
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 16 June 2022
Sec. Vascular Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.913454
This article is part of the Research TopicNew Strategies for the Treatment and Prevention of Cardiovascular and Cerebrovascular Diseases: Recent Progress in Basic and Clinical ResearchView all 8 articles
Background: This study aimed to investigate whether advanced interatrial block (IAB) is a predictor of recurrent atrial fibrillation (AF) and/or ischemic stroke in elderly patients with AF and hypertension.
Methods and objectives: Five hundred and sixteen elderly inpatients (mean age 85.53 ± 9.08 years; 5.43% women) with concurrent paroxysmal AF and hypertension were enrolled in this retrospective observational study. Data on comorbidity, medication, digital electrocardiograms (ECG), and outcomes were obtained from the medical records and follow-up examinations. IAB was classified as partial IAB or advanced IAB according to 12-lead surface ECG analysis on admission. Advanced IAB was defined as a maximum P wave duration of >120 ms with biphasic (±) morphology in leads II, Ⅲ, and aVF by two blinded investigators. The endpoints were recurrent AF and ischemic stroke.
Results: We enrolled 120 patients (23.26%) with partial IAB and 187 (36.24%) with advanced IAB. The mean follow-up duration was 19 months. A total of 320 patients (62.02%) developed AF recurrence, and 31 (6.01%) experienced ischemic stroke. Significant predictors of advanced IAB in multivariate analysis were older age (>80 years), increased left atrial diameter (>40 mm), and being overweight (body mass index >25 kg/m2). In the multivariable comprehensive Cox regression analyses, partial IAB was associated with AF recurrence. Advanced IAB was an independent predictor of increased risk of AF recurrence and ischemic stroke.
Conclusion: Both partial and advanced IAB are associated with AF recurrence in elderly patients with hypertension. Furthermore, advanced IAB is an independent predictor of ischemic stroke.
Atrial fibrillation (AF) is one of the most common types of cardiac arrhythmias in elderly patients with hypertension and is associated with significant ischemic stroke and cardiovascular mortalities. Interatrial block (IAB) often occurs when a conduction delay occurs over Bachmann’s bundle or between the activation of the atrium. IAB was initially classified by Bayes de Luna and has recently often been defined according to IAB severity (Bayés de Luna et al., 2018; van der Does et al., 2021). Partial IAB is defined as a P-wave duration over 120 ms, and advanced IAB is defined as a P-wave morphology (positive/negative) in II, III, and aVF with a duration ≥120 ms. Advanced IAB has been reported as being associated with both new-onset and recurrent atrial fibrillation, left atrial electromechanical dysfunction, ischemic stroke, and cardiovascular mortality in some clinical scenarios (O'Neal et al., 2016a; Fujimoto et al., 2018; Rubio Campal et al., 2018; Lacalzada-Almeida et al., 2019). However, there is little data related to the prognosis of elderly patients with hypertension. The current study aimed to evaluate the role of advanced IAB in predicting recurrent AF and/or ischemic stroke in elderly patients with hypertension.
This was a retrospective observational study conducted at Chinese PLA general hospital in Beijing. All eligible patients were aged ≥65 years, of Chinese ethnicity, and diagnosed with hypertension and paroxysmal non-valvular atrial fibrillation between April 2014 and May 2016. Patients with the following conditions were excluded: 1) valvular AF such as post-mechanical valve replacement or moderate-to-severe rheumatic mitral valve stenosis; 2) severe renal insufficiency (estimated glomerular filtration rate of <30 ml/min/1.73 m2); 3) severe obstructive sleep apnea syndrome (apnea-hypopnea index of >10); 4) hyperthyroidism; 5) radiofrequency catheter ablation procedures for all types of atrial arrhythmia; 6) left atrial appendage occlusion procedures; and 7) cardiac pacemaker implantation. All participants provided written informed consent, and the study was approved by the institutional ethics committee.
A surface standard 12-lead ECG (filter 150 Hz, 25 mm/s, 10 mm/mV) was recorded for each study patient at baseline and subsequent follow-up examinations and measured with digital calipers using the Muse Cardiology Information System. We amplified the ECG images up to 10 times their original size to define the P-wave duration and PR interval. IAB was classified according to the baseline 12-lead ECG by two independent blinded investigators as follows: 1) partial IAB was defined as P-wave duration longer than 120 ms without biphasic morphology in leads II, III, and aVF; and 2) advanced IAB was defined as P-wave duration longer than 120 ms and presented biphasic morphology in leads II, III, and aVF (Bayés de Luna et al., 2018) (Figure 1).
