
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 06 July 2022
Sec. Renal Physiology and Pathophysiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.909569
This article is part of the Research TopicInflammation in Diabetes and its Secondary ComplicationsView all 8 articles
Diabetic kidney disease (DKD) is a common complication of diabetes mellitus and a major cause of end-stage kidney disease (ESKD). The pathogenesis of DKD is very complex and not completely understood. Recently, accumulated evidence from in vitro and in vivo studies has demonstrated that inflammation plays an important role in the pathogenesis and the development of DKD. It has been well known that a variety of pro-inflammatory cytokines and related signaling pathways are involved in the procession of DKD. Additionally, some anti-hyperglycemic agents and mineralocorticoid receptor antagonists (MRAs) that are effective in alleviating the progression of DKD have anti-inflammatory properties, which might have beneficial effects on delaying the progression of DKD. However, there is currently a lack of systematic overviews. In this review, we focus on the novel pro-inflammatory signaling pathways in the development of DKD, including the nuclear factor kappa B (NF-κB) signaling pathway, toll-like receptors (TLRs) and myeloid differentiation primary response 88 (TLRs/MyD88) signaling pathway, adenosine 5′-monophosphate-activated protein kinase (AMPK) signaling pathways, inflammasome activation, mitochondrial DNA (mtDNA) release as well as hypoxia-inducible factor-1(HIF-1) signaling pathway. We also discuss the related anti-inflammation mechanisms of metformin, finerenone, sodium-dependent glucose transporters 2 (SGLT2) inhibitors, Dipeptidyl peptidase-4 (DPP-4) inhibitors, Glucagon-like peptide-1 (GLP-1) receptor agonist and traditional Chinese medicines (TCM).
Diabetic kidney disease (DKD), which is also known as diabetic nephropathy before, is the most common microvascular complication of diabetes mellitus (Thomas et al., 2015; Doshi and Friedman, 2017; Sun et al., 2022). The number of DKD patients has increased year by year, which has led to a huge burden on global public health. Recent studies show that 21.8%–40% of diabetic patients may progress to DKD, which is the main cause of end-stage renal disease (ESRD) (Zhang J. et al., 2020; Mottl et al., 2022). In addition, nearly 38.8% of ESRD in 2018 was attributable to DKD in America (Johansen et al., 2021). In China and India, the proportion of ESRD in DKD patients was about 1/5 (23%) and 1/3 (31.2%), respectively (Hussain et al., 2019; Zhang L. et al., 2019). On the other hand, treatment of DKD mainly includes lifestyle interventions, such as exercise, weight loss, smoking cessation and sodium restriction, and medication to control hyperglycemia as well as blood pressure (Doshi and Friedman, 2017; Anders et al., 2018). However, current treatments cannot completely delay the progression to ESRD. Therefore, it is still necessary to further explore the pathogenesis of DKD and find new therapeutic targets.
Previous studies demonstrate that many factors are implicated in the progression of DKD, such as metabolic abnormalities and hemodynamic changes caused by hyperglycemia and insulin resistance, etc (Forbes and Thorburn, 2018). It has been well-known that overproduction of reactive oxygen species (ROS), abnormal autophagy, senescence are also involved in the development of DKD (Ren et al., 2020; Sun et al., 2022). Recently, accumulated evidence suggested that inflammation plays a key role in the pathophysiology of DKD. For example, oxidative stress (Huang W. et al., 2020), abnormal autophagy (Han et al., 2021), and senescence (Xiong and Zhou, 2019) could lead to the abnormal immune-inflammation in DKD. Furthermore, several clinical trials found that non-steroidal selective mineralocorticoid receptor antagonists (MRA) could delay the progression of DKD by inhibiting inflammation (Donath, 2014; Komada and Muruve, 2019). These data strongly suggest that inflammation plays an important role in the development of DKD.
Inflammation is a biologically conserved immune defense mechanism (Cronkite and Strutt, 2018). In human, inflammation plays a dual role in various disease. Early inflammatory responses may promote tissue repair. However, severe inflammation would lead to secondary injury resulting in tissue damage or fibrosis (Nathan and Ding, 2010; Rathinam and Chan, 2018). In addition, increasing evidence indicates that in chronic non-infectious diseases, such as diabetes and its complications including DKD and atherosclerosis, persistent systemic or local inflammation plays a critical role in the progression of disease (Pichler et al., 2017; Tang and Yiu, 2020; Engelen et al., 2022; Rohm et al., 2022). A large number of infiltrated macrophages were observed in the renal biopsy specimens in patients with DKD, and the expression of inflammatory cytokines such as Interleukin 6 (IL-6), Interleukin 1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α) were increased (Navarro-Gonzalez et al., 2011; Araujo et al., 2020). In recent years, the microarray analysis has also showed that the expression of pro-inflammatory genes and fibrosis-related genes were significantly increased in patients with DKD or animal models, which was accompanied with increased levels of inflammatory cytokines in peripheral blood specimens (Wilson et al., 2019; Levin et al., 2020; Sur et al., 2021). These data further indicate that there is a notable association between inflammation and the development of DKD.
In this review, we discuss the role of some novel and key pro-inflammatory signaling pathways in the progression of DKD, such as the nuclear factor kappa B (NF-κB) signaling pathway, toll-like receptors (TLRs) and myeloid differentiation primary response 88 (TLRs/MyD88) signaling pathway, adenosine 5′-monophosphate-activated protein kinase (AMPK) signaling pathways, inflammasome activation, mitochondrial DNA (mtDNA) release and hypoxia-inducible factor-1(HIF) signaling pathway. We also introduce the role of anti-hyperglycemic drugs, non-steroidal selective MRA and traditional Chinese medicine in anti-inflammation during the treatment of DKD.
Hyperglycemia and insulin resistance are the most common pathological characteristics in patients with DKD and can lead to systemic low-grade inflammation (Saraheimo et al., 2003; Leehey, 2020). Hyperglycemia can lead to overproduction of ROS and mitochondrial DNA release, which further cause inflammation in renal parenchymal cells by different signaling pathways (Sun et al., 2022).
The transcription factor NF-κB is a key mediator of inflammation and NF-κB remains inactive while binding to the proteins of the IkappaB (IκB) family (mainly IκBα) (Sun, 2011, 2017; Zhang Q. et al., 2017). There are two pathways for the activation of NF-κB: the canonical pathway and the non-canonical pathway (Zhang S. et al., 2017). Under the stimulation of various immune receptors, such as pattern recognition receptors (PRR), TNF receptors (TNFR), T cell receptors (TCR) and B-cell receptors, intracellular IκBα is degraded and the canonical NF-κB pathway is activated (Sun, 2017). The non-canonical NF-κB activation is primarily associated with ligands of a subset of TNFR superfamily members, such as the lymphotoxin beta receptor (LTβR), B-cell activating factor receptor (BAFFR), Cluster of differentiation 40 (CD40), and Receptor activator of nuclear factor κ B (RANK) (Sun, 2017; Hou et al., 2018). NF-κB mediates the activation of downstream signaling pathways including inflammasome activation, HIF signaling pathways and AMPK signaling pathways (Rius et al., 2008; Afonina et al., 2017). In addition, the activation of NF-kB signaling pathway inhibits the anti-inflammatory pathway, Sirtuin 1 (SIRT1) signaling pathway, which is closely related to the development of non-infectious chronic diseases such as atherosclerosis and DKD (Stein et al., 2010; Kauppinen et al., 2013; Sun et al., 2021). Overall, the NF-kB signaling pathway plays a key role in the inflammatory response.
It has been observed that activated NF-κB signaling pathway is associated with inflammation and fibrosis in DKD (Mezzano et al., 2004; Schmid et al., 2006; Liu P. et al., 2014). Foresto-Neto et al. found that the activation of NF-κB signaling pathway increased the expression of the inflammatory cytokines IL-6, which preceded the kidney damage in the db/db mice (Foresto-Neto et al., 2020). Microarray analysis of renal biopsies in patients with DKD also suggested that a number of NF-κB targets were significantly upregulated (Schmid et al., 2006). These data suggest the key role of abnormal activation of NF-κB in the progression of DKD. A recent study found that delivery of Smad7 siRNA to the kidney through ultrasound microbubbles could reduce the production of IL-1β and monocyte chemoattractant protein 1 (MCP1) in the renal tissue of db/db mice by inhibiting NF-κB signaling pathway (Ka et al., 2012). Sun et al. demonstrated that dephosphorylated P65 NF-κB could attenuate renal inflammation-related damage in STZ-induced diabetic mice by downregulating the expression levels of TNF-α, IL-1β, and cyclooxygenase 2 (COX-2). (Zhang M. et al., 2019). These data further confirm that the NF-κB signaling pathway plays a critical role in the development of DKD and targeting NF-κB signaling pathway might be a potential therapeutic strategy for DKD.
Toll-like receptors (TLRs) is an evolutionarily ancient family of pattern recognition receptors and an important component of innate immunity (Tang and Yiu, 2020). Activation of TLRs in response to pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) could lead to inflammation in injured tissue (Fitzgerald and Kagan, 2020; Tang and Yiu, 2020). Myeloid differentiation factor 88 (MyD88) is a critical adaptor protein in innate immunity and central hub in inflammatory response (Xu et al., 2000; Deguine and Barton, 2014), which could respond to the activation of TLRs. The activation of TLRs-MyD88 signaling pathway might initiate the translocation of NF-κB into the nucleus, thus promoting the transcription of pro-inflammatory factors and chemokines, such as IL-1β, IL-6, MCP-1 etc. (Warner and Nunez, 2013; Fitzgerald and Kagan, 2020). These studies suggest that TLRs-MyD88 signaling pathway plays a key role in the inflammatory response.
It has been demonstrated that the high glucose (HG) could activate TLRs-MyD88 signaling pathway in the kidneys of DKD, which is the initiator of renal interstitial fibrosis (Lin and Tang, 2014; Liu and Zen, 2021). Liu et al. found that the mRNA and protein levels of TLR4 and MyD88 increased in renal tubular epithelial cells treated with HG, which was accompanied with the activation of NF-κB and increased expression of chemokine MCP-1 (Liu R. et al., 2014). Zhang et al. found that pharmacological inhibition of MyD88 by LM8, a new small-molecule inhibitor of MyD88, could alleviate the progression of renal fibrosis by inhibiting the activation of NF-kB and reducing the expression of TNF-α and IL-1β in both streptozotocin (STZ)-induced diabetic mice and db/db mice (Zhang et al., 2022). These data indicate that targeting TLRs-MyD88 might be a potential therapeutic option to reduce renal fibrosis in DKD. In addition, there are a series of Chinese herbal medicines to reduce renal fibrosis by inhibiting TLRs-MyD88 signaling-mediated inflammation (Lu et al., 2019; Arigela et al., 2021; Guo et al., 2021).
Inflammasome was first proposed by Tschopp et al., in 2002, which could modulate inflammation by the activation of caspases (Martinon et al., 2002; Schroder and Tschopp, 2010; Guo et al., 2015). There are canonical inflammasomes and non-canonical inflammasomes. The former are caspase-1-dependent inflammasomes and the non-canonical inflammasomes depend on caspase-11 in mouse and caspase-4 or caspase-5 (the homologue of caspase-11) in humans (Stowe et al., 2015; Van Opdenbosch and Lamkanfi, 2019). It is well known that the activation of the inflammasome is associated with a series of pattern recognition receptors (PRRs) in the membrane and cytoplasm, which include nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), Toll-like receptors (TLRs), C-type lectin receptors (CLRs), Rig-I-like receptors (RLRs), and absent in melanoma 2 (AIM2)-like receptors (ALRs) (Schroder and Tschopp, 2010; Strowig et al., 2012; Evavold and Kagan, 2019). This series of PRRs could rapidly respond to danger signals such as PAMPs or DAMPs to activate inflammasomes (Guo et al., 2015).
Accumulated data suggests that the inflammasome contributes to the progression of DKD (Strowig et al., 2012; Masood et al., 2015). Upregulated expression of IL-1β and Interleukin 18 (IL-18) was observed in the peripheral blood or renal tissue of DKD patients (Uzu et al., 2011; Lei et al., 2019). In addition, the activation of the NLRP3 inflammasome was not only present in the renal infiltrating macrophages (Shahzad et al., 2015; Zhang J. et al., 2021), but also in podocytes (Xiong et al., 2020; Wu et al., 2021), tubular epithelial cells (Han et al., 2019; Xie et al., 2019) and mesangial cells (Yi et al., 2017; Tung et al., 2018). Inhibition or knockout of NLRP3 has also been shown to be effective in delaying the progression of DKD. It has been reported that the NLRP3-specific small molecule inhibitor MCC950 could alleviate renal damage and fibrosis in db/db mice (Wang et al., 2017; Zhang W. et al., 2019; Ding et al., 2021) and knockdown of NLRP3 has a significant reno-protective effect in STZ-induced diabetic mice (Wu et al., 2018). In addition to the NLRP3 inflammasome, NLRP1 and NLRC4 inflammasome were also activated in the kidneys of DKD mice and associated with increased urinary albumin excretion and renal damage (Yuan et al., 2016; Soares et al., 2018). Furthermore, Luan et al. demonstrated that NLRC5 gene deficiency also reduced the inflammation and renal damage and delayed the progression of renal fibrosis in STZ-induced diabetic mice (Luan et al., 2018).
