- 1Division of Training and Movement Sciences, Research Focus Cognition Sciences, University of Potsdam, Potsdam, Germany
- 2Department of Individual Sports, Higher Institute of Sport and Physical Education of Ksar Said, University of Manouba, Tunis, Tunisia
- 3Department of Health Sciences, CUNY Lehman College, Bronx, NY, United States
- 4Rehabilitation and Exercise Sciences, School of Sport, University of Essex, Colchester, United Kingdom
- 5Department of Physical Activity Sciences, Universidad de Los Lagos, Osorno, Chile
- 6Exercise and Rehabilitation Sciences Laboratory, Faculty of Rehabilitation Sciences, School of Physical Therapy, Universidad Andres Bello, Santiago, Chile
- 7Department of Sport Science, Institute III, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
- 8Department of Orthopedics, University Medicine Rostock, Rostock, Germany
- 9Exercise Biology Group, Faculty of Sport and Health Sciences, Technical University of Munich, Munich, Germany
- 10School of Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom
- 11Institute of Sport, Exercise and Health, University College London, London, United Kingdom
- 12Department of Sports and Health Sciences, Faculty of Human Sciences, University of Potsdam, Potsdam, Germany
Objective: To examine the effect of plyometric jump training on skeletal muscle hypertrophy in healthy individuals.
Methods: A systematic literature search was conducted in the databases PubMed, SPORTDiscus, Web of Science, and Cochrane Library up to September 2021.
Results: Fifteen studies met the inclusion criteria. The main overall finding (44 effect sizes across 15 clusters median = 2, range = 1–15 effects per cluster) indicated that plyometric jump training had small to moderate effects [standardised mean difference (SMD) = 0.47 (95% CIs = 0.23–0.71); p < 0.001] on skeletal muscle hypertrophy. Subgroup analyses for training experience revealed trivial to large effects in non-athletes [SMD = 0.55 (95% CIs = 0.18–0.93); p = 0.007] and trivial to moderate effects in athletes [SMD = 0.33 (95% CIs = 0.16–0.51); p = 0.001]. Regarding muscle groups, results showed moderate effects for the knee extensors [SMD = 0.72 (95% CIs = 0.66–0.78), p < 0.001] and equivocal effects for the plantar flexors [SMD = 0.65 (95% CIs = −0.25–1.55); p = 0.143]. As to the assessment methods of skeletal muscle hypertrophy, findings indicated trivial to small effects for prediction equations [SMD = 0.29 (95% CIs = 0.16–0.42); p < 0.001] and moderate-to-large effects for ultrasound imaging [SMD = 0.74 (95% CIs = 0.59–0.89); p < 0.001]. Meta-regression analysis indicated that the weekly session frequency moderates the effect of plyometric jump training on skeletal muscle hypertrophy, with a higher weekly session frequency inducing larger hypertrophic gains [β = 0.3233 (95% CIs = 0.2041–0.4425); p < 0.001]. We found no clear evidence that age, sex, total training period, single session duration, or the number of jumps per week moderate the effect of plyometric jump training on skeletal muscle hypertrophy [β = −0.0133 to 0.0433 (95% CIs = −0.0387 to 0.1215); p = 0.101–0.751].
Conclusion: Plyometric jump training can induce skeletal muscle hypertrophy, regardless of age and sex. There is evidence for relatively larger effects in non-athletes compared with athletes. Further, the weekly session frequency seems to moderate the effect of plyometric jump training on skeletal muscle hypertrophy, whereby more frequent weekly plyometric jump training sessions elicit larger hypertrophic adaptations.
1 Introduction
Plyometric jump training is a popular form of physical conditioning in both general (Moran et al., 2018; Izquierdo et al., 2021) and athletic populations (Ramirez-Campillo et al., 2018; Ramirez-Campillo et al., 2020b). It constitutes a suitable training option in a resource-constrained setting as it can be carried out without the need for equipment. Plyometric exercises involve the physiological phenomenon called the “stretch-shortening cycle” (Ishikawa and Komi, 2008; Taube et al., 2012), which consists of a rapid eccentric action of the agonistic muscle-tendon unit immediately followed by rapid concentric action of that same muscle-tendon unit (Komi, 1984; Taube et al., 2012). The main advantage of the stretch-shortening cycle compared with isolated concentric or eccentric muscle actions is the storage and subsequent release of kinetic energy eliciting greater power production (Dietz et al., 1979; Voigt et al., 1998). Generally, the efficiency of the stretch-shortening cycle is underpinned by a complex interaction of multiple hierarchical levels of the central nervous system including the coordination of anticipated (feedforward) and reflex (feedback) mechanisms [for more insights see Taube et al. (2012)].
There is persuasive evidence on the effectiveness of plyometric jump training on a wide range of measures of physical fitness (e.g., muscle strength, muscle power, sprint speed, and balance) regardless of age, sex, and training experience (Bedoya et al., 2015; Ramírez-Campillo et al., 2015; Chaabene and Negra, 2017; Moran et al., 2018; Chaabene et al., 2019; Vetrovsky et al., 2019; Ramirez-Campillo et al., 2020a; Ramirez-Campillo et al., 2021a; Ramirez-Campillo et al., 2021b). Additionally, plyometric jump training benefits many parameters of health (e.g., bone mineral density, injury prevention, and fall prevention) (Markovic and Mikulic, 2010; Kish et al., 2015; Gómez-Bruton et al., 2017; Vlachopoulos et al., 2018; Vetrovsky et al., 2019; Bull et al., 2020; Huang et al., 2020). The benefits of plyometric jump training are mainly attributable to an increased neural drive to the active muscles (Häkkinen et al., 1985; Häkkinen et al., 1990; Chimera et al., 2004; Kyröläinen et al., 2005; Fouré et al., 2012; Suchomel et al., 2018). More specifically, the increase in muscle strength and power following plyometric jump training has usually been attributed to increased neuromuscular activation (e.g., motor unit recruitment, firing frequency, synchronization, etc.) and better inter-muscular coordination (e.g., decreased co-activation of the antagonist).
Unlike traditional resistance training, the effects of plyometric jump training on skeletal muscle hypertrophy have received little attention in the literature. Skeletal muscle hypertrophy can be defined as an increase in muscle mass and cross-sectional area (CSA) at the level of the entire muscle as well as individual muscle fibers (Russell et al., 2000; Schoenfeld et al., 2021). It can be directly measured using macroscopic [e.g., B-mode ultrasound, magnetic resonance imaging (MRI)] or microscopic (e.g., biopsy) assessment methods (Haun et al., 2019). Additionally, an indirect method has also been developed and applied using a prediction equation to assess muscle volume and CSA (Chelly et al., 2006). Of note, the effects of plyometric jump training on skeletal muscle hypertrophy seem to be equivocal in the existing literature. For instance, Kyröläinen et al. (2005) examined the effects of plyometric jump training on fiber CSA of the lateral gastrocnemius muscle in recreationally active males aged 24 years and reported no changes after 15 weeks of training. Likewise, Fouré et al. (2012) studied the effects of 14 weeks of plyometric jump training on the CSA of the gastrocnemius muscles in healthy active males aged 20 years and revealed no significant changes after training. In contrast, Kubo et al. (2007) reported a significant increase in plantar flexor muscle volume (∼5%) following 12 weeks of plyometric jump training in healthy untrained males aged 22 years. Additionally, Malisoux et al. (2006b) examined the effects of 8 weeks of plyometric jump training on single fiber CSA in recreationally active males aged 23 years. These authors revealed a significant increase in the CSA of type I (+23%), type IIa (+22%), and type IIa/IIx fibers (+30%) in the vastus lateralis muscle. Markovic and Mikulic (2010) conducted a comprehensive review of the effects of plyometric jump training on neuromuscular and performance outcomes and concluded that plyometric jump training has the potential to enhance skeletal muscle hypertrophy but to a lesser extent compared with traditional resistance training. Recently, Ramírez-delaCruz et al. (2022) conducted a systematic review and meta-analysis on the effects of plyometric jump training on lower body muscle architecture in healthy adults (≥18 years). The authors revealed that plyometric jump training is an effective method to increase muscle thickness of the vastus lateralis, vastus medialis, rectus femoris, and triceps surae. They additionally concluded that plyometric jump training is effective in increasing fascicle length of the vastus lateralis and rectus femoris muscles, and pennation angle of the rectus femoris muscle. However, the study suffers from several methodological flaws pertaining to the included studies, which would lead to biased outcomes. For example, some studies have used plyometric jump training with increased or reduced body mass (Hirayama et al., 2017; Ullrich et al., 2018; Stien et al., 2020), others used a combination of plyometric jump training with traditional resistance training (Hunter and Marshall, 2002; Kijowksi et al., 2015) and some of the included studies actually did not use plyometric training at all (Helland et al., 2017; Horwath et al., 2019; Kudo et al., 2020; van der Zwaard et al., 2021). Additionally, the authors calculated within-group pre-post effect size (Ramírez-delaCruz et al., 2022). Of note, such an approach has been criticized as it results in biased outcomes (Cuijpers et al., 2017).
Given the inconsistent outcomes as to the effects of plyometric jump training on skeletal muscle hypertrophy from individual studies and the methodological shortcomings in a recent meta-analysis (Ramírez-delaCruz et al., 2022), there is a need to systematically summarize the literature and aggregate data from the available studies to draw more conclusive evidence (Higgins, 2011). Additionally, factors such as age, sex, and training experience as well as different training variables like weekly session frequency, number of jumps per session, and training duration appear to moderate the effects of plyometric jump training on measures of physical fitness (de Villarreal et al., 2009; Sáez-Sáez de Villarreal et al., 2010; Asadi et al., 2016), yet are not well elucidated for skeletal muscle hypertrophy. Therefore, the primary aim of this systematic review with multilevel meta-analysis was to examine the effect of plyometric jump training on skeletal muscle hypertrophy in healthy individuals. The secondary objective was to identify the factors (i.e., age, sex, and training experience) and plyometric jump training variables (i.e., total training period, weekly session frequency, single session duration, and number of jumps per week) that potentially moderate the effect on skeletal muscle hypertrophy to help guide training prescription.
2 Materials and Methods
This systematic review was conducted per the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statements (Page et al., 2021). The current study was pre-registered in the International Prospective Register of Systematic Reviews (PROSPERO) on 13 August 2021 under the registration number “CRD42021265213.”
2.1 Search Strategy
A literature search was performed separately and independently by two of the coauthors (AF and CH) in PubMed, SPORTDiscus, Web of Science, and Cochrane Library databases up to September 2021. The search was conducted using a Boolean search strategy with the operators “AND” and “OR” and a combination of the following keywords: (“plyometric” OR “stretch-shortening cycle” OR “stretch shortening cycle*” OR “jump training” OR jump) AND (“hypertrophy” OR “muscle size” OR “muscle mass” OR “muscle fiber” OR “muscle fibre” OR “lean body mass” OR “fat-free mass” OR “cross-sectional area” OR “quadriceps size” OR “circumference”). Keywords were determined through expert opinion, literature review, and controlled vocabulary (e.g., Medical Subject Headings). In addition, corresponding meta-analyses, as well as studies that were eligible for inclusion, were searched for additional publications in so-called “snowball” searches (Greenhalgh and Peacock, 2005). Only peer-reviewed studies written in English were considered for inclusion.
2.2 Inclusion and Exclusion Criteria (Study Selection)
We used the PICOS (Population, Intervention, Comparison, Outcome, Study Design) approach to identify eligible studies (Moher et al., 2009). The following inclusion criteria were defined a priori: 1) Population: a cohort of healthy participants with no restriction related to age, sex, or training experience, 2) Intervention: plyometric jump training (i.e., jump exercises soliciting the stretch-shortening cycle), with a minimum duration of 4 weeks (Monti et al., 2020), 3) Comparison: active/passive control group, 4) Outcome: at least one measure of skeletal muscle hypertrophy (e.g., muscle/single fiber cross-sectional area, muscle thickness, lean body mass, muscle circumference), and 5) Study design: (randomized) controlled trials with baseline and follow up measures. We excluded studies involving individuals with pre-existing health problems (e.g., diabetes, hypertension, and asthma), an absence of a passive/active control group, plyometric jump training interventions in combination with additional interventions (e.g., nutrition), and/or lack of baseline and follow-up data.
