
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 06 December 2022
Sec. Invertebrate Physiology
Volume 13 - 2022 | https://doi.org/10.3389/fphys.2022.1061817
This article is part of the Research TopicPhysiological Events Associated with Pesticide-ResistanceView all 6 articles
The mirid bugs Apolygus lucorum and Adelphocoris suturalis are considered serious pests of many crops in China, the host plant recognition of these pests remains unclear. The current study investigated the vital odor cues of two mirid bugs and evaluated the role of olfactory recognition in host recognition. The GC-EAD response of mirid bugs to volatiles of their host plant Phaseolus vulgaris was tested. Tetradecane, 2-propyl-1-pentanol, and dodecanal elicited strong EAG responses by mirid bugs and were tested with field experiments. The results indicated tetradecane was significantly more attractive than other attractants, yielding 30.33 ± 2.19 mirid bugs trapped during 7 days. The selected response rates to tetradecane were above 60%, which was most attractive to female A. lucorum at 1.5 mg/ml. Among seven tetradecane derivatives, tetradecane and tetradecanoic acid were the most potent attractants to A. lucorum and A. suturalis. Tetradecane was present in the volatiles of 10 common hosts, and their difference in relative content was significant. The presence of tetradecane seemed relevant to the olfactory response intensity of two mirid bugs towards the different host plants. The artificial supplement of tetradecane increased the attractive effect of host plants. These results suggested that tetradecane plays a vital role in the olfactory selection by two mirid bugs, and it can be made into field baits as a novel ecological strategy to manage these pests with widely reported pesticide resistance. However, results suggested host recognition is not entirely dependent on odor cues. We demonstrated that A. suturalis and A. lucorum adults have similar olfactory recognition mechanisms to their hosts in long-distance host selection. While, the differences in host plant selection between the two pests should occur in close range due to differences in gustatory or tactile sensory organs of A. lucorum and A. suturalis.
The mirid bugs Apolygus lucorum (Meyer-Dür) and Adelphocoris suturalis (Jakovlev) (Hemiptera: Miridae) attack a wide range of cultivated crops, including cash crops such as cotton, jujube, and the kidney bean (Blackmer et al., 2004; Pan et al., 2015a). These bugs were originally minor cotton pests in China. However, since the introduction of transgenic cotton in the late 1990s (Lu et al., 2010; Lu et al., 2012), mirid populations have rapidly increased, becoming significant cotton pests in major cotton-growing regions of the country (Lu and Wu, 2011).
Currently, A. lucorum and A. suturalis are managed mainly through synthetic insecticides that may negatively impact the environment, non-target insects, and human health. Moreover, these bugs have quickly developed resistance against many insecticides (Snodgrass et al., 2009; Zhen et al., 2016). Some previous studies discovered that A. lucorum population in China has moderate resistance to several insecticides (Guo et al., 2010; Zhang et al., 2015). All this limit the use of chemical control. There is, therefore, an urgent need to explore and test alternative management methods for mirid bugs.
Many studies have documented the “chemical conversation” with the surrounding environment by insects using their remarkable olfactory organs (Del Socorro et al., 2010; Gregg et al., 2010; Robert and Iain, 2010; Knight and Light, 2013). Insect pests can select suitable food sources or oviposition sites by odorous cues provided by plants in the form of volatile emissions announcing their physiological status in the natural environment (Shiojiri and Karban, 2008; Weiss et al., 2011; Sun et al., 2012; Cha et al., 2017; Hosseini et al., 2017). Previous study has found some important odorous cues recognized by A. lucorum including butyl acrylate, butyl propionate, diethyl phthalate and methyl levulinate in the host plant volatiles (Pan et al., 2015a; Yin et al., 2021). However, compared to the enormous knowledge about lepidopteran and coleopteran pests, very little is known about the responses of mirid bugs, especially A. suturalis to plant volatiles (Pan et al., 2015a; Pan et al., 2015b). The two species of mirid bugs have many common host plants we tried to verify whether A. suturalis have similar olfactory recognition mechanisms with that of A. lucorum or not. Thus in this study, we hope to find the vital odor cues in a wide range of shared host plants of two mirid bugs. In addition, previous studies mainly focused on olfactory recognition we attempt to demonstrate whether host selection is entirely dependent on odor cues by seeking evidence that other sensory organs are involved in host identification. We hope this study will contribute to understanding the host plant recognition mechanism of A. lucorum and A. suturalis which can be a theoretical basis for developing safe and effective potential field baits for both pests.
The current study tested GC-EAD responses of A. lucorum and A. suturalis to volatiles of the common bean, Phaseolus vulgaris (Fabacea), an important host plant of these pests. The volatiles were identified by gas chromatography and mass spectroscopy (GC-MS). In field tests and laboratory trials, lure trapping and two-way competition experiments were used to examine the relative attractiveness of substances that produced EAD responses. Finally, the attractive effects of ten other host plants on the insects were also evaluated by ‘strictly olfactory’ assays and normal assays. Then we analysed the relationship between behavioral responses and the composition of volatiles. The key objective of this study was to understand the mechanisms of host plant identification used by two mirid bugs towards developing specific trapping protocols for controlling field infestations.
Synthetic analogues of the volatiles identified from P. vulgaris were purchased from Shanghai Macklin Biochemical Co., Ltd., and Aladdin Industrial Corporation, China. A three-arm olfactometer (Brand: YMM3-150), a GC-EAG measurement system, and a QP 2010 SE GC-MS system were purchased from Shanghai Yuming Instrument Co., Ltd., Syntech Limited, and Shimadzu Corporation, respectively. The adsorption column consisted of a glass tube (15 cm long and 0.7 cm in diameter) with an airflow outlet and inlet equipped with 200 mg SuperQ adsorbent, purchased from Altec, United States.
About 15 P. vulgaris pods were placed inside a glass jar (35 × 20 cm – height and diameter) provided with an airflow inlet and outlet at the top. An adsorption column was attached to the outlet exit, while an activated carbon filter (air flow rate: 600 L/min) was attached prior to the inlet. After collecting air volatiles for 8 h, the adsorption column was removed to be eluted with GC-MS grade hexane.
