
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 08 March 2022
Sec. Invertebrate Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.844820
This article is part of the Research Topic Endocrine Regulation of Insect Diapause View all 8 articles
Within the United States and Canada, the primary pollinator of alfalfa is the alfalfa leafcutting bee (ALCB), Megachile rotundata. Our previous findings showed that overwintering conditions impacted gene expression profile in ALCB prepupae that entered diapause early in the season. However, ALCB are a bivoltine species, which begs the question of whether bees entering diapause later in the season also show this trend. To better understand the effects of the timing of diapause initiation, we analyzed mRNA copy number of genes known to be involved in diapause regulation in early and late season diapausing ALCB that were overwintered in field conditions or using current agricultural management conditions. We hypothesized that overwintering conditions for late diapausing bees also affects gene expression profiles. Our results showed that expression profiles were altered by both overwintering condition and timing of diapause initiation, with bees that entered diapause earlier in the season showing different expression patterns than those that entered diapause later in the season. This trend was seen in expression of members of the cyclin family and several targets of the insulin signaling pathway, including forkhead box protein O (FOXO), which is known to be important for diapause regulation and stress responses. But, of the genes screened, the proto-oncogene, Myc, was the most impacted by the timing of diapause initiation. Under field conditions, there were significant differences in Myc expression between the early and late season samples in all months except for November and February. This same general trend in Myc expression was also seen in the laboratory-maintained bees with significant difference in expression in all months except for November, February, and May. These results support previous conclusions from our research showing that the molecular regulation of diapause development in ALCB is not a simple singular cascade of gene expression but a highly plastic response that varies between bees depending upon their environmental history.
Diapause is a state of developmental dormancy that many insects undergo to survive changes in resource availability and below optimal temperatures in the winter months. Diapause can be divided into several ecophysiological phases: induction, preparation, initiation, maintenance, termination, and post-diapause quiescence, with finally resuming development (Kostal, 2006). These phases of diapause are regulated by both exogeneous and endogenous controls, including thermoperiod, photoperiod, and hormonal titers (Bell, 1968; Denlinger, 2002; Denlinger et al., 2005, 2012; Kostal, 2006; Sim and Denlinger, 2008, 2013). During diapause initiation, direct development ceases and is usually followed by metabolic suppression, processes which are both regulated by hormones (Denlinger et al., 2005, 2012), and possibly heat-shock proteins (Hayward et al., 2005; Rinehart et al., 2007). Although environmental conditions may still be favorable, diapausing individuals will maintain their course through endogenous controls, although environmental stimuli (ex. long-day photoperiod) may aid in diapause maintenance (Kostal, 2006; Kostal et al., 2017). The regulation of diapause termination is still not well understood but has been shown to be regulated by a combination of external and internal cues, such as chilling and change in photoperiod sensitivity, along with tissue sensitivity to stimuli (Kostal et al., 2000; Rinehart et al., 2001; Hodek, 2002). If environmental conditions are favorable, insects may resume direct development after diapause termination but most remain in post-diapause quiescence, a stage, that is, exogenously controlled by environmental conditions, such as day length and temperature (Kostal, 2006; Kostal et al., 2017). Finally, insects will resume direct development when they receive the appropriate environmental cues.
A key event in diapause is cell cycle arrest (Nakagaki et al., 1991; Denlinger, 2002; Denlinger et al., 2005, 2012; Kostal, 2006; Kostal et al., 2009, 2017; Hahn and Denlinger, 2011; Shimizu et al., 2018a,b). Cells are arrested at various points in the cell cycle by decreases in cell cycle regulators called cyclins (Vermeulen et al., 2003). Expression of these genes is controlled by several transcription factors, including proto-oncogene Myc, which activates transcription of cyclin substrates, cyclin-dependent kinases (CDK; Vermeulen et al., 2003). Myc is a downstream target of the Wnt/β-catenin pathway, a development-related signaling cascade (Chen and Xu, 2014). The Wnt/β-catenin pathway also interacts with targets of the insulin pathway, another pathway known to regulate insect diapause (Sim and Denlinger, 2008, 2013; Denlinger et al., 2012). Glycogen synthase kinase-3 beta-like (GSK-3β) is an antagonistic regulator of Wnt signaling and a downstream target of the insulin signaling pathway (IIS; Lin et al., 2009). A downstream target of Wnt/β-catenin signaling, β-catenin, interacts with Forkhead box protein O (FOXO), a downstream target of the insulin pathway that has been shown to be crucial for diapause regulation and stress responses (Sim and Denlinger, 2008; Sim et al., 2015). The insulin signaling pathway plays a role in creating diapause phenotypes through reduced metabolism (Hahn and Denlinger, 2011), enhanced stress tolerance (Wu and Brown, 2006; Sim and Denlinger, 2013; Matsunaga et al., 2016), and energy reserve accumulation (Satake et al., 1997). Previous studies showed that downregulation of the insulin signaling pathway plays a role in diapause regulation in both the mosquito Culex pipiens, and the alfalfa leafcutting bee (ALCB) Megachile rotundata (Sim and Denlinger, 2007, 2008; Cambron et al., 2021).
Our previous findings showed that ALCB overwintering conditions impacted the gene expression profile of bees that entered diapause early in the season, with temperature impacting expression levels of insulin pathway genes (Cambron et al., 2021). However, ALCB are a bivoltine species, with summer-emerging adults generating progeny (second generation) in the late summer that enter diapause in August/September (Yocum et al., 2018). This creates two diapause cohorts with the first entering diapause in June/July, and the second in the late summer, which begs the question of whether bees entering diapause later in the season also show similar expression profiles. Because temperature impacted IIS pathway gene expression in early season overwintering bees but late season bees are not exposed to summer temperatures, therefore expression levels for temperature-regulated genes, such as Samui and targets of the IIS pathway, may be differentially regulated. We hypothesize that expression profiles of bees entering diapause later in the season will differ from those in the early season. To better understand the effects of the timing of diapause initiation, we analyzed mRNA copy number of genes known to be involved in diapause regulation in early and late season diapausing ALCB.