Other novel inflammasomes such as NLRP2, NLRP6, NLRP10, NLRP12, and AIM2 are also found to be involved in the regulation of inflammation and their expressions are upregulated in various kidney diseases (Yuan et al., 2016; Luan et al., 2018; Wu et al., 2018). It has been shown that the above inflammasomes participate in the pathogenesis of non-diabetic kidney diseases (Lech et al., 2010; Komada et al., 2018; Valino-Rivas et al., 2020). Valiño-Rivas et al. found that NLRP6 deficiency aggravated ischemia-reperfusion (I/R) induced acute kidney injury (Valino-Rivas et al., 2020). The AIM2 inflammasome is a sensor for endogenous Double Stranded DNA (dsDNA) that mediates canonical and non-canonical inflammasome activation. The expression level of AIM2 inflammasome is also associated with the progression of hepatitis B-related glomerulonephritis and lupus nephritis (Zhang et al., 2013; Zhen et al., 2014; Komada et al., 2018). These data suggested that the inflammasomes might also have potential role in the pathogenesis of chronic kidney disease (CKD). However, there is currently a lack of studies on investigating the effect of inflammasomes above in DKD, thus it needs to be further explored in future.
AMP-activated protein kinase (AMPK) is a serine/threonine kinase that is involved in regulating cellular metabolism by activating energy-producing pathways (Cordero et al., 2018; Lyons and Roche, 2018; Larabi et al., 2020) (Wang et al., 2018), In addition, AMPK plays a key role in regulating autophagy (Li and Chen, 2019) and other biological functions. Recent studies have also suggested that AMPK is involved in inflammation, particularly the activation of NLRP3 inflammasome (Youm et al., 2015; Xian et al., 2021). It is wellknown that the AMPK dysfunction is associated with a variety of diseases including diabetes mellitus (Chen et al., 2021) and cancers (Wang et al., 2016).
Interestingly, AMPK signaling pathway was related to the renal inflammation (Garcia and Shaw, 2017). Previous study by our group showed that DsbA-L could inhibit the activation of NLRP3 by activating AMPK phosphorylation in the kidney of DKD mice, while the expression levels of IL-18 and IL-1β were increased in that of DKD mice treated with compound C (an AMPK inhibitor) (Yang et al., 2021a). AMPK also inhibits the expression of inducible nitric oxide synthase (iNOS), thereby inhibiting the activation of NLRP3 inflammasome (Pilon et al., 2004; Liao et al., 2021). These studies confirmed the reno-protective and anti-inflammatory effects of AMPK signaling pathway in DKD. In addition, AMPK activation induced mitophagy, which could clear damaged mitochondria in cells and reduce the production of ROS and activation of NLRP3 inflammasome (Seabright et al., 2020; Yang et al., 2021b; Drake et al., 2021). What’s more, a variety of drugs for the treatments of DKD such as metformin, could also activate phosphorylation of AMPK to inhibit renal inflammation (Zhang X.-X. et al., 2020). Therefore, understanding mechanisms of AMPK signaling pathway would be critical to improving the strategy of DKD prevention and treatment.
When mitochondria are dysfunctional, the mitochondrial DNA (mtDNA) translocates to cytoplasm and initiates the inflammation through various signaling pathways (Figure 1) (Zhang et al., 2010; Fang et al., 2016; Riley and Tait, 2020). First, mtDNA, as an endogenous DAMPs, could directly activate the inflammasomes (Nakahira et al., 2011), which conclude NLRP3 (Shimada et al., 2012; Zhong et al., 2018) and AIM2 (Bae et al., 2019; Xu et al., 2021). In addition, mtDNA could initiate the cGAS-STING signaling pathway. The mtDNA is translocated to the cytoplasm and associated with the DNA receptor cyclic GMP-AMP Synthase (cGAS), causing the activation of cGAS, which catalyzes ATP and GTP into the second messenger, cyclic GMP-AMP (cGAMP) (Wu et al., 2013). Stimulator of interferon genes (STING) was localized in the endoplasmic reticulum and activated by cGAMP. STING then translocated to the Golgi apparatus to initiate interferon regulatory factor 3 (IRF3) and canonical NF-κB signaling pathway (Ishikawa and Barber, 2008; Hopfner and Hornung, 2020). Recently, it has also been found that the hypomethylated mtDNA is the ligands of TLR9 for regulating inflammation (Goulopoulou et al., 2012; Rodriguez-Nuevo et al., 2018). When cells are under stress, TLR9 is translocated to endo-lysosome from endoplasmic reticulum (Latz et al., 2004; Leifer et al., 2004; Fang et al., 2016), where hypomethylated mtDNA binds to TLR9 to activate TLR9/NF-κB and TLR9/MyD88 signaling pathways, finally resulting in increased production of TNF-α, IL-6, IL-1β, and MCP-1 (Zhang et al., 2014; Wei et al., 2015; Rodriguez-Nuevo et al., 2018). These data indicat that mtDNA release after mitochondria damage plays a key role in the inflammatory response.
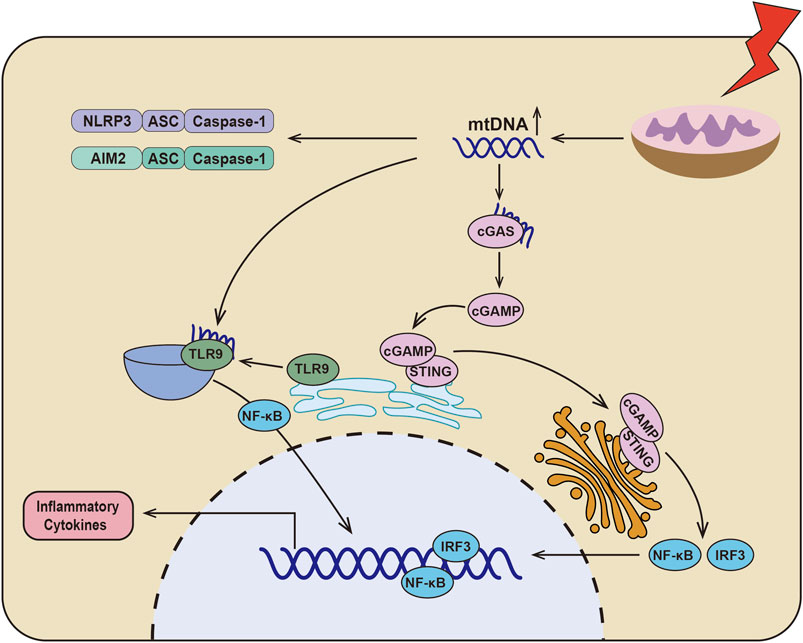
FIGURE 1. Schematic depicting the relationship between dysfunctional mitochondria and inflammation. In DKD, there are serious mitochondrial dysfunction and mtDNA leakage, which could directly activate NLRP3 inflammasome and AIM2 inflammasome. Additionally, mtDNA could be sensed by cGAS to catalyze ATP and GTP into the second messenger cyclic GMP-AMP (cGAMP). And then cGAMP binds to STING and translocation of the Golgi apparatus to initiate IRF3 and canonical NF-κB signaling pathway. Furthermore, mtDNA activates TLR9/NF-κB signaling pathway by binding to TLR9, which results in the production of inflammatory cytokines.
As we know that proximal tubular cells of the kidney are rich in mitochondria and mitochondrial dysfunction and mtDNA release have been recognized in various kidney disease (Forbes and Thorburn, 2018; Ahmad et al., 2021). A large amount of mtDNA was detected in the blood and urine of patients with DKD, which is also related to the severity of interstitial fibrosis (Malik et al., 2009; Huang Y. et al., 2020; Jin et al., 2021). The level of mtDNA was increased in primary mesangial cells treated with high glucose, which is accompanied with enhanced TLR9 activation (Czajka et al., 2015). Chung et al. confirmed that STING gene deficiency significantly reduced renal fibrosis and the expression of TNF-α, IL-1β, IL-6 and MCP1 (Chung et al., 2019). There is no doubt that the activation of the mtDNA-cGAS-STING signaling pathway is related to renal fibrosis in DKD. Meanwhile, Myakala et al. also found that sacubitril/valsartan could regulate mitochondrial function and alleviate mtDNA release to inhibit mtDNA-cGAS-STING signaling pathway, ultimately delaying the progression of DKD. This data indicated that targeting mtDNA may be a powerful solution for the treatment of DKD. In addition, it has been reported that mitophagy inhibits the activation of NLRP3 inflammasome and the secretion of inflammatory cytokines such as IL-1β in DKD (Han et al., 2021). These data suggest that mitochondrial homeostasis disruption and mtDNA release are important initiator in renal inflammation, and it might be a potential therapeutic target for the treatment of DKD.
Hypoxia-inducible factor (HIF) is conserved in biological evolution and is an important mediator of inflammation (Palazon et al., 2014; McGettrick and O'Neill, 2020). The structure of HIF contains α and β subunits, which include three α subunits (HIF-1α, HIF-2α, HIF-3α) and three β subunits (HIF-1β, HIF-2β, HIF- 3β). It has been reported that HIF-1α and HIF-2α are related to the inflammation (Keith et al., 2011). A large amount of evidence shows that the activation of HIF-1α under hypoxia regulates the transcription level of NF-κB (Palazon et al., 2014). Additionally, the fatty acid oxidation (FAO)-mediated NLRP3 inflammasome is activated when the HIF-2α is deficient in macrophage (Li et al., 2021). Of note, inflammation also results in the activation of HIF signaling pathway (Watts and Walmsley, 2019; McGettrick and O'Neill, 2020). Zhang et al. found that SARS-CoV-2 infection induced nuclear translocation of NF-κB to upregulate the mRNA level of HIF-1α via IL18/IL18R1 (Zhang L. et al., 2021). In addition, endogenous DAMPs could upregulate expression of HIF-1α and induced the production of IL-1β (Tannahill et al., 2013). It could be seen that the HIF signaling pathway and inflammation are interdependent in multiple dimensions.
Recent studies have shown that HIF signaling pathway is closely related to the development of renal interstitial fibrosis in DKD (Li et al., 2019; Jiang et al., 2020). The activation of HIF signaling indicates that the kidney is intolerant to hypoxia, which contributes to the initial adaptive response to hypoxia and tissue repair (Isoe et al., 2010; Garcia-Pastor et al., 2019). Our team has found that HIF-1α activation exerts a reno-protective effect by regulating mitochondrial dynamics through HO-1 in the early stage of DKD in STZ-induced diabetic mice (Jiang et al., 2020). Yu et al. also found that HIF-1α/Parkin/PINK1-mediated mitophagy improved mitochondrial function in renal tubular epithelial cells, whereas inhibition of HIF-1α by YC-1 (a specific inhibitor of HIF-1α) promoted inflammation and increased the production of IL-1β and IL-18 (Yu et al., 2021). These data indicate the reno-protective effect of HIF in the early stage of DKD. However, HIF signaling pathway could also promote chronic inflammation and interstitial fibrosis in CKD including the development of DKD (Wang et al., 2014; Catrina and Zheng, 2021). Co-treatment of mesangial cells with HG and HIF-1α siRNA reduce the production of inflammation and fibrosis factors such as endothelin-1, TGF-β1, CTGF and VEGF (Shao et al., 2016). It has been reported that high doses of MK-8617 (hypoxia-inducible factor-prolyl hydroxylase inhibitor) can promote the tubulointerstitial fibrosis by activating HIF-1α/KLF5/TGF-β1 axis (Li et al., 2019). These data indicate that persistent activation of HIF signaling pathway is an important mediator of the progression of renal interstitial fibrosis and is closely related to the inflammation of DKD (Figure 2).
