
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 29 March 2022
Sec. Exercise Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.809029
This article is part of the Research TopicSynergistic Effects of Exercise and Nutrition on Body Weight ControlView all 4 articles
This study aimed to compare vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness in middle-aged Korean women according to obesity defined using body mass index (BMI). A total of 32 Korean women aged between 34 and 60 years (16 without obesity, mean age 46.31 ± 7.49 years and 16 with obesity, mean age 49.68 ± 6.69 years) participated in this study. Obesity was defined as BMI ≥ 25 kg/m2. The body composition, vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness of all participants were measured. Statistical differences in the dependent parameters between individuals with and without obesity were analyzed, and the correlations between BMI and the dependent variables were verified. The obese group showed significantly worse results (p < 0.05) for body composition (significantly higher weight, BMI, fat mass, and percent body fat), vascular function [significantly higher branchial ankle pulse wave velocity (baPWV) and lower flow-mediated vasodilation (FMD)], cardiometabolic parameters [significantly higher insulin and homeostatic model assessment for insulin resistance (HOMA-IR)], hemorheological function (significantly lower erythrocyte deformability and higher aggregation), and cardiorespiratory fitness [significantly lower maximal oxygen uptake (VO2max)] compared to the non-obese group. In addition, BMI showed a significant positive correlation (p < 0.05) with baPWV (r = 0.430); total cholesterol (r = 0.376), triglyceride (r = 0.411), low-density lipoprotein cholesterol (r = 0.462), and insulin (r = 0.477) levels; HOMA-IR (r = 0.443); and erythrocyte aggregation (r = 0.406), and a significant negative correlation (p < 0.05) with VO2max (r = −0.482) and FMD (r = −0.412). Our study confirmed that obesity is a major determinant for deterioration of vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness.
Obesity can be classified into primary and secondary obesity according to the cause (Hoelscher et al., 2015). Primary obesity, in which weight and body fat are increased due to an imbalance between energy expenditure and energy intake, is caused by various factors such as diet, lifestyle, age, race, socioeconomic factors, genetics, intestinal microflora, and environmental chemicals (Nammi et al., 2004; Hoelscher et al., 2015). Secondary obesity is caused by genetic disorders, congenital disorders, neuroendocrine disorders, mental disorders, and drugs (Nammi et al., 2004; Hoelscher et al., 2015).
Obesity is recognized as a global health issue and can be described as a new-world syndrome. Obesity is a multi-factorial disorder, which is often associated with many other significant diseases such as hypertension, other cardiovascular diseases, diabetes, osteoarthritis, and certain cancers (Nammi et al., 2004; Park et al., 2018, 2019b). Patients with obesity have a higher risk of morbidity and mortality compared to those with an ideal body weight (Choi, 2012). Many previous studies have shown that a 5–10% reduction in the initial body weight induces significant improvements in a wide range of comorbid conditions in obese patients (Nammi et al., 2004; Wang et al., 2008; Choi, 2012; Jarolimova et al., 2013). In 2016, the World Health Organization reported that more than 1.9 billion adults were overweight and more than 650 million were obese, the prevalence of obesity increased by 50% in 2016 compared to that in 2000, and the prevalence of obesity in women increased by 70% in 2016 compared to that in 2000 (Mohanty and Mohanty, 2021). In South Korea, the prevalence of obesity among adults continued to increase from 29.7% in 2009 to 35.7% in 2018 (Nam et al., 2020).
Previous studies have described in detail the effects of obesity on pathophysiological aspects such as cardiometabolic risk, vascular function, hemorheological function, and cardiorespiratory fitness (Bravo et al., 2006; Vassallo, 2007; Guiraudou et al., 2013; Simonds et al., 2014; Zhang et al., 2014; Schwartz et al., 2017; Cercato and Fonseca, 2019; Bennie et al., 2020). Several cardiometabolic parameters, such as serum glucose, insulin, fatty acid, triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high density lipoprotein cholesterol (HDL-C) levels, and adipocytes are involved in the pathogenesis of obesity, in addition to the gastric and nervous systems that regulate appetite and control food intake (Vassallo, 2007; Zhang et al., 2014; Schwartz et al., 2017; Cercato and Fonseca, 2019). Obesity also induced increased blood pressure and attenuated vascular function. The increase in leptin production due to obesity worsens the function of vascular endothelial cells responsible for vasodilation by interfering with nitric oxide production and activates the sympathetic nervous system to induce vasoconstriction and increase blood pressure (Bravo et al., 2006; Simonds et al., 2014; Cercato and Fonseca, 2019). Guiraudou et al. (2013) verified that obesity is highly correlated with increased red blood cell (RBC) aggregation and blood viscosity due to a rise in hematocrit, and reported that deterioration of hemorheological function increases the risk of diabetes and cardiovascular disease. In addition, they reported that cardiorespiratory fitness was associated with a lower prevalence of obesity, and the associations were more pronounced in groups with higher obesity (Bennie et al., 2020). Another study showed that increased physical activity and improved cardiorespiratory fitness were related to decreased prevalence of obesity and cardiometabolic risk factors (Park et al., 2021b).
However, most previous studies analyzing the relationship between obesity and pathophysiological function were conducted using public data, such as public health surveillance data (Soriano-Maldonado et al., 2016; Park et al., 2020b). Few previous studies have comprehensively reviewed the effects of obesity on dependent variables, such as cardiometabolic risk, vascular function, hemorheological function, and cardiorespiratory fitness, via a cross-sectional study based on measured results under strict control.
Therefore, this study aimed to compare vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness between middle-aged Korean women with and without obesity. We hypothesized that obesity negatively affects vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness, and that body mass index (BMI) is significantly correlated with these dependent variables.
