- 1Guizhou Provincial Key Laboratory for Rare Animal and Economic Insect of the Mountainous Region, College of Biology and Environmental Engineering, Guiyang University, Guiyang, China
- 2Institute of South China Karst, Guizhou Normal University, Guiyang, China
RNAi was used to downregulate the expression of insulin-like peptides (ILP2), with air-modulation, and high-concentration CO2 stress, in the larvae of Tribolium castaneum. We assessed the changes in carbohydrate-related content, trehalase activity, and the expression levels of trehalose pathway genes. And pupation, adult emergence, pupation rate, and mortality were assessed. There was a significant change in the expression of ILPs in T. castaneum, at a certain concentration of CO2. ILP2 RNAi did not alter the trehalose content significantly, however, the glycogen and glucose content increased significantly. High-concentration CO2 stress altered the trehalose content and reduced the glycogen and glucose content. The expression levels of TPS and TRE2 were up-regulated by hypoxia/hypercapnia and dsILP2 combination, with the increase of CO2 concentration, other trehalase genes begin to respond successively. ILP2 knockout raised the mortality and reduced the pupation rate and eclosion rate in CO2. Understanding the insulin pathway responses to hypoxic stress induced by a high concentration of CO2 would further elucidate the mechanisms underlying trehalose metabolism in insects.
Introduction
The red flour beetle, Tribolium castaneum (Herbst) is a coleopteran belonging to the family, Tenebrionidae. It is distributed in the warmer regions and is an extremely important universal pest of stored grains (Shelby, 1977; Campbell et al., 2010; Wei, 2013). It damages a variety of commodities (such as flour, beans, nuts, Chinese medicinal materials, even meat) and causes tremendous economic losses, because of its fast reproduction, long life span, complex eating habits, and strong adaptability. When the population density of pest is high, T. castaneum chestnut secretes benzoquinones, which imparts a pungent odor to the commodity that render it unfit for consumption (Campbell and Runnion, 2003). T. castaneum in stored products and grain is primarily controlled using fumigants and sprays, but long-term use has resulted in the emergence of resistant populations (Pimentel et al., 2010; Boyer et al., 2012; Opit et al., 2012; Perkin and Oppert, 2019). Furthermore, usage of insecticides on food is a concerned due to the safe of human health and environment would expose to harm. Therefore, it is essential to identify new and effective methods to control T. castaneum.
Hermetic storage technology, a chemical-free approach, is used to protect stored grains and pulses against insect pests (Kharel et al., 2019). Although some grain-feeding insects were control by air-tight packaging successfully, a number of storage pests show remarkable air resistance to hypoxia, including T. castaneum and Callosobruchus maculatus, can survive days of low oxygen treatment (Hoback and Stanley, 2001; Chi et al., 2011), which means hypoxia alone is not enough. Oxidative injury can be induced by too low O2 level in organisms which result in morbidity and mortality, whereas CO2 toxicity raises in a concentration-dependent manner (Cao et al., 2015a,b), Several studies have looked at less than 3% oxygen or more than 60% carbon dioxide is effective in controlling most eggs and adults of storage pests (Sauer and Shelton, 2002; Navarro, 2006; Azzam et al., 2010; Kharel et al., 2019). As a consequence, a modified atmospheres with depleted O2 accompanied by elevated CO2 maybe can control stored grain insect pests effectively, become an environmentally friendly alternative to fumigants, it can be used for gas controlled grain storage in the warehouses. (Cheng et al., 2012). This inhibits insect respiration, therefore, controls insect pests and enables green grain storage.
Trehalose is the most important carbohydrate in the insect hemolymph, accounting for 80–90% of the total insect hemolymph sugar, and is therefore, called the “blood sugar” of insects (Elbein et al., 2003; Chen et al., 2010). The trehalose concentration in the hemolymph is not regulated by a steady state in its body, but by a stress metabolite. A drastic change in the nutritional status or the external environment induces changes in the trehalose concentration in the hemolymph, for adapting to the environment and enabling growth (Zhu et al., 2018). It has been reported that trehalose protects Drosophila and mammalian cells from hypoxia and anoxic injury (Chen et al., 2003). Trehalose-6-phosphate synthase (TPS), which synthesize trehalose, is essential for insect growth and development, and overexpression of TPS increases trehalose levels and insect pest tolerance to anoxic (Chen et al., 2003; Chen et al., 2018). Trehalose plays a very important role in the physiological life activities and dealing with abiotic stresses of insects, one of the key regulatory hormones is insulin (Yu et al., 2008; Shukla et al., 2015; Tang et al., 2018). In insects, the insulin/insulin-like growth factor signaling (IIS) pathway regulates carbohydrate and lipid metabolism and it associate with trehalose. Insulin is responsible for lowering blood sugar and promotes synthesis of glycogen, fat, and protein. Multiple insulin-like peptides (ILPs) and a single insulin receptor (InR), in coordination with the other pathways, control the physiological activities in different tissues (He, 2015). Among of them, ILPs are a crucial controller of carbohydrate reserve depletion in insects. ILPs are involved in the regulation of hemolymph trehalose levels in various insects (Satake et al., 1997; Broughton et al., 2005; Xu et al., 2013). ILP2 has been proven that regulate carbohydrate, protein, and lipid metabolism during starvation (Xu et al., 2013; Hun et al., 2019), so we chose to interfere with ILP2 for research.
High carbon dioxide concentration being toxic to insects while also can effectively control most storage pests (Cao et al., 2019). It is an indisputable fact that changes in trehalose levels can help insects acclimatize to adverse environments, however, whether ILP2 could counteract hypoxia/hypercapnia by regulating trehalose metabolism have rarely been reported. T. castaneum is a model insect used for genetic research, which has the most complete sequenced and annotated genome, thus providing the most advanced genetic model of coleopteran pests (Lorenzen et al., 2007). In our study, hypoxia stress induced by hypoxia/hypercapnia and combined with RNAi of ILP2 were assessed for its effects on trehalose metabolism.