2.3 Data Extraction
The first author (AF) extracted the data from the included studies in a standardised template created with Microsoft Excel. A second author (MA) cross-verified the extracted data. In case of disagreement pertaining data extraction or study eligibility, co-author CH was consulted for clarification.
Of note, we included data for all skeletal muscle hypertrophy measures reported in the studies and for all time points at which they were measured. More specifically, if a study reported multiple skeletal muscle hypertrophy measures, they were all included, and if a study reported measurements during and after the training period, they were all included as well (dependence between effect sizes was handled using a multilevel robust variance estimation approach—see statistical analyses). If data were not reported in a way that was conducive to extraction for our analysis, we contacted the respective authors to request appropriate data [i.e., mean ± standard deviation (SD), raw data]. When multiple studies were published using the same data set (Skurvydas and Brazaitis, 2010; Skurvydas et al., 2010), only one study was considered (Skurvydas and Brazaitis, 2010). In cases where the authors did not respond to our request for raw data, we used WebPlotDigitizer (v4.3, Ankit Rohatgi; https://apps.automeris.io/wpd/) to extract relevant data in studies that only reported graphical data (Drevon et al., 2017).
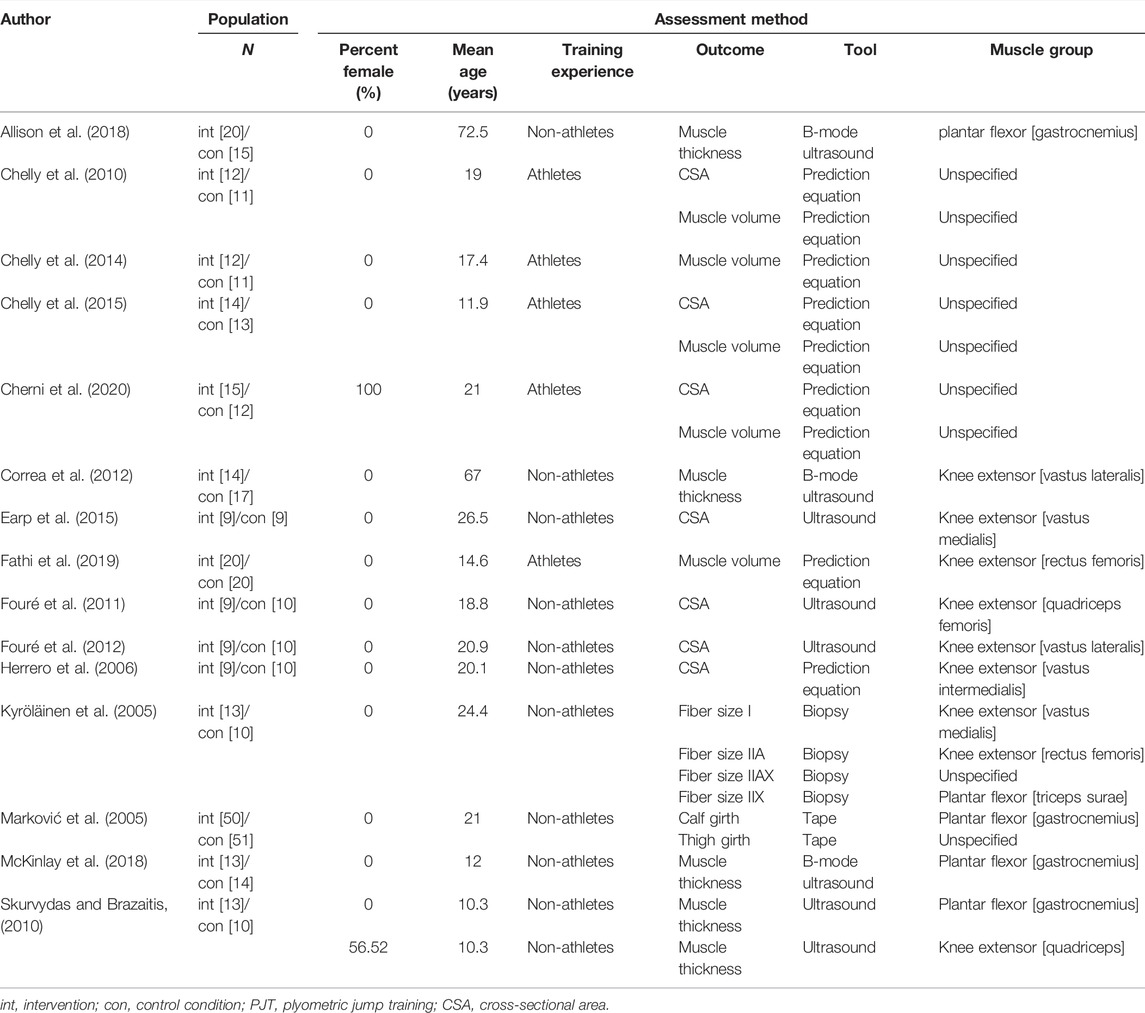
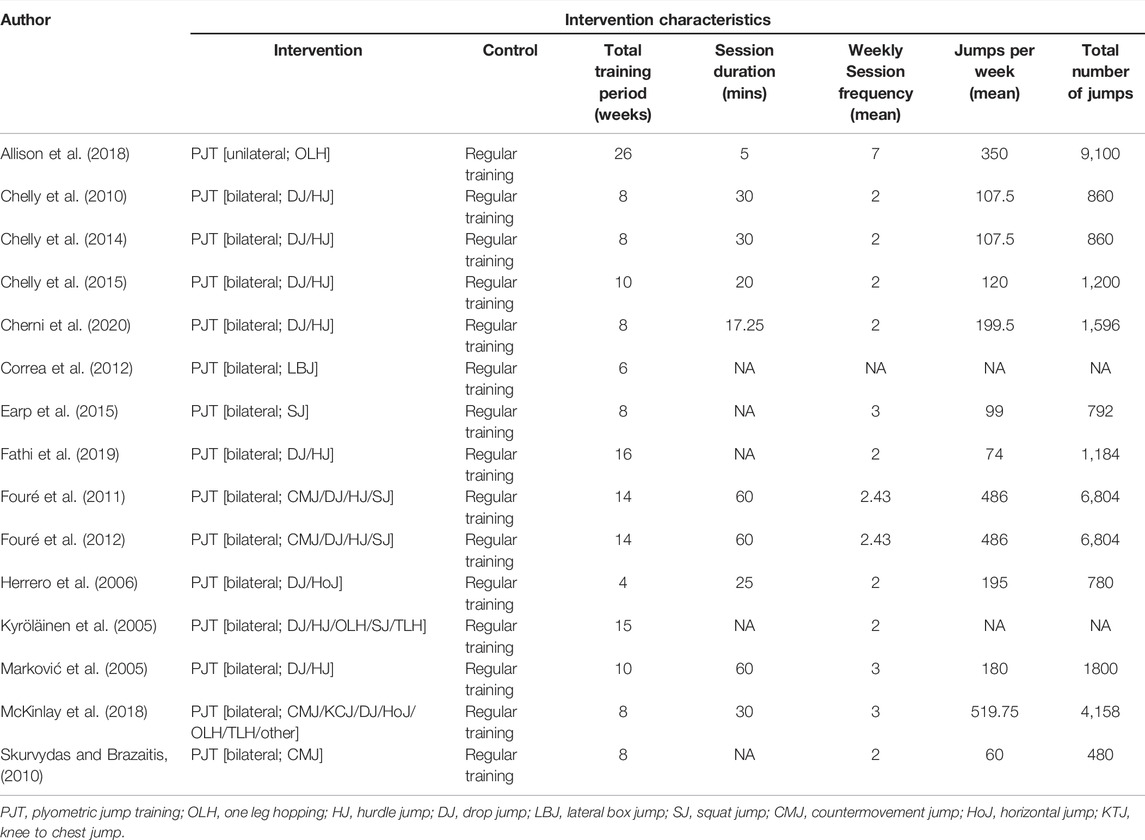
From all included studies, we extracted 1) author and year of publication; 2) mean age of participants; 3) percentage of females in the sample; 4) training experience [i.e., athlete vs. non-athlete (see footnote1)], 5) muscle group investigated [i.e., knee extensors (e.g., vastus medialis and rectus femoris) vs., plantar flexors (e.g., gastrocnemius)], 6) assessment method (i.e., ultrasound and prediction equation), 7) total training period (weeks), 8) weekly session frequency, 9) single session duration, 10) total number of jumps per week, and 11) type of jumping. The characteristics of the included studies are displayed in Tables 1, 2.
2.4 The Methodological Quality of the Included Studies
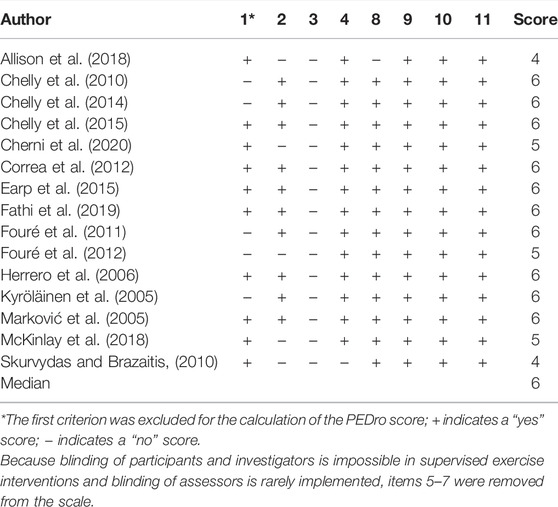
The Physiotherapy Evidence Database (PEDro) scale was used to evaluate the methodological quality of the included studies. The validity and reliability of the PEDro scale have been established in previous studies (Maher et al., 2003; de Morton, 2009). Additionally, its agreement with other assessment tools such as the Cochrane risk of bias tool has been established (Moseley et al., 2019). As blinding of participants and investigators is not feasible in exercise interventions and blinding of assessors is rarely implemented, items 5–7 were removed from the scale consistent with previous systematic reviews (Grgic et al., 2017; Schoenfeld et al., 2017). Hence the methodological quality of the included studies was rated on a scale from 0 to 7. In accordance with previous systematic reviews (Kümmel et al., 2016; Schoenfeld et al., 2017), the quality of the included studies was categorized as “poor” = 0–3, “moderate” = 4, “good” = 5, and “excellent” = 6–7 (Table 3). Additionally, to visually estimate publication bias, a contour-enhanced funnel plot was used (Harrer et al., 2019).
2.5 Synthesis and Analyses
The meta-analysis was performed using the “metafor” (Viechtbauer, 2010) and “tidyverse” (Wickham et al., 2019) packages in R (v 4.0.2; R Core Team, https://www.r-project.org/). All analyses are available in the supplementary documentation (https://osf.io/bf478/). The standardised mean difference (SMD) was calculated by subtracting the standardised mean change of the intervention group minus the standardised mean change of the control group. The respective variance was calculated by pooling the pre-test standard deviations of both groups, an approach deemed appropriate to provide a comparatively unbiased estimate of the population effect size (Morris, 2008). The magnitude of standardised effect sizes was interpreted in accordance with Cohen’s thresholds (Cohen, 1988): trivial (< 0.2), small (0.2 to 0.5), moderate (0.5 to 0.8), and large (≥ 0.8).
Due to the nested structure of the calculated effect sizes (i.e., effects nested within groups nested within studies), multilevel mixed-effects meta-analyses with study and intra-study groups as random effects were calculated to examine the effect of plyometric jump training on skeletal muscle hypertrophy. Further, cluster robust point estimates using 95% compatibility (confidence) intervals (CIs) were calculated (Hedges et al., 2010) and weighted by inverse sampling variance to account for the within- and between-study variance (tau-squared). Restricted maximal likelihood estimation was applied in all models. A main model was created containing all effect sizes. Additional exploratory subgroup comparisons and meta-regression analyses of moderator variables were performed, including mean age, proportion of females per group, training experience, and muscle group studied, as well as training characteristics such as total training period, training frequency, session duration, and number of jumps per week. For training experience and muscle group examined, multilevel models with subgroups were calculated and robust estimates were produced. Meta-regressions were calculated for mean age, proportion of females per study, and the aforementioned training characteristics.