Overwintered M. rotundata samples were obtained from a previously published experiment (Yocum et al., 2018). In the summer of 2010 M. rotundata were reared as described in Yocum et al. (2018). Briefly, adults were released into an on-farm facility (Logan, UT, 41°47′37.04″N; 112°8′18.35″W). Nests that were removed from nesting blocks between the 30th of June and the 19th of July 2010 were first generation and designated as “early” season nests, and those removed on the 1st of September were second generation and designated “late” season nests. Both early and late offspring were placed into temperature treatment groups in October of 2010, consisting of 16°C for 2 weeks and then transferred to either a laboratory setting of 4–5°C and darkness (Constant), or left in the field shelter exposed to naturally fluctuating conditions (Field; Supplementary Figure S1). A HOBO Datalogger (Onset Computer Corp., Bourne, MA, United States) was used to record temperatures outside and inside the field shelter, showing that field samples were exposed to temperatures ranging from −18°C (January 2011) to 35°C (May 2011; Supplementary Figure S1; Yocum et al., 2018). Every month, individual bees were collected from both seasonal nests within each temperature treatment, flash-frozen in liquid nitrogen, and stored in −80°C until used in RNA extraction.
RNA was extracted from frozen prepupae ground in liquid nitrogen and the resulting frozen powder was transferred to a 1.5 ml microcentrifuge containing TRIzol (Invitrogen, Life Technologies, Grand Island, NY, United States). The samples were extracted according to manufacturer’s instructions. RNA samples from all months (November, December, January, February, March, April, May, and June), early and late season, and temperature treatments were used. RNA pellets were prepared and quantified as previously described (Cambron et al., 2021). Four bees per treatment and seasonal group were used for nCounter analysis.
The same custom probe set from Cambron et al. (2021) was used for this study. Genes from the insulin pathway, downstream proto-oncogenes, and cell cycle regulators were measured (Supplementary Table S1; Cambron et al., 2021). RNA samples were prepared for nCounter analysis as previously described (Cambron et al., 2021), and shipped on dry ice to the University of Minnesota Genomics Center (Minneapolis, MN) for processing with nCounter Analysis System (NanoString Technologies Inc.). Resulting copy numbers were normalized to the geometric mean of the 10 reference genes used previously (Cambron et al., 2021). Normalized data set (Supplementary Table S2) is provided.
JMP Pro software (v.15.2.1, SAS Institute Inc., 2019, Cary, NC, United States) and SAS (v.9.4 SAS/STAT 15.1, SAS Institute Inc., 2018, Cary, NC, United States) were used for statistical analyses. Hierarchical cluster analysis with a Ward linkage method was performed with JMP Pro (v.15.2.1, SAS Institute Inc., 2018, Cary, NC, United States). To explore the relationship between season and temperature treatment, an additional variable was created (season + treatment, ST) since a season × temperature treatment interaction in our model would have only allowed for one post hoc comparison and an incomplete estimation of the effect due to month. The linear relationship between copy number (gene expression level) and the interaction of month and ST for each gene was modeled with a random coefficient growth curve model (RCGCM; Vonesh, 2012), and each gene’s regression line was determined as previously described (Cambron et al., 2021). Post hoc analyses were conducted by comparing upper and lower confidence limits between seasonal groups for each temperature treatment group across months. Limits that did not overlap were considered significantly different with 95% confidence. Copy number means +/− SEM and 95% CI are reported.
Of the 30 genes tested, 73% showed a significant interaction of month and ST, and 5% were significant only by season (Table 1). Insulin-like growth factor I was not significantly different by any effect (Table 1), and AKTIP and PTEN were unable to be fitted to the model.
When looking at overall expression profiles, two-way hierarchical cluster analysis showed different profiles for early and late season diapausers (Figure 1A). When comparing individual season profiles side by side, it was clearly shown that gene regulation is impacted by season as shown by distinctly different expression patterns (Figures 1B,C). Additionally, each season had different clustering of expression by months. In the early season, months grouped into two clusters for each temperature treatment groups, with the field temperature group dividing into (1) November to March and (2) April to June, while the constant temperature treatment group divided into (1) November to December, and (2) January to June (Figure 1B). For the late season, there were three clusters for each treatment group. Field temperature group broke up into (1) November to January, (2) February, March, June, and (3) April to May, while the constant temperature treatment group broke up into (1) November, (2) December to February, and (3) March to June (Figure 1C). Clustering of genes was also different by season, with early season branching from Samui and cyclin E (Figure 1B), but late season branching from p85 and FOXO (Figure 1C).
