
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 24 March 2022
Sec. Environmental, Aviation and Space Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.846229
This article is part of the Research TopicRising Stars in Environmental, Aviation and Space Physiology: 2022View all 8 articles
Women are more prone to orthostatic intolerance compared to men and have a greater vasodilatory capacity. We investigated the hypothesis that women would have greater peripheral flow-mediated dilation (FMD) while in the upright posture compared to men, which could contribute to this phenomenon. In young healthy women (age: 20 ± 3, BMI: 27 ± 5 kg/m2, n = 10) and men (age = 21 ± 2, BMI: 27 ± 8 kg/m2, n = 8), we assessed FMD of the brachial artery and hemodynamics to determine endothelial function during the supine and 70° head-up tilt postures (randomized). The brachial artery was kept at heart level in both trials. We observed that FMD increased in both sexes during tilt (Women: 11.9 ± 5.3 to 15.7 ± 5.6%; Men: 8.4 ± 3.2 to 14.6 ± 3.4%, Main effect of tilt p = 0.005) which was not due to changes in blood pressure or shear stress. There were no interaction effects between sex and posture. In a second cohort of women (age: 22 ± 3, BMI: 23 ± 3 kg/m2, n = 9) and men (age: 22 ± 2, BMI: 25 ± 8 kg/m2, n = 8), we investigated reactive hyperemia by peripheral arterial tonometry (LnRHI) via EndoPAT. Interestingly, we found that the EndoPAT response was decreased in both sexes during tilt (LnRHI: Men: 0.70 ± 0.28 to 0.59 ± 0.40, Women: 0.52 ± 0.23 to 0.30 ± 0.32, Main effect of tilt p = 0.037). We previously found that FMD is related to coronary responses to acetylcholine and adenosine whereas EndoPAT is related to coronary responses to dobutamine. Therefore, we suggest that sympathetic mediated dilation is attenuated in the upright posture while the increased vasodilatory response as measured by FMD in the tilt posture could be attributed to increasing metabolite production from postural muscles.
Orthostatic tolerance is well-known to be lower in women compared to men, yet the mechanisms are still being investigated. Previous studies have focused on hemodynamics and vasoconstrictor capacity, yet few have investigated vasodilatory capacity. Vasodilatory capacity could be an important consideration in orthostatic tolerance studies due to potential functional sympatholysis while upright. Greater vasodilation or sympatholysis while upright would reduce peripheral resistance and thus blood pressure, potentially contributing to orthostatic intolerance. A standard non-invasive measurement to assess vasodilatory capacity is brachial artery flow-mediated dilation (FMD). FMD has been shown to be enhanced in women compared to men (Hashimoto et al., 1995; Harris et al., 2012), and estradiol has been shown to upregulate the production of endothelial nitric oxide synthase (Rosenfeld et al., 2003; Kan et al., 2008). However, the sex differences in FMD have recently been disputed based on controlling for women having a smaller baseline diameter (i.e., allometric scaling, Shenouda et al., 2018; Johns et al., 2020). It has also been observed that women have greater activity/sensitivity of β2-adrenergic receptors, which cause peripheral vasodilation (Kneale et al., 2000). Since Fu et al. have observed similar sympathetic nerve responses during upright tilt between the sexes (Fu et al., 2005, 2010); the greater β2-adrenergic activity/sensitivity in women may result in greater adrenergic induced vasodilation.
Thijssen et al. (2014) observed that FMD was attenuated following simulated orthostatic stress via lower body negative pressure (LBNP), and Dyson et al. (2006) found that FMD was not influenced during LBNP, yet only men were examined in these studies and LBNP is conducted in the supine posture without skeletal muscle activation. It is currently unknown if being in the upright posture influences FMD or β2-adrenergic responsiveness in men and/or women. Indeed, Dyson et al. (2010) concurrently found that muscle chemoreflex activation via post-exercise circulatory occlusion of the legs did enhance the brachial FMD response, yet they attributed this enhanced dilation to baseline constriction obscuring the hyperemic dilatory response. It is important to note that Dyson et al. (2010) controlled for shear stress by changing the length of time that the forearm was occluded in all trials. During upright posture (sitting and standing), brachial vascular resistance increases in healthy men and women (Edgell et al., 2012), potentially indicating a reduction of shear rate and FMD. A reduction of FMD while upright could contribute to the maintenance of blood pressure while upright due to increased peripheral resistance.
Our lab group recently found that brachial FMD correlated with measures of adenosine or acetylcholine mediated coronary resistance in patients with suspected cardiac microvascular disease (Nardone et al., 2020). At the same time, we found that the natural logarithm of the reactive hyperemia index (LnRHI) as measured by the EndoPAT device correlated with dobutamine mediated coronary resistance (Nardone et al., 2020). Therefore, we are using FMD and LnRHI in the current study as markers of these vasodilatory processes in the supine and upright postures. We hypothesized that (1) in the upright posture, brachial artery shear stress would be lower and therefore FMD responses would be attenuated; (2) due to greater adrenergic responses in the upright posture, LnRHI would be enhanced while upright; and (3) both FMD and LnRHI would be augmented in women compared to men.
All procedures were approved by the York University Research Ethics Board and all participants gave written informed consent. We adhered to the Declaration of Helsinki and Title 45, US Code of Federal Results, Part 46, Protection of Human Subjects, and all subsequent revisions and amendments. Two unique cohorts of participants were recruited as the EndoPAT protocols were completed as a follow-up study to the FMD protocol. The FMD protocol consisted of 10 women and nine men, while the EndoPAT protocol included nine women and eight men (Table 1). Participants were excluded if they suffered from any previously diagnosed cardiovascular or pulmonary diseases. Women must have never taken oral contraceptives or have stopped taking them for a period of at least 3 months prior to participating in the study. Women were also excluded if they had been using a hormonal intrauterine device, contraceptive patches, or any other form of hormonal contraceptives. Women were tested in the early follicular phase during days 2–5 of the menstrual cycle. For 12 h before testing, all participants were asked to refrain from: smoking (e.g., cigarettes, vaping, and marijuana), drinking alcohol, drinking caffeine (e.g., coffee and tea), heavy exercise (including sports, resistance training, and moderate to intense aerobic exercises), and eating fatty foods. Height and weight were measured with a standard stadiometer and age, sex, and weekly episodes of moderate to vigorous exercise were by self-report. Estimated VO2max was calculated using anthropometrics and the frequency of exercise using the Ainsworth equation (Ainsworth et al., 1993).
Heart rate (HR) was measured using a single lead ECG. Blood pressure (BP) was measured using beat-to-beat finger photoplethysmography (NexFin, BMEYE, Amsterdam, Netherlands), which was calibrated to an automated BP measurement (BPTru Medical Devices, Canada) using the right arm in the supine position. Stroke volume (SV) and subsequently calculated cardiac output (Q) and total peripheral resistance (TPR) were collected using the Modelflow algorithm of Nexfin. SV (and thus Q and TPR) were normalized to body surface area using the Du Bois formula (Du Bois and Du Bois, 1916). All hemodynamic signals were obtained using a Powerlab data acquisition device (1,000 Hz) and LabChart Pro software (ADInstruments, Colorado Springs, United States).