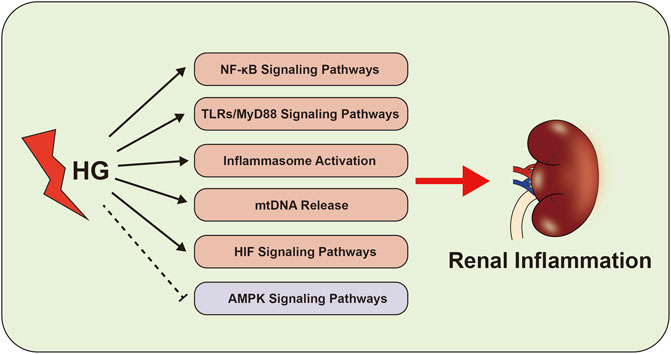
FIGURE 2. Inflammation-related signaling pathways in the development of DKD. The abnormal of NF-κB signaling pathways, TLRs-MyD88 signaling pathways, inflammasome activation, AMPK signaling pathways, mtDNA and HIF signaling pathway activate the renal inflammation, which cause to kidney damage in DKD.
In addition to be involved in the pathogenesis of DKD, inflammation has recently been shown to play a role in diabetic cardiomyopathy (Quagliariello et al., 2020). Under hyperglycemia condition, it has been demonstrated that the heart would be more susceptible to drug damage or ischemia-reperfusion injury because of hyperglycemia-induced inflammation (Peng et al., 2015; Quagliariello et al., 2020). Quagliariello et al. found that hyperglycemia could activate the NLRP3 inflammasome in cardiomyocytes and enhance the cardiotoxicity of ipilimumab (an anti-cancer drug for breast cancer) (Quagliariello et al., 2020). Additionally, diabetic cardiomyopathy might result in low myocardial reserve (Moir et al., 2006). Treatment of kaempferol to hyperglycemia-induced myocardial damage might reduce ROS production and the expression of TNF-α by inhibiting the NF-κB signaling pathway (Chen et al., 2018). These data suggest that HG leads to cardiac damage and decreases myocardial reserve through the inflammatory signaling pathways.
The treatments of DKD mainly include management of hyperglycemia, hypertension and hyperlipidemia. Actually, these drugs especially the nonsteroidal selective mineralocorticoid receptor antagonist (MRA) finerenone and some Chinese traditional medicine could alleviate the development of DKD by regulating the inflammation.
Metformin has been used for treatment of diabetes mellitus since 1950s, and is now the most widely used anti-hyperglycemic drug (Flory and Lipska, 2019). There’s a lot of evidence that metformin has not only anti-hyperglycemic properties, but also reno-protective effect. A cohort study showed that treatment of 10,426 patients with type 2 diabetes and chronic kidney disease at stage 3B with metformin significantly reduced the risk of all-cause mortality and ESRD events (Kwon et al., 2020). Similar result was observed in metformin treated DKD animal models, which also showed improved renal function and alleviated renal tubulointerstitial fibrosis (Zhang Q. et al., 2017). Recently, studies have shown that the reno-protective effect of metformin might relate to the mechanisms of anti-inflammation. Among them, AMPK signaling pathway might be the most important anti-inflammatory mechanism of metformin. Recently, Ma et al. found that metformin inhibited v-ATPase activity by inducing the binding of PEN2 to the ATP6AP1 subunit, and then activated lysosomal AMPK (Ma et al., 2022). The work of Han et al. suggested that metformin maintained mitochondrial homeostasis through AMPK-mediated mitophagy and inhibited the activation of NLRP3 inflammasome in DKD (Han et al., 2021). In addition to AMPK signaling pathway, metformin could also regulate renal inflammation by HIF signaling pathway (Takiyama et al., 2011), intestinal flora (Induri et al., 2022), and autophagy (Bharath et al., 2020).
Nonsteroidal selective MRA is a novel drug with cardio-renal protection. Compared with traditional MRAs such as spironolactone and eplerenone, nonsteroidal selective MRA has significantly improved efficacy and safety. Finerenone (also known as BAY 94-8862) is currently approved by FDA for treatment of DKD. A series of clinical studies confirmed the reno-protective effect of finerenone in DKD. The phase III FIDELIO-DKD trial has shown that finerenone significantly delayed renal failure and eGFR decline. In addition to finerenone, several nonsteroidal MRAs are currently in development (Patel et al., 2021), such as AZD9977 (phase I) (Whittaker et al., 2020), apararenone (phase II) (Wada et al., 2021) and esaxerenone (phase III) (Ito et al., 2020), which might also have reno-protective effect in DKD by regulating inflammation.
It has been well known that overactivation of MR is a key event for chronic inflammation by increasing the recruitment of neutrophil, macrophage and Th1 & Th17 cells and upregulating the expression of pro-inflammatory factors and fibrotic-related factors including TGF-α, endothelin 1, PAI-1, CTGF (Young and Rickard, 2015). There is sufficient evidence to suggest that treatment with MRA including finerenone might alleviate the progression of chronic kidney disease and eliminate the renal inflammation by reducing the expression of pro-inflammatory cytokines, such as MCP-1, TNF-α and Matrix metalloproteinase-12 (MMP-12) (Han et al., 2006; Huang et al., 2014). Martínez et al. found that finerenone regulated the activation of NF-kB signaling pathway through neutrophil gelatinase-associated lipocalin (NGAL) and inhibited the inflammation in cardiac remodeling after myocardial infarction (Martinez-Martinez et al., 2017). Bhuiyan et al. also found that esaxerenone, another nonsteroidal selective, significantly reduced the expression of MCP-1 and inflammatory cell infiltration in DKD by inhibiting ROS (Bhuiyan et al., 2019). These studies suggest that nonsteroidal selective MRA can alleviate the progression of DKD through targeting inflammatory factors.
Sodium-glucose cotransporter 2 inhibitor (SGLT2i) is a novel anti-hyperglycemic drug by inhibiting reabsorption of glucose in the kidney (Ferrannini, 2017). Several clinical trials have confirmed that SGLT2i have excellent cardio-renal benefit and low risk of hypoglycemia (Perkovic et al., 2019). Interestingly, the SGLT2i could reduce the tissue low-grade inflammation (Bonnet and Scheen, 2018; Packer, 2020). A retrospective study showed that compared to glimepiride, SGLT2i canagliflozin significantly reduced the expression levels of inflammatory markers and fibrosis markers, such as tumor necrosis factor receptor 1 (TNFR1), matrix metalloproteinase-7 (MMP7), Fibronectin (FN) (Heerspink et al., 2019) and IL-6. The anti-inflammatory mechanism of SGLT2i might relate to HIF-1α in DKD (Bessho et al., 2019). In addition, SGLT2i also can regulate mitochondrial energy metabolism through reducing mitochondrial Ca2+ concentration and activating AMPK signaling pathway (Hawley et al., 2016; Baartscheer et al., 2017). Recent study demonstrates that Canagliflozin exerts the anti-inflammatory effect by activating AMPK-Akt-eNOS pathway (Heerspink et al., 2019). Interestingly, it has also been reported that SGLT2i could inhibit inflammasome activation. Kim et al. found that compared to sulfonamide, SGLT2i significantly inhibited the activation of NLRP3 inflammasome and reduced the production of IL-1β (Kim et al., 2020). Birnbaum et al. also found that SGLT2i could inhibit the activation of NLRP3 inflammasome and reduce the mRNA expression of IL-1β, IL-6, TNF-α in BTBR ob/ob mice (Birnbaum et al., 2018). In conclusion, the reno-protective effect of SGLT2i in DKD might be related to anti-inflammation.
Glucagon-like peptide 1 (GLP-1) is an incretin hormone that stimulates insulin secretion in response to food intake (Baggio and Drucker, 2007). GLP-1 binds to the GLP-1 receptor in the pancreas and then regulates blood sugar by promoting insulin secretion. The half-life of natural GLP-1 is very short (1–2 min) because it is rapidly degraded by the ubiquitous proteolytic enzyme DPP-4 (Bell et al., 1983; Hansen et al., 1999). Recently, dipeptidyl peptidase-4 inhibitor (DDP-4i) and GLP-1 receptor agonists (GLP1RA) have been widely used in anti-hyperglycemic medications by interfering with GLP-1 expression (McGuire et al., 2019; Shaman et al., 2022). Recent studies demonstrated that DDP-4i and GLP-1RA could not only control blood sugar by regulating the secretion of insulin, but also exert anti-inflammatory and reno-protective effects in DKD (Gangadharan et al., 2016; Coppolino et al., 2018; Shaman et al., 2022). Many studies have suggested that DDP-4i could reduce the secretion of MCP-1 from renal parenchymal cells and inhibit the chemotaxis and activation of mono-macrophages (Ishibashi et al., 2011; Shah et al., 2011; Coppolino et al., 2018). DDP-4i could also inhibit the activity of NF-κB signaling pathway in renal tissue and reduce the expression of TNF-α in STZ-induced diabetic mice (Kodera et al., 2014; Gangadharan et al., 2016). Additionally, supplementation of exendin-4 (a GLP-1 analog) could reduce the secretion of pro-inflammatory factors in the kidney of diabetic mouse, including TNF-α, IL-1β, MCP-1, Intercellular Adhesion Molecule 1 (ICAM-1), etc. and alleviate the progression of DKD (Hendarto et al., 2012; Sancar-Bas et al., 2015). Furthermore, Hasan et al. found that in GLP-1R-deficient mice with 5/6 nephrectomy, linagliptin (a DDP-4 inhibitor) also significantly inhibited the expression of TGF-β, Collagen I, and Phospho-Mothers against decapentaplegic homolog 3 (pSMAD3) and reduced renal fibrosis (Hasan et al., 2019). These studies suggest that DPP-4i and GLP-1RA might have a reno-protective role in DKD through anti-inflammation, which is independent of the anti-hyperglycemia effect. The role of GLP-1 and DDP-4i on anti-inflammation have been confirmed, as well. Zobel el al. found liraglutide treatment (1.8 mg/day) could reduce the gene expression of TNF-α, IL-1β in the peripheral blood mononuclear cells (PBMC) of patients with type 2 diabetes (Zobel et al., 2021). The study of Tremblay et al. also indicated that the treatment with sitagliptin significantly reduced the plasma levels of C-reactive protein (CRP), IL-6, IL-18, secreted phospholipase-A2 (sPLA2), soluble intercellular adhesion molecule-1 and E-selectin (Tremblay et al., 2014).
Traditional Chinese medicines (TCM) such as Astragalus, Cordyceps sinensis, Tripterygium wilfordii, Fructus arctii, Panax notoginseng, Berberine, etc. are also powerful weapons against DKD (Wen et al., 2017; Zhong et al., 2019). A systematic review and meta-analysis including 29 trials suggested that TCM significantly reduced urinary protein excretion rate and urinary protein (Xiao et al., 2013). Furthermore, Chinese herbal medicines combined with ACEI or ARB have a better effect in reducing urinary protein in patients with DKD compared to ACEI or ARB therapy alone? (Xiao et al., 2013). Some studies indicate that the mechanism of TCM in DKD might be related to anti-inflammation. The study by Zhong et al. suggested that arctigenin, an extract of burdock, reduced proteinuria in diabetic mice through inhibiting NF-κB-mediated inflammation by binding to protein phosphatase 2 A (PP2A) in podocytes exposed to HG ambiance (Zhong et al., 2019). The Tripterygium wilfordii Hook. f (GTW) could inhibit the activation of NF-κB signaling pathway and reduce inflammatory cell infiltration as well as the mRNA expression of IL-1β and TNF-α in STZ-induced diabetic mice combined with unilateral nephrectomy (Wu et al., 2017). Guo et al. found that maackiain could reduce renal inflammation and the expression of MCP-1 and TNF-α by inhibiting TLRs-MyD88 signaling pathway in STZ-induced diabetic rat (Guo et al., 2021). In addition, the anti-inflammatory effect of TCM is also related to reducing ROS, regulating autophagy and inhibiting epithelial-mesenchymal transition (Li et al., 2017; Zhang et al., 2021a; Zhang et al., 2021b). All of these researches demonstrate that TCM might be a potential weapon for the treatment of DKD by anti-inflammation.
In addition to TCM, nutraceuticals derived from Chinese herbal medicines could prevent DKD, for example, boswellic acid, curcumin and quercetin (Lu et al., 2015; Lu et al., 2017; Asgharpour and Alirezaei, 2021). A systematic review and Meta-analysis revealed that curcumin supplementation significantly improved blood sugar, lipid and blood pressure control and decreased the serum creatinine in DKD patients (Jie et al., 2021). Lu et al. also found that curcumin could delay fibrosis progression in DKD mice by inhibiting the initiation of NLRP3 inflammasome and reduce the expression of IL-1β in renal tissue (Lu et al., 2017). Additionally, the present studies have revealed the protective effect of quercetin, a natural AMPK activator, in STZ-induced diabetic rat (Zhang J. et al., 2021). Lu et al. also found that treatment of quercetin could alleviate the progression of renal fibrosis in STZ-induced diabetic rat (Lu et al., 2015).
