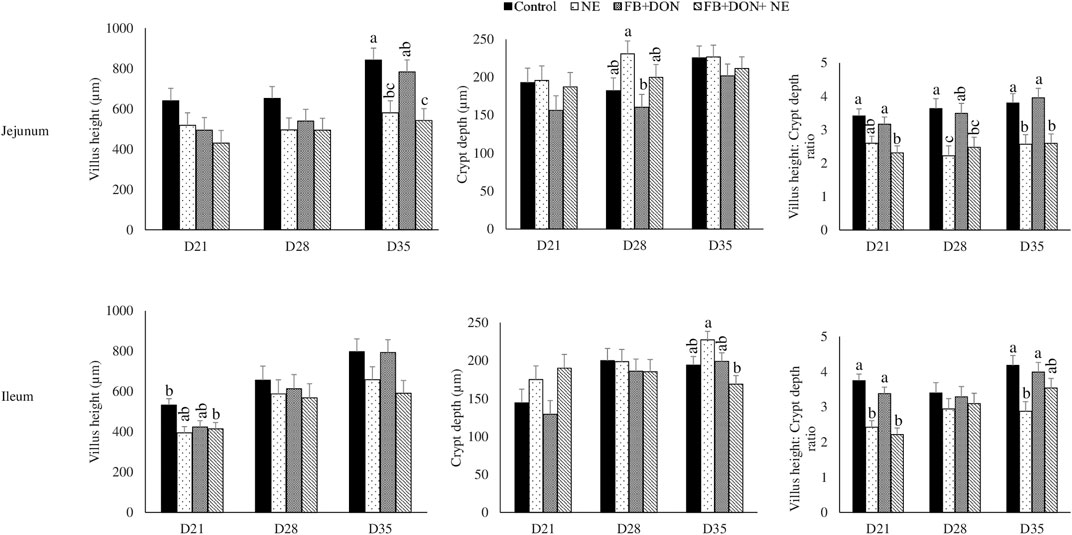
FIGURE 6. Effect of subclinical dose of FB + DON and E. maxima/C. perfringens challenge on jejunal and ileal histomorphology. Day-old chicks were distributed into four treatment groups: control, necrotic enteritis (NE), fumonisin + deoxynivalenol (FB + DON), and FB + DON + NE groups. Birds in the NE and FB + DON + NE groups received 2.5 × 103 Eimeria maxima oocyst per bird on day 14. All birds received 1 × 108 CFU/bird of Clostridium perfringens on days 19, 20, and 21. Jejunal and ileal sections were stained with hematoxylin and eosin. Villi height and crypt depth were measured using cellSens Imaging software, and villi height:crypt depth ratio was calculated. Bars (+SEM) without a common superscript differ significantly (p < 0.05). n = 8 (8 pens of 15 birds/pen).
On days 21, 28, and 35, birds in the NE, FB + DON, and FB + DON + NE groups had an approximately 1.3 Log increase in C. perfringens loads in the cecal tonsils compared to the birds in the control group (Figure 7).
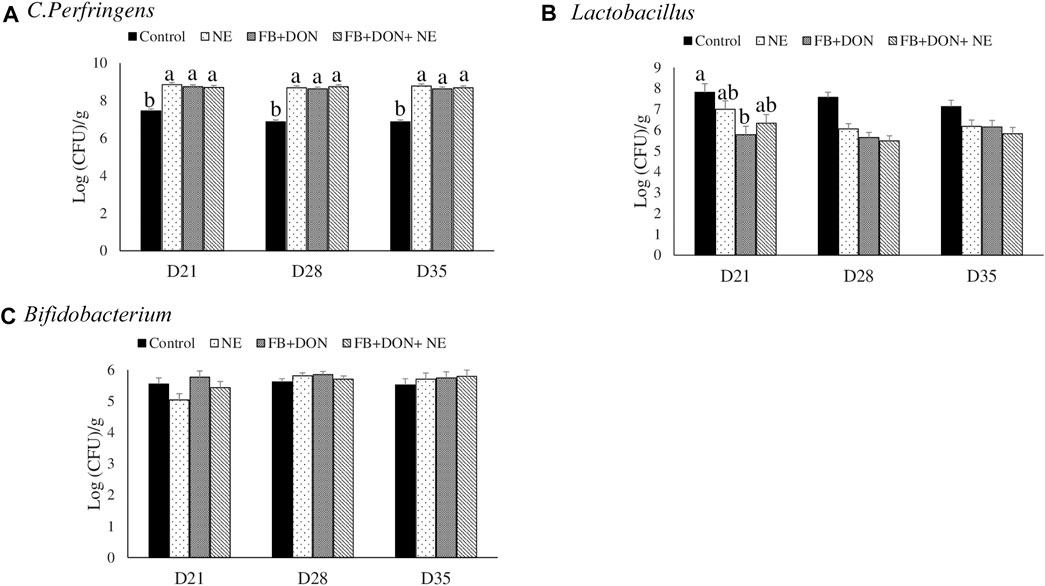
FIGURE 7. Effect of subclinical dose of FB + DON and E. maxima/C. perfringens challenge on C. perfringens, total Lactobacillus, and total Bifidobacteria loads in the cecal content. Day-old chicks were distributed into four treatment groups: control, necrotic enteritis (NE), fumonisin + deoxynivalenol (FB + DON), and FB + DON + NE groups. Birds in the NE and FB + DON + NE groups received 2.5 × 103 Eimeria maxima oocyst per bird on day 14. All birds received 1 × 108 CFU/bird of Clostridium perfringens on days 19, 20, and 21. Cecal content was analyzed for C. perfringens, (A) total Lactobacillus, (B) and total Bifidobacteria (C) through PCR. Bars (+SEM) without a common superscript differ significantly (p < 0.05). n = 8 (8 pens of 15 birds/pen).
On day 21, birds in the FB + DON group had lower (p < 0.05) Lactobacillus spp. compared to the birds in the control group.
The negative value of Pearson’s coefficient indicated that IL-1β and IL10- mRNA expression on days 21 and 28 were inversely related to body weight (Figure 8).