To avoid dichotomizing the existence of an effect in our models, we reported absolute p-values but did not employ traditional null hypothesis significance testing (Amrhein et al., 2019a; Amrhein et al., 2019b; McShane et al., 2019). We also focused on the point estimate in the interpretation with the greatest emphasis on the effects from the lower to the upper limit of the interval estimates (Nakagawa and Cuthill, 2007; Lee, 2016; Van Calster et al., 2018). The risk of small study bias was visualised through contour-enhanced funnel plots. Further, Q and I2 statistics were produced and reported (Higgins et al., 2003). A significant Q statistic is usually taken as an indicator that the effects are unlikely to come from a common population. I2 values indicate the degree of heterogeneity of effects as follows: 0%–40% indicates no heterogeneity, 30%–60% moderate heterogeneity, 50%–90% substantial heterogeneity, and 75%–100% considerable heterogeneity (Higgins et al., 2019). For within-participant effects, pre-post correlations for measures have rarely been reported; therefore, we adopted a range of values for correlation coefficients (r = 0.5, 0.7, and 0.9) and examined the sensitivity of the results to each of these values. Since the overall results were relatively insensitive to this range, we reported the results for r = 0.7 here and included the results for the other assumed correlation coefficients in the Supplementary Material is available on the following link: (https://osf.io/bf478/).
3 Results
3.1 Study Characteristics
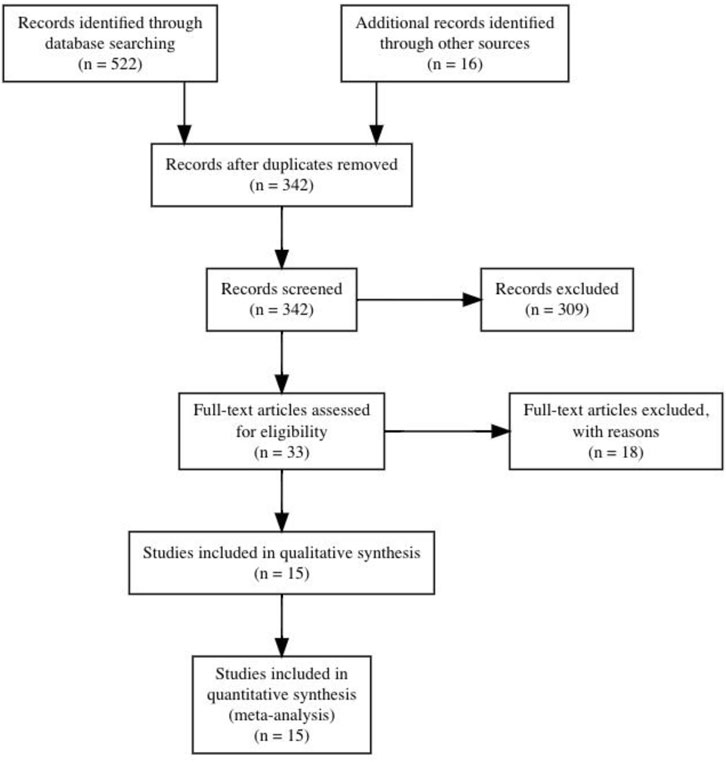
After initial searches and screening, nine studies were identified that met inclusion criteria. Supplementary search approaches identified six additional eligible studies. Thus, a total of 15 studies were ultimately included for analysis. All studies included active control groups. Details of the search and inclusion process are shown in the flow chart (Figure 1; https://osf.io/3u567/). The total number of participants across the included studies is 478 (range = 18–101; median = 23), comprising 245 INT (range = 9–50, median = 13) and 233 CON (range = 5–51, median = 11). The majority of the studies used different types of jumps in their training programmes (e.g., bilateral/unilateral vertical and horizontal jumps) (Kyröläinen et al., 2005; Marković et al., 2005; Herrero et al., 2006; Chelly et al., 2010; Fouré et al., 2011; Fouré et al., 2012; Chelly et al., 2014; Chelly et al., 2015; McKinlay et al., 2018; Fathi et al., 2019; Cherni et al., 2020). Four studies used one single type of jump such as bilateral countermovemt jump or lateral box jump (Skurvydas and Brazaitis, 2010; Correa et al., 2012; Earp et al., 2015; Allison et al., 2018). The mean age across studies ranged from 10.3 to 72.5 years with a median of 20.1 years. Two studies examined the effects of plyometric jump training on hypertrophy in female participants (Correa et al., 2012; Cherni et al., 2020), while one study included a mixed intervention group but used a male-only control group (Skurvydas and Brazaitis, 2010). The remaining twelve studies included male participants. Two studies included older adults (Correa et al., 2012; Allison et al., 2018). As to training experience, five studies recruited athletes (Chelly et al., 2010; Chelly et al., 2014; Chelly et al., 2015; Fathi et al., 2019; Cherni et al., 2020), while ten studies investigated the effects of plyometric jump training on skeletal muscle hypertrophy in non-athletes (Kyröläinen et al., 2005; Marković et al., 2005; Herrero et al., 2006; Skurvydas and Brazaitis, 2010; Fouré et al., 2011; Fouré et al., 2012; Correa et al., 2012; Earp et al., 2015; Allison et al., 2018; McKinlay et al., 2018). Regarding the muscle group investigated, four studies examined hypertrophy in the knee extensors (Skurvydas and Brazaitis, 2010; Correa et al., 2012; Earp et al., 2015; McKinlay et al., 2018) and four in the plantar flexor (Kyröläinen et al., 2005; Fouré et al., 2011; Fouré et al., 2012; Allison et al., 2018). In the seven remaining studies, the muscle group investigated was not specified (e.g., assessment of thigh muscle volume or thigh/calf girth) (Marković et al., 2005; Herrero et al., 2006; Chelly et al., 2010; Chelly et al., 2014; Chelly et al., 2015; Fathi et al., 2019; Cherni et al., 2020). Seven studies used ultrasound imaging technique (Skurvydas and Brazaitis, 2010; Fouré et al., 2011; Fouré et al., 2012; Correa et al., 2012; Earp et al., 2015; Allison et al., 2018; McKinlay et al., 2018), while six studies used a prediction equation to assess muscle hypertrophy (Herrero et al., 2006; Chelly et al., 2010; Chelly et al., 2014; Chelly et al., 2015; Fathi et al., 2019; Cherni et al., 2020). One study assessed muscle hypertrophy using muscle biopsy (Kyröläinen et al., 2005) and one study used tape (Marković et al., 2005). The median duration of plyometric jump training was 8 weeks (range from 4–26) and the median weekly session frequency was two (range from 2–7). The median session duration was 30 min and ranged from 5 to 60 min. However, session duration was not reported in two studies (Kyröläinen et al., 2005; Correa et al., 2012). The median of the number of jumps performed per week was 230 (range = 60–520). Full details of all included studies can be seen in Tables 1, 2. Regarding the methodological quality of the included studies, the PEDro scores ranged from 4 to 6 with a median score of 6 (Table 3).
3.2 Main Model—All Effects
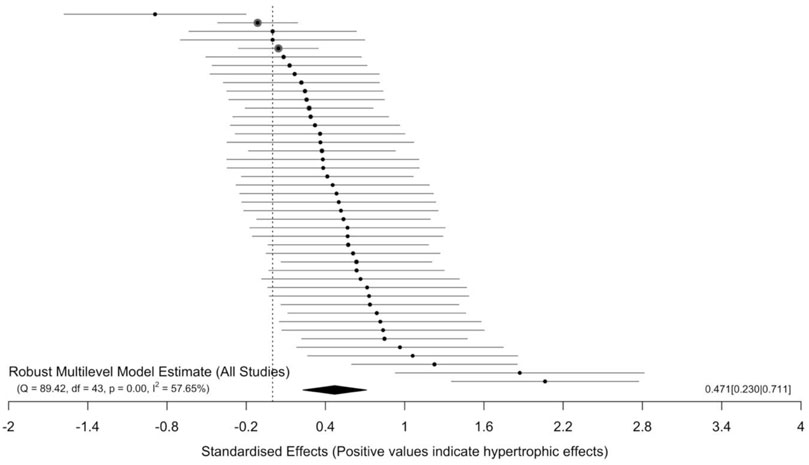
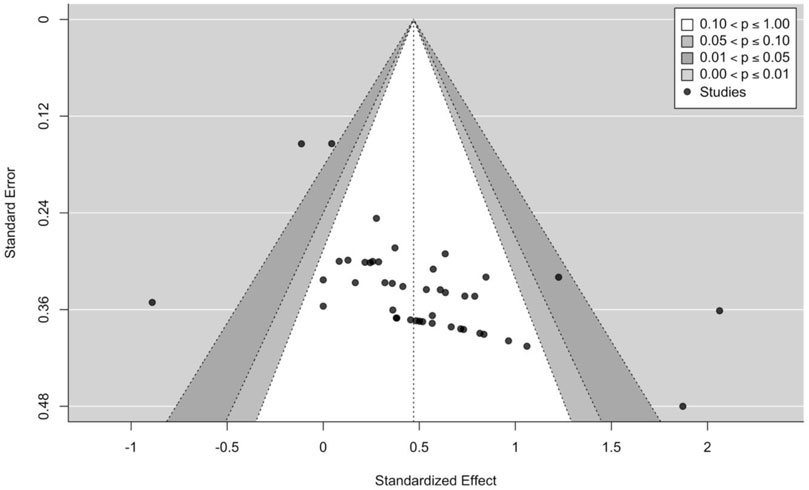
The main model (44 effect sizes across 15 clusters median = 2, range = 1–15 effects per cluster) yielded small to moderate effects with a small point estimate [SMD = 0.47 (95% CIs = 0.23–0.71); p < 0.001] and moderate to substantial heterogeneity (I2 = 57.53%). All effect sizes and interval estimates are presented in an ordered caterpillar plot (Figure 2; https://osf.io/csnrq/). The visual inspection of the funnel plot indicated a seemingly symmetrical distribution pattern of the effects that might be reflective of an apparently absence of publication bias (Figure 3; https://osf.io/gv7xq/).
3.3 Subgroup and Meta-Regression Analyses
3.3.1 Muscle Group
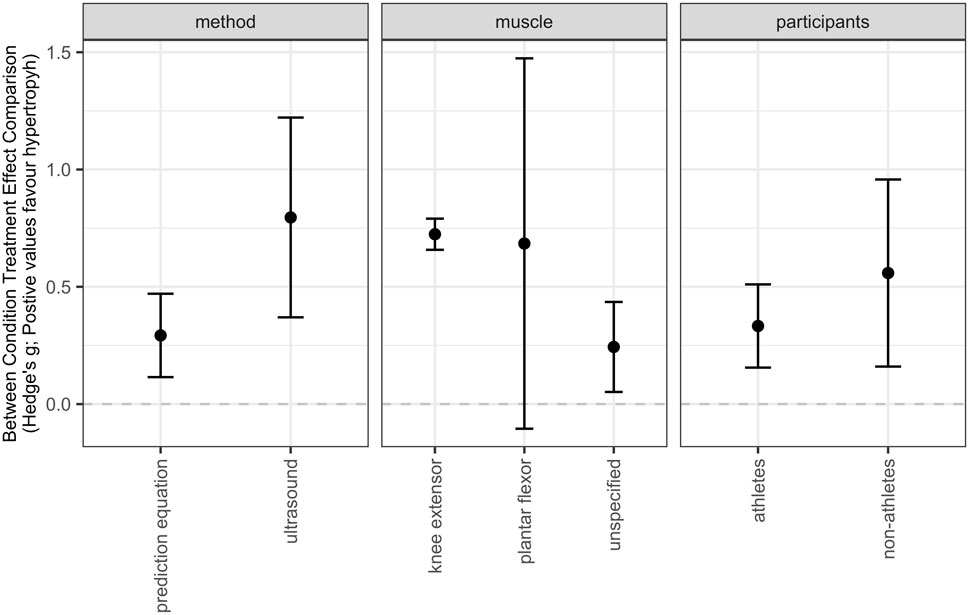
Subgroup models showed moderate effects for the knee extensors with a moderate point estimate [SMD = 0.72 (95% CIs = 0.66–0.78); p < 0.001] and an equivocal effect for the plantar flexors with a moderate point estimate [SMD = 0.64 (95% CIs = −0.25 to 1.55); p = 0.142]. Additionally, trivial to moderate effects with a small point estimate were observed for unspecified muscle groups [SMD = 0.23 (95% CIs = 0.04–0.43); p = 0.024]. The difference between sub-groups was notable (p = 0.001). The level of heterogeneity was moderate (I2 = 48.25%). This subgroup analysis is presented in a point-range plot in Figure 4 (https://osf.io/wn2xv/).
