- 1Division of Biopesticides and Environmental Toxicology, Sri Paramakalyani Centre for Excellence in Environmental Sciences, Manonmaniam Sundaranar University, Tirunelveli, Tamil Nadu, India
- 2Department of Entomology, University of Kentucky, Lexington, KY, United States
- 3Department of Zoology, Sri Paramakalyani College, Tirunelveli, Tamil Nadu, India
- 4Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 5Department of Plant Protection, Faculty of Agriculture Saba Basha, Alexandria University, Alexandria, Egypt
- 6Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand
- 7Innovative Agriculture Research Center, Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand
Spodoptera litura (Fabricius) is an agriculturally significant polyphagous insect pest that has evolved a high level of resistance to conventional insecticides. A dietary assay was used in this work to assess the resilience of field populations of S. litura to λ-cyhalothrin. Analysis of the function and expression of the cytochrome P450 gene was used to test the sensitivity of S. litura larvae to sub-lethal concentrations of the insecticidal plant chemical Precocene 1, both by itself and in combination with λ-cyhalothrin. The activity of esterase enzymes (α and β) was found to decrease 48 h post treatment with Precocene 1. The activity of GST enzyme and cytochrome P450 increased with Precocene 1 treatment post 48 h, however. Expression studies revealed the modulation by Precocene 1 of cytochrome P450 genes, CYP4M16, CYP4M15, CYP4S8V4, CYP4G31, and CYP4L10. While CYP4M16 expression was stimulated the most by the synergistic Precocene 1 + λ–cyhalothrin treatment, expression of CYP4G31 was the most down-regulated by Precocene 1 exposure. Hence, it is evident that λ–cyhalothrin-resistant pest populations are still sensitive to Precocene 1 at a sublethal concentration that is nevertheless capable of hindering their development. Precocene 1 can therefore be considered a potent candidate for the effective management of insecticide-resilient S. litura.
Introduction
Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) is a cosmopolitan insect pest, widely distributed throughout tropical and subtropical regions (Senthil-Nathan and Kalaivani 2005,2006; Senthil-Nathan, 2015) due to its high levels of reproduction, detoxification mechanisms, and migratory patterns (Liu et al., 2010; Amala et al., 2021). These insects infect more than 40 plant families, including 180 host plants. The phytophagous pest resists insecticide attack through various mechanisms, such as by lowering the quantity of allelochemicals, de-activating xenobiotics metabolically (Peng et al., 2017), skillfully avoiding the defense-induced area of the leaf surface of plants (Senthil-Nathan, 2019), developing insensitivity in plants toward protease inhibitors (Senthil-Nathan, 2019), and by intensively eliminating allelochemicals through ATP-binding cassette transporters (ABC transporters) (Meena et al., 2022).
Pest control practices via indiscriminate application of synthetic insecticides lead to resistance development and hence the failure of the control measure (Murali-Baskaran et al., 2021; Subaharan et al., 2021). Resistance to insecticides in lepidopteran pests is mainly by detoxification and target site insensitivity, involving enzymes such as esterase, glutathione complex, and cytochrome P450 (Tang et al., 2022). Organophosphate (OP) and carbamate insecticides inhibit the activity of AChE in insects through the process of phosphorylating or carbamylating the serine residues at the target site (Cao et al., 2020). Resistance to organophosphates and carbamates in Helicoverpa armigera and S. litura has already been reported (Selin-Rani et al., 2016). The epsilon class GSTs of S. litura are good enough to detoxify DDT (dichloro-diphenyl-trichloroethane) and deltamethrin (Deng et al., 2009). Excessive application of cypermethrin and pyrethroid develops resistance in S. litura (Shyam-Sundar et al., 2021a, 2021b) by way of decreased penetration, target site sensitivity alteration, and enhancement of detoxification enzyme activity, such as that of cytochrome P450 monooxygenase (P450), carboxylesterase (CarE), and glutathione S-transferase (GST) (Ahmad and McCaffery, 1999; Dong et al., 2016). Esterase exhibits a broad spectrum of accuracy, capable of cleaving tri-ester-phosphates, halides, esters, thioesters, amides, and peptides. The modality of esterases in detoxifying insecticides is well reported (Saleem and Shakoori, 1996). These enzymes are synthesized in the biochemical pathway during the developmental stages of the larva (Huang and Han, 2007). Increase in esterase activity when exposed to synthetic pesticide is one of the main resistance mechanisms in pests (Kranthi et al., 2002). Glutathione-S-transferase (GST) belongs to a protein family, and plays a major role in the detoxification of xenobiotics by converting them into less toxic water-soluble products (Singh et al., 2001). GST develops resistance against organophosphorus and pyrethroid insecticides in a wide range of insect pests (Senthil-Nathan, 2020). Insecticide-resistant strains of various insects reveal a correlation, in terms of an upsurge in expression of gene and GST activity, as part of their insecticide resistance (Li et al., 2007).
All organisms contain the cytochrome P450 monooxygenase (P450 or CYP) family, with a varied functional group of hemoproteins (Li W. et al., 2004; Guo et al., 2011; Nelson, 2011; Cheng et al., 2017). In insects, the role of P450 enzymes involves growth, development, biosynthesis, the regulation of hormones, and the metabolism of the xenobiotics (Nelson, 2011; Pottier et al., 2012) and insecticides that create resistance (Zhou et al., 2010; Guo et al., 2011; Sparks et al., 2021). Insect P450 genes consist of CYP2, CYP3, and CYP4 and the mitochondrial CYP clades (Feyereisen, 2011; Schuler and Berenbaum, 2013). Moreover, Clade 3 has the CYP6 and CYP9 families (Li et al., 2007), and particularly the CYP6 family, which are connected to the biochemical pathways that break down the derivatives of plant allelochemicals and lead to changes in feeding behavior and growth patterns (Li X. C. et al., 2004; Despre et al., 2007; Zeng et al., 2007; Niu et al., 2011; Dawkar et al., 2013).
The mechanism of the detoxifying P450 enzyme in insects helps them resist phytoconstituents and chemical insecticides (Sasabe et al., 2004; Mao et al., 2006; Despre et al., 2007; Bautista et al., 2009; Niu et al., 2011; Pentzold et al., 2014; Lu et al., 2021). Various P450s are capable of metabolizing a single substrate, and a single P450 is able to metabolize multiple substrates (Meunier et al., 2004). Phytochemical-inducible P450s are required for the development of cross-tolerance in insecticides (Li et al., 2000). The overexpression of P450s results in increased insecticide resistance, and tolerance to allelochemicals has been reported in various orders of insects, such as Lepidoptera, Diptera, Coleoptera, Hemiptera, and Hymenoptera (Bass et al., 2011; Johnson et al., 2012; Liang et al., 2015; Chen C. et al., 2017; Chen Y. et al., 2017; Wang R. L. et al., 2018; Wang X. et al., 2018; Hass et al., 2022). The role of cytochrome P450 genes and enzymes in S. litura for detoxifying host plant allelochemicals and other xenobiotics has not been much explored.
The major damage to agricultural crops results mainly from attack by lepidopteran insects (Shu, 2012; Selin-Rani et al., 2016). S. litura, in particular, develops resistance when exposed to synthetic pesticides (Jahan et al., 2008; Shyam-Sundar et al., 2021a, 2021b). The present study is aimed at finding the strategy of using allelochemicals for developing resistance when exposed to insecticides. In light of the previously mentioned, the present study scrutinizes the impact of Precocene 1, and its influence on detoxifying enzyme activity, and on the expression levels of five P450 genes in S. litura larvae (CYP4M16, CYP4M15, CYP4S8V4, CYP4G31, and CYP4L10), upon exposure to λ-cyhalothrin.