Materials and Methods
Insect Culture
The T. castaneum, continuously bred in the laboratory on whole wheat flour containing 5% yeast in an incubator at 28 ± 1°C and 65 ± 5% relative humidity under a constant 24 h dark (0L:24D). The T. castaneum were treated with 25% CO2 + 75% air, 50% CO2 + 50% air, 75% CO2 + 25% air and 95% CO2 + 5% air. The group treated with normal concentration of CO2 was the control group (CK). T. castaneum on the first day of eighth instar larvae were cultured under different levels of CO2 for 48 h and assay ILP genes (ILP1, ILP2, ILP3 and ILP4) relative expression (Pan et al., 2020). T. castaneum on the first day of the eighth instar larvae was used in this study.
Injection of dsRNA and Samples Collection
Premier 5.0 software was used to design specific dsRNA primers for TcILP2, following homologous alignment, and the production was cloned using T cloning, that dsRNA was synthesized using primers containing T7 promoter sequences, and the control dsGFP was synthesized similarly. dsILP2 or dsGFP (200 ng of each) was injected into the soft part between the third and fourth abdominal segments of T. castaneum on the first day of eighth instar larvae. The larvae were collected 48 h after dsRNA (dsILP2 or dsGFP) injection combined with different concentration CO2 treatment, and stored at −80°C. Fifteen insects each were used for the analysis of interferent-effect of dsRNA, gene expression, sugar content, and activity of the enzymes in the glucose metabolism pathways. All assays were performed in triplicate.
The mortality observed within 24 h of the injection was recorded as mechanical death. The mortality after 48 h of processing, the pupal rate, and the emergence rate were recorded.
Total RNA Extraction and First Strand cDNA Synthesis
Total RNA was isolated from each sample by using the MiniBEST Universal Extraction Kit (TaKaRa, Dalian, China), following the manufacturer’s instructions. The RNA integrity was verified by 1% agarose gel electrophoresis, and the RNA concentration was measured by a NanoDrop2000 spectrophotometer (Thermo Scientific, Waltham, MA, United States). First-strand complementary DNA (cDNA) synthesis was performed using the PrimeScript® RT Reagent Kit (TaKaRa, Dalian, China) following manufacturer’s instructions.
Quantitative Real-Time Polymerase Chain Reaction
The relative expression levels of TcILP2 were assessed using qRT-PCR. The insects from each treatment were collected 48 h after dsRNA injection, for assessing the efficiency of the RNAi, using qPCR. Following TcILP2 knockdown under different concentrations of CO2, the transcript levels of six genes pertaining to the trehalose metabolic pathway, including five trehalase genes (TcTre1-1, TcTre1-2, TcTre1-3, TcTre1-4, and TcTre2) and trehalose-6-phosphate synthase gene (TcTPS), were analyzed. The qPCR was carried out on a CFX-96 real-time detection system (Bio-Rad, Hercules, CA, United States) in a 20 µL reaction containing 1 µL (100 ng/μL) cDNA, 1 µL (10 µM) of each primer, 7 µL nuclease-free water, and 10 µL of GoTaq qPCR MasterMix (Promega). The reaction was performed under the following conditions: pre-incubation at 95°C for 2 min, followed by 40 cycles of 95°C for 30 s and annealing at 60°C for 30 s, with melting curves obtained at 65–95°C. Ribosomal protein L13a (RPL13a) was the internal control. Specific primers shown in Table 1. The experiments were performed in triplicate, with three technical replicates each. Relative gene expression was analyzed using the 2−△△CT method (Livak and Schmittgen, 2001).
Trehalase Activity and Sugar Content Following RNAi Combined With High Concentration CO2 Treatment
Following the dsRNA injection, the insects were cultured under different levels of CO2, and the samples were collected. T. castaneum samples were homogenized through ultrasonication, mixed using centrifugation, and suspended in PBS. The concentrations of glucose, trehalose, and glycogen were evaluated, the activities of soluble trehalase and membrane-bound trehalase were detected using absorption/emission at different wavelengths. Standard glucose curve and trehalase activity curve were prepared according to the kit instructions (Sigma-Aldrich, St. Louis, MO, United States). Trehalose was quantified using the anthrone-sulfuric acid method (Leyva et al., 2008). Glucose and glycogen content were quantified, as described previously (Zhang et al., 2017).
Statistical Analyses
Analyses were carried out with SPSS Statistics 21 software (IBM Japan). Bars represent mean ± standard error (SE). Data were statistically evaluated using the unpaired two-tailed Student’s t test for two groups, and values were considered statistically significant when p < 0.05.
Results
The Expression of Insulin Pathway-Related Genes After High-Concentration CO2 Treatment for 48 h and the Different Expression of TcILP2 Gene of T. castaneum After RNAi
High concentration of CO2 stress influenced the expression of the insulin pathway genes. The expression of TcILP1 was upregulated in 25% CO2 + 75% air group and 95% CO2 + 5% air, TcILP2 was upregulated in 25% CO2 + 75% air group, and TcILP3 was upregulated in 25% CO2 + 75% air group, while that of TcILP4 was downregulated in 50% CO2 + 50% air group (Figure 1A). The relative expression levels of TcILP2 in response to RNAi were detected using qPCR. The expression of TcILP2 gene was downregulated in CK group after RNAi, indicating that dsRNA successfully inhibited the expression of the target gene, TcILP2. When combined with high-concentration CO2 stress treatment, highly expressed under the 25% CO2 + 75% air treatment, while, TcILP2 was significantly inhibited under other concentrations of CO2 (Figure 1B).