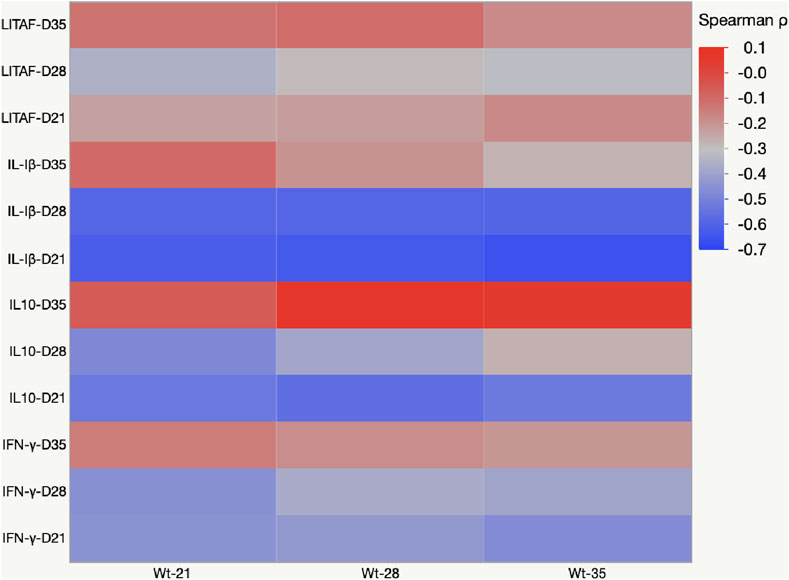
FIGURE 8. Heat map representing Pearson’s r correlation coefficient matrix between cytokine amounts and body weight gain. Heat map showing the transcriptional fold change of LITAF, IL-1β, IL-10, and IFN-γ in the cecal tonsils of birds fed mycotoxin contaminated diet and induced with necrotic enteritis. The color scale, −1 (blue) to +1 (red), and the negative value of the coefficient indicate that increased IL-1β and IL-10 mRNA expression levels are inversely affected the body weight.
Corn is the major energy source in poultry feed and constitutes 50%–80% of the finished poultry feeds in the United States and Europe (Guerre, 2016). Mycotoxins are ubiquitous in nature (Shimshoni et al., 2013), and under practical conditions, it is difficult to produce clean corn without mycotoxin contamination. In this study, the starter basal diets in the control group were naturally contaminated with 40 μg/kg aflatoxin, 400 μg/kg FB, and 100 μg/kg DON, and the finisher basal diets in the control group were contaminated with 3 μg/kg aflatoxin, 1,500 μg/kg FB, and 200 μg/kg DON. Because there is an increase in the occurrence of mycotoxins contamination of poultry feed under field conditions, there is a growing concern regarding the negative effects of combined mycotoxins, even when present at sub-clinical doses, on gut health. Hence, this study aimed to identify whether the combined presence of FB and DON at subclinical concentration predisposed broiler chickens to NE and acerbated the severity of NE lesions.
In the current study, a combined dose of 3 mg/kg FB and 4 mg/kg DON decreased the chickens’ body weight on day 14 even before the birds were inoculated with E. maxima. In birds that were induced with NE, FB and DON further decreased the body weight gain. Our data suggest that a combined dose of 3 mg/kg FB and 4 mg/kg DON in the poultry diet increased gut permeability and decreased villi height to crypt depth ratio, which can be expected to decrease body weight and increase the FCR. An earlier study identified that combination of FB and DON either at 20 and 1.5 mg/kg or 20 and 5.0 mg/kg feed, respectively, increases the feed conversion ratio. A similar result was observed in piglets when feeding 6 mg/kg FB and 3 mg/kg DON in combination, which decreased the production performance (Grenier and Oswald, 2011). Broilers exposed to multiple mycotoxins at subclinical doses in the starter to finisher diets exhibit decreased production broiler performance and impaired health (Wang et al., 2005). Earlier reports have identified that poultry feed contaminated with 5 mg/kg DON alone did not alter the chicken production performance (Awad et al., 2011). Similarly, FB alone at 300 mg (Brown et al., 1992) or 50 mg (Yu et al., 2022) did not cause a decrease in production performance in broiler birds. Considering that when FB or DON was individually fed, they did not decrease the production performance even when present at 300 mg/kg and 5 mg/kg. It should be noted that subclinical doses of FB + DON had numerical changes, rather than statistical significance, on production performances. It has been suggested that the interpretation of p values should not be a dichotomous conclusion as either significant or nonsignificant, but it should be interpreted based on the real-world implication of the observed change in the data points (Andrade, 2019). FB + DON decreased the body weight gain by 87 g, further decreased the body weight by 97 g, and worsened the FCR by 3 points in birds induced with NE on day 35. FB + DON at subclinical dose can thus lead to a loss of up to 184 g per bird, which accounts for approximately 10.5% of live body weight. Thus, it can be concluded that in this present study, the combined dose of DON and FB had a synergistic negative effect on body weight gain and feed conversion ratio.
The presence of both FB and DON increased the severity of the NE lesions in birds induced with NE. However, FB and DON did not increase NE mortality (23.3% and 21.2% mortality in FB + DON and FB + DON + NE group). In the NE model studied, the control group was inoculated with C. perfringens and, hence, the C. perfringens loads were approximately 7 logs/g of cecal content. In the absence of accompanying intestinal wall damage because of E. maxima or mycotoxins, the control group had no NE lesions. These findings suggest that combined subclinical doses of FB and DON increase the severity of the NE lesion without increasing the associated mortality. NE lesion scores, but not the associated mortality, should be used to assess the cost of subclinical doses of FB and DON under field conditions.
Previous studies have identified that chronic exposure to FB1 at 100 mg/kg concentration for 28 days or 300 mg/kg for 14 days decreases the jejunum villus height and villus: crypt depth ratio and causes mild villus atrophy and goblet cell hyperplasia in broiler chicks (Rauber et al., 2013). This study identified that the presence of FB and DON combination decreased villi height to crypt depth ratio similar to that in the NE group. The villi length to crypt depth ratio is an indicator of the intestinal renovation rate and a higher villi to crypt ratio indicates a lower intestinal turnover (Brown et al., 1992; Van Nevel et al., 2005). Thus, subclinical doses of FB and DON combination increased the intestinal turnover and contributed to the observed decrease in FCR and loss in body weight gain during NE.