The brachial artery was imaged by author KH approximately 3–5 cm proximal from the antecubital fossa using a linear array high resolution ultrasound transducer (9L-RS; 3–10 MHz) using Duplex ultrasound to concurrently measure blood flow velocity and vessel diameter (Vivid i, GE Healthcare Systems, Mississauga, Canada). Continuous ultrasound images were recorded using a video grabber device (AV.io HD, Epiphan Video) and the blood velocity, brachial artery diameter, and resultant shear rates (baseline and maximal) were analyzed using automated edge-detection software (Cardiovascular Suite, Quipu, Italy). Shear rate (SR) was calculated as SR = 4*(velocity/diameter), where 4 assumes a constant blood viscosity. The FMD protocol consisted of 2 min of baseline measurement, 5 min of forearm occlusion ~50 mmHg over systolic blood pressure, and 3 min of reactive hyperemia. Low-flow mediated constriction was calculated by comparing the brachial diameter at baseline to the brachial diameter during the last 30 s of occlusion.
The EndoPAT device (EndoPAT, Itamar Medical, Israel) was used according to manufacturer’s instructions except that the occlusion cuff was placed on the forearm rather than the upper arm for concurrent measurements of FMD. This technique has been used previously by our group (Nardone et al., 2020, 2021). Briefly, two tonometry finger cuffs were placed on the index finger of each hand and pulse waveforms throughout each cardiac cycle and were relayed to the device and an automated algorithm quantified the reactive hyperemia index (RHI). The EndoPAT protocol consisted of 5 min of baseline measurement, 5 min of forearm occlusion ~50 mmHg over systolic blood pressure, and 5 min of reactive hyperemia. Since the RHI is not normally distributed (Hamburg and Benjamin, 2009), the software computed the natural logarithm of the RHI (Ln-RHI). The EndoPAT 2000 also calculated the augmentation index (AI), typically used as an indirect marker of systemic arterial stiffness, and calculated the AI at a normalized HR of 75 bpm, which serves to allow for comparisons across different populations and postures.
Each FMD or EndoPAT protocol (one while supine and one while 70° upright) consisted of baseline measurements, forearm occlusion/ischemia, and reperfusion measurements as described above. A standard blood pressure cuff was placed on the forearm of the right arm and for all trials both hands/arms were kept at the level of the heart. For tilted trials, the EndoPAT trial began immediately upon achieving upright posture (~2 min) whereas the FMD trials began within ~5 min due to the necessity of obtaining an adequate ultrasound image in the upright posture. Since the baseline period for the EndoPAT trial is 5 min and the baseline period for the FMD trials is 2 min, we planned for cuff release at approximately the same time between the trials. Supine and upright trials within each methodology were randomized and conducted with a 30-min break between them to minimize any serial effect of the occlusion periods. The protocol is shown in Figure 1.
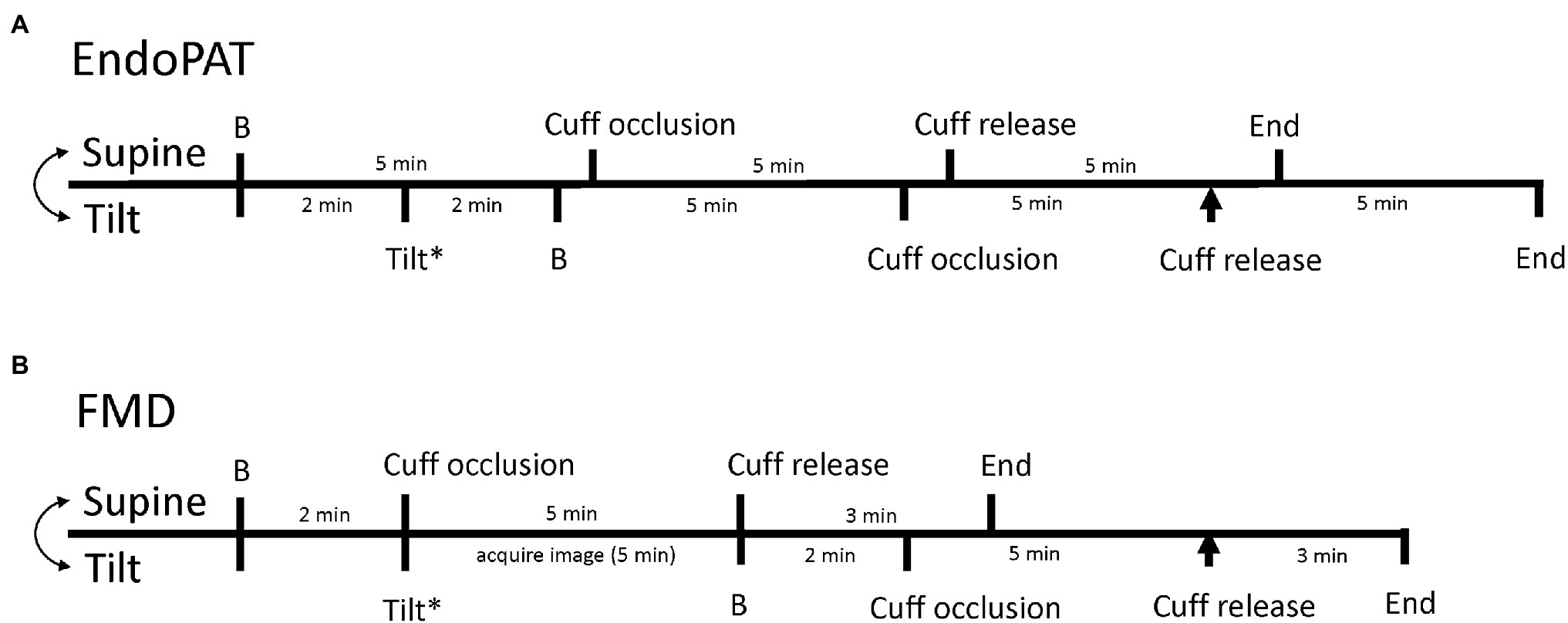
Figure 1. Timeline of data collection for EndoPAT trials (A) and flow-mediated dilation (FMD) trials (B). The timelines for the supine trials are on the top of each line and the timeline for the tilted trials are on the bottom of each line. The double headed arrow indicates that the supine and tilted trials are randomized. B Indicates baseline, the asterisk highlights the time of upright tilt, and the arrowhead indicates the time of reactive hyperemia/cuff release during tilt.