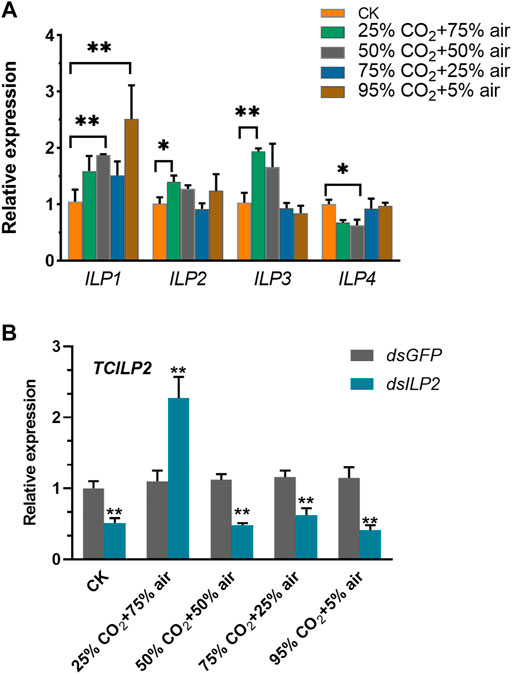
FIGURE 1. Relative expression of TcILP in different concentration CO2 treatment (A) and TcILP2 (B) detection of RNAi interference effect after normal concentration and high concentration CO2 respectively. T. castaneum larvae were divided into three groups (each group covered 10 larvae) and used to treat with 25% CO2 + 75% air, 50% CO2 + 50% air, 75% CO2 + 25% air and 95% CO2 + 5% air, respectively. Samples were collected for 48 h treatment and mRNA expression level were detected by q-PCR. The mRNA level was normalized to that of TcRPL13a mRNA. Significant differences were identified by Student’s t-test (p < 0.05 was expressed by *, p < 0.01 was expressed by **), the same below.
Effects of TcILP2 Knockdown Combined With Different Concentrations of CO2 on the Content of Trehalose, Glucose, and Glycogen
Injection of dsILP2 did not influence the trehalose content significantly (Figure 2A), but the concentrations of glycogen and glucose increased significantly (Figures 2B,C). When combined with the high-concentration CO2 stress treatment, the concentrations of trehalose, glycogen, and glucose decreased, however, the changes in trehalose concentration were not significant, when compared with that in the dsGFP group.
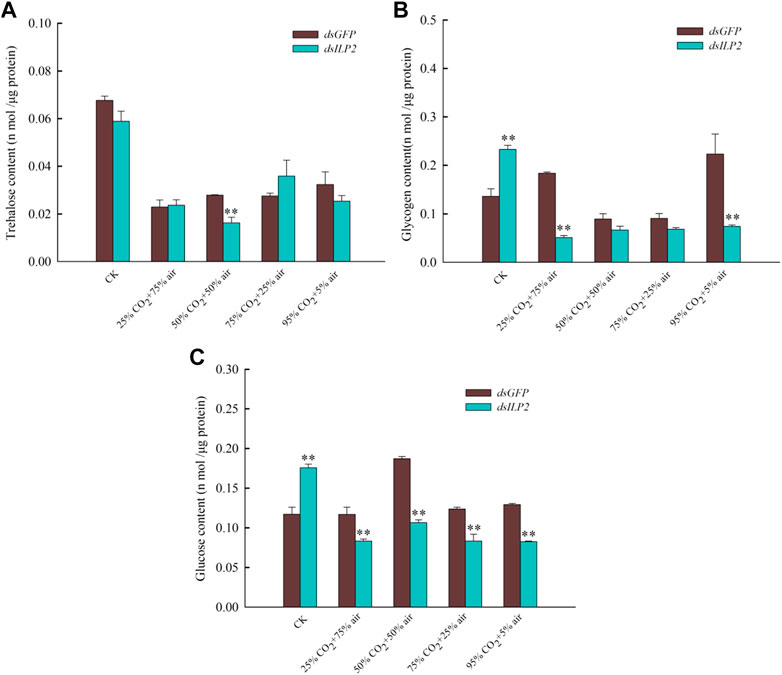
FIGURE 2. Effects of TcILP2 knockdown on content of trehalose (A), glycogen (B), and glucose (C). T. castaneum larvae were divided into three groups (each group covered 15 larvae) and injected with dsILP2 and dsGFP and combined with treatments of 25% CO2 + 75% air, 50% CO2 + 50% air, 75% CO2 + 25% air and 95% CO2 + 5% air, respectively. Insects were collected and used to detect trehalose, glycogen, and glucose content after treatments for 48 h.
Effects of TcILP2 Knockdown Combined With Different Concentrations of CO2 on Trehalase Activity
The result shown that knockdown TcILP2 could use to increase content of soluble trehalose activity (Figure 3A). When RNAi was combined with high-concentration CO2 treatment, the soluble trehalase activity did not change significantly under 50% CO2 + 50% air and 75% CO2 + 25% air treatments. However, it increased significantly in response to the other treatments (Figure 3A). The activity of the membrane-bound trehalase decreased significantly under the stress of 75% CO2 + 25% air and 95% CO2 + 5% air, but there was no significant change under other treatments (Figure 3B).
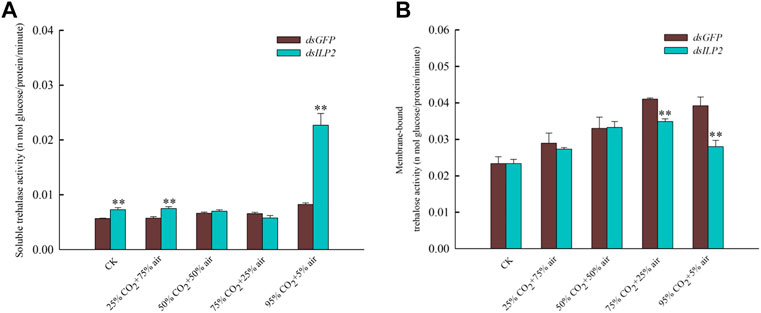
FIGURE 3. Changes of trehalase activity after RNAi combined with high concentration CO2. (A) soluble trehalase activity; (B) membrane-bound trehalase activity. After dsRNA (dsILP2 and dsGFP) injection, T. castaneum larvae were treated with 25% CO2 + 75% air, 50% CO2 + 50% air, 75% CO2 + 25% air and 95% CO2 + 5% air for 48 h. Insects were collected and divided into three groups (each group covered 15 larvae), and used to detect two trehalase enzyme activity.