Two 4.5 cm long glass capillaries were filled with 0.9% sodium chloride saline and inserted with two 4.5 cm long clean silver wires as electrodes. One glass tube was used as the measuring pole and the other one served as the reference pole.
Antennae of adult bugs were excised at the base using a scalpel under a dissection microscope and chipped at their tip. One antenna was attached to the reference pole by the surface tension of the saline solution and connected to the measuring pole under a stereo microscope. When the baseline readings of the EAD remained stable and the GC oven was ready. 4 μl of plant volatile solution was injected into the GC port. Obtained data were recorded and analyzed with GC-EAD 2014 V1.2.5 software.
The procedure for collecting volatiles was as previously stated. Gas chromatograph settings included an Agilent DB-wax chromatographic column (30 m. 0.25 mm; 0.25 µm, Agilent Technologies). The injector temperature was set to 225°C, carrier gas was helium, and injections were made on splitless mode. The oven temperature was programmed to three steps: 1) 40°C hold for 5 min; 2) increase at the rate of 5°C/min to 110°C, hold for 1 min; 3) increased at the rate of 10°C/min to 240°C and hold for 10 min. The mass spectrometer ion source temperature was set to 300°C, and the scanning speed used at 2500, with a 0.3 s interval. Obtained mass spectra were compared with external synthetic standards using Labsolution software to confirm the chemical identity of the main volatiles recovered from P. vulgaris pods.
Volatiles eliciting significantly stronger EAG responses were selected for attractiveness field tests using synthetic analogues. The field tests were conducted from 28 August to 3 September 2020 in a cotton field measuring about 4,000 m2 in Ezhou, Hubei, China. Respective synthetic compounds were diluted to 150 mg/ml with ddH2O, and 1 ml solution was added to individual micro centrifuge tubes sealed with plastic film, minutely punctured to prevent quick evaporation. The tubes were taped to the middle of a yellow board (RAL 1016 Sulfur yellow according to the international standard colour metric scale). Glue was spread on the outer face of the yellow board to capture the bugs. Each compound was tested three times using different yellow boards. Three boards were presented parallel as controls containing no attractants, using ddH2O as a control. Treatment and control groups were randomly distributed, distanced from each other by at least 20 m, where each board was held suspended at about 20 cm above a cotton plant. After 7 days, the numbers and species of mirid bugs captured on the yellow boards were counted.
Volatiles presenting a positive attractiveness response in field experiments were further tested by a two-way competition experimental design in laboratory conditions. Prior to the tests, male and female adults of A. suturalis and A. lucorum were starved for 4 h and submitted to olfactory behavior assays using a three-arm olfactometer (Figure 1). The arms were ventilated for 5 min before the experiment, with the airflow rate set to 2 L/min. The synthetic volatiles were diluted to 1.5 mg/ml and 15 mg/ml in ddH2O and added 30 μl anhydrous ethanol to assist in complete solubilization. One of the olfactometer arms was blocked with absorbent cotton, and the other two were connected to glass bottles containing either 50 μl volatile solution or ddH2O with 30 μl anhydrous ethanol as a control group. Ten adult bugs were placed in the response chamber from an opening at the top. The test was repeated nine times for each species, totaling 90 male and female adults assayed for each species. All behavioral response assays were done in the dark, being recorded for 30 min once insects were introduced. Any insects moving beyond 50% of an arm’s length were considered to have shown a positive attractiveness response to the tested volatile. In setting up each replicate, the olfactometer parts were washed with ethanol, and the direction of odor sources were randomized.
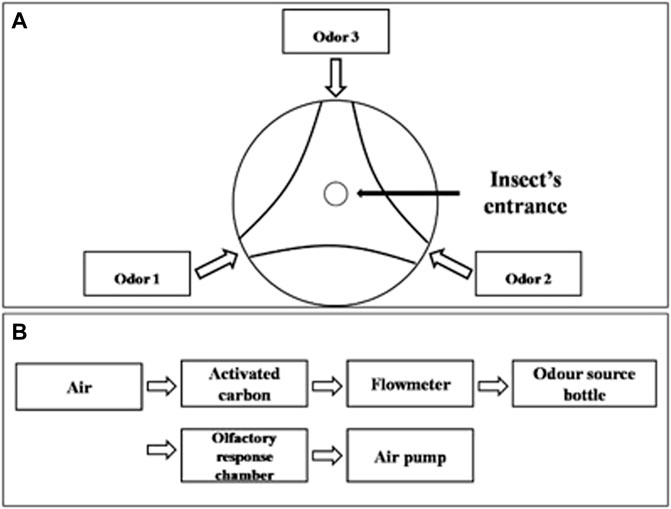
FIGURE 1. Schematic diagram of YMM 3–150 olfactometer. (A) Top view of olfactory reaction chamber of olfactometer, (B) connections diagram of olfactometer.
The selected response rate and selection coefficient were calculated as follows (Zhou et al., 2009):
To test the attractiveness effects of tetradecane analogues, seven analogues, including aldehydes, aldehydes, alcohols and acids, were employed for two-way competition experiments of female adults as described above. The analogues were 7-tetradecene, 1-tetradecene, tetradecanal, 2-tetradecanol, 1-tetradecanol, 2-tetradecenoic acid and tetradecanoic acid.
Fruits of Vitis vinifera, Zea mays, Pyrus bretschneideri, Malus pumila and seedlings of Pisum sativum (100 g each) were placed in individual glass jars to collect volatiles, as previously described. Five different plant parts of cotton, including buds, bolls, seedling leaves, bolls leaves, and bud leaves, were obtained from Xinzhou in 2019 and stored in afrigerator at −80°C as ground powders. Fifteen grams of each different plant part sample were likewise used to collect volatiles.
Volatile compounds were identified by GCMS, but using a Rtx-5 MS chromatographic column (30 m × 0.25 mm x 0.25 µm), injection port temperature was 250°C, helium as the carrier gas, injections done in splitless mode. The oven temperature program was set to three steps: 1) 40°C for 1 min; 2) increasing at the rate of 4°C/min to 130°C hold for 5 min; 3) increasing at the rate of 10°C/min to 250°C, final hold for 5 min. The mass spectrometer ion source temperature was set to 250°C; the scanning speed was 2500 with a 0.3 s interval. Runs were repeated three times, and the relative content of tetradecane in each sample was based on obtained peak areas in respective chromatograms.