Materials and methods
Insects
The larvae of S. litura were obtained from agricultural land in Kadayam (latitude 8.8213° N, longitude 77.3741° E), Tenkasi District, India, and cultured in the Biopesticides and Ecotoxicology Laboratory (BET Lab), SPKCEES, Manonmaniam Sundaranar University, Alwarkurichi. To maintain generation, the larvae were kept in an insectary at 27 ± 1°C, with relative humidity (RD) of 85%, under a 12:12 L:D photoperiod schedule. Castor leaves were given as feed, and the adults that emerged were placed in a container (10 × 10 × 7 cm), with castor leaves for pairing (1 male: 2 females). A 10% honey solution was given to adults for oviposition, and a sanitary black cloth was used to cover the cage.
Chemicals
Precocene 1, phenyl methylsulfonyl fluoride (PMSF), and NADPH were purchased from Sigma-Aldrich (Mumbai, India). The λ-cyhalothrin was purchased commercially from a local chemical company in India. Dithiothreitol (DTT), glycerol, and Tris were obtained from Himedia Chemicals, Hyderabad, India, while 7-ethoxycoumarin, 7-hydroxycoumarin, EDTA, and bovine serum albumin were obtained from Sigma-Aldrich, Mumbai, India. Reagent grade chemicals and solvents were used.
Preparation of chemically supplemented diets
Preparation of an artificial and chemically supplemented diet followed previously published protocols (Karthi and Shivakumar, 2015; Chen C. et al., 2017). The Precocene 1 was dissolved in 1% dimethyl sulfoxide (DMSO). For the control, the artificial feed was made with the addition of 1% of DMSO. Distilled water containing 0.1% (v/v) Triton X-100 and 1% DMSO was used to dilute the λ-cyhalothrin insecticide, plus 1% DMSO, and treated as stock solution. For bioassays, 15 ml of stock solution was pipetted and sterilized in plastic cups of 4.0 cm in diameter × 3.0 cm in height. Agar was added and stirred into the liquid artificial diet for 2 min, which was then allowed to solidify at 40–45°C. A similar protocol was carried out for the preparation of the control diet, but without adding insecticides, and the control was then maintained at 4°C prior to use.
Toxicity bioassay
Third-instar larvae were used to find the effects of Precocene 1 uptake and tolerance to λ-cyhalothrin for all instances, as this developmental stage allows for observation of weight gain and mortality. 0.2% Precocene 1 was added to the artificial diet for 48 h before bioassay, and the control larvae were given the artificial diet containing the 0.1% DMSO solution. In total, twenty-five third-instar larvae were used for the experiment with five replications. In addition, in order to study the toxicity of λ-cyhalothrin, a diet merger methodology was followed using third-instar larvae (Karthi and Shivakumar, 2015). A standard solution (2,500 mg/L) of λ-cyhalothrin was dissolved in deionized water, having 0.1% (v/v) Triton X-100 and 1% DMSO for bioassays at concentrations of 150, 180, 210, 240, and 270 mg/L. A known volume of insecticide from the abovementioned solutions was added to tiny 20-ml sterilized plastic cups (4.0 cm × 3.0 cm), into which the artificial liquid diet was incorporated and stirred for 2 min. For the control, the same quantity of 0.1% (v/v) Triton X-100 and 1% DMSO was included in the diet and the cups were covered with perforated lids for the purpose of ventilation. For every bioassay series, and for each insecticide concentration, twenty-five larvae from the pretreated 0.2% Precocene 1 group, and from the non-exposed group, were used. The diet without chemical treatment was given to the control larvae. Mortality was recorded at 72 h post-treatment, and five replications were carried out in each experimental study.
Synergistic impact of PBO on the toxic nature of insecticide
Piperonyl butoxide (PBO) was used as the synergist, and its presence or absence was evaluated as described earlier. S. litura larvae were fed with an artificial diet with or without 0.2% Precocene 1 for 48 h. To obtain the concentration of 25 mg/L, the PBO was dissolved in acetone. Using a micro-syringe, 10 µg of PBO/larva was applied topically in the dorsal prothorax region of individual larvae of S. litura. After sterilization, plastic cups were used to place the 2 h PBO-exposed larvae of S. litura, to which were added different concentrations of insecticide solutions, namely, 150, 180, 210, 240, and 270 of λ-cyhalothrin mg/L, with or without 0.2% Precocene 1, to assess the toxicity of λ-cyhalothrin. The diet with Precocene 1 was fed to the larvae for 48 h, without the pre-treated PBO of λ-cyhalothrin, and the larvae were kept in cups with perforated lids. Every bioassay was conducted in triplicate.
Whole-body homogenate preparation for enzyme assay
Ten fourth-instar larvae were treated with Precocene 1 and then homogenized on ice with 0.1 M phosphate buffer (7.2 pH), containing 1 mM EDTA, 1 mM DTT, 1 mM PTU, 1 mM PMSF, and 20% glycerol. Tissues were collected after 24 and 48 h periods of homogenization in 2 ml of the buffer, and centrifugation was performed at 4°C, 10,000 g for 15 min. Solid debris and cellular materials were separated and the supernatant was transferred into Eppendorf tubes. These were placed on ice for the assay of carboxyl esterase, glutathione-S-transferase, and cytochrome P450. The total content of protein was then estimated using the procedure of Lowry et al. (1951).
Carboxyl esterase assays
The α and β-carboxylesterase activity was determined (Kranthi, 2005) in the larval extracts prepared with phosphate potassium buffer (0.1 M: pH 7.2), and with 20 μl; 84 μg protein. The extract was added to 500 μl buffer (0.3 mMα- or β-naphthyl acetate in 0.1 M phosphate potassium at pH 7.2, containing 1% acetone), and followed by incubation at 30°C for 20 min. To this, 0.3 and 3.3 percentages of Fast Blue B and sodium dodecyl sulfate (SDS) were added, respectively. After centrifugation (3,000x g, 28°C), the supernatant was collected and absorbance was recorded at 590 nm. One unit of enzyme activity determines the quantity of enzyme required to generate 1 μmol of α- or β-naphthol per minute.
Glutathione-S-transferase activity
Glutathione S-transferase assays were carried out, following the protocol of Kao et al. (1989), with reduced glutathione GSH as the substrate with 1-chloro 2,4-dinitrobenzene. Fourth-instar larvae were homogenized with 250 μl of sodium phosphate buffer (50 mM: pH 7.2) and centrifuged at 15,000×g at 4°C for 20 min. The union of the thiol group of glutathione with the substrate comprising 1-chloro-2, 4-dinitrobenzene (CDNB) was estimated using the Sigma-Aldrich (Catalog 0410, Bangalore) GST assay kit. Each well was loaded with 20 μl of the homogenate, along with 200 μl of Dulbecco’s phosphate buffer (Sigma-Aldrich, Bangalore, IN), reduced glutathione (4 mM), and CDNB (2 mM). The GST activity was conjugated as a μmol/mg protein/min substrate.
Cytochrome P450 activity
Determination of Cyt P450 activity was carried out by peroxidation of a TMBZ assay following the protocol of Brogdon et al. (1989) with minimal alteration. After adding 250 μl of 0.05 M potassium phosphate buffer (pH 7.0) to 50 μl microfuge supernatant and 500 μl TMBZ solution (0.05% 3,3′,5,5′ tetramethyl benzidine, i.e., TMBZ +5 ml methanol +15 ml sodium acetate buffer 0.25 M pH 5.0), the mixture was prepared by adding 200 μl of 3% hydrogen peroxide and incubated at room temperature for 30 min. The reading was taken at 630 nm absorbance and calculated by comparison with the standard curve of cytochrome C.