3.3.2 Training Experience
Subgroup models indicated trivial to moderate effects with a small point estimate for athletes [SMD = 0.33 (95% CIs = 0.16–0.51); p = 0.001] and trivial to large effects with a moderate point estimate for non-athletes [SMD = 0.55 (95% CIs = 0.18–0.93); p = 0.007] with no differences between subgroups (p = 0.270). The level of heterogeneity was substantial (I2 = 58.84%). The subgroup analysis is presented in a point-range plot in Figure 4 (https://osf.io/wn2xv/).
3.3.3 Assessment Method
Subgroup models revealed trivial to small effects with a small point estimate for prediction equations [SMD = 0.29 (95% CIs = 0.16–0.42); p < 0.001] and moderate to large effects with a moderate point estimate for ultrasound imaging [SMD = 0.74 (95% CIs = 0.59–0.89); p < 0.001] with a clear difference between subgroups (p < 0.001). Analysis further revealed no heterogeneity (I2 = 0%). The subgroup analysis is presented in a point-range plot in Figure 4 (https://osf.io/wn2xv/).
3.3.4 Mean Age of Participants
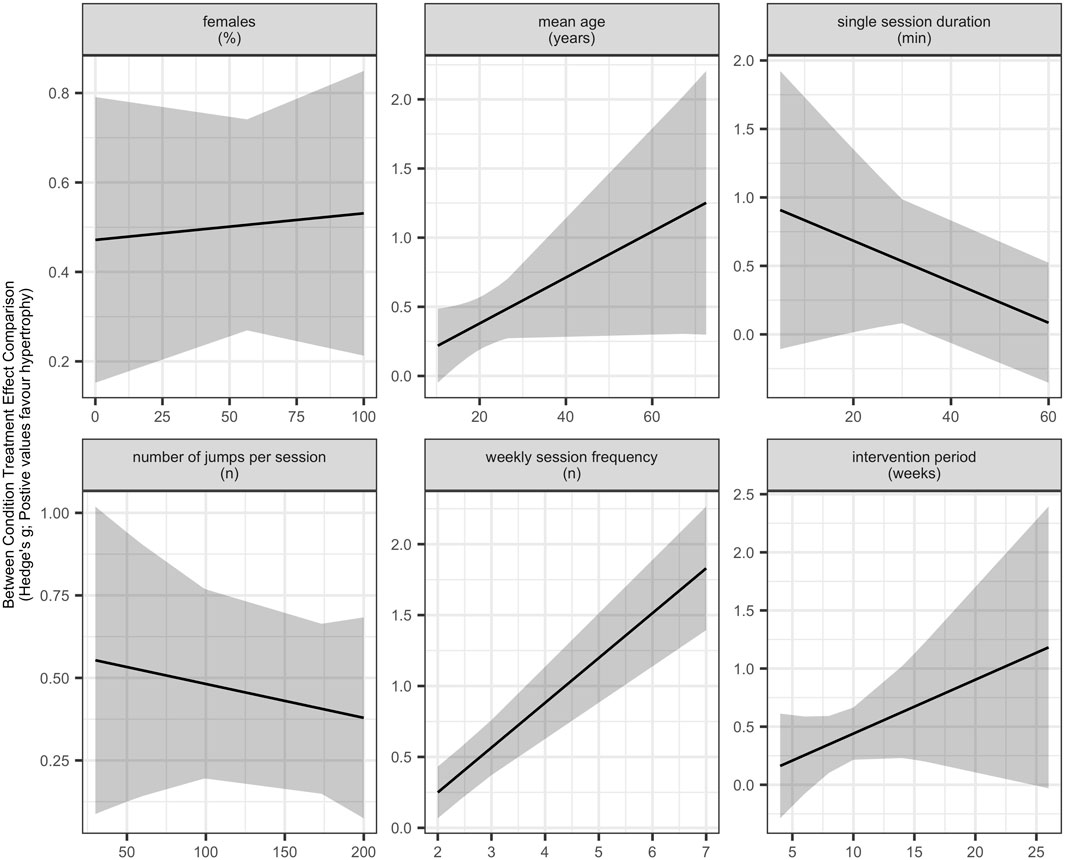
Meta-regression analyses did not indicate clear evidence that age moderates skeletal muscle hypertrophy adaptations in response to plyometric jump training [β = 0.0149 (95% CIs = −0.0033 to 0.0332); p = 0.101]. The level of heterogeneity was moderate (I2 = 46.90%). The regression analysis is depicted in a meta-analytic plot in Figure 5 (https://osf.io/g8aet/).
3.3.5 Percentage of Females in the Sample
Meta-regression analyses did not detect clear evidence that the percentage of females in the sample moderates the effects of plyometric jump training on skeletal muscle hypertrophy [β = 0.0006 (95% CIs = −0.0036 to 0.0049); p = 0.751]. The level of heterogeneity was substantial (I2 = 60.43%). The subgroup analysis is presented in a meta-analytic plot in Figure 5 (https://osf.io/g8aet/).
3.3.6 Total Training Period
Meta-regression analyses did not indicate clear evidence that total training period moderates the effect of plyometric jump training on skeletal muscle hypertrophy [β = 0.0433 (95% CIs = −0.0347 to 0.1215); p = 0.253). The level of heterogeneity was moderate to substantial (I2 = 56.78%). The subgroup analysis is illustrated in a meta-analytic plot in Figure 5 (https://osf.io/g8aet/).
3.3.7 Weekly Session Frequency
Meta-regression analyses showed that the weekly session frequency moderates the effect of plyometric jump training on skeletal muscle hypertrophy, with more sessions per week inducing larger gains in hypertrophy [β = 0.3233 (95% Cis = 0.2040–0.4425); p < 0.001]. The analysis further revealed no to moderate heterogeneity (I2 = 38.63%). The subgroup analysis is presented in a meta-analytic plot in Figure 5 (https://osf.io/g8aet/).
3.3.8 Single Session Duration
Meta-regression analyses revealed no clear evidence that single session duration moderates the effects of plyometric jump training on skeletal muscle hypertrophy [β = −0.0133 (95% CIs = −0.0387 to 0.0120); p = 0.261). The analysis further revealed substantial heterogeneity (I2 = 70.36%). The subgroup analysis is depicted in a meta-analytic plot in Figure 5 (https://osf.io/g8aet/).
3.3.9 Number of Jumps Per Week
Meta-regression analyses revealed no clear evidence that the number of jumps per week moderates the effects of plyometric jump training on skeletal muscle hypertrophy [β = 0.0009 (95% CIs = −0.0006 to 0.0025) p = 0.214]. The analysis showed substantial heterogeneity (I2 = 60.57%). The subgroup analysis is presented in a meta-analytic plot in Figure 5 (https://osf.io/g8aet/).
4 Discussion
This systematic review with multilevel meta-analysis aimed to 1) examine the effects of plyometric jump training on skeletal muscle hypertrophy in healthy individuals across different age ranges and 2) identify potentially important plyometric jump training variables relevant for promoting hypertrophic adaptations to help guide training prescription. The main findings indicated that plyometric jump training elicits small to moderate effects on skeletal muscle hypertrophy, regardless of sex, age, or training experience. Additionally, subgroup analyses showed relatively larger effects in non-athletes compared with athletes, and moderate effects for the knee extensors with an equivocal effect for the plantar flexors. Moreover, we found no clear evidence that age or sex moderated the effects of plyometric jump training on skeletal muscle hypertrophy. Furthermore, meta-regression analyses suggested that the effects on skeletal muscle hypertrophy were moderated by the weekly session frequency, with more frequent weekly plyometric jump training sessions resulting in larger hypertrophic adaptations. We found no clear evidence that total training period, single session duration, or the number of jumps per week moderate the effects of plyometric jump training on skeletal muscle hypertrophy.
4.1 Main Effect
The main findings of the present meta-analysis indicated small to moderate effects of plyometric jump training on skeletal muscle hypertrophy [SMD = 0.47 (95% CIs = 0.23–0.71)], regardless of sex, age, and training experience. The current outcomes corroborate the results of a recently published systematic review with meta-analysis where authors reported a moderate effect of plyometric jump training on muscle thickness (SMD = 0.59) and fascicle length (SMD = 0.51) in healthy adults (Ramírez-delaCruz et al., 2022). However, our results are relatively more conservative, which we attribute to our more stringent inclusion criteria for plyometric jump training as well as the comparison of the plyometric jump training to an active/passive control group. In sum, the findings of the current study as well as those of recent ones (Grgic et al., 2020; Ramírez-delaCruz et al., 2022) question the common belief, indicating that plyometric jump training can indeed increase not only the motor drive to the active muscles but also skeletal muscle hypertrophy.
The previously claimed limited potential for plyometric jump training-related hypertrophic adaptations has been attributed to the relatively short time under tension during the jumps and, therefore, a reduced mechanical stimulus for muscle protein synthesis (Schoenfeld, 2010; Wackerhage et al., 2019). In addition, some researchers have proposed that the inability to continually provide an overload stimulus during plyometric jump training is another potential limitation from a hypertrophy standpoint (Suchomel et al., 2018). In this context, while extra loads additional to body mass may be used with plyometric jump exercises (e.g., weighted vests) (Negra et al., 2020), caution must be taken given that heavier loads may result in greater impact forces and delay the transition time between eccentric and concentric muscle actions, which could harm the overall training stimulus (Suchomel et al., 2018). However, our findings seem to refute these claims. Indeed, the small to moderate effects of plyometric jump training on skeletal muscle hypertrophy observed in this study indicate that the high contraction velocity seems to contribute to skeletal muscle hypertrophy.
Earlier studies showed that high-velocity lengthening actions (i.e., rapid eccentric phase during movements under the stretch-shortening cycle) tend to hypertrophy type II compared with type I muscle fibers (Potteiger et al., 1999; Shepstone et al., 2005; Malisoux et al., 2006b). For example, Shepstone et al. (2005) studied the effects of two modes of resistance training programmes, fast vs. slow isokinetic lengthening action of the elbow flexors, on muscle fiber hypertrophy in healthy untrained individuals aged 24 years. The results of the study demonstrated greater hypertrophy in type IIa muscle fibers following fast (+13%) compared with slow muscle lengthening (+3%). Authors further demonstrated greater (+185%) Z-line disruption following fast compared with slow muscle lengthening. It should be noted that Z-line disruption is considered a prominent marker of muscle protein remodeling (Yu and Thornell, 2002; Yu et al., 2004). Additionally, there is direct evidence based on muscle biopsy that a single bout of plyometric exercise induces preferential damage (e.g., loss in dystrophin staining, Z-line disruption) to type II muscle fibers (Macaluso et al., 2012). Of note, exercise-induced damage to muscle tissues is discussed as a potential mechanism for skeletal muscle hypertrophy, perhaps mediated by stimulating satellite cell activity (Vierck et al., 2000; Schoenfeld, 2010; Schoenfeld, 2012). In fact, satellite cells represent the resident stem cells of skeletal muscle (Scharner and Zammit, 2011) and lead to increased muscle regeneration (Barton-Davis et al., 1999). Existing evidence indicated larger satellite cell activation and proliferation after exercise that induced muscle damage (Crameri et al., 2007).