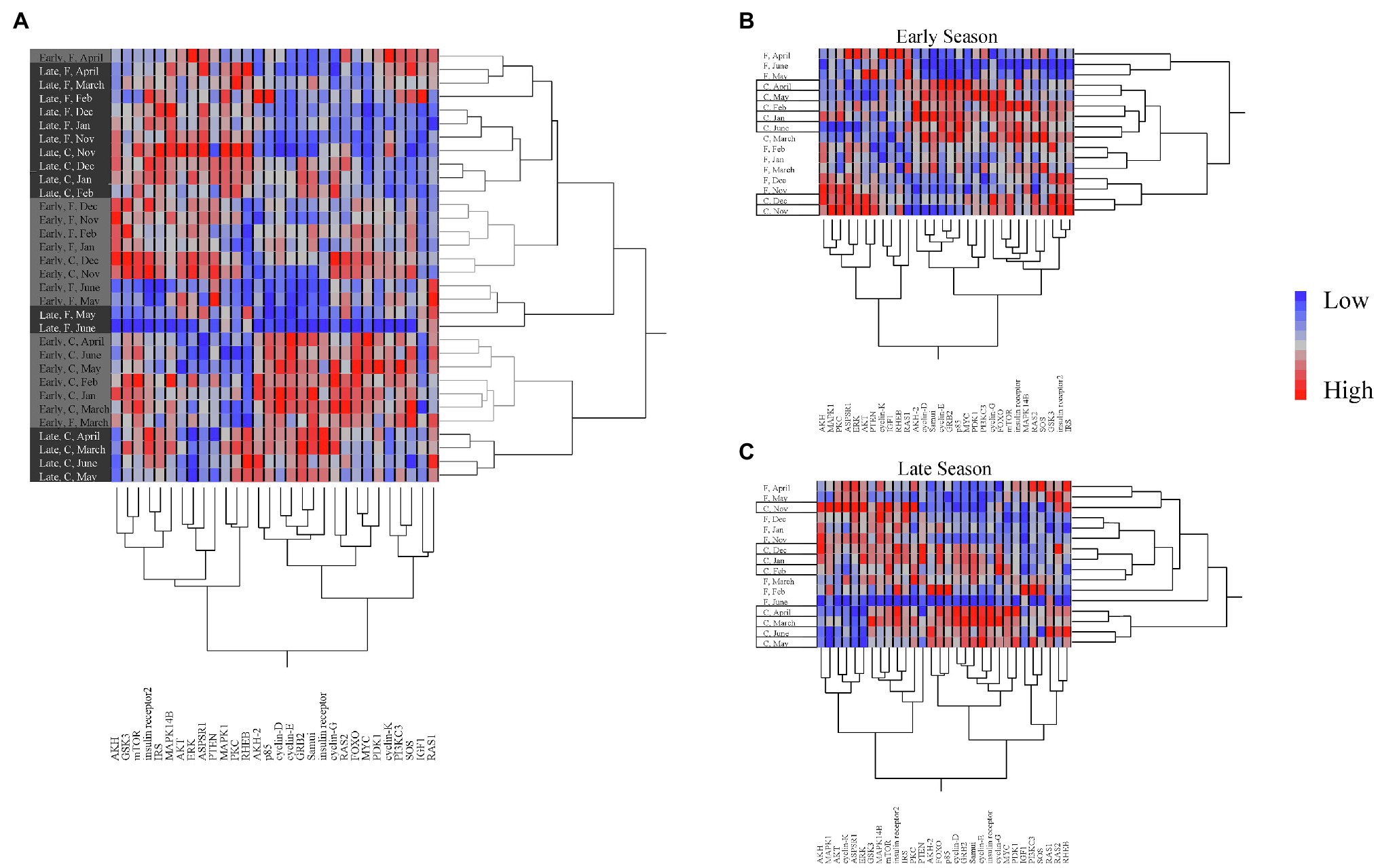
Figure 1. Two-way cluster analysis of gene expression showing (A) between seasons and (B) only Early season and (C) only Late season. Seasons are color-coded as light gray for Early season and dark gray for Late season. Temperature treatments are shown between seasons as field (F) and constant (C), and within seasons as field in open text and constant in boxed text. Expression gradient of blue to red indicates low to high expression, respectively.
Five downstream targets of the insulin signaling pathway exhibited significantly different expression by season. Levels of GSK3β were significantly lower in late season bees for the month of November in the constant temperature treatment group (Figure 2A). FOXO also showed lower levels of expression in late season diapausing bees for January, March, April, and June of the field temperature group and November, April, May, and June of the constant temperature treatment group (Figure 2B). December expression levels of p85 were lower for late season diapausers overwintered in field temperatures (Figure 2C). SOS expression levels were significantly lower in November for late season diapausers that were overwintered in constant temperatures (Figure 2D). Lastly, expression levels for RAS1 were also significantly lower in late season diapausers in December that were overwintered under field temperatures (Figure 2E).
