- 1Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand
- 2Department of Mathematics, Universitat Autònoma de Barcelona, Bellaterra, Spain
- 3Bristol Heart Institute, University of Bristol, Bristol, United Kingdom
- 4HU Liryc, Electrophysiology and Heart Modeling Institute, Fondation Bordeaux Université, Bordeaux, France
- 5Centre de Recherche Cardio-Thoracique de Bordeaux, Université Bordeaux, Bordeaux, France
- 6INSERM, Centre de Recherche Cardio-Thoracique de Bordeaux, Bordeaux, France
- 7Auckland City Hospital, Auckland, New Zealand
- 8Department of Medicine, University of Auckland, Auckland, New Zealand
- 9Department of Physiology, University of Auckland, Auckland, New Zealand
- 10Libin Cardiovascular Research Institute, Calgary University, Calgary, AB, Canada
Atrial fibrillation (AF) is the most common cardiac dysrhythmia and percutaneous catheter ablation is widely used to treat it. Panoramic mapping with multi-electrode catheters has been used to identify ablation targets in persistent AF but is limited by poor contact and inadequate coverage of the left atrial cavity. In this paper, we investigate the accuracy with which atrial endocardial surface potentials can be reconstructed from electrograms recorded with non-contact catheters. An in-silico approach was employed in which “ground-truth” surface potentials from experimental contact mapping studies and computer models were compared with inverse potential maps constructed by sampling the corresponding intracardiac field using virtual basket catheters. We demonstrate that it is possible to 1) specify the mixed boundary conditions required for mesh-based formulations of the potential inverse problem fully, and 2) reconstruct accurate inverse potential maps from recordings made with appropriately designed catheters. Accuracy improved when catheter dimensions were increased but was relatively stable when the catheter occupied >30% of atrial cavity volume. Independent of this, the capacity of non-contact catheters to resolve the complex atrial potential fields seen in reentrant atrial arrhythmia depended on the spatial distribution of electrodes on the surface bounding the catheter. Finally, we have shown that reliable inverse potential mapping is possible in near real-time with meshless methods that use the Method of Fundamental Solutions.
Introduction
Intracardiac catheters can acquire electrograms simultaneously at multiple sites on or close to the heart wall and have been used to construct panoramic maps of electrical activity in patients during persistent atrial fibrillation (AF) (Narayan et al., 2012; Pathik et al., 2018). While macro-scale atrial activation is disorganized in AF, it is argued that repeated patterns of local electrical reentry in such maps may provide targets for the percutaneous catheter ablation procedures used to treat this dysrhythmia (Narayan et al., 2012; Haissaguerre et al., 2016). Effective contact mapping with multi-electrode catheters presents challenges. The spatial distribution of electrodes in the 8-spline basket catheters that have been used for intra-atrial mapping is inherently non-uniform, with greater density along splines than around the equator of these devices when fully deployed (Pathik et al., 2018). Deformation of basket catheter splines in contact with the wall can exacerbate sampling heterogeneity (Pathik et al., 2018). Furthermore, experimental and modelling studies indicate incomplete wall coverage, with ∼50% only of electrodes close to the atrial wall (<5 mm from endocardium) in typical studies of the left atrium (LA) (Oesterlein et al., 2016; Martinez-Mateu et al., 2018; Pathik et al., 2018).
Inverse methods can be used to reconstruct potential maps on the heart surface from electrograms recorded with electrodes that are not in contact with it (Johnson and Bronzino, 2000; Pullan et al., 2005). This requires information about the geometry of the heart surface, the 3D locations of the electrodes and the electrical properties of the volume between them. Mesh-based solutions of the inverse potential problem have been widely used for non-invasive electrocardiographic imaging (ECGi) (Barr et al., 1977; Johnson and Bronzino, 2000; Ramanathan and Rudy, 2001; Pullan et al., 2005; Cluitmans et al., 2017; Duchateau et al., 2019) but also for non-contact intracardiac potential mapping with electrodes arrays mounted on the surface of inflatable balloons (Khoury et al., 1995). To solve this problem, it is necessary to specify Cauchy boundary conditions; that is to assign both potentials and normal potential gradients at points across the boundary on which electrical recordings are made (Johnson and Bronzino, 2000; Pullan et al., 2005). This presents no difficulties for ECGi or for intracardiac inverse potential mapping if electrodes are mounted on an inflatable balloon. Sampling surfaces are insulating in both instances and the normal potential gradient is zero everywhere on them. This is not the case, however, for a multi-electrode basket catheter and normal potential gradients must be estimated on the virtual surface that bounds the electrodes to solve mesh-based formulations of the inverse potential problem. While reliable solutions of the inverse potential problem can in principle be obtained with mesh-based methods such as the finite element method (FEM) or boundary element method (BEM) if appropriate input information is provided (Johnson and Bronzino, 2000; Pullan et al., 2005), meshless methods that employ the Method of Fundamental Solutions (MFS) (Fairweather and Karageorghis, 1998) offer a simpler alternative. The latter approach has been used for ECGi (Wang and Rudy, 2006; Bear et al., 2018) and was recently proposed for non-contact intracardiac potential mapping (Meng et al., 2022).
Here, we provide a systematic in silico analysis of mesh-based and meshless methods for solving the intracardiac inverse potential problem—for the first time as far as we are aware. The mathematical bases of the approaches used in this setting are summarized and a simple method for estimating Cauchy boundary conditions from electrograms recorded with a multi-electrode basket catheter is outlined. This is tested in a simplified 2D domain and then used for an FEM-based investigation of inverse potential mapping in the 3D atria. The extent to which accuracy is affected by catheter dimensions, electrode distribution and noise are considered. Finally, we compare the efficacy of this mesh-based approach with meshless methods that use the MFS.
This study demonstrates that reliable non-contact potential mapping can be achieved across a wide range of basket catheter dimensions using mesh-based inverse methods if the electrode distribution is sufficient to provide representative samples of the intracardiac potential field. It also shows that the MFS is equally accurate over most of this range but computationally more efficient.
Mathematical Background
The electrostatic potential
where σ is the electrical conductivity tensor and
where
A Mesh-Based Inverse Approach
A representation of the potential problem is given in Figure 1A. If the potential on the endocardial surface
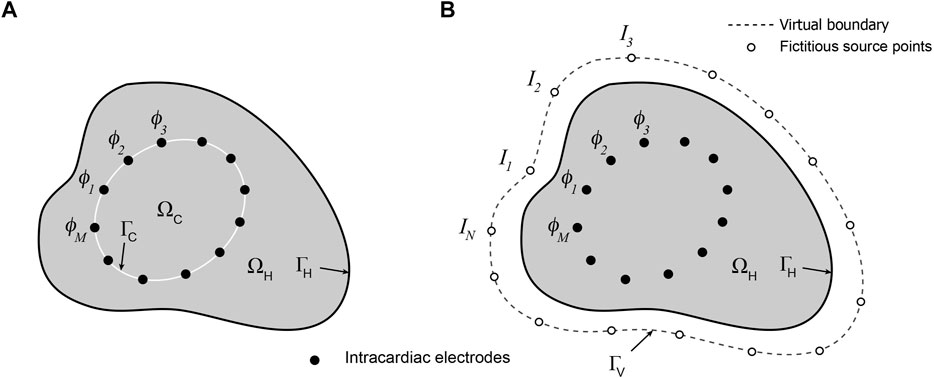
FIGURE 1. Schematic representations of (A) mesh-based and (B) meshless/MFS formulations of the intracardiac inverse potential problem which seeks to map the potential distribution on the surface
The objective of the corresponding inverse problem is to reconstruct
where
Both problems can be solved numerically using finite difference, finite element and finite volume methods or, because the problem can be reduced to the boundaries alone since
where the first term is the sum of squared residuals from Eq. 3 and the second penalizes lack of smoothness of the solution vector. With zero-order Tikhonov regularization
To solve the intracardiac inverse problem, it is necessary to specify appropriate boundary conditions at
That is
where in and out indicate inner and outer sides of
For a balloon catheter,
Meshless Inverse Methods That Use the Method of Fundamental Solutions
The Method of Fundamental Solutions (MFS) provides a means of solving partial differential equations such as the Laplace equation without the need to set up connected internal meshes in the solution domain (Fairweather and Karageorghis, 1998). This approach was applied to ECGi by Wang and Rudy (Wang and Rudy, 2006) and here we extend it to intracardiac inverse potential mapping.
The meshless/MFS formulation of the intracardiac problem is presented in Figure 1B. Potentials
At any instant, the potential
where
where
This results in an M
This system is inherently under-determined because the number of electrodes M is generally less than N, the number of fictitious sources needed to map potentials faithfully onto
Methods
A well-established computational approach (Ramanathan and Rudy, 2001) was used to quantify the accuracy with which potentials around an external boundary can be reconstructed from non-contact potentials sampled within the corresponding domain using inverse solution methods. The basic steps were as follows. First, “ground-truth” potential distributions, one simple and one more complex, were specified on the external boundary. The corresponding internal field was then determined by numerical solution of Laplace’s equation and this potential field was sampled at points corresponding to different catheter dimensions and electrode distributions. Finally, potentials on the outer boundary were reconstructed using the sampled potentials and compared with ground-truth potentials to assess the accuracy of inverse mapping. Key features of our mesh-based inverse approach were tested first with simple 2D problems and then extended to a more realistic 3D FEM analysis using atrial endocardial boundary geometry and representative potential distributions on this anatomy based on experimental measurement and simulation. Finally, the efficacy of inverse potential mapping using a meshless/MFS approach was compared with a representative mesh-based FEM analysis.
2D Analysis
Aspects of the approach employed here are illustrated in Figure 2. Two different arbitrary ground-truth potential distributions were specified on the boundary
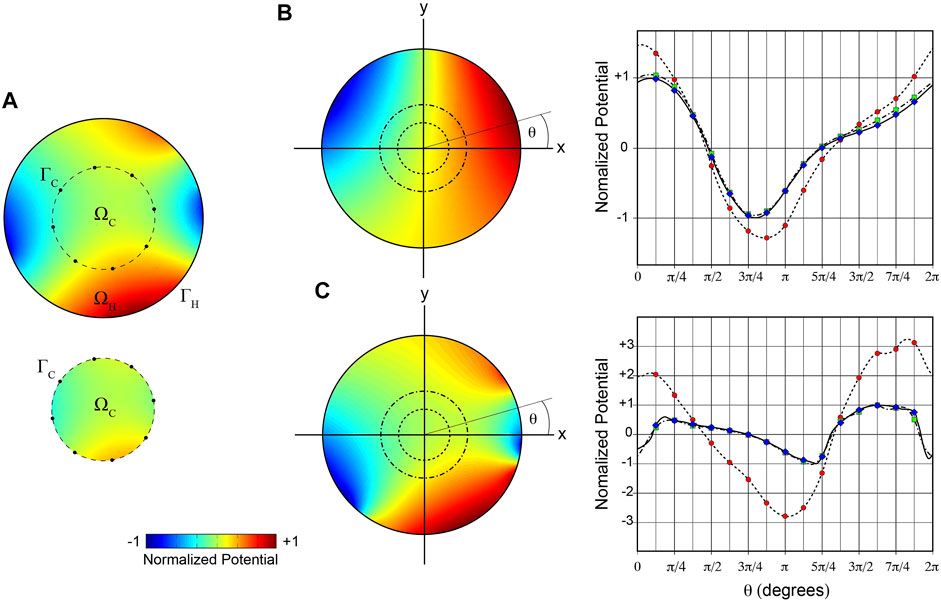
FIGURE 2. 2D illustration of mesh-based inverse potential mapping. (A) Schematic of steps in specifying Cauchy boundary conditions on
Cauchy boundary conditions on
3D Analyses
Anatomic and experimental data used for 3D analyses were acquired from an anesthetized closed-chest sheep employing methods summarized below. All procedures were approved by the Animal Ethics Committee of the University of Auckland and conform to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85–23).
Gadolinium-enhanced (Gd-DTPA 0.2 mmol kg) ECG-gated magnetic resonance images (MRIs) of the atria (1.0 mm2 × 1.0 mm2 in-plane resolution approximately parallel to the atrio-ventricular valve plane and 1.6 mm between slices) were acquired with a 3T Siemens Magnetom Skyra scanner in late diastole with lungs inflated. Atrial electrical activation was subsequently mapped using 38 and 48 mm 64-electrode Constellation™ catheters (Boston Scientific) introduced percutaneously into the atria via the jugular vein under fluoroscopic guidance. Catheters were positioned in the LA using a guide wire and sheath introduced by trans-septal puncture. Electrograms from LA catheters (bandlimited to 0.5–1,500 Hz and sampled at 3 kHz) were recorded simultaneously in sinus rhythm (SR) using a multi-channel acquisition system (UnEmap, Auckland UniServices) with catheters in different locations. Serial biplane ciné X-ray views of the catheters (LAO/RAO, 25 frames/second, with concurrent Lead II ECG added for synchronization) were acquired immediately after each electrical recording. The ventilator was switched off during fluoroscopy to minimize respiratory motion.
Endocardial surface geometry from a representative LA was segmented from serial MRI using Amira 5.4 (Thermo Fisher Scientific) and reconstructed in 3D with the atrial appendage cropped (see Figure 2). LA electro-anatomic maps were reconstructed for this heart from recordings in SR with 3D electrode locations estimated from biplane X-ray records (Meng et al., 2017). Ground-truth potential distributions in SR were constructed at selected activation times by interpolating potentials around the activation wavefront from recorded electrograms. Ground truth data representing reentrant atrial activation were simulated. Meandering spiral wave reentry was simulated on an isotropic 2D monodomain with Fenton Karma activation kinetics (Fenton and Karma, 1998) using a standard cross-field S1-S2 stimulus protocol (Pandit et al., 2005). Points on the 2D domain were sampled and mapped onto the 3D surface mesh so that surface area was similar in both, with a contour adjacent to the boundary in the former assigned to the mitral valve orifice. Extracellular potentials were approximated from the transmembrane currents computed at each 3D point at a sampling rate of 1 kHz.
The open-source software environment SCIRun (Burton et al., 2011) was used for FEM solutions of 3D forward problems. A triangular surface mesh (1,529 nodes) was fitted to the LA and
SCIRun was also used for FEM solutions of 3D inverse problems. The methods outlined above for estimating Cauchy boundary conditions for the 2D case were extended to 3D as follows. Intracardiac fields were sampled at points corresponding to electrodes on specified intracardiac catheters. A triangular mesh was fitted to
Inverse solutions with the MFS were run with purpose-written code and a more detailed account of the methods used is given in Meng et al. (Meng et al., 2022). In brief, the virtual boundary Γv was formed by uniform radial inflation of the atrial surface mesh
Correspondence between ground-truth and reconstructed potential maps were quantified by evaluating normalized root-mean-squared error (nRMSE) and correlation coefficient (CC).
where N is the number of surface points compared,
Activation times (ATs) for ground-truth and reconstructed electrograms were estimated as maximum negative rate of potential change and the activation time difference ΔT at each surface point was evaluated as the difference between the ground-truth and reconstructed ATs
SCIRun was used for 3D FEM forward and inverse calculations and for visualization of all 3D results. Meshless/MFS inverse solutions were run in purpose-written C code. All other computation (2D analysis, estimation of potential gradients, regularization and evaluation of correspondence measures), was implemented in the MATLAB programming language (The Mathworks, Natick, Massachusetts).
Results
2D Analysis of Mesh-Based Intracardiac Potential Mapping
We used a simple 2D analysis initially to test the feasibility of our methods for estimating intracardiac Cauchy boundary conditions. Figure 2A illustrates the steps involved. It shows that the ground truth potential field in
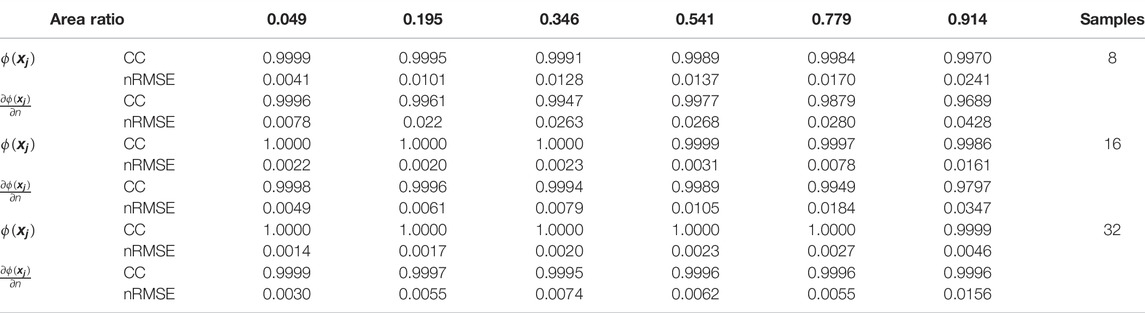
TABLE 1. Effects of number of points on sampling boundary
In this figure, we also compare ground-truth potentials on
3D Analysis of Mesh-Based Intracardiac Potential Mapping Accuracy
Figure 3 presents the results of an in silico analysis of the accuracy with which LA surface potential distributions can be reconstructed from non-contact electrograms recorded in SR using 64-channel basket catheters. The ground truth endocardial potential distribution at one instant (43.9 msec after onset of atrial activation) is shown in Figure 3A with the 3D locations of basket catheter electrodes superimposed (the volume ratio of the catheter with respect to LA cavity was 0.67). The corresponding inverse reconstruction of atrial surface potentials in Figure 3B is qualitatively similar to the ground-truth map, while reference and inverse electrograms at a representative site (point 1 in Figure 3B) correspond closely throughout the activation cycle (Figure 3C). Figures 3D–F show acceptable non-contact mapping accuracy for a wide range of catheter dimensions (median: CC >0.96; nRMSE <0.12; ΔT = 3 ms for catheter-atrial volume ratios >0.3). However, error accumulates progressively when catheter dimensions are decreased below this range.
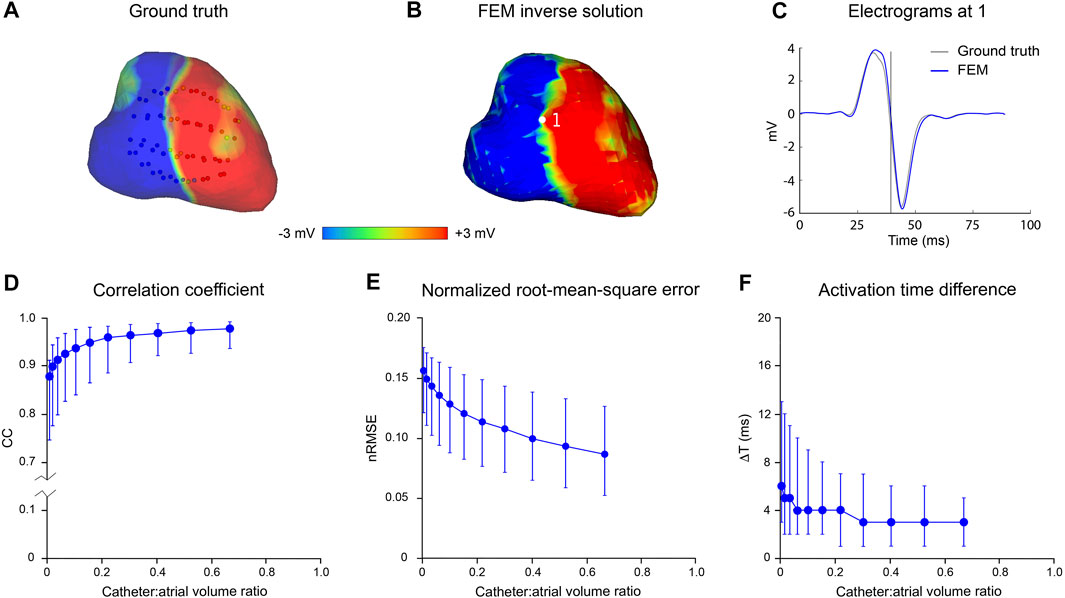
FIGURE 3. Effect of catheter size on accuracy of inverse potential mapping. Comparison of ground truth potential maps on endocardial surface of LA in SR with inverse maps reconstructed using FEM. Inverse potential maps were reconstructed from electrograms “sampled” using a 64-electrode basket catheter, with centroids of catheter and LA chamber aligned. The upper panel presents typical results for a catheter which bounds a volume fraction of 0.67 relative to LA volume. These include (A) ground-truth surface potential distribution 43.9 msec after onset of activation with basket catheter superimposed, and (B) corresponding potential maps reconstructed using FEM. Finally, in (C) a ground-truth electrogram (black) at point 1 is compared with corresponding electrograms reconstructed using FEM (blue). In the lower panel, (D) correlation coefficient (CC) (E) normalized root-mean-squared error (nRMSE), and (F) activation time difference (ΔT) are presented as functions of relative catheter volume for FEM. Median values and interquartile range are given. Abbreviations: FEM, finite element method; SR sinus rhythm.
Figure 4 presents the error introduced when the normal potential gradient on the surface bounding the electrodes,
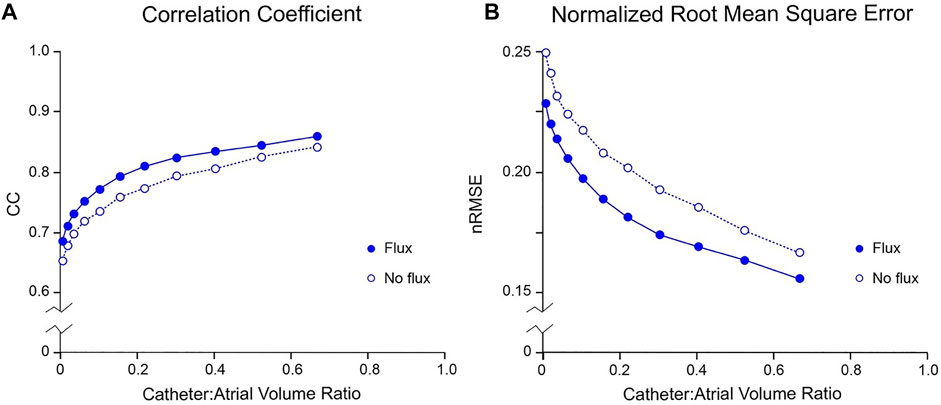
FIGURE 4. Effect of boundary value specification on accuracy of inverse potential mapping using FEM. Comparison of ground truth potential maps on endocardial surface of LA in SR 43.9 msec after onset of activation with inverse maps reconstructed using FEM from potentials sampled with centrally located internal basket catheters with 64 equi-spaced electrodes. In (A) and (B), respectively, relative root-mean-squared error (nRMSE) and correlation coefficient (CC) are presented as functions of catheter volume relative to LA. Additional error introduced by not estimating normal potential gradients on the virtual surface bounding electrodes is indicated by the no flux results (open circles) in which normal potential gradients are set to zero. Abbreviations: FEM, finite element method; SR, sinus rhythm.
Results of an analysis of inverse mapping accuracy for more complex atrial rhythms in the presence of noise are presented in Figure 5. In this case, a simulated rotor with a moving core was used as ground-truth. Three activation cycles were sampled with a 130-electrode basket catheter and Gaussian noise at RMS voltages of 18, 56 and 178 µV was added to these records. The upper panel shows representative results for a catheter-atrial volume ratio of 0.67. Ground-truth surface potential maps (Figure 5A) were reconstructed with reasonable accuracy in the absence of noise (see Figure 5B). Median results were CC = 0.92, nRMSE = 0.11 and ΔT = 2 ms; clearly better than the corresponding result with a 64-electrode catheter (CC = 0.83, nRMSE = 0.14 and ΔT = 3 ms). At this catheter dimension also, inverse mapping was robust in the presence of realistic levels of electrical noise. Results with systematic variation of relative catheter dimension and noise are shown in Figures 5D–F. Accuracy was relatively invariant despite increasing noise as catheter-atrial volume ratio was reduced from 0.67 to ∼0.2. At dimensions less than this, however, there was a progressive increase in error which scaled with noise level. It is noteworthy that activation time estimates were markedly degraded by noise at reduced catheter dimensions.
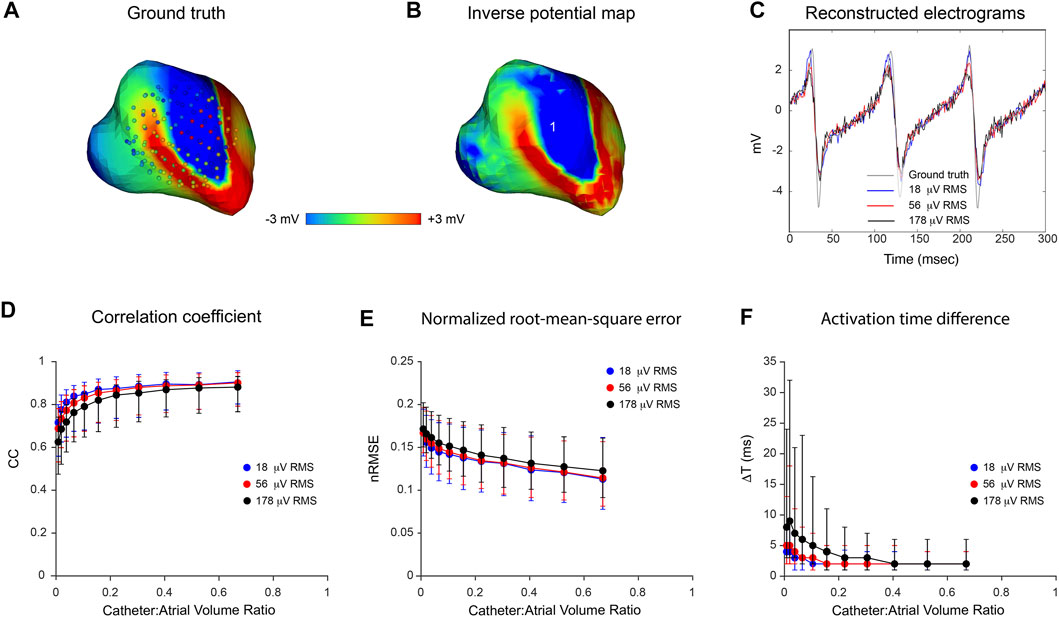
FIGURE 5. Effects of catheter dimension and noise on inverse potential maps reconstructed during macro-reentry using FEM. LA surface potentials during 3 cycles of simulated atrial flutter are reconstructed from electrograms sampled inside the LA cavity with 130-electrode basket catheters and compared with ground-truth data. The upper panel presents typical results for catheters that bound a volume fraction of 0.67 relative to LA volume. These include (A) the ground-truth surface potential distribution at one instant with catheter electrodes overlaid (B) corresponding potential map reconstructed using electrograms “sampled” with a 130-electrode basket catheter, and (C) electrograms reconstructed at location 1 from sampled records with 18 µV RMS (blue), 56 µV RMS (red) and 178 µV RMS (black) of added Gaussian noise compared with the ground truth electrogram (grey) at the same site. In the lower panel, (D) correlation coefficient (CC) (E) normalized root-mean-squared error (nRMSE), and (F) activation time difference (ΔT) are presented as functions of catheter-atrial volume ratio for these levels of added noise. Median values and interquartile range are given. Abbreviation: FEM, finite element method.
An important final observation is that the transfer matrices used for 3D FEM analyses were over-determined in all cases, with the LA represented by a 1529-node triangular surface mesh while a 6720-node triangular mesh was fitted to the catheter. This was necessary to achieve stable solutions.
Comparison of FEM and MFS Inverse Solutions
In Figure 6, we compare the performance of mesh-based inverse mapping employing a FEM solver with a meshless approach that employs the MFS. We used the simulated rotor in Figure 5 as ground-truth and again “sampled” 3 activation cycles with 130-electrode basket catheters of different dimensions. FEM inverse solutions matched ground-truth maps quite well, with median values of CC = 0.91 and nRMSE = 11.3% across the activation sequence at a catheter-atrial volume ratio of 0.67. Corresponding results for the meshless/MFS approach were 0.95 and 4.9%, but activation time differences with ground truth were the same for both. While CC was marginally better with MFS than FEM for catheter-atrial volume ratios >0.3, this measure decreased more rapidly with the MFS when catheter dimensions were reduced further (see Figure 6A). Likewise, ΔT was greater with the MFS for catheter-atrial volume ratios <0.3. In contrast, nRMSE was substantially less for MFS than FEM inverse results across the full volume range.
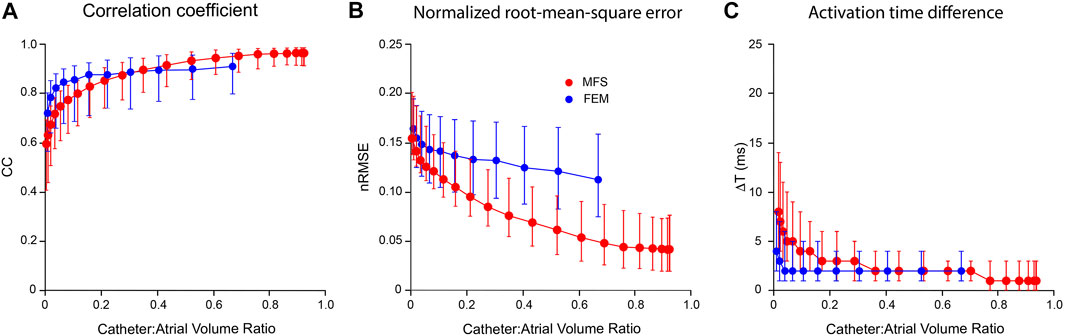
FIGURE 6. Comparison of inverse potential maps reconstructed during macro-reentry using FEM and meshless methods that employ the MFS. LA surface potentials throughout 3 activation cycles in simulated atrial flutter reconstructed from electrograms sampled inside LA cavity with 130-electrode basket catheters and compared with ground-truth data. (A) Correlation coefficient (CC) (B) normalized root-mean-squared error (nRMSE), and (C) activation time difference (ΔT) are presented as functions of catheter-atrial volume ratio for FEM (blue) and meshless/MFS (red). Median values and interquartile range are given. Abbreviations: FEM, finite element method; MFS method of fundamental solutions.
The main difference between methods was that the MFS was much more efficient computationally than the FEM in our hands. Transfer matrices were simple to set up and inverse solutions were obtained in near real-time using purpose-written code. Finally, the meshless/MFS formulation was robust, with stable inverse solutions despite the fact that transfer matrices were inherently under-determined.
Discussion
Summary
In this paper, we present the results of a computational analysis of the accuracy with which endocardial potential maps can be reconstructed from non-contact multi-electrode basket catheter recordings. This inverse problem is addressed initially using a mesh-based approach where transfer relationships are formulated between potentials on the two boundaries involved. This is accurate in principle because assumptions made about the electrical properties of the solution domain are limited (and inherently realistic). However, it requires Cauchy conditions to be specified on the surface
Mesh-Based Inverse Potential Mapping
With the mesh-based inverse solvers used in this analysis, it is necessary to specify potentials at sufficient points on the surface
Our 2D analysis demonstrates that intracardiac potential fields in the vicinity of
Our analyses show that the accuracy of inverse potential mapping decreases when catheter dimensions are reduced and this becomes more marked as noise levels are increased. In the 3D examples presented here (Figures 3, 5), error remains relatively low as catheter-atrial volume ratios decrease to ∼0.3 but increases exponentially with further reduction. These findings are intuitively reasonable. With increasing distance from the heart surface, intracardiac potentials are progressively attenuated and smoothed. The extent to which high temporal frequencies on
The 3D analyses above also show that the accuracy with which potentials on
Comparison of Mesh-Based and Meshless Inverse Potential Mapping
As noted at the start of the Discussion, we opted to use mesh-based inverse potential mapping as the reference method in this study because assumptions made about the electrical properties of the solution domain with this approach are minimal. We argue that the correspondence of the 3D FEM solutions presented here with ground truth and the stability of these results support this strategy. In contrast, the meshless/MFS alternative with which it is compared employs a much simpler representation of the intracardiac forward problem but introduces additional assumptions about the current sources that give rise to intracardiac potential distributions. The fact that the MFS approach performs better for catheter-atrial volume ratios >0.3 (Figure 6) warrants further consideration. It is likely that much of the apparent improvement with meshless/MFS is due to the compact support for linear interpolation in the FEM implementation used. This gives rise to discontinuities across element boundaries (see Figure 5B) whereas potentials on the heart surface are continuous with meshless inverse mapping. We note that there is no difference in ΔT for catheter-atrial volume ratios >0.3 and argue that meshless/MFS inverse potential mapping is at least as accurate as mesh-based inverse methods over this range.
The major advantage of meshless/MFS methods in this setting is that the forward transfer function is computationally simple and can be assembled very rapidly. In contrast, with mesh-based alternatives, such as FEM, the forward transfer function is complex and time consuming to assemble and invert. Furthermore, our results indicate that the meshless/MFS representation of the intracardiac problem is much better conditioned and therefore more robust than FEM. This is reflected by the fact that an over-determined transfer matrix was needed for stable inverse solutions with FEM, whereas accurate solutions were obtained with MFS despite the fact that transfer matrices were under-determined.
Potential Clinical Impact of These Findings
Non-contact intracardiac mapping systems that have been used clinically have utilized balloon-mounted multi-electrode array for potential mapping (Khoury et al., 1995; Khoury et al., 1998; Schilling et al., 1999) or have reconstructed membrane charge density from electrograms recorded with an open basket catheter (Willems et al., 2019). While the inverse problem techniques used are different, one would expect the information recovered to be affected similarly by electrode density and positioning, and catheter size, i.e. the number of recording electrodes, their physical spacing on the catheter and proximity of the electrodes to the atrial wall once the catheter is fully deployed. Validation studies have shown that the accuracy with which endocardial electrograms are constructed with the first of these approaches is inversely related to the distance from the electrodes array to corresponding points on the cavity surface (Earley et al., 2006). As far as we are aware, an equivalent systematic validation has not been completed for the second. This study indicates that reliable non-contact potential mapping can also be performed using multi-electrode catheters and could be carried out in near real-time using meshless methods that employ the MFS.
In terms of optimal catheter design, greater electrode density and more uniform distribution would be expected to provide higher resolution. However, the question of how much is enough has only started to be addressed recently. Martinez et al. (Martinez-Mateu et al., 2018) showed computationally that methods used to transform basket electrogram signals back into catheter surface potential maps may result in the creation of fictitious repetitive activation patterns resembling AF rotors when the input information was too sparsely sampled. Williams et al. (Williams et al., 2018) on the other hand defined optimal endocardial sampling densities, both computationally and in-vivo, required to resolve activation patterns of varying complexities. They report that a minimum endocardial sampling density of 1.0–1.5 points/cm2 is required, with higher densities needed to resolve spiral wave activity. Whilst they were looking at endocardial interpolation of contact recordings not inverse solutions, it is evident from our work here that potential pitfalls in inverse mapping also need to be addressed with good catheter design and mechanistic insight.
Limitations
It could be argued that the BEM is better matched to the mesh-based inverse potential problem addressed here (Oostendorp and van Oosterom, 1991; Johnson and Bronzino, 2000; Pullan et al., 2005). The FEM generates sparse transfer matrices and is computationally expensive, while BEMs reduce the solution domain to the boundaries only giving rise to compact transfer matrices that can reduce computational overheads and improve accuracy (Johnson and Bronzino, 2000; Pullan et al., 2005). However, our purpose here was to benchmark the mesh-based approach and we opted to use FEM to avoid possible instability that can occur when boundaries are geometrically complex as is the case in the atria. We note that our mesh-based analysis has proved stable and that the meshless/MFS methods with which they are compared are much more efficient computationally than either FEM or BEM. A further limitation is that although our ground-truth data represent atrial rhythms of increasing complexity they do not replicate the spatio-temporal disorder that characterizes AF.
Conclusion
This computational analysis indicates that potentials on the endocardial surface of a cardiac chamber can be reconstructed with intracardiac multi-electrode basket catheters using inverse solution methods provided that the boundary geometry is specified and the 3D location of catheters with respect to it are known. These data are now available clinically. Panoramic electro-anatomic maps can therefore be generated at successive time steps from non-contact recordings. Mapping accuracy is determined by 1) the distance of recording electrodes from the endocardium, 2) their distribution within the subdomain sampled, and 3) rhythm complexity. These issues should be factored into the design of future non-contact multi-electrode basket catheters. We conclude that reliable non-contact potential mapping can be carried out in near real-time using meshless methods that employ the MFS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the All procedures were approved by the Animal Ethics Committee of the University of Auckland and conform to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85–23).
Author Contributions
SM and NS jointly completed all aspects of the analyses presented here and developed the computational pipeline used. JC-S is responsible for our use of MFS, completed 2D prototype studies using this approach and was influential in the direction taken in this work. LB wrote our initial 3D MFS code. SM, BS, IL, NL, AG, GS, and JZ designed, performed and analyzed animal experiments which provided data for this study. JZ contributed to the simulation of reentrant arrhythmia and provided phase mapping code. SM, NS and BS generated the figures. SM, NS, GS, and BS wrote the manuscript. BS and DB were responsible for funding and direction of the project. All authors contributed to manuscript revision, read, and approved the version submitted.
Funding
This work was supported in part by the Health Research Council of New Zealand (NZ) under Programme Grant 13-799, by the NZ Ministry of Business and Innovation Endeavour Fund Grant CONT-50916-ENDSI-UOA and by Return on Sciences, NZ.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barr R. C., Ramsey M., Spach M. S. (1977). Relating Epicardial to Body Surface Potential Distributions by Means of Transfer Coefficients Based on Geometry Measurements. IEEE Trans. Biomed. Eng. 24, 1–11. doi:10.1109/TBME.1977.326201
Bear L. R., LeGrice I. J., Sands G. B., Lever N. A., Loiselle D. S., Paterson D. J., et al. (2018). How Accurate Is Inverse Electrocardiographic Mapping? Circ Arrhythmia Electrophysiol. 11, e006108. doi:10.1161/CIRCEP.117.006108
Burton B. M., Tate J. D., Erem B., Swenson D. J., Wang D. F., Steffen M., et al. (2011). “A Toolkit for Forward/inverse Problems in Electrocardiography within the SCIRun Problem Solving Environment,” in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society EMBS, Boston, MA, USA, 30 August 2011-03 September 2011, 267–270. doi:10.1109/IEMBS.2011.6090052
Chamorro-Servent J., Dubois R., Coudière Y. (2019). Considering New Regularization Parameter-Choice Techniques for the Tikhonov Method to Improve the Accuracy of Electrocardiographic Imaging. Front. Physiol. 10, 273. doi:10.3389/fphys.2019.00273
Cluitmans M. J. M., Bonizzi P., Karel J. M. H., Das M., Kietselaer B. L. J. H., de Jong M. M. J., et al. (2017). In Vivo Validation of Electrocardiographic Imaging. JACC Clin. Electrophysiol. 3, 232–242. doi:10.1016/j.jacep.2016.11.012
Duchateau J., Sacher F., Pambrun T., Derval N., Chamorro-Servent J., Denis A., et al. (2019). Performance and Limitations of Noninvasive Cardiac Activation Mapping. Heart rhythm. 16, 435–442. doi:10.1016/j.hrthm.2018.10.010
Earley M. J., Abrams D. J. R., Sporton S. C., Schilling R. J. (2006). Validation of the Noncontact Mapping System in the Left Atrium during Permanent Atrial Fibrillation and Sinus Rhythm. J. Am. Coll. Cardiol. 48, 485–491. doi:10.1016/j.jacc.2006.04.069
Fairweather G., Karageorghis A. (1998). The Method of Fundamental Solutions for Elliptic Boundary Value Problems. Adv. Comput. Math. 9, 69–95. doi:10.1023/A:1018981221740
Fenton F., Karma A. (1998). Vortex Dynamics in Three-Dimensional Continuous Myocardium with Fiber Rotation: Filament Instability and Fibrillation. Chaos 8, 20–47. doi:10.1063/1.166311
Haissaguerre M., Shah A. J., Cochet H., Hocini M., Dubois R., Efimov I., et al. (2016). Intermittent Drivers Anchoring to Structural Heterogeneities as a Major Pathophysiological Mechanism of Human Persistent Atrial Fibrillation. J. Physiol. 594, 2387–2398. doi:10.1113/JP270617
Hansen P. C. (2010). “Front Matter,” in Discrete Inverse Problems (Society for Industrial and Applied Mathematics) (Philadelphia: Society for Industrial and Applied Mathematics), i–xii. doi:10.1137/1.9780898718836.fm
Johnson C. R. (2000). “Numerical Methods for Bioelectric Field Problems,” in The Biomedical Engineering Handbook. Editor J. D. Bronzino. 2nd Edition (Boca Raton: CRC Press LLC).
Khoury D. S., Berrier K. L., Badruddin S. M., Zoghbi W. A., Zoghbi W. A. (1998). Three-dimensional Electrophysiological Imaging of the Intact Canine Left Ventricle Using a Noncontact Multielectrode Cavitary Probe: Study of Sinus, Paced, and Spontaneous Premature Beats. Circulation 97, 399–409. doi:10.1161/01.cir.97.4.399
Khoury D. S., Taccardi B., Lux R. L., Ershler P. R., Rudy Y. (1995). Reconstruction of Endocardial Potentials and Activation Sequences from Intracavitary Probe Measurements: Localization of Pacing Sites and Effects of Myocardial Structure. Circulation 91, 845–863. doi:10.1161/01.CIR.91.3.845
Martinez-Mateu L., Romero L., Ferrer-Albero A., Sebastian R., Rodríguez Matas J. F., Jalife J., et al. (2018). Factors Affecting Basket Catheter Detection of Real and Phantom Rotors in the Atria: A Computational Study. PLoS Comput. Biol. 14, e1006017. doi:10.1371/journal.pcbi.1006017
Meng S., Sunderland N., Chamorro-Servent J., Bear L. R., Lever N. A., Sands G. B., et al. (2022). Intracardiac Inverse Potential Mapping Using the Method of Fundamental Solutions. Front. Physiol. 13, 873049. doi:10.3389/fphys.2022.873049
Meng S., Zhao J., Lever N., Sands G., Bear L., Gillis A., et al. (2017). “Atrial Electro-Anatomic Mapping with a Novel Noncontact Approach,” in Computing in Cardiology, Rennes, France, 24-27 September 2017, 1–4. doi:10.22489/CinC.2017.105-291
Narayan S. M., Krummen D. E., Rappel W.-J. (2012). Clinical Mapping Approach to Diagnose Electrical Rotors and Focal Impulse Sources for Human Atrial Fibrillation. J. Cardiovasc Electrophysiol. 23, 447–454. doi:10.1111/j.1540-8167.2012.02332.x
Oesterlein T., Frisch D., Loewe A., Seemann G., Schmitt C., Dössel O., et al. (2016). Basket-type Catheters: Diagnostic Pitfalls Caused by Deformation and Limited Coverage. BioMed Res. Int. 2016, 1–13. doi:10.1155/2016/5340574
Oostendorp T., van Oosterom A. (1991). The Potential Distribution Generated by Surface Electrodes in Inhomogeneous Volume Conductors of Arbitrary Shape. IEEE Trans. Biomed. Eng. 38, 409–417. doi:10.1109/10.81559
Pandit S. V., Berenfeld O., Anumonwo J. M. B., Zaritski R. M., Kneller J., Nattel S., et al. (2005). Ionic Determinants of Functional Reentry in a 2-D Model of Human Atrial Cells During Simulated Chronic Atrial Fibrillation. Biophysical J. 88, 3806–3821. doi:10.1529/biophysj.105.060459
Pathik B., Kalman J. M., Walters T., Kuklik P., Zhao J., Madry A., et al. (2018). Absence of Rotational Activity Detected Using 2-dimensional Phase Mapping in the Corresponding 3-dimensional Phase Maps in Human Persistent Atrial Fibrillation. Heart rhythm. 15, 182–192. doi:10.1016/j.hrthm.2017.09.010
Pullan A. J., Cheng L. K., Buist M. L. (2005). Mathematically Modelling the Electrical Activity of the Heart: From Cell to Body Surface and Back Again. Singapore: World Scientific Publishing Company Pte Ltd. ISBN: 978-981-256-373-6. doi:10.1142/5859
Ramanathan C., Rudy Y. (2001). Electrocardiographic Imaging: II. Effect of Torso Inhomogeneities on Noninvasive Reconstruction of Epicardial Potentials, Electrograms, and Isochrones. J. Cardiovasc. Electrophysiol. 12, 241–252. doi:10.1046/j.1540-8167.2001.00241.x
Rice S. O. (1950). Communication in the Presence of Noise-Probability of Error for Two Encoding Schemes. Bell Syst. Tech. J. 29, 60–93. doi:10.1002/j.1538-7305.1950.tb00933.x
Schilling R. J., Peters N. S., Davies D. W. (1999). Feasibility of a Noncontact Catheter for Endocardial Mapping of Human Ventricular Tachycardia. Circulation 99, 2543–2552. doi:10.1161/01.CIR.99.19.2543
Tikhonov A. N., Arsenin V. Y. (1977). Solutions of Ill-Posed Problems. Washington DC: Winston and Sons. Available at: https://www.ams.org/bull/1979-01-03/S0273-0979-1979-14602-0/(Accessed May 4, 2021).
Wang Y., Rudy Y. (2006). Application of the Method of Fundamental Solutions to Potential-Based Inverse Electrocardiography. Ann. Biomed. Eng. 34, 1272–1288. doi:10.1007/s10439-006-9131-7
Willems S., Verma A., Betts T. R., Murray S., Neuzil P., Ince H., et al. (2019). Targeting Nonpulmonary Vein Sources in Persistent Atrial Fibrillation Identified by Noncontact Charge Density Mapping: UNCOVER AF Trial. Circ Arrhythmia Electrophysiol. 12, e007233. doi:10.1161/CIRCEP.119.007233
Keywords: atrial fibrillation, open basket catheters, inverse problem, non-contact mapping, endocardial potentials
Citation: Meng S, Chamorro-Servent J, Sunderland N, Zhao J, Bear LR, Lever NA, Sands GB, LeGrice IJ, Gillis AM, Budgett DM and Smaill BH (2022) Non-Contact Intracardiac Potential Mapping Using Mesh-Based and Meshless Inverse Solvers. Front. Physiol. 13:873630. doi: 10.3389/fphys.2022.873630
Received: 11 February 2022; Accepted: 07 June 2022;
Published: 07 July 2022.
Edited by:
Matthijs Cluitmans, Maastricht University, NetherlandsReviewed by:
Jake Bergquist, The University of Utah, United StatesPeter Michael Van Dam, University Medical Center Utrecht, Netherlands
Copyright © 2022 Meng, Chamorro-Servent, Sunderland, Zhao, Bear, Lever, Sands, LeGrice, Gillis, Budgett and Smaill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Meng, c2h1Lm1lbmdAYXVja2xhbmQuYWMubno=
 Shu Meng
Shu Meng