Participants were randomly sampled through posters and flyers. A total of 32 Korean women aged between 34 and 60 years (16 without obesity, mean age 46.31 ± 7.49 years and 16 with obesity, mean age 49.68 ± 6.69 years) with no smoking history, orthopedic or cardiovascular diseases, and low levels of activity (<600 MET min/week) in the past year. The menopausal status of the study participants was not assessed. The physical characteristics of the participants are shown in Table 1. Participants were assigned to the non-obese group (n = 16; BMI, 22.85 ± 1.05 kg/m2) or the obese group (n = 16; BMI, 28.28 ± 3.43 kg/m2) based on a cutoff value of BMI = 25 kg/m2. BMI, a measure of weight corrected for height, reflects the total body fat, and is the most accepted parameter for defining obesity (Taylor et al., 1998). All Korean women completed the study, and their measured data were used in the analyses. We explained the experiments and possible side effects to all participants prior to the start of the study and obtained written consent for participation. The present study was approved by the Institutional Review Board (7001355-201909-HR-334) and was conducted in accordance with the provisions of the Declaration of Helsinki.
The present study was designed to have 2 consecutive days of testing sessions. On the first testing day, all participants fasted for ≥8 h and after stabilization, the following tests were performed sequentially from 7:00 am: blood pressure (BP) and body composition were measured, and 8 ml of venous blood was collected for the measurement of cardiometabolic parameters and hemorheological function. After sufficient rest and meals, the cardiorespiratory fitness was measured. On the second testing day, pulse wave velocity (PWV) and flow-mediated dilation (FMD), which are indicators of vascular function, were measured in order in the morning (7:00 am) after fasting for ≥8 h (Jung et al., 2020a).
All participants underwent body composition (height, weight, BMI, free fat mass, fat mass, and body fat percentage), vascular function [systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), pulse pressure (PP), brachial-ankle PWV (baPWV), and FMD], cardiometabolic parameters [TC, HDL-C, LDL-C, TG, free fatty acid (FFA), glucose, and insulin levels; homeostatic model assessment for insulin resistance (HOMA-IR); and homeostasis model assessment of β-cell function (HOMA-β)], hemorheological function (RBC deformability and aggregation), and cardiorespiratory fitness [estimated maximal oxygen uptake (VO2max)] measurements.
All measurements were performed in the Konkuk University Metabolic and Environmental Chamber (NCTC-1, Nara control, Seoul, South Korea) at a temperature of 23 ± 1°C and a humidity of 50 ± 5%.
Body composition parameters including height, weight, BMI, free fat mass, fat mass, and body fat percentage of all the participants were estimated using a stadiometer (YM-1, KDS, Seoul, Korea) and bioelectrical impedance analyzer (Inbody 770, Inbody, Seoul, South Korea). All participants wore lightweight clothing and were asked to remove all metal items from their bodies.
Measurement of vascular function was conducted based on previous studies (Jung et al., 2020b; Park et al., 2020b). After the participants rested for at least 20 min, BP (SBP, DBP, MAP, and PP), baPWV, and FMD were sequentially measured to evaluate vascular function.
The BP in the right branchial artery was measured twice using an automatic BP monitor (HBP-9020, Omron, Tokyo, Japan), and the average value was used for analysis. If the results of the first and second measurements were significantly different, the measurement was repeated after a 10-min rest period.
The baPWV was measured using an automatic oscillometer (VP-1000plus, Omron, Osaka, Japan). The VP-1000plus simultaneously records the baPWV and brachial and ankle BPs on the left and right sides, produces an electrocardiogram, and records the heart sounds. Electrocardiography electrodes were placed on both wrists and cuffs were placed on the brachium and ankles bilaterally. A microphone for detecting heart sounds was placed on the left edge of the sternum. The cuffs were connected to a plethysmographic sensor, which determined the volume pulse form and an oscillometric pressure sensor that measured BP. The brachial and ankle pulse volume waveforms were recorded using a semiconductor pressure sensor. The baPWV values on the right and left sides were obtained and averaged for analysis (Park et al., 2020b).
The FMD in the right branchial artery was measured using noninvasive Doppler ultrasound (UNEX-EF, Tokyo, Japan). During FMD measurement, we fixed the ultrasound instrument in the brachial artery region 3–5 cm above the elbow and measured the diameter of the medial muscle artery. Next, blood flow was stopped for 5 min by increasing the cuff pressure by 50 mmHg more than the resting BP. After 5 min, the arterial diameter at deflation was automatically recorded for the next 2 min, and the calculated values of FMD [FMD = (reactive hyperemia diameter − baseline diameter) × 100%] were used to evaluate the diameter and blood flow rate (Jung et al., 2020b).
Measurement of cardiometabolic parameters was conducted based on previous studies (Jung et al., 2020a). All cardiometabolic parameters (TC, HDL-C, LDL-C, TG, FFA, glucose, and insulin levels; HOMA-IR; and HOMA-β) were analyzed by the Seegene Medical Foundation (an organization certified by the Korean government). Eight milliliters of venous blood was collected in a serum separating tube (SST). Clot formation was ensured in the SST by centrifuging the sample at 3500 rpm for 10 min. TC level was determined using an enzymatic kinetic assay using Cobas C702 (Roche, Mannheim, Germany). HDL-C and LDL-C levels were detected using a homogeneous enzymatic colorimetric assay using Cobas C702 (Roche, Mannheim, Germany). The glucose level was determined using an enzymatic kinetic assay using Cobas8000 C702 (Roche, Mannheim, Germany), and the insulin level was detected using an electrochemiluminescence immunoassay (ECLIA) using Cobas8000 e602 (Roche, Mannheim, Germany). HOMA-IR and HOMA-β were calculated using the following formula: HOMA-IR = [glucose (mg/dl) × insulin (μU/ml)]/405, HOMA-β = [360 × insulin (μU/ml)/glucose (mg/dl) − 63] (Jung et al., 2020a).