Effects of TcILP2 Knockdown Combined With Different Concentrations of CO2 on the Expression of Genes in the Trehalose Metabolic Pathway
The expression level of TcTRE1-2 and TcTPS were increased significantly at 48 h after TcILP2 inhibition (p < 0.05) in CK group (Figures 4B,F), on the contrary the expression level of TcTRE1-1 was downregulated significantly (p < 0.05) (Figure 4A), expression level of other genes had no change. Contrary to the relative expression level of TcTRE1-1, TcTRE1-3, and TcTRE1-4 were decreased significantly (p < 0.01) (Figures 4A,C,D), the relative expression level of TcTRE1-2, TcTRE2, and TcTPS were increased significantly (p < 0.01) (Figures 4B,E,F) after treatment of dsILP2 combined with 25% CO2 + 75% air. The relative expression level of TcTRE1-2 and TcTRE1-4 were significant decrease (p < 0.05) (Figures 4B,D), and TcTRE1-3, TcTRE2 and TcTPS were significant increase (p < 0.05) (Figures 4C,E,F) when cultivate T. castaneum under 50% CO2 + 50% air after dsILP2 injection. 75% CO2 + 25% air treatments group after treatment of dsILP2, the expression level of TcTRE1-1 and TcTRE1-4 decreased significantly (p < 0.05) (Figures 4A,D), on the contrary, TcTRE1-2, TcTRE1-3, TcTRE2, and TcTPS were upregulated significantly (p < 0.05) (Figures 4B,C,E,F). As for dsILP2 combined with 95% CO2 + 5% air treatments, the expression of TcTRE1-1 only has any change, while other genes (TcTRE1-2, TcTRE1-3, TcTRE1-4, TcTRE2, TcTPS) increased significantly (p < 0.05) (Figures 4B–F).
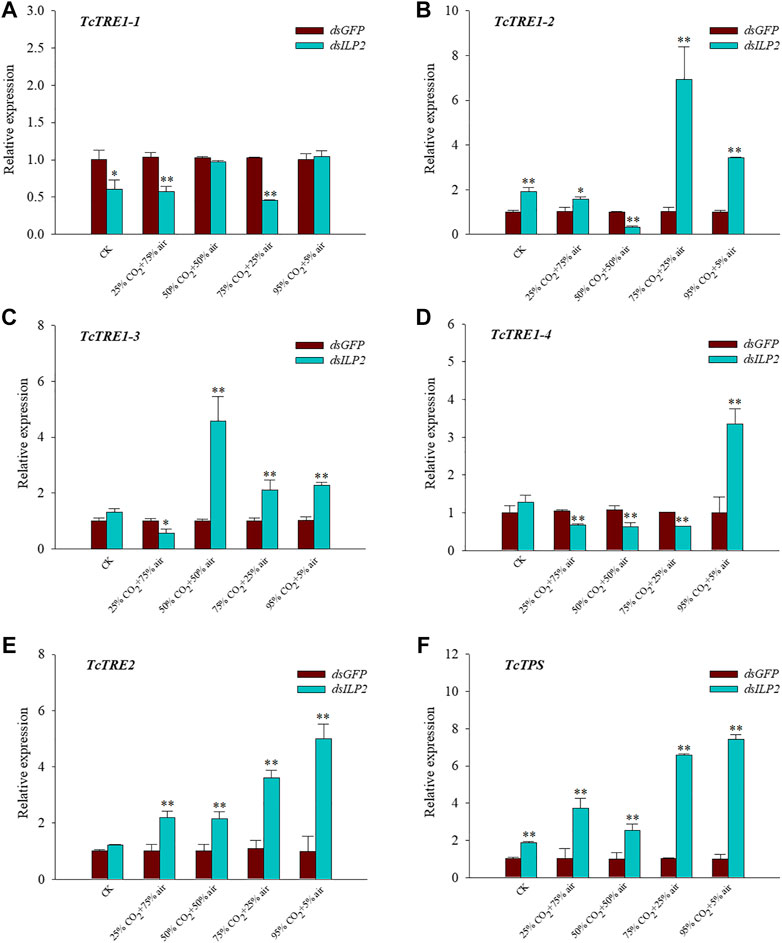
FIGURE 4. Effects of TcILP2 knockdown on the expressions of six trehalose metabolic pathway genes. T. castaneum larvae were divided into three groups (each group covered 10 larvae) and injected with dsILP2 and dsGFP, and 48 h combined with treatments of 25% CO2 + 75% air, 50% CO2 + 50% air, 75% CO2 + 25% air and 95% CO2 + 5% air, respectively. Samples were collected and mRNA expression level were detected by q-PCR. The mRNA level was normalized to that of TcRPL13a mRNA. Five trehalase genes including TcTre1-1, TcTre1-2, TcTre1-3, TcTre1-4, TcTre2 (A–E) and one trehalose-6-phosphate synthases gene [TcTPS, (F)].
Effects of TcILP2 Knockdown Combined With Different Concentrations of CO2 for 48 h on the Death, Pupation, and Emergence of the T. castaneum
The mortality increased in both the dsGFP and the dsILP2 treatments 48 h with the increase in the CO2 concentration, the mortality rate had been reached 100% at 50% CO2 + 50% air concentration (Figure 5A). In addition, at normal CO2 concentration, the mortality rate of the dsILP2 group was significantly higher than that of the control group, however, there was no significant difference between the groups at other concentrations. The pupation rate and emergence rate decreased with the increase in CO2 concentration (Figures 5B,C). The pupation rate was nearly 0 at 75% CO2 + 20% air concentration (Figure 5B), while the feathering rate was nearly 0 at 50% CO2 + 50% air concentration (Figure 5C).