Extraction of total RNA and synthesis of cDNA
The total content of the RNA in S. litura larvae was extracted by TRIzol (Invitrogen). Synthesis of cDNA was performed in 20 µl of reactant, having 1 µl of total RNA, 0.6 µl of forward primer (10 pmol), 0.6 µl of reverse primer (10 pmol), and 20 units of RNase inhibitor, 1 µl of dNTP mixture (10 mM each), and 1 µl oligo (dT)18 primer (50 µM) at 42°C , for 1 h by reverse transcription. The concentration of RNA was quantified at 260–280 nm absorbance. The quality was assessed by agarose gel electrophoresis, and staining was performed with ethidium bromide (EB).
Analysis of real-time RT-PCR
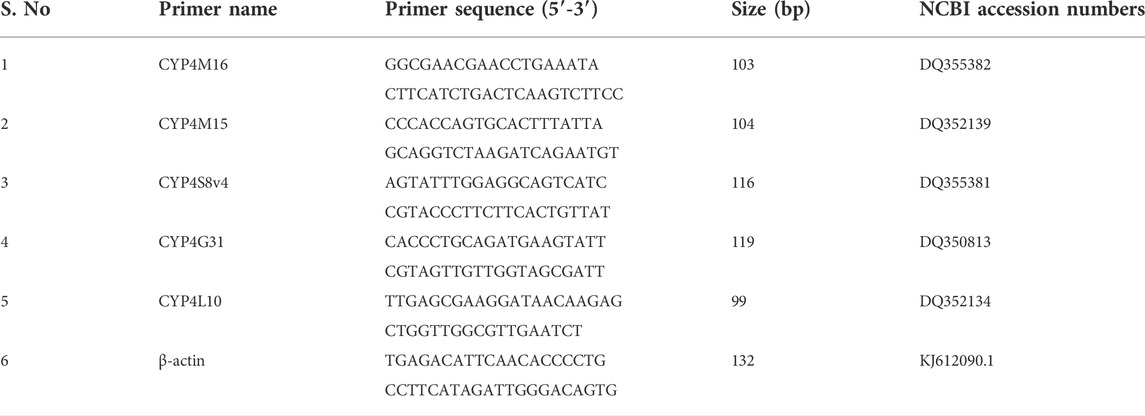
To estimate the amount of RNA using agarose gel electrophoresis, the reverse transcription to cDNA from 1 µg of total RNA was conducted with a PrimeScript® RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Japan) according to the manufacturer’s protocol. To study the RT-PCR, primers (Table 1) were planned using Primer 3 software (Applied Biosystems). SYBR Green qPCR was carried out in a 0.2 ml PCR 8-tube strip with flat 8-cap strips (Axygen, USA), employing an iQ5 real-time PCR detection system (Bio-Rad, United States). The prepared 20 µl PCR reaction consisted of 2 µl cDNA template, 10 µl 2 X SYBR® Premix Ex Taq™ II (Perfect Real Time) (TaKaRa, Japan), 0.8 µl of each primer (10 pmol/µl), and 6.4 µl ddH2O. The RT-PCR program was carried out using the melting curve dissociation methodology (from 55°C to 95°C), and by following the thermal conditions: initial denaturation 95°C for 30 s, subsequently 40 cycles of 95°C for 5 s, and 60°C for 30 s. A melting curve analysis was then performed to assess the specificity and consistency of the PCR products. The expression levels of target genes were calculated with the 2−ΔΔCT method and normalized to the internal housekeeping gene β-actin.
Statistical analysis
Data are represented in mean ± SD. Resulting pairs were compared using the Student’s t-test. One-way ANOVA was applied to determine the significant differences (p < 0.05) for different groups by using the Tukey test. Statistical analysis was carried out with SPSS 11.5 software.
Results
λ-cyhalothrin toxicity to S. litura larvae and synergist activity
The impact of Precocene 1 and its synergistic effect with PBO against the tolerance and sensitivity of S. litura to λ-cyhalothrin is presented in Table 2. The LC50 value of larvae of S. litura treated with PBO was 91.89 mg/L. The effect of Precocene 1 alone, tested for LC50 in the larvae of S. litura, was found to be lower (78.05 mg ai/L), a considerable decrease due to the synergistic effect of PBO with Precocene 1 (61.06 mg ai/L).
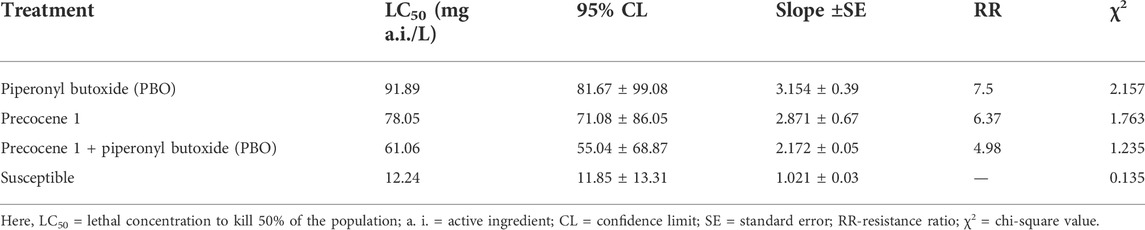
TABLE 2. Synergistic effect of piperonyl butoxide on the susceptibility of third-instar larvae of S. litura to lambda-cyhalothrin after the ingestion of Precocene 1.
The influence of diet with Precocene 1 on detoxification enzymes in S. litura larvae
The activity of the α-esterase enzyme in the control, the effect of Precocene 1 only, of λ-cyhalothrin, and of the synergistic effect of Precocene 1 with λ-cyhalothrin in the larva of S. litura at 24 and 48 h after exposure is reported in Figure 1. The higher enzyme activity of S. litura, due to the various treatments, is shown in the order of Precocene 1 + λ-cyhalothrin > Precocene 1>λ-cyhalothrin > control and was 1.95, 1.5, 1.1, and 0.8, respectively, in the said order at 24 h of exposure. At 48 h of exposure, profound activity was observed in Precocene 1 + λ-cyhalothrin (2.7), followed by λ-cyhalothrin, Precocene 1, and the control (2.4, 1.9, and 1.2).
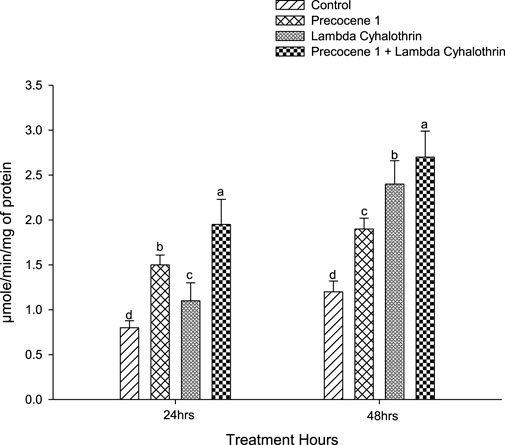
FIGURE 1. Effects of Precocene 1 on Spodoptera litura larvae tolerance to λ-cyhalothrin in α-esterase activity after 24 and 48 h. Data in the figure are means ± SE. Different letters above bars indicate significant differences (p < 0.05) according to the Tukey HSD test.
The β-esterase enzyme activity in the larvae of S. litura at 24 and 48 h of exposure in the control, Precocene 1, λ-cyhalothrin, and the combined effect of Precocene 1 + λ-cyhalothrin is shown in Figure 2. A similar trend in the enzyme activity in S. litura as in the case of alpha esterase was observed, and the values were 2.01, 1.7, 1.4, and 1.15 at 24 h of exposure. Similarly, in the larva of S. litura, the enzyme activity at 48 h of exposure was also found to be of the same order as reported in the alpha esterase activity in S. litura at 48 h, with values of 2.89, 2.6, 2.10, and 1.33.
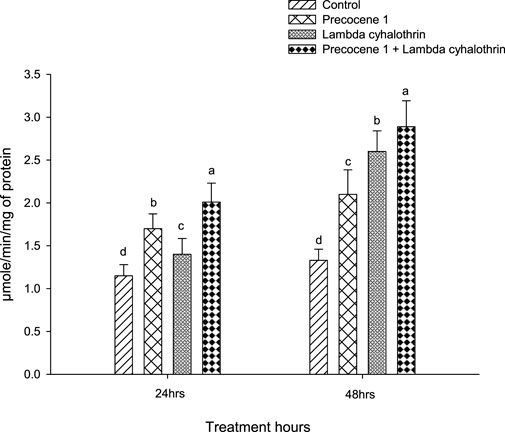
FIGURE 2. Effects of Precocene 1 on Spodoptera litura larvae tolerance to λ-cyhalothrin in β-esterase activity after 24 and 48 h. Data in the figure are means ± SE. Different letters above bars indicate significant differences (p < 0.05) according to the Tukey HSD test.
Regarding GST enzyme activity, both at 24 and 48 h of exposure to the synergistic effect of Precocene 1 + λ-cyhalothrin, the activity of the enzymes was found to be greater, being 1.89 and 2.11 in the larva of S. litura. The enzyme activity of S. litura was followed in the order of Precocene 1, λ-cyhalothrin, and the control as 1.29, 1.13, and 0.98 at 24 h of exposure, while a similar rate of enzyme activity was seen in S. litura larva at 48 h of exposure (Figure 3).
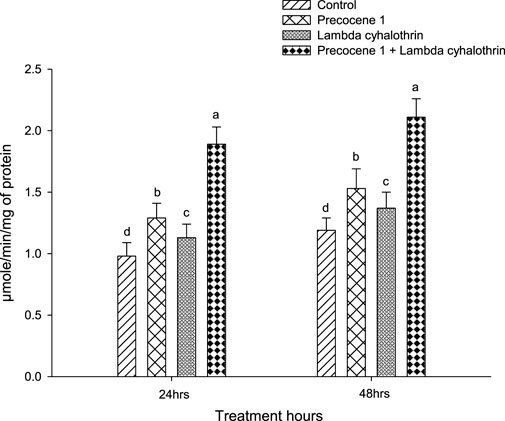
FIGURE 3. Effects of Precocene 1 on Spodoptera litura larvae tolerance to λ-cyhalothrin in glutathione-S-transferase activity after 24 and 48 h. Data in the figure are means ± SE. Different letters above bars indicate significant differences (p < 0.05) according to the Tukey HSD test.
The cytochrome P450 enzyme activity of S. litura in the control, Precocene 1, λ-cyhalothrin, and the synergistic effect of Precocene 1 + the λ-cyhalothrin treated group at 24 h was 1.21, 1.98, 1.82, and 2.35, whereas the values were 1.64, 2.31, 2.53, and 2.94 at 48 h of exposure, respectively, as shown in Figure 4.
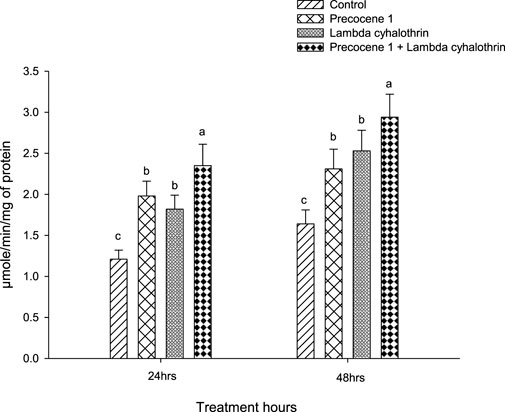
FIGURE 4. Effects of Precocene 1 on Spodoptera litura larvae tolerance to λ-cyhalothrin in cytochrome P450 activity after 24 and 48 h. Data in the figure are means ± SE. Different letters above bars indicate significant differences (p < 0.05) according to the Tukey HSD test.
Expression responses of cytochrome P450 genes in S. litura on exposure to Precocene 1
The expressions of the genes CYP4M16, CYP4M15, CYP4S8V4, CYP4G31, and CYP4L10 of S. litura at 24 h of exposure in the control, Precocene 1, λ-cyhalothrin, and Precocene 1 + λ-cyhalothrin groups are reported in Figure 5. Regarding CYP4M16, the gene expression of the larva was found to increase (1.01, 2.22, 2.68, and 2.97-fold). A similar increasing pattern of gene expression was noticed in CYP4L10, where the values were 1.01, 1.77 1.89, and 1.98-fold. When compared with the control in all other families, the gene expression was found to increase, whereas a changing pattern of gene expression was observed among the three groups. Among the five families studied, the CYP4M16 showed higher gene expression due to the combined effect of Precocene 1 with λ-cyhalothrin at 24 h of exposure.
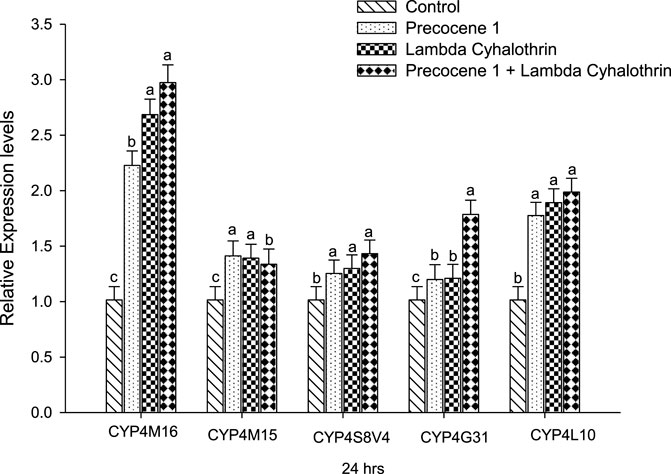
FIGURE 5. Effect of Precocene 1 on Spodoptera litura larvae tolerance to λ-cyhalothrin and relative expression levels of cytochrome P450s genes after 24 h. The transcription levels of three cytochrome P450s genes are determined by quantitative real-time PCR, normalized to different genes. Each bar indicates the mean of transcription levels (±SE), each being replicated. Different letters above bars indicate significant differences (p < 0.05) according to the Tukey HSD test.
The CYP4M16, CYP4M15, CYP4S8V4, CYP4G31, and CYP4L10 expressions of S. litura after an exposure period of 48 h in the control, Precocene 1, λ-cyhalothrin, and Precocene 1 + λ-cyhalothrin groups are reported in Figure 6. A high level of gene expression was found in CYP4M16, with Precocene 1 + λ-cyhalothrin (2.65); the lowest level of gene expression (1.20) was observed in the Precocene 1-treated CYP4G31 of S. litura; and no variation in the gene expression pattern was noticed in the control.
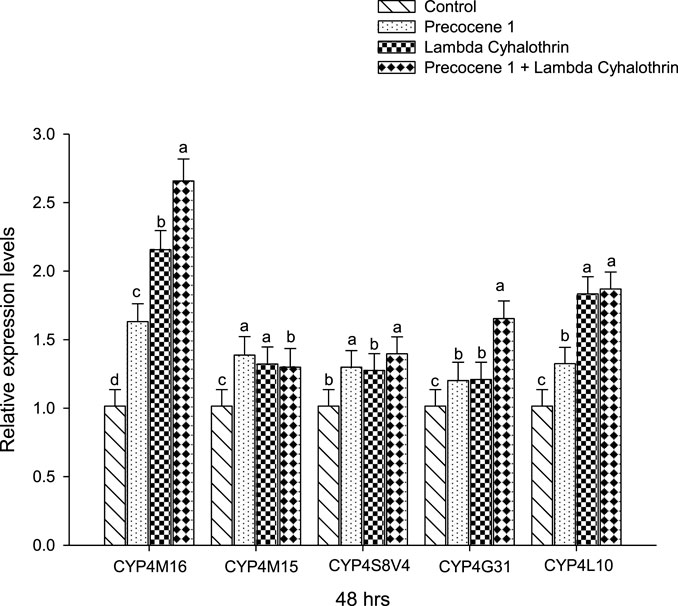
FIGURE 6. Effect of Precocene 1 on Spodoptera litura larvae tolerance to λ-cyhalothrin and relative expression levels of cytochrome P450s genes after 48 h. The transcription levels of three cytochrome P450s genes are determined by quantitative real-time PCR, normalized to different genes. Each bar indicates the mean of transcription levels (±SE), each being replicated. Different letters above bars indicate significant differences (p < 0.05) according to the Tukey HSD test.
Discussion
Plants produce a variety of secondary metabolites, or allelochemicals, which play a defensive role against plant eaters and pathogens. Furthermore, the natural predators of herbivores are attracted by these allelochemicals (Takabayashi et al., 1991; War et al., 2011). In the meanwhile, such feeding behavior paves the way to persistent development of resistance against pesticides in the agricultural field (Li et al., 2007; Zhu et al., 2016). The success of phytophagous insects is affected by the way they modulate their defensive strategies against the changing biotic stress of various kinds of secondary metabolites present in the plant. They do this by using the detoxification enzyme to detoxify or eliminate harmful components (Hafeez et al., 2018) Cytochrome P450 monooxygenases (P450s), esterases, and glutathione S-transferases (GST), are the prime detoxification enzymes to disarm insecticides and phytotoxins (Scott and Wen, 2001; Feyereisen, 2005; Li et al., 2007; Schuler, 2012; Liu et al., 2013). The present study investigated the effect of a diet incorporating Precocene 1 on the tolerance of S. litura larvae in response to λ-cyhalothrin. Furthermore, the impact of Precocene 1 on the activity of P450, esterases, and glutathione S-transferases, and the relative gene expression levels of cytochrome genes (CYP4M16, CYP4M15, CYP4S8V4, CYP4G31, and CYP4L10) were also assessed.
Concerning the synergistic ratio, findings on the individual effect of Precocene1 in an enhanced state, as opposed to the synergistic and lone effect of PBO, deviate from the results of Chen et al. (2018), while at the same time they coincide well with the findings of Hafeez et al. (2020). Adoption of the molecular strategy of upregulating P450 genes may be the mechanism behind the resistance (Elzaki et al., 2015). The enhanced α- and β-esterase activity at 24 and 48 h of exposure to the various treatments in the present study supports the results of other researchers (Mukherjee, 2003; Usha Rani and Pratyusha, 2013; Karthi and Shivakumar, 2016). Generally, the allelochemicals, be they either the secondary metabolites of the plant or the synthetic pesticides, developed toxicity when exposed to the pest, and in response, the pest nullified the same. The pest exhibited enhanced detoxifying enzyme activity, which may be the reason for the results of the present study. The increased levels of the detoxifying enzyme GST found in this study are in accordance with the reports of Dhivya et al. (2018), and Manjula et al. (2020), who identify the crucial role the multifunctional enzyme GST has in metabolizing the treatment of toxic plant allelochemicals, namely, hexane extract of Prosopis juliflora, and petroleum benzene leaf extract of Manihot esculenta, in S. litura. In the present study, the diet incorporating Precocene 1 led to an increased level of tolerance to the insecticide, λ-cyhalothrin, in S. litura. A similar kind of resistance to λ-cyhalothrin was noticed in S. exigua (Hafeez et al., 2020) and H. armigera (Chen et al., 2018), due to quercetin ingestion, and in H. zea forα-cypermethrin when exposed to xanthotoxin (Li et al., 2000). Likewise, tolerance to deltamethrin was observed in S. exigua fed with gossypol (Hafeez et al., 2018). The enhanced action of the P450 enzyme from 24 to 48 h, noticed in Precocene 1, λ-cyhalothrin, and Precocene 1 + λ-cyhalothrin, fed to larvae of S. litura, is corroborated by the results of Chen et al. (2018) and Tao et al. (2012) concerning H. armigera when fed with gossypol on exposure to pyrethroid, and quercetin to λ-cyhalothrin, respectively. In the result of RT-PCR at 24 and 48 h, the transcriptional levels of the CYP4M16, CYP4M15, CYP4S8V4, CYP4G31, and CYP4L10 enzymes of S. litura increased more markedly in all treatments other than in the control, and such activity resulted in the enhancement of P450 gene expression. The results of the present study accord with those of Li X. C. et al. (2004); Liu et al. (2006); and Rupasinghe et al. (2007), across a broad spectrum of compounds such as xanthotoxin, quercetin, and rutin, as well as the synthetic insecticides cypermethrin, diazinon, and aldrin.
Conclusion
The impact of active compounds isolated from a natural plant could elevate sensitivity to insecticides by enhancing the activity of detoxification enzymes in agricultural insects. Furthermore, the cytochrome P450 enzyme system certainly plays a vital role in ways insects resist the plant’s chemical defense mechanisms. The current investigation assessed the impact of Precocene 1 alone, PBO, and their combined effect on the synergistic potential of S. litura, the role of plant allelochemicals in the activity of the detoxification enzymes viz., esterase, GST, and Cytochrome P450, and also the gene expression levels of CYP4M16, CYP4M15, CYP4S8V4, CYP4G31, and CYP4L10 in response to λ-cyhalothrin. It is obvious from the results that the S. litura showed different degrees of resistance, and after Precocene 1 treatment, particularly, the P450 gene showed a low level of expression, as in the control. Research is needed to discover the candidate genes that respond specifically to the natural toxins of plants, and also the impact of insecticides against such pests, in order to improve pest management strategies.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
NS-S: study conception and design, data collection, data analysis, interpretation of results, and drafted manuscript preparation. RR: study conception and design, data collection, data analysis, interpretation of results, and drafted manuscript preparation. SK: study conception and design, data collection, data analysis, interpretation of results, and drafted manuscript preparation. SS-N: supervision, data analysis, interpretation of results, and drafted manuscript preparation. KM-PC: data collection, analysis, interpretation of results, and drafted manuscript preparation. HS: data analysis, interpretation of results, and drafted manuscript preparation. VS-R: data analysis, interpretation of results, and drafted manuscript preparation. GR: data analysis, interpretation of results, and drafted manuscript preparation, KN: data analysis, interpretation of results, and drafted manuscript preparation. SM: data analysis, interpretation of results, and drafted manuscript preparation. KA-G: data analysis, interpretation of results, and drafted manuscript preparation. AA-M: data analysis, interpretation of results, and drafted manuscript preparation. PK: data analysis, interpretation of results, and drafted manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
The research was supported by the DST-FIST program under the grant no. SR/FST/LS-1/2019/522. The authors extend their appreciation to the researchers supporting project number RSP-2021/93, King Saud University, Riyadh, Saudi Arabia. This research work was partially supported by Chiang Mai University, Thailand.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.900570/full#supplementary-material
References
Ahmad M., McCaffery A. R. (1999). Penetration and metabolism of trans cypermethrinin a susceptible and a pyrethroid-resistant strain of Helicoverpa armigera. Pestic. Biochem. Physiol. 65, 6–14. doi:10.1006/pest.1999.2420
Amala K., Karthi S., Ganesan R., Radhakrishnan N., Srinivasan K., Mostafa A. E. Z. M., et al. (2021). Bioefficacy of Epaltes divaricata (L.) n-Hexane extracts and their major metabolites against the Lepidopteran pests Spodoptera litura (fab.) and dengue mosquito Aedes aegypti (Linn.). Molecules 26 (12), 3695. doi:10.3390/molecules26123695
Bass C., Carvalho R. A., Oliphant L., Puinean A. M., Field L. M., Nauen R., et al. (2011). Overexpression of a cytochrome P450 monooxygenase, CYP6ER1, is associated with resistance to imidacloprid in the Brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 20, 763–773. doi:10.1111/j.1365-2583.2011.01105.x
Bautista M. A. M., Miyata T., Miura K., Tanaka T. (2009). RNA interference-mediated knockdown of a cytochrome P450, CYP6BG1, from the diamondback moth, Plutella xylostella, reduces larval resistance to permethrin. Insect biochem. Mol. Biol. 39, 38–46. doi:10.1016/j.ibmb.2008.09.005
Brogdon W. G., McAllister J. C., Vulule J. (1989). Heme peroxidase activity measured in single mosquitoes identifies individuals expressing an elevated oxidase for insecticide resistance. J. Am. Mosq. Control Assoc. 13, 233–237.
Cao J., Wang M., Yu H., She Y., Cao Z., Ye J., et al. (2020). An overview on the mechanisms and applications of enzyme inhibition-based methods for determination of organophosphate and carbamate pesticides. J. Agric. Food Chem. 68 (28), 7298–7315. doi:10.1021/acs.jafc.0c01962
Chen C., Han P., Yan W., Wang S., Shi X., Zhou X., et al. (2018). Uptake of quercetin reduces larval sensitivity to lambda-cyhalothrin in Helicoverpa armigera. J. Pest Sci. 91, 919–926. doi:10.1007/s10340-017-0933-1
Chen C., Liu Y., Shi X., Desneux N., Han P., Gao X. (2017a). Elevated carboxylesterase activity contributes to the lambda-cyhalothrin insensitivity in quercetin fed Helicoverpa armigera (Hübner). PLoS One 12, e0183111. doi:10.1371/journal.pone.0183111
Chen Y., Xiang X., Gong C., Wang X. (2017b). Effects of sublethal doses of chlorantraniliprole on the detoxification enzymes activities and the growth and reproduction of Spodoptera exigua. Sci. Agri. Sin. 50, 1440–1451.
Cheng T., Wu J., Wu Y., Chilukuri R. V., Huang L., Yamamoto K., et al. (2017). Genomic adaptation to polyphagy and insecticides in a major East Asian noctuid pest. Nat. Ecol. Evol. 1, 1747–1756. doi:10.1038/s41559-017-0314-4
Dawkar V. V., Chikate Y. R., Lomate P. R., Dholakia B. B., Gupta V. S., Giri A. P. (2013). Molecular insights into resistance mechanisms of Lepidopteran insect pests against toxicants. J. Proteome Res. 12, 4727–4737. doi:10.1021/pr400642p
Deng H., Huang Y., Feng Q., Zheng S. (2009). Two epsilon glutathione S-transferase cDNAs from the common cutworm, Spodoptera litura: Characterization and developmental and induced expression by insecticides. J. Insect Physiol. 55, 1174–1183. doi:10.1016/j.jinsphys.2009.08.017
Despre L., David J. P., Gallet C. (2007). The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 22, 298–307. doi:10.1016/j.tree.2007.02.010
Dhivya K., Vengateswari G., Arunthirumeni M., Karthi S., Senthil-Nathan S., Shivakumar M. S. (2018). Bioprospecting of Prosopis juliflora (Sw.) DC seed pod extract effect on antioxidant and immune system of Spodoptera litura (Lepidoptera: Noctuidae). Physiol. Mol. Plant Pathol. 101, 45–53. doi:10.1016/j.pmpp.2017.09.003
Dong W., Zhang X., Zhang X., Wu H., Zhang M., Ma E., et al. (2016). Susceptibility and potential biochemical mechanism of Oedaleus asiaticus to beta cypermethrin and deltamethrin in the Inner Mongolia, China. Pestic. Biochem. Physiol. 132, 47–52. doi:10.1016/j.pestbp.2015.11.011
Elzaki M. E. A., Zhang W. F., Feng A., Qiu X. Y., Zhao W. X., Han Z. J. (2015). Constitutive overexpression of cytochrome P450 associated with imidacloprid resistance in Laodelphax striatellus (Fallen). Pest Manag. Sci. 72, 1051–1058. doi:10.1002/ps.4155
Feyereisen R. (2011). Arthropod CYPomes illustrate the tempo and mode in P450evolution. Biochim. Biophys. Acta 1814, 19–28. doi:10.1016/j.bbapap.2010.06.012
Feyereisen R. (2005). “Insect cytochrome P450,” in Comprehensive molecular insect science, 1–77. doi:10.1016/b0-44-451924-6/00049-1
Guo Y., Zhang J., Yu R., Zhu K. Y., Guo Y., Ma E. (2011). Identification of two new cytochrome P450 genes and RNA interference to evaluate their roles in detoxification of commonly used insecticides in Locusta migratoria. Chemosphere 87, 709–717. doi:10.1016/j.chemosphere.2011.12.061
Haas J., Hayward A., Buer B., Maiwald F., Nebelsiek B., Glaubitz J., et al. (2022). Phylogenomic and functional characterization of an evolutionary conserved cytochrome P450-based insecticide detoxification mechanism in bees. Proc. Natl. Acad. Sci. U. S. A. 119, e2205850119. doi:10.1073/pnas.2205850119
Hafeez M., Liu S., Jan S., Ali B., Shahid M., Fernandez-Grandon G. M., et al. (2018). Gossypol-induced fitness gain and increased resistance to deltamethrin in beet armyworm, Spodoptera exigua (Hubner). Pest Manag. Sci. 75, 683–693. doi:10.1002/ps.5165
Hafeez M., Qasim M., Ali S., Yousaf H. K., Waqas M., Ali E., et al. (2020). Expression and functional analysis of P450 gene induced tolerance/resistance to lambda-cyhalothrin in quercetin fed larvae of beet armyworm Spodoptera exigua (Hubne). Saudi J. Biol. Sci. 27 (1), 77–87. doi:10.1016/j.sjbs.2019.05.005
Huang S. J., Han Z. J. (2007). Mechanisms for multiple resistances in field populations of common cutworm, Spodoptera litura (Fabricius) in China. Pestic. Biochem. Physiol. 87, 14–22. doi:10.1016/j.pestbp.2006.05.002
Jahan F. N., Rahman A., Mohammad S., Rashid A. R. (2008). Antimicrobial activity and toxicity of Quisqualis indica. Orient. Pharm. Exp. Med. 8 (1), 53–58. doi:10.3742/opem.2008.8.1.053
Johnson R. M., Mao W., Pollock H. S., Niu M. A., Schuler M. A., Berenbaum M. R. (2012). Ecologically appropriate xenobiotics induce cytochrome P450s in Apis Mellifera. PLoS One 7, e31051. doi:10.1371/journal.pone.0031051
Kao C. H., Hung C. F., Sun C. N. (1989). Parathion and methyl parathion resistance in diamondback moth (Lepidoptera: Plutellidae) larvae. J. Econ. Entomol. 82, 1299–1304. doi:10.1093/jee/82.5.1299
Karthi S., Shivakumar M. S. (2015). The protective effect of melatonin against cypermethrin-induced oxidative stress damage in Spodoptera litura (Lepidoptera: Noctuidae). Biol. Rhythm Res. 46, 1–12. doi:10.1080/09291016.2013.870758
Karthi S., Shivakumar M. S. (2016). Time-of-day specific changes in pesticide detoxification ability of Spodoptera litura (Lepidoptera: Noctuidae). Biol. Rhythm Res. 47 (2), 303–314. doi:10.1080/09291016.2015.1116738
Kranthi K. R. (2005). Insecticide resistance – monitoring, mechanisms and management manual. Nagpur: Central Institute for Cotton Research.
Kranthi K. R., Jadhav D. R., Wanjari R. R., Ali S. S., Russel D., Russell D. (2002). Insecticide resistance in five major insect pests of cotton in India. Crop Prot. 21, 449–460. doi:10.1016/s0261-2194(01)00131-4
Li W., Zangerl A. R., Schuler M. A., Berenbaum M. R. (2004a). Characterization and evolution of furanocoumarin-inducible cytochrome P450s in the parsnip webworm, Depressaria pastinacella. Insect Mol. Biol. 13, 603–613. doi:10.1111/j.0962-1075.2004.00518.x
Li X. C., Baudry J., Berenbaum M. R., Schuler M. A. (2004b). Structural and functional divergence of insect CYP6B proteins: From specialist to generalist cytochromeP450. Proc. Natl. Acad. Sci. U. S. A. 101, 2939–2944. doi:10.1073/pnas.0308691101
Li X., Schuler M. A., Berenbaum M. R. (2007). Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52, 231–253. doi:10.1146/annurev.ento.51.110104.151104
Li X., Zangerl A. R., Schuler M. A., Berenbaum M. R. (2000). Cross-resistance to a cypermethrin after xanthotoxin ingestion in Helicoverpa zea (Lepidoptera: Noctuidae). J. Econ. Entomol. 93, 18–25. doi:10.1603/0022-0493-93.1.18
Liang X., Xiao D., He Y., Yao J., Zhu G., Zhu K. Y. (2015). Insecticide-mediated up regulation of cytochrome P450 genes in the red flour beetle (Tribolium castaneum). Int. J. Mol. Sci. 16, 2078–2098. doi:10.3390/ijms16012078
Liu J., Zheng S., Liu L., Li L., Feng Q. (2010). Protein profiles of the midgut of spodoptera litura larvae at the sixth instar feeding stage by shotgun ESI-MS approach. J. Proteome Res. 9 (5), 2117–2147. doi:10.1021/pr900826f
Liu X. N., Liang P., Gao X., Shi X. Y. (2006). Induction of the cytochrome P450 activity by plant allelochemicals in the cotton bollworm, Helicoverpa armigera (Hübner). Pestic. Biochem. Physiol. 84, 127–134. doi:10.1016/j.pestbp.2005.06.002
Liu X., Zhang L., Zhang X., Gao X. W. (2013). Molecular cloning and recombinant expression of cytochrome P450 CYP6B6 from Helicoverpa armigera in Escherichia coli. Mol. Biol. Rep. 40, 1211–1217. doi:10.1007/s11033-012-2163-1
Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275. doi:10.1016/s0021-9258(19)52451-6
Lu K., Song Y., Zeng R. (2021). The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 43, 103–107. doi:10.1016/j.cois.2020.11.004
Manjula P., Vengateswari G., Patil J., Nathan S. S., Lalitha K., Shivakumar M. S. (2020). Effect of Manihot esculenta (Crantz) leaf extracts on antioxidant and immune system of Spodoptera litura (Lepidoptera: Noctuidae). Biocatal. Agric. Biotechnol. 23, 101476. doi:10.1016/j.bcab.2019.101476
Mao W., Rupasinghe S., Zangerl A. R., Schuler M. A., Berenbaum M. R. (2006). Remarkable substrate-specificity of CYP6AB3 in Depressaria pastinacella, highly specialized caterpillar. Insect Mol. Biol. 15, 169–179. doi:10.1111/j.1365-2583.2006.00623.x
Meena S. S., Mankoti M., Rout P. R., Mohanty A. (2022). “Plant-microbe interactions and its effect on crop productivity,” in Advances in agricultural and industrial microbiology (Singapore: Springer), 29–60.
Meunier B., de Visser S. P., Shaik S. (2004). Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem. Rev. 104, 3947–3980. doi:10.1021/cr020443g
Mukherjee S. (2003). Influence of plant allelochemicals on growth rate, nutritional physiology and mid-gut esterase activity in fifth instar larvae of Spodoptera litura (F.) (Lepidoptera: Noctuidae). Invertebr. Reprod. Dev. 43, 125–132. doi:10.1080/07924259.2003.9652531
Murali-Baskaran R. K., Senthil-Nathan S., Hunter W. B. (2021). Anti-herbivore activity of soluble silicon for crop protection in agriculture: A review. Environ. Sci. Pollut. Res. Int. 28 (3), 2626–2637. doi:10.1007/s11356-020-11453-0
Nelson D. R. (2011). Progress in tracing the evolutionary paths of cytochrome P450. Biochim. Biophys. Acta 1814, 14–18. doi:10.1016/j.bbapap.2010.08.008
Niu G., Rupasinghe S. G., Zangerl A. R., Siegel J. P., Schuler M. A., Berenbaum M. R. (2011). A substrate-specific cytochrome P450 monooxygenase, CYP6AB11, from the polyphagous navel orange worm (Amyelois transitella). Insect biochem. Mol. Biol. 41, 244–253. doi:10.1016/j.ibmb.2010.12.009
Peng L., Zhao Y., Wang H., Song C., Shangguan X., Ma Y., et al. (2017). Functional study of cytochrome P450 enzymes from the Brown planthopper (Nilaparvata lugens Stål) to analyze its adaptation to BPH-resistant rice. Front. Physiol. 8, 972. doi:10.3389/fphys.2017.00972
Pentzold S., Zagrobelny M., Rook F., Bak S. (2014). How insects overcome two-component plant chemical defence: Plant β-glucosidases as the main target for herbivore adaptation. Biol. Rev. Camb. Philos. Soc. 89, 531–551. doi:10.1111/brv.12066
Pottier M. A., Bozzolan F., Chertemps T., Jacquin-Joly E., Lalouette L., Siaussat D., et al. (2012). Cytochrome P450s and cytochrome P450 reductase in the olfactory organ of the cotton leafworm Spodoptera littoralis. Insect Mol. Biol. 21, 21568–21580. doi:10.1111/j.1365-2583.2012.01160.x
Rupasinghe S. G., Wen Z. M., Chiu T. L., Schuler M. A. (2007). Helicoverpa zea CYP6B8 and CYP321A1: Different molecular solutions to the problem of metabolizing plant toxins and insecticides. Protein Eng. Des. Sel. 20, 615–624. doi:10.1093/protein/gzm063
Saleem M. A., Shakoori A. R. (1996). Biochemical studies in talcord 10EC effect on some enzyme activities and macromolecules of 6th instar larvae of Tribolium castaneum, Pakistan. J. Zool. 28, 75–83.
Sasabe M., Wen Z., Berenbaum M. R., Schuler M. A. (2004). Molecular analysis of CYP321A1, a novel cytochrome P450 involved in metabolism of plant allelochemicals (furanocoumarins) and insecticides (cypermethrin) in Helicoverpazea. Gene 338, 163–175. doi:10.1016/j.gene.2004.04.028
Schuler M. A., Berenbaum M. R. (2013). Structure and function of cytochrome P450S in insect adaptation to natural and synthetic toxins: Insights gained from molecular modeling. J. Chem. Ecol. 39, 1232–1245. doi:10.1007/s10886-013-0335-7
Schuler M. A. (2012). Insect P450s: Mounted for battle in their war against toxins. Mol. Ecol. 21, 4157–4159. doi:10.1111/j.1365-294X.2012.05657.x
Scott J. G., Wen Z. M. (2001). Cytochromes P450 of insects: The tip of the iceberg. Pest Manag. Sci. 57, 958–967. doi:10.1002/ps.354
Selin-Rani S., Senthil-Nathan S., Thanigaivel A., Vasantha-Srinivasan P., Edwin E. S., Ponsankar A., et al. (2016). Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere 165, 257–267. doi:10.1016/j.chemosphere.2016.08.136
Senthil-Nathan S. (2015). “A review of biopesticides and their mode of action against insect pests,” in Environmental sustainability (New Delhi: Springer), 49–63. doi:10.1007/978-81-322-2056-5_3
Senthil-Nathan S. (2020). A review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals, and essential oils as alternative larvicidal agents against mosquitoes. Front. Physiol. 10, 1591. doi:10.3389/fphys.2019.01591
Senthil-Nathan S., Kalaivani K. (2006). Combined effects of azadirachtin and nucleopolyhedrovirus (SpltNPV) on Spodoptera litura Fabricius (Lepidoptera: Noctuidae) larvae. Biol. Control 39 (1), 96–104. doi:10.1016/j.biocontrol.2006.06.013
Senthil-Nathan S., Kalaivani K. (2005). Efficacy of nucleopolyhedrovirus and azadirachtin on Spodoptera litura fabricius (Lepidoptera: Noctuidae). Biol. Control 34 (1), 93–98. doi:10.1016/j.biocontrol.2005.03.001
Senthil‐Nathan S. (2019). Effect of methyl jasmonate (MeJA) ‐induced defenses in rice against the rice leaffolder Cnaphalocrocis medinalis (guenèe)(Lepidoptera: Pyralidae). Pest Manag. Sci. 75 (2), 460–465. doi:10.1002/ps.5139
Shu Y., Zhang G. (2012). JResponse of the common cutworm spodoptera litura to zinc stress: Zn accumulation, metallothionein and cell ultrastructure of the midgut. Sci. Total Environ. 438, 210–217. doi:10.1016/j.scitotenv.2012.06.065
Shyam-Sundar N., Sivanesh H., Karthi S., Thanigaivel A., Stanley-Raja V., Chanthini K. M. P., et al. (2021a). Developmental response of Spodoptera litura Fab in response to plant extract of Desmostachya bipinnata (L.) and its effect on non-target organism, earthworm (Eisenia fetida). Environ. Sci. Pollut. Res. Int. 28 (7), 7870–7882. doi:10.1007/s11356-020-11015-4
Shyam-Sundar N. S., Karthi S., Sivanesh H., Stanley-Raja V., Chanthini K. M. P., Ramasubramanian R., et al. (2021b). Efficacy of Precocene I from Desmosstachya bipinnata as an effective bioactive molecules against the Spodoptera litura fab. and its impact on Eisenia fetida Savigny. Molecules 26 (21), 6384. doi:10.3390/molecules26216384
Singh S. P., Coronella J. A., Benes H., Cochrane B. J., Zimniak P. (2001). Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST-2) in conjugation of lipid peroxidation end products. Eur. J. Biochem. 268, 2912–2923. doi:10.1046/j.1432-1327.2001.02179.x
Sparks T. C., Storer N., Porter A., Slater R., Nauen R. (2021). Insecticide resistance management and industry: The origins and evolution of the insecticide resistance action committee (IRAC) and the mode of action classification scheme. Pest Manag. Sci. 77, 2609–2619. doi:10.1002/ps.6254
Subaharan K., Senthoorraja R., Manjunath S., Thimmegowda G. G., Pragadheesh V. S., Bakthavatsalam N., et al. (2021). Toxicity, behavioural and biochemical effect of Piper betle L. essential oil and its constituents against housefly, Musca domestica L. Pestic. Biochem. Physiol. 174, 104804. doi:10.1016/j.pestbp.2021.104804
Takabayashi J., Dicke M., Posthumus M. A. (1991). Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: Relative influence of plant and herbivore. Chemoecology 2, 1–6. doi:10.1007/bf01240659
Tang B., Xu K., Liu Y., Zhou Z., Karthi S., Yang H., et al. (2022). A review of physiological resistance to insecticide stress in Nilaparvata lugens. Biotech. 12 (3), 84–88. doi:10.1007/s13205-022-03137-y
Tao X. Y., Xue X. Y., Huang Y. P., Chen X. Y., Mao Y. B. (2012). Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide. Mol. Ecol. 21, 4371–4385. doi:10.1111/j.1365-294X.2012.05548.x
Usha-Rani P., Pratyusha S. (2013). Defensive role of Gossypium hirsutum L. anti-oxidative enzymes and phenolic acids in response to Spodoptera litura F. feeding. J. Asia. Pac. Entomol. 16, 131–136. doi:10.1016/j.aspen.2013.01.001
Wang R. L., Liu S. W., Baerson S. R., Qin Z., Ma Z. H., Su Y. J., et al. (2018a). Identification and functional analysis of a novel cytochrome P450 gene CYP9A105 associated with pyrethroid detoxification in Spodoptera exigua Hubner. Int. J. Mol. Sci. 19, 737. doi:10.3390/ijms19030737
Wang X., Chen Y., Gong C., Yao X., Jiang C., Yang Q. (2018b). Molecular identification of four novel cytochrome P450 genes related to the development of resistance of Spodoptera exigua (Lepidoptera: Noctuidae) to chlorantraniliprole. Pest Manag. Sci. 74, 1938–1952. doi:10.1002/ps.4898
War A. R., Paulraj M. G., War M. Y., Ignacimuthu S. (2011). Herbivore- and elicitor induced resistance in groundnut to Asian armyworm, Spodoptera litura, (Fab.) (Lepidoptera: Noctuidae). Plant Signal. Behav. 6, 1769–1777. doi:10.4161/psb.6.11.17323
Zeng R. S., Wen Z., Niu G., Schuler M. R. (2007). Berenbaum, Allelochemical induction of cytochrome P450 monooxygenases and amelioration of xenobiotic toxicity in Helicoverpa zea. J. Chem. Ecol. 33, 449–461. doi:10.1007/s10886-006-9238-1
Zhou X. J., Ma C. X., Li M., Sheng C. F., Liu H. X., Qiu X. H. (2010). CYP9A12 and CYP9A17 in the cotton bollworm, Helicoverpa armigera: Sequence similarity expression profile and xenobiotic response. Pest Manag. Sci. 66, 65–73. doi:10.1002/ps.1832
Keywords: phytochemicals, Precocene 1, pyrethroid, growth regulator, anti-juvenile hormone, cytochrome P450, qRT-PCR, gene expression
Citation: Shyam-Sundar N, Ramasubramanian R, Karthi S, Senthil-Nathan S, Chanthini KM-P, Sivanesh H, Stanley-Raja V, Ramkumar G, Narayanan KR, Mahboob S, Al-Ghanim KA, Abdel-Megeed A and Krutmuang P (2022) Effects of phytocompound Precocene 1 on the expression and functionality of the P450 gene in λ-cyhalothrin-resistant Spodoptera litura (Fab.). Front. Physiol. 13:900570. doi: 10.3389/fphys.2022.900570
Received: 20 March 2022; Accepted: 13 September 2022;
Published: 10 November 2022.
Edited by:
Arash Zibaee, University of Guilan, IranReviewed by:
Jalal Jalali Sendi, University of Guilan, IranCheng Qin, Zunyi Vocational and Technical College, China
Copyright © 2022 Shyam-Sundar, Ramasubramanian, Karthi, Senthil-Nathan, Chanthini, Sivanesh, Stanley-Raja, Ramkumar, Narayanan, Mahboob, Al-Ghanim, Abdel-Megeed and Krutmuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sengottayan Senthil-Nathan, c2VudGhpbEBtc3VuaXYuYWMuaW4=; Patcharin Krutmuang, cGF0Y2hhcmluazI2QGdtYWlsLmNvbQ==
 Narayanan Shyam-Sundar1
Narayanan Shyam-Sundar1 Sengodan Karthi
Sengodan Karthi Sengottayan Senthil-Nathan
Sengottayan Senthil-Nathan Vethamonickam Stanley-Raja
Vethamonickam Stanley-Raja Govindaraju Ramkumar
Govindaraju Ramkumar Ahmed Abdel-Megeed
Ahmed Abdel-Megeed Patcharin Krutmuang
Patcharin Krutmuang