Anthropometric data were compared with an unpaired t-test between the sexes. One minute hemodynamic averages were taken at baseline (i.e., 1-min prior to cuff inflation while supine or tilted, as appropriate), and 15 s averages were taken at the time of maximal brachial artery dilation (FMD) or the time of maximal finger blood volume (EndoPAT) after cuff-release/reperfusion. In the tilted posture, the baseline values were taken at least 2 min after onset of tilt. Our lab group previously observed that in healthy young men and women blood pressure stabilized after 2 min of 70° upright tilt (Joshi and Edgell, 2019). Changes in hemodynamics were calculated as the change from baseline to the time of maximum dilation. A two-way mixed model ANOVA was used to compare hemodynamic data across the two postures (sex and posture as factors, posture is a repeated measure). Analysis of vascular variables (i.e., FMD, SR, SRAUC, and Ln-RHI) was also done via a two-way mixed model ANOVA while accounting for sex and posture (repeated measure) as factors. The normality of distribution was assessed via the Spiro-Wilks test of normality. Tukey’s post hoc analysis was used when significance was found. Correlations between the change in FMD between postures and the blood pressure responses were conducted with linear regression. Significance was defined as p < 0.05. All statistical analyses were performed via Sigmaplot 13.2 (San Jose, California, United States) statistical software. Data in tables and text are presented as Mean ± SD. Data in figures are presented as median and the 25 and 75th percentiles.
For both the FMD and EndoPAT trials, women were smaller than men and had a lower estimated VO2max (Table 1; all p < 0.05). The baseline brachial artery was smaller in women but did not differ between posture trials (Sex: p = 0.002, Posture: p = 0.43, and Interaction: p = 0.81). At baseline in the tilted posture, both women and men had higher HR, lower mean arterial pressure (MAP), and lower stroke volume index (SVi) compared to the baseline in the supine position (Table 2, all p < 0.005). Men had higher baseline cardiac output index (Qi) and SVi compared to women in both supine and tilted trials (Table 2, all p < 0.05). There were no effects of sex or posture on total peripheral resistance index (TPRi) or baseline brachial artery shear rate/blood velocity (Table 2, all p > 0.05).
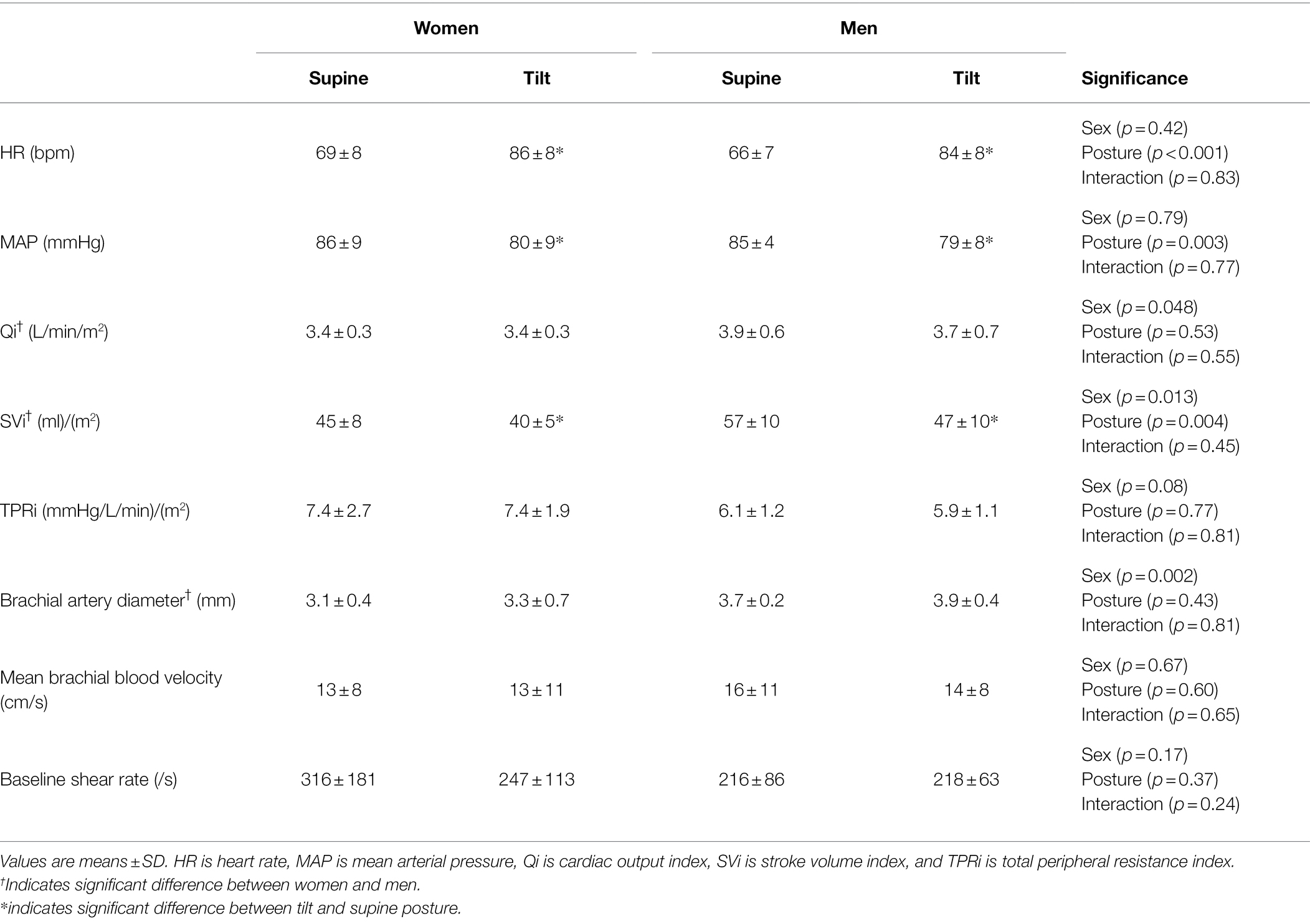
Table 2. Baseline hemodynamics and brachial artery diameter of women and men prior to starting the FMD protocol in the supine and tilt posture.
The change in brachial artery diameter from baseline to immediately before reperfusion (indicating low-flow mediated constriction) was not different between sexes or postures (Women supine: −0.01 ± 0.14 mm, Women tilt: −0.01 ± 0.34 mm; Men supine: −0.03 ± 0.11 mm, Men tilt: +0.14 ± 0.23 mm; Sex: p = 0.20, Posture: p = 0.37, and Interaction: p = 0.38). Upright tilt enhanced the FMD response in both men and women (Figure 2A; p = 0.005) despite no change in shear stress while upright (Figure 2B; p = 0.31). Men had lower shear stress during both trials compared to women (Figure 2B; p = 0.005). Both sexes had a greater reduction of MAP from the beginning to the end of the FMD trial during the tilted trial compared to the supine trial (Figure 2C; p = 0.003). When FMD was normalized to maximal shear rate by division, a significant increase was still observed in both sexes during the upright posture (Women supine: 0.010 ± 0.004 au, Women tilt: 0.015 ± 0.007 au; Men supine: 0.009 ± 0.005 au, Men tilt: 0.019 ± 0.006 au; Sex: p = 0.48, Posture: p < 0.001, and Interaction: p = 0.19). From the beginning to the end of the trials, women had a greater increase of HR regardless of posture (Table 3, p = 0.046), and men had a greater reduction of SVi over the course of the trial in the tilted posture compared to the supine posture which was not seen in women (Table 3, Interaction effect p = 0.047). There were no effects of sex or tilt on the change in Qi or TPRi over the course of the trials (Table 3, all p > 0.05).