FB and DON acerbated the loss in the tight junction protein and increase in gut permeability associated with NE. FB and DON combination decreased the jejunal claudin-1, claudin-2, and zona-occluden-1 in the intestine. The decrease in the jejunal tight junction protein owing to subclinical mycotoxin was comparable to the loss in the tight junction in the birds induced with NE. Earlier studies have identified that chronic exposure to FB decreases the proliferation of intestinal epithelial cells and breaks down the gut barrier in pigs (Bouhet et al., 2004). Tight junction proteins are comprised of transmembrane proteins such as claudins and occludens, and cytoplasmic proteins, such as zona occludens (Findley and Koval, 2009). Tight junction proteins act as a barrier to pathogens and harmful toxins while permitting the entry of nutrients, ions, and water (Tomaszewska et al., 2021). Caco-2 cells exposed to a combination of aflatoxin and ochratoxin had significantly decreased tight junction proteins (Gao et al., 2018). FB inhibits ceramide synthase, which results in the accumulation of sphingoid bases and their metabolites, leading to the depletion of complex sphingolipids (Wang et al., 1991). In addition, FB leads to the accumulation of sphinganine (Riley et al., 1999) and increases calmodulin, an apoptotic protein. Alteration in the sphingolipid metabolic products, sphingosine content, and calmodulin can be expected to decrease intestinal cell viability and loss in tight junction proteins (Bouhet et al., 2004). Furthermore, chronic exposure to FB + DON enhances the claudin-1, claudin-2, and zona-occluden internalization by endocytosis (Fujita et al., 2000). This results in the reduction of claudins at a cellular level and a lack of new molecules to replace the damaged tight junction proteins (Hopkins et al., 2003).
During NE infection, the integrity of intestinal epithelial cells is compromised due to either inflammation or toxins or the associated gut dysbiosis. Quantification of serum FITC-d is commonly used as an indicator for assessing intestinal paracellular permeability and magnitude of severity (Kuttappan et al., 2015). The oral administration of FITC-D passes through the disrupted intestinal epithelium and enters systemic circulation, which can be quantified in the blood (Liu et al., 2021). In this current study, the presence of FB and DON caused a loss in gut integrity, and this loss in gut integrity was acerbated in birds challenged with NE. The observed increase in serum FITC-D level correlated with decreased tight junction proteins in the ileum. A decrease in the tight junction proteins of the intestine leads to a loss in gut integrity and an increase in gut permeability, and it can explain the observed increase in serum FITC-D concentration. This current study suggests that chronic exposure to even subclinical doses of mycotoxins could adversely damage the intestinal gut epithelium.
FB and DON increased the cecal tonsil IL-1β, an inflammatory cytokine. Upregulation of interleukins is observed normally during various bacterial and parasitic infections (Mensikova et al., 2013). Immune system activation includes changes in cytokines such as tumor necrosis factor (TNF-α, IL-1β, IFN-γ, and IL-10 (Wallach et al., 2014). Activated macrophages secrete IL-1β to induce inflammation (Bhat and Fitzgerald, 2014). In mice, a single dose of in vivo DON exposure increases TNF-α, IL-1β, IFN-γ, and IL-10 in CD4+ cells isolated from spleen and Peyer’s patches (Zhou et al., 1997). In vitro treatment of chicken splenocytes with DON increases the concentrations of IL-1β, IL-10, and IFN-γ (Azcona-Olivera et al., 1995; Ren et al., 2015). In this present study, birds exposed to FB and DON had increased cecal tonsil IFN-γ mRNA transcription at levels similar to that in the birds undergoing a NE challenge. IFN-γ plays an important role in the host’s defense against intracellular pathogens such as coccidiosis. This increased IFN-γ mRNA transcription at D21 in the combined toxin group suggests that FB and DON could have had a synergistic effect on IFN-γ mRNA transcription. Cecal tonsils of Eimeria-challenged birds had an increase in IFN-γ mRNA transcription when chickens were fed Fusarium mycotoxins contaminated diet (Girgis et al., 2010). Chronic exposure to combined FB + DON activates the NF-kB pathway to upregulate pro-inflammatory cytokines (Pinton and Oswald, 2014; Taranu et al., 2015). Several studies have identified that the dietary mycotoxins, at doses even below EU guidance, could upregulate both pro and anti-inflammatory cytokines in the duodenum and jejunum (Bracarense et al., 2012; Lucke et al., 2018; Guo et al., 2021). Similarly, in this present study, 4 mg/kg DON and 3 mg/kg FB increased the pro-and anti-inflammatory cytokines, suggesting that combined toxins could have adverse effects on intestinal epithelial cells to modify the cecal tonsils cytokines expression in broilers. Furthermore, Pearson’s correlation analysis identified significant negative correlations (p < 0.05) between IL-1β, IL-10, and body weight. The negative coefficient indicated that the chronic exposure to mycotoxins increased the IL-1β and IL-10 mRNA transcripts, coinciding with an ultimate decrease in body weight gain. Activation of the immune system and cytokines production requires energy resources and affects the production performance, resulting in a trade-off between immune function and growth (van der Most et al., 2011). NE infection by itself increased proinflammatory cytokines, and further synergism between FB, DON, and NE acerbated the loss in body weight gain in the FB + DON + NE group.
T cell proliferation involves the activation and differentiation of T cells into effector and memory subsets which is critical for the adaptive immune system. CD8+: CD4+ ratio is a marker of immune dysfunction (Roitt, 1992; Martin et al., 2016). The impairment in CD4+ T cell regeneration and persistent elevation of CD8+ T cells are indicators of inflammation that involves gut microbial translocation (Hirakawa et al., 2020; Ruhnau et al., 2020). In this present study, the presence of FB + DON decreased the CD8+: CD4+ cell ratio in the cecal tonsils, and this effect was acerbated in the FB + DON + NE group compared to the control group. Similar results were observed in chickens’ peripheral mononuclear cells (PBMCs) when they were fed contaminated diets containing up to 3.8 μg/g deoxynivalenol (DON), 0.3 μg/g 15-acetyl DON, and 0.2 μg/g zearalenone (Girgis et al., 2008). Furthermore, broilers fed 20 mg/kg FB and 1.5 mg/kg of DON had an increased percentage of T lymphocytes, and CD4+CD25+ in the cecal tonsils (Grenier et al., 2016). In bovine and porcine PBMCs, a similar kind of trend was observed when they were fed DON contaminated diet. In porcine PBMCs, in vitro studies with DON at 0.4 mM or higher concentration have decreased the proliferation of CD8+ and CD4+ cells (Novak et al., 2018). Similarly, beef cattle exposed to 1.7 mg DON and 3.5 mg FB for 21 days has significantly decreased the CD8+: CD4+ ratio (Duringer et al., 2020; Roberts et al., 2021). Our studies demonstrated that combined subclinical doses of FB and DON negatively affected the proliferation of the CD8+ and CD4+ T cells. FB and DON target the cell with high protein turnover and inhibit protein synthesis. CD8+ and CD4+ cells are considered highly proliferative cells (Overgaard et al., 2015) and are likely highly sensitive to FB and DON (Taranu et al., 2010; Daenicke et al., 2011). Impaired CD8+ and CD4+ cell proliferation can be expected to compromise the immune response to NE. Changes in T-helper and cytotoxic T cell profiles, along with changes in inflammatory cytokines, suggest that the chicken immune system is altered by chronic exposure to Fusarium mycotoxins even at a subclinical dose in broiler chickens leading to impaired resistance to NE. Our results suggest that the CD8+: CD4+ ratio could be a potential biomarker of early Fusarium mycotoxin exposure.