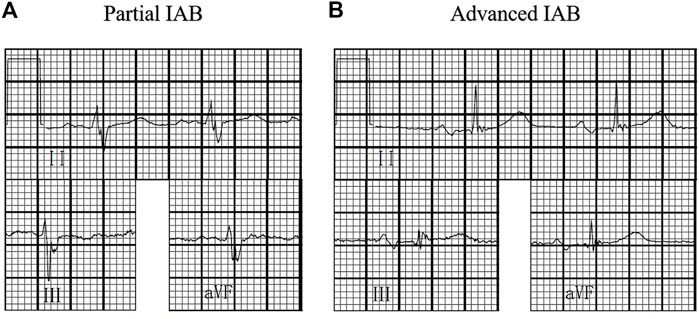
FIGURE 1. Interatrial block (IAB) degree. (A) partial IAB:P wave ≥120 ms without bimodal morphology;(B) advanced IAB:P wave ≥120 ms with bimodal morphology in inferior leads.
All patients underwent two-dimensional transthoracic echocardiographic evaluation on admission. Echocardiographic parameters, such as ventricular ejection fraction (LVEF) and left atrial volume (LAV), were derived from the echocardiographic data. LVEF and LAV were measured using Simpson’s method and the area-length method, respectively. The left atrial volume index (LAVI) was calculated as the left atrial volume divided by the body surface area of each patient.
Baseline demographic characteristics, relevant medical histories, therapeutic procedures, cardiovascular events, and laboratory and imaging data were extracted from hospital electronic medical records. All patients were followed up at regular intervals or during all-cause hospitalization, with additional visits as required in the case of decompensation. The regular schedule of visits included a minimum of quarterly visits by the cardiovascular specialists. A 12-lead ECG was recorded at every visit or at any time when the patient had palpitations. A 24 h ECG monitor (Holter) was evaluated at least twice in patients with no recurrence, as necessary. Information on the endpoints was collected from the medical records and questionnaires answered by the patients. The primary endpoint was recurrent AF and was defined using ECGs or 24 h Holter electrocardiogram results. The secondary endpoint was AF-related ischemic stroke, which was defined as a sudden onset of neurological deficits or other relative symptoms lasting over 24 h and was associated with AF and confirmed using computed tomography or magnetic resonance imaging.
All statistical data were evaluated using IBM SPSS Statistics for Windows version 23.0 (IBM, NY, United States). Continuous variables are represented as mean ± standard deviation. Univariate analysis was performed using analysis of variance for continuous variables and the chi-square test for categorical variables. Kaplan–Meier estimates were used to compute the cumulative incidence of recurrent AF. Follow-up time was defined as the time between the baseline visit and recurrence of AF, time to loss to follow-up, death, or the end of the study period. We conducted multivariate logistic regression analyses to screen the independent risk factors for IAB. The Cox proportional hazard model was used to calculate the adjusted hazard ratio (HR) of risk factors for recurrent AF and ischemic stroke. Adjusted HRs with 95% confidence intervals (CIs) are reported separately. Statistical significance was set at p < 0.05.
A total of 516 patients with paroxysmal AF and hypertension (women, 5.43%; mean age at baseline, 85.53 ± 9.08 years) were included in the study. During a mean follow-up of 19 months, 320 patients (62.02%) experienced AF recurrence, and 31 (6.01%) experienced ischemic stroke. The baseline characteristics of the patients, according to their IAB degree, are listed in Table 1. In this cohort, 209 (40.50%) patients had normal atrial conduction, 120 had partial IAB (23.26%), and 187 had advanced IAB (36.24%). In general, the more advanced the IAB, the higher the burden of the comorbidity. Patients with advanced IAB were significantly older compared to those with normal atrial conduction (87.95 ± 7.15 vs. 83.39 ± 10.12; p < 0.01). Participants with advanced IAB were more likely to have coronary artery disease, heart failure, or prior ischemic stroke than those with normal atrial conduction. They were also more likely to take diuretics and had higher values of PR intervals, P-wave duration, body mass index (BMI), left atrial diameter, and right atrial diameter. Participants with partial IAB were more likely to have a higher PR interval and P-wave duration than those with normal atrial conduction. Similarly, participants with advanced IAB were more likely to have anemia, P-wave prolongation, a larger left atrial diameter, a larger right atrial diameter, and a higher BMI than those with partial IAB. There was no significant difference in the use of antiarrhythmic drugs (including amiodarone and propafenone) and anticoagulants (recommended dose of rivaroxaban or dabigatran) among the three groups (partial/advanced and no IAB groups) and during follow-up.