Exercise training needs to cause positive net protein balance to induce skeletal muscle hypertrophy. To our knowledge, muscle protein synthetic responses to plyometric jump training in humans have never been examined in the literature (Grgic et al., 2020). Relevant outcomes from an animal study indicated that rats exposed to plyometric jump training showed a positive net protein balance compared with a control condition (Watt et al., 1982). Lim et al. (2017) examined the effects of one bout of single-mode traditional resistance training vs. one bout of combined traditional resistance training and plyometric jump training on satellite cell activity and anabolic signaling in elite male weightlifters. Their results revealed an increase in satellite cell activation and myofibrillar protein synthesis following both exercise modes. However, the same authors reported that single-mode traditional resistance training resulted in higher satellite cell activity with a tendency for higher expression of mTOR (mammalian target of rapamycin) and p70S6K (ribosomal protein S6 kinase) compared with combined traditional resistance training and plyometric jump training (Lim et al., 2017). Despite these intriguing results, this study does not provide insights into the effects of single-mode plyometric jump training on the anabolic signaling pathway. Therefore, researchers should seek to fill this gap in the literature. Overall, contrary to the previous speculation, plyometric jump training appears to contribute to skeletal muscle hypertrophy, regardless of sex, age, and training experience. These findings have both important scientific and practical implications.
4.2 Moderating Variables
Our findings indicate moderate effects of plyometric jump training on knee extensor hypertrophy [SMD = 0.72 (95% CIs = 0.66–0.78)]. However, the heterogeneity of the outcomes across studies yielded an equivocal effect of plyometric jump training on plantar flexor hypertrophy (SMD = 0.64; 95% CIs = −0.25 to 1.55). It has been shown that jumping exercises principally solicit activation of the knee extensors (i.e., quadriceps) and plantar flexors (e.g., gastrocnemius) but not hamstrings (Ebben et al., 2008). As such, we would expect larger hypertrophic adaptations in these muscles. Monti et al. (2020) investigated the effects of 6 weeks of plyometric jump training on knee extensor muscle mass in healthy males aged 25 years. They demonstrated increased knee extensor power (+19.7%), which was accompanied by increases in quadriceps femoris (+5.8%) and vastus lateralis (+9.6%) volume as well as mean CSA of the quadriceps femoris (+5.8%) after plyometric jump training. The same authors reported significant positive correlations between mean CSA and volume of quadriceps femoris and muscle power (R2 = 0.46 and 0.44, respectively). Additionally, McKinlay et al. (2018) examined the effects of 8 weeks of plyometric jump training on knee extensor hypertrophy and found an 8.1% post-study increase in vastus lateralis muscle thickness in adolescent soccer players aged 11–13 years. Furthermore, Váczi et al. (2014) indicated a 20.5% increase in quadriceps CSA measured via MRI in older adults following 10 weeks of plyometric jump training. In sum, plyometric jump training appears to be an effective means to improve knee extensor hypertrophy. Conversely, our findings do not support consistent hypertrophic effects in the plantar flexors. This could be due to the different mechanical properties between the patellar tendon and Achilles tendon, resulting in different hypertrophic effects on the quadriceps (knee extensor) and gastrocnemius (plantar flexor). In fact, the patellar tendon has been shown to be stiffer and, therefore, mechanically better suited to effectively transmit muscle force compared with the Achilles tendon (Wiesinger et al., 2016). Nevertheless, given the relative paucity of research, future studies should be further redirected towards the assessment of plyometric jump training on plantar flexor hypertrophy to achieve more conclusive inferences.
In regard to training experience, results showed a relatively larger effects for non-athletes [0.55 (95% CIs = 0.18–0.93)] compared with athletes [0.33 (95% CIs = 0.16–0.51)], with a substantial degree of heterogeneity observed across studies. It is well-established that previous training history moderates adaptations to further training interventions (Faigenbaum, 2000; Rhea et al., 2003; Harries et al., 2015; Figueiredo et al., 2018; Suchomel et al., 2018). Indeed, a larger magnitude of adaptation to training would be expected in individuals with less, compared with more, training experience (Faigenbaum, 2000; Suchomel et al., 2018). In this context, earlier studies (Rhea et al., 2003; Figueiredo et al., 2018) demonstrated that adaptations to traditional resistance training are moderated by the magnitude of adaptation that has already been achieved by the individual, implying that the so-called “ceiling effect” attenuates continued adaptations. To the authors’ knowledge, none of the available studies have contrasted the effects of plyometric jump training on skeletal muscle hypertrophy of athletes vs. non-athletes, highlighting a void in the current literature. Nevertheless, results from separate studies indicate large effects of plyometric jump training on skeletal muscle hypertrophy (11.5%–18.8% increase of quadriceps femoris CSA) in non-athletes (Earp et al., 2015) but only a relatively small effect in athletes (9.9% increase in thigh CSA) (Cherni et al., 2020). This is in agreement with the findings of the present study, given that trivial to large effects were observed for non-athletes and trivial to moderate effects were noted for athletes. This suggests that to achieve comparable or larger gains, individuals with greater training experience may need to increase their training volume/intensity to a level that exceeds those who are less experienced and/or fit (Suchomel et al., 2018; Chaabene et al., 2020). In summary, plyometric jump training appears to be more effective to improve skeletal muscle hypertrophy in non-athletes compared with athletes. Future studies should seek to explore the specific mechanisms that facilitate larger gains in non-athletes compared with athletes.
For the assessment methods of skeletal muscle hypertrophy, results show larger effects for ultrasound imaging [SMD = 0.74 (95% CIs = 0.59–0.89)] compared with prediction equation [SMD = 0.29 (95% CIs = 0.16–0.42)] with a clear difference between subgroups. Ultrasound is an easy, non-invasive, and rapid tool to assess muscle thickness, which in turn informs about skeletal muscle hypertrophy (Haun et al., 2019). Muscle thickness evaluated using ultrasound is highly reliable in a range of muscles (Thoirs and English, 2009). However, the prediction equation represents a valid tool that affords a crude estimate of skeletal muscle hypertrophy (Chelly et al., 2006). The major drawback of the prediction equation though is that it does not allow for the differentiation between muscle tissue, fat tissue, and bone (Chelly et al., 2006; Grgic et al., 2019). For the reasons above, it is advisable to favor using ultrasound over the prediction equation to provide more accurate insights about skeletal muscle hypertrophy. Nevertheless, the affordability of the prediction equation could make it further useful, when equipment such as ultrasound is not available. Furthermore, the hypertrophic improvement following plyometric jump training seems to be stable across other assessment methods such as biopsies or MRI. For example, Malisoux et al. (2006a) studied the effects of plyometric jump training on single muscle fiber diameters of the vastus lateralis measured using biopsies in healthy active males aged 23 years. Participants demonstrated a significant increase in type I (+11%), type IIa (+10%), and type IIa/IIx (+15%) fibers following training. The same authors reported that fiber force increased for all fiber types, in part due to increased fiber diameter (Malisoux et al., 2006a). Furthermore, Vissing et al. (2008) compared the effects of plyometric jump training vs. traditional resistance training on skeletal muscle hypertrophy via MRI in untrained males aged 25 years and revealed an increase in the CSA of the quadriceps, hamstrings, and adductor muscles (+7%–10%).
Regarding training frequency, our findings indicate that the effects of plyometric jump training on skeletal muscle hypertrophy were moderated by the weekly session frequency [β = 0.3233 (95% CIs = 0.2040–0.4425)] with a higher weekly session frequency inducing larger hypertrophic gains. Of note, most of the included studies used biweekly or triweekly plyometric jump training sessions. Specifically, three sessions of plyometric jump training per week showed large increases in skeletal muscle hypertrophy (12.8%–25.8% increase in vastus lateralis CSA) (Earp et al., 2015) compared with smaller gains following two weekly sessions (14% increase in thigh muscle volume) (Fathi et al., 2019). This is in agreement with the literature about traditional resistance training (Wernbom et al., 2007; Schoenfeld et al., 2019; Schoenfeld et al., 2021). More specifically, in a systematic review with meta-analysis of the effects of traditional resistance training frequency on skeletal muscle hypertrophy in healthy individuals, Schoenfeld et al. (2019) reported a slightly larger effect of higher compared with lower frequencies of training on hypertrophic outcomes when training volume was not equated between conditions. However, under equated-volume conditions, no additional effects of higher compared with lower frequencies of training were reported (Schoenfeld et al., 2019). The same conclusion was made in a recent consensus review in that a higher number of traditional resistance training sessions per week (e.g., 3 sessions vs. 1 session) is recommended to gain more muscle mass (Schoenfeld et al., 2021). The same authors attributed the larger benefits of manipulating training frequency to its potential effect on the distribution of the weekly training volume (Schoenfeld et al., 2021). Future studies should endeavor to better understand the interaction between plyometric jump training frequency and hypertrophic adaptations, particularly in context with alterations in volume and intensity.
With respect to age, the positive point estimate suggests that older participants tend to achieve larger hypertrophic adaptations (Figure 5; https://osf.io/g8aet/). The same observation was noted for total training period (Figure 5; https://osf.io/g8aet/), with longer exposure to plyometric jump training appearing to induce larger hypertrophic gains. However, for a single session duration (Figure 5; https://osf.io/g8aet/), there is a tendency for shorter sessions to induce larger hypertrophic gains. It should be noted though that these observations are not conclusive and hence need to be confirmed in future studies.
4.3 Limitations
Some limitations of this meta-analysis need to be acknowledged. The lack of a standardised method to assess skeletal muscle hypertrophy can constitute a limitation given that different assessment techniques may disagree with one another (e.g., macroscopic vs. microscopic) (Haun et al., 2019). Indeed, studies included in this analysis have used a wide range of methods to assess skeletal muscle hypertrophy. More specifically, the included studies relied upon macroscopic assessment methods (e.g., B-mode ultrasound) (Skurvydas and Brazaitis, 2010; Fouré et al., 2011; Fouré et al., 2012; Correa et al., 2012; Earp et al., 2015; Allison et al., 2018; McKinlay et al., 2018), microscopic methods (e.g., biopsy) (Kyröläinen et al., 2005), and a prediction equation to assess muscle volume and CSA (Chelly et al., 2010; Chelly et al., 2014; Chelly et al., 2015; Fathi et al., 2019). In fact, the prediction equation represents an indirect tool that provides a crude estimate of skeletal muscle hypertrophy and does not allow for the differentiation between muscle tissue, fat tissue, and bone (Chelly et al., 2006; Grgic et al., 2019). Additionally, although skeletal muscle hypertrophy was a primary outcome in most of the included studies (Kyröläinen et al., 2005; Marković et al., 2005; Herrero et al., 2006; Correa et al., 2012; Chelly et al., 2014; Chelly et al., 2015; Earp et al., 2015; Allison et al., 2018), it was in some other studies (Chelly et al., 2010; Skurvydas and Brazaitis, 2010; Fouré et al., 2011; Fouré et al., 2012; McKinlay et al., 2018; Fathi et al., 2019; Cherni et al., 2020) a secondary outcome. As such, caution must be taken when interpreting the present findings. Furthermore, moderator analyses were computed independently, ignoring any potential interdependency (interaction) between variables. Therefore, the results of univariate analyses must be interpreted with caution. Finally, although we have included studies that used athletic samples, the resistance training expertise of these participants is generally not clear. Thus, results cannot necessarily be generalized to well-trained individuals. Further studies are warranted to determine the effects of plyometric jump training on skeletal muscle hypertrophy in those with significant resistance training experience.
5 Conclusion
Contrary to common belief, plyometric jump training seems to induce skeletal muscle hypertrophy, albeit to a small to moderate magnitude. Such an effect appears to be consistent across different ages, sexes, and training experiences. Furthermore, there is evidence of relatively larger hypertrophic adaptations in non-athletes compared with athletes, with no clear evidence that either age or sex moderated the effects of plyometric jump training on skeletal muscle hypertrophy. Regarding the assessment methods, it is advisable to favor the use of ultrasound and other validated site-specific imaging modalities over the prediction equation to provide more accurate insights into skeletal muscle hypertrophy. Moreover, meta-regression analyses suggest that the effects on skeletal muscle hypertrophy are moderated by the weekly session frequency with higher frequencies inducing larger gains in skeletal muscle hypertrophy. However, there is no clear evidence that total training period, single session duration, and the number of jumps per week moderated the effects of plyometric jump training on skeletal muscle hypertrophy.