Further 15–20 g of Vitis vinifera, Zea mays fruits, and 5-7 cotton leaves were employed to test for attractiveness to mirid bugs, but these tests were designed as ‘strictly olfactory’ assays and normal assays using female adults. In the case of the ‘strictly olfactory’ assays, three different plants were presented as host options inside separate glass bottles connected to three arms of the olfactory response chamber and the odor of host plants was blown into the response chamber from glass bottles by filtered air so the mirid bugs could smell them but could not reach the host plant through the hose that connected the response chamber and the glass bottles. Hence, they were unable to access them visually or physically. Evaluation methods were as described above.
In the case of normal assays, the three samples of different plant parts were offered as host options directly inside the different arms so that mirid bugs could evaluate hosts by smell, touch and gustation cues because in this case there is no barrier between the host plants and pests. Twenty adult A. suturalis and A. lucorum were placed into the olfactory response chamber from an opening at the top centre in each replicate and left overnight between 17:00–8:00. Lights were switched off to maintain dark conditions. Each assay was repeated three times, where the positions of host options were changed and randomized. Response rates were calculated as described previously.
Plants presenting the highest and lowest tetradecane contents were selected for this experiment. The plant with the lowest tetradecane content was supplemented with added synthetic tetradecane to quantify any relative attractiveness changes using the three-arm olfactometer described above. In this experiment, one treatment group and two control groups were set up and 15–20 g of the host plants were used in each group. The treatment group contained host plants with the lowest tetradecane content, and the plants were artificially supplemented with 50 μl of tetradecane solution. Control group 1 included host plants containing the highest tetradecane contents, and Control group 2 included host plants containing the lowest tetradecane content but no artificial tetradecane supplement. Three different concentrations of tetradecane supplementation were tested: undiluted solution, 15 mg/ml and 1.5 mg/ml. Treatment and two control groups were thus allocated to the three glass bottles connected to the olfactometer to be assayed as described above in ‘strictly olfactory’ experiments.
The Least Significant Difference (LSD) test was employed to check for differences in the numbers of mirid bugs trapped by boards presented in the field, in the content of tetradecane in each examined plant bouquet, and in the relative attractiveness of different plants to hosts. Olfactory responses to volatiles and analogues were analyzed using the chi-square test. All analyses were conducted using SPSS software, while data of GC-EAD were analyzed with GC-EAD 2014 V1.2.5 software.
The results of GC-EAD indicated three compounds from P. vulgaris pods inducing antennal responses in males of A. suturalis, and females of A. suturalis and A. lucorum. Their respective retention times were 11.39 min, 17.70 min and 22.61 min (Figure 2), corresponding to 2-propyl-1-pentanol, tetradecane and dodecanal (Table 1), respectively. Interestingly, 2-propyl-1-pentanol did not induce any EAG response in A. lucorum males.
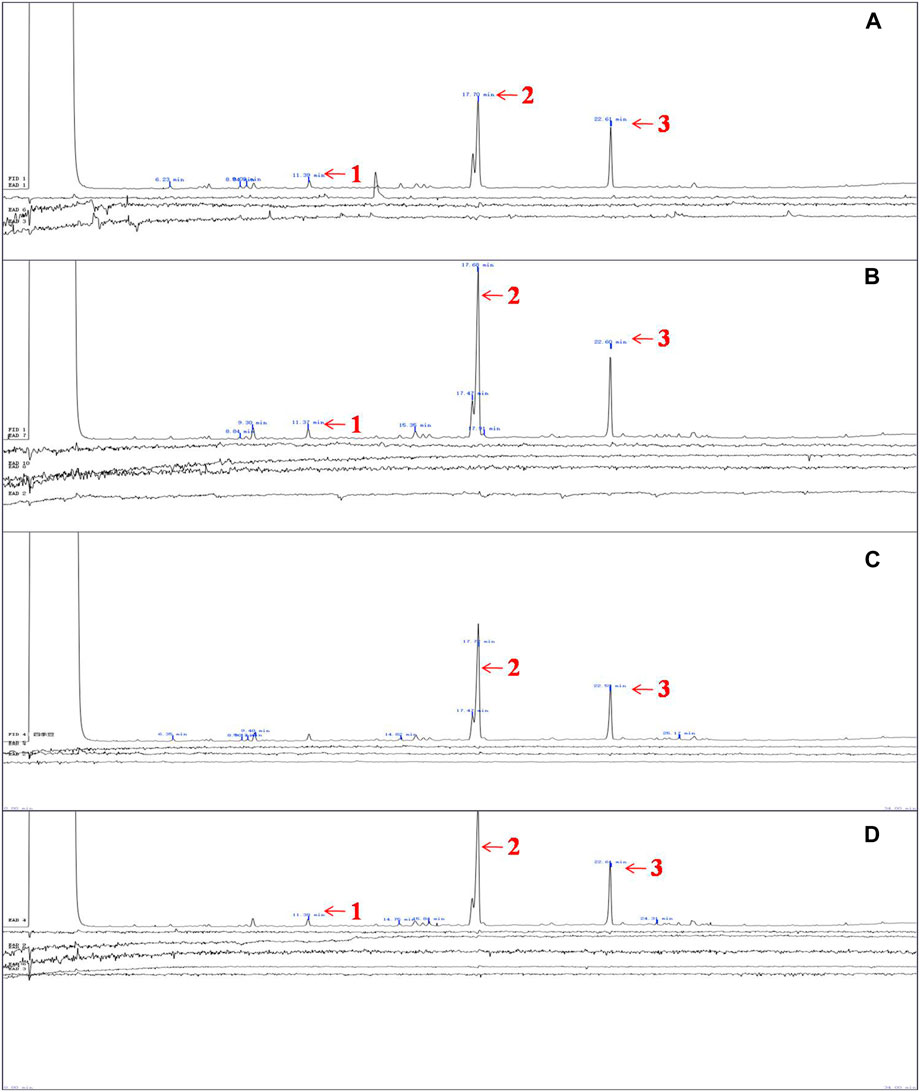
FIGURE 2. Measured EAG responses of A. lucorum and A. suturalis to volatiles from Phaseolus vulgaris. (A) The EAG responses of female A. lucorum to volatiles from P. vulgaris, (B) The EAG responses of female A. suturalis to volatiles from P. vulgaris, (C) The EAG responses of male A. lucorum to volatiles from P. vulgaris, (D) The EAG responses of male A. suturalis to volatiles from P. vulgaris 1, 2,3 indicated FID peaks of three volatiles at 11.39 min, 17.70 min, and 22.61 min, approximately, which induced EAG responses of (A) lucorum and A. suturalis adults.