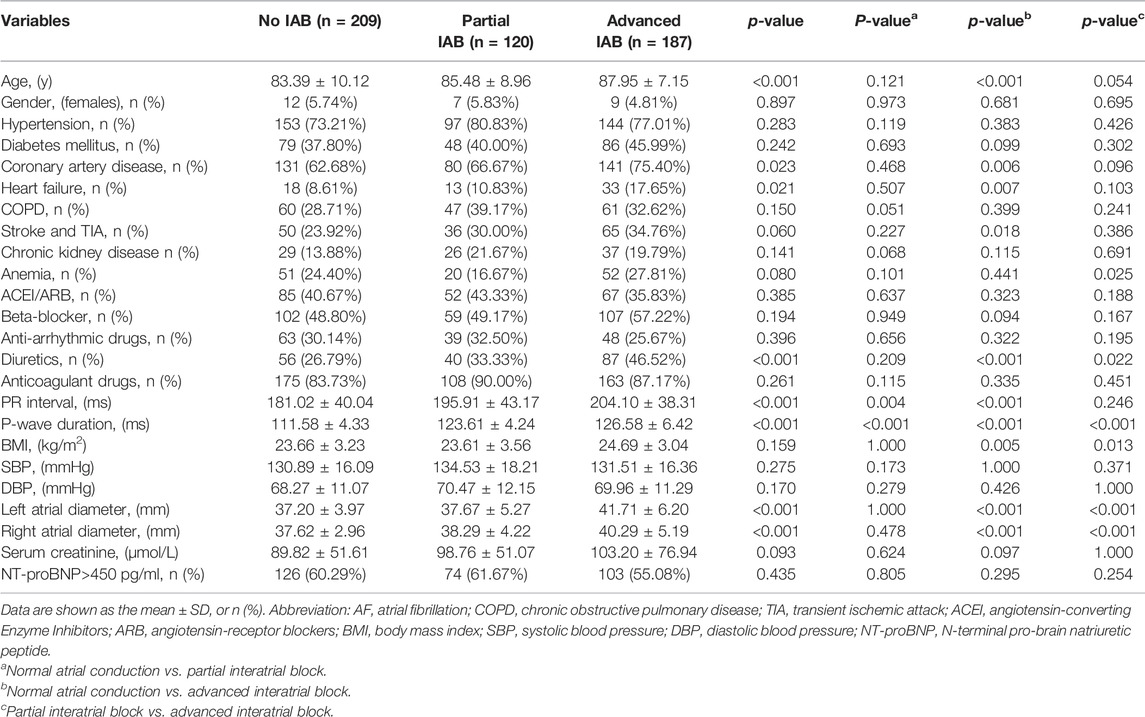
TABLE 1. Baseline characteristics of the study participants with AF and hypertension according to the interatrial conduction.
Table 2 shows the unadjusted and adjusted risk factors for advanced IAB. The strongest predictors of advanced IAB on admission in multivariate analysis were age of >80 years (odds ratio [OR] = 2.731, 95% CI: 1.629–4.577; p < 0.001), left atrial enlargement (left atrial diameter of >40 mm) (OR = 3.264; 95% CI: 2.206–4.829; p < 0.001), and BMI of >25 kg/m2 (OR = 1.700; 95% CI: 1.133–2.551; p = 0.010). Overall, this model explained 69.6% of the variability in the prevalence of advanced IAB.
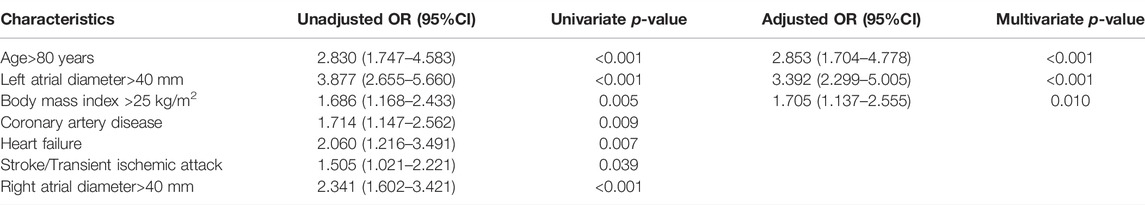
TABLE 2. Results of univariate and multivariate logistic regression analysis for identifying predictors of advanced interatrial block.
The annual incidence of recurrent AF in patients with advanced IAB and hypertension was approximately 57.71 per 1,000 person-years, which declined stepwise to 35.02 per 1,000 person-years, and 15.92 per 1,000 person-years in patients with partial IAB and those with normal atrial conduction. Moreover, the annual incidence of ischemic stroke was highest in patients with advanced IAB (rate of 5.95 per 1,000 person-years), intermediate in patients with partial IAB (rate of 3.42 per 1,000 person-years), and lowest in patients with normal atrial conduction (rate of 1.31 per 1,000 person-years) (Table 3). There was no significant difference in the proportion of anticoagulant use between the stroke event and control groups (90.32% vs. 86.19%; p >0.05).