However, TCM has limitations such as low bioavailability, which greatly limits its broad application in the treatment of DKD (Liu et al., 2013; Zhang C. et al., 2019). The study of Rosso et al. suggested that hyaluronic acid (HA-NPs) and sub-micron particles (a CD44-targeted vector) were effective means to deliver bio-actives to targeted tissue in a specific and controlled manner (Rosso et al., 2013). This suggests that delivering TCM to the kidney might be a potential strategy to improve the bioavailability. Importantly, the expression of CD44 is low in the normal kidney and would specifically increase during renal damage (Zhao et al., 2019). The study of Hu et al. confirmed that CD44-targeted hyaluronic acid-curcumin prodrug could reduce the ROS in renal tubular epithelial cell. Moreover, Wang et al. have found the bioavailability and therapeutic effect of rhein, an active ingredient of TCM, were significantly improved in STZ-induced diabetic mice through kidney-targeted rhein-loaded liponanoparticles (Wang et al., 2019). This study further confirmed the feasibility of kidney-targeted therapies. Thus, it might be effective to transport TCM to the kidney through hyaluronic vector, liponanoparticle, etc. However, this conjecture needs be verified through further experiments.
Mesenchymal Stem Cells (MSCs) and extracellular vesicles (EVs) are current research hotspots and pre-clinical agents for the treatment of DKD (Tang et al., 2019; Savio-Silva et al., 2020; Shen et al., 2020). Previous studies suggested that systemic injection of MSCs improved glycemic control but showing no renal protection (Savio-Silva et al., 2020). Recent evidence suggests that proteinuria is decreased after inducing renal homing of MSC by ultrasound-targeted micro-bubble destruction (UTMD) (Marquez-Curtis and Janowska-Wieczorek, 2013). Xiang et al. found that intravenous injection of human umbilical cord-derived MSCs could reduce the expression of inflammatory factors such as IL-6, IL-1β and TNF-α in the kidney and blood, and alleviate the renal interstitial fibrosis in the STZ-induced diabetic rat (Xiang et al., 2020). These studies suggest that MSCs treatment might be beneficial to reduce the renal inflammation and alleviate the progression of DKD.
The EVs include exosomes, microvesicles and apoptotic bodies. It has been found that the EVs play an important role in the progression of DKD (Tang et al., 2019). Under the stimulation of exosomes secreted from HG-treated macrophages, the mRNA and protein levels of TNF-α, IL-1β and MCP-1 in renal mesangial cells increased. Furthermore, intravenous injection of the exosomes into C57BL/6 mice resulted in the increased expression of TNF-α, pro-IL-1β and IL-1β and renal interstitial fibrosis (Zhu et al., 2019). This data suggested that EVs secreted from damaged macrophage or renal cells could promote renal inflammation and interstitial fibrosis in DKD. Additionally, Jiang et al. have found that intravenous injection of the exosomes secreted by human urine-derived stem cells could inhibit the apoptosis of podocytes and renal tubular epithelial cells, and reduce urinary microalbumin excretion in STZ-induced diabetic rat (Jiang et al., 2016). This suggests that EVs derived from MSCs may be excellent biological agents to alleviate the progression of interstitial fibrosis in DKD.
Inflammation plays an important role in the progression of DKD (Perez-Morales et al., 2019). In this review, we have discussed and summarized the role of NF-κB signaling pathways, AMPK signaling pathways, TLRs-MyD88 signaling pathway, inflammasome activation, mtDNA release and HIFsignaling pathway in the inflammation-induced renal damage of DKD. To date, drugs directly targeting inflammation are limited in treatment of patients with DKD, but the commonly used anti-hyperglycemic drugs such as metformin, SGLT2i, DPP-4i, GLP-1AR and TCM also exhibit excellent anti-inflammatory effect. Interestingly, finerenone (a reno-protective nonsteroidal selective MRA) also exhibits strong anti-inflammatory efficacy to protect the kidney and heart in patients with DKD. This data suggested that attention should be paid to inflammation and anti-Inflammation therapy in DKD in future research.
In addition, MSCs and EVs have shown the curative effect and anti-inflammatory effect in the treatment of DKD animal models (Tang et al., 2019). However, both MSCs and EVs are mainly used for animal experiments currently and their efficacy and safety are uncertain in human body.
CBL and MY wrote the first draft of the manuscript. SLL, JFY, and CRL provided consultations on the preparation of the work. During the revision, LL and HFL participated in editing and revising the manuscript. LS contributed to manuscript revision and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81730018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
I would like to thank the National Natural Science Foundation of China for the support and thank Frontiers in Physiology for the invite.
Afonina I. S., Zhong Z., Karin M., Beyaert R. (2017). Limiting Inflammation-The Negative Regulation of NF-κB and the NLRP3 Inflammasome. Nat. Immunol. 18, 861–869. doi:10.1038/ni.3772
Ahmad A. A., Draves S. O., Rosca M. (2021). Mitochondria in Diabetic Kidney Disease. Cells 10, 2945. doi:10.3390/cells10112945
Anders H.-J., Huber T. B., Isermann B., Schiffer M. (2018). CKD in Diabetes: Diabetic Kidney Disease versus Nondiabetic Kidney Disease. Nat. Rev. Nephrol. 14, 361–377. doi:10.1038/s41581-018-0001-y
Araújo L. S., Torquato B. G. S., da Silva C. A., dos Reis Monteiro M. L. G., dos Santos Martins A. L. M., da Silva M. V., et al. (2020). Renal Expression of Cytokines and Chemokines in Diabetic Nephropathy. BMC Nephrol. 21, 308. doi:10.1186/s12882-020-01960-0
Arigela C. S., Nelli G., Gan S. H., Sirajudeen K. N. S., Krishnan K., Abdul Rahman N., et al. (2021). Bitter Gourd Honey Ameliorates Hepatic and Renal Diabetic Complications on Type 2 Diabetes Rat Models by Antioxidant, Anti-inflammatory, and Anti-apoptotic Mechanisms. Foods 10, 2872. doi:10.3390/foods10112872
Asgharpour M., Alirezaei A. (2021). Herbal Antioxidants in Dialysis Patients: A Review of Potential Mechanisms and Medical Implications. Ren. Fail. 43, 351–361. doi:10.1080/0886022X.2021.1880939
Baartscheer A., Schumacher C. A., Wüst R. C. I., Fiolet J. W. T., Stienen G. J. M., Coronel R., et al. (2017). Empagliflozin Decreases Myocardial Cytoplasmic Na+ through Inhibition of the Cardiac Na+/H+ Exchanger in Rats and Rabbits. Diabetologia 60, 568–573. doi:10.1007/s00125-016-4134-x
Bae J. H., Jo S. I., Kim S. J., Lee J. M., Jeong J. H., Kang J. S., et al. (2019). Circulating Cell-free mtDNA Contributes to AIM2 Inflammasome-Mediated Chronic Inflammation in Patients with Type 2 Diabetes. Cells 8, 328. doi:10.3390/cells8040328
Baggio L. L., Drucker D. J. (2007). Biology of Incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157. doi:10.1053/j.gastro.2007.03.054
Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C. (1983). Exon Duplication and Divergence in the Human Preproglucagon Gene. Nature 304, 368–371. doi:10.1038/304368a0
Bessho R., Takiyama Y., Takiyama T., Kitsunai H., Takeda Y., Sakagami H., et al. (2019). Hypoxia-inducible Factor-1α Is the Therapeutic Target of the SGLT2 Inhibitor for Diabetic Nephropathy. Sci. Rep. 9, 14754. doi:10.1038/s41598-019-51343-1
Bharath L. P., Agrawal M., McCambridge G., Nicholas D. A., Hasturk H., Liu J., et al. (2020). Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 32, 44–55. doi:10.1016/j.cmet.2020.04.015
Bhuiyan A. S., Rafiq K., Kobara H., Masaki T., Nakano D., Nishiyama A. (2019). Effect of a Novel Nonsteroidal Selective Mineralocorticoid Receptor Antagonist, Esaxerenone (CS-3150), on Blood Pressure and Renal Injury in High Salt-Treated Type 2 Diabetic Mice. Hypertens. Res. 42, 892–902. doi:10.1038/s41440-019-0211-0
Birnbaum Y., Bajaj M., Yang H.-C., Ye Y. (2018). Combined SGLT2 and DPP4 Inhibition Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Nephropathy in Mice with Type 2 Diabetes. Cardiovasc Drugs Ther. 32, 135–145. doi:10.1007/s10557-018-6778-x
Bonnet F., Scheen A. J. (2018). Effects of SGLT2 Inhibitors on Systemic and Tissue Low-Grade Inflammation: The Potential Contribution to Diabetes Complications and Cardiovascular Disease. Diabetes & Metabolism 44, 457–464. doi:10.1016/j.diabet.2018.09.005
Catrina S.-B., Zheng X. (2021). Hypoxia and Hypoxia-Inducible Factors in Diabetes and its Complications. Diabetologia 64, 709–716. doi:10.1007/s00125-021-05380-z
Chen M., Huang N., Liu J., Huang J., Shi J., Jin F. (2021). AMPK: A Bridge between Diabetes Mellitus and Alzheimer's Disease. Behav. Brain Res. 400, 113043. doi:10.1016/j.bbr.2020.113043
Chen X., Qian J., Wang L., Li J., Zhao Y., Han J., et al. (2018). Kaempferol Attenuates Hyperglycemia-Induced Cardiac Injuries by Inhibiting Inflammatory Responses and Oxidative Stress. Endocrine 60, 83–94. doi:10.1007/s12020-018-1525-4
Chung K. W., Dhillon P., Huang S., Sheng X., Shrestha R., Qiu C., et al. (2019). Mitochondrial Damage and Activation of the STING Pathway Lead to Renal Inflammation and Fibrosis. Cell Metab. 30, 784–799. doi:10.1016/j.cmet.2019.08.003
Coppolino G., Leporini C., Rivoli L., Ursini F., di Paola E. D., Cernaro V., et al. (2018). Exploring the Effects of DPP-4 Inhibitors on the Kidney from the Bench to Clinical Trials. Pharmacol. Res. 129, 274–294. doi:10.1016/j.phrs.2017.12.001
Cordero M. D., Williams M. R., Ryffel B. (2018). AMP-activated Protein Kinase Regulation of the NLRP3 Inflammasome during Aging. Trends Endocrinol. Metabolism 29, 8–17. doi:10.1016/j.tem.2017.10.009
Cronkite D. A., Strutt T. M. (2018). The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J. Immunol. Res. 2018, 1–14. doi:10.1155/2018/1467538
Czajka A., Ajaz S., Gnudi L., Parsade C. K., Jones P., Reid F., et al. (2015). Altered Mitochondrial Function, Mitochondrial DNA and Reduced Metabolic Flexibility in Patients with Diabetic Nephropathy. EBioMedicine 2, 499–512. doi:10.1016/j.ebiom.2015.04.002
Deguine J., Barton G. M. (2014). MyD88: A Central Player in Innate Immune Signaling. F1000Prime Rep. 6, 97. doi:10.12703/P6-97