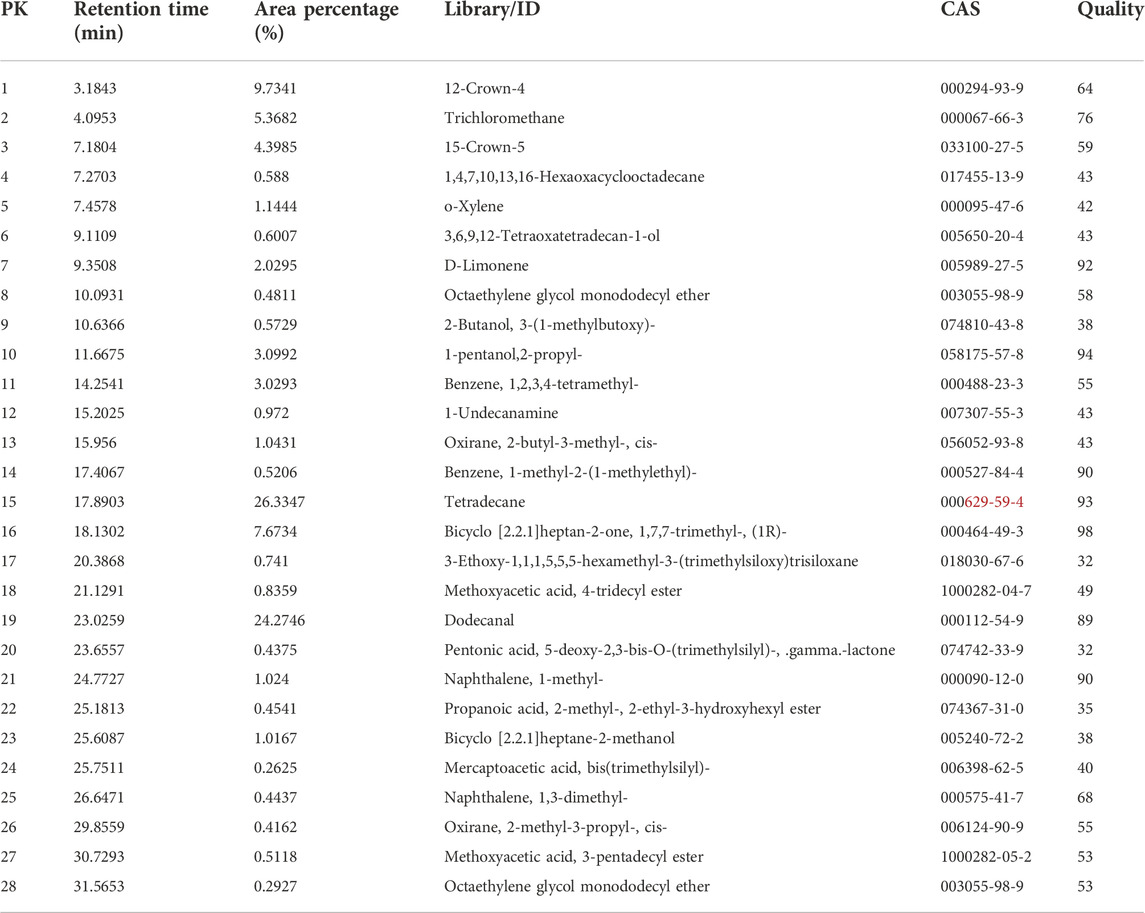
TABLE 1. Volatiles from common beans Phaseolus vulgaris pods, identified by gas-chromatography coupled with mass spectrometry.
Tetradecane, 2-propyl-1-pentanol, and dodecanal were obtained as synthetic compounds for field tests as baits on yellow board traps. As shown in Figure 3, tetradecane yielded high attractiveness: within 7 days, the average amount of captured mirid bugs was 30.33 ± 2.19, significantly more than with other attractants (p < 0.01) and controls (p < 0.05). In fact, the number of captured mirid bugs trapped by 2-propyl-1-pentanol and dodecanal was significantly lower than in controls (p < 0.05).
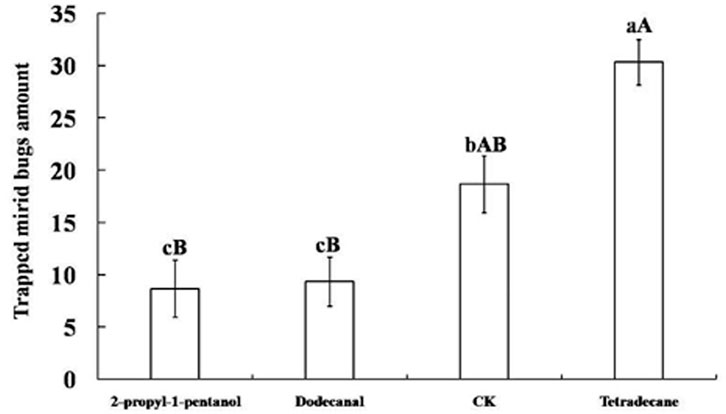
FIGURE 3. Mirid bugs trapped by yellow boards with different attractant. Different uppercase and lowercase letters indicated extremely significant (p < 0.01) and significant (p < 0.05) differences of mirid bugs amount that trapped by yellow boards with three different attractants and control group (Least significant difference test).
In field experiments, tetradecane appeared as the most attractive volatile to bugs; nevertheless, many uncontrollable factors may have influenced these findings, necessitating confirmation in a laboratory setting. In two-choice olfactory tests, volatile concentrations of 1.5 mg/ml and 15 mg/ml were used. Results showed that tetradecane significantly increased female mirid bugs’ attractiveness, with selected response rates above 60%. Tetradecane was most effective in attracting females of both bug species at a concentration of 1.5 mg/ml. While tetradecane was less attractive to males, it showed no significant olfactory response at the concentration of 1.5 mg/ml (Table 2).