6 Future Research Perspectives
Given that skeletal muscle hypertrophy was a secondary outcome in many of the included studies, future investigations of high methodological quality (e.g., randomized-controlled trials) where skeletal muscle hypertrophy is the primary endpoint are required to substantiate the present findings. In addition, the effects of plyometric jump training on muscle protein synthesis are still unknown. Therefore, future research should explore the mechanisms by which plyometric jump training induces skeletal muscle hypertrophy. That said, researchers’ attention should be redirected toward the effects of single-mode plyometric jump training on the anabolic signaling pathway. Such studies will provide novel insights into the mechanisms of plyometric jump training-related hypertrophic adaptations in humans. Further, there is a need for future longitudinal studies to compare and contrast the effects of different plyometric jump training volumes, frequencies, and intensities on skeletal muscle hypertrophy. Also, we were able to locate only three studies that included female participants (Skurvydas and Brazaitis, 2010; Correa et al., 2012; Cherni et al., 2020) and only two studies that included older adults (Correa et al., 2012; Allison et al., 2018). Therefore, future investigations should recruit females as well as older adults to fill this gap in the literature. Furthermore, the effects of plyometric jump training vs. traditional resistance training on skeletal muscle hypertrophy have never been meta-analyzed. Of note, there is only one previous review on the topic, but it is descriptive and included only six studies (Grgic et al., 2020), limiting the veracity of its main findings. As such, there is a need to aggregate data from the available literature to draw statistical inferences on the effects of plyometric jump training vs. traditional resistance training on skeletal muscle hypertrophy when there are a sufficient number of studies on the topic. Moreover, given that the combination between plyometric jump training and traditional resistance training favors an anabolic hormonal milieu (Beaven et al., 2011; Ali et al., 2019), it would be relevant to determine optimal combination strategies between plyometric jump training and traditional resistance training to maximize skeletal muscle hypertrophy.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://osf.io/bf478/.
Author Contributions
AF collected the data, analysed the data, and wrote the manuscript. MB wrote the manuscript. MA analysed the data and wrote the manuscript. SB wrote the manuscript. MJ wrote the manuscript. RRC wrote the manuscript. BM wrote the manuscript. BP wrote the manuscript. ERM wrote the manuscript. HL wrote the manuscript and CH collected the data, analysed the data and wrote the manuscript.
Conflict of Interest
BJS serves on the scientific advisory board of Tonal Corporation, a manufacturer of exercise equipment.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All authors met the authorship criteria for this journal and each author made a significant contribution to the final version of this paper. This study is supported by the Open Access Publishing Fund of University of Potsdam, Germany. The funders had no role in study design or preparation of the manuscript.
Footnotes
1Training experience was determined with regard to the context from which participants were recruited. Athletes were recruited from specific sport settings (e.g., sports clubs or teams) and were actively participating in competitive events while non-athletes were recruited outside this setting [e.g., educational institutions (school, university) or rural/urban communities] and were not actively participating in sports competition (Araújo and Scharhag, 2016).
References
Ali K., Verma S., Ahmad I., Singla D., Saleem M., Hussain M. E. (2019). Comparison of Complex versus Contrast Training on Steroid Hormones and Sports Performance in Male Soccer Players. J. Chiropr. Med. 18, 131–138. doi:10.1016/j.jcm.2018.12.001
Allison S. J., Brooke-Wavell K., Folland J. (2018). High and Odd Impact Exercise Training Improved Physical Function and Fall Risk Factors in Community-Dwelling Older Men. J. Musculoskelet. Neuronal Interact. 18, 100
Amrhein V., Greenland S., Mcshane B. B. (2019b). Statistical Significance Gives Bias a Free Pass. Eur. J. Clin. Invest. 49, e13176. doi:10.1111/eci.13176
Amrhein V., Greenland S., Mcshane B. (2019a). Scientists Rise up against Statistical Significance. London: Nature Publishing Group.
Araújo C., Scharhag J. (2016). Athlete: a Working Definition for Medical and Health Sciences Research. Scand. J. Med. Sci. sports 26, 4–7. doi:10.1111/sms.12632
Asadi A., Arazi H., Young W. B., de Villarreal E. S. (2016). The Effects of Plyometric Training on Change-Of-Direction Ability: A Meta-Analysis. Int. J. Sports Physiol. Perform. 11, 563–573. doi:10.1123/ijspp.2015-0694
Barton-Davis E. R., Shoturma D. I., Sweeney H. L. (1999). Contribution of Satellite Cells to IGF-I Induced Hypertrophy of Skeletal Muscle. Acta Physiol. Scand. 167, 301–305. doi:10.1046/j.1365-201x.1999.00618.x
Beaven C. M., Gill N. D., Ingram J. R., Hopkins W. G. (2011). Acute Salivary Hormone Responses to Complex Exercise Bouts. J. Strength Cond. Res. 25, 1072–1078. doi:10.1519/jsc.0b013e3181bf4414
Bedoya A. A., Miltenberger M. R., Lopez R. M. (2015). Plyometric Training Effects on Athletic Performance in Youth Soccer Athletes: A Systematic Review. J. Strength Cond. Res. 29, 2351–2360. doi:10.1519/jsc.0000000000000877
Bull F. C., Al-Ansari S. S., Biddle S., Borodulin K., Buman M. P., Cardon G., et al. (2020). World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 54, 1451–1462. doi:10.1136/bjsports-2020-102955
Chaabene H., Negra Y., Moran J., Prieske O., Sammoud S., Ramirez-Campillo R., et al. (2019). Plyometric Training Improves Not Only Measures of Linear Speed, Power, and Change-Of-Direction Speed but Also Repeated Sprint Ability in Female Young Handball Players. J. Strength Cond. Res. 35, 2230–2235.
Chaabene H., Negra Y. (2017). The Effect of Plyometric Training Volume on Athletic Performance in Prepubertal Male Soccer Players. Int. J. Sports Physiol. Perform. 12, 1205–1211. doi:10.1123/ijspp.2016-0372
Chaabene H., Prieske O., Moran J., Negra Y., Attia A., Granacher U. (2020). Effects of Resistance Training on Change-Of-Direction Speed in Youth and Young Physically Active and Athletic Adults: A Systematic Review with Meta-Analysis. Sports Med. 50, 1483–1499. doi:10.1007/s40279-020-01293-w
Chelly M., Chamari K., Verney J., Denis C. (2006). Comparison of Muscle Mechanical and Histochemical Properties between Young and Elderly Subjects. Int. J. Sports Med. 27, 885–893. doi:10.1055/s-2006-923773
Chelly M. S., Ghenem M. A., Abid K., Hermassi S., Tabka Z., Shephard R. J. (2010). Effects of In-Season Short-Term Plyometric Training Program on Leg Power, Jump- and Sprint Performance of Soccer Players. J. Strength. Cond. Res. 24, 2670–2676. doi:10.1519/jsc.0b013e3181e2728f
Chelly M. S., Hermassi S., Aouadi R., Shephard R. J. (2014). Effects of 8-week In-Season Plyometric Training on Upper and Lower Limb Performance of Elite Adolescent Handball Players. J. Strength. Cond. Res. 28, 1401–1410. doi:10.1519/jsc.0000000000000279
Chelly M. S., Hermassi S., Shephard R. J. (2015). Effects of In-Season Short-Term Plyometric Training Program on Sprint and Jump Performance of Young Male Track Athletes. J. Strength. Cond. Res. 29, 2128–2136. doi:10.1519/jsc.0000000000000860
Cherni Y., Hammami M., Jelid M. C., Aloui G., Suzuki K., Shephard R. J., et al. (2020). Neuromuscular Adaptations and Enhancement of Physical Performance in Female Basketball Players after 8 Weeks of Plyometric Training. Front. Physiol. 11, 588787. doi:10.3389/fphys.2020.588787
Chimera N. J., Swanik K. A., Swanik C. B., Straub S. J. (2004). Effects of Plyometric Training on Muscle-Activation Strategies and Performance in Female Athletes. J. Athl. Train. 39, 24
Cohen J. (1988). Statistical Power Analysis for the Behaviors Science. New Jersey: Laurence Erlbaum Associates, Publishers.
Correa C., Laroche D., Cadore E., Reischak-Oliveira A., Bottaro M., Kruel L. F., et al. (2012). 3 Different Types of Strength Training in Older Women. Int. J. Sports Med. 33, 962–969. doi:10.1055/s-0032-1312648
Crameri R. M., Aagaard P., Qvortrup K., Langberg H., Olesen J., Kjaer M. (2007). Myofibre Damage in Human Skeletal Muscle: Effects of Electrical Stimulationversusvoluntary Contraction. J. Physiol. 583, 365–380. doi:10.1113/jphysiol.2007.128827
Cuijpers P., Weitz E., Cristea I. A., Twisk J. (2017). Pre-post Effect Sizes Should Be Avoided in Meta-Analyses. Epidemiol. Psychiatr. Sci. 26, 364–368. doi:10.1017/s2045796016000809
De Morton N. A. (2009). The PEDro Scale Is a Valid Measure of the Methodological Quality of Clinical Trials: a Demographic Study. Aust. J. Physiother. 55, 129–133. doi:10.1016/s0004-9514(09)70043-1
De Villarreal E. S.-S., Kellis E., Kraemer W. J., Izquierdo M. (2009). Determining Variables of Plyometric Training for Improving Vertical Jump Height Performance: a Meta-Analysis. J. Strength Cond. Res. 23, 495–506. doi:10.1519/jsc.0b013e318196b7c6
Dietz V., Schmidtbleicher D., Noth J. (1979). Neuronal Mechanisms of Human Locomotion. J. Neurophysiology 42, 1212–1222. doi:10.1152/jn.1979.42.5.1212
Drevon D., Fursa S. R., Malcolm A. L. (2017). Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 41, 323–339. doi:10.1177/0145445516673998
Earp J. E., Newton R. U., Cormie P., Blazevich A. J. (2015). Inhomogeneous Quadriceps Femoris Hypertrophy in Response to Strength and Power Training. Med. Sci. Sports Exerc 47, 2389–2397. doi:10.1249/mss.0000000000000669
Ebben W. P., Simenz C., Jensen R. L. (2008). Evaluation of Plyometric Intensity Using Electromyography. J. Strength. Cond. Res. 22, 861–868. doi:10.1519/jsc.0b013e31816a834b
Faigenbaum A. (2000). Age-and Sex-Related Differences and Their Implications for Resistance Exercise. Essentials strength Train. Cond. 3, 142–158.
Fathi A., Hammami R., Moran J., Borji R., Sahli S., Rebai H. (2019). Effect of a 16-week Combined Strength and Plyometric Training Program Followed by a Detraining Period on Athletic Performance in Pubertal Volleyball Players. J. Strength Cond. Res. 33, 2117–2127. doi:10.1519/jsc.0000000000002461
Figueiredo V. C., De Salles B. F., Trajano G. S. (2018). Volume for Muscle Hypertrophy and Health Outcomes: the Most Effective Variable in Resistance Training. Sports Med. 48, 499–505. doi:10.1007/s40279-017-0793-0
Fouré A., Nordez A., Mcnair P., Cornu C. (2011). Effects of Plyometric Training on Both Active and Passive Parts of the Plantarflexors Series Elastic Component Stiffness of Muscle-Tendon Complex. Eur. J. Appl. Physiol. 111, 539–548. doi:10.1007/s00421-010-1667-4
Fouré A., Nordez A., Cornu C. (2012). Effects of Plyometric Training on Passive Stiffness of Gastrocnemii Muscles and Achilles Tendon. Eur. J. Appl. Physiol. 112, 2849–2857. doi:10.1007/s00421-011-2256-x
Gómez-Bruton A., Matute-Llorente Á., González-Agüero A., Casajús J. A., Vicente-Rodríguez G. (2017). Plyometric Exercise and Bone Health in Children and Adolescents: a Systematic Review. World J. Pediatr. 13, 112–121. doi:10.1007/s12519-016-0076-0
Greenhalgh T., Peacock R. (2005). Effectiveness and Efficiency of Search Methods in Systematic Reviews of Complex Evidence: Audit of Primary Sources. Bmj 331, 1064–1065. doi:10.1136/bmj.38636.593461.68
Grgic J., Lazinica B., Mikulic P., Krieger J. W., Schoenfeld B. J. (2017). The Effects of Short versus Long Inter-set Rest Intervals in Resistance Training on Measures of Muscle Hypertrophy: A Systematic Review. Eur. J. sport Sci. 17, 983–993. doi:10.1080/17461391.2017.1340524
Grgic J., Schoenfeld B. J., Latella C. (2019). Resistance Training Frequency and Skeletal Muscle Hypertrophy: A Review of Available Evidence. J. Sci. Med. sport 22, 361–370. doi:10.1016/j.jsams.2018.09.223
Grgic J., Schoenfeld B. J., Mikulic P. (2020). Effects of Plyometric vs. Resistance Training on Skeletal Muscle Hypertrophy: A Review. J. Sport Health Sci. 10 (5), 530–536. doi:10.1016/j.jshs.2020.06.010
Häkkinen K., Komi P. V., Alén M. (1985). Effect of Explosive Type Strength Training on Isometric Force- and Relaxation-Time, Electromyographic and Muscle Fibre Characteristics of Leg Extensor Muscles. Acta Physiol. Scand. 125, 587–600. doi:10.1111/j.1748-1716.1985.tb07759.x
Häkkinen K., Pakarinen A., Kyröläinen H., Cheng S., Kim D., Komi P. (1990). Neuromuscular Adaptations and Serum Hormones in Females during Prolonged Power Training. Int. J. Sports Med. 11, 91–98. doi:10.1055/s-2007-1024769
Harrer M., Cuijpers P., Furukawa T. A., Ebert D. D. (2019). Doing Meta-Analysis with R: A Hands-On Guide. New York: CRC Press.
Harries S. K., Lubans D. R., Callister R. (2015). Systematic Review and Meta-Analysis of Linear and Undulating Periodized Resistance Training Programs on Muscular Strength. J. Strength Cond. Res. 29, 1113–1125. doi:10.1519/jsc.0000000000000712
Haun C. T., Vann C. G., Roberts B. M., Vigotsky A. D., Schoenfeld B. J., Roberts M. D. (2019). A Critical Evaluation of the Biological Construct Skeletal Muscle Hypertrophy: Size Matters but So Does the Measurement. Front. Physiol. 10, 247. doi:10.3389/fphys.2019.00247
Hedges L. V., Tipton E., Johnson M. C. (2010). Robust Variance Estimation in Meta-Regression with Dependent Effect Size Estimates. Res. Synth. Method 1, 39–65. doi:10.1002/jrsm.5
Helland C., Hole E., Iversen E., Olsson M. C., Seynnes O., Solberg P. A., et al. (2017). Training Strategies to Improve Muscle Power: Is Olympic-style Weightlifting Relevant? Med. Sci. Sports Exerc 49, 736–745. doi:10.1249/mss.0000000000001145
Herrero J., Izquierdo M., Maffiuletti N., García-López J. (2006). Electromyostimulation and Plyometric Training Effects on Jumping and Sprint Time. Int. J. Sports Med. 27, 533–539. doi:10.1055/s-2005-865845
Higgins J. P., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., et al. (2019). Cochrane Handbook for Systematic Reviews of Interventions. UniM INTERNET Resource: John Wiley & Sons
Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring Inconsistency in Meta-Analyses. Bmj 327, 557–560. doi:10.1136/bmj.327.7414.557
Hirayama K., Iwanuma S., Ikeda N., Yoshikawa A., Ema R., Kawakami Y. (2017). Plyometric Training Favors Optimizing Muscle-Tendon Behavior during Depth Jumping. Front. Physiol. 8, 16. doi:10.3389/fphys.2017.00016
Horwath O., Paulsen G., Esping T., Seynnes O., Olsson M. C. (2019). Isokinetic Resistance Training Combined with Eccentric Overload Improves Athletic Performance and Induces Muscle Hypertrophy in Young Ice Hockey Players. J. Sci. Med. sport 22, 821–826. doi:10.1016/j.jsams.2018.12.017
Huang Y.-L., Jung J., Mulligan C. M. S., Oh J., Norcross M. F. (2020). A Majority of Anterior Cruciate Ligament Injuries Can Be Prevented by Injury Prevention Programs: A Systematic Review of Randomized Controlled Trials and Cluster-Randomized Controlled Trials with Meta-Analysis. Am. J. Sports Med. 48, 1505–1515. doi:10.1177/0363546519870175
Hunter J. P., Marshall R. N. (2002). Effects of Power and Flexibility Training on Vertical Jump Technique. Med. Sci. Sports Exerc. 34, 478–486. doi:10.1097/00005768-200203000-00015
Ishikawa M., Komi P. V. (2008). Muscle Fascicle and Tendon Behavior during Human Locomotion Revisited. Exerc Sport Sci. Rev. 36, 193–199. doi:10.1097/jes.0b013e3181878417
Izquierdo M., Merchant R., Morley J., Anker S., Aprahamian I., Arai H., et al. (2021). International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health. Aging 5, 824–853. doi:10.1007/s12603-021-1665-8
Kijowksi K. N., Capps C. R., Goodman C. L., Erickson T. M., Knorr D. P., Triplett N. T., et al. (2015). Short-term Resistance and Plyometric Training Improves Eccentric Phase Kinetics in Jumping. J. Strength Cond. Res. 29, 2186–2196. doi:10.1519/jsc.0000000000000904
Kish K., Mezil Y., Ward W. E., Klentrou P., Falk B. (2015). Effects of Plyometric Exercise Session on Markers of Bone Turnover in Boys and Young Men. Eur. J. Appl. Physiol. 115, 2115–2124. doi:10.1007/s00421-015-3191-z
Komi P. V. (1984). Physiological and Biomechanical Correlates of Muscle Function: Effects of Muscle Structure and Stretch-Shortening Cycle on Force and Speed. Exerc. Sport Sci. Rev. 12, 81–122. doi:10.1249/00003677-198401000-00006
Kubo K., Morimoto M., Komuro T., Yata H., Tsunoda N., Kanehisa H., et al. (2007). Effects of Plyometric and Weight Training on Muscle-Tendon Complex and Jump Performance. Med. Sci. sports Exerc. 39, 1801–1810. doi:10.1249/mss.0b013e31813e630a
Kudo S., Sato T., Miyashita T. (2020). Effect of Plyometric Training on the Fascicle Length of the Gastrocnemius Medialis Muscle. J. Phys. Ther. Sci. 32, 277–280. doi:10.1589/jpts.32.277
Kümmel J., Kramer A., Giboin L. S., Gruber M. (2016). Specificity of Balance Training in Healthy Individuals: a Systematic Review and Meta-Analysis. Sports Med. 46, 1261–1271. doi:10.1007/s40279-016-0515-z
Kyröläinen H., Avela J., Mcbride J. M., Koskinen S., Andersen J. L., Sipilä S., et al. (2005). Effects of Power Training on Muscle Structure and Neuromuscular Performance. Scand. J. Med. Sci. Sports 15, 58–64. doi:10.1111/j.1600-0838.2004.00390.x
Lee D. K. (2016). Alternatives to P Value: Confidence Interval and Effect Size. Korean J. Anesthesiol. 69, 555–562. doi:10.4097/kjae.2016.69.6.555
Lim C. H., Luu T. S., Phoung L. Q., Jeong T. S., Kim C. K. (2017). Satellite Cell Activation and mTOR Signaling Pathway Response to Resistance and Combined Exercise in Elite Weight Lifters. Eur. J. Appl. Physiol. 117, 2355–2363. doi:10.1007/s00421-017-3722-x
Macaluso F., Isaacs A. W., Myburgh K. H. (2012). Preferential Type II Muscle Fiber Damage from Plyometric Exercise. J. Athl. Train. 47, 414–420. doi:10.4085/1062-6050-47.4.13
Maher C. G., Sherrington C., Herbert R. D., Moseley A. M., Elkins M. (2003). Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 83, 713–721. doi:10.1093/ptj/83.8.713
Malisoux L., Francaux M., Nielens H., Renard P., Lebacq J., Theisen D. (2006a). Calcium Sensitivity of Human Single Muscle Fibers Following Plyometric Training. Med. Sci. sports Exerc. 38, 1901–1908. doi:10.1249/01.mss.0000232022.21361.47
Malisoux L., Francaux M., Nielens H., Theisen D. (2006b). Stretch-shortening Cycle Exercises: an Effective Training Paradigm to Enhance Power Output of Human Single Muscle Fibers. J. Appl. physiology 100, 771–779. doi:10.1152/japplphysiol.01027.2005
Marković G., Jukić I., Milanović D., Metikoš D. (2005). Effects of Sprint and Plyometric Training on Morphological Characteristics in Physically Active Men. Kinesiology 37, 32
Markovic G., Mikulic P. (2010). Neuro-musculoskeletal and Performance Adaptations to Lower-Extremity Plyometric Training. Sports Med. 40, 859–895. doi:10.2165/11318370-000000000-00000
Mckinlay B. J., Wallace P., Dotan R., Long D., Tokuno C., Gabriel D. A., et al. (2018). Effects of Plyometric and Resistance Training on Muscle Strength, Explosiveness, and Neuromuscular Function in Young Adolescent Soccer Players. J. Strength Cond. Res. 32, 3039–3050. doi:10.1519/jsc.0000000000002428
Mcshane B. B., Gal D., Gelman A., Robert C., Tackett J. L. (2019). Abandon Statistical Significance. Am. Statistician 73, 235–245. doi:10.1080/00031305.2018.1527253
Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Ann. Intern Med. 151, 264–269. doi:10.7326/0003-4819-151-4-200908180-00135
Monti E., Franchi M. V., Badiali F., Quinlan J. I., Longo S., Narici M. V. (2020). The Time-Course of Changes in Muscle Mass, Architecture and Power during 6 Weeks of Plyometric Training. Front. Physiol. 11, 946. doi:10.3389/fphys.2020.00946
Moran J., Ramirez-Campillo R., Granacher U. (2018). Effects of Jumping Exercise on Muscular Power in Older Adults: A Meta-Analysis. Sports Med. 48, 2843–2857. doi:10.1007/s40279-018-1002-5
Morris S. B. (2008). Estimating Effect Sizes from Pretest-Posttest-Control Group Designs. Organ. Res. methods 11, 364–386. doi:10.1177/1094428106291059
Moseley A. M., Rahman P., Wells G. A., Zadro J. R., Sherrington C., Toupin-April K., et al. (2019). Agreement between the Cochrane Risk of Bias Tool and Physiotherapy Evidence Database (PEDro) Scale: A Meta-Epidemiological Study of Randomized Controlled Trials of Physical Therapy Interventions. PLoS One 14, e0222770. doi:10.1371/journal.pone.0222770
Nakagawa S., Cuthill I. C. (2007). Effect Size, Confidence Interval and Statistical Significance: a Practical Guide for Biologists. Biol. Rev. 82, 591–605. doi:10.1111/j.1469-185x.2007.00027.x
Negra Y., Chaabene H., Sammoud S., Prieske O., Moran J., Ramirez-Campillo R., et al. (2020). The Increased Effectiveness of Loaded versus Unloaded Plyometric Jump Training in Improving Muscle Power, Speed, Change of Direction, and Kicking-Distance Performance in Prepubertal Male Soccer Players. Int. J. Sports Physiol. Perform. 15, 189–195. doi:10.1123/ijspp.2018-0866
Page M. J., Mckenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Potteiger J. A., Lockwood R. H., Haub M. D., Dolezal B. A., Almuzaini K. S., Schroeder J. M., et al. (1999). Muscle Power and Fiber Characteristics Following 8 Weeks of Plyometric Training. J. strength Cond. Res. 13, 275–279. doi:10.1519/00124278-199908000-00016
Ramirez-Campillo R., Moran J., Chaabene H., Granacher U., Behm D. G., García-Hermoso A., et al. (2020b). Methodological Characteristics and Future Directions for Plyometric Jump Training Research: A Scoping Review Update. Scand. J. Med. Sci. Sports 30, 983–997. doi:10.1111/sms.13633
Ramirez-Campillo R., Álvarez C., García-Hermoso A., Ramírez-Vélez R., Gentil P., Asadi A., et al. (2018). Methodological Characteristics and Future Directions for Plyometric Jump Training Research: A Scoping Review. Sports Med. 48, 1059–1081. doi:10.1007/s40279-018-0870-z
Ramirez-Campillo R., García-De-Alcaraz A., Chaabene H., Moran J., Negra Y., Granacher U. (2021a). Effects of Plyometric Jump Training on Physical Fitness in Amateur and Professional Volleyball: a Meta-Analysis. Front. Physiology 12, 636140. doi:10.3389/fphys.2021.636140
Ramirez-Campillo R., Garcia-Hermoso A., Moran J., Chaabene H., Negra Y., Scanlan A. T. (2020a). The Effects of Plyometric Jump Training on Physical Fitness Attributes in Basketball Players: A Meta-Analysis. J. Sport Health Sci. S2095-2546 (20), 30169. doi:10.1016/j.jshs.2020.12.005
Ramirez-Campillo R., Garcia-Pinillos F., Chaabene H., Moran J., Behm D. G., Granacher U. (2021b). Effects of Plyometric Jump Training on Electromyographic Activity and its Relationship to Strength and Jump Performance in Healthy Trained and Untrained Populations: A Systematic Review of Randomized Controlled Trials. J. Strength & Cond. Res. 35, 2053–2065. doi:10.1519/JSC.0000000000004056
Ramírez-Campillo R., Henríquez-Olguín C., Burgos C., Andrade D. C., Zapata D., Martínez C., et al. (2015). Effect of Progressive Volume-Based Overload during Plyometric Training on Explosive and Endurance Performance in Young Soccer Players. J. Strength. Cond. Res. 29, 1884–1893. doi:10.1519/JSC.0000000000000836
Ramírez-Delacruz M., Bravo-Sánchez A., Esteban-García P., Jiménez F., Abián-Vicén J. (2022). Effects of Plyometric Training on Lower Body Muscle Architecture, Tendon Structure, Stiffness and Physical Performance: A Systematic Review and Meta-Analysis. Sports Med. Open 8, 40. doi:10.1186/s40798-022-00431-0
Rhea M. R., Alvar B. A., Burkett L. N., Ball S. D. (2003). A Meta-Analysis to Determine the Dose Response for Strength Development. Med. Sci. Sports Exerc. 35, 456–464. doi:10.1249/01.mss.0000053727.63505.d4
Russell B., Motlagh D., Ashley W. W. (2000). Form Follows Function: How Muscle Shape Is Regulated by Work. J. Appl. Physiology 88, 1127–1132. doi:10.1152/jappl.2000.88.3.1127
Sáez-Sáez De Villarreal E., Requena B., Newton R. U. (2010). Does Plyometric Training Improve Strength Performance? A Meta-Analysis. J. Sci. Med. Sport 13, 513–522. doi:10.1016/j.jsams.2009.08.005
Scharner J., Zammit P. S. (2011). The Muscle Satellite Cell at 50: the Formative Years. Skelet. Muscle 1, 28. doi:10.1186/2044-5040-1-28
Schoenfeld B., Fisher J., Grgic J., Haun C., Helms E., Phillips S., et al. (2021). Resistance Training Recommendations to Maximize Muscle Hypertrophy in an Athletic Population: Position Stand of the IUSCA. Int. J. Strength Cond. 1, 81. doi:10.47206/ijsc.v1i1.81
Schoenfeld B. J. (2012). Does Exercise-Induced Muscle Damage Play a Role in Skeletal Muscle Hypertrophy? J. Strength Cond. Res. 26, 1441–1453. doi:10.1519/jsc.0b013e31824f207e
Schoenfeld B. J., Grgic J., Krieger J. (2019). How Many Times Per Week Should a Muscle Be Trained to Maximize Muscle Hypertrophy? A Systematic Review and Meta-Analysis of Studies Examining the Effects of Resistance Training Frequency. J. sports Sci. 37, 1286–1295. doi:10.1080/02640414.2018.1555906
Schoenfeld B. J., Grgic J., Ogborn D., Krieger J. W. (2017). Strength and Hypertrophy Adaptations between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-Analysis. J. Strength & Cond. Res. 31, 3508–3523. doi:10.1519/jsc.0000000000002200
Schoenfeld B. J. (2010). The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength & Cond. Res. 24, 2857–2872. doi:10.1519/jsc.0b013e3181e840f3
Shepstone T. N., Tang J. E., Dallaire S., Schuenke M. D., Staron R. S., Phillips S. M. (2005). Short-term High- vs. Low-Velocity Isokinetic Lengthening Training Results in Greater Hypertrophy of the Elbow Flexors in Young Men. J. Appl. Physiology 98, 1768–1776. doi:10.1152/japplphysiol.01027.2004
Skurvydas A., Brazaitis M. (2010). Plyometric Training Does Not Affect Central and Peripheral Muscle Fatigue Differently in Prepubertal Girls and Boys. Pediatr. Exerc. Sci. 22, 547–556. doi:10.1123/pes.22.4.547
Skurvydas A., Brazaitis M., Streckis V., Rudas E. (2010). The Effect of Plyometric Training on Central and Peripheral Fatigue in Boys. Int. J. Sports Med. 31, 451–457. doi:10.1055/s-0030-1251991
Stien N., Strate M., Andersen V., Saeterbakken A. H. (2020). Effects of Overspeed or Overload Plyometric Training on Jump Height and Lifting Velocity. Sports Med. Int. Open 4, E32–e38. doi:10.1055/a-1116-0749
Suchomel T. J., Nimphius S., Bellon C. R., Stone M. H. (2018). The Importance of Muscular Strength: Training Considerations. Sports Med. 48, 765–785. doi:10.1007/s40279-018-0862-z
Taube W., Leukel C., Gollhofer A. (2012). How Neurons Make Us Jump: The Neural Control of Stretch-Shortening Cycle Movements. Exerc Sport Sci. Rev. 40, 106–115. doi:10.1097/jes.0b013e31824138da
Thoirs K., English C. (2009). Ultrasound Measures of Muscle Thickness: Intra-examiner Reliability and Influence of Body Position. Clin. physiology Funct. imaging 29, 440–446. doi:10.1111/j.1475-097x.2009.00897.x
Ullrich B., Pelzer T., Pfeiffer M. (2018). Neuromuscular Effects to 6 Weeks of Loaded Countermovement Jumping with Traditional and Daily Undulating Periodization. J. Strength Cond. Res. 32, 660–674. doi:10.1519/jsc.0000000000002290
Váczi M., Nagy S. A., Kőszegi T., Ambrus M., Bogner P., Perlaki G., et al. (2014). Mechanical, Hormonal, and Hypertrophic Adaptations to 10 Weeks of Eccentric and Stretch-Shortening Cycle Exercise Training in Old Males. Exp. Gerontol. 58, 69
Van Calster B., Steyerberg E. W., Collins G. S., Smits T. (2018). Consequences of Relying on Statistical Significance: Some Illustrations. Eur. J. Clin. Invest. 48, e12912. doi:10.1111/eci.12912
Van Der Zwaard S., Koppens T. F. P., Weide G., Levels K., Hofmijster M. J., De Koning J. J., et al. (2021). Training-induced Muscle Adaptations during Competitive Preparation in Elite Female Rowers. Front. Sports Act. Living 3, 781942. doi:10.3389/fspor.2021.781942
Vetrovsky T., Steffl M., Stastny P., Tufano J. J. (2019). The Efficacy and Safety of Lower-Limb Plyometric Training in Older Adults: A Systematic Review. Sports Med. 49, 113–131. doi:10.1007/s40279-018-1018-x
Viechtbauer W. (2010). Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 36, 1–48. doi:10.18637/jss.v036.i03
Vierck J., O'reilly B., Hossner K., Antonio J., Byrne K., Bucci L., et al. (2000). Satellite Cell Regulation Following Myotrauma Caused by Resistance Exercise. Cell. Biol. Int. 24, 263–272. doi:10.1006/cbir.2000.0499
Vissing K., Brink M., Lønbro S., Sørensen H., Overgaard K., Danborg K., et al. (2008). Muscle Adaptations to Plyometric vs. Resistance Training in Untrained Young Men. J. Strength Cond. Res. 22, 1799–1810. doi:10.1519/jsc.0b013e318185f673
Vlachopoulos D., Barker A. R., Ubago-Guisado E., Williams C. A., Gracia-Marco L. (2018). The Effect of a High-Impact Jumping Intervention on Bone Mass, Bone Stiffness and Fitness Parameters in Adolescent Athletes. Arch. Osteoporos. 13, 128. doi:10.1007/s11657-018-0543-4
Voigt M., Dyhre‐poulsen P., Simonsen E. B. (1998). Modulation of Short Latency Stretch Reflexes during Human Hopping. Acta Physiol. Scand. 163, 181–194. doi:10.1046/j.1365-201x.1998.00351.x
Wackerhage H., Schoenfeld B. J., Hamilton D. L., Lehti M., Hulmi J. J. (2019). Stimuli and Sensors that Initiate Skeletal Muscle Hypertrophy Following Resistance Exercise. J. Appl. Physiology 126, 30–43. doi:10.1152/japplphysiol.00685.2018
Watt P. W., Kelly F. J., Goldspink D. F., Goldspink G. (1982). Exercise-induced Morphological and Biochemical Changes in Skeletal Muscles of the Rat. J. Appl. physiology 53, 1144–1151. doi:10.1152/jappl.1982.53.5.1144
Wernbom M., Augustsson J., Thome? R. (2007). The Influence of Frequency, Intensity, Volume and Mode of Strength Training on Whole Muscle Cross-Sectional Area in Humans. Sports Med. 37, 225–264. doi:10.2165/00007256-200737030-00004
Wickham H., Averick M., Bryan J., Chang W., Mcgowan L., François R., et al. (2019). Welcome to the Tidyverse. Joss 4, 1686. doi:10.21105/joss.01686
Wiesinger H.-P., Rieder F., Kösters A., Müller E., Seynnes O. R. (2016). Are Sport-specific Profiles of Tendon Stiffness and Cross-Sectional Area Determined by Structural or Functional Integrity? PLoS One 11, e0158441. doi:10.1371/journal.pone.0158441
Yu J.-G., Carlsson L., Thornell L.-E. (2004). Evidence for Myofibril Remodeling as Opposed to Myofibril Damage in Human Muscles with DOMS: an Ultrastructural and Immunoelectron Microscopic Study. Histochem. Cell. Biol. 121, 219–227. doi:10.1007/s00418-004-0625-9
Keywords: muscle tissue, muscle strength, stretch shortening cycle exercise, muscle growth, human physical conditioning, youth sports, aged
Citation: Arntz F, Mkaouer B, Markov A, Schoenfeld BJ, Moran J, Ramirez-Campillo R, Behrens M, Baumert P, Erskine RM, Hauser L and Chaabene H (2022) Effect of Plyometric Jump Training on Skeletal Muscle Hypertrophy in Healthy Individuals: A Systematic Review With Multilevel Meta-Analysis. Front. Physiol. 13:888464. doi: 10.3389/fphys.2022.888464
Received: 02 March 2022; Accepted: 18 May 2022;
Published: 27 June 2022.
Edited by:
Wolfgang Kemmler, Friedrich-Alexander-University Erlangen-Nürnberg, GermanyCopyright © 2022 Arntz, Mkaouer, Markov, Schoenfeld, Moran, Ramirez-Campillo, Behrens, Baumert, Erskine, Hauser and Chaabene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: H. Chaabene, Y2hhYWJlbmVAdW5pLXBvdHNkYW0uZGU=
 F. Arntz1
F. Arntz1 B. Mkaouer
B. Mkaouer A. Markov
A. Markov B. J. Schoenfeld
B. J. Schoenfeld J. Moran
J. Moran R. Ramirez-Campillo
R. Ramirez-Campillo M. Behrens
M. Behrens H. Chaabene
H. Chaabene