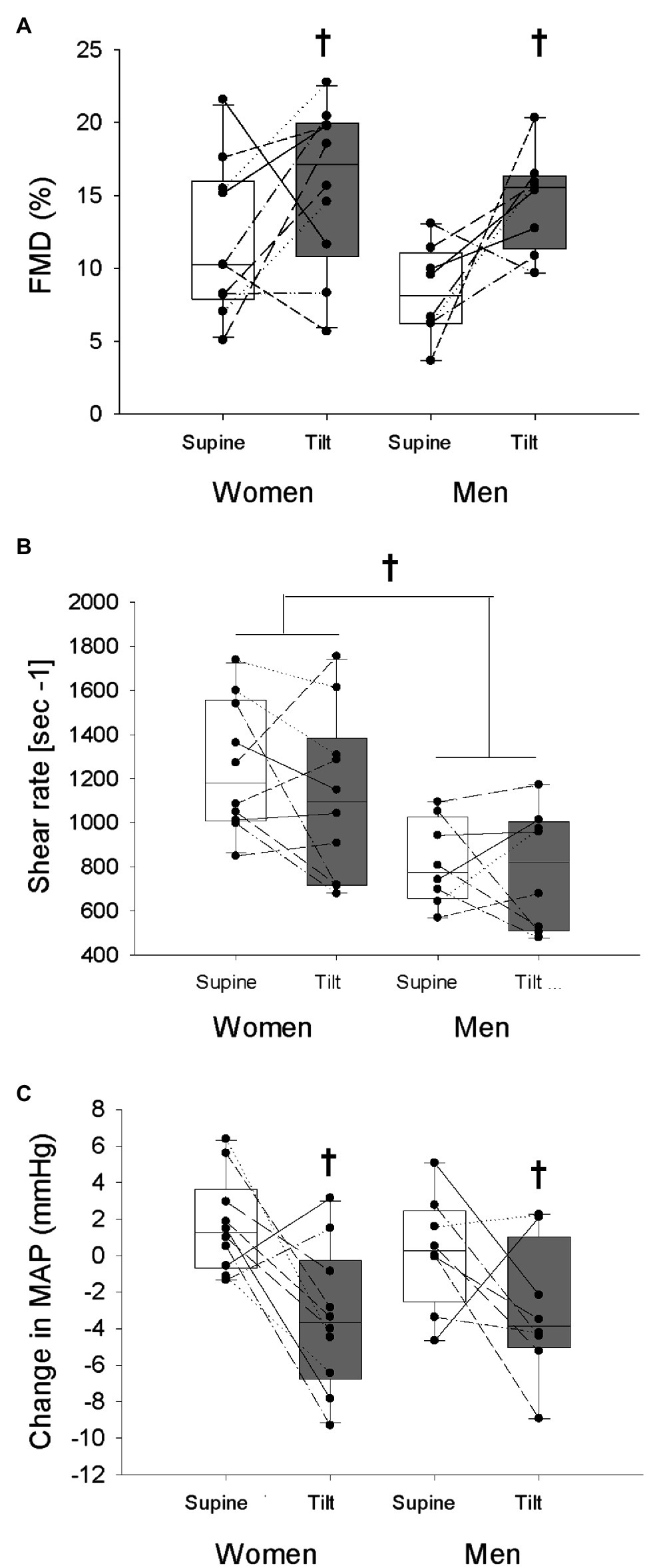
Figure 2. Flow-mediated dilation (FMD; A), maximal shear rate (B), and the change in mean arterial pressure (MAP) from the beginning of each trial to the time of maximal brachial artery diameter response (C) is shown in women and men in the supine and tilted postures. White bars indicate supine posture, grey bars indicate upright posture. †indicates a main effect of posture (p < 0.05). *Indicates a main effect of sex (p < 0.05).

Table 3. Hemodynamic changes from baseline to the time of maximal dilation of women and men across the supine and tilt in the FMD testing.
To investigate if the observed fall in MAP over the course of the tilt trial was from the observed increase of FMD, we examined their relationship, yet found none in our combined group of participants [Change in BP = −2.832 − (0.297 × Change in FMD), R2 = 0.15, p = 0.12]. To investigate potential sex differences, the group was separated by sex. The equation for men was [Change in BP = −1.990 − (0.200 × Change in FMD), R2 = 0.05, p = 0.59], and for women was [Change in BP = −3.549 − (0.412 × Change in FMD), R2 = 0.34, p = 0.08].
For the EndoPAT trials, women had higher TPRi compared to men at baseline (Table 4, p < 0.001), both women and men had higher HR and lower SVi at baseline in the tilted trial compared to baseline in the supine trial (Table 4, p < 0.001), and there were no effects of sex or posture on baseline MAP, Qi, or TPRi (Table 4, p > 0.30). Both men and women displayed a reduction of LnRHI in the tilted posture compared to the supine posture (Figure 3A, p = 0.037), yet no posture effect on the change in MAP over the course of the trials (Figure 3B, p = 0.78). All participants had a greater increase of HR over the course of the tilted trial compared to the supine trial (Table 5, p = 0.049), and there were no effects of sex or posture on the change of Qi, SVi, or TPRi over the course of the trials (Table 5, p > 0.05). AI@75 bpm was not different between sexes or with posture change (Women Supine: −10.0 ± 11%, Women Tilt: −8.0 ± 8%, Men Supine: −20 ± 8%, Men Tilt: −16 ± 13%; Sex p = 0.070, Posture p = 0.13, and Sex × Posture p = 0.54).
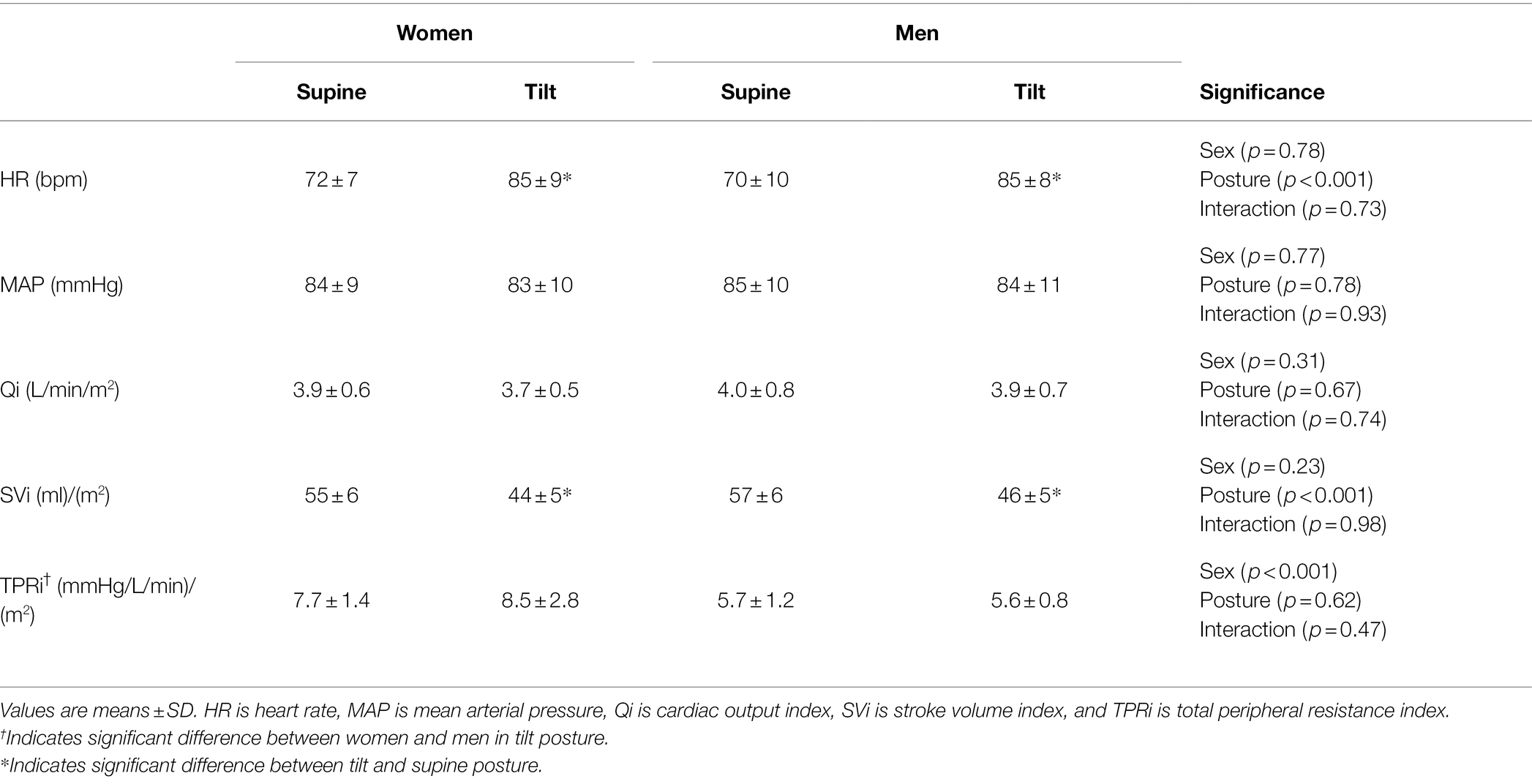
Table 4. Baseline hemodynamics of women and men prior to starting the EndoPAT protocol in the supine and tilt posture.
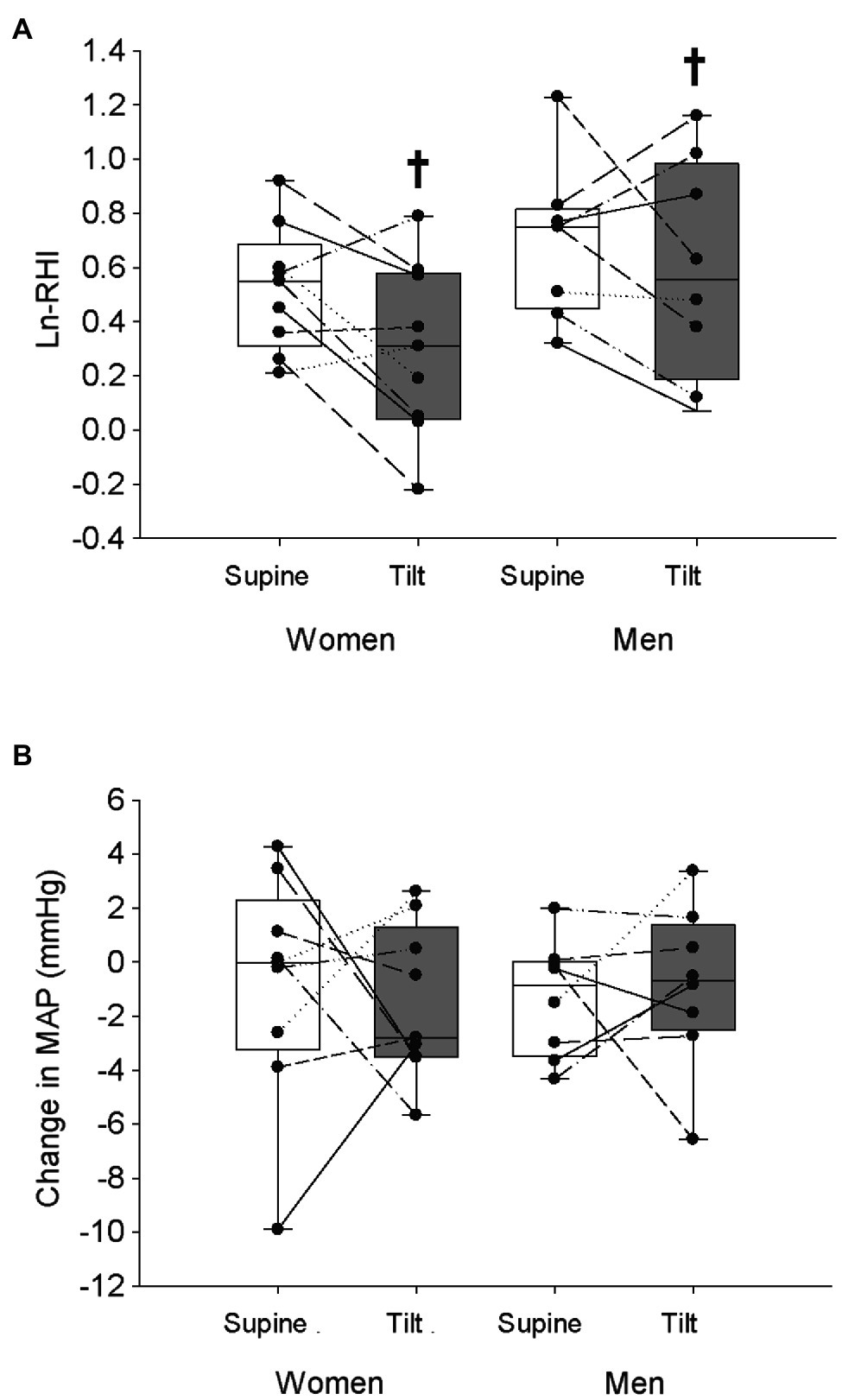
Figure 3. Natural logarithm of the reactive hyperemia index (LnRHI; A) and the change in MAP (B) from the beginning of each trial to the time of maximal hyperemic response is shown in women and men in the supine and tilted postures. White bars indicate supine posture, grey bars indicate upright posture. †Indicates a main effect of posture ( p < 0.05).