Lactobacillus spp. and Bifidobacterium spp. are considered to be beneficial bacteria in the chicken gut. In this present study, the subclinical dose of FB and DON decreased the Lactobacillus spp. load in the ceca. Similar results were found when chickens were exposed to DON 5 mg/kg diet (Antonissen et al., 2015; Guo et al., 2021). Chronic exposure to subclinical doses of FB and DON increased the C. perfringens load and caused intestinal dysbiosis, and hence, this current study identified that FB and DON mycotoxins can be predisposing factors for C. perfringens-induced NE in chickens. Increased C. perfringens altered the balance between intestinal microbiota, with major changes observed in Lactobacillus spp. (Antonissen et al., 2016; Zhang et al., 2018; Hernandez-Patlan et al., 2019). The chronic exposure to FB + DON increased the cecal C. perfringens load but had no effect on Bifidobacterium spp. (Lucke et al., 2018). Therefore, it can be concluded that chronic exposure to subclinical doses of combined FB + DON affected the relative abundance of Lactobacillus spp. and exacerbated the NE by enhancing intestinal inflammation and shifting the gut microbiome towards pathogenic microorganisms (Yang et al., 2021).
The findings reported here have significant practical importance and reflect the real-world problem because of the common occurrence of Fusarium mycotoxins in poultry feeds and subclinical necrotic enteritis occurrence in the field. According to the FDA, the recommended level for FB and DON in the poultry finished diet is 50 mg/kg and 5 mg/kg (FDA, 2001; FDA, 2010). The level of FB and DON in the experimental diets of the current study was much lower than the FDA tolerance levels. The findings of this study represent the effects of chronic exposure to the subclinical levels of FB and DON in broiler chickens and their role in inducing subclinical necrotic enteritis. Our findings identified the mechanism through which FB and DON exhibited synergistic effects and predicted the specific thresholds of combined toxins and their adverse effects in chickens. Our data suggested that Fusarium mycotoxins not only directly affected the production performance but also influenced chicken health by inducing NE and acerbated the severity of NE.
Our data demonstrated that chronic feeding of a combined dose of 3 mg/kg FB and 4 mg/kg DON in the poultry diet downregulates the tight junction proteins and increased the severity of NE in broiler chickens. Chicken diets with FB and DON contamination, even at subclinical levels, induced a negative impact on performance, altered small intestinal morphology, and significantly increased the incidence of NE. In conclusion, the presence of FB and DON decreased the BWG, increased the FCR, increased gut permeability, decreased jejunal tight junction protein, increased inflammatory cytokines in the cecal tonsil, decreased CD8+:CD4+ ratio in the cecal tonsil and spleen, increased C. perfringens load in the cecal content, and decreased Lactobacillus spp. loads in the cecal content and predisposed broiler birds to NE.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the SPRG IACUC committee.
RS: conceptualization, investigation, methodology, data curation, and writing—original draft preparation. DA: methodology and writing—review and editing. SR: funding acquisition. RM: funding acquisition. TA: conceptualization and writing—review and editing. SC: methodology. AP-A: methodology and writing—review and editing. AG: resources and writing—review and editing.
This work was funded by USDA ARS award number 6040-42000-045-00D.
SR and RM were employed by the company DSM Animal Nutrition and Health.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge D. Olson (USDA) for preparing and growing the fungal culture material producing FB and DON; T. Mitchell (USDA) and J. Hawkins (USDA) for quantifying FB and DON in culture materials; Romer Labs Inc. for independent quantification of mycotoxins in the poultry feed; L. Fuller (UGA) for providing Eimeria maxima sporulated oocysts, and C. McDonough (USDA), C. Miller (USDA), and H. Yeh (USDA) for their assistance in sampling. The authors thank M. Jones (Southern Poultry Research Group) for NE lesion scoring and C. Hofacre (SPRG) and other crew members at SPRG for assistance in animal trial.