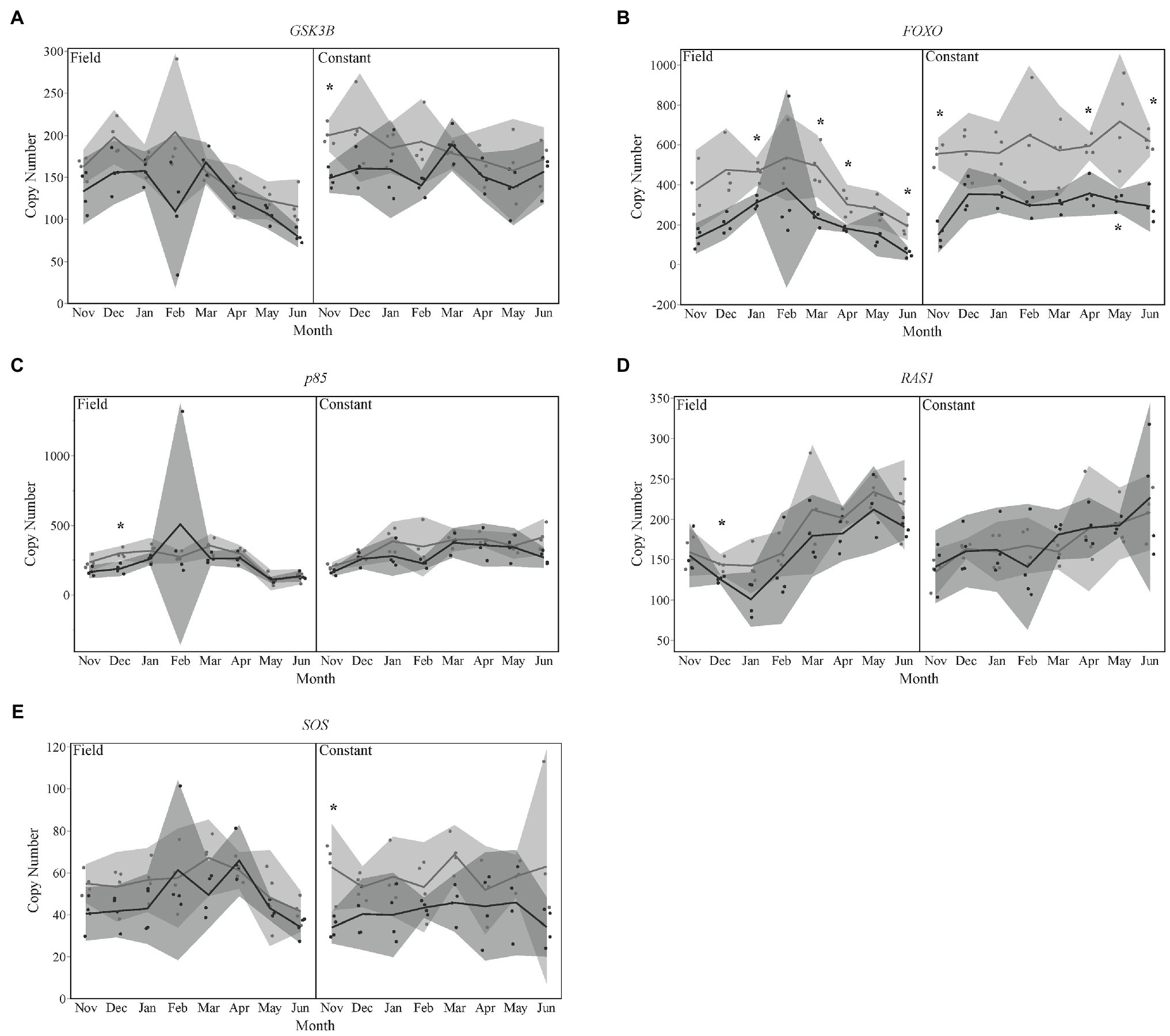
Figure 2. Gene expression for insulin signaling pathway targets (A) GSK3β, (B) FOXO, (C) p85, (D) RAS1, and (E) SOS. Seasons are color-coded as light gray for Early season and dark gray for Late season. Shaded areas indicate 95% CIs. Asterisks indicate months that were statistically significant between seasons.
For bees that were overwintered under field temperatures, those that entered diapause later in the season had significantly lower expression levels of Samui in the months of November through January (Figure 3A). Expression levels of Myc were significantly lower for late season of both temperature treatment groups for the months of December, January, March, April, May (field treatment only), and June (Figure 3B).

Figure 3. Gene expression levels for transcription factors (A) Samui and (B) Myc over time, for each temperature treatment. Seasons are color-coded as light gray for Early season and dark gray for Late season. Shaded areas indicate 95% CIs. Asterisks indicate months that were statistically significant between seasons.
Cyclins D, E, and K were all significantly different by season for a given treatment temperature and month (Figure 4). The late season diapausers had significantly lower expression levels of cyclin D (Figure 4A) in November, January, April, and June of the field temperature group, as well as in May of the constant temperature treatment group. This pattern of late season having lower expression levels was also true for cyclin E (Figure 4B) and cyclin K (Figure 4C) for several months in both temperature treatment groups.
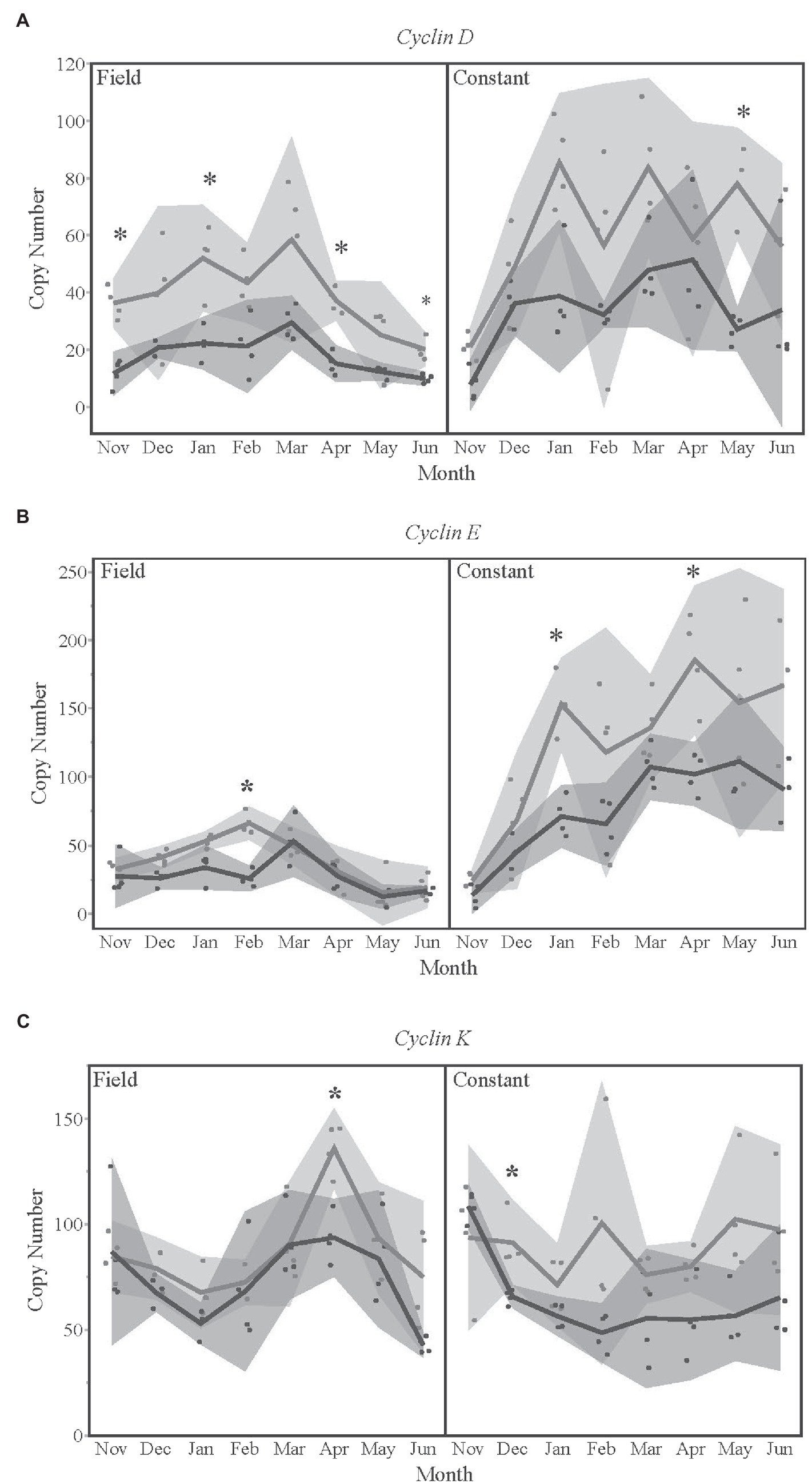
Figure 4. Gene expression levels for (A) cyclin D, (B) cyclin E, and (C) cyclin K over time, for each temperature treatment. Seasons are color-coded as light gray for Early season and dark gray for Late season. Shaded areas indicate 95% CIs. Asterisks indicate months that were statistically significant between seasons.
The objective of this study was to investigate the impact of the timing of diapause initiation and the overwintering environmental conditions on gene expression over the course of diapause development in the ALCB. Our results showed that the transcription profiles of bees at a specific time-point during winter depends on when the bee initiated diapause during summer (i.e., early or late) as well as the temperature conditions where it overwintered (i.e., field vs. constant). These differences in expression profiles included cell cycle regulator genes, transcription factors, and downstream targets of the IIS pathway. Although early season diapausing prepupae were already 1–2 months older than late season prepupae when we collected samples, we were surprised to find similar results to our previous findings with early season diapausing bees of only downstream targets and not the whole IIS pathway changing during overwintering (Figure 2). Two transcription factors, Myc and Samui were significantly different by season and over time (Figure 3), which made sense given their roles in regulating development. Our results demonstrated that at any given time point over the course of diapause development, gene expression profiles may vary between two individual bees due to timing of diapause initiation and their overwintering thermal history (Figure 1).
When comparing early and late season data together, the branching comes between cyclin D and cyclin E (Figure 1A). Cyclins have different functions throughout the cell cycle, with D types being present throughout actively dividing cells and E types only being present in cells that are in mid-G1 to mid-S phase (Lents and Baldassare, 2016; Shimizu et al., 2018b). Looking at overall expression profiles, we observed that gene expression in early season diapausing bees cluster into two groups by temperature treatment (Figure 1B), whereas late season diapausing bees showed more variation with grouping more similar to the ecophysiological phases of diapause (Figure 1C; Kostal, 2006). This clustering was originally seen in early season diapausing bees (Figure 2; Cambron et al., 2021), but we did not see the same pattern this time possibly due to needing a slightly different model to account for season. However, we did still see gene clusters main branching between Samui and cyclin E as shown previously (Cambron et al., 2021). Interestingly, the main branch for late season bees was between FOXO and p85 (Figure 1C), both which regulate insulin signaling (Kops et al., 2002; Ueki et al., 2003; Luong et al., 2006; Sim and Denlinger, 2008; Grewal, 2009). These differences in branching may indicate that early and late season diapausing bees sampled at the same time points are physiologically different from each other.
Constant temperature exposures are not ecologically relevant to ALCB’s life history and have been shown to be detrimental. Exposing developing ALCB to constant temperatures results in the adults emerging randomly throughout the day and over more days than bees exposed to a fluctuating thermal regime (Yocum et al., 2016; Bennett et al., 2018). Whereas bees exposed to some form of fluctuating thermal regime display synchronous emergence around the beginning of the photophase and emerge over fewer days than bees under constant temperatures. Bees exposed to constant low temperatures during prepupal to adult development are less cold tolerant than bees exposed to some form of fluctuating thermal regime (Yocum et al., 2019). Finally, constant low-temperature exposure has been demonstrated to induce sublethal effects that negatively impact the bee fitness (Bennett et al., 2015). At the transcription level, bees exposed to constant temperature treatment display a different gene expression profile than bees exposed to field temperatures (Torson et al., 2015, 2017; Melicher et al., 2019; Cambron et al., 2021).
It would be expected that both temperature treatment groups of bees (constant vs. field) would experience different forms of stress, or degree of stress of a common stress, and therefore result in different gene expression patterns. Indeed, this was the case demonstrated in this investigation. Based on our previous results, we conclude that constant temperature exposure should be considered as a form of physiological stress. For example, Samui is a cold-induced gene, and in our study, both early and late season bees overwintered in field conditions increased expression levels with increasing cold exposure (Figure 3A). However, overwintering bees at a constant temperature in a laboratory setting led to expression levels of Samui to be constant with high variability (Figure 3A). Although Samui has been shown to be temperature-regulated (Moribe et al., 2010), the interaction of diapause regulation and temperature on Samui expression is still unclear.