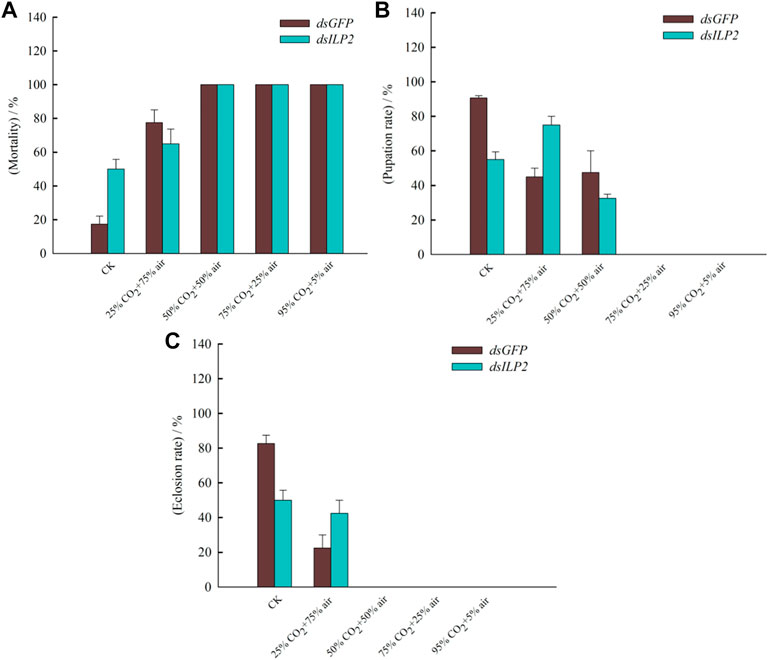
FIGURE 5. The mortality, pupation rate, and emergence rate of the red rice pirate treated with RNAi combined with high concentration CO2. (A) mortality; (B) pupation rate; (C) emergence rate. T. castaneum larvae were divided into three groups, each group covered 150 larvae. The samples used to be counted mortality, pupation, and emergence rate.
Discussion
There are four ILP(ILP1-LIP4) genes in T. castaneum (Li et al., 2008), the gene expression levels of ILPs were changed significantly with different concentrations of carbon dioxide when T. castaneum treated by four air and high CO2 combinations. It evidenced that changes of CO2 concentration level have a significant effect on IIS pathway. Exposed to starvation Drosophila, dilp2 was upregulated and dilp3 was downregulated (Birse et al., 2011). AccILP-2 might play an important role in the response to abiotic stress including light, H2O2, heavy metals (HgCl2 and CdCl2) and pesticides (dichlorvos, paraquat and cyhalothrin) in Apis cerana cerana (Chi et al., 2018). Those proves that insect stress resistance requires the participation of ILPs. Therefore, we inferred that the endogenous metabolites in the larvae of the T. castaneum under different carbon dioxide stress changed to resist adversity. In current research, TcILP2 was inhibited significantly in CK group which indicated dsRNA effective noticeably, it is worth noting that, only 25% CO2 + 75% air group appear TcILP2 upregulation significantly, it was the same with Figure 1A, it indicated at 25% CO2 + 75% air could still stimulate the TcILP2 transcription when RNAi was effective. In contrast, TcILP2 was also significantly inhibited in stress of other high CO2 concentrations after gene interference successfully. AccILP-2 expression was downregulated at 44°C (Chi et al., 2018), we inferred that high stress coupled with dsRNA could also repress target gene.
RNAi of TcILP2 up-regulated glycogen and glucose levels in the hemolymph of T. castaneum rather trehalose levels, in line with previous reports (Nässel and Broeck, 2016; Xue et al., 2020). It suggests that TcILP2 is responsible for downregulation glycogen and glucose levels in the T. castaneum. mRNA expression levels of other IIS genes elevated by the reduction of ILPs (Grönke et al., 2010; Walsh and Smith, 2011; Fu et al., 2016). In our results, though knockout TcILP2 can increase TPS transcript level and soluble trehalase activity, trehalose content had no obvious change, thus trehalose content may be regulated by other ILPs in T. castaneum. The decrease in glycogen and glucose content levels in 25% CO2 + 75% air group was likely due to TcILP2 upregulation. However, we trended towards the interaction of CO2 and ILP2, the sudden increase in CO2 is clearly harmful to pest insects, and explaining the dramatic rise in mortality.
The addition of hypercapnia made a complex alteration on the hypoxia response of Callosobruchus chinensis transcriptome, carbohydrate metabolism was one of the most highly enriched pathways for genes significantly changed (Cui et al., 2017; 2019). Furthermore, JH regulates the expression of genes encoding trehalase and TRE through the ILP2 and IIS pathways, further affects trehalose homeostasis in T. castaneum (Ling and Raikhel, 2021). Further detected relative expression levels of trehalose metabolism genes we discovered the high expression for TPS and TRE2 in any modified atmosphere. In Drosophila, overexpression of TPS was found toward increase trehalose levels and tolerance to hypoxia (Chen et al., 2003). It was reported that glucose and glycogen are used to synthesize trehalose by TPS in insect tissue, TRE2 mainly resides in fat body functions to hydrolyze membrane-bound trehalase for contribute energy, and TcILP2 is mainly in the brain and fat body (Nardelli et al., 2019; Chowański et al., 2021). Then we postulated that T. castaneum enhance the expressions of TPS and TRE resist hypoxia/hypercapnia under adverse conditions. Interestingly, trehalose and glucose contents were decrease rather than increase. While the effect of TcILP2 was effectively eliminated trehalose pathway genes begin to respond successively in order to endure increasing concentrations of CO2 (TRE 1-3 expressed first followed by TRE 1-2 then TRE 1-4). Those results revealed, T. castaneum tried to strengthen the trehalose or glucose content at the transcript levels of the trehalose pathway genes, but in high CO2 concentrations, fat body consumes too much energy bring on the content of trehalose or glucose extremely lower than the normal.
We found that under 25% CO2 + 75% air mix, the T. castaneum can still survive partially, and the mortality rate reaches 100% at 50% CO2 + 50% air, 75% CO2 + 25% air, and 95% CO2 + 5% air. The matching situation was 25% under CO2, dsILP2 not only has a lower mortality rate than dsGFP, but also a significant increase in pupation rate and emergence rate—may be attributed to the increase in TcILP2 expression—which is similar to Xu’s research results (Xu et al., 2013). Insulin signaling pathway plays a role in the material, sugar, and energy metabolism of insects. It has important regulatory effects on various physiological processes such as metabolism, growth and development, reproduction, and stress resistance (Yuan et al., 2018; Nässel and Zandawala, 2019; Strilbytska et al., 2020; Leyria et al., 2021). Moreover, high CO2 stress causes hypoxia, which directly limits energy supply and free radical damage and other processes, and ultimately leads to insect death (Nystul and Roth, 2004; Helenius et al., 2009). Combination of ILP2 knockout and hypoxia/hypercapnia could accelerate the carbohydrates consumption and affect survival and development of insects. Thus, we hypothesized that the TcILP2 could participate in the regulation of the trehalose metabolism during hypoxia to maintain insects alive.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
CL and K-KX designed the overall study and provided financial support. MZ and Y-HS conducted statistical analyses of the data. Y-YW, X-YZ, X-RM, and MZ involved in the whole study. Y-YW, X-YZ, and XL prepared the manuscript. All authors reviewed and approved the final manuscript.