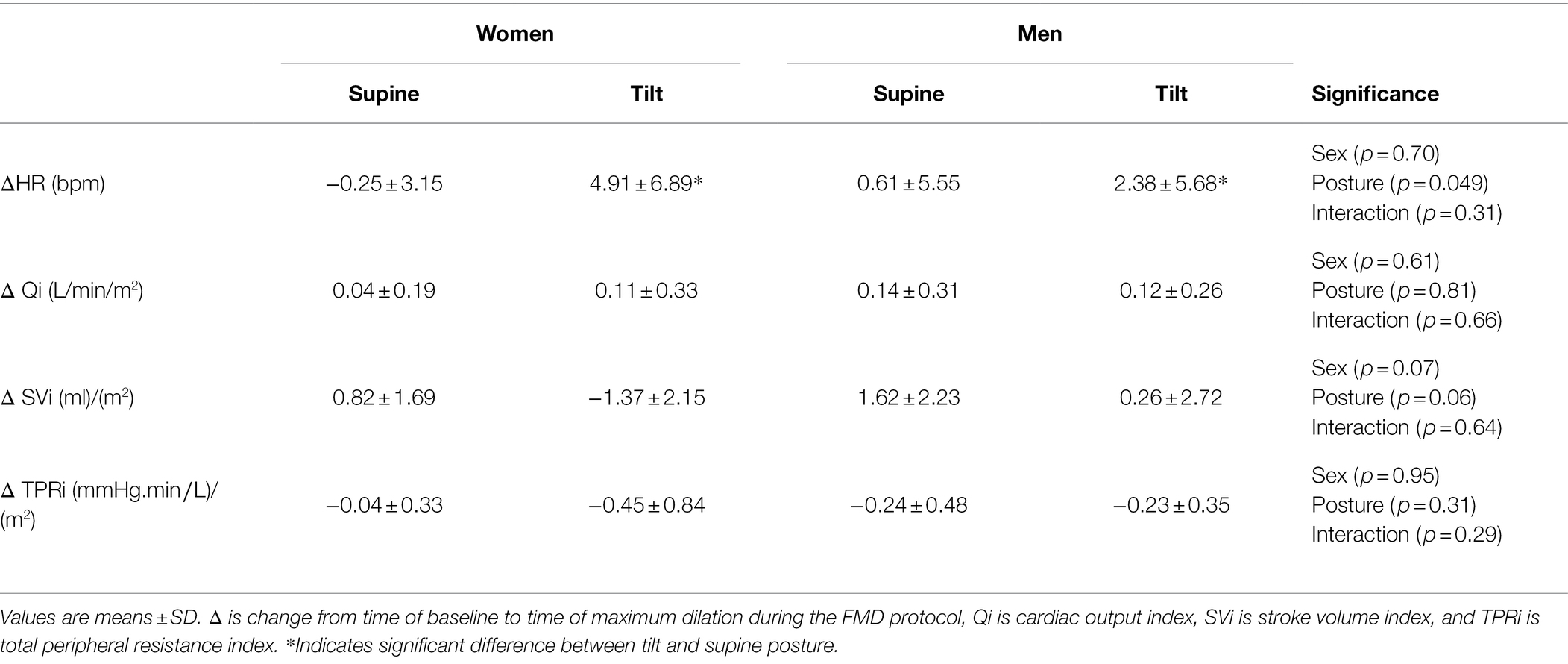
Table 5. Hemodynamic changes from baseline to the time of maximal hyperemia of women and men across the supine and tilt in the EndoPAT testing.
We found that in men and women vasodilatory responses change in the upright posture equally. Specifically, we observed that the FMD response is enhanced yet the EndoPAT response is attenuated. These changes do not appear to be due to changes in shear stress or hemodynamics.
We hypothesized that FMD would be lower in the tilt position due to reduced shear stress; however, we found no change in shear stress and an improved FMD while upright. We also did not observe any sex differences in FMD in either posture. While FMD can be influenced by other contributing factors, FMD is typically proportional to shear stress as its primary mechanism (Pyke et al., 2004). However, in the current study, we observed an increased FMD in the upright posture with no change in shear stress and a reduction in blood pressure. Hence, our results suggest a mechanism other than shear stress causing the increased vasodilation. Further, while we hypothesized that a reduction of FMD while upright would be protective of blood pressure while upright, these results suggest that enhanced FMD while upright could in fact be a contributing factor to reductions of blood pressure while upright.
Guazzi et al. (2005) previously explored brachial FMD while 60° upright in a healthy group of primarily men (10/12 men). However, shear stress was not calculated nor was continuous arterial diameter and blood pressure (measures were taken every 15 s). Nonetheless, our results in both women and men correspond with their findings. Guazzi et al. (2005) also measured brachial diameter in a subset of participants in the contralateral arm during and after hyperemia in tilt and found no changes; however, they combined healthy controls with those with pathophysiologic conditions such as hypertension and type 2 diabetes. Therefore, it remains unknown if the observed enhancement of vasodilation is local or systemic in healthy men and women. Indeed, Dietz et al. (1997) observed that during syncope, sympathetic withdrawal was not sufficient to explain the vasodilation that occurs, and that the vasodilation may act through mechanisms independent of β2-adrenergic and nitric oxide mediated mechanisms.
Rubini et al. (2012) conducted a study investigating the effect of the upright posture on the metabolic, cardiovascular, and electromyographic (EMG) activity of postural muscles (soleus and gastrocnemius) and they observed that the EMG activity of both muscles increased while upright. These results indicate increased muscle activation and presumably increased metabolite production while upright. However, it is important to note that they used a standing model rather than tilt table testing. We suggest that metabolites produced via upright posture (and potentially from deltoid and rotator cuff muscles from the elevated arm position of the imaged arm despite support) enter the ischemic forearm upon reperfusion causing an enhanced vasodilatory effect mediated by FMD related mechanisms such as adenosine and potentially lactate. Indeed, enhanced adenosine production is known to lead to syncope (Saadjian et al., 2002) and lactate stays in circulation for approximately 14 min (Almenoff et al., 1989). Hence, it is feasible that metabolites such as lactate stay in the general circulation long enough to enter the ischemic arm upon cuff release and possibly cause the increased non-shear stress dependent vasodilatory effect observed in our results. Systemic plasma concentrations of adenosine and lactate are needed during tilt table testing to determine their role in enhanced vasodilation.