For univariate analysis, we observed significant differences in the Kaplan–Meier estimates of cumulative incidences of recurrent AF and ischemic stroke according to IAB severity (p < 0.01) (Figure 2).
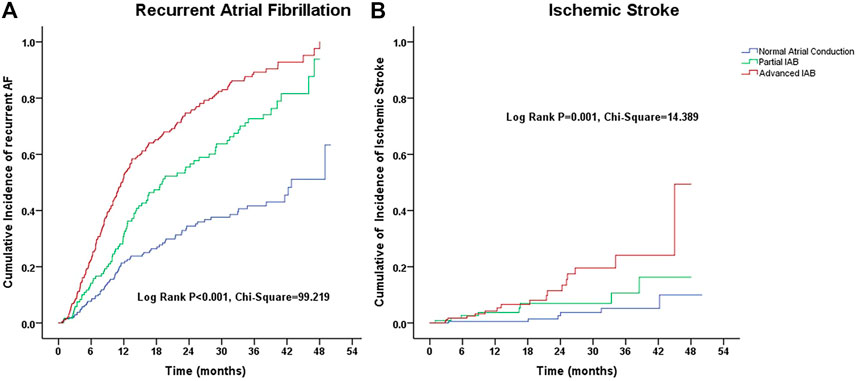
FIGURE 2. Kalpana-Meier estimate of cumulative incidences of (A) recurrent atrial fibrillation and (B) ischemic stroke according to the interatrial block (IAB) degree.
A multivariate Cox regression model was applied, including IAB severity, age, sex, PR interval, BMI, hypertension, diabetes mellitus, coronary artery disease, heart failure, chronic obstructive pulmonary disease, prior stroke/transient ischemic attack, chronic kidney disease stage, anemia, left atrial diameter, right atrial diameter, N-terminal pro b-type natriuretic peptide (NT-proBNP) levels, and clinical medications. During the observation period, the crude HRs for recurrence of AF and ischemic stroke in the elderly AF patients with advanced IAB were higher than those with normal atrial conduction and remained significant (HR: 2.87, 95% CI: 2.12–3.88, p < 0.001; and HR: 4.57, 95% CI: 1.76–11.89, p = 0.002, respectively) after adjusting for potential confounders and demographics. Similarly, using multivariate analysis, we observed that partial IAB was also an independent risk factor associated with AF recurrence (HR: 2.165, 95% CI: 1.569–2.987; p < 0.001) but not for ischemic stroke (HR: 2.740; 95% CI: 0.944–7.958; p = 0.064) after adjusting for other risk factors. We observed no significant correlation between anticoagulant use and the risk of ischemic stroke (HR: 0.842; 95% CI: 0.255–2.773; p > 0.05). Additionally, age of ≥80 years, heart failure, left atrial diameter of >40 mm, right atrial diameter of >40 mm, anemia, and NT-proBNP levels of >450 pg/ml were all independent risk factors for AF recurrence (p < 0.05) (Table 4).
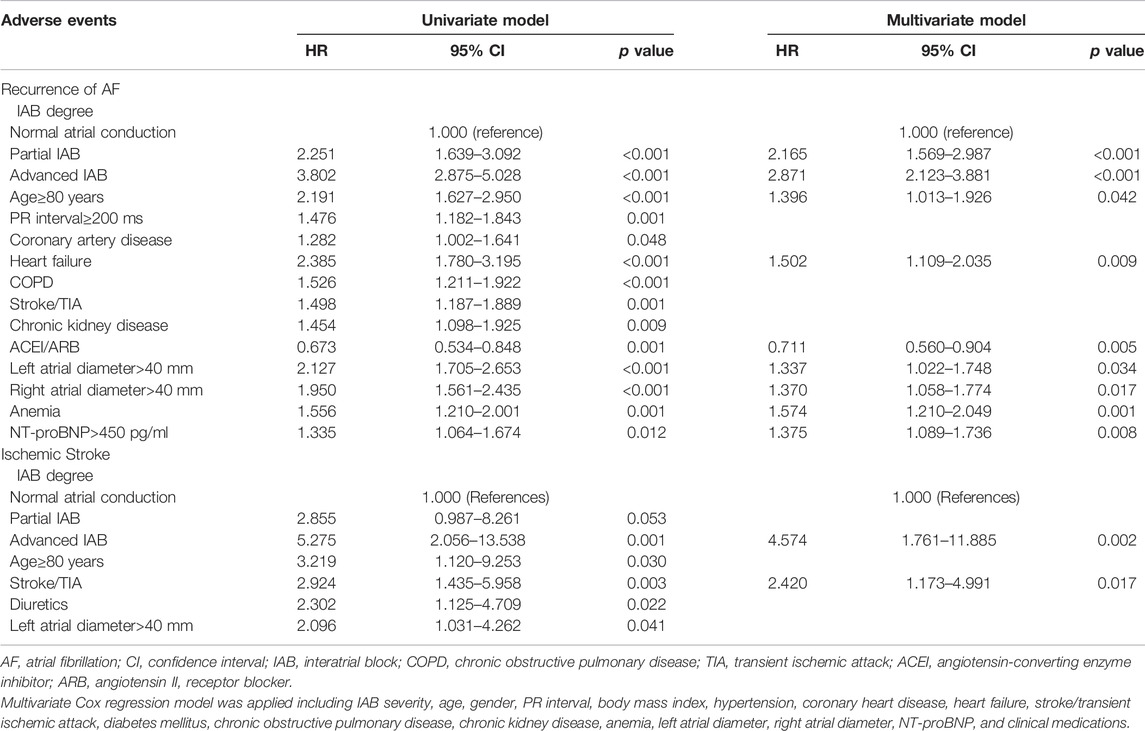
TABLE 4. Univariate and Multivariate Cox Regression Analyses of Risk Factors for Recurrent AF and Ischemic Stroke in elderly patients with hypertension during follow-up.