Surprisingly, of all the differentially expressed genes, the proto-oncogene Myc was the most impacted by timing of diapause initiation. Myc plays a pivotal role in mitochondrial biogenesis (Li et al., 2005). In the cotton bollworm Helicoverpa armigera, hypoxia-inducible factor (HIF-1α) suppresses Myc activity, decreasing mitochondrial activity and leading to developmental arrest (Lin et al., 2016). Interestingly, there is another mechanism for diapause initiation involving Myc. In response to low levels of ecdysone, Myc downregulates the activity of hexokinase, a gene important for insect development and metabolic activity (Lin and Xu, 2016). In our study, Myc expression levels were higher in early season diapausing bees, regardless of temperature treatment (Figure 3B). The month of February showed several significant differences between seasons and even within seasons, especially for Myc. Upon further investigation of our temperature data (Supplementary Table S3), the month of February showed a peak in maximum daily temperatures, therefore increasing daily mean temperatures and potentially causing drastic changes in gene expression. For Myc, the month of February was not significantly different due to such high variability (Figure 2B). Future studies would need to also include ecdysone and hexokinase to investigate the differences in Myc expression between seasons. Our study highlights the role of Myc in seasonal impacts on expression profiles, but more studies will be needed to understand the mechanism behind the role of Myc in diapause regulation between seasons, and whether it impacts thermal tolerance.
Myc, FOXO, and cyclin genes were the most differentially expressed genes between early and late season diapausing bees (Supplementary Table S1). The majority of genes impacted by the timing of diapause were not in the IIS pathway but rather regulate it or regulate development. This provides further support showing that the IIS pathway does not change during overwintering and that the IIS pathway suppression is a key process in diapause regulation (Denlinger, 2002; Sim and Denlinger, 2008, 2009, 2013). However, diapause is a complex network of regulatory processes that can vary drastically depending on the environmental conditions experienced prior to and during diapause, and many more factors that remain insufficiently investigated.
Our investigation demonstrated that at the time points, we measured the early and late season bees were transcriptionally different as determined by differential gene expression patterns. Obvious questions arise from these results, such as (1) what is the mechanism driving these differences, (2) can the genes found to be differentially regulated over the course of diapause development be subdivided into those primarily regulated by environmental factors and those that directly regulate diapause development, (3) how or do these two groups of genes interact to shape the diapause phenotype, and (4) are these differences biologically significant? One possible mechanism for the differences, we found is that the bees are at different points in their diapause development. Therefore, the early season bees are further along in their diapause development than the late season bees. As attractive as this explanation is, it has been demonstrated in other insect species that the timing of diapause initiation regulates the duration of diapause (Danks, 1987). Entering diapause later in the season resulted in a shorter diapause thereby aiding in synchronizing the post-diapause emerging insects. To start to resolve these questions in future investigations, we will need well-defined physiological landmarks to ensure that the insects being compared are at the same point in their diapause development. For an exemplary example of using physiological landmarks to investigate the molecular regulation of diapause, see Ragland et al. (2011).
The gene expression datasets generated for this study can be found on Dryad (doi 10.5061/dryad.h9w0vt4kb).
LC-K, GY, and KG designed the experiment and involved in funding. LC-K collected data, wrote first draft of the manuscript, and created all figures. LC-K, GY, and KY conducted statistical analysis. KG, GY, and KY edited the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by a National Science Foundation Graduate Research Fellowship and a Graduate Research Internship with the USDA-ARS Fargo, ND, to LC-K, an NSF EPSCoR-1826834 to KG, and USDA-ARS funding 3060-21220-032-00D to GY.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Theresa Pitts-Singer at the USDA-ARS Logan, Utah for supplying the bees used in this study. We would like to thank the reviewers for their insightful comments and feedback on this manuscript. Special thanks to our laboratory technician Marnie Larson (USDA-ARS) for her guidance and help on sample processing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.844820/full#supplementary-material
Bell, R. A. (1968). Photoperiodic induction of diapause in tobacco hornworm Manduca sexta. Am. Zool. 8:791.
Bennett, M. M., Cook, K. M., Rinehart, J. P., Yocum, G. D., Kemp, W. P., and Greenlee, K. J. (2015). Exposure to suboptimal temperatures during metamorphosis reveals a critical developmental window in the solitary bee, Megachile rotundata. Physiol. Biochem. Zool. 88, 508–520. doi: 10.1086/682024
Bennett, M. M., Rinehart, J. P., Yocum, G. D., Doetkott, C., and Greenlee, K. J. (2018). Cues for cavity nesters: investigating relevant zeitgebers for emerging leafcutting bees, Megachile rotundata. J. Exp. Biol. 221:jeb175406. doi: 10.1242/jeb.175406
Cambron, L. D., Yocum, G. D., Yeater, K. M., and Greenlee, K. J. (2021). Overwintering conditions impact insulin pathway gene expression in diapausing Megachile rotundata. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 256:110937. doi: 10.1016/j.cbpa.2021.110937
Chen, W., and Xu, W.-H. (2014). Wnt/β-catenin signaling regulates Helicoverpa armigera pupal development by up-regulating c-Myc and AP-4. Insect Biochem. Mol. Biol. 53, 44–53. doi: 10.1016/j.ibmb.2014.07.004
Danks, H. V. (1987). Insect Dormancy: An Ecological Perspective. Biological Survey of Canada (Terrestrial Arthropods). Ottawa: Entomological Society of Canada.