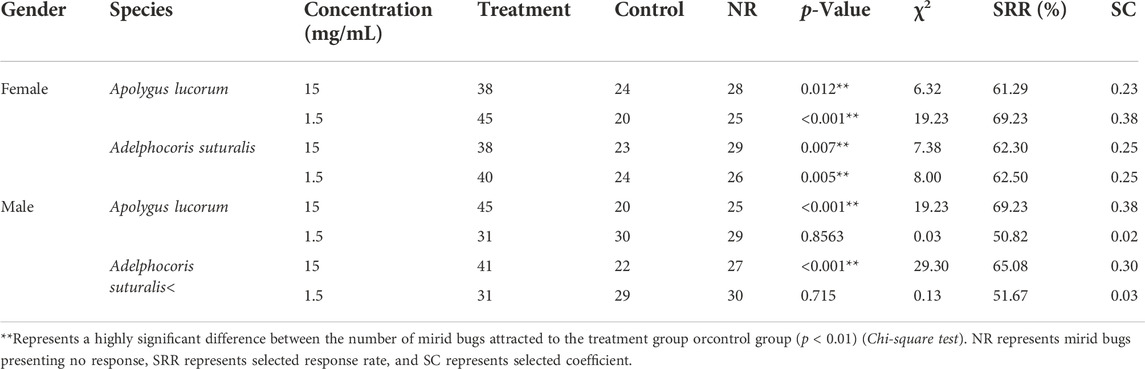
TABLE 2. The olfactory responses of two pest mirid bugs to synthetic tetradecane, measured in laboratory tests.
Seven synthetic derivatives from various chemical groups, including alcohols, aldehydes, acids and olefins, were chosen for the two-way competition experiment to assess the relative attractiveness of tetradecane analogues to A. lucorum and A. suturalis. As tetradecane proved comparatively less attractive to males, we tested the olfactory response of females only. Overall, tetradecane derivatives proved most attractive to A. lucorum than to A. suturalis, especially at the lowest concentrations. Four analogues showed stronger attractiveness effects on A. lucorum (p < 0.001) compared to controls at the concentration of 1.5 mg/ml. Among them, tetradecanal was the most attractive to A. lucorum, eliciting a higher selected response rate than tetradecane. Only tetradecanoic acid yielded a higher attraction (p < 0.001) of A. suturalis at the concentration of 15 mg/ml (Table 3).
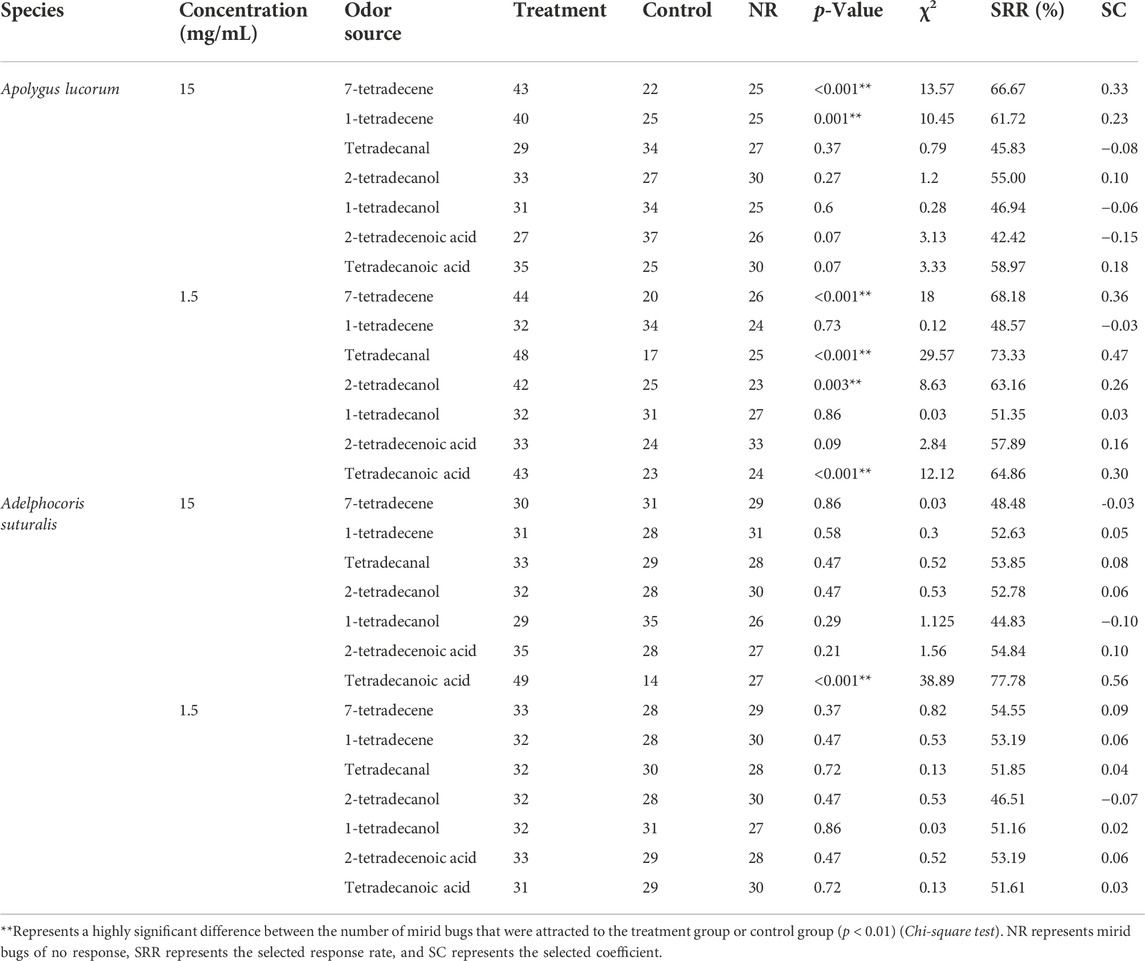
TABLE 3. The olfactory responses of two pest mirid bugs to synthetictetradecane derivatives, measured in laboratory tests.