Alqaisi O., Ndambi O. A., Williams R. B. (2017). Time Series Livestock Diet Optimization: Cost-Effective Broiler Feed Substitution Using the Commodity Price Spread Approach. Agric. Food Econ. 5 (1), 1–19. doi:10.1186/s40100-017-0094-9
Altpeter F., Posselt U. K. (1994). Production of High Quantities of 3-acetyldeoxynivalenol and Deoxynivalenol. Appl. Microbiol. Biotechnol. 41 (4), 384–387. doi:10.1007/bf01982524
Amit-Romach E., Sklan D., Uni Z. (2004). Microflora Ecology of the Chicken Intestine Using 16S Ribosomal DNA Primers. Poult. Sci. 83 (7), 1093–1098. doi:10.1093/ps/83.7.1093
Andrade C. (2019). The P Value and Statistical Significance: Misunderstandings, Explanations, Challenges, and Alternatives. Indian J. Psychol. Med. 41 (3), 210–215. doi:10.4103/IJPSYM.IJPSYM_193_19
Antonissen G., Croubels S., Pasmans F., Ducatelle R., Eeckhaut V., Devreese M., et al. (2015). Fumonisins Affect the Intestinal Microbial Homeostasis in Broiler Chickens, Predisposing to Necrotic Enteritis. Vet. Res. 46, 98. doi:10.1186/s13567-015-0234-8
Antonissen G., Eeckhaut V., Van Driessche K., Onrust L., Haesebrouck F., Ducatelle R., et al. (2016). Microbial Shifts Associated with Necrotic Enteritis. Avian Pathol. 45 (3), 308–312. doi:10.1080/03079457.2016.1152625
Antonissen G., Van Immerseel F., Pasmans F., Ducatelle R., Haesebrouck F., Timbermont L., et al. (2014). The Mycotoxin Deoxynivalenol Predisposes for the Development of Clostridium Perfringens-Induced Necrotic Enteritis in Broiler Chickens. PLoS One 9 (9), e108775. doi:10.1371/journal.pone.0108775
Awad W. A., Vahjen W., Aschenbach J. R., Zentek J. (2011). A Diet Naturally Contaminated with the Fusarium Mycotoxin Deoxynivalenol (DON) Downregulates Gene Expression of Glucose Transporters in the Intestine of Broiler Chickens. Livest. Sci. 140 (1-3), 72–79. doi:10.1016/j.livsci.2011.02.014
Azcona-Olivera J. I., Ouyang Y.-L., Warner R. L., Linz J. E., Pestka J. J. (1995). Effects of Vomitoxin (Deoxynivalenol) and Cycloheximide on IL-2, 4, 5 and 6 Secretion and mRNA Levels in Murine CD4+ Cells. Food Chem. Toxicol. 33 (6), 433–441. doi:10.1016/0278-6915(95)00012-q
Bhat N., Fitzgerald K. A. (2014). Recognition of Cytosolic DNA by cGAS and Other STING-dependent Sensors. Eur. J. Immunol. 44 (3), 634–640. doi:10.1002/eji.201344127
Biomin (2021). World Mycotoxin Survey Impact 2021. [Online]. Available: https://www.biomin.net/science-hub/world-mycotoxin-survey-impact-2021 [Accessed].
Bouhet S., Hourcade E., Loiseau N., Fikry A., Martinez S., Roselli M., et al. (2004). The Mycotoxin Fumonisin B1 Alters the Proliferation and the Barrier Function of Porcine Intestinal Epithelial Cells. Toxicol. Sci. 77 (1), 165–171. doi:10.1093/toxsci/kfh006
Bracarense A.-P. F. L., Lucioli J., Grenier B., Drociunas Pacheco G., Moll W.-D., Schatzmayr G., et al. (2012). Chronic Ingestion of Deoxynivalenol and Fumonisin, Alone or in Interaction, Induces Morphological and Immunological Changes in the Intestine of Piglets. Br. J. Nutr. 107 (12), 1776–1786. doi:10.1017/s0007114511004946
Broom L. J. (2017). Necrotic Enteritis; Current Knowledge and Diet-Related Mitigation. World's Poult. Sci. J. 73 (2), 281–292. doi:10.1017/s0043933917000058
Brown T. P., Rottinghaus G. E., Williams M. E. (1992). Fumonisin Mycotoxicosis in Broilers: Performance and Pathology. Avian Dis. 36, 450–454. doi:10.2307/1591528
Chen Y. P., Cheng Y. F., Li X. H., Yang W. L., Wen C., Zhuang S., et al. (2017). Effects of Threonine Supplementation on the Growth Performance, Immunity, Oxidative Status, Intestinal Integrity, and Barrier Function of Broilers at the Early Age. Poult. Sci. 96 (2), 405–413. doi:10.3382/ps/pew240
NRC (1994). Nutrient Requirements of PoultryNinth Revised Edition. Washington, DC: National Academies Press, 19–34.
Daenicke S., Keese C., Goyarts T., Döll S. (2011). Effects of Deoxynivalenol (DON) and Related Compounds on Bovine Peripheral Blood Mononuclear Cells (PBMC) In Vitro and In Vivo. Mycotox Res. 27 (1), 49–55. doi:10.1007/s12550-010-0074-3
Dänicke S., Gareis M., Bauer J. (2001). Orientation Values for Critical Concentrations of Deoxynivalenol and Zearalenone in Diets for Pigs, Ruminants and Gallinaceous Poultry. Proc. Soc. Nutr. Physiol., 171–174.
Duringer J. M., Roberts H. L., Doupovec B., Faas J., Estill C. T., Jiang D., et al. (2020). Effects of Deoxynivalenol and Fumonisins Fed in Combination on Beef Cattle: Health and Performance Indices. World Mycotoxin J. 13 (4), 533–543. doi:10.3920/wmj2020.2567
Filazi A., Yurdakok-Dikmen B., Kuzukiran O., Sireli U. T. (2017). “Mycotoxins in Poultry,” in Poultry Science. Editor Dr. Milad Manafi (USA: InTech), 73–92.
Findley M. K., Koval M. (2009). Regulation and Roles for Claudin-Family Tight Junction Proteins. IUBMB life 61 (4), 431–437. doi:10.1002/iub.175
Food and Administration (2010). Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed. Food and Drug Administration: Rockville, MD.
FDA (2001). Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds. Washington DC: United States Food and Drug Administration.