Denlinger, D. L. (2002). Regulation of diapause. Annu. Rev. Entomol. 47, 93–122. doi: 10.1146/annurev.ento.47.091201.145137
Denlinger, D. L., Yocum, G. D., and Rinehart, J. P. (2005). “3.12—Hormonal control of diapause,” in Comprehensive Molecular Insect Science. ed. Gilbert, L. I. (Amsterdam: Elsevier), 615–650.
Denlinger, D. L., Yocum, G. D., and Rinehart, J. P. (2012). “10—Hormonal control of diapause,” in Insect Endocrinology. ed. Gilbert, L. I. (San Diego: Academic Press), 430–463.
Grewal, S. S. (2009). Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int. J. Biochem. Cell Biol. 41, 1006–1010. doi: 10.1016/j.biocel.2008.10.010
Hahn, D. A., and Denlinger, D. L. (2011). Energetics of insect diapause. Annu. Rev. Entomol. 56, 103–121. doi: 10.1146/annurev-ento-112408-085436
Hayward, S. A., Pavlides, S. C., Tammariello, S. P., Rinehart, J. P., and Denlinger, D. L. (2005). Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J. Insect Physiol. 51, 631–640. doi: 10.1016/j.jinsphys.2004.11.009
Hodek, I. (2002). Controversial aspects of diapause development. Eur. J. Entomol. 99, 163–173. doi: 10.14411/eje.2002.024
Kops, G., Dansen, T. B., Polderman, P. E., Saarloos, I., Wirtz, K. W. A., Coffer, P. J., et al. (2002). Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321. doi: 10.1038/nature01036
Kostal, V. (2006). Eco-physiological phases of insect diapause. J. Insect Physiol. 52, 113–127. doi: 10.1016/j.jinsphys.2005.09.008
Kostal, V., Shimada, K., and Hayakawa, Y. (2000). Induction and development of winter larval diapause in a drosophilid fly, Chymomyza costata. J. Insect Physiol. 46, 417–428. doi: 10.1016/S0022-1910(99)00124-9
Kostal, V., Šimůnková, P., Kobelková, A., and Shimada, K. (2009). Cell cycle arrest as a hallmark of insect diapause: changes in gene transcription during diapause induction in the drosophilid fly, Chymomyza costata. Insect Biochem. Mol. Biol. 39, 875–883. doi: 10.1016/j.ibmb.2009.10.004
Kostal, V., Stetina, T., Poupardin, R., Korbelova, J., and Bruce, A. W. (2017). Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomic profiling. Proc. Natl. Acad. Sci. U. S. A. 114, 8532–8537. doi: 10.1073/pnas.1707281114
Lents, N. H., and Baldassare, J. J. (2016). “Cyclins and cyclin-dependent kinases,” in Encyclopedia of Cell Biology. eds. Bradshaw, R. A., and Stahl, P. D. (Waltham: Academic Press), 423–431.
Li, F., Wang, Y., Karen, I. Z., James, J. P., Diane, R. W., Kathryn, A. O., et al. (2005). Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell. Biol. 25, 6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005
Lin, J.-L., Lin, P.-L., and Gu, S.-H. (2009). Phosphorylation of glycogen synthase kinase-3β in relation to diapause processing in the silkworm, Bombyx mori. J. Insect Physiol. 55, 593–598. doi: 10.1016/j.jinsphys.2009.03.007
Lin, X.-W., Tang, L., Yang, J., and Xu, W.-H. (2016). HIF-1 regulates insect lifespan extension by inhibiting c-Myc-TFAM signaling and mitochondrial biogenesis. Biochim. Biophys. Acta 1863, 2594–2603. doi: 10.1016/j.bbamcr.2016.07.007
Lin, X.-W., and Xu, W.-H. (2016). Hexokinase is a key regulator of energy metabolism and ROS activity in insect lifespan extension. Aging 8, 245–259. doi: 10.18632/aging.100885
Luong, N., Davies, C. R., Wessells, R. J., Graham, S. M., King, M. T., Veech, R., et al. (2006). Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 4, 133–142. doi: 10.1016/j.cmet.2006.05.013
Matsunaga, Y., Honda, Y., Honda, S., Iwasaki, T., Qadota, H., Benian, G. M., et al. (2016). Diapause is associated with a change in the polarity of secretion of insulin-like peptides. Nat. Commun. 7:10573. doi: 10.1038/ncomms10573
Melicher, D., Torson, A. S., Anderson, T. J., Yocum, G. D., Rinehart, J. P., and Bowsher, J. H. (2019). Immediate transcriptional response to a temperature pulse under a fluctuating thermal regime. Integr. Comp. Biol. 59, 320–337. doi: 10.1093/icb/icz096
Moribe, Y., Oka, K., Niimi, T., Yamashita, O., and Yaginuma, T. (2010). Expression of heat shock protein 70a mRNA in Bombyx mori diapause eggs. J. Insect Physiol. 56, 1246–1252. doi: 10.1016/j.jinsphys.2010.03.023
Nakagaki, M., Takei, R., Nagashima, E., and Yaginuma, T. (1991). Cell cycles in embryos of the silkworm, Bombyx mori: G2-arrest at diapause stage. Rouxs Arch. Dev. Biol. 200, 223–229. doi: 10.1007/BF00361341
Ragland, G. J., Egan, S. P., Feder, J. L., Berlocher, S. H., and Hahn, D. A. (2011). Developmental trajectories of gene expression reveal candidates for diapause termination: a key life-history transition in the apple maggot fly Rhagoletis pomonella. J. Exp. Biol. 214, 3948–3960. doi: 10.1242/jeb.061085
Rinehart, J., Cikra-Ireland, R., Flannagan, R., and Denlinger, D. (2001). Expression of ecdysone receptor is unaffected by pupal diapause in the flesh fly, Sarcophaga crassipalpis, while its dimerization partner, USP, is downregulated. J. Insect Physiol. 47, 915–921. doi: 10.1016/S0022-1910(01)00064-6