GC-MS analysis showed tetradecane is present as a volatile in the other ten plant species commonly exploited by mirid bugs and their relative amounts are significantly different (p < 0.05). The concentration of tetradecane was the highest in V. vinifera grapes, reaching 1.07 ± 0.06%, followed by P. sativum seedlings and Z. mays fruits (p < 0.05). Other species had statistically similar, lower amounts, including five different plant parts of G. herbaceum (p < 0.01) (Figure 4).
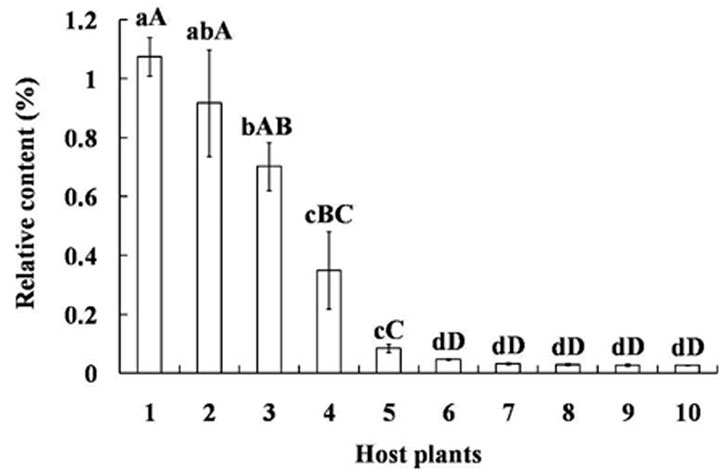
FIGURE 4. Relative content of tetradecane among volatiles of different plants exploited as hosts by mirid bugs. Different uppercase and lowercase letters indicated highly significant (p < 0.01) and significant (p < 0.05) differences in the relative contents of tetradecane among ten host plants (Least significant difference test). Numbers on the horizontal axis represent: 1. Fruits of Vitis vinifera, 2. Seedling of Pisum sativum, 3. Fruits of Zea mays, 4. Fruits of Pyrus bretschneideri, 5. Fruits of Malus pumila, 6. Buds of Gossypium herbaceum, 7. Bolls of G. herbaceum, 8. Leaves at seedling stage of G. herbaceum, 9. Leaves on the bolls of G. herbaceum, 10. Leaves on the buds of G. herbaceum.
Therefore, fruits of V. vinifera and Z. mays and seedling leaves of G. herbaceum were selected to test for attractiveness to A. suturalis and A. lucorum adults as representative of significantly different tetradecane concentrations. Since tetradecane proved most attractive to females, experiments were designed with females only. Selected response rates of mirid bugs to three host plants in a “strictly olfactory” experiment were correlated with the tetradecane concentration of their volatiles. The selected response rate of A. lucorum to V. vinifera was the highest and significantly higher than that of G. herbaceum (p < 0.01), reaching 44.44 ± 0.80%. While for A. suturalis, there were no significant differences between the selected response rates for V. vinifera and Z. mays, reaching 37.98 ± 2.00% and 38.04 ± 0.54%, respectively. The selected response rate of G. herbaceum was significantly lower than that of the other two host plants (p < 0.01), reaching 23.97 ± 1.60%.
Interestingly, the selected response rates in normal assays (i.e., where bugs can select hosts using multiplecues) were irrelevant with relative contents of tetradecane in the volatiles from three host plants: A. lucorum preferred Zea mays fruits, while A. suturalis preferred G. herbaceum leaves, with respective selected response rates of 56.33 ± 3.30% and 57.92 ± 5.61% (Figure 5).
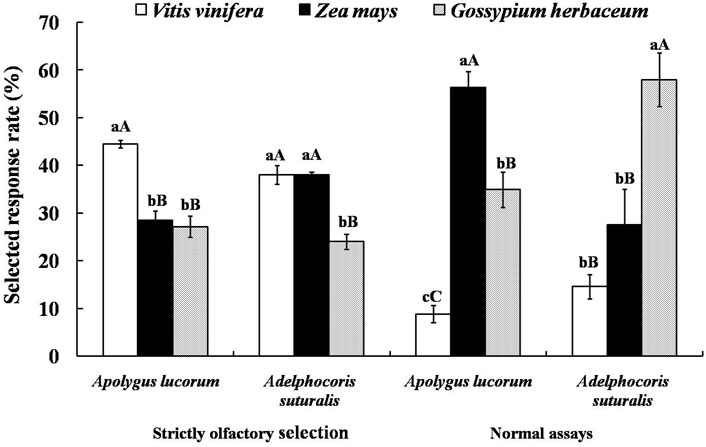
FIGURE 5. Attractive effects of different host plants on A. suturalis and A. lucorum. Different uppercase and lowercase letters showed extremely significant (p < 0.01) and significant (p < 0.05) differences of the selected response rate of mirid bugs to different hosts in two modes of behaviour tests respectively (Least Significant Difference test).
As shown in Figure 6, supplementation with the undiluted solution of tetradecane significantly increased the olfactory selected response rates of both A. lucorum and A. suturalis to G. herbaceum leaves (46.41 ± 3.15% and 45.31 ± 3.86%, respectively). This response was significantly higher (p < 0.01) than the pertinent control group and more elevated than V. vinifera fruits. On the other hand, when tetradecane was added at concentrations of either 15 mg/ml or 1.5 mg/ml to G. herbaceum leaves, response rates did not change significantly (p > 0.05).