Interestingly Guazzi et al. (2005) found a strong positive association between the change of HR during the tilt trial and the change of brachial artery diameter potentially linking peripheral vasodilation and the tachycardiac response to tilt. However, in the current study, even though MAP decreased and FMD increased during tilt, we did not observe a significant relationship between the changes in blood pressure and FMD. Contrary to our expectations we did not find evidence that women had enhanced FMD compared to men in either posture, yet they did have higher shear stress which is likely due to having smaller brachial arteries. Conflicting evidence about sex differences in the FMD response exist. Johns et al. (2020) suggested these discrepancies could be due to consideration of baseline brachial arterial diameter. For example, Juonala et al. (2008) found that women have greater FMD responses in comparison to men, however, after normalizing for baseline diameter, they found no significant differences in FMD between women and men and after similar normalization Shenouda et al. (2018) found lower FMD in women compared to men. Allometric scaling in the current study would not influence our primary results (i.e., change of FMD in upright posture) as there were no differences in baseline diameter in between postures. We also suggest that measurements of fitness or VO2 should be conducted in future studies. In the current study, since the significantly higher shear stimulus in women compared to men did not elicit higher FMD in women, we suggest that our cohort of women have reduced endothelial function compared to our male cohort due to less times exercising per week leading to a lower estimated VO2max. VO2max has been shown to be correlated with endothelial health (Buscemi et al., 2013).
While the EndoPAT has been used to assess overall endothelial function, we used it as an index of assessment for β2-mediated dilation of the microvasculature, as our lab group previously observed that LnRHI is correlated with dobutamine induced vasodilation in the coronary artery (Nardone et al., 2020). We hypothesized that (1) LnRHI would be enhanced in the upright posture because of the increased β2 adrenergic receptor binding from sympathetic activation associated with the upright posture, and (2) in both postures, women would have higher LnRHI compared to men. Neither hypothesis was supported by our findings.
Goswami et al. (2013) investigated LnRHI and posture change in men and women previously and found no effect of sex or posture, however, they measured LnRHI after returning to the supine posture rather than while upright. For the current study, LnRHI was lower in the upright posture in both sexes suggesting reduced β2-mediated dilation in light of enhanced adrenergic responses to tilt. We speculate that prior to cuff release, the β2-receptors were saturated with norepinephrine which entered the ischemic arm via sympathetic nerve activity. We suggest that this was not observed as vasodilation prior to reperfusion (i.e., baseline brachial artery diameter did not change in the FMD trials when tilted) due to concurrent vasoconstrictor signals from α1-receptor stimulation. However, upon reperfusion and the associated stimuli, there was a reduced capacity for the β2-receptors to cause further dilation which would have been measured by LnRHI.
We did not observe a sex difference in resting LnRHI which was unexpected as Davis et al. (2020) found that women have greater Ln-RHI scores in comparison to age-matched men. This discrepancy could again be due to the underlying fitness differences of our participants. The men and women in Davis et al. (2020) were matched on the history of regular resistance exercise, and we did not control for fitness or physical activity. Hence, reduced fitness in women for the current study could have been a confounding variable responsible for attenuated vascular function.
Blood pressure fell over the course of the FMD trial, but not throughout the EndoPAT trial. This could have been due to any delays in the length of time that it took to find an adequate brachial artery image upon tilt (despite attempting to control for the time of cuff release). However, despite this fall in blood pressure, shear stress was not affected during reperfusion and FMD increased regardless. For the EndoPAT trials, the relatively higher (i.e., unchanged from baseline) blood pressure compared to the FMD trials could have been expected to increase LnRHI while upright, however, the opposite was observed.
We did not include measurements of muscle metabolism, plasma nitrates/nitrites, and oxidative stress markers, such as peroxynitrite, catecholamines, or other vasoactive substances. In order to test our hypothesis that the increased FMD in the upright posture is due to activation of postural muscles, future studies should consider measuring potentially vasoactive substances, such as lactate, adenosine, O2 and CO2, nitrates/nitrites, as well as electromyography of postural and shoulder muscles. Along with these measurements of muscle metabolism, we suggest conducting a true cardiopulmonary exercise test to determine fitness, as we used a VO2 estimate via the Ainsworth equation. Lastly, we suggest measurements of alternate vascular beds (e.g., renal and splanchnic) to determine their role in the maintenance of blood pressure in light of the greater vasodilatory capacity in skeletal muscle beds while upright.
Our calculations of the relationships between the reduction of blood pressure of the course of the tilt FMD trial and the increase of FMD were underpowered. Greater sample numbers would help to strengthen any assumptions, which may be made using those data. Similarly, the statistically significant reduction of LnRHI in the upright posture was underpowered at β = 0.48; however, all other statistically significant comparison reached sufficient power of β > 0.8.
We have provided evidence of changes in two distinct vasodilatory pathways that have opposite responses while in the upright posture. FMD increases while LnRHI decreases while upright. These results indicate that while endothelial-dependent vasodilation improves, endothelial-independent vasodilation is impaired. To support these observations, an investigation of the arterial response to sublingual nitroglycerin while upright would be beneficial. Despite the hypothesis that these changes could be partially responses for sex differences in orthostatic tolerance, sex differences were not observed in these cohorts. Larger studies with in-depth investigations of metabolism and fitness are required.
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request.
The studies involving human participants were reviewed and approved by York University Research Ethics Board. The patients/participants provided their written informed consent to participate in this study.
KH and HE had substantial contributions to the conception or design of the work. KH, BF, and HE contributed to the acquisition, analysis, or interpretation of data for the work, contributed to drafting the work or revising it critically for important intellectual content, provided approval for publication of the content, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
This study was funded by the Natural Sciences and Engineering Research Council of Canada (grant number: 2016-05289).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to acknowledge the technical assistance of Kevin R. Murray for help with data collection. This work is a part of the M.Sc. thesis of author KH.
Ainsworth, B., Richardons, M., Jacobs, D., and Leon, A. (1993). Prediction of cardiorespiratory fitness using physical activity questionnaires. Med. Exerc. Nutr. Health 1, 75–82.
Almenoff, P. L., Leavy, J., Weil, M. H., Goldberg, N. B., Vega, D., and Rackow, E. C. (1989). Prolongation of the half-life of lactate after maximal exercise in patients with hepatic dysfunction. Crit. Care Med. 17, 870–873. doi: 10.1097/00003246-198909000-00004
Buscemi, S., Canino, B., Batsis, J. A., Buscemi, C., Calandrino, V., Mattina, A., et al. (2013). Relationships between maximal oxygen uptake and endothelial function in healthy male adults: a preliminary study. Acta Diabetol. 50, 135–141. doi: 10.1007/s00592-010-0229-x
Davis, D. W., Garver, M. J., Stone, W. J., Penumetcha, M., Hair, J. N., and Philipp, N. M. (2020). Endothelial function and arterial stiffness in young adults with histories of chronic resistance activity. J. Hum. Sport Exerc. 17, 1–12. doi: 10.14198/jhse.2022.172.11
Dietz, N. M., Halliwill, J. R., Spielmann, J. M., Lawler, L. A., Papouchado, B. G., Eickhoff, T. J., et al. (1997). Sympathetic withdrawal and forearm vasodilation during vasovagal syncope in humans. J. Appl. Physiol. 82, 1785–1793. doi: 10.1152/jappl.1997.82.6.1785
Du Bois, D., and Du Bois, E. F. (1916). A formula to estimate the approximate surface area if height and weight be known. Arch. Intern. Med. XVII, 863–871. doi: 10.1001/archinte.1916.00080130010002
Dyson, K. S., Shoemaker, J. K., Arbeille, P., and Hughson, R. L. (2010). Modelflow estimates of cardiac output compared with Doppler ultrasound during acute changes in vascular resistance in women. Exp. Physiol. 95, 561–568. doi: 10.1113/expphysiol.2009.050815
Dyson, K. S., Shoemaker, J. K., and Hughson, R. L. (2006). Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am. J. Physiol. Heart Circ. Physiol. 290, H1446–H1453. doi: 10.1152/ajpheart.00771.2005
Edgell, H., Robertson, A. D., and Hughson, R. L. (2012). Hemodynamics and brain blood flow during posture change in younger women and postmenopausal women compared with age-matched men. J. Appl. Physiol. 112, 1482–1493. doi: 10.1152/japplphysiol.01204.2011
Fu, Q., Vangundy, T. B., Shibata, S., Auchus, R. J., Williams, G. H., and Levine, B. D. (2010). Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension 56, 82–90. doi: 10.1161/HYPERTENSIONAHA.110.151787
Fu, Q., Witkowski, S., Okazaki, K., and Levine, B. D. (2005). Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R109–R116. doi: 10.1152/ajpregu.00013.2005
Goswami, N., Gorur, P., Pilsl, U., Anyaehie, B., Green, D. A., Bondarenko, A. I., et al. (2013). Effect of orthostasis on endothelial function: a gender comparative study. PLoS One 8:e71655. doi: 10.1371/journal.pone.0071655
Guazzi, M., Lenatti, L., Tumminello, G., and Guazzi, M. D. (2005). Effects of orthostatic stress on forearm endothelial function in normal subjects and in patients with hypertension, diabetes, or both diseases. Am. J. Hypertens. 18, 986–994. doi: 10.1016/j.amjhyper.2005.02.018
Hamburg, N. M., and Benjamin, E. J. (2009). Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc. Med. 19, 6–11. doi: 10.1016/j.tcm.2009.03.001
Harris, R. A., Tedjasaputra, V., Zhao, J., and Richardson, R. S. (2012). Premenopausal women exhibit an inherent protection of endothelial function following a high-fat meal. Reprod. Sci. 19, 221–228. doi: 10.1177/1933719111418125
Hashimoto, M., Akishita, M., Eto, M., Ishikawa, M., Kozaki, K., Toba, K., et al. (1995). Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92, 3431–3435. doi: 10.1161/01.CIR.92.12.3431
Johns, J. A., O’brien, M. W., Bungay, A., and Kimmerly, D. S. (2020). Sex and light physical activity impact popliteal, but not brachial artery flow-mediated dilation in physically active young adults. Appl. Physiol. Nutr. Metab. 45, 1387–1395. doi: 10.1139/apnm-2020-0308
Joshi, H., and Edgell, H. (2019). Sex differences in the ventilatory and cardiovascular response to supine and tilted metaboreflex activation. Phys. Rep. 7:e14041. doi: 10.14814/phy2.14041
Juonala, M., Kähönen, M., Laitinen, T., Hutri-Kähönen, N., Jokinen, E., Taittonen, L., et al. (2008). Effect of age and sex on carotid intima-media thickness, elasticity and brachial endothelial function in healthy adults: the cardiovascular risk in young Finns study. Eur. Heart J. 29, 1198–1206. doi: 10.1093/eurheartj/ehm556
Kan, W. H., Hsu, J. T., Ba, Z. F., Schwacha, M. G., Chen, J., Choudhry, M. A., et al. (2008). p38 MAPK-dependent eNOS upregulation is critical for 17beta-estradiol-mediated cardioprotection following trauma-hemorrhage. Am. J. Physiol. Heart Circ. Physiol. 294, H2627–H2636. doi: 10.1152/ajpheart.91444.2007
Kneale, B. J., Chowienczyk, P. J., Brett, S. E., Coltart, D. J., and Ritter, J. M. (2000). Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J. Am. Coll. Cardiol. 36, 1233–1238. doi: 10.1016/S0735-1097(00)00849-4
Nardone, M., Miner, S., Mccarthy, M., Ardern, C. I., and Edgell, H. (2020). Noninvasive microvascular indices reveal peripheral vascular abnormalities in patients With suspected coronary microvascular dysfunction. Can. J. Cardiol. 36, 1289–1297. doi: 10.1016/j.cjca.2019.12.003
Nardone, M., Miner, S., Mccarthy, M., and Edgell, H. (2021). Standard exercise stress testing attenuates peripheral microvascular function in patients with suspected coronary microvascular dysfunction. BMC Sports Sci. Med. Rehabil. 13:18. doi: 10.1186/s13102-021-00246-8
Pyke, K. E., Dwyer, E. M., and Tschakovsky, M. E. (2004). Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J. Appl. Physiol. 97, 499–508. doi: 10.1152/japplphysiol.01245.2003
Rosenfeld, C. R., Chen, C., Roy, T., and Liu, X. (2003). Estrogen selectively up-regulates eNOS and nNOS in reproductive arteries by transcriptional mechanisms. J. Soc. Gynecol. Investig. 10, 205–215. doi: 10.1016/S1071-55760300049-2
Rubini, A., Paoli, A., and Parmagnani, A. (2012). Body metabolic rate and electromyographic activities of antigravitational muscles in supine and standing postures. Eur. J. Appl. Physiol. 112, 2045–2050. doi: 10.1007/s00421-011-2180-0
Saadjian, A. Y., Levy, S., Franceschi, F., Zouher, I., Paganelli, F., and Guieu, R. P. (2002). Role of endogenous adenosine as a modulator of syncope induced during tilt testing. Circulation 106, 569–574. doi: 10.1161/01.CIR.0000023924.66889.4C
Shenouda, N., Priest, S. E., Rizzuto, V. I., and Macdonald, M. J. (2018). Brachial artery endothelial function is stable across a menstrual and oral contraceptive pill cycle but lower in premenopausal women than in age-matched men. Am. J. Physiol. Heart Circ. Physiol. 315, H366–H374. doi: 10.1152/ajpheart.00102.2018
Keywords: flow-mediated dilation, reactive hyperemia, orthostatic stress, sex differences, hemodynamics
Citation: Habib K, Fallah B and Edgell H (2022) Effect of Upright Posture on Endothelial Function in Women and Men. Front. Physiol. 13:846229. doi: 10.3389/fphys.2022.846229
Received: 30 December 2021; Accepted: 21 February 2022;
Published: 24 March 2022.
Edited by:
Takuro Washio, Institute for Exercise and Environmental Medicine, United StatesReviewed by:
Artur Fedorowski, Lund University, SwedenCopyright © 2022 Habib, Fallah and Edgell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heather Edgell, ZWRnZWxsQHlvcmt1LmNh, orcid.org/0000-0001-8865-1921
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.