Rinehart, J. P., Li, A., Yocum, G. D., Robich, R. M., Hayward, S. A. L., and Denlinger, D. L. (2007). Up-regulation of heat shock proteins is essentail for cold survival during insect diapause. Proc. Natl. Acad. Sci. U. S. A. 104, 11130–11137. doi: 10.1073/pnas.0703538104
Satake, S., Masumura, M., Ishizaki, H., Nagata, K., Kataoka, H., Suzuki, A., et al. (1997). Bombyxin, an insulin-related peptide of insects, reduces the major storage carbohydrates in the silkworm Bombyx mori. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 118, 349–357. doi: 10.1016/S0305-0491(97)00166-1
Shimizu, Y., Mukai, A., and Goto, S. G. (2018a). Cell cycle arrest in the jewel wasp Nasonia vitripennis in larval diapause. J. Insect Physiol. 106, 147–152. doi: 10.1016/j.jinsphys.2016.11.011
Shimizu, Y., Tamai, T., and Goto, S. G. (2018b). Cell cycle regulator, small silencing RNA, and segmentation patterning gene expression in relation to embryonic diapause in the band-legged ground cricket. Insect Biochem. Mol. Biol. 102, 75–83. doi: 10.1016/j.ibmb.2018.09.012
Sim, C., and Denlinger, D. L. (2007). RNA interference of the insulin receptor in Culex pipiens arrests ovarian development and simulate diapause. Am. J. Trop. Med. Hyg. 77:67. doi: 10.4269/ajtmh.2007.77.50
Sim, C., and Denlinger, D. L. (2008). Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. U. S. A. 105, 6777–6781. doi: 10.1073/pnas.0802067105
Sim, C., and Denlinger, D. L. (2009). A shut-down in expression of an insulin-like peptide, ILP-1, halts ovarian maturation during the overwintering diapause of the mosquito Culex pipiens. Insect Mol. Biol. 18, 325–332. doi: 10.1111/j.1365-2583.2009.00872.x
Sim, C., and Denlinger, D. L. (2013). Insulin signaling and the regulation of insect diapause. Front. Physiol. 4:189. doi: 10.3389/fphys.2013.00189
Sim, C., Kang, D. S., Kim, S., Bai, X., and Denlinger, D. L. (2015). Identification of FOXO targets that generate diverse features of the diapause phenotype in the mosquito Culex pipiens. Proc. Natl. Acad. Sci. U. S. A. 112, 3811–3816. doi: 10.1073/pnas.1502751112
Torson, A. S., Yocum, G. D., Rinehart, J. P., Kemp, W. P., and Bowsher, J. H. (2015). Transcriptional responses to fluctuating thermal regimes underpinning differences in survival in the solitary bee Megachile rotundata. J. Exp. Biol. 218, 1060–1068. doi: 10.1242/jeb.113829
Torson, A. S., Yocum, G. D., Rinehart, J. P., Nash, S. A., Kvidera, K. M., and Bowsher, J. H. (2017). Physiological responses to fluctuating temperatures are characterized by distinct transcriptional profiles in a solitary bee. J. Exp. Biol. 220, 3372–3380. doi: 10.1242/jeb.156695
Ueki, K., Fruman, D. A., Yballe, C. M., Fasshauer, M., Klein, J., Asano, T., et al. (2003). Positive and negative roles of p85 alpha and p85 beta regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J. Biol. Chem. 278, 48453–48466. doi: 10.1074/jbc.M305602200
Vermeulen, K., Van Bockstaele, D. R., and Berneman, Z. N. (2003). The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 36, 131–149. doi: 10.1046/j.1365-2184.2003.00266.x
Vonesh, E. F. (2012). Generalized Linear and Nonlinear Models for Correlated Data: Theory and Applications Using SAS®. Cary, NC: SAS Institute Inc.
Wu, Q., and Brown, M. R. (2006). Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 51, 1–24. doi: 10.1146/annurev.ento.51.110104.151011
Yocum, G. D., Childers, A. K., Rinehart, J. P., Rajamohan, A., Pitts-Singer, T. L., Greenlee, K. J., et al. (2018). Environmental history impacts gene expression during diapause development in the alfalfa leafcutting bee, Megachile rotundata. J. Exp. Biol. 221:jeb173443. doi: 10.1242/jeb.173443
Yocum, G. D., Rinehart, J. P., Rajamohan, A., Bowsher, J. H., Yeater, K. M., and Greenlee, K. J. (2019). Thermoprofile parameters affect survival of Megachile rotundata during exposure to low-temperatures. Integr. Comp. Biol. 59, 1089–1102. doi: 10.1093/icb/icz126
Keywords: solitary bee, diapause, overwintering, gene expression, insulin signaling pathway, seasonal variation, Megachile rotundata
Citation: Cambron-Kopco LD, Yocum GD, Yeater KM and Greenlee KJ (2022) Timing of Diapause Initiation and Overwintering Conditions Alter Gene Expression Profiles in Megachile rotundata. Front. Physiol. 13:844820. doi: 10.3389/fphys.2022.844820
Received: 28 December 2021; Accepted: 31 January 2022;
Published: 08 March 2022.
Edited by:
Wen Liu, Huazhong Agricultural University, ChinaCopyright © 2022 Cambron-Kopco, Yocum, Yeater and Greenlee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizzette D. Cambron-Kopco, bGNhbWJyb24wMUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.