Measurement of hemorheological function was conducted based on previous studies (Jung et al., 2020a,b; Park et al., 2020b). All participants were evaluated for RBC deformability and aggregation as hemorheological function parameters to evaluate microvascular circulation function. We analyzed RBC deformability and aggregation using Rheoscan-D under environmental conditions of 25°C and 3 Pa shear stress within 4–6 h after blood collection (Uyuklu et al., 2009; Jung et al., 2020a). RBC deformability was measured using the elongation index. For this, we transferred the sample to a 2-mL microseparation tube and diluted it in 700 μl of 5.5% polyvinylpyrrolidone (360 kDa) dissolved in 1 mmol phosphate buffered saline (pH 7.4; osmolality, 300 mOsmol/kg) in a K3-ethylenediaminetetraacetic acid tube (Greiner Bio-one, Chon Nuri, Thailand). Thereafter, 0.5 ml of this solution was analyzed using a D-test kit according to the instructions provided by Rheo Meditech Inc. The accuracy of the RBC elongation index was measured using a Lineweaver–Burk plot model (Baskurt and Meiselman, 2004). RBC aggregation was measured using the aggregation index. For this, we analyzed 8 μl of whole blood using an A-test kit according to the instructions provided by Rheo Meditech Inc. (Kim et al., 2019).
Measurement of cardiorespiratory fitness was conducted based on previous studies (Jung et al., 2020a). All participants were evaluated for estimated VO2max as an indicator of cardiorespiratory fitness. A graded exercise test using an electrically braked cycle ergometer (Aerobike 75XLIII, Konami, Japan) was performed to measure the estimated VO2max. A heart rate (HR) sensor was attached to the participants’ earlobes and their personal information (height, weight, and age) was entered into the cycle ergometer. Participants performed the graded exercise test after stabilization of their HR. All participants exercised on the cycle ergometer at a rate of 50 rpm until the HR reached 75% of the maximal heart rate (HRmax) using the Lamb protocol (15 W/min; men HRmax: 206–0.69 × age, women HRmax: 205–0.75 × age). The estimated VO2max was calculated using the regression equation (estimated VO2max = 9.386 W + 289.6; Miyashita et al., 1985).
The means and standard deviations were calculated for all dependent parameters. The normality of distribution of all outcome parameters was verified using the Shapiro–Wilk W-test prior to parametric tests. An independent t-test was used to verify the statistical differences between the non-obese and obese groups. Pearson’s correlation analysis was used to analyze the correlations between BMI and all dependent parameters. All analyses were performed using the Statistical Package for the Social Sciences version 23.0 (IBM Corporation, Armonk, NY, United States). A priori, the level of significance was set at p < 0.05.