In this single-center, respective observational study, the major findings were as follows: 1) the significant predictors of advanced IAB were age of >80 years, left atrial diameter of >40 mm, and BMI >25 kg/m2; 2) advanced IAB was significantly associated with an increased risk of recurrent AF and ischemic stroke in elderly patients with paroxysmal AF and hypertension; 3) partial IAB was also an independent predictor of recurrent AF but not of ischemic stroke.
IAB is rarely considered an independent, routine, and important electrocardiographic diagnosis despite its high prevalence and ease of diagnosis. Previous studies have found that the prevalence of IAB increased with age and could increase from 26% to 44% in patients with cardiovascular risk factors, rising to 50% in octogenarians (Martínez-Sellés, 2017; Escobar-Robledo et al., 2018; Skov et al., 2018). In the present study, the mean age of the participants was over 80 years. In our cohort, 187 patients (36.24%) had advanced IAB, and 120 (23.26%) had partial IAB. We further observed that IAB was often associated with increased P-wave duration and left atrial diameter in elderly patients. We believe that the high prevalence of advanced IAB in the present study could be due to the high prevalence of octogenarians and recognized stroke risk factors encompassed in the CHA2DS2-VASc (congestive heart failure, hypertension, age of ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, and sex) risk score.
In the present study, we found that the significant predictors of advanced IAB were the age of >80 years, left atrial enlargement (left atrial diameter of >40 mm), and BMI of >25 kg/m2, which supports and extends the results of previous studies. A study by Bryce Alexander et al. demonstrated BMI of >30 kg/m2, male sex, and increased age (per 10 years increase) (OR = 1.46, 95% CI: 1.14–1.88) were significant predictors of IAB in patients with carotid and coronary artery disease (Alexander et al., 2018). The Atherosclerosis Risk in Communities (ARIC) study showed that age, male sex, BMI, and systolic blood pressure were all risk factors for advanced IAB development (O'Neal et al., 2016b). Another study revealed that an increased CHADS2 (congestive heart failure, hypertension, age >75 years, diabetes mellitus, prior stroke) score, coronary artery disease, and increased left atrial diameter were independently associated with IAB development. The association between IAB and degenerative diseases due to aging has been extensively studied. The mechanisms of the higher prevalence of IAB in obese patients than in those with normal weight may partly be due to an unhealthy lifestyle, diffuse ischemia, obstructive sleep apnea, and sympathetic nervous activation status (Baranchuk et al., 2017). Previous studies have confirmed the relationship between IAB and left atrial enlargement. In the present study, a left atrial diameter of >40 mm may be a more significant predictor of advanced IAB than other risk factors, as left atrial enlargement may be more directly related to atrial fibrosis and atrial conduction velocity, which are considered anatomical substrates of advanced IAB. Advanced IAB is probably a composite of impaired atrial conduction velocity and left atrial enlargement.