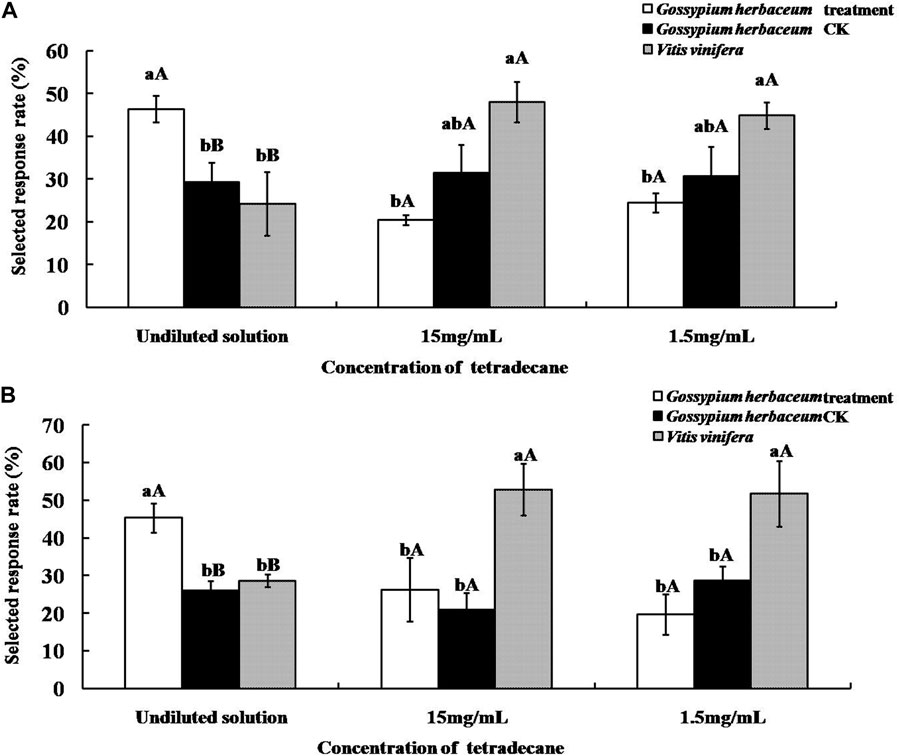
FIGURE 6. Selected response rates of Apolygus lucorum (A) and Adelphocoris suturalis (B) to host plants supplemented with tetradecane. Different uppercase and lowercase letters showed highly significant (p < 0.01) and significant (p < 0.05) differences in the selected responserate of mirid bugs to different hosts treated by tetradecane of different concentration respectively (Least Significant Difference test).
The current study used laboratory and field experiments to test the volatiles of host plants’ attractiveness in two mirid bugs that might be useful in an attract-and-kill system for these species. Results showed that three volatiles from P. vulgaris pods successfully induced EAG responses of A. suturalis and A. lucorum adults. We further confirmed the attractive effect of tetradecane, which was a common volatile among ten host plants exploited by mirid bugs, by field trapping studies and olfactory lab testing. Additionally, we found that artificially supplementing with tetradecane improved the response rate of host plants with low tetradecane contents. However, it was valid only for olfactory selection.
Previous studies on attractants of mirid bugs are relatively few and mainly focused on A. lucorum. Some compounds, including cis-formic acid-3-hexene ester, m-xylene, and 3-ethyl benzene have been demonstrated and potentially used to control A. lucorum (Sun et al., 2014; Pan et al., 2015a; Pan et al., 2015b). However, the host recognition mechanisms of mirid bugs, especially A. suturalis have remained unclear.
The current study showed that tetradecane is a common volatile of the host plants that mirid bugs exploit. Tetradecane is a straight-chain alkane that has a role as a plant metabolite and a volatile component. It also functions as a potent defence induction signal that prepares neighbouring plants for incoming attacks from insect pests (Pan et al., 2022). Some herbivore-infested plants produce tetradecane, which acts as kairomone to attract the pest’s natural enemies (Li et al., 2022). It has been reported as an attractive plant volatile for several insect pests (Mu et al., 2012; Mitra et al., 2020; Chen et al., 2022; Guo et al., 2022). There is, however, scant evidence from earlier investigations that it is an attractant for mirid bugs. When A. suturalis and A. lucorum were allowed to select hosts solely by olfaction in ‘strictly olfactory’ assays, attractiveness seems closely related to their relative amounts of tetradecane in volatiles, supporting the hypothesis that tetradecane is an important olfactory clue for mirid bugs. Increased selected response rates further confirmed this after artificial supplementation with tetradecane in host plants presenting low tetradecane concentrations. A possibility for creating attractants centred on a similar chemical structure was also indicated by some attraction displayed by other tetradecane derivatives. We also discovered that male and female mirid beetles react differently to tetradecane. This agrees with previous studies showing that host plant volatiles were more attractive to female adults, while sex pheromones were often more strongly responded by males than by females in locating their mating partners (Groot et al., 2005; Nurkomar et al., 2017).
However, host recognition by insects is a complex process involving different sensory organs at different spatial scales (Bruce et al., 2005; Henze et al., 2018). In the current study, we found that the selected response rates of mirid bugs to three host plants were unrelated to tetradecane contents when mirid bugs were able to contact or taste the hosts contrary to the results in ‘strictly olfactory’ assays. It proved that olfactory recognition is only one aspect of host recognition by mirid bugs, and other sensory organs may also be crucial in host recognition. We considered that different sensory organs are used in field orlaboratory tests except for olfactory organs. In a field experiment, physical signals recognized by visual organs can synergistically enhance host plants’ attractiveness due to the existence of natural light (Kerr et al., 2017). A previous study showed that A. lucorum was more attracted to cotton plants containing green lights, and the selected response was significantly higher than that of olfactory or visual cues alone (Pan et al., 2015c). Thus, we believe yellow boards may have contributed synergistically to the attractive effect of compounds in our field experiments.
However, visual cues likely had minimum relevance in our laboratory tests because the experiments were conducted in the dark. In the quite different scenario from the field experiments, gustatory and tactile cues may play a critical role in host recognition which has been demonstrated for several species of herbivorous arthropods (Zhang et al., 2010; Ambrosio and Brooks, 2011). We hypothesize that mirid bugs locate hosts by olfactory cues and settle their selection depending on the contact or taste of a prospective host at close range in the laboratory tests. In this study, we demonstrated that A. suturalis and A. lucorum adults used the same odor cue, tetradecane, in long-distance host selection, indicating that the two pests have similar olfactory recognition mechanisms to their hosts. However, the differences in host selection between the two pests to Zea mays fruits and G. herbaceum leaves should occur in close range after contacting or tasting them, which may indicate differences in gustatory and tactile sensory organs of two mirid bugs. Future studies could focus on establishing the role of the various sensory organs, especially gustation and tactile receptors, in recognizing plant hosts towards developing even more effective field traps.