Fujita K., Katahira J., Horiguchi Y., Sonoda N., Furuse M., Tsukita S. (2000). Clostridium Perfringensenterotoxin Binds to the Second Extracellular Loop of Claudin-3, a Tight Junction Integral Membrane Protein. FEBS Lett. 476 (3), 258–261. doi:10.1016/s0014-5793(00)01744-0
Gao Y., Li S., Wang J., Luo C., Zhao S., Zheng N. (2018). Modulation of Intestinal Epithelial Permeability in Differentiated Caco-2 Cells Exposed to Aflatoxin M1 and Ochratoxin A Individually or Collectively. Toxins (Basel) 10 (1), 13. doi:10.3390/toxins10010013
Girgis G. N., Barta J. R., Girish C. K., Karrow N. A., Boermans H. J., Smith T. K. (2010). Effects of Feed-Borne Fusarium Mycotoxins and an Organic Mycotoxin Adsorbent on Immune Cell Dynamics in the Jejunum of Chickens Infected with Eimeria Maxima. Veterinary Immunol. Immunopathol. 138 (3), 218–223. doi:10.1016/j.vetimm.2010.07.018
Girgis G. N., Sharif S., Barta J. R., Boermans H. J., Smith T. K. (2008). Immunomodulatory Effects of Feed-Borne Fusarium Mycotoxins in Chickens Infected with Coccidia. Exp. Biol. Med. (Maywood) 233 (11), 1411–1420. doi:10.3181/0805-rm-173
Glenn A. E. (2007). Mycotoxigenic Fusarium Species in Animal Feed. Animal Feed Sci. Technol. 137 (3), 213–240. doi:10.1016/j.anifeedsci.2007.06.003
Grenier B., Dohnal I., Shanmugasundaram R., Eicher S., Selvaraj R., Schatzmayr G., et al. (2016). Susceptibility of Broiler Chickens to Coccidiosis when Fed Subclinical Doses of Deoxynivalenol and Fumonisins-Special Emphasis on the Immunological Response and the Mycotoxin Interaction. Toxins 8 (8), 231. doi:10.3390/toxins8080231
Grenier B., Oswald I. (2011). Mycotoxin Co-contamination of Food and Feed: Meta-Analysis of Publications Describing Toxicological Interactions. World Mycotoxin J. 4 (3), 285–313. doi:10.3920/wmj2011.1281
Guerre P. (2016). Worldwide Mycotoxins Exposure in Pig and Poultry Feed Formulations. Toxins 8 (12), 350. doi:10.3390/toxins8120350
Guo H., Chang J., Wang P., Yin Q., Liu C., Li S., et al. (2021). Detoxification of Aflatoxin B1 in Broiler Chickens by a Triple-Action Feed Additive. Food Addit. Contam. Part A 38 (9), 1583–1593. doi:10.1080/19440049.2021.1957159
Hernandez-Patlan D., Solis-Cruz B., Pontin K. P., Hernandez-Velasco X., Merino-Guzman R., Adhikari B., et al. (2019). Impact of a Bacillus Direct-Fed Microbial on Growth Performance, Intestinal Barrier Integrity, Necrotic Enteritis Lesions, and Ileal Microbiota in Broiler Chickens Using a Laboratory Challenge Model. Front. Vet. Sci. 6, 108. doi:10.3389/fvets.2019.00108
Hirakawa R., Nurjanah S., Furukawa K., Murai A., Kikusato M., Nochi T., et al. (2020). Heat Stress Causes Immune Abnormalities via Massive Damage to Effect Proliferation and Differentiation of Lymphocytes in Broiler Chickens. Front. Vet. Sci. 7, 46. doi:10.3389/fvets.2020.00046
Hofacre C. L., Froyman R., Gautrias B., George B., Goodwin M. A., Brown J. (1998). Use of Aviguard and Other Intestinal Bioproducts in Experimental Clostridium Perfringens-Associated Necrotizing Enteritis in Broiler Chickens. Avian Dis. 42, 579–584. doi:10.2307/1592685
Hofacre C. L., Smith J. A., Mathis G. F. (2018). An Optimist's View on Limiting Necrotic Enteritis and Maintaining Broiler Gut Health and Performance in Today's Marketing, Food Safety, and Regulatory Climate. Poult. Sci. 97 (6), 1929–1933. doi:10.3382/ps/pey082
Hopkins A. M., Walsh S. V., Verkade P., Boquet P., Nusrat A. (2003). Constitutive Activation of Rho Proteins by CNF-1 Influences Tight Junction Structure and Epithelial Barrier Function. J. cell Sci. 116 (4), 725–742. doi:10.1242/jcs.00300
Immerseel F. V., Buck J. D., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. (2004). Clostridium Perfringensin Poultry: an Emerging Threat for Animal and Public Health. Avian Pathol. 33 (6), 537–549. doi:10.1080/03079450400013162
Kuttappan V. A., Berghman L. R., Vicuña E. A., Latorre J. D., Menconi A., Wolchok J. D., et al. (2015). Poultry Enteric Inflammation Model with Dextran Sodium Sulfate Mediated Chemical Induction and Feed Restriction in Broilers. Poult. Sci. 94 (6), 1220–1226. doi:10.3382/ps/pev114
Langendijk P. S., Schut F., Jansen G. J., Raangs G. C., Kamphuis G. R., Wilkinson M. H., et al. (1995). Quantitative Fluorescence In Situ Hybridization of Bifidobacterium Spp. With Genus-specific 16S rRNA-Targeted Probes and its Application in Fecal Samples. Appl. Environ. Microbiol. 61 (8), 3069–3075. doi:10.1128/aem.61.8.3069-3075.1995
Li Y., Zhang H., Chen Y. P., Yang M. X., Zhang L. L., Lu Z. X., et al. (2015). Bacillus Amyloliquefaciens Supplementation Alleviates Immunological Stress and Intestinal Damage in Lipopolysaccharide-Challenged Broilers. Animal Feed Sci. Technol. 208, 119–131. doi:10.1016/j.anifeedsci.2015.07.001
Liu J., Teng P.-Y., Kim W. K., Applegate T. J. (2021). Assay Considerations for Fluorescein Isothiocyanate-Dextran (FITC-D): An Indicator of Intestinal Permeability in Broiler Chickens. Poult. Sci. 100 (7), 101202. doi:10.1016/j.psj.2021.101202
Livak K. J., Schmittgen T. D. (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. methods 25 (4), 402–408. doi:10.1006/meth.2001.1262
Lucke A., Böhm J., Zebeli Q., Metzler-Zebeli B. U. (2018). Dietary Deoxynivalenol and Oral Lipopolysaccharide Challenge Differently Affect Intestinal Innate Immune Response and Barrier Function in Broiler Chickens. J. Anim. Sci. 96 (12), 5134–5143. doi:10.1093/jas/sky379
Markazi A. D., Luoma A., Shanmugasundaram R., Murugesan R., Mohnl M., Selvaraj R. (2019). Effect of Acidifier Product Supplementation in Laying Hens Challenged with Salmonella. J. Appl. Poult. Res. 28 (4), 919–929. doi:10.3382/japr/pfz053
Martin S. J., Burton D. R., Roitt I. M., Delves P. J. (2016). Roitt's Essential Immunology. New Jersey: John Wiley & Sons.
McGlone J. (2010). Guide for the Care and Use of Agricultural Animals in Teaching and Research. United States: American Dairy Science Association.