Funding
This work was supported in part by the National Natural Science Foundation of China (No. 31960542), Academician Workstation of Guiyang University, Guizhou Province [QKHRCP No. (2019) 5605], the special funding of Guiyang science and technology bureau and Guiyang University [GYU-KY-(2021)], the Project of Graduate Research Project of Guiyang University [GYU-YJS(2019)-13], Special key laboratory of higher education institution in Guizhou province [QJHKYZ(2011)001], and the Innovation Group Project of Education Department of Guizhou Province [(2021)013].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the volunteers participating in the study for their consent and participation in this study.
References
Azzam Z. S., Sharabi K., Guetta J., Bank E. M., Gruenbaum Y. (2010). The Physiological and Molecular Effects of Elevated CO2 levels. Cell Cycle 9 (8), 1528–1532. doi:10.4161/cc.9.8.11196
Birse R. T., Söderberg J. A. E., Luo J., Winther Å. M. E., Nässel D. R. (2011). Regulation of Insulin-Producing Cells in the Adult Drosophila Brain via the Tachykinin Peptide Receptor DTKR. J. Exp. Biol. 214 (Pt 24), 4201–4208. doi:10.1242/jeb.062091
Boyer S., Zhang H., Lempérière G. (2012). A Review of Control Methods and Resistance Mechanisms in Stored-Product Insects. Bull. Entomol. Res. 102 (2), 213–229. doi:10.1017/s0007485311000654
Broughton S. J., Piper M. D. W., Ikeya T., Bass T. M., Jacobson J., Driege Y., et al. (2005). Longer Lifespan, Altered Metabolism, and Stress Resistance in Drosophila from Ablation of Cells Making Insulin-like Ligands. Proc. Natl. Acad. Sci. U.S.A. 102 (8), 3105–3110. doi:10.1073/pnas.0405775102
Campbell J. F., Runnion C. (2003). Patch Exploitation by Female Red Flour Beetles, Tribolium castaneum. J. Insect Sci. (Online) 3, 20. doi:10.1093/jis/3.1.20
Campbell J. F., Toews M. D., Arthur F. H., Arbogast R. T. (2010). Long-term Monitoring of Tribolium castaneum Populations in Two Flour Mills: Rebound after Fumigation. ec 103 (3), 1002–1011. doi:10.1603/ec09348
Cao Y., Wu X. Y., Lu L. H., Deng J., Li C. (2015a). Toxicity of CO2 to Stegobium Paniceum and Comparison of its Energy Metabolism. Chin. J. Biol. Control. 31, 57–63. doi:10.16409/j.cnki.2095-039x.2015.01.009
Cao Y., Xu K., Zhu X., Bai Y., Yang W., Li C. (2019). Role of Modified Atmosphere in Pest Control and Mechanism of its Effect on Insects. Front. Physiol. 10, 206. doi:10.3389/fphys.2019.00206
Cao Y., Yan Y. F., Yang W. J., Xiong Z. L., Wang L. J., Li C. (2015b). Influence of Carbon Dioxide Controlled Atmosphere on Oryzaephilus Surinamensis Linne and its Utilization of Energy Substances. J. Zhejiang Univ. 41, 631–640. doi:10.3785/j.issn.1008-9209.2015.04.072
Chen J., Tang B., Chen H., Yao Q., Huang X., Chen J., et al. (2010). Different Functions of the Insect Soluble and Membrane-Bound Trehalase Genes in Chitin Biosynthesis Revealed by RNA Interference. PLoS One 5 (4), e10133. doi:10.1371/journal.pone.0010133
Chen Q., Behar K. L., Xu T., Fan C., Haddad G. G. (2003). Expression of Drosophila Trehalose-Phosphate Synthase in HEK-293 Cells Increases Hypoxia Tolerance. J. Biol. Chem. 278 (49), 49113–49118. doi:10.1074/jbc.m308652200
Chen Q. W., Jin S., Zhang L., Shen Q. D., Wei P., Wei Z. M., et al. (2018). Regulatory Functions of Trehalose-6-Phosphate Synthase in the Chitin Biosynthesis Pathway in Tribolium castaneum (Coleoptera: Tenebrionidae) Revealed by RNA Interference. Bull. Entomol. Res. 108 (3), 388–399. doi:10.1017/s000748531700089x
Cheng W., Lei J., Ahn J.-E., Liu T.-X., Zhu-Salzman K. (2012). Effects of Decreased O2 and Elevated CO2 on Survival, Development, and Gene Expression in Cowpea Bruchids. J. Insect Physiol. 58 (6), 792–800. doi:10.1016/j.jinsphys.2012.02.005
Chi X., Wei W., Zhang W., Wang H., Liu Z., Xu B. (2018). Molecular Cloning and Characterization under Different Stress Conditions of Insulin-like Peptide 2 Gene (AccILP-2) from Apis cerana Cerana. J. Asia-Pacific Entomol. 21 (2), 474–481. doi:10.1016/j.aspen.2018.02.007
Chi Y. H., Ahn J.-E., Yun D.-J., Lee S. Y., Liu T.-X., Zhu-Salzman K. (2011). Changes in Oxygen and Carbon Dioxide Environment Alter Gene Expression of Cowpea Bruchids. J. Insect Physiol. 57 (1), 220–230. doi:10.1016/j.jinsphys.2010.11.011
Chowański S., Walkowiak-Nowicka K., Winkiel M., Marciniak P., Urbański A., Pacholska-Bogalska J. (2021). Insulin-like Peptides and Cross-Talk with Other Factors in the Regulation of Insect Metabolism. Front. Physiol. 12, 701203. doi:10.3389/fphys.2021.701203
Cui S. F., Wang L., Ma L., Wang Y. L., Qiu J. P., Liu Z. C., et al. (2019). Comparative Transcriptome Analyses of Adzuki Bean Weevil (Callosobruchus Chinensis) Response to Hypoxia and Hypoxia/hypercapnia. Bull. Entomol. Res. 109 (2), 266–277. doi:10.1017/s0007485318000512
Cui S., Wang L., Qiu J., Liu Z., Geng X. (2017). Comparative Metabolomics Analysis of Callosobruchus Chinensis Larvae under Hypoxia, Hypoxia/hypercapnia and Normoxia. Pest Manag. Sci. 73 (6), 1267–1276. doi:10.1002/ps.4455
Elbein A. D., Pan Y. T., Pastuszak I., Carroll D. (2003). New Insights on Trehalose: a Multifunctional Molecule. Glycobiology 13, 17R–27R. doi:10.1093/glycob/cwg047
Fu K.-Y., Zhu T.-T., Guo W.-C., Ahmat T., Li G.-Q. (2016). Knockdown of a Putative Insulin-like Peptide Gene LdILP2 in Leptinotarsa decemlineata by RNA Interference Impairs Pupation and Adult Emergence. Gene 581 (2), 170–177. doi:10.1016/j.gene.2016.01.037
Grönke S., Clarke D.-F., Broughton S., Andrews T. D., Partridge L. (2010). Molecular Evolution and Functional Characterization of Drosophila Insulin-like Peptides. Plos Genet. 6 (2), e1000857. doi:10.1371/journal.pgen.1000857
He S. F. (2015). Study on Insulin Gene Analysis and Function of Brown Planthopper. Hangzhou: Zhejiang University. [dissertation/master’s thesis]. [Zhejiang (CN)].
Helenius I. T., Krupinski T., Turnbull D. W., Gruenbaum Y., Silverman N., Johnson E. A., et al. (2009). Elevated CO2 Suppresses Specific Drosophila Innate Immune Responses and Resistance to Bacterial Infection. Proc. Natl. Acad. Sci. U.S.A. 106 (44), 18710–18715. doi:10.1073/pnas.0905925106
Hoback W. W., Stanley D. W. (2001). Insects in Hypoxia. J. Insect Physiol. 47 (6), 533–542. doi:10.1016/s0022-1910(00)00153-0
Hun L. V., Luckhart S., Riehle M. A. (2019). Increased Akt Signaling in the Fat Body of Anopheles stephensi Extends Lifespan and Increases Lifetime Fecundity through Modulation of Insulin-like Peptides. J. Insect Physiol. 118, 103932. doi:10.1016/j.jinsphys.2019.103932
Kharel K., Mason L. J., Murdock L. L., Baributsa D. (2019). Efficacy of Hypoxia against Tribolium castaneum (Coleoptera: Tenebrionidae) throughout Ontogeny. J. Econ. Entomol. 112 (3), 1463–1468. doi:10.1093/jee/toz019
Leyria J., Orchard I., Lange A. B. (2021). The Involvement of insulin/ToR Signaling Pathway in Reproductive Performance of Rhodnius prolixus. Insect Biochem. Mol. BiologyInsect Biochem. Mol. Biol. 130, 103526. doi:10.1016/j.ibmb.2021.103526
Leyva A., Quintana A., Sánchez M., Rodríguez E. N., Cremata J., Sánchez J. C. (2008). Rapid and Sensitive Anthrone-Sulfuric Acid Assay in Microplate Format to Quantify Carbohydrate in Biopharmaceutical Products: Method Development and Validation. Biologicals 36, 134–141. doi:10.1016/j.biologicals.2007.09.001
Li B., Predel R., Neupert S., Hauser F., Tanaka Y., Cazzamali G., et al. (2008). Genomics, Transcriptomics, and Peptidomics of Neuropeptides and Protein Hormones in the Red Flour Beetle Tribolium castaneum. Genome Res. 18 (1), 113–122. doi:10.1101/gr.6714008
Ling L., Raikhel A. S. (2021). Cross-talk of Insulin-like Peptides, Juvenile Hormone, and 20-hydroxyecdysone in Regulation of Metabolism in the Mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 118 (6), e2023470118. doi:10.1073/pnas.2023470118
Livak K. J., Schmittgen T. D. (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408. doi:10.1006/meth.2001.1262
Lorenzen M. D., Kimzey T., Shippy T. D., Brown S. J., Denell R. E., Beeman R. W. (2007). PiggyBac-based Insertional Mutagenesis in Tribolium castaneum Using Donor/helper Hybrids. Insect Mol. Biol. 16 (3), 265–275. doi:10.1111/j.1365-2583.2007.00727.x
Nardelli A., Vecchi M., Mandrioli M., Manicardi G. C. (2019). The Evolutionary History and Functional Divergence of Trehalase (Treh) Genes in Insects. Front. Physiol. 10, 62. doi:10.3389/fphys.2019.00062
Nässel D. R., Broeck J. V. (2016). Insulin/IGF Signaling in Drosophila and Other Insects: Factors that Regulate Production, Release and post-release Action of the Insulin-like Peptides. Cell. Mol. Life Sci. 73 (2), 271–290. doi:10.1007/s00018-015-2063-3
Nässel D. R., Zandawala M. (2019). Recent Advances in Neuropeptide Signaling in Drosophila, from Genes to Physiology and Behavior. Prog. Neurobiol. 179, 101607. doi:10.1016/j.pneurobio.2019.02.003
Navarro S. (2006). Modified Atmospheres for the Control of Stored-Product Insects and Mites. Insect Manage. Food Storage Process. 101, 105–145. doi:10.1016/B978-1-891127-46-5.50016-7
Nystul T. G., Roth M. B. (2004). Carbon Monoxide-Induced Suspended Animation Protects against Hypoxic Damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 101 (24), 9133–9136. doi:10.1073/pnas.0403312101
Opit G. P., Phillips T. W., Aikins M. J., Hasan M. M. (2012). Phosphine Resistance in Tribolium castaneum and Rhyzopertha dominica from Stored Wheat in oklahoma. Jnl. Econ. Entom. 105 (4), 1107–1114. doi:10.1603/ec12064
Pan B.-y., Xu K.-k., Luo Y.-j., Wang Y.-y., Zhou M., Li C., et al. (2020). The Sequence Characteristics and Functions on Regulating Trehalose Metabolism of Two PTP Genes in Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 89, 101692. doi:10.1016/j.jspr.2020.101692
Perkin L. C., Oppert B. (2019). Gene Expression in Tribolium castaneum Life Stages: Identifying a Species-specific Target for Pest Control Applications. Peer J. 7, e6946. doi:10.7717/peerj.6946
Pimentel M. A. G., Faroni L. R. D. A., Silva F. H. d., Batista M. D., Guedes R. N. C. (2010). Spread of Phosphine Resistance Among Brazilian Populations of Three Species of Stored Product Insects. Neotrop. Entomol. 39, 101–107. doi:10.1590/s1519-566x2010000100014
Satake S. I., Masumura M., Ishizaki H., Nagata K., Kataoka H., Suzuki A., et al. (1997). Bombyxin, an Insulin-Related Peptide of Insects, Reduces the Major Storage Carbohydrates in the Silkworm Bombyx mori. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 118 (2), 349–357. doi:10.1016/s0305-0491(97)00166-1
Sauer J. A., Shelton M. D. (2002). High-temperature Controlled Atmosphere for post-harvest Control of Indian Meal Moth (Lepidoptera: Pyralidae) on Preserved Flowers. J. Econ. Entomol. 95 (5), 1074–1078. doi:10.1093/jee/95.5.1074
Shelby C. E. (1977). The Biology of Tribolium with Special Emphasis on Genetic Aspects. Bull. Entomol. Soc. America 23 (4), 298–299. doi:10.1093/besa/23.4.298a
Shukla E., Thorat L. J., Nath B. B., Gaikwad S. M. (2015). Insect Trehalase: Physiological Significance and Potential Applications. Glycobiology 25 (4), 357–367. doi:10.1093/glycob/cwu125
Strilbytska O. M., Semaniuk U. V., Storey K. B., Yurkevych I. S., Lushchak O. (2020). Insulin Signaling in Intestinal Stem and Progenitor Cells as an Important Determinant of Physiological and Metabolic Traits in Drosophila. Cells 9 (4), 803. doi:10.3390/cells9040803
Tang B., Zhang L., Xiong X. P., Wang H. J., Wang S. G. (2018). Advances in Trehalose Metabolism and its Regulation of Insect Chitin Synthesis. Chin. Agric. Sci. (in China) 51 (04), 697–707. doi:10.3864/j.issn.0578-1752.2018.04.009
Walsh A. L., Smith W. A. (2011). Nutritional Sensitivity of Fifth Instar Prothoracic Glands in the Tobacco Hornworm, Manduca Sexta. J. Insect Physiol. 57 (6), 809–818. doi:10.1016/j.jinsphys.2011.03.009
Wei P. (2013). Regulating Functions of Trehalase in the Pathway of Energy Metabolism and Chitin Biosynthesis in Tribolium castaneum Revealed by RNA Interference. Hangzhou: Hangzhou Normal University. [dissertation/master’s thesis]. [Hangzhou (CN)].
Xu J., Sheng Z., Palli S. R. (2013). Juvenile Hormone and Insulin Regulate Trehalose Homeostasis in the Red Flour Beetle, Tribolium castaneum. Plos Genet. 9 (6), e1003535. doi:10.1371/journal.pgen.1003535
Xue W. H., Liu Y. L., Jiang Y. Q., He S. F., Wang Q. Q., Yang Z. N., et al. (2020). Molecular Characterization of Insulin‐like Peptides in the Brown Planthopper, Nilaparvata Lugens (Hemiptera: Delphacidae). Insect Mol. Biol. 29 (3), 309–319. doi:10.1111/imb.12636
Yu C. H., Lu D., Lin R. H., Yang X. X., Jiang H., Zhao F. (2008). Trehalose-the Blood Sugar in Insects. Chin. Bull. Entomol. 45 (5), 832–837.
Yuan Z., Chen Z., Li G. B., Hu G. Y. (2018). Research Progress of Protein Tyrosine Phosphatase 1B and its Inhibitors. J. China Pharm. Univ. 49 (01), 1–9.
Zhang L., Qiu L.-Y., Yang H.-L., Wang H.-J., Zhou M., Wang S.-G., et al. (2017). Study on the Effect of Wing Bud Chitin Metabolism and its Developmental Network Genes in the Brown Planthopper, Nilaparvata Lugens, by Knockdown of TRE Gene. Front. Physiol. 8, 750. doi:10.3389/fphys.2017.00750
Keywords: Tribolium castaneum, insulin pathway, carbon dioxide induced hypoxia stress, ILP2, trehalose metabolism
Citation: Wang Y-Y, Zhang X-Y, Mu X-R, Li X, Zhou M, Song Y-H, Xu K-K and Li C (2022) Insulin-Like ILP2 Regulates Trehalose Metabolism to Tolerate Hypoxia/Hypercapnia in Tribolium castaneum. Front. Physiol. 13:857239. doi: 10.3389/fphys.2022.857239
Received: 18 January 2022; Accepted: 21 March 2022;
Published: 20 April 2022.
Edited by:
Peng He, Guizhou University, ChinaReviewed by:
Bimalendu B. Nath, Savitribai Phule Pune University, IndiaKai Liu, Zhongkai University of Agriculture and Engineering, China
Copyright © 2022 Wang, Zhang, Mu, Li, Zhou, Song, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Can Li, bGljYW43OTAxMDhAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yuan-Yuan Wang1†
Yuan-Yuan Wang1† Yue-Hua Song
Yue-Hua Song Kang-Kang Xu
Kang-Kang Xu Can Li
Can Li