The body composition data between the non-obese and obese groups are presented in Table 1. The obese group showed a significantly higher weight (p < 0.001), BMI (p < 0.001), fat mass (p < 0.001), and body fat percentage (p < 0.001) than the non-obese group.
Table 2 presents the vascular function (SBP, DBP, MAP, PP, baPWV, and FMD) data of the non-obese and obese groups. No significant differences between non-obese and obese patients were observed with regard to BP parameters. However, significant differences between both groups were noted in baPWV (p = 0.006) and FMD (p = 0.024), and the obese group showed a significantly higher baPWV and a lower FMD than the non-obese group.
As shown in Table 3, a significant difference between the non-obese and obese groups was found with regard to insulin levels (p = 0.015) and HOMA-IR (p = 0.022) among cardiometabolic parameters. The obese group showed significantly higher insulin levels and HOMA-IR than the non-obese group. However, there was no significant difference between the groups in the remaining cardiometabolic parameters (TC, HDL-C, LDL-C, TG, FFA, and glucose levels and HOMA-ß).
Figure 1 depicts the hemorheological function (RBC deformability and aggregation) data for the non-obese and obese groups. Statistical analysis showed a significant difference in RBC deformability (p = 0.024) and aggregation (p = 0.004) between the groups. The obese group showed significantly lower RBC deformability and higher RBC aggregation than the non-obese group.
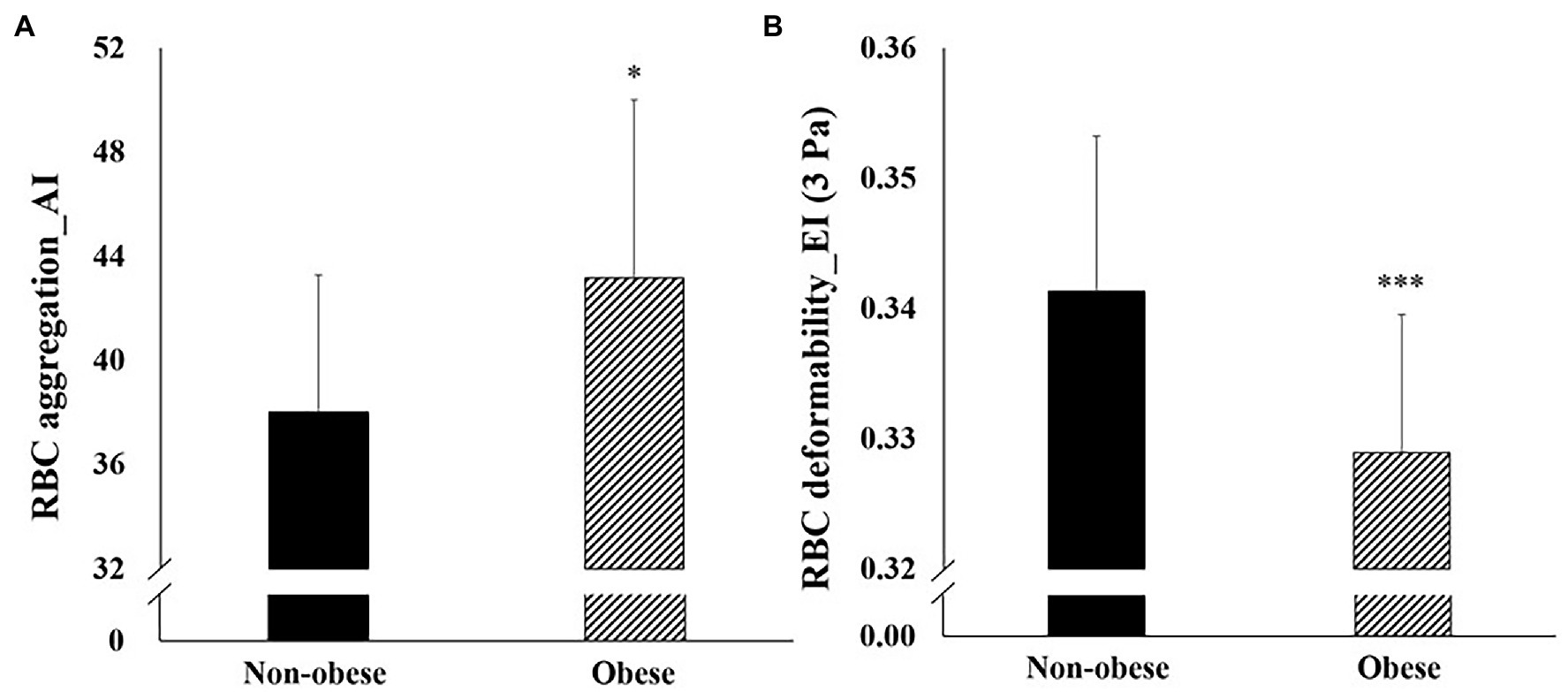
Figure 1. Difference in hemorheological function (mean ± SD) between non-obese and obese. SD, standard deviation; RBC, red blood cell; EI, elongation index; and AI, aggregation index. *p < 0.05; **p < 0.01; and ***p < 0.001 vs. non-obese.
The cardiorespiratory fitness in both groups is shown in Figure 2. A significant difference between the non-obese and obese groups was observed in VO2max (p = 0.049). The obese group showed significantly lower VO2max than the non-obese group.
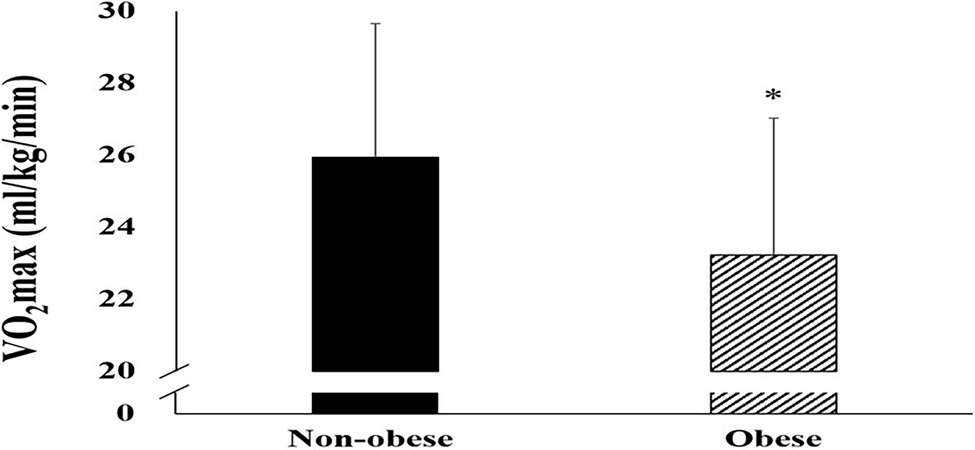
Figure 2. Difference in maximal oxygen uptake (mean ± SD) between non-obese and obese. SD, standard deviation and VO2max, maximal oxygen uptake. *p < 0.05; **p < 0.01; ***p < 0.001 vs. non-obese.
Figure 3 shows a significant correlation between the vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness and BMI. BMI was significantly negatively correlated with VO2max (r = −0.482, p = 0.005) and FMD (r = −0.412, p = 0.019). On the other hand, BMI was significantly positively correlated with baPWV (r = 0.430, p = 0.014), TC (r = 0.376, p = 0.034), TG (r = 0.411, p = 0.020), LDL-C (r = 0.462, p = 0.008), insulin (r = 0.477, p = 0.006), HOMA-IR (r = 0.443, p = 0.011), and RBC aggregation (r = 0.406, p = 0.021).
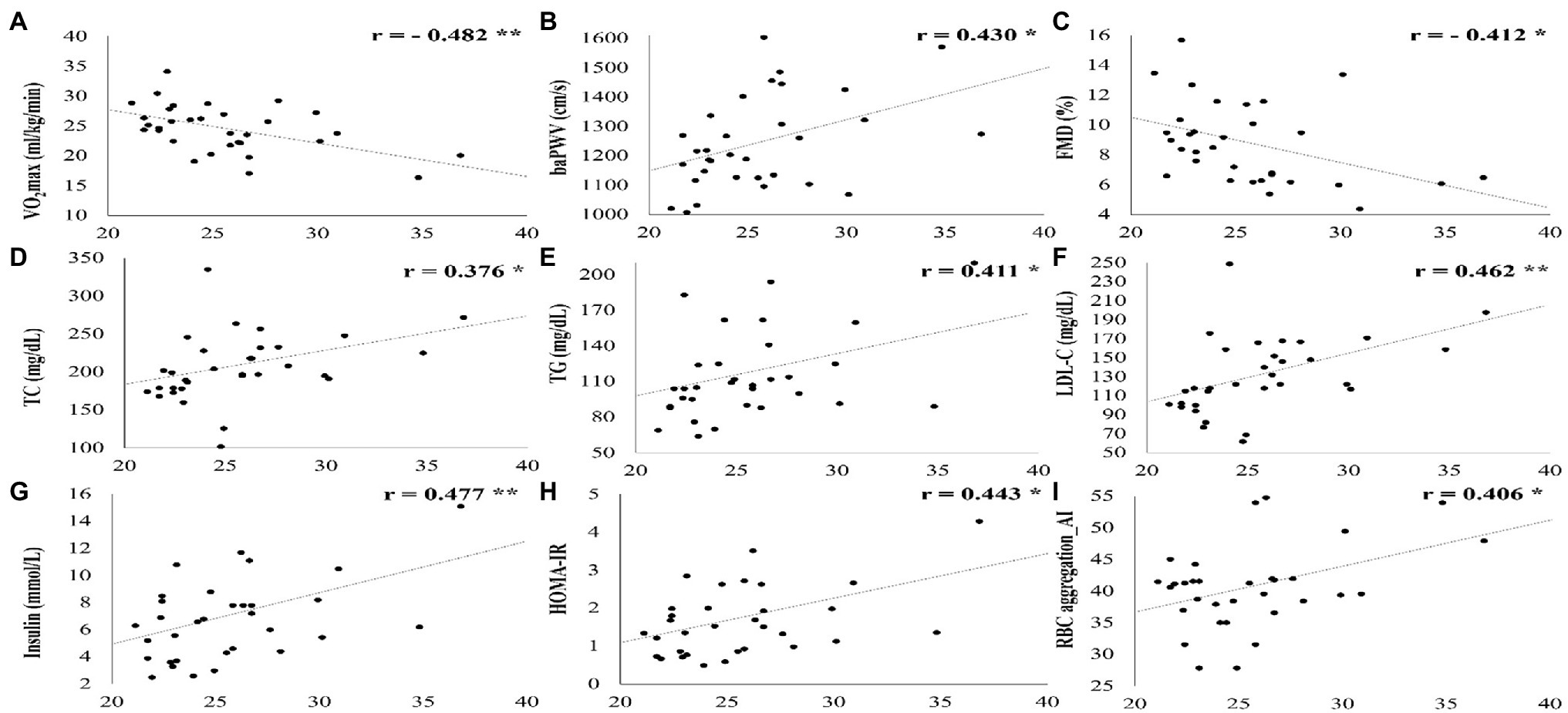
Figure 3. All parameters that showed a significant correlation with BMI. SD, standard deviation; VO2max, maximal oxygen uptake; baPWV, branchial ankle pulse wave velocity; FMD, flow-mediated vasodilation; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance, RBC, red blood cell; and AI, aggregation index. *p < 0.05; **p < 0.01; ***p < 0.001.
In the present study, we hypothesized that individuals with obesity may show attenuated vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness compared with individuals without obesity and that BMI may be significantly correlated with these dependent variables. Our study verified that obesity is a major risk factor for the deterioration of vascular function (a higher baPWV and a lower FMD), cardiometabolic parameters (higher insulin levels and HOMA-IR), hemorheological function (lower RBC deformability and higher aggregation), and cardiorespiratory fitness (lower VO2max). We also demonstrated that BMI was significantly correlated with various pathophysiological parameters (negatively correlation: VO2max and FMD; positively correlation: baPWV, TC, TG, LDL-C, insulin, HOMA-IR, and RBC aggregation).
Regarding the relationship between BMI and body composition, Ranasinghe et al. (2013) analyzed the relationship between BMI and body fat percentage in a group of South Asian adults who had a different body composition than the ethnic group in the present study. They reported that BMI shows a strong correlation with the body fat percentage measured using bioelectrical impedance in this sub-population of South Asian adults. On the other hand, Meeuwsen et al. (2010) examined the relationship between age, sex, and the influence of age and sex on BMI and body fat percentage over a wide range of BMI and age, and reported that the association between BMI and body fat percentage is not strong. Particularly, in the desirable BMI range, it is curvilinear rather than linear and is affected by age. Although our study was a pilot study with a small sample size, we confirmed a significant difference in the fat mass and body fat percentage between the non-obese and obese groups, and a strong correlation between BMI and fat mass or body fat percentage. These results are consistent with those of a previous study (Ranasinghe et al., 2013).
Obesity promotes the development of several major cardiovascular risk factors (Park et al., 2019a). Obesity may impair vascular endothelial function, a key factor in the pathogenesis of atherosclerosis and in triggering acute ischemic events, and is associated with increased BP and attenuated vascular function (Brook, 2006). Especially, increased leptin secretion from adipocytes in obese individuals attenuates vascular endothelial function, which is responsible for vasodilation, by interfering with nitric oxide production and activates the sympathetic nervous system to induce vasoconstriction and increase BP (Bravo et al., 2006; Brook, 2006; Simonds et al., 2014; Cercato and Fonseca, 2019). Therefore, we investigated the difference in BP, baPWV (a predictive indicator of arterial stiffness), and FMD (vasodilation of arteriole due to the release of nitric oxide from endothelial cells that occurs when blood flow in it increases) between the non-obese and obese groups. Tang et al. assessed the association between BMI and baPWV in Chinese individuals who were overweight/obese and reported that individuals who were overweight/obese had significantly higher baPWV than those with normal weight (Tang et al., 2020). Son et al. (2017) analyzed the effect of obesity on BP and PWV in middle-aged Korean women and reported that obesity is closely related to blood pressure and arterial stiffness. Davison et al. (2010) investigated the relationship between obesity and arterial endothelial function and found that the obese group showed a lower FMD than the non-obese group, and FMD was impaired in patients with obesity, which may contribute to arterial and metabolic dysfunction. Ne et al. (2017) analyzed the relationship between obesity and arterial endothelial function using a meta-analysis, and demonstrated that obesity was associated with lower flow-mediated dilatation. In the present study, the obese group showed a higher PWV and lower FMD than the non-obese group, and BMI was significantly correlated with PWV and FMD, which is consistent with the results of previous studies. These results suggest that obesity induces arterial stiffness and deteriorates endothelial function.