Several previous studies have found that IAB, especially advanced IAB, is strongly correlated with rapid atrial arrhythmias in different clinical scenarios and may predict ineffective cardioversion of atrial fibrillation (Relander et al., 2021). For example, Enriquez et al. reported that the overall recurrent AF in 61 patients without structural heart disease following pharmacological cardioversion was 36%, with a 90.9% recurrence in patients with advanced IAB (Enriquez et al., 2014). The ARIC study found that the incidence rate for advanced IAB was 2.27 per 1,000 person-years in 14,625 participants and confirmed a significant association between advanced IAB and an increased risk for AF (O'Neal et al., 2016). Recently, Wu et al. found the recurrence rate of paroxysmal AF after catheter ablation was higher in patients with advanced IAB than in those without advanced IAB (46.3% vs. 26.4%) (Wu et al., 2016). Furthermore, Skov et al. found that patients with advanced IAB and no cardiovascular disease (hypertension, heart failure, and ischemic heart disease) had a higher risk of AF than those with cardiovascular disease and no IAB (Skov et al., 2018). They also found that the addition of advanced IAB to a conventional risk model, including traditional cardiovascular risk factors, showed a high discriminative performance in the prediction of AF. In the present study, the incidence of recurrent AF in elderly patients with advanced IAB and hypertension was higher than that in those with normal atrial conduction (HR: 2.87, 95% CI: 2.12–3.88, p < 0.001), which supports and extends the results from previous studies. The mechanisms underlying the higher risk for recurrence of AF among patients with advanced IAB may partly be attributed to atrial fibrosis and atrial remodeling, which are considered anatomical substrates of advanced IAB, and play important roles in determining heterogeneous electrophysiological changes in atrial cardiomyocytes (Acampa et al., 2018; Bayés-de-Luna et al., 2020). IAB might result in electrical heterogeneity and impaired mechanical function of the left atrium and increased susceptibility to interatrial desynchrony and AF. Furthermore, cardiac ischemia, degenerative disease due to aging, infiltrative diseases, proinflammatory cytokines, the renin-angiotensin-aldosterone system, and oxidative stress may all disrupt the Bachmann’s bundle and interatrial desynchronization, thus promoting atrial cardiopathy and recurrence of AF (Kaireviciute et al., 2011; Martínez-Sellés, 2017; Escobar-Robledo et al., 2018). Some studies have further suggested that compromised left atrial function and heterogeneous electrophysiological changes might share a genetic underpinning with cardiomyopathy. The genetic risk of atrial fibrillation influences the left atrial structure prior to the diagnosis of AF (Ahlberg et al., 2021). The P-wave morphology distribution in atrial electrophysiology was also common in the early stage of arrhythmia and may be a cause rather than a consequence of AF (Holmqvist et al., 2011).
In this study, we observed a higher incidence of ischemic stroke in patients with advanced IAB than in those with normal atrial conduction. Advanced IAB, but not partial IAB, was an independent predictor of ischemic stroke after adjusting for potential confounders and demographics. Recent reports have shown that advanced IAB is associated with ischemic stroke. Evidence from prior studies has suggested that AF might serve as an intermediate event between advanced IAB and stroke. Subsequent rapid atrial arrhythmia may further lead to high risks of atrial cardiopathy, atrial dysfunction, and left atrial thrombosis (Lacalzada-Almeida et al., 2019; Istolahti et al., 2020). Patients with advanced IAB are usually more likely to have traditional risk factors for ischemic stroke and thromboembolism, such as hypertension, heart failure, diabetes, and aging. Some previous studies demonstrated that advanced IAB was associated with CHADS2 score, which was also a common independent predictor of IAB development and ischemic stroke (Wu et al., 2018). Furthermore, a recent study demonstrated that advanced IAB could predict all-cause mortality in ischemic stroke survivors with and without additional cardiovascular comorbidities (Baturova et al., 2019; Jacobsson et al., 2020; Prasitlumkum et al., 2020).
The current study has several limitations. First, the retrospective nature of this study and the relatively small sample size from a single center could have introduced selection bias. Second, recurrent AF was defined by ECGs and clinical examinations at every visit and 24-h Holter electrocardiograms in patients with no AF recurrence during follow-up. However, the recurrence rate of AF may have been underestimated, and more episodes of asymptomatic AF may be detected by longer rhythm monitoring. Third, IAB was classified according to the baseline 12-lead ECG, and changes in the performance of IAB during follow-up were not considered. Fourth, there is insufficient evidence to support our finding that advanced IAB could further improve the prediction efficiency of the CHA2DS2-VASc risk scoring system. Finally, we acknowledge that residual confounding remains a possibility, similar to that in other epidemiologic studies. Large-scale, multicenter, prospective studies with longer follow-up periods are required to validate our findings.
Our results support the hypothesis that IAB is prevalent in elderly patients with hypertension. Both partial and advanced IAB were associated with AF recurrence in elderly patients with paroxysmal AF and hypertension. Furthermore, advanced IAB is an independent predictor of ischemic stroke.
The data analyzed in this study is subject to the following licenses/restrictions: Confidentiality needs of patients’ clinical data. Requests to access these datasets should be directed to Haijun Wang, d2FuZ2hqMzAxQDE2My5jb20=.
The studies involving human participants were reviewed and approved by the ethics committee of Chinese PLA General Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HW, LC, QS and YS worked on the conception of the study. LC, YG and LS contributed to the data collection and data analysis. HW and YS checked the data. HW and QS drafted the manuscript. HW, LC, YG, LS, QS and YS reviewed the manuscript. All authors have read and approved the final version of the manuscript.
This work was supported in part by the Health Care Projects of Chinese PLA General Hospital (NLBJ-2019013 and 2019MBD-018), the Projects of Chinese Military Health Care (20BJZ44, 14BJZ08, and 17BJZ49), and the Foundation of National Clinical Research Center for Geriatric Diseases (NCRCG-PLAGH-2019021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acampa M., Lazzerini P. E., Martini G. (2018). Atrial Cardiopathy and Sympatho-Vagal Imbalance in Cryptogenic Stroke: Pathogenic Mechanisms and Effects on Electrocardiographic Markers. Front. Neurol. 9, 469. doi:10.3389/fneur.2018.00469
Ahlberg G., Andreasen L., Ghouse J., Bertelsen L., Bundgaard H., Haunsø S., et al. (2021). Genome-wide Association Study Identifies 18 Novel Loci Associated with Left Atrial Volume and Function. Eur. Heart J. 42 (44), 4523–4534. doi:10.1093/eurheartj/ehab466
Alexander B., Baranchuk A., Haseeb S., van Rooy H., Kuchtaruk A., Hopman W., et al. (2018). Interatrial Block Predicts Atrial Fibrillation in Patients with Carotid and Coronary Artery Disease. J. Thorac. Dis. 10 (7), 4328–4334. doi:10.21037/jtd.2018.06.53
Baranchuk A., Enriquez A., Antiperovitch P., Alexander B., Çinier G. (2017). Advanced Interatrial Block as a Key Marker for Atrial Fibrillation Recurrence: Bayés' Syndrome. J. Geriatr. Cardiol. 14 (3), 169–173. doi:10.11909/j.issn.1671-5411.2017.03.005
Baturova M. A., Lindgren A., Shubik Y. V., Carlson J., Platonov P. G. (2019). Interatrial Block in Prediction of All-Cause Mortality after First-Ever Ischemic Stroke. BMC Cardiovasc Disord. 19 (1), 1–9. doi:10.1186/s12872-019-1015-5
Bayés de Luna A., Escobar-Robledo L. A., Aristizabal D., Weir Restrepo D., Mendieta G., Massó van Roessel A., et al. (2018). Atypical Advanced Interatrial Blocks: Definition and Electrocardiographic Recognition. J. Electrocardiol. 51 (6), 1091–1093. doi:10.1016/j.jelectrocard.2018.09.004
Bayés-de-Luna A., Martínez-Sellés M., Elosua R., Bayés-Genís A., Mendieta G., Baranchuk A., et al. (2020). Relation of Advanced Interatrial Block to Risk of Atrial Fibrillation and Stroke. Am. J. Cardiol. 125 (11), 1745–1748. doi:10.1016/j.amjcard.2020.02.034
Enriquez A., Conde D., Hopman W., Mondragon I., Chiale P. A., de Luna A. B., et al. (2014). Advanced Interatrial Block Is Associated with Recurrence of Atrial Fibrillation Post Pharmacological Cardioversion. Cardiovasc Ther. 32 (2), 52–56. doi:10.1111/1755-5922.12063
Escobar-Robledo L. A., Bayés-de-Luna A., Lupón J., Baranchuk A., Moliner P., Martínez-Sellés M., et al. (2018). Advanced Interatrial Block Predicts New-Onset Atrial Fibrillation and Ischemic Stroke in Patients with Heart Failure: The "Bayes' Syndrome-HF" Study. Int. J. Cardiol. 271, 174–180. doi:10.1016/j.ijcard.2018.05.050
Fujimoto Y., Yodogawa K., Maru Y.-j., Oka E., Hayashi H., Yamamoto T., et al. (2018). Advanced Interatrial Block Is an Electrocardiographic Marker for Recurrence of Atrial Fibrillation after Electrical Cardioversion. Int. J. Cardiol. 272, 113–117. doi:10.1016/j.ijcard.2018.07.135
Holmqvist F., Olesen M. S., Tveit A., Enger S., Tapanainen J., Jurkko R., et al. (2011). Abnormal Atrial Activation in Young Patients with Lone Atrial Fibrillation. Europace 13 (2), 188–192. doi:10.1093/europace/euq352
Istolahti T., Eranti A., Huhtala H., Lyytikäinen L.-P., Kähönen M., Lehtimäki T., et al. (2020). The Prevalence and Prognostic Significance of Interatrial Block in the General Population. Ann. Med. 52 (3-4), 63–73. doi:10.1080/07853890.2020.1731759
Jacobsson J., Carlson J., Reitan C., Borgquist R., Platonov P. G. (2020). Interatrial Block Predicts Atrial Fibrillation and Total Mortality in Patients with Cardiac Resynchronization Therapy. Cardiology 145 (11), 720–729. doi:10.1159/000509916
Kaireviciute D., Lip G. Y. H., Balakrishnan B., Uzdavinys G., Norkunas G., Kalinauskas G., et al. (2011). Intracardiac Expression of Markers of Endothelial Damage/dysfunction, Inflammation, Thrombosis, and Tissue Remodeling, and the Development of Postoperative Atrial Fibrillation. J. Thromb. Haemost. 9 (12), 2345–2352. doi:10.1111/j.1538-7836.2011.04523.x
Lacalzada‐Almeida J., Izquierdo‐Gómez M. M., García‐Niebla J., Elosua R., Jiménez‐Sosa A., Baranchuk A., et al. (2019). Advanced Interatrial Block Is a Surrogate for Left Atrial Strain Reduction Which Predicts Atrial Fibrillation and Stroke. Ann. Noninvasive Electrocardiol. 24, e12632. doi:10.1111/anec.12632
Martínez-Sellés M. (2017). Prevalence and Incidence of Interatrial Block in Global Population and in Different Clinical Situations. J. Geriatr. Cardiol. 14 (3), 158–160. doi:10.11909/j.issn.1671-5411.2017.03.006
O'Neal W. T., Kamel H., Zhang Z.-M., Chen L. Y., Alonso A., Soliman E. Z. (2016a). Advanced Interatrial Block and Ischemic Stroke: The Atherosclerosis Risk in Communities Study. Neurology 87 (4), 352–356. doi:10.1212/WNL.0000000000002888
O'Neal W. T., Zhang Z.-M., Loehr L. R., Chen L. Y., Alonso A., Soliman E. Z. (2016b). Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am. J. Cardiol. 117 (11), 1755–1759. doi:10.1016/j.amjcard.2016.03.013
Prasitlumkum N., Cheungpasitporn W., Mekritthikrai R., Thongprayoon C., Bathini T., Vallabhajosyula S., et al. (2020). Interatrial Block and its Association with an Increased Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis. J. Electrocardiol. 61, 92–98. doi:10.1016/j.jelectrocard.2020.06.011
Relander A., Hellman T., Vasankari T., Nuotio I., Airaksinen J. K. E., Kiviniemi T. (2021). Advanced Interatrial Block Predicts Ineffective Cardioversion of Atrial Fibrillation: a FinCV2 Cohort Study. Ann. Med. 53, 722–729. doi:10.1080/07853890.2021.1930139
Rubio Campal J. M., Benezet-Mazuecos J., Iglesias Bravo J. A., Sánchez Borque P., Miracle Blanco Á., de la Vieja Alarcón J. J., et al. (2018). P-wave and Interatrial Block: New Predictor for Atrial High Rate Episodes in Patients with Cardiac Implantable Electronic Devices. Pacing Clin. Electrophysiol. 41 (3), 223–228. doi:10.1111/pace.13268
Skov M. W., Ghouse J., Kühl J. T., Platonov P. G., Graff C., Fuchs A., et al. (2018). Risk Prediction of Atrial Fibrillation Based on Electrocardiographic Interatrial Block. Jaha 7 (11), e008247. doi:10.1161/JAHA.117.008247
van der Does W. F. B., Heida A., van der Does L. J. M. E., Bogers A. J. J. C., de Groot N. M. S. (2021). Conduction Disorders during Sinus Rhythm in Relation to Atrial Fibrillation Persistence. J. Clin. Med. 10 (13), 2846. doi:10.3390/jcm10132846
Wu J.-T., Fan X.-W., Yang H.-T., Yan L.-J., Xu X.-J., Wang S.-L., et al. (2018). Association between CHADS2 Score and the Development of Interatrial Block. Int. Heart J. 59 (6), 1261–1265. doi:10.1536/ihj.17-616
Keywords: atrial fibrillation, interatrial block, elderly, hypertension, stroke
Citation: Wang H, Cai L, Guo Y, Shuai L, Shi Y and Si Q (2022) Advanced Interatrial Block Predicts Recurrence of Atrial Fibrillation and Ischemic Stroke in Elderly Patients With Hypertension. Front. Physiol. 13:913454. doi: 10.3389/fphys.2022.913454
Received: 05 April 2022; Accepted: 27 May 2022;
Published: 16 June 2022.
Edited by:
Yabin Wang, People ‘s Liberation Army General Hospital, ChinaReviewed by:
Morten Salling Olesen, University of Copenhagen, DenmarkCopyright © 2022 Wang, Cai, Guo, Shuai, Shi and Si. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Shi, c2hpeTIwMTVAc2luYS5jbg==; Quanjin Si, cXVhbmppbjMwMUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.