Ding H., Li J., Li Y., Yang M., Nie S., Zhou M., et al. (2021). MicroRNA-10 Negatively Regulates Inflammation in Diabetic Kidney via Targeting Activation of the NLRP3 Inflammasome. Mol. Ther. 29, 2308–2320. doi:10.1016/j.ymthe.2021.03.012
Donath M. Y. (2014). Targeting Inflammation in the Treatment of Type 2 Diabetes: Time to Start. Nat. Rev. Drug Discov. 13, 465–476. doi:10.1038/nrd4275
Doshi S. M., Friedman A. N. (2017). Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 12, 1366–1373. doi:10.2215/CJN.11111016
Drake J. C., Wilson R. J., Laker R. C., Guan Y., Spaulding H. R., Nichenko A. S., et al. (2021). Mitochondria-localized AMPK Responds to Local Energetics and Contributes to Exercise and Energetic Stress-Induced Mitophagy. Proc. Natl. Acad. Sci. U.S.A. 118. doi:10.1073/pnas.2025932118
Engelen S. E., Robinson A. J. B., Zurke Y.-X., Monaco C. (2022). Therapeutic Strategies Targeting Inflammation and Immunity in Atherosclerosis: How to Proceed? Nat. Rev. Cardiol., 1–21. doi:10.1038/s41569-021-00668-4
Evavold C. L., Kagan J. C. (2019). Inflammasomes: Threat-Assessment Organelles of the Innate Immune System. Immunity 51, 609–624. doi:10.1016/j.immuni.2019.08.005
Fang C., Wei X., Wei Y. (2016). Mitochondrial DNA in the Regulation of Innate Immune Responses. Protein Cell 7, 11–16. doi:10.1007/s13238-015-0222-9
Ferrannini E. (2017). Sodium-Glucose Co-transporters and Their Inhibition: Clinical Physiology. Cell Metab. 26, 27–38. doi:10.1016/j.cmet.2017.04.011
Fitzgerald K. A., Kagan J. C. (2020). Toll-like Receptors and the Control of Immunity. Cell 180, 1044–1066. doi:10.1016/j.cell.2020.02.041
Forbes J. M., Thorburn D. R. (2018). Mitochondrial Dysfunction in Diabetic Kidney Disease. Nat. Rev. Nephrol. 14, 291–312. doi:10.1038/nrneph.2018.9
Foresto-Neto O., Albino A. H., Arias S. C. A., Faustino V. D., Zambom F. F. F., Cenedeze M. A., et al. (2020). NF-κB System Is Chronically Activated and Promotes Glomerular Injury in Experimental Type 1 Diabetic Kidney Disease. Front. Physiol. 11, 84. doi:10.3389/fphys.2020.00084
Gangadharan Komala M., Gross S., Zaky A., Pollock C., Panchapakesan U. (2016). Saxagliptin Reduces Renal Tubulointerstitial Inflammation, Hypertrophy and Fibrosis in Diabetes. Nephrology 21, 423–431. doi:10.1111/nep.12618
Garcia D., Shaw R. J. (2017). AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 66, 789–800. doi:10.1016/j.molcel.2017.05.032
García-Pastor C., Benito-Martínez S., Moreno-Manzano V., Fernández-Martínez A. B., Lucio-Cazaña F. J. (2019). Mechanism and Consequences of the Impaired Hif-1α Response to Hypoxia in Human Proximal Tubular HK-2 Cells Exposed to High Glucose. Sci. Rep. 9, 15868. doi:10.1038/s41598-019-52310-6
Goulopoulou S., Matsumoto T., Bomfim G. F., Webb R. C. (2012). Toll-like Receptor 9 Activation: A Novel Mechanism Linking Placenta-Derived Mitochondrial DNA and Vascular Dysfunction in Pre-eclampsia. Clin. Sci. (Lond) 123, 429–435. doi:10.1042/CS20120130
Guo H., Callaway J. B., Ting J. P.-Y. (2015). Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 21, 677–687. doi:10.1038/nm.3893
Guo J., Li J., Wei H., Liang Z. (2021). Maackiain Protects the Kidneys of Type 2 Diabetic Rats via Modulating the Nrf2/HO-1 and TLR4/NF-κB/Caspase-3 Pathways. Dddt 15, 4339–4358. doi:10.2147/DDDT.S326975
Han S.-Y., Kim C.-H., Kim H.-S., Jee Y.-H., Song H.-K., Lee M.-H., et al. (2006). Spironolactone Prevents Diabetic Nephropathy through an Anti-inflammatory Mechanism in Type 2 Diabetic Rats. J. Am. Soc. Nephrol. 17, 1362–1372. doi:10.1681/ASN.2005111196
Han Y.-c., Tang S.-q., Liu Y.-t., Li A.-m., Zhan M., Yang M., et al. (2021). AMPK Agonist Alleviate Renal Tubulointerstitial Fibrosis via Activating Mitophagy in High Fat and Streptozotocin Induced Diabetic Mice. Cell Death Dis. 12, 925. doi:10.1038/s41419-021-04184-8
Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X., et al. (20192018). Corrigendum to 'Reactive Oxygen Species Promote Tubular Injury in Diabetic Nephropathy: The Role of the Mitochondrial Ros-Txnip-Nlrp3 Biological axis. Redox Biol. 16, 32–46. doi:10.1016/j.redox.2019.101216
Hansen L., Deacon C. F., Ørskov C., Holst J. J. (1999). Glucagon-Like Peptide-1-(7-36)Amide Is Transformed to Glucagon-like Peptide-1-(9-36)Amide by Dipeptidyl Peptidase IV in the Capillaries Supplying the L Cells of the Porcine Intestine1. Endocrinology 140, 5356–5363. doi:10.1210/endo.140.11.7143
Hasan A. A., von Websky K., Reichetzeder C., Tsuprykov O., Gaballa M. M. S., Guo J., et al. (2019). Mechanisms of GLP-1 Receptor-independent Renoprotective Effects of the Dipeptidyl Peptidase Type 4 Inhibitor Linagliptin in GLP-1 Receptor Knockout Mice with 5/6 Nephrectomy. Kidney Int. 95, 1373–1388. doi:10.1016/j.kint.2019.01.010
Hawley S. A., Ford R. J., Smith B. K., Gowans G. J., Mancini S. J., Pitt R. D., et al. (2016). The na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes 65, 2784–2794. doi:10.2337/db16-0058
Heerspink H. J. L., Perco P., Mulder S., Leierer J., Hansen M. K., Heinzel A., et al. (2019). Canagliflozin Reduces Inflammation and Fibrosis Biomarkers: A Potential Mechanism of Action for Beneficial Effects of SGLT2 Inhibitors in Diabetic Kidney Disease. Diabetologia 62, 1154–1166. doi:10.1007/s00125-019-4859-4
Hendarto H., Inoguchi T., Maeda Y., Ikeda N., Zheng J., Takei R., et al. (2012). GLP-1 Analog Liraglutide Protects against Oxidative Stress and Albuminuria in Streptozotocin-Induced Diabetic Rats via Protein Kinase A-Mediated Inhibition of Renal NAD(P)H Oxidases. Metabolism 61, 1422–1434. doi:10.1016/j.metabol.2012.03.002
Hopfner K.-P., Hornung V. (2020). Molecular Mechanisms and Cellular Functions of cGAS-STING Signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521. doi:10.1038/s41580-020-0244-x
Hou Y., Liang H., Rao E., Zheng W., Huang X., Deng L., et al. (2018). Non-canonical NF-κB Antagonizes STING Sensor-Mediated DNA Sensing in Radiotherapy. Immunity 49, 490–503. doi:10.1016/j.immuni.2018.07.008
Huang L. L., Nikolic-Paterson D. J., Han Y., Ozols E., Ma F. Y., Young M. J., et al. (2014). Myeloid Mineralocorticoid Receptor Activation Contributes to Progressive Kidney Disease. J. Am. Soc. Nephrol. 25, 2231–2240. doi:10.1681/ASN.2012111094
Huang W., Man Y., Gao C., Zhou L., Gu J., Xu H., et al. (2020). Short-Chain Fatty Acids Ameliorate Diabetic Nephropathy via GPR43-Mediated Inhibition of Oxidative Stress and NF-κB Signaling. Oxidative Med. Cell. Longev. 2020, 1–21. doi:10.1155/2020/4074832
Huang Y., Chi J., Wei F., Zhou Y., Cao Y., Wang Y. (2020). Mitochondrial DNA: A New Predictor of Diabetic Kidney Disease. Int. J. Endocrinol. 2020, 1–7. doi:10.1155/2020/3650937
Hussain S., Habib A., Najmi A. K. (2019). Limited Knowledge of Chronic Kidney Disease Among Type 2 Diabetes Mellitus Patients in India. Ijerph 16, 1443. doi:10.3390/ijerph16081443
Induri S. N. R., Kansara P., Thomas S. C., Xu F., Saxena D., Li X. (2022). The Gut Microbiome, Metformin, and Aging. Annu. Rev. Pharmacol. Toxicol. 62, 85–108. doi:10.1146/annurev-pharmtox-051920-093829
Ishibashi Y., Nishino Y., Matsui T., Takeuchi M., Yamagishi S.-i. (2011). Glucagon-like Peptide-1 Suppresses Advanced Glycation End Product-Induced Monocyte Chemoattractant Protein-1 Expression in Mesangial Cells by Reducing Advanced Glycation End Product Receptor Level. Metabolism 60, 1271–1277. doi:10.1016/j.metabol.2011.01.010
Ishikawa H., Barber G. N. (2008). STING Is an Endoplasmic Reticulum Adaptor that Facilitates Innate Immune Signalling. Nature 455, 674–678. doi:10.1038/nature07317
Isoe T., Makino Y., Mizumoto K., Sakagami H., Fujita Y., Honjo J., et al. (2010). High Glucose Activates HIF-1-Mediated Signal Transduction in Glomerular Mesangial Cells through a Carbohydrate Response Element Binding Protein. Kidney Int. 78, 48–59. doi:10.1038/ki.2010.99
Ito S., Kashihara N., Shikata K., Nangaku M., Wada T., Okuda Y., et al. (2020). Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN). Clin. J. Am. Soc. Nephrol. 15, 1715–1727. doi:10.2215/CJN.06870520
Jiang N., Zhao H., Han Y., Li L., Xiong S., Zeng L., et al. (2020). HIF‐1α Ameliorates Tubular Injury in Diabetic Nephropathy via HO‐1-Mediated Control of Mitochondrial Dynamics. Cell Prolif. 53, e12909. doi:10.1111/cpr.12909
Jiang Z.-z., Liu Y.-m., Niu X., Yin J.-y., Hu B., Guo S.-c., et al. (2016). Exosomes Secreted by Human Urine-Derived Stem Cells Could Prevent Kidney Complications from Type I Diabetes in Rats. Stem Cell Res. Ther. 7, 24. doi:10.1186/s13287-016-0287-2
Jie Z., Chao M., Jun A., Wei S., LiFeng M. (2021). Effect of Curcumin on Diabetic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Clinical Trials. Evidence-Based Complementary Altern. Med. 2021, 1–14. doi:10.1155/2021/6109406
Jin L., Yu B., Armando I., Han F. (2021). Mitochondrial DNA-Mediated Inflammation in Acute Kidney Injury and Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2021, 1–12. doi:10.1155/2021/9985603
Johansen K. L., Chertow G. M., Foley R. N., Gilbertson D. T., Herzog C. A., Ishani A., et al. (2021). US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the united states. Am. J. Kidney Dis. 77, A7–A8. doi:10.1053/j.ajkd.2021.01.002
Ka S. M., Yeh Y. C., Huang X. R., Chao T. K., Hung Y. J., Yu C. P., et al. (2012). Kidney-targeting Smad7 Gene Transfer Inhibits Renal TGF-β/MAD Homologue (SMAD) and Nuclear Factor κB (NF-κB) Signalling Pathways, and Improves Diabetic Nephropathy in Mice. Diabetologia 55, 509–519. doi:10.1007/s00125-011-2364-5
Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. (2013). Antagonistic Crosstalk between NF-κB and SIRT1 in the Regulation of Inflammation and Metabolic Disorders. Cell. Signal. 25, 1939–1948. doi:10.1016/j.cellsig.2013.06.007
Keith B., Johnson R. S., Simon M. C. (2011). HIF1α and HIF2α: Sibling Rivalry in Hypoxic Tumour Growth and Progression. Nat. Rev. Cancer 12, 9–22. doi:10.1038/nrc3183
Kim S. R., Lee S.-G., Kim S. H., Kim J. H., Choi E., Cho W., et al. (2020). SGLT2 Inhibition Modulates NLRP3 Inflammasome Activity via Ketones and Insulin in Diabetes with Cardiovascular Disease. Nat. Commun. 11, 2127. doi:10.1038/s41467-020-15983-6
Kodera R., Shikata K., Takatsuka T., Oda K., Miyamoto S., Kajitani N., et al. (2014). Dipeptidyl Peptidase-4 Inhibitor Ameliorates Early Renal Injury through its Anti-inflammatory Action in a Rat Model of Type 1 Diabetes. Biochem. Biophysical Res. Commun. 443, 828–833. doi:10.1016/j.bbrc.2013.12.049
Komada T., Chung H., Lau A., Platnich J. M., Beck P. L., Benediktsson H., et al. (2018). Macrophage Uptake of Necrotic Cell DNA Activates the AIM2 Inflammasome to Regulate a Proinflammatory Phenotype in CKD. J. Am. Soc. Nephrol. 29, 1165–1181. doi:10.1681/ASN.2017080863
Komada T., Muruve D. A. (2019). The Role of Inflammasomes in Kidney Disease. Nat. Rev. Nephrol. 15, 501–520. doi:10.1038/s41581-019-0158-z
Kwon S., Kim Y. C., Park J. Y., Lee J., An J. N., Kim C. T., et al. (2020). The Long-Term Effects of Metformin on Patients with Type 2 Diabetic Kidney Disease. Diabetes Care 43, 948–955. doi:10.2337/dc19-0936
Larabi A., Barnich N., Nguyen H. T. T. (2020). New Insights into the Interplay between Autophagy, Gut Microbiota and Inflammatory Responses in IBD. Autophagy 16, 38–51. doi:10.1080/15548627.2019.1635384
Latz E., Schoenemeyer A., Visintin A., Fitzgerald K. A., Monks B. G., Knetter C. F., et al. (2004). TLR9 Signals after Translocating from the ER to CpG DNA in the Lysosome. Nat. Immunol. 5, 190–198. doi:10.1038/ni1028
Lech M., Avila-Ferrufino A., Skuginna V., Susanti H. E., Anders H.-J. (2010). Quantitative Expression of RIG-like Helicase, NOD-like Receptor and Inflammasome-Related mRNAs in Humans and Mice. Int. Immunol. 22, 717–728. doi:10.1093/intimm/dxq058
Leehey D. J. (2020). Targeting Inflammation in Diabetic Kidney Disease: Is There a Role for Pentoxifylline? Kidney360 1, 292–299. doi:10.34067/KID.0001252019
Lei Y., Devarapu S. K., Motrapu M., Cohen C. D., Lindenmeyer M. T., Moll S., et al. (2019). Interleukin-1β Inhibition for Chronic Kidney Disease in Obese Mice with Type 2 Diabetes. Front. Immunol. 10, 1223. doi:10.3389/fimmu.2019.01223
Leifer C. A., Kennedy M. N., Mazzoni A., Lee C., Kruhlak M. J., Segal D. M. (2004). TLR9 Is Localized in the Endoplasmic Reticulum Prior to Stimulation. J. Immunol. 173, 1179–1183. doi:10.4049/jimmunol.173.2.1179
Levin A., Reznichenko A., Witasp A., Liu P., Greasley P. J., Sorrentino A., et al. (2020). Novel Insights into the Disease Transcriptome of Human Diabetic Glomeruli and Tubulointerstitium. Nephrol. Dial. Transpl. 35, 2059–2072. doi:10.1093/ndt/gfaa121
Li X.-y., Wang S.-s., Han Z., Han F., Chang Y.-p., Yang Y., et al. (2017). Triptolide Restores Autophagy to Alleviate Diabetic Renal Fibrosis through the miR-141-3p/PTEN/Akt/mTOR Pathway. Mol. Ther. - Nucleic Acids 9, 48–56. doi:10.1016/j.omtn.2017.08.011
Li X., Zhang X., Xia J., Zhang L., Chen B., Lian G., et al. (2021). Macrophage HIF-2α Suppresses NLRP3 Inflammasome Activation and Alleviates Insulin Resistance. Cell Rep. 36, 109607. doi:10.1016/j.celrep.2021.109607
Li Y., Chen Y. (2019). AMPK and Autophagy. Adv. Exp. Med. Biol. 1206, 85–108. doi:10.1007/978-981-15-0602-4_4
Li Z. L., Lv L. L., Wang B., Tang T. T., Feng Y., Cao J. Y., et al. (2019). The Profibrotic Effects of MK‐8617 on Tubulointerstitial Fibrosis Mediated by the KLF5 Regulating Pathway. FASEB J. 33, 12630–12643. doi:10.1096/fj.201901087RR
Liao K., Lv D.-Y., Yu H.-L., Chen H., Luo S.-X. (2021). iNOS Regulates Activation of the NLRP3 Inflammasome through the sGC/cGMP/PKG/TACE/TNF-α axis in Response to Cigarette Smoke Resulting in Aortic Endothelial Pyroptosis and Vascular Dysfunction. Int. Immunopharmacol. 101, 108334. doi:10.1016/j.intimp.2021.108334
Lin M., Tang S. C. W. (2014). Toll-like Receptors: Sensing and Reacting to Diabetic Injury in the Kidney. Nephrol. Dial. Transplant. 29, 746–754. doi:10.1093/ndt/gft446
Liu J.-Y., Lee K.-F., Sze C.-W., Tong Y., Tang S. C.-W., Ng T.-B., et al. (2013). Intestinal Absorption and Bioavailability of Traditional Chinese Medicines: A Review of Recent Experimental Progress and Implication for Quality Control. J. Pharm. Pharmacol. 65, 621–633. doi:10.1111/j.2042-7158.2012.01608.x
Liu M., Zen K. (2021). Toll-Like Receptors Regulate the Development and Progression of Renal Diseases. Kidney Dis. 7, 14–23. doi:10.1159/000511947
Liu P., Li F. A., Qiu M., He L. (2014). Expression and Cellular Distribution of TLR4, MyD88, and NF-κB in Diabetic Renal Tubulointerstitial Fibrosis, In Vitro and In Vivo. Diabetes Res. Clin. Pract. 105, 206–216. doi:10.1016/j.diabres.2014.04.020
Liu R., Zhong Y., Li X., Chen H., Jim B., Zhou M.-M., et al. (2014). Role of Transcription Factor Acetylation in Diabetic Kidney Disease. Diabetes 63, 2440–2453. doi:10.2337/db13-1810
Lu M., Yin N., Liu W., Cui X., Chen S., Wang E. (2017). Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signaling. BioMed Res. Int. 2017, 1–10. doi:10.1155/2017/1516985
Lu Q., Ji X.-J., Zhou Y.-X., Yao X.-Q., Liu Y.-Q., Zhang F., et al. (2015). Quercetin Inhibits the mTORC1/p70S6K Signaling-Mediated Renal Tubular Epithelial-Mesenchymal Transition and Renal Fibrosis in Diabetic Nephropathy. Pharmacol. Res. 99, 237–247. doi:10.1016/j.phrs.2015.06.006
Lu S., Zhang H., Wei X., Huang X., Chen L., Jiang L., et al. (2019). 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione Isolated from Averrhoa Carambola L. Root Ameliorates Diabetic Nephropathy by Inhibiting the TLR4/MyD88/NF-κB Pathway. Dmso Vol. 12, 1355–1363. doi:10.2147/DMSO.S209436
Luan P., Zhuang J., Zou J., Li H., Shuai P., Xu X., et al. (2018). NLRC5 Deficiency Ameliorates Diabetic Nephropathy through Alleviating Inflammation. FASEB J. 32, 1070–1084. doi:10.1096/fj.201700511RR
Lyons C., Roche H. (2018). Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Ijms 19, 3092. doi:10.3390/ijms19103092
Ma T., Tian X., Zhang B., Li M., Wang Y., Yang C., et al. (2022). Low-dose Metformin Targets the Lysosomal AMPK Pathway through PEN2. Nature 603, 159–165. doi:10.1038/s41586-022-04431-8
Malik A. N., Shahni R., Iqbal M. M. (2009). Increased Peripheral Blood Mitochondrial DNA in Type 2 Diabetic Patients with Nephropathy. Diabetes Res. Clin. Pract. 86, e22–e24. doi:10.1016/j.diabres.2009.07.002
Marquez-Curtis L. A., Janowska-Wieczorek A. (2013). Enhancing the Migration Ability of Mesenchymal Stromal Cells by Targeting the SDF-1/CXCR4 axis. BioMed Res. Int. 2013, 1–15. doi:10.1155/2013/561098
Martínez-Martínez E., Buonafine M., Boukhalfa I., Ibarrola J., Fernández-Celis A., Kolkhof P., et al. (2017). Aldosterone Target NGAL (Neutrophil Gelatinase-Associated Lipocalin) Is Involved in Cardiac Remodeling after Myocardial Infarction through NFκB Pathway. Hypertension 70, 1148–1156. doi:10.1161/HYPERTENSIONAHA.117.09791
Martinon F., Burns K., Tschopp J. (2002). The Inflammasome. Mol. Cell 10, 417–426. doi:10.1016/s1097-2765(02)00599-3
Masood H., Che R., Zhang A. (2015). Inflammasomes in the Pathophysiology of Kidney Diseases. Kidney Dis. 1, 187–193. doi:10.1159/000438843
McGettrick A. F., O’Neill L. A. J. (2020). The Role of HIF in Immunity and Inflammation. Cell Metab. 32, 524–536. doi:10.1016/j.cmet.2020.08.002
McGuire D. K., Alexander J. H., Johansen O. E., Perkovic V., Rosenstock J., Cooper M. E., et al. (2019). Linagliptin Effects on Heart Failure and Related Outcomes in Individuals with Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation 139, 351–361. doi:10.1161/CIRCULATIONAHA.118.038352
Mezzano S., Aros C., Droguett A., Burgos M. E., Ardiles L., Flores C., et al. (2004). NF- B Activation and Overexpression of Regulated Genes in Human Diabetic Nephropathy. Nephrol. Dial. Transplant. 19, 2505–2512. doi:10.1093/ndt/gfh207
Moir S., Hanekom L., Fang Z. Y., Haluska B., Wong C., et al. (2006). Relationship between Myocardial Perfusion and Dysfunction in Diabetic Cardiomyopathy: A Study of Quantitative Contrast Echocardiography and Strain Rate Imaging. Heart 92, 1414–1419. doi:10.1136/hrt.2005.079350
Mottl A. K., Alicic R., Argyropoulos C., Brosius F. C., Mauer M., Molitch M., et al. (2022). KDOQI US Commentary on the KDIGO 2020 Clinical Practice Guideline for Diabetes Management in CKD. Am. J. Kidney Dis. 79, 457–479. doi:10.1053/j.ajkd.2021.09.010
Nakahira K., Haspel J. A., Rathinam V. A. K., Lee S.-J., Dolinay T., Lam H. C., et al. (2011). Autophagy Proteins Regulate Innate Immune Responses by Inhibiting the Release of Mitochondrial DNA Mediated by the NALP3 Inflammasome. Nat. Immunol. 12, 222–230. doi:10.1038/ni.1980
Nathan C., Ding A. (2010). Nonresolving Inflammation. Cell 140, 871–882. doi:10.1016/j.cell.2010.02.029
Navarro-González J. F., Mora-Fernández C., de Fuentes M. M., García-Pérez J. (2011). Inflammatory Molecules and Pathways in the Pathogenesis of Diabetic Nephropathy. Nat. Rev. Nephrol. 7, 327–340. doi:10.1038/nrneph.2011.51
Packer M. (2020). Role of Impaired Nutrient and Oxygen Deprivation Signaling and Deficient Autophagic Flux in Diabetic CKD Development: Implications for Understanding the Effects of Sodium-Glucose Cotransporter 2-Inhibitors. J. Am. Soc. Nephrol. 31, 907–919. doi:10.1681/ASN.2020010010
Palazon A., Goldrath A. W., Nizet V., Johnson R. S. (2014). HIF Transcription Factors, Inflammation, and Immunity. Immunity 41, 518–528. doi:10.1016/j.immuni.2014.09.008
Patel V., Joharapurkar A., Jain M. (2021). Role of Mineralocorticoid Receptor Antagonists in Kidney Diseases. Drug Dev. Res. 82, 341–363. doi:10.1002/ddr.21760
Peng J., Li X., Zhang D., Chen J.-K., Su Y., Smith S. B., et al. (2015). Hyperglycemia, P53, and Mitochondrial Pathway of Apoptosis Are Involved in the Susceptibility of Diabetic Models to Ischemic Acute Kidney Injury. Kidney Int. 87, 137–150. doi:10.1038/ki.2014.226
Pérez-Morales R. E., del Pino M. D., Valdivielso J. M., Ortiz A., Mora-Fernández C., Navarro-González J. F. (2019). Inflammation in Diabetic Kidney Disease. Nephron 143, 12–16. doi:10.1159/000493278
Perkovic V., Jardine M. J., Neal B., Bompoint S., Heerspink H. J. L., Charytan D. M., et al. (2019). Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 380, 2295–2306. doi:10.1056/NEJMoa1811744
Pichler R., Afkarian M., Dieter B. P., Tuttle K. R. (2017). Immunity and Inflammation in Diabetic Kidney Disease: Translating Mechanisms to Biomarkers and Treatment Targets. Am. J. Physiology-Renal Physiology 312, F716–F731. doi:10.1152/ajprenal.00314.2016
Pilon G., Dallaire P., Marette A. (2004). Inhibition of Inducible Nitric-Oxide Synthase by Activators of AMP-Activated Protein Kinase. J. Biol. Chem. 279, 20767–20774. doi:10.1074/jbc.M401390200
Quagliariello V., De Laurentiis M., Cocco S., Rea G., Bonelli A., Caronna A., et al. (2020). NLRP3 as Putative Marker of Ipilimumab-Induced Cardiotoxicity in the Presence of Hyperglycemia in Estrogen-Responsive and Triple-Negative Breast Cancer Cells. Ijms 21, 7802. doi:10.3390/ijms21207802
Rathinam V. A. K., Chan F. K.-M. (2018). Inflammasome, Inflammation, and Tissue Homeostasis. Trends Mol. Med. 24, 304–318. doi:10.1016/j.molmed.2018.01.004
Riley J. S., Tait S. W. (2020). Mitochondrial DNA in Inflammation and Immunity. EMBO Rep. 21, e49799. doi:10.15252/embr.201949799
Rius J., Guma M., Schachtrup C., Akassoglou K., Zinkernagel A. S., Nizet V., et al. (2008). NF-κB Links Innate Immunity to the Hypoxic Response through Transcriptional Regulation of HIF-1α. Nature 453, 807–811. doi:10.1038/nature06905
Rodríguez‐Nuevo A., Díaz‐Ramos A., Noguera E., Díaz‐Sáez F., Duran X., Muñoz J. P., et al. (2018). Mitochondrial DNA and TLR9 Drive Muscle Inflammation upon Opa1 Deficiency. Embo J. 37. doi:10.15252/embj.201796553
Rohm T. V., Meier D. T., Olefsky J. M., Donath M. Y. (2022). Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 55, 31–55. doi:10.1016/j.immuni.2021.12.013
Rosso F., Quagliariello V., Tortora C., Di Lazzaro A., Barbarisi A., Iaffaioli R. V. (2013). Cross-linked Hyaluronic Acid Sub-micron Particles: In Vitro and In Vivo Biodistribution Study in Cancer Xenograft Model. J. Mater Sci. Mater Med. 24, 1473–1481. doi:10.1007/s10856-013-4895-4
Sancar-Bas S., Gezginci-Oktayoglu S., Bolkent S. (2015). Exendin-4 Attenuates Renal Tubular Injury by Decreasing Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Mice. Growth factors. 33, 419–429. doi:10.3109/08977194.2015.1125349
Saraheimo M., Teppo A.-M., Forsblom C., Fagerudd J., Groop P.-H. (2003). Diabetic Nephropathy Is Associated with Low-Grade Inflammation in Type 1 Diabetic Patients. Diabetologia 46, 1402–1407. doi:10.1007/s00125-003-1194-5
Sávio-Silva C., Beyerstedt S., Soinski-Sousa P. E., Casaro E. B., Balby-Rocha M. T. A., Simplício-Filho A., et al. (2020). Mesenchymal Stem Cell Therapy for Diabetic Kidney Disease: A Review of the Studies Using Syngeneic, Autologous, Allogeneic, and Xenogeneic Cells. Stem Cells Int. 2020, 1–28. doi:10.1155/2020/8833725
Schmid H., Boucherot A., Yasuda Y., Henger A., Brunner B., Eichinger F., et al. (2006). Modular Activation of Nuclear Factor-κB Transcriptional Programs in Human Diabetic Nephropathy. Diabetes 55, 2993–3003. doi:10.2337/db06-0477
Schroder K., Tschopp J. (2010). The Inflammasomes. Cell 140, 821–832. doi:10.1016/j.cell.2010.01.040
Seabright A. P., Fine N. H. F., Barlow J. P., Lord S. O., Musa I., Gray A., et al. (2020). AMPK Activation Induces Mitophagy and Promotes Mitochondrial Fission while Activating TBK1 in a PINK1‐Parkin Independent Manner. FASEB J. 34, 6284–6301. doi:10.1096/fj.201903051R
Shah Z., Kampfrath T., Deiuliis J. A., Zhong J., Pineda C., Ying Z., et al. (2011). Long-term Dipeptidyl-Peptidase 4 Inhibition Reduces Atherosclerosis and Inflammation via Effects on Monocyte Recruitment and Chemotaxis. Circulation 124, 2338–2349. doi:10.1161/CIRCULATIONAHA.111.041418
Shahzad K., Bock F., Dong W., Wang H., Kopf S., Kohli S., et al. (2015). Nlrp3-inflammasome Activation in Non-myeloid-derived Cells Aggravates Diabetic Nephropathy. Kidney Int. 87, 74–84. doi:10.1038/ki.2014.271
Shaman A. M., Bain S. C., Bakris G. L., Buse J. B., Idorn T., Mahaffey K. W., et al. (2022). Effect of the Glucagon-like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients with Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation 145, 575–585. doi:10.1161/CIRCULATIONAHA.121.055459
Shao Y., Lv C., Wu C., Zhou Y., Wang Q. (2016). Mir-217 Promotes Inflammation and Fibrosis in High Glucose Cultured Rat Glomerular Mesangial Cells via Sirt1/HIF-1α Signaling Pathway. Diabetes Metab. Res. Rev. 32, 534–543. doi:10.1002/dmrr.2788
Shen A.-R., Zhong X., Tang T.-T., Wang C., Jing J., Liu B.-C., et al. (2020). Integrin, Exosome and Kidney Disease. Front. Physiol. 11, 627800. doi:10.3389/fphys.2020.627800
Shimada K., Crother T. R., Karlin J., Dagvadorj J., Chiba N., Chen S., et al. (2012). Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 36, 401–414. doi:10.1016/j.immuni.2012.01.009
Soares J. L. S., Fernandes F. P., Patente T. A., Monteiro M. B., Parisi M. C., Giannella-Neto D., et al. (2018). Gain-of-function Variants in NLRP1 Protect against the Development of Diabetic Kidney Disease: NLRP1 Inflammasome Role in Metabolic Stress Sensing? Clin. Immunol. 187, 46–49. doi:10.1016/j.clim.2017.10.003
Stein S., Lohmann C., Schäfer N., Hofmann J., Rohrer L., Besler C., et al. (2010). SIRT1 Decreases Lox-1-Mediated Foam Cell Formation in Atherogenesis. Eur. Heart J. 31, 2301–2309. doi:10.1093/eurheartj/ehq107
Stowe I., Lee B., Kayagaki N. (2015). Caspase-11: Arming the Guards against Bacterial Infection. Immunol. Rev. 265, 75–84. doi:10.1111/imr.12292
Strowig T., Henao-Mejia J., Elinav E., Flavell R. (2012). Inflammasomes in Health and Disease. Nature 481, 278–286. doi:10.1038/nature10759
Sun H.-J., Xiong S.-P., Cao X., Cao L., Zhu M.-Y., Wu Z.-Y., et al. (2021). Polysulfide-mediated Sulfhydration of SIRT1 Prevents Diabetic Nephropathy by Suppressing Phosphorylation and Acetylation of P65 NF-κB and STAT3. Redox Biol. 38, 101813. doi:10.1016/j.redox.2020.101813
Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B. B., et al. (2022). IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi:10.1016/j.diabres.2021.109119
Sun S.-C. (2011). Non-canonical NF-κB Signaling Pathway. Cell Res. 21, 71–85. doi:10.1038/cr.2010.177
Sun S.-C. (2017). The Non-canonical NF-κB Pathway in Immunity and Inflammation. Nat. Rev. Immunol. 17, 545–558. doi:10.1038/nri.2017.52
Sur S., Nguyen M., Boada P., Sigdel T. K., Sollinger H., Sarwal M. M. (2021). FcER1: A Novel Molecule Implicated in the Progression of Human Diabetic Kidney Disease. Front. Immunol. 12, 769972. doi:10.3389/fimmu.2021.769972
Takiyama Y., Harumi T., Watanabe J., Fujita Y., Honjo J., Shimizu N., et al. (2011). Tubular Injury in a Rat Model of Type 2 Diabetes Is Prevented by Metformin. Diabetes 60, 981–992. doi:10.2337/db10-0655
Tang S. C. W., Yiu W. H. (2020). Innate Immunity in Diabetic Kidney Disease. Nat. Rev. Nephrol. 16, 206–222. doi:10.1038/s41581-019-0234-4
Tang T.-T., Lv L.-L., Lan H.-Y., Liu B.-C. (2019). Extracellular Vesicles: Opportunities and Challenges for the Treatment of Renal Diseases. Front. Physiol. 10, 226. doi:10.3389/fphys.2019.00226
Tannahill G. M., Curtis A. M., Adamik J., Palsson-McDermott E. M., McGettrick A. F., Goel G., et al. (2013). Succinate Is an Inflammatory Signal that Induces IL-1β through HIF-1α. Nature 496, 238–242. doi:10.1038/nature11986
Thomas M. C., Brownlee M., Susztak K., Sharma K., Jandeleit-Dahm K. A. M., Zoungas S., et al. (2015). Diabetic Kidney Disease. Nat. Rev. Dis. Prim. 1, 15018. doi:10.1038/nrdp.2015.18
Tremblay A. J., Lamarche B., Deacon C. F., Weisnagel S. J., Couture P. (2014). Effects of Sitagliptin Therapy on Markers of Low-Grade Inflammation and Cell Adhesion Molecules in Patients with Type 2 Diabetes. Metabolism 63, 1141–1148. doi:10.1016/j.metabol.2014.06.004
Tung C.-W., Hsu Y.-C., Shih Y.-H., Chang P.-J., Lin C.-L. (2018). Glomerular Mesangial Cell and Podocyte Injuries in Diabetic Nephropathy. Nephrology 23 (Suppl. 4), 32–37. doi:10.1111/nep.13451
Uzu T., Yokoyama H., Itoh H., Koya D., Nakagawa A., Nishizawa M., et al. (2011). Elevated Serum Levels of Interleukin-18 in Patients with Overt Diabetic Nephropathy: Effects of Miglitol. Clin. Exp. Nephrol. 15, 58–63. doi:10.1007/s10157-010-0343-7
Valiño-Rivas L., Cuarental L., Nuñez G., Sanz A. B., Ortiz A., Sanchez-Niño M. D. (2020). Loss of NLRP6 Expression Increases the Severity of Acute Kidney Injury. Nephrol. Dial. Transpl. 35, 587–598. doi:10.1093/ndt/gfz169
Van Opdenbosch N., Lamkanfi M. (2019). Caspases in Cell Death, Inflammation, and Disease. Immunity 50, 1352–1364. doi:10.1016/j.immuni.2019.05.020
Wada T., Inagaki M., Yoshinari T., Terata R., Totsuka N., Gotou M., et al. (2021). Apararenone in Patients with Diabetic Nephropathy: Results of a Randomized, Double-Blind, Placebo-Controlled Phase 2 Dose-Response Study and Open-Label Extension Study. Clin. Exp. Nephrol. 25, 120–130. doi:10.1007/s10157-020-01963-z
Wang G., Li Q., Chen D., Wu B., Wu Y., Tong W., et al. (2019). Kidney-targeted Rhein-Loaded Liponanoparticles for Diabetic Nephropathy Therapy via Size Control and Enhancement of Renal Cellular Uptake. Theranostics 9, 6191–6208. doi:10.7150/thno.37538
Wang Q., Liu S., Zhai A., Zhang B., Tian G. (2018). AMPK-mediated Regulation of Lipid Metabolism by Phosphorylation. Biol. Pharm. Bull. 41, 985–993. doi:10.1248/bpb.b17-00724
Wang S., Li Y., Fan J., Zhang X., Luan J., Bian Q., et al. (2017). Interleukin-22 Ameliorated Renal Injury and Fibrosis in Diabetic Nephropathy through Inhibition of NLRP3 Inflammasome Activation. Cell Death Dis. 8, e2937. doi:10.1038/cddis.2017.292
Wang Z., Wang N., Liu P., Xie X. (2016). AMPK and Cancer. Exp. Suppl. 107, 203–226. doi:10.1007/978-3-319-43589-3_9
Wang Z., Zhu Q., Li P.-L., Dhaduk R., Zhang F., Gehr T. W., et al. (2014). Silencing of Hypoxia-Inducible Factor-1α Gene Attenuates Chronic Ischemic Renal Injury in Two-Kidney, One-Clip Rats. Am. J. Physiology-Renal Physiology 306, F1236–F1242. doi:10.1152/ajprenal.00673.2013
Warner N., Núñez G. (2013). MyD88: A Critical Adaptor Protein in Innate Immunity Signal Transduction. J. I. 190, 3–4. doi:10.4049/jimmunol.1203103
Watts E. R., Walmsley S. R. (2019). Inflammation and Hypoxia: HIF and PHD Isoform Selectivity. Trends Mol. Med. 25, 33–46. doi:10.1016/j.molmed.2018.10.006
Wei X., Shao B., He Z., Ye T., Luo M., Sang Y., et al. (2015). Cationic Nanocarriers Induce Cell Necrosis through Impairment of Na+/K+-ATPase and Cause Subsequent Inflammatory Response. Cell Res. 25, 237–253. doi:10.1038/cr.2015.9
Wen Y., Yan M., Zhang B., Li P. (2017). Chinese Medicine for Diabetic Kidney Disease in China. Nephrology 22 (Suppl. 4), 50–55. doi:10.1111/nep.13149
Whittaker A., Kragh Å. M., Hartleib‐Geschwindner J., Albayaty M., Backlund A., Greasley P. J., et al. (2020). Safety, Tolerability, and Pharmacokinetics of the Mineralocorticoid Receptor Modulator AZD9977 in Healthy Men: A Phase I Multiple Ascending Dose Study. Clin. Transl. Sci. 13, 275–283. doi:10.1111/cts.12705
Wilson P. C., Wu H., Kirita Y., Uchimura K., Ledru N., Rennke H. G., et al. (2019). The Single-Cell Transcriptomic Landscape of Early Human Diabetic Nephropathy. Proc. Natl. Acad. Sci. U.S.A. 116, 19619–19625. doi:10.1073/pnas.1908706116
Wu J., Sun L., Chen X., Du F., Shi H., Chen C., et al. (2013). Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science 339, 826–830. doi:10.1126/science.1229963
Wu M., Han W., Song S., Du Y., Liu C., Chen N., et al. (2018). NLRP3 Deficiency Ameliorates Renal Inflammation and Fibrosis in Diabetic Mice. Mol. Cell. Endocrinol. 478, 115–125. doi:10.1016/j.mce.2018.08.002
Wu M., Yang Z., Zhang C., Shi Y., Han W., Song S., et al. (2021). Inhibition of NLRP3 Inflammasome Ameliorates Podocyte Damage by Suppressing Lipid Accumulation in Diabetic Nephropathy. Metabolism 118, 154748. doi:10.1016/j.metabol.2021.154748
Wu W., Yang J.-J., Yang H.-M., Huang M.-M., Fang Q.-J., Shi G., et al. (2017). Multi-glycoside of Tripterygium Wilfordii Hook. F. Attenuates Glomerulosclerosis in a Rat Model of Diabetic Nephropathy by Exerting Anti-microinflammatory Effects without Affecting Hyperglycemia. Int. J. Mol. Med. 40, 721–730. doi:10.3892/ijmm.2017.3068
Xian H., Liu Y., Rundberg Nilsson A., Gatchalian R., Crother T. R., Tourtellotte W. G., et al. (2021). Metformin Inhibition of Mitochondrial ATP and DNA Synthesis Abrogates NLRP3 Inflammasome Activation and Pulmonary Inflammation. Immunity 54, 1463–1477. doi:10.1016/j.immuni.2021.05.004
Xiao Y., Liu Y., Yu K., Zhou L., Bi J., Cheng J., et al. (2013). The Effect of Chinese Herbal Medicine on Albuminuria Levels in Patients with Diabetic Nephropathy: A Systematic Review and Meta-Analysis. Evidence-Based Complementary Altern. Med. 2013, 1–11. doi:10.1155/2013/937549
Xie C., Wu W., Tang A., Luo N., Tan Y. (2019). lncRNA GAS5/miR-452-5p Reduces Oxidative Stress and Pyroptosis of High-Glucose-Stimulated Renal Tubular Cells. Dmso 12, 2609–2617. doi:10.2147/DMSO.S228654
Xiong W., Meng X.-F., Zhang C. (2020). Inflammasome Activation in Podocytes: A New Mechanism of Glomerular Diseases. Inflamm. Res. 69, 731–743. doi:10.1007/s00011-020-01354-w
Xiong Y., Zhou L. (20192019). The Signaling of Cellular Senescence in Diabetic Nephropathy. Oxidative Med. Cell. Longev. 2019, 1–16. doi:10.1155/2019/7495629
Xu L., Zhou J., Che J., Wang H., Yang W., Zhou W., et al. (2021). Mitochondrial DNA Enables AIM2 Inflammasome Activation and Hepatocyte Pyroptosis in Nonalcoholic Fatty Liver Disease. Am. J. Physiology-Gastrointestinal Liver Physiology 320, G1034–G1044. doi:10.1152/ajpgi.00431.2020
Xu Y., Tao X., Shen B., Horng T., Medzhitov R., Manley J. L., et al. (2000). Structural Basis for Signal Transduction by the Toll/interleukin-1 Receptor Domains. Nature 408, 111–115. doi:10.1038/35040600
Yang M., Luo S., Jiang N., Wang X., Han Y., Zhao H., et al. (2021a). DsbA-L Ameliorates Renal Injury through the AMPK/NLRP3 Inflammasome Signaling Pathway in Diabetic Nephropathy. Front. Physiol. 12, 659751. doi:10.3389/fphys.2021.659751
Yang M., Wang X., Han Y., Li C., Wei L., Yang J., et al. (2021b). Targeting the NLRP3 Inflammasome in Diabetic Nephropathy. Cmc 28, 8810–8824. doi:10.2174/0929867328666210705153109
Yi H., Peng R., Zhang L.-y., Sun Y., Peng H.-m., Liu H.-d., et al. (2017). LincRNA-Gm4419 Knockdown Ameliorates NF-κB/NLRP3 Inflammasome-Mediated Inflammation in Diabetic Nephropathy. Cell Death Dis. 8, e2583. doi:10.1038/cddis.2016.451
Youm Y.-H., Nguyen K. Y., Grant R. W., Goldberg E. L., Bodogai M., Kim D., et al. (2015). The Ketone Metabolite β-hydroxybutyrate Blocks NLRP3 Inflammasome-Mediated Inflammatory Disease. Nat. Med. 21, 263–269. doi:10.1038/nm.3804
Young M. J., Rickard A. J. (2015). Mineralocorticoid Receptors in the Heart: Lessons from Cell-Selective Transgenic Animals. J. Endocrinol. 224, R1–R13. doi:10.1530/JOE-14-0471
Yu L., Wang Y., Guo Y. H., Wang L., Yang Z., Zhai Z. H., et al. (2021). HIF-1α Alleviates High-Glucose-Induced Renal Tubular Cell Injury by Promoting Parkin/PINK1-Mediated Mitophagy. Front. Med. 8, 803874. doi:10.3389/fmed.2021.803874
Yuan F., Kolb R., Pandey G., Li W., Sun L., Liu F., et al. (2016). Involvement of the NLRC4-Inflammasome in Diabetic Nephropathy. PLoS One 11, e0164135. doi:10.1371/journal.pone.0164135
Zhang C., Zhu X., Li L., Ma T., Shi M., Yang Y., et al. (2019). A Small Molecule Inhibitor MCC950 Ameliorates Kidney Injury in Diabetic Nephropathy by Inhibiting NLRP3 Inflammasome Activation. Dmso 12, 1297–1309. doi:10.2147/DMSO.S199802
Zhang J., Huang L., Shi X., Yang L., Hua F., Ma J., et al. (2020). Metformin Protects against Myocardial Ischemia-Reperfusion Injury and Cell Pyroptosis via AMPK/NLRP3 Inflammasome Pathway. Aging 12, 24270-24287. doi:10.18632/aging.202143
Zhang J.-Z., Liu Z., Liu J., Ren J.-X., Sun T.-S. (2014). Mitochondrial DNA Induces Inflammation and Increases TLR9/NF-κB Expression in Lung Tissue. Int. J. Mol. Med. 33, 817–824. doi:10.3892/ijmm.2014.1650
Zhang J., Yang X., Zhang X., Lu D., Guo R. (2021). Electro-Acupuncture Protects Diabetic Nephropathy-Induced Inflammation through Suppression of NLRP3 Inflammasome in Renal Macrophage Isolation. Emiddt 21, 2075–2083. doi:10.2174/1871530321666210118161721
Zhang L., Zhao M.-H., Zuo L., Wang Y., Yu F., Zhang H., et al. (2019). China Kidney Disease Network (CK-NET) 2015 Annual Data Report. Kidney Int. Suppl. 9, e1-e81. doi:10.1016/j.kisu.2018.11.001
Zhang L., Li M., Wang Z., Sun P., Wei S., Zhang C., et al. (2021). Cardiovascular Risk after SARS-CoV-2 Infection Is Mediated by IL18/IL18R1/HIF-1 Signaling Pathway axis. Front. Immunol. 12, 780804. doi:10.3389/fimmu.2021.780804
Zhang M., Chen Y., Yang M. j., Fan X. R., Xie H., Zhang L., et al. (2019). Celastrol Attenuates Renal Injury in Diabetic Rats via MAPK/NF‐κB Pathway. Phytotherapy Res. 33, 1191–1198. doi:10.1002/ptr.6314
Zhang Q.-y., Xu S.-j., Qian J.-c., Yang L.-b., Chen P.-q., Wang Y., et al. (2022). Pharmacological Inhibition of MyD88 Suppresses Inflammation in Tubular Epithelial Cells and Prevents Diabetic Nephropathy in Experimental Mice. Acta Pharmacol. Sin. 43, 354–366. doi:10.1038/s41401-021-00766-6
Zhang Q., Lenardo M. J., Baltimore D. (2017). 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 168, 37–57. doi:10.1016/j.cell.2016.12.012
Zhang Q., Liu X., Sullivan M. A., Shi C., Deng B. (2021a). Protective Effect of Yi Shen Pai Du Formula against Diabetic Kidney Injury via Inhibition of Oxidative Stress, Inflammation, and Epithelial-To-Mesenchymal Transition in Db/db Mice. Oxidative Med. Cell. Longev. 2021, 1–15. doi:10.1155/2021/7958021
Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., et al. (2010). Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury. Nature 464, 104–107. doi:10.1038/nature08780
Zhang Q., Song W., Zhao B., Xie J., Sun Q., Shi X., et al. (2021b). Quercetin Attenuates Diabetic Peripheral Neuropathy by Correcting Mitochondrial Abnormality via Activation of AMPK/PGC-1α Pathway In Vivo and In Vitro. Front. Neurosci. 15, 636172. doi:10.3389/fnins.2021.636172
Zhang S., Xu H., Yu X., Wu Y., Sui D. (2017). Metformin Ameliorates Diabetic Nephropathy in a Rat Model of Low-Dose Streptozotocin-Induced Diabetes. Exp. Ther. Med. 14, 383–390. doi:10.3892/etm.2017.4475
Zhang W., Cai Y., Xu W., Yin Z., Gao X., Xiong S. (2013). AIM2 Facilitates the Apoptotic DNA-Induced Systemic Lupus Erythematosus via Arbitrating Macrophage Functional Maturation. J. Clin. Immunol. 33, 925–937. doi:10.1007/s10875-013-9881-6
Zhang W., Huai Y., Miao Z., Qian A., Wang Y. (2019). Systems Pharmacology for Investigation of the Mechanisms of Action of Traditional Chinese Medicine in Drug Discovery. Front. Pharmacol. 10, 743. doi:10.3389/fphar.2019.00743
Zhang X.-X., Kong J., Yun K. (2020). Prevalence of Diabetic Nephropathy Among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies. J. Diabetes Res. 2020, 1–11. doi:10.1155/2020/2315607
Zhao X., Chen X., Chima A., Zhang Y., George J., Cobbs A., et al. (2019). Albumin Induces CD44 Expression in Glomerular Parietal Epithelial Cells by Activating Extracellular Signal‐regulated Kinase 1/2 Pathway. J. Cell. Physiology 234, 7224–7235. doi:10.1002/jcp.27477
Zhen J., Zhang L., Pan J., Ma S., Yu X., Li X., et al. (2014). AIM2 Mediates Inflammation-Associated Renal Damage in Hepatitis B Virus-Associated Glomerulonephritis by Regulating Caspase-1, IL-1β, and IL-18. Mediat. Inflamm. 2014, 1–9. doi:10.1155/2014/190860
Zhong Y., Lee K., Deng Y., Ma Y., Chen Y., Li X., et al. (2019). Arctigenin Attenuates Diabetic Kidney Disease through the Activation of PP2A in Podocytes. Nat. Commun. 10, 4523. doi:10.1038/s41467-019-12433-w
Zhong Z., Liang S., Sanchez-Lopez E., He F., Shalapour S., Lin X.-j., et al. (2018). New Mitochondrial DNA Synthesis Enables NLRP3 Inflammasome Activation. Nature 560, 198–203. doi:10.1038/s41586-018-0372-z
Zhu Q. J., Zhu M., Xu X. X., Meng X. M., Wu Y. G. (2019). Exosomes from High Glucose-Treated Macrophages Activate Glomerular Mesangial Cells via TGF‐β1/Smad3 Pathway In Vivo and In Vitro. FASEB J. 33, 9279–9290. doi:10.1096/fj.201802427RRR
Keywords: diabetic kidney disease, inflammation, signaling pathway, anti-Inflammation, anti-hyperglycemic, mineralocorticoid receptor antagonists, traditional Chinese medicine
Citation: Liu C, Yang M, Li L, Luo S, Yang J, Li C, Liu H and Sun L (2022) A Glimpse of Inflammation and Anti-Inflammation Therapy in Diabetic Kidney Disease. Front. Physiol. 13:909569. doi: 10.3389/fphys.2022.909569
Received: 04 April 2022; Accepted: 18 May 2022;
Published: 06 July 2022.
Edited by:
Paul Edward Squires, University of Lincoln, United KingdomReviewed by:
Vincenzo Quagliariello, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyCopyright © 2022 Liu, Yang, Li, Luo, Yang, Li, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Sun, c3VubGluQGNzdS5lZHUuY24=; Huafeng Liu, aGYtbGl1QDI2My5uZXQ=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.