Many previous studies have clinically confirmed the relationship between obesity and cardiometabolic parameters. Soriano-Maldonado et al. (2016) analyzed the effect of severe/morbid obesity (BMI ≥ 35) on cardiometabolic parameters and concluded that severe/morbid obesity is highly correlated with more adverse cardiometabolic risk factors in both male and female adults. Nyangasa et al. (2019) investigated the association between obesity indices and cardiovascular risk factors and reported that the obese group showed higher BP, LDL-C levels, and glycated hemoglobin levels and lower HDL-C levels than the non-obese group. They concluded that increased BMI, waist circumference, and body fat percentage were strongly associated with hypertension, with individuals with high waist circumference being twice as likely to have hypertension. In our study, only insulin levels and HOMA-IR among the cardiometabolic parameters were higher in the obese group than in the non-obese group. This can be explained by the fact that the BMI of the obese group in the present study was 28.28 ± 3.43, and the participants did not have severe obesity. However, as there was a significant correlation between BMI and most cardiometabolic parameters (TC, TG, LDL-C, and insulin levels and HOMA-IR), it was confirmed that an increase in BMI could increase metabolic disease, as reported in previous studies.
RBC deformability is a key feature of erythrocytes that allows them to pass through blood vessels smaller than themselves, and decreased RBC deformability results in decreased blood flow to the capillaries, which can result in a variety of microvascular diseases (Shin et al., 2009; Kim et al., 2019). In contrast, erythrocytes tend to aggregate to form linear stacking shapes known as rouleaux and exacerbated RBC aggregation in the microcirculation by excessive rouleaux formation causes diabetic microcirculatory diseases (Kim et al., 2019; Lee et al., 2019). Wiewióra et al. (2010) compared hemorheological properties between non-obese and obese groups, and examined the correlation between BMI and hemorheological parameters to determine the relationship between obesity and hemorheological disturbances. They reported that obesity may attenuate hemorheological properties by increasing whole blood viscosity and RBC aggregation. We investigated the effect of obesity, which was defined as BMI ≥ 25, on hemorheological properties and the correlation between BMI and hemorheological function. Our results showed that obesity was significantly correlated with RBC deformability and aggregation, and obesity induced deterioration of hemorheological function via decreased RBC deformability and increased RBC aggregation. These results are consistent with those of previous studies that reported that changes in rheological properties of blood are closely related to obesity and diabetic microcirculatory diseases (Shin et al., 2009; Wiewióra et al., 2010; Kim et al., 2019; Lee et al., 2019).
Cardiorespiratory fitness reflects the ability of the cardiorespiratory system to transport oxygen to skeletal muscles during exercise and is the most important clinical indicator for evaluating pathological conditions, including metabolic and cardiovascular diseases (Ross et al., 2016; Park et al., 2021a). Cardiorespiratory fitness is an index that reflects risk factors and disease burden over an individual’s lifetime, and a low cardiorespiratory fitness increases the risk of obesity, diabetes, hypertension, and cardiovascular disease, thereby increasing morbidity and mortality (Kodama et al., 2009; Gray et al., 2015; Park et al., 2021a). Therefore, we evaluated the correlation between BMI and VO2max, and analyzed the difference in VO2max between the non-obese and obese groups. We observed that the obese group showed a lower VO2max than the non-obese group, and BMI had a significant negative correlation with VO2max. These results are consistent with those of previous studies that showed that obesity worsens cardiorespiratory fitness, which increases the prevalence of diabetes, hypertension, and cardiovascular disease, thereby increasing morbidity and mortality (Kodama et al., 2009; Gray et al., 2015; Park et al., 2020a, 2021a).
Our study confirmed the normal distribution using the Shapiro–Wilk W-test, its limitation was the relatively small sample size. Thus, to utilize the results of this study in the field of public health, it is necessary to comprehensively evaluate the correlation between vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness and obesity (BMI ≥25) using a larger sample size. Also, we included only adult women in the study. Men and women have different physiological characteristics and need further research on adult men. Our study also did not evaluate extraneous variables that could affect dependent variables such as the ovarian cycle, female sex hormones, diet, and exercise.
In conclusion, the present study suggests that obesity, defined as BMI ≥25 kg/m2, is a major risk factor that deteriorates vascular function, cardiometabolic parameters, hemorheological function, and cardiorespiratory fitness, and BMI is significantly correlated with these dependent variables in adult women.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board (7001355-201909-HR-334) and was conducted in accordance with the provisions of the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
H-YP: conception and study design, investigation, and writing-original draft preparation. S-WK, W-SJ, and KJ: statistical analysis, data interpretation, and writing-review and editing. KL: supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2019S1A5B8099542).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This paper was supported by the Konkuk University (KU) Research Professor Program.
Baskurt, O. K., and Meiselman, H. J. (2004). Analyzing shear stress-elongation index curves: comparison of two approaches to simplify data presentation. Clin. Hemorheol. Microcirc. 31, 23–30.
Bennie, J. A., De Cocker, K., Pavey, T., Stamatakis, E., Biddle, S. J. H., and Ding, D. (2020). Muscle strengthening, aerobic exercise, and obesity: a pooled analysis of 1.7 million US adults. Obesity (Silver Spring) 28, 371–378. doi: 10.1002/oby.22673
Bravo, P. E., Morse, S., Borne, D. M., Aguilar, E. A., and Reisin, E. (2006). Leptin and hypertension in obesity. Vasc. Health Risk Manag. 2, 163–169. doi: 10.2147/vhrm.2006.2.2.163
Brook, R. D. (2006). Obesity, weight loss, and vascular function. Endocrine 29, 21–26. doi: 10.1385/endo:29:1:21
Cercato, C., and Fonseca, F. A. (2019). Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 11:74. doi: 10.1186/s13098-019-0468-0
Choi, J. Y. (2012). Prevalence of overweight and obesity among US immigrants: results of the 2003 new immigrant survey. J. Immigr. Minor. Health 14, 1112–1118. doi: 10.1007/s10903-011-9560-8
Davison, K., Bircher, S., Hill, A., Coates, A. M., Howe, P. R., and Buckley, J. D. (2010). Relationships between obesity, cardiorespiratory fitness, and cardiovascular function. J. Obes. 2010:191253. doi: 10.1155/2010/191253
Gray, B. J., Stephens, J. W., Williams, S. P., Davies, C. A., Turner, D., and Bracken, R. M. (2015). Cardiorespiratory fitness is a stronger indicator of cardiometabolic risk factors and risk prediction than self-reported physical activity levels. Diab. Vasc. Dis. Res. 12, 428–435. doi: 10.1177/1479164115599907
Guiraudou, M., Varlet-Marie, E., Raynaud de Mauverger, E., and Brun, J. F. (2013). Obesity-related increase in whole blood viscosity includes different profiles according to fat localization. Clin. Hemorheol. Microcirc. 55, 63–73. doi: 10.3233/ch-131690
Hoelscher, D. M., Butte, N. F., Barlow, S., Vandewater, E. A., Sharma, S. V., Huang, T., et al. (2015). Incorporating primary and secondary prevention approaches to address childhood obesity prevention and treatment in a low-income, ethnically diverse population: study design and demographic data from the Texas childhood obesity research demonstration (TX CORD) study. Child. Obes. 11, 71–91. doi: 10.1089/chi.2014.0084
Jarolimova, J., Tagoni, J., and Stern, T. A. (2013). Obesity: its epidemiology, comorbidities, and management. Prim. Care Companion CNS Disord. 15:PCC.12f01475. doi: 10.4088/PCC.12f01475
Jung, K., Kim, J., Park, H. Y., Jung, W. S., and Lim, K. (2020a). Hypoxic Pilates intervention for obesity: a randomized controlled trial. Int. J. Environ. Res. Public Health 17:7186. doi: 10.3390/ijerph17197186
Jung, K., Seo, J., Jung, W. S., Kim, J., Park, H. Y., and Lim, K. (2020b). Effects of an acute Pilates program under hypoxic conditions on vascular endothelial function in pilates participants: a randomized crossover trial. Int. J. Environ. Res. Public Health 17:2584. doi: 10.3390/ijerph17072584
Kim, S. W., Jung, W. S., Park, W., and Park, H. Y. (2019). Twelve weeks of combined resistance and aerobic exercise improves cardiometabolic biomarkers and enhances red blood cell hemorheological function in obese older men: a randomized controlled trial. Int. J. Environ. Res. Public Health 16:5020. doi: 10.3390/ijerph16245020
Kodama, S., Saito, K., Tanaka, S., Maki, M., Yachi, Y., Asumi, M., et al. (2009). Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301, 2024–2035. doi: 10.1001/jama.2009.681
Lee, H., Na, W., Lee, S. B., Ahn, C. W., Moon, J. S., Won, K. C., et al. (2019). Potential diagnostic hemorheological indexes for chronic kidney disease in patients with type 2 diabetes. Front. Physiol. 10:1062. doi: 10.3389/fphys.2019.01062
Meeuwsen, S., Horgan, G. W., and Elia, M. (2010). The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin. Nutr. 29, 560–566. doi: 10.1016/j.clnu.2009.12.011
Miyashita, M., Mutoh, Y., Yoshika, Y., and Sadamoto, T. (1985). Effects of physical training. Med. Sci. Sports 10, 3–5.
Mohanty, S. S., and Mohanty, P. K. (2021). Obesity as potential breast cancer risk factor for postmenopausal women. Genes Dis. 8, 117–123. doi: 10.1016/j.gendis.2019.09.006
Nam, G. E., Kim, Y. H., Han, K., Jung, J. H., Rhee, E. J., Lee, S. S., et al. (2020). Obesity fact sheet in Korea, 2019: prevalence of obesity and abdominal obesity from 2009 to 2018 and social factors. J. Obes. Metab. Syndr. 29, 124–132. doi: 10.7570/jomes20058
Nammi, S., Koka, S., Chinnala, K. M., and Boini, K. M. (2004). Obesity: an overview on its current perspectives and treatment options. Nutr. J. 3:3. doi: 10.1186/1475-2891-3-3
Ne, J. Y. A., Cai, T. Y., Celermajer, D. S., Caterson, I. D., Gill, T., Lee, C. M. Y., et al. (2017). Obesity, arterial function and arterial structure - a systematic review and meta-analysis. Obes. Sci. Pract. 3, 171–184. doi: 10.1002/osp4.108
Nyangasa, M. A., Buck, C., Kelm, S., Sheikh, M. A., Brackmann, K. L., and Hebestreit, A. (2019). Association between cardiometabolic risk factors and body mass index, waist circumferences and body fat in a Zanzibari cross-sectional study. BMJ Open 9:e025397. doi: 10.1136/bmjopen-2018-025397
Park, W., Jung, W. S., Hong, K., Kim, Y. Y., Kim, S. W., and Park, H. Y. (2020b). Effects of moderate combined resistance- and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int. J. Environ. Res. Public Health 17. doi: 10.3390/ijerph17197233
Park, H. Y., Jung, W. S., Kim, J., Hwang, H., and Lim, K. (2019a). Changes in the paradigm of traditional exercise in obesity therapy and application of a new exercise modality: a narrative review article. Iran. J. Public Health 48, 1395–1404. doi: 10.18502/ijph.v48i8.2978
Park, H. Y., Jung, W. S., Kim, J., Hwang, H., and Lim, K. (2020a). Twelve weeks of aerobic exercise at the lactate threshold improves autonomic nervous system function, body composition, and aerobic performance in women with obesity. J. Obes. Metab. Syndr. 29, 67–75. doi: 10.7570/jomes19063
Park, H. Y., Jung, W. S., Kim, J., and Lim, K. (2019b). Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: a randomized controlled trial. Geriatr Gerontol Int 19, 311–316. doi: 10.1111/ggi.13625
Park, H. Y., Jung, W. S., Kim, S. W., and Lim, K. (2021b). Relationship between sarcopenia, obesity, osteoporosis, and cardiometabolic health conditions and physical activity levels in Korean older adults. Front. Physiol. 12:706259. doi: 10.3389/fphys.2021.706259
Park, H. Y., Jung, H., Lee, S., Kim, J. W., Cho, H. L., and Nam, S. S. (2021a). Estimated artificial neural network modeling of maximal oxygen uptake based on multistage 10-m shuttle run test in healthy adults. Int. J. Environ. Res. Public Health 18:8510. doi: 10.3390/ijerph18168510
Park, H. Y., Kim, J., Park, M. Y., Chung, N., Hwang, H., Nam, S. S., et al. (2018). Exposure and exercise training in hypoxic conditions as a new obesity therapeutic modality: a mini review. J. Obes. Metab. Syndr. 27, 93–101. doi: 10.7570/jomes.2018.27.2.93
Ranasinghe, C., Gamage, P., Katulanda, P., Andraweera, N., Thilakarathne, S., and Tharanga, P. (2013). Relationship between body mass index (BMI) and body fat percentage, estimated by bioelectrical impedance, in a group of Sri Lankan adults: a cross sectional study. BMC Public Health 13:797. doi: 10.1186/1471-2458-13-797
Ross, R., Blair, S. N., Arena, R., Church, T. S., Després, J. P., Franklin, B. A., et al. (2016). Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134, e653–e699. doi: 10.1161/cir.0000000000000461
Schwartz, M. W., Seeley, R. J., Zeltser, L. M., Drewnowski, A., Ravussin, E., Redman, L. M., et al. (2017). Obesity pathogenesis: an Endocrine Society scientific statement. Endocr. Rev. 38, 267–296. doi: 10.1210/er.2017-00111
Shin, S., Yang, Y., and Suh, J. S. (2009). Measurement of erythrocyte aggregation in a microchip stirring system by light transmission. Clin. Hemorheol. Microcirc. 41, 197–207. doi: 10.3233/ch-2009-1172
Simonds, S. E., Pryor, J. T., Ravussin, E., Greenway, F. L., Dileone, R., Allen, A. M., et al. (2014). Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416. doi: 10.1016/j.cell.2014.10.058
Son, W. M., Kim, D. Y., Kim, Y. S., and Ha, M. S. (2017). Effect of obesity on blood pressure and arterial stiffness in middle-aged Korean women. Osong. Public Health Res. Perspect. 8, 369–372. doi: 10.24171/j.phrp.2017.8.6.02
Soriano-Maldonado, A., Aparicio, V. A., Félix-Redondo, F. J., and Fernández-Bergés, D. (2016). Severity of obesity and cardiometabolic risk factors in adults: sex differences and role of physical activity. The HERMEX study. Int. J. Cardiol. 223, 352–359. doi: 10.1016/j.ijcard.2016.07.253
Tang, B., Luo, F., Zhao, J., Ma, J., Tan, I., Butlin, M., et al. (2020). Relationship between body mass index and arterial stiffness in a health assessment Chinese population. Medicine 99:e18793. doi: 10.1097/md.0000000000018793
Taylor, R. W., Keil, D., Gold, E. J., Williams, S. M., and Goulding, A. (1998). Body mass index, waist girth, and waist-to-hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am. J. Clin. Nutr. 67, 44–49. doi: 10.1093/ajcn/67.1.44
Uyuklu, M., Cengiz, M., Ulker, P., Hever, T., Tripette, J., Connes, P., et al. (2009). Effects of storage duration and temperature of human blood on red cell deformability and aggregation. Clin. Hemorheol. Microcirc. 41, 269–278. doi: 10.3233/ch-2009-1178
Wang, Y., Beydoun, M. A., Liang, L., Caballero, B., and Kumanyika, S. K. (2008). Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity 16, 2323–2330. doi: 10.1038/oby.2008.351
Wiewióra, M., Sosada, K., Slowinska, L., Piecuch, J., Glück, M., Zurawinski, W., et al. (2010). Sex-dependent differences in rheological properties and the relation of blood viscosity to erythrocyte aggregation indices among morbidly obese patients. Clin. Hemorheol. Microcirc. 44, 259–267. doi: 10.3233/ch-2010-1275
Keywords: obesity, vascular function, cardiometabolic parameters, hemorheological function, cardiorespiratory fitness
Citation: Park H-Y, Jung W-S, Kim S-W, Jung K and Lim K (2022) Comparison of Vascular Function, Cardiometabolic Parameters, Hemorheological Function, and Cardiorespiratory Fitness Between Middle-Aged Korean Women With and Without Obesity—A Pilot Study. Front. Physiol. 13:809029. doi: 10.3389/fphys.2022.809029
Received: 04 November 2021; Accepted: 22 February 2022;
Published: 29 March 2022.
Edited by:
Martin Burtscher, University of Innsbruck, AustriaReviewed by:
Daroonwan Suksom, Chulalongkorn University, ThailandCopyright © 2022 Park, Jung, Kim, Jung and Lim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiwon Lim, ZXhlcmNpc2VAa29ua3VrLmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.