Mensikova M., Stepanova H., Faldyna M. (2013). Interleukin-17 in Veterinary Animal Species and its Role in Various Diseases: a Review. Cytokine 64 (1), 11–17. doi:10.1016/j.cyto.2013.06.002
Mora Z. V.-d. l., Macías-Rodríguez M. E., Arratia-Quijada J., Gonzalez-Torres Y. S., Nuño K., Villarruel-López A. (2020). Clostridium perfringens as Foodborne Pathogen in Broiler Production: Pathophysiology and Potential Strategies for Controlling Necrotic Enteritis. Animals 10 (9), 1718. doi:10.3390/ani10091718
Novak B., Vatzia E., Springler A., Pierron A., Gerner W., Reisinger N., et al. (2018). Bovine Peripheral Blood Mononuclear Cells Are More Sensitive to Deoxynivalenol Than Those Derived from Poultry and Swine. Toxins 10 (4), 152. doi:10.3390/toxins10040152
Ogbuewu I. (2011). Effects of Mycotoxins in Animal Nutrition: A Review. Asian J. Anim. Sci. 5, 19–33. doi:10.3923/ajas.2011.19.33
Overgaard N. H., Jung J.-W., Steptoe R. J., Wells J. W. (2015). CD4+/CD8+double-positive T Cells: More Than Just a Developmental Stage? J. Leukoc. Biol. 97 (1), 31–38. doi:10.1189/jlb.1ru0814-382
Pinton P., Oswald I. (2014). Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins 6 (5), 1615–1643. doi:10.3390/toxins6051615
Rauber R. H., Oliveira M. S., Mallmann A. O., Dilkin P., Mallmann C. A., Giacomini L. Z., et al. (2013). Effects of Fumonisin B1 on Selected Biological Responses and Performance of Broiler Chickens. Pesq. Vet. Bras. 33 (9), 1081–1086. doi:10.1590/s0100-736x2013000900006
Ren Z., Wang Y., Deng H., Deng Y., Deng J., Zuo Z., et al. (2015). Deoxynivalenol-induced Cytokines and Related Genes in Concanavalin A-Stimulated Primary Chicken Splenic Lymphocytes. Toxicol. Vitro 29 (3), 558–563. doi:10.1016/j.tiv.2014.12.006
Riley R. T., Voss K. A., Norred W. P., Bacon C. W., Meredith F. I., Sharma R. P. (1999). Serine Palmitoyltransferase Inhibition Reverses Anti-proliferative Effects of Ceramide Synthase Inhibition in Cultured Renal Cells and Suppresses Free Sphingoid Base Accumulation in Kidney of BALBc Mice. Environ. Toxicol. Pharmacol. 7 (2), 109–118. doi:10.1016/s1382-6689(98)00047-7
Roberts H. L., Bionaz M., Jiang D., Doupovec B., Faas J., Estill C. T., et al. (2021). Effects of Deoxynivalenol and Fumonisins Fed in Combination to Beef Cattle: Immunotoxicity and Gene Expression. Toxins 13 (10), 714. doi:10.3390/toxins13100714
Roitt I. (1992). Essential Immunology. Rev. Inst. Med. Trop. S. Paulo 34, 32. doi:10.1590/s0036-46651992000100014
Ruhnau D., Hess C., Grenier B., Doupovec B., Schatzmayr D., Hess M., et al. (2020). The Mycotoxin Deoxynivalenol (DON) Promotes Campylobacter Jejuni Multiplication in the Intestine of Broiler Chickens with Consequences on Bacterial Translocation and Gut Integrity. Front. Veterinary Sci. 1027, 573894. doi:10.3389/fvets.2020.573894
Šefcová M., Larrea-Álvarez M., Larrea-Álvarez C., Revajová V., Karaffová V., Koščová J., et al. (2020). Effects of Lactobacillus Fermentum Supplementation on Body Weight and Pro-inflammatory Cytokine Expression in Campylobacter Jejuni-Challenged Chickens. Veterinary Sci. 7 (3), 121. doi:10.3390/vetsci7030121
Shanmugasundaram R., Acevedo K., Mortada M., Akerele G., Applegate T. J., Kogut M. H., et al. (2021). Effects of Salmonella enterica Ser. Enteritidis and Heidelberg on Host CD4+CD25+ Regulatory T Cell Suppressive Immune Responses in Chickens. Plos one 16 (11), e0260280. doi:10.1371/journal.pone.0260280
Shanmugasundaram R., Kogut M. H., Arsenault R. J., Swaggerty C. L., Cole K., Reddish J. M., et al. (2015). Effect of Salmonella Infection on Cecal Tonsil Regulatory T Cell Properties in Chickens. Poult. Sci. 94 (8), 1828–1835. doi:10.3382/ps/pev161
Shanmugasundaram R., Markazi A., Mortada M., Ng T. T., Applegate T. J., Bielke L. R., et al. (2020). Research Note: Effect of Synbiotic Supplementation on Caecal Clostridium perfringens Load in Broiler Chickens with Different Necrotic Enteritis Challenge Models. Poult. Sci. 99 (5), 2452–2458. doi:10.1016/j.psj.2019.10.081
Shanmugasundaram R., Mortada M., Cosby D. E., Singh M., Applegate T. J., Syed B., et al. (2019a). Synbiotic Supplementation to Decrease Salmonella Colonization in the Intestine and Carcass Contamination in Broiler Birds. Plos one 14 (10), e0223577. doi:10.1371/journal.pone.0223577
Shanmugasundaram R., Mortada M., Murugesan G. R., Selvaraj R. K. (2019b). In Vitro characterization and Analysis of Probiotic Species in the Chicken Intestine by Real-Time Polymerase Chain Reaction. Poult. Sci. 98 (11), 5840–5846. doi:10.3382/ps/pez188
Shanmugasundaram R., Selvaraj R. K. (2012). Effect of Killed Whole Yeast Cell Prebiotic Supplementation on Broiler Performance and Intestinal Immune Cell Parameters. Poult. Sci. 91 (1), 107–111. doi:10.3382/ps.2011-01732
Shanmugasundaram R., Wick M., Lilburn M. S. (2019c). Effect of a Post-hatch Lipopolysaccharide Challenge in Turkey Poults and Ducklings after a Primary Embryonic Heat Stress. Dev. Comp. Immunol. 101, 103436. doi:10.1016/j.dci.2019.103436
Shanmugasundaram R., Wick M., Lilburn M. S. (2018). Effect of Embryonic Thermal Manipulation on Heat Shock Protein 70 Expression and Immune System Development in Pekin Duck Embryos. Poult. Sci. 97 (12), 4200–4210. doi:10.3382/ps/pey298
Shimshoni J. A., Cuneah O., Sulyok M., Krska R., Galon N., Sharir B., et al. (2013). Mycotoxins in Corn and Wheat Silage in Israel. Food Addit. Contam. Part A 30 (9), 1614–1625. doi:10.1080/19440049.2013.802840
Smith J. A. (2019). Broiler Production without Antibiotics: United States Field Perspectives. Animal Feed Sci. Technol. 250, 93–98. doi:10.1016/j.anifeedsci.2018.04.027
Taranu I., Marin D. E., Burlacu R., Pinton P., Damian V., Oswald I. P. (2010). Comparative Aspects Ofin Vitroproliferation of Human and Porcine Lymphocytes Exposed to Mycotoxins. Archives Animal Nutr. 64 (5), 383–393. doi:10.1080/1745039x.2010.492140
Taranu I., Marin D. E., Pistol G. C., Motiu M., Pelinescu D. (2015). Induction of Pro-inflammatory Gene Expression by Escherichia coli and Mycotoxin Zearalenone Contamination and Protection by a Lactobacillus Mixture in Porcine IPEC-1 Cells. Toxicon 97, 53–63. doi:10.1016/j.toxicon.2015.01.016
Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. (2011). Necrotic Enteritis in Broilers: an Updated Review on the Pathogenesis. Avian Pathol. 40 (4), 341–347. doi:10.1080/03079457.2011.590967
Tomaszewska E., Rudyk H., Dobrowolski P., Donaldson J., Świetlicka I., Puzio I., et al. (2021). Changes in the Intestinal Histomorphometry, the Expression of Intestinal Tight Junction Proteins, and the Bone Structure and Liver of Pre-laying Hens Following Oral Administration of Fumonisins for 21 Days. Toxins 13 (6), 375. doi:10.3390/toxins13060375
van der Most P. J., de Jong B., Parmentier H. K., Verhulst S. (2011). Trade‐off between Growth and Immune Function: a Meta‐analysis of Selection Experiments. Funct. Ecol. 25 (1), 74–80. doi:10.1111/j.1365-2435.2010.01800.x
Van Nevel C. J., Decuypere J. A., Dierick N. A., Molly K. (2005). Incorporation of Galactomannans in the Diet of Newly Weaned Piglets: Effect on Bacteriological and Some Morphological Characteristics of the Small Intestine. Archives Animal Nutr. 59 (2), 123–138. doi:10.1080/17450390512331387936
Wallach D., Kang T.-B., Kovalenko A. (2014). Concepts of Tissue Injury and Cell Death in Inflammation: a Historical Perspective. Nat. Rev. Immunol. 14 (1), 51–59. doi:10.1038/nri3561
Wang E., Norred W. P., Bacon C. W., Riley R. T., Merrill A. H. (1991). Inhibition of Sphingolipid Biosynthesis by Fumonisins. Implications for Diseases Associated with Fusarium Moniliforme. J. Biol. Chem. 266 (22), 14486–14490. doi:10.1016/s0021-9258(18)98712-0
Wang R. F., Cao W. W., Cerniglia C. E. (1996). PCR Detection and Quantitation of Predominant Anaerobic Bacteria in Human and Animal Fecal Samples. Appl. Environ. Microbiol. 62 (4), 1242–1247. doi:10.1128/aem.62.4.1242-1247.1996
Wang R. J., Fui S. X., Miao C. H., Feng D. Y. (2005). Effects of Different Mycotoxin Adsorbents on Performance, Meat Characteristics and Blood Profiles of Avian Broilers Fed Mold Contaminated Corn. Asian Australas. J. Anim. Sci. 19 (1), 72–79. doi:10.5713/ajas.2006.72
Yang Q., Liu J., Wang X., Robinson K., Whitmore M. A., Stewart S. N., et al. (2021). Identification of an Intestinal Microbiota Signature Associated with the Severity of Necrotic Enteritis. Front. Microbiol. 12, 703693. doi:10.3389/fmicb.2021.703693
Yu S., Jia B., Lin H., Zhang S., Yu D., Liu N., et al. (2022). Effects of Fumonisin B and Hydrolyzed Fumonisin B on Growth and Intestinal Microbiota in Broilers. Toxins 14 (3), 163. doi:10.3390/toxins14030163
Zhang B., Lv Z., Li Z., Wang W., Li G., Guo Y. (2018). Dietary L-Arginine Supplementation Alleviates the Intestinal Injury and Modulates the Gut Microbiota in Broiler Chickens Challenged by Clostridium perfringens. Front. Microbiol. 9, 1716. doi:10.3389/fmicb.2018.01716
Zhang C., Zhao X. H., Yang L., Chen X. Y., Jiang R. S., Jin S. H., et al. (2017). Resveratrol Alleviates Heat Stress-Induced Impairment of Intestinal Morphology, Microflora, and Barrier Integrity in Broilers. Poult. Sci. 96 (12), 4325–4332. doi:10.3382/ps/pex266
Keywords: fumonisins, deoxynivalenol, necrotic enteritis, tight junction proteins, immune response, broiler chicken
Citation: Shanmugasundaram R, Adams D, Ramirez S, Murugesan GR, Applegate TJ, Cunningham S, Pokoo-Aikins A and Glenn AE (2022) Subclinical Doses of Combined Fumonisins and Deoxynivalenol Predispose Clostridium perfringens–Inoculated Broilers to Necrotic Enteritis. Front. Physiol. 13:934660. doi: 10.3389/fphys.2022.934660
Received: 02 May 2022; Accepted: 13 June 2022;
Published: 22 July 2022.
Edited by:
Francesca Soglia, University of Bologna, ItalyReviewed by:
Marco Zampiga, University of Bologna, ItalyCopyright © 2022 Shanmugasundaram, Adams, Ramirez, Murugesan, Applegate, Cunningham, Pokoo-Aikins and Glenn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Shanmugasundaram, cmV2YXRoaS5zaGFuQHVzZGEuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.