- The First Endocrine Department of Shengjing Hospital of China Medical University, Shenyang, China
Objective: To elucidate the relationship between CYP17A1/CYP19A1/SHBG gene polymorphisms and PCOS susceptibility.
Methods: We searched multiple databases from inception to December 2020 and meta analysis was conducted to elucidate the relationship between gene polymorphisms and PCOS risk.
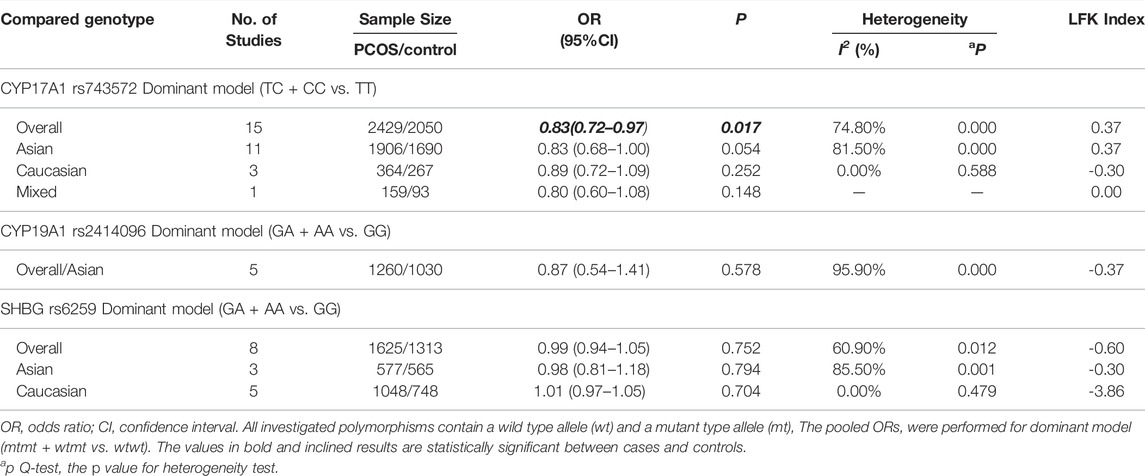
Results: 26 studies were included, comprising 4860 PCOS and 4043 controls. CYP17A1 rs743572 polymorphisms were found to be negatively associated with PCOS risk under dominant model (p = 0.017, OR = 0.83, 95%CI 0.72–0.97, I2 = 74.80%, Pheterogeneity = 0.000) in the general population while neither CYP19A1 rs2414096 polymorphisms (p = 0.578, OR = 0.87, 95%CI 0.54–1.41, I2 = 95.90%, Pheterogeneity = 0.000) nor SHBG rs6529 polymorphisms (p = 0.752, OR = 0.99, 95%CI 0.94–1.05, I2 = 60.90%, Pheterogeneity = 0.012) was associated with PCOS susceptibility under dominant model in the general population.
Conclusion: CYP17A1 rs7435721 polymorphisms might be protective factors against PCOS in general populations.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#myprospero, identifier CRD4202122640.
Introduction
Polycystic ovary syndrome (PCOS) is a common reproductive endocrine disorder that occurs in approximately 5–10% of the women of childbearing age (Goodarzi et al., 2011; Dadachanji et al., 2018). PCOS is typically characterized by chronic anovulation, elevated androgen levels, a distorted luteinizing hormone/follicle-stimulating hormone (LH/FSH) ratio, irregular menstrual cycle, appearance of polycystic ovaries, and insulin resistance (Goodarzi et al., 2011).
Hyperandrogenemia is a key indicator of PCOS, and increased androgen concentrations including testosterone (T) and androstenedione have been observed in most patients, while increased dehydroepiandrosterone sulfate has been observed in a minority [∼25%] of the patients (Livadas et al., 2014). Androgens are produced in the ovaries and adrenal glands as the final products of a series of enzymatic reactions involving the conversion of cholesterol into dehydroepiandrosterone and androstenedione. In both locations, the rate of sex steroid synthesis is limited by certain crucial enzymes (Wawrzkiewicz-Jałowiecka et al., 2020). The heterogeneity of the androgen phenotype in the steroid synthesis pathway may be attributed to differences in the activity of important enzymes; for example, in hyperandrogenic PCOS patients, the activities of 17 and 20 lyases and 3β-hydroxysteroid dehydrogenase II (3β-HSD) increase, while aromatase activity bound to the Δ4 pathway decreases (de Medeiros et al., 2015). Mutations in steroid pathway genes, such as CYP1A, CYP19, CYP17, CYP3, CYP11, and CYP21, may affect androgen synthesis (de Medeiros et al., 2015; Ajmal et al., 2019). As a transporter of sex hormones, sex hormone binding globulin (SHBG), produced in the liver, combines with circulating steroids with a high affinity to regulate the bioavailability and concentration of bioactive sex hormones in the blood (Hammond et al., 2012). As SHBG shows a high affinity for T and low affinity for estradiol, it can effectively regulate the levels of bioactive free T in the body (Somboonporn and Davis, 2004). T has no biological effect when combined with SHBG, and only approximately 1–2% of the total T has biological activity in normal women. Therefore, SHBG can be used to judge the severity of hyperandrogenemia and evaluate the therapeutic effect in PCOS women (Zhu et al., 2019).
Previous genetic association studies have found that a large number of genetic variations are related to PCOS susceptibility, and genetic factors may greatly impact the occurrence of PCOS (Saddick, 2020). Single nucleotide polymorphisms (SNPs) might reveal functional changes caused by amino acid variation or gene expression regulation. Candidate gene investigations provide insight into different frequency distributions in healthy and diseased populations (Douma et al., 2019); however, previous studies exploring the potential relationship between PCOS susceptibility and CYP17A1/CYP19A1/SHBG gene polymorphisms used statistically insufficient samples sizes and reported inconsistent findings (Li et al., 2012; Liao and Cao, 2020; Li et al., 2021; Sharma et al., 2021). Thus, The search acronym (PICO) for our meta-analysis to elucidate the relationship between CYP17A1/CYP19A1/SHBG gene polymorphisms in both wild type and mutant type and PCOS susceptibility in general populations in a larger study cohort.
Materials and Methods
Literature Search
This meta-analysis was registered with the PROSPERO international prospective register of systematic reviews (registration number CRD42021226402). We searched medical literature for relevant studies using PubMed, EMBASE, the Cochrane Library, Web of Science, WanFang Database, and China National Knowledge Infrastructure (CNKI) from their date of establishment to December 2020. A total of 463 records were identified using electronic search strategies; in addition, one relevant record was obtained from the reference lists of the included studies. We used the search terms: “Steroid 17-alpha-Hydroxylase or CYP17,” “aromatase or CYP19,” “sex hormone binding globulin or SHBG,” “Single nucleotide polymorphism (SNP) or polymorphisms or genotype or genetic or mutation or variant,” and “Polycystic ovary syndrome or PCOS.” We limited the publication type to case-control studies, and there were no language or location restrictions. We also tried to search in grey literature, but no new relevant cohort studies were found.
Inclusion and Exclusion Criteria
To be included in this meta-analysis, the studies needed to meet the following inclusion criteria: (a) originated from a case-control study design; (b) reported the cases of PCOS patients diagnosed with one of the following three diagnostic criteria: National Institutes of Health (NIH) 1999, Rotterdam 2003, and AE-PCOS Society 2006; (c) evaluated the association between SHBG or CYP17 or CYP19 gene polymorphisms and PCOS; (d) reported odds ratios (ORs) and corresponding 95% confidence intervals (CIs) or provided the distribution of sufficient genotypic and allelic data for estimation in cases and controls.
Exclusion criteria included the following: (a) insufficient data on genotyping; (b) no control population; (c) study included disorders other than PCOS; (d) case reports, case series, abstracts, reviews, meta-analysis, comments, editorial articles, or letters without original data; (e) duplicate data; (f) the SNP was reported in less than 5 case-control study.
Data Extraction and Quality Assessment
For published articles, two investigators (CX and HZ) independently extracted data and assessed methodological quality. Data were extracted from the included studies to collect the following necessary information: first author, year of publication, country of origin, ethnicity, number of cases and controls, PCOS diagnostic criteria, genotype method, genotype data, and evidence of Hardy-Weinberg equilibrium (HWE). Genotype data of case and control studies were extracted to calculate the OR with 95% CI and p-value of HWE in the control group. As described elsewhere, quality, internal validity, and risk of bias of the included studies were assessed using the validated quality of genetic association studies checklist (Q-Genie) (Santana et al., 2020). The Q-genie tool consists of 11 questions, which address the following aspects of study methodologies: study rationale, outcome, comparability, exposure, bias, sample size, analyses, statistical methods and control for confounding, inferences for genetic analyses, and inferences drawn from results. Each question has seven possible answers as follows: “1 (poor),” “2,” “3 (good),” “4,” “5 (very good),” “6,” “7 (excellent).” The overall quality of studies is classified as “poor quality” if score is <35, a score of 36–45 indicates “moderate quality” and a score of >45 indicates “good quality.” Discrepancies were discussed or adjudicated with the third author (H.B.), until a satisfactory consensus was reached.
Statistical Analysis
Pooled ORs with corresponding 95% CIs were calculated to identify the potential association between susceptibility to PCOS and gene polymorphisms for the following genotypic models: dominant model (mtmt + wtmt vs. wtwt), recessive model (mtmt vs. wtwt + wtmt), co-dominant model (mtmt vs. wtwt) or (wtmt vs. wtwt), and complete overdominant model (mtmt + wtwt vs. wtmt) for rs743572 of CYP17 gene polymorphisms, rs2414096 of CYP19 gene polymorphisms, rs6529 of SHBG gene polymorphisms. In order to synthesize the best genetic model, we refer to the method of screening the best genetic model in the study of Thakkinstian et al. (2005). Data from each study is extracted as the number of subjects with each genotype (AA, Aa, and aa) in the case and control groups. The gene effects of each study are defined as OR, which are OR1, OR2, and OR3 for AA versus aa, Aa versus aa, and AA versus Aa, respectively. The pooled ORs are calculated by the inverse variance method. To determine the overall gene effect, the model that includes gene is compared with the model without gene. If the overall gene effect is statistically significant, further comparisons of OR1, OR2, and OR3 are explored. These comparisons should be performed using residual variance obtained from the regression model. These pairwise differences can be used to indicate the most appropriate genetic model, as outlined below (assuming that the risk allele is A): (a) If OR1 = OR3 = 1 and OR2 = 1, then a recessive model is suggested; (b) If OR1 = OR2 = 1 and OR3 = 1, then a dominant model is suggested; (c) If OR2 = 1 = OR3 = 1 and OR1 = 1, then a complete overdominant model is suggested; (d) If OR1 > OR2 > 1 and OR1 > OR3 > 1 (or OR1 < OR2 < 1 and OR1 < OR3 < 1), then a co-dominant model is suggested. The heterogeneity between the included studies was estimated using the Q test and the I-squared statistic. Values of p < 0.1 and I2 > 50% were considered to indicate significant heterogeneity (Higgins et al., 2003), using of the quality effects models. Subgroup analysis was performed for gene with more than five different studies according to PCOS, control, and ethnicity. LFK index and Doi plots were used to statistically assess publication bias (Furuya-Kanamori et al., 2018). For all tests, p < 0.05 was considered statistically significant. Stata version 16.0 (Stata Corp, College Station, TX) was used to perform the statistical analysis.
Results
Literature Identification
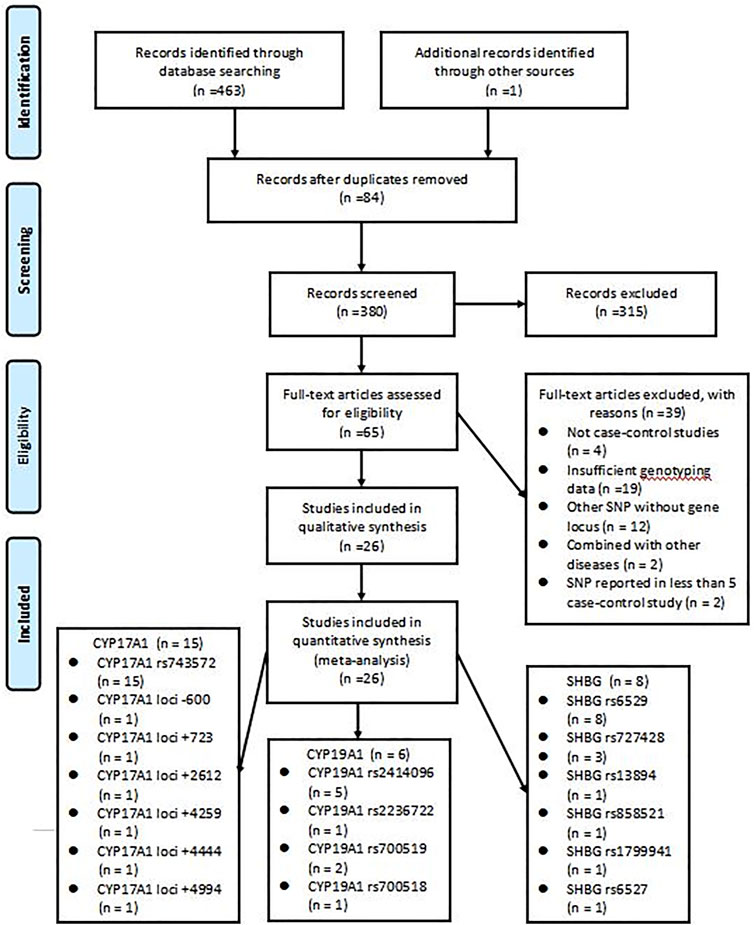
The initial search strategy retrieved a total of 464 studies from all electronic databases. After reading the titles, abstracts, and full texts, we selected 26 studies (Diamanti-Kandarakis et al., 1999; Marszalek et al., 2001; Cousin et al., 2004; Kahsarmiller et al., 2004; Tan et al., 2005; Bendlová et al., 2007; Ferk et al., 2007; Echiburú et al., 2008; Park et al., 2008; Jin et al., 2009; Unsal et al., 2009; Martinez-Garcia et al., 2012; Dasgupta et al., 2014; Dai et al., 2015; Li et al., 2015; Abu-Hijleh et al., 2016; Banerjee et al., 2016; Mehdizadeh et al., 2017; Wu et al., 2017; Jiao et al., 2018; Kaur et al., 2018; Bhatnager et al., 2019; Liu et al., 2019; Rahimi and Mohammadi, 2019; Munawar Lone et al., 2020; Ashraf et al., 2021), which included a total of 8,903 women, for inclusion in the analysis. The remaining studies were excluded: 84 were duplicates, 315 were of apparent irrelevance based on a review of abstracts and titles, four were not case-control studies, 19 lacked sufficient genotyping data, 12 included other SNPs without gene locus, two were combined with other diseases, and two had SNPs reported in less than 5 case-control study. The literature screening process and results are shown in Figure 1, followed the PRISMA flowchart.
Characteristics of Included Studies
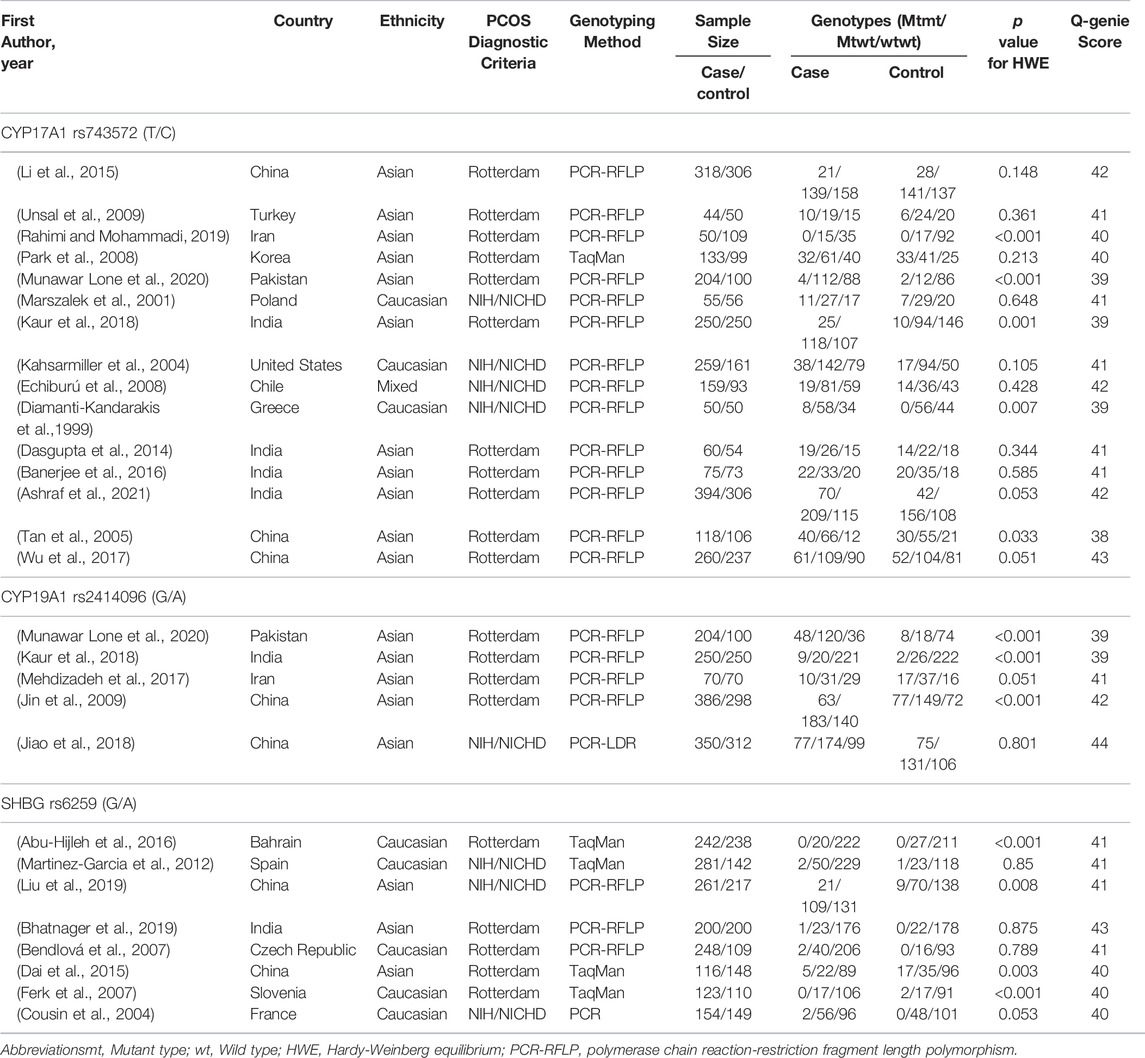
Table 1 presents the characteristics of included studies. During the search no language restriction was applied. Trials were performed in European, American, and Asian countries and were published from 1999 to 2020. Participants in these trials were recruited from outpatient clinics, hospitals, or medical centers and were diagnosed with PCOS according to either the NIH or Rotterdam criteria. Participants aged 18–49 years were included in all 26 studies exploring the association between CYP17A1 (Diamanti-Kandarakis et al., 1999; Marszalek et al., 2001; Kahsarmiller et al., 2004; Tan et al., 2005; Echiburú et al., 2008; Park et al., 2008; Unsal et al., 2009; Dasgupta et al., 2014; Li et al., 2015; Banerjee et al., 2016; Wu et al., 2017; Kaur et al., 2018; Rahimi and Mohammadi, 2019; Munawar Lone et al., 2020; Ashraf et al., 2021), CYP19A1 (Jin et al., 2009; Mehdizadeh et al., 2017; Jiao et al., 2018; Kaur et al., 2018; Munawar Lone et al., 2020), and SHBG (Cousin et al., 2004; Bendlová et al., 2007; Ferk et al., 2007; Martinez-Garcia et al., 2012; Dai et al., 2015; Abu-Hijleh et al., 2016; Bhatnager et al., 2019; Liu et al., 2019) gene polymorphisms and PCOS risk. There were 15 studies of CYP17A1 gene polymorphisms, including 15 studies of CYP17A1 rs743572 and one study of CYP17A1 loci -600, CYP17A1 loci +723, CYP17A1 loci +2612, CYP17A1 loci +4259, CYP17A1 loci +4444 and CYP17A1 loci +4994; there were six studies of CYP19A1 gene polymorphisms, including five studies of CYP19A1 rs2414096, one study of CYP19A1 rs2236722, two studies of CYP19A1 rs700519 and one study of CYP19A1 rs700518; there were eight studies of SHBG gene polymorphisms, including eight studies of SHBG rs6529, three studies of SHBG rs727428, one study of SHBG rs13894, one study of SHBG rs858521, one study of SHBG rs1799941 and one study of SHBG rs6527. There were 17 studies of Asian patients (Tan et al., 2005; Park et al., 2008; Jin et al., 2009; Unsal et al., 2009; Dasgupta et al., 2014; Dai et al., 2015; Li et al., 2015; Banerjee et al., 2016; Mehdizadeh et al., 2017; Wu et al., 2017; Jiao et al., 2018; Kaur et al., 2018; Bhatnager et al., 2019; Liu et al., 2019; Rahimi and Mohammadi, 2019; Munawar Lone et al., 2020; Ashraf et al., 2021), eight studies of Caucasian patients (Diamanti-Kandarakis et al., 1999; Marszalek et al., 2001; Cousin et al., 2004; Kahsarmiller et al., 2004; Bendlová et al., 2007; Ferk et al., 2007; Martinez-Garcia et al., 2012; Abu-Hijleh et al., 2016), and one study of mixed patients (Echiburú et al., 2008), which included 4860 PCOS cases and 4043 controls overall. All studies extracted DNA from peripheral blood, and PCR, PCR-RFLP, PCR-LDR, and TaqMan assays were used. The genotype distributions among the controls of all studies were consistent with HWE in most studies. With regard to the quality of the included studies, the Q-Genie scores were of medium quality, with an average Q-Genie score of 40.88 (range 38–44).
Quantitative Analysis
For a quantitative analysis, at least two studies are required for each polymorphism. For there were few cohort studies on specific loci polymorphisms (such as only one study on CYP17A1 loci -600, CYP17A1 loci +723, CYP17A1 loci +2612, CYP17A1 loci +4259, CYP17A1 loci +4444, CYP17A1 loci +4994, CYP19A1 rs2236722, CYP19A1 rs700518, SHBG rs13894, SHBG rs858521, SHBG rs1799941 and SHBG rs6527; two studies on CYP19A1 rs700519; three studies on SHBG rs727428), to make the conclusions more reliable, we only included loci with no less than five cohort studies for quantitative analysis.
Association Between the CYP17A1 (rs743572) Polymorphisms and PCOS Risk
In the current meta-analysis, 15 case-control studies involving 2429 PCOS patients and 2050 control women were included to estimate the relationship between the CYP17A1 (rs743572) polymorphisms and PCOS risk. As shown in Table 2, the CYP17A1 rs743572 polymorphisms was found to be negatively associated with PCOS risk under dominant model (p = 0.017, OR = 0.83, 95%CI 0.72–0.97, I2 = 74.80%, Pheterogeneity = 0.000) in the general population. In subgroup analyses by ethnicity, however, no significant association between the CYP17A1 rs743572 polymorphisms and the PCOS susceptibility was found in the Asian (p = 0.054, OR = 0.83, 95%CI 0.68–1.00), Caucasian (p = 0.252, OR = 0.87, 95%CI 0.72–1.09) and mixed (p = 0.148, OR = 0.80, 95%CI 0.60–1.08) population. We evaluated the overall potential publication biases with LFK index = 0.37 and Doi plots showing no asymmetry (Figure 2A).
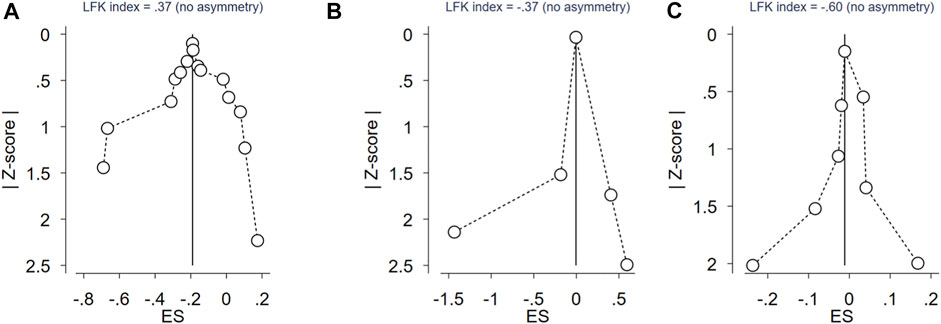
FIGURE 2. Doi plot of rs743572 of CYP17 gene polymorphisms, rs2414096 of CYP19 gene polymorphisms, rs6529 of SHBG gene polymorphisms and PCOS risk. (A) Doi plot of rs743572 of CYP17 gene polymorphisms and PCOS risk; (B) Doi plot of rs2414096 of CYP19 gene polymorphisms and PCOS risk; (C) Doi plot of rs6529 of SHBG gene polymorphisms and PCOS risk.
Association Between the CYP19A1 (rs2414096) Polymorphisms and PCOS Risk
In the current meta-analysis, five case-control studies involving 1260 PCOS patients and 1030 control women were included to estimate the relationship between the CYP19A1 (rs2414096) polymorphisms and PCOS risk. All of the five studies were conducted in the Asian population (Table 2), no significant association between the CYP19A1 rs2414096 polymorphisms and the PCOS susceptibility was found under dominant model (p = 0.578, OR = 0.87, 95%CI 0.54–1.41, I2 = 95.90%, Pheterogeneity = 0.000). We evaluated the overall potential publication biases with LFK index = -0.37 and Doi plots showing no asymmetry (Figure 2B).
Association Between the SHBG (rs6529) Polymorphisms and PCOS Risk
In the current meta-analysis, we explored the relationship between the SHBG (rs6529) polymorphisms and PCOS risk. As shown in Table 2, for SHBG rs6529 polymorphisms and the PCOS susceptibility, eight case-control studies involving 1625 PCOS patients and 1313 control women were included, with no significant association found under dominant model (p = 0.752, OR = 0.99, 95%CI 0.94–1.05, I2 = 60.90%, Pheterogeneity = 0.012) in the general population. In subgroup analyses by ethnicity, no significant association between the SHBG rs6529 polymorphisms and the risk of PCOS susceptibility was found in the Asian (p = 0.794, OR = 0.98, 95%CI 0.81–1.18) and Caucasian (p = 0.704, OR = 1.01, 95%CI 0.97–1.05) population. The overall potential publication biases were evaluated with LFK index = -0.60 and Doi plots showing no asymmetry (Figure 2C).
Discussion
Our meta-analysis included 4860 PCOS patients and 4043 control participants from 26 case-control studies to evaluate CYP17A1, CYP19A1, and SHBG gene polymorphisms. The results showed that CYP17A1 rs743572 polymorphisms were found to be negatively associated with PCOS risk under dominant model in the general population while neither CYP19A1 rs2414096 polymorphisms nor SHBG rs6529 polymorphisms was associated with PCOS susceptibility under dominant model in the general population.
The CYP17A1 gene encodes a cytochrome P450 enzyme on chromosome 10q24.3. The enzyme converts pregnenolone and progesterone into 17-hydroxyprogesterone and 17-hydroxyprogesterone, respectively, through the activity of 17-hydroxylase. These steroids are converted to dehydroepiandrosterone (DHEA) and 4-androstenedione through the activity of 17, 20-lyase (Mykhalchenko et al., 2017). An additional Sp1 transcription factor binding site is produced at position-34 (- 34T/C) of the promoter, which regulates the expression of CYP17A1 and thus the androgen levels (Sharp et al., 2004). Many studies have explored the relationship between CYP17A1 gene polymorphisms and PCOS risk, and all of them have focused on the T > C polymorphisms in the promoter region. Pusalkar et al. showed that the frequency of the C allele increases in Indian women with PCOS, which may affect their hyperandrogenic phenotype (Pusalkar et al., 2009). However, a meta-analysis published by Li et al., in 2012 showed that in the overall analyses and some subgroup analyses (by race and country), the CYP17A1 rs743572 T > C polymorphisms were not associated with PCOS risk, but a significant increase in the PCOS risk was observed in studies within HWE and in small sample studies (Li et al., 2012). Compared to the study by Li et al., our meta-analysis included more case-control studies and confirmed that the CYP17A1 rs743572 gene polymorphisms were negatively associated with the risk of PCOS under dominant model. In the current study, neither LFK index nor Doi plots showed a publication bias.
The CYP19A1 gene is located on chromosome 15q21.2 and encodes aromatase P450, which plays an important role in the synthesis of estrogens from androgens (Dadachanji et al., 2018). Aromatase activity is less in thin and obese women with PCOS, which may be further inhibited by hyperandrogenemia (Somboonporn and Davis, 2004). The hyperandrogenic follicular environment may be a key factor leading to the downregulation of the expression of aromatase in luteinized granulosa cells in women with PCOS (Yang et al., 2015). One study demonstrated hypermethylation of the promoter and decreased levels of CYP19A1 mRNA and protein in PCOS ovaries, which indicated that the expression of aromatase was inhibited (Yu et al., 2013). However, some results are contradictory. A meta-analysis by Sharma et al. showed that there was a significant association between the CYP19A1 rs2414096 gene polymorphisms and PCOS risk in non-Indian populations, while no association was found in Indian populations (Sharma et al., 2021). However, our meta-analysis found no significant association between the CYP19A1 rs2414096 gene polymorphisms and PCOS risk under dominant model. Additionally, Rahimi et al (Park et al., 2008) and Kaur et al. (Kahsarmiller et al., 2004) also found that CYP19A1 rs2236722 and rs700519 did not show significant association with PCOS. As there are few studies on CYP19A1 gene polymorphisms and all of them are on Asian people, studies at a larger scale with different ethnicities are required to confirm the relationship between CYP19 gene polymorphisms and PCOS risk in the future.
SHBG is mainly synthesized in the liver, binding androgens in a high-fidelity manner, and thereby making it biologically inaccessible to target tissues. Several polymorphisms of the SHBG gene on chromosome 17 have been shown to alter SHBG liver biosynthesis, plasma levels, and plasma clearance efficiency, thereby regulating the distribution of androgens (Hammond, 2016). In patients with PCOS, SHBG concentrations are usually low because of elevated androgen levels, and hyperandrogenemia promotes compensatory hyperinsulinemia and insulin resistance by increasing lipoprotein and reducing insulin clearance (Shorakae et al., 2018). Hyperinsulinemia and hyperandrogenemia can hinder the secretion and synthesis of SHBG in the liver (Lim et al., 2013). Women with an SHBG deficiency showed truncated SHBG synthesis and abnormal glycosylation, which led to a significant decrease in the SHBG levels and an increase in the circulating free T levels. The D327N (rs6259) SNP was the first reported SHBG gene polymorphism, and it was found to increase the half-life of the SHBG and decrease its clearance rate, resulting in an overall increase in SHBG concentration (Cousin et al., 1998). Several studies have explored the relationship between the SHBG rs6259 SNP and serum SHBG concentration and PCOS risk, but they present conflicting results (Cousin et al., 2004; Bendlová et al., 2007; Ferk et al., 2007; Martinez-Garcia et al., 2012; Dai et al., 2015; Abu-Hijleh et al., 2016; Bhatnager et al., 2019; Liu et al., 2019). Our results showed that SHBG rs6259 was not associated with PCOS risk, which is in agreement with the results of studies by Li et al. and Liao et al. (Liao and Cao, 2020; Li et al., 2021). Additionally, Liao et al. also found that a null link between the SHBG rs727428 polymorphism and the risk of PCOS was also found under any genetic models (Liao and Cao, 2020). Due to the limited sample size and a high degree of heterogeneity among study groups, the reliability and consistency of the results may be affected.
Our meta results confirmed that androgen synthesis related gene polymorphisms may affect ovarian function and thus affect the susceptibility of PCOS. Neuroendocrine dysfunction is a component of PCOS, mainly manifested by increased secretion of LH. LH excess associated with PCOS may be secondary to the peripheral events within the ovary, the mechanism of neuroendocrine dysfunction resulting in an elevated LH in PCOS may be an uncoupling of hypothalamic estradiol inhibition by elevated ovarian androstenedione, while elevated LH may cause hyperandrogenemia (Doi et al., 2005; Doi, 2008). Herein, we also tried to explore the relationship between serum LH level and androgen synthesis related gene polymorphism, but due to limited data, meta-analysis was not possible. Li (Li et al., 2015) and Wu (Wu et al., 2017) studied the relationship between serum LH and CYP17A1 gene polymorphisms, but reached a repulsive conclusion. Only Jin (Jin et al., 2009) studied the relationship between serum LH and CYP19A1 gene polymorphisms and found that in PCOS patients the serum LH of AA type was lower than that of GA or GG type.
As hyperandrogenemia is a key characteristics seen in PCOS patients, we focused on the CYP17A1 and CYP19A1 gene polymorphisms that affect androgen synthesis and the SHBG gene polymorphisms that affect SHBG synthesis to determine the relationship between gene polymorphisms and PCOS risk through a comprehensive literature search, so as to draw more comprehensive, accurate, and reliable conclusions. In addition, the studies that we included here were conducted in different countries and using different ethnic groups, making the pooled results more universal. Finally, according to the quality evaluation system, all the articles included in this study were of medium quality.
The present study has several limitations. First, in this meta-analysis, the combined OR was estimated by the number of genotypes or alleles in the case and control subjects, without adjusting for other confounding factors, which might cause bias. Second, although the study group was large, some subgroup analyses were performed with quite small sample sizes. Despite the sensitivity analysis, our results might still be affected by a type II error. Thus, future research encompassing a larger and more detailed sample set is necessary. Third, we did not search for gray literature, the publication bias might still affect the results of our meta-analysis. Fourth, some polymorphic studies have significant inter-study heterogeneity, and some studies have a deviation from the genotype distribution of the HWE. Finally, this meta-analysis does not discuss gene-environment or gene-gene interactions. However, the conclusions and limitations of this study provide some direction for the design of future studies.
Conclusion
In summary, this meta-analysis showed that CYP17A1 rs7435721 polymorphisms might serve as a protective factor against PCOS in general populations. Given that the pathogenesis of PCOS is extremely complex, the probability that specific gene polymorphisms could significantly contribute to its development is low, and we strongly recommend further studies with a larger sample size to comprehensively explore the potential roles of gene-gene and gene-environmental interactions in the development of PCOS.
Summary
CYP17A1 rs743572 polymorphisms were found to be negatively associated with PCOS risk under dominant model in the general population while neither CYP19A1 rs2414096 polymorphisms nor SHBG rs6529 polymorphisms was associated with PCOS susceptibility under dominant model in the general population. CYP17A1 rs7435721 polymorphisms in general populations might be protective factors against PCOS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
CX designed the research, collected data, analyzed data, and wrote the manuscript. CX and HZ screened and evaluated the literature. JZ collected materials. BH reviewed and edited the manuscript. All the authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81570765).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Hijleh T. M., Gammoh E., Al-Busaidi A. S., Malalla Z. H., Madan S., Mahmood N., et al. (2016). Common Variants in the Sex Hormone-Binding Globulin (SHBG) Gene Influence SHBG Levels in Women with Polycystic Ovary Syndrome. Ann. Nutr. Metab. 68, 66–74. doi:10.1159/000441570
Ajmal N., Khan S. Z., Shaikh R. (2019). Polycystic Ovary Syndrome (PCOS) and Genetic Predisposition: A Review Article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 3, 100060. doi:10.1016/j.eurox.2019.100060
Ashraf S., Rasool S. U. A., Nabi M., Ganie M. A., Jabeen F., Rashid F., et al. (2021). CYP17 Gene Polymorphic Sequence Variation Is Associated with Hyperandrogenism in Kashmiri Women with Polycystic Ovarian Syndrome. Gynecol. Endocrinol. 37, 230–234. doi:10.1080/09513590.2020.1770724
Banerjee U., Dasgupta A., Khan A., Ghosh M., Roy P., Rout J., et al. (2016). A Cross-Sectional Study to Assess Any Possible Linkage of C/T Polymorphism in CYP17A1 Gene with Insulin Resistance in Non-obese Women with Polycystic Ovarian Syndrome. Indian J. Med. Res. 143, 739–747. doi:10.4103/0971-5916.191990
Bendlová B., Zavadilová J., Vaňková M., Vejražková D., Lukášová P., Včelák J., et al. (2007). Role of D327N Sex Hormone-Binding Globulin Gene Polymorphism in the Pathogenesis of Polycystic Ovary Syndrome. J. Steroid Biochem. Mol. Biol. 104, 68–74. doi:10.1016/j.jsbmb.2006.10.002
Bhatnager R., Senwal A., Nanda S., Dang A. S. (2019). Association of Rs6259 Polymorphism with SHBG Levels and Poly Cystic Ovary Syndrome in Indian Population: a Case Control Study. Mol. Biol. Rep. 46, 2131–2138. doi:10.1007/s11033-019-04665-2
Cousin P., Calemard-Michel L., Lejeune H., Raverot G., Yessaad N., Emptoz-Bonneton A., et al. (2004). Influence ofSHBGGene Pentanucleotide TAAAA Repeat and D327N Polymorphism on Serum Sex Hormone-Binding Globulin Concentration in Hirsute Women. J. Clin. Endocrinol. Metab. 89, 917–924. doi:10.1210/jc.2002-021553
Cousin P., Déchaud H., Grenot C., Lejeune H., Pugeat with the technical assistanc M., Baret C., et al. (1998). Human Variant Sex Hormone-Binding Globulin (SHBG) with an Additional Carbohydrate Chain Has a Reduced Clearance Rate in Rabbit1. J. Clin. Endocrinol. Metab. 83, 235–240. doi:10.1210/jcem.83.1.4515
Dadachanji R., Shaikh N., Mukherjee S. (2018). Genetic Variants Associated with Hyperandrogenemia in PCOS Pathophysiology. Genet. Res. Int. 2018, 1–12. doi:10.1155/2018/7624932
Dai J. F., Fan J., Sun B. Z. (2015). Asp327Asn Polymorphism in Patients with Polycystic Ovarian Syndrome. Acta Acad. Qindao Univ., 51, 157–162.
Dasgupta A., Banerjee U., Roy P., Khan A., Ghosh M., Chowdhuri K. M. (2014). Assessment of CYP 17 Gene Polymorphism in Subjects with Polycystic Ovarian Syndrome and Central Obesity in an Indian Subpopulation. Int. J. Hum. Genet. 14, 33–41. doi:10.1080/09723757.2014.11886225
de Medeiros S. F., Barbosa J. S., Yamamoto M. M. W. (2015). Comparison of Steroidogenic Pathways Among Normoandrogenic and Hyperandrogenic Polycystic Ovary Syndrome Patients and normal Cycling Women. J. Obstet. Gynaecol. Res. 41, 254–263. doi:10.1111/jog.12524
Diamanti-Kandarakis E., Bartzis M. I., Zapanti E. D., Spina G. G., Filandra F. A., Tsianateli T. C., et al. (1999). Polymorphism T→C (−34 Bp) of Gene CYP17 Promoter in Greek Patients with Polycystic Ovary Syndrome. Fertil. Sterility 71, 431–435. doi:10.1016/s0015-0282(98)00512-3
Doi S. A. R., Al-Zaid M., Towers P. A., Scott C. J., Al-Shoumer K. A. S. (2005). Ovarian Steroids Modulate Neuroendocrine Dysfunction in Polycystic Ovary Syndrome. J. Endocrinol. Invest. 28 (10), 882–892. doi:10.1007/BF03345319
Doi S. A. R. (2008). Neuroendocrine Dysfunction in PCOS: a Critique of Recent Reviews. Clin. Med. Res. 6 (2), 47–53. doi:10.3121/cmr.2008.796
Douma Z., Lautier C., Haydar S., Mahjoub T., Grigorescu F. (2019). Portability of Gwas Results between Ethnic Populations: Genetic Markers for Polycystic Ovary Syndrome (Pcos) in Mediterranean Area. Acta Endo (Buc) 15, 364–371. doi:10.4183/aeb.2019.364
Echiburú B., Pérez-Bravo F., Maliqueo M., Sánchez F., Crisosto N., Sir-Petermann T. (2008). Polymorphism T → C (−34 Base Pairs) of Gene CYP17 Promoter in Women with Polycystic Ovary Syndrome Is Associated with Increased Body Weight and Insulin Resistance: a Preliminary Study. Metabolism 57, 1765–1771. doi:10.1016/j.metabol.2008.08.002
Ferk P., Teran N., Gersak K. (2007). The (TAAAA)n Microsatellite Polymorphism in the SHBG Gene Influences Serum SHBG Levels in Women with Polycystic Ovary Syndrome. Hum. Reprod. 22, 1031–1036. doi:10.1093/humrep/del457
Furuya-Kanamori L., Barendregt J. J., Doi S. A. R. (2018). A New Improved Graphical and Quantitative Method for Detecting Bias in Meta-Analysis. Int. J. Evid. Based Healthc. 16 (4), 195–203. doi:10.1097/XEB.0000000000000141
Goodarzi M. O., Dumesic D. A., Chazenbalk G., Azziz R. (2011). Polycystic Ovary Syndrome: Etiology, Pathogenesis and Diagnosis. Nat. Rev. Endocrinol. 7, 219–231. doi:10.1038/nrendo.2010.217
Hammond G. L. (2016). Plasma Steroid-Binding Proteins: Primary Gatekeepers of Steroid Hormone Action. J. Endocrinol. 230, R13–R25. doi:10.1530/JOE-16-0070
Hammond G. L., Wu T.-S., Simard M. (2012). Evolving Utility of Sex Hormone-Binding Globulin Measurements in Clinical Medicine. Curr. Opin. Endocrinol. Diabetes Obes. 19, 183–189. doi:10.1097/MED.0b013e328353732f
Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327, 557–560. doi:10.1136/bmj.327.7414.557
Jiao X., Chen W., Zhang J., Wang W., Song J., Chen D., et al. (2018). Variant Alleles of the ESR1, PPARG, HMGA2, and MTHFR Genes Are Associated with Polycystic Ovary Syndrome Risk in a Chinese Population: A Case-Control Study. Front. Endocrinol. 9, 504. doi:10.3389/fendo.2018.00504
Jin J.-L., Sun J., Ge H.-J., Cao Y.-X., Wu X.-K., Liang F.-J., et al. (2009). Association between CYP19 Gene SNP Rs2414096 Polymorphism and Polycystic Ovary Syndrome in Chinese Women. BMC Med. Genet. 10, 139. doi:10.1186/1471-2350-10-139
Kahsarmiller M., Boots L., Bartolucci A., Azziz R. (2004). Role of a CYP17 Polymorphism in the Regulation of Circulating Dehydroepiandrosterone Sulfate Levels in Women with Polycystic Ovary Syndrome. Fertil. Sterility 82, 973–975. doi:10.1016/j.fertnstert.2004.05.068
Kaur R., Kaur T., Kaur A. (2018). Genetic Association Study from North India to Analyze Association of CYP19A1 and CYP17A1 with Polycystic Ovary Syndrome. J. Assist. Reprod. Genet. 35, 1123–1129. doi:10.1007/s10815-018-1162-0
Li L., Gu Z.-P., Bo Q.-M., Wang D., Yang X.-S., Cai G.-H. (2015). Association of CYP17A1gene -34T/C Polymorphism with Polycystic Ovary Syndrome in Han Chinese Population. Gynecol. Endocrinol. 31, 40–43. doi:10.3109/09513590.2014.947948
Li Y., Fang L., Yan Y., Wang Z., Wu Z., Jia Q., et al. (2021). Association between Human SHBG Gene Polymorphisms and Risk of PCOS: a Meta-Analysis. Reprod. BioMedicine Online 42, 227–236. doi:10.1016/j.rbmo.2020.10.003
Li Y., Liu F., Luo S., Hu H., Li X.-H., Li S.-W. (2012). Polymorphism T→C of Gene CYP17 Promoter and Polycystic Ovary Syndrome Risk: A Meta-Analysis. Gene 495, 16–22. doi:10.1016/j.gene.2011.12.048
Liao X., Cao S. (2020). Association of the Genetic Polymorphisms Rs6259 and Rs727428 of the SHBG Gene with Polycystic Ovary Syndrome Risk: A Meta-Analysis. Genet. Test. Mol. Biomarkers 24, 492–501. doi:10.1089/gtmb.2019.0229
Lim S. S., Norman R. J., Davies M. J., Moran L. J. (2013). The Effect of Obesity on Polycystic Ovary Syndrome: a Systematic Review and Meta-Analysis. Obes. Rev. 14, 95–109. doi:10.1111/j.1467-789X.2012.01053.x
Liu Y., Zhao X. X., Hu X. J., Yang F., Lin P., Cui S. C., et al. (2019). Effect of Sex Hormone-Binding Globulin Polymorphisms on the Outcome of In Vitro Fertilization‐embryo Transfer for Polycystic Ovary Syndrome Patients: A Case‐control Study. J. Cel Biochem 120, 4675–4686. doi:10.1002/jcb.27756
Livadas S., Pappas C., Karachalios A., Marinakis E., Tolia N., Drakou M., et al. (2014). Prevalence and Impact of Hyperandrogenemia in 1,218 Women with Polycystic Ovary Syndrome. Endocrine 47, 631–638. doi:10.1007/s12020-014-0200-7
Marszalek B., Lacinski M., Babych N., Capla E., Biernacka-Lukanty J., Warenik-Szymankiewicz A., et al. (2001). Investigations on the Genetic Polymorphism in the Region of CYP17 Gene Encoding 5'-UTR in Patients with Polycystic Ovarian Syndrome. Dgye 15, 123–128. doi:10.1080/gye.15.2.123.12810.1080/713602803
Martinez-Garcia M. A., Gambineri A., Alpañés M., Sanchón R., Pasquali R., Escobar-Morreale H. F. (2012). Common Variants in the Sex Hormone-Binding Globulin Gene (SHBG) and Polycystic Ovary Syndrome (PCOS) in Mediterranean Women. Hum. Reprod. 27, 3569–3576. doi:10.1093/humrep/des335
Mehdizadeh A., Kalantar S. M., Sheikhha M. H., Aali B. S., Ghanei A. (2017). Association of SNP rs.2414096 CYP19 Gene with Polycystic Ovarian Syndrome in Iranian Women. Ijrm 15, 491–496. doi:10.29252/ijrm.15.8.491
Munawar Lone N., Babar S., Sultan S., Malik S., Nazeer K., Riaz S. (2020). Association of the CYP17 and CYP19 Gene Polymorphisms in Women with Polycystic Ovary Syndrome from Punjab, Pakistan. Gynecol. Endocrinol. 37, 456–461. doi:10.1080/09513590.2020.1822803
Mykhalchenko K., Lizneva D., Trofimova T., Walker W., Suturina L., Diamond M. P., et al. (2017). Genetics of Polycystic Ovary Syndrome. Expert Rev. Mol. Diagn. 17, 723–733. doi:10.1080/14737159.2017.1340833
Park J. M., Lee E. J., Ramakrishna S., Cha D. H., Baek K. H. (2008). Association Study for Single Nucleotide Polymorphisms in the CYP17A1 Gene and Polycystic Ovary Syndrome. Int. J. Mol. Med. 22, 249–254.
Pusalkar M., Meherji P., Gokral J., Chinnaraj S., Maitra A. (2009). CYP11A1 and CYP17 Promoter Polymorphisms Associate with Hyperandrogenemia in Polycystic Ovary Syndrome. Fertil. Sterility 92, 653–659. doi:10.1016/j.fertnstert.2008.07.016
Rahimi Z., Mohammadi E. (2019). The CYP17 MSP AI (T-34C) and CYP19A1 (Trp39Arg) Variants in Polycystic Ovary Syndrome: A Case-Control Study. Ijrm 17, 201–208. doi:10.18502/ijrm.v17i3.4519
Saddick S. Y. (2020). Identifying Genes Associated with the Development of Human Polycystic Ovary Syndrome. Saudi J. Biol. Sci. 27, 1271–1279. doi:10.1016/j.sjbs.2020.01.012
Santana L. G., Flores-Mir C., Iglesias-Linares A., Pithon M. M., Marques L. S. (2020). Influence of Heritability on Occlusal Traits: a Systematic Review of Studies in Twins. Prog. Orthod. 21 (1), 29. doi:10.1186/s40510-020-00330-8
Sharma P., Kaur M., Khetarpal P. (2021). CYP19 Gene Rs2414096 Variant and Differential Genetic Risk of Polycystic Ovary Syndrome: a Systematic Review and Meta-Analysis. Gynecol. Endocrinol. 37, 126–131. doi:10.1080/09513590.2020.1813274
Sharp L., Cardy A. H., Cotton S. C., Little J. (2004). CYP17 Gene Polymorphisms: Prevalence and Associations with Hormone Levels and Related Factors. A HuGE Review. Am. J. Epidemiol. 160, 729–740. doi:10.1093/aje/kwh287
Shorakae S., Ranasinha S., Abell S., Lambert G., Lambert E., de Courten B., et al. (2018). Inter-related Effects of Insulin Resistance, Hyperandrogenism, Sympathetic Dysfunction and Chronic Inflammation in PCOS. Clin. Endocrinol. 89, 628–633. doi:10.1111/cen.13808
Somboonporn W., Davis S. R. (2004). Testosterone Effects on the Breast: Implications for Testosterone Therapy for Women. Endocr. Rev. 25, 374–388. doi:10.1210/er.2003-0016
Tan L., Zhang L., Zhu G. J. (2005). Polymorphism of CYP17 Gene in Patients with Polycystic Ovary Syndrome of Han Nationality in China. J. Zhengzhou Univ. 40, 438–441.
Thakkinstian A., McElduff P., D'Este C., Duffy D., Attia J. (2005). A Method for Meta-Analysis of Molecular Association Studies. Statist. Med. 24 (9), 1291–1306. doi:10.1002/sim.2010
Unsal T., Konac E., Yesilkaya E., Yilmaz A., Bideci A., Ilke Onen H., et al. (2009). Genetic Polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 Genes in Adolescent Girls with Polycystic Ovary Syndrome. J. Assist. Reprod. Genet. 26, 205–216. doi:10.1007/s10815-009-9308-8
Wawrzkiewicz-Jałowiecka A., Kowalczyk K., Trybek P., Jarosz T., Radosz P., Setlak M., et al. (2020). In Search of New Therapeutics-Molecular Aspects of the PCOS Pathophysiology: Genetics, Hormones, Metabolism and beyond. Ijms 21, 7054. doi:10.3390/ijms21197054
Wu M. M., Lu Y. L., Han L., Liu N., WanUyhan L. (2017). Han Nationality Patients with Polycystic Ovary Syndrome in Xinjiang: Polymorphism of CYP17 Gene and its Relationship with Pathogenesis. Prog. Mod. Biomed. 17, 5297–5308.
Yang F., Ruan Y.-C., Yang Y.-j., Wang K., Liang S.-s., Han Y.-b., et al. (2015). Follicular Hyperandrogenism Downregulates Aromatase in Luteinized Granulosa Cells in Polycystic Ovary Syndrome Women. Reproduction 150, 289–296. doi:10.1530/REP-15-0044
Yu Y.-Y., Sun C.-X., Liu Y.-K., Li Y., Wang L., Zhang W. (2013). Promoter Methylation ofCYP19A1Gene in Chinese Polycystic Ovary Syndrome Patients. Gynecol. Obstet. Invest. 76, 209–213. doi:10.1159/000355314
Keywords: polycystic ovary syndrome, single nucleotide polymorphism, CYP19, CYP17, sex hormone binding globulin
Citation: Xing C, Zhao H, Zhang J and He B (2022) The Association of CYP17A1, CYP19A1, and SHBG Gene Polymorphisms in Polycystic Ovary Syndrome Susceptibility: A Systematic Review and Meta-Analysis. Front. Physiol. 13:741285. doi: 10.3389/fphys.2022.741285
Received: 04 August 2021; Accepted: 18 March 2022;
Published: 09 May 2022.
Edited by:
Ravinder Anand-Ivell, University of Nottingham, United KingdomReviewed by:
Hou-De Zhou, Central South University, ChinaKarina Braga Gomes, Federal University of Minas Gerais, Brazil
Copyright © 2022 Xing, Zhao, Zhang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing He, aGViaW5nNzU1N0AxNjMuY29tLw==
 Chuan Xing
Chuan Xing Han Zhao
Han Zhao Jiaqi Zhang
Jiaqi Zhang