To date, insect attractants proven effective in pest management are mainly sex pheromones (Rizvi et al., 2021). While being more attractive to female mirid bugs, we think tetradecane might also supplement the effects of some sex pheromones in field applications. The baits yielded significant attractiveness when applied with yellow boards in the field. To further enhance the impact as a attract-and-kill products, it is an excellent strategy to combine this attractant with sex pheromones (Kendra et al., 2017; Baroffio et al., 2018; Tasin et al., 2018). Previous studies showed that sex pheromones enhance the attractiveness of host plant volatiles to males. The host plants’ volatiles can increase males’ EAG response to sex pheromones and promote mating behaviour (Binyameen et al., 2013; Ian et al., 2017). However, further work would be needed to optimize the blends of pheromones and plant volatiles, their stability in the field and their effectiveness. We hope the current study contributes to developing more efficient and safer pest control strategies against mirid bugs in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
HY and MX did the experiment. HY wrote the main manuscript text. All authors reviewed the manuscript.
This work was supported by the National Science Foundation of China (32001966).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.1061817/full#supplementary-material
Ambrosio L. J., Brooks W. R. (2011). Recognition and use of ascidian hosts, and mate acquisition by the symbiotic pea crab Tunicotheres moseri (rathbun, 1918): The role of chemical, visual and tactile cues. Symbiosis 53, 53–61. doi:10.1007/s13199-011-0112-8
Baroffio C., Sigsgaard L., Ahrenfeldt E., Borg-Karlson A. K., Bruun S., Cross J., et al. (2018). Combining plant volatiles and pheromones to catch two insect pests in the same trap: Examples from two berry crops. Crop Prot. 109, 1–8. doi:10.1016/j.cropro.2018.02.025
Binyameen M., Hussain A., Yousefi F., Birgersson G., Schlyter F. (2013). Modulation of reproductive behaviors by non-host volatiles in the polyphagous Egyptian cotton leafworm, Spodoptera littoralis. J. Chem. Ecol. 39, 1273–1283. doi:10.1007/s10886-013-0354-4
Blackmer J. L., Naranjo S. E., Williams L. H. (2004). Tethered and untethered flight by Lygus hesperus and Lygus lineolaris (heteroptera: Miridae). Environ. Entomol. 33, 1389–1400. doi:10.1603/0046-225x-33.5.1389
Bruce T. J., Wadhams L. J., Woodcock C. M. (2005). Insect host location: A volatile situation. Trends Plant Sci. 10, 269–274. doi:10.1016/j.tplants.2005.04.003
Cha D. H., Olsson S. B., Yee W. L., Goughnour R. B., Hood G. R., Mattsson M., et al. (2017). Identification of host fruit volatiles from snowberry (Symphoricarpos albus), attractive to Rhagoletis zephyria flies from the Western United States. J. Chem. Ecol. 43, 188–197. doi:10.1007/s10886-016-0814-8
Chen Z. L., Huang C., Li X. S., Li G. C., Yu T. H., Fu G. J., et al. (2022). Behavioural regulator and molecular reception of a double-edge-sword hunter beetle. Pest Manag. Sci. 78, 2693–2703. doi:10.1002/ps.6901
Del Socorro A. P., Gregg P. C., Alter D., Moore C. J. (2010). Development of a synthetic plant volatile-based attracticide for female noctuid moths. I. Potential sources of volatiles attractive to Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 49, 10–20. doi:10.1111/j.1440-6055.2009.00733.x
Gregg P. C., Del Socorro A. P., Henderson G. S. (2010). Development of a synthetic plant volatile-based attracticide for female noctuid moths. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 49, 21–30. doi:10.1111/j.1440-6055.2009.00734.x
Groot A., Gemeno C., Brownie C., Gould F., Schal C. (2005). Male and female antennal responses in Heliothis virescens and H. subflexa to conspecific and heterospecific sex pheromone compounds. Environ. Entomol. 34, 256–263. doi:10.1603/0046-225x-34.2.256
Guo H., Miao S., Ai P., Zhang M., Yan Z., Yan Z., et al. (2022): Bioactive volatile compounds from penicillium digitatum-infected apples: Oviposition attractants for yellow peach moth Conogethes punctiferalis (Lepidoptera: Crambidae). (Preprint). doi:10.21203/rs.3.rs-1619268/v1
Guo T., Zhang Z., Zhou C., Liu F., Mu W. (2010). Susceptibilities of Lygus lucorum meyer-dür (Hemiptera: Miridae) from five cotton-growing regions in shandong, China to selected insecticides. Acta Entomol. Sin. 53, 993–1000.
Henze M. J., Lind O., Mappes J., Rojas B., Kelber A. (2018). An aposematic colour-polymorphic moth seen through the eyes of conspecifics and predators–Sensitivity and colour discrimination in a tiger moth. Funct. Ecol. 32, 1797–1809. doi:10.1111/1365-2435.13100
Hosseini S. A., Goldansaz S. H., Menken S. B., van Wijk M., Roessingh P., Groot A. T. (2017). Field attraction of carob moth to host plants and conspecific females. J. Econ. Entomol. 110, 2076–2083. doi:10.1093/jee/tox218
Ian E., Kirkerud N. H., Galizia C. G., Berg B. G. (2017). Coincidence of pheromone and plant odor leads to sensory plasticity in the heliothine olfactory system. PloS one 12, e0175513. doi:10.1371/journal.pone.0175513
Kendra P. E., Owens D., Montgomery W. S., Narvaez T. I., Bauchan G. R., Schnell E. Q., et al. (2017). α-Copaene is an attractant, synergistic with quercivorol, for improved detection of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae). PLoS One 12, e0179416. doi:10.1371/journal.pone.0179416
Kerr J. L., Kelly D., Bader M. K. F., Brockerhoff E. G. (2017). Olfactory cues, visual cues, and semiochemical diversity interact during host location by invasive forest beetles. J. Chem. Ecol. 43, 17–25. doi:10.1007/s10886-016-0792-x
Knight A. L., Light D. M. (2013). Adding micro encapsulated pear ester to insecticides for control of Cydia pomonella (Lepidoptera: Tortricidae) in apple. Pest Manag. Sci. 69 (1), 66–74. doi:10.1002/ps.3363
Li M., Xia S., Zhang T., Williams L., Xiao H., Lu Y. (2022). Volatiles from cotton plants infested by Agrotis segetum (Lep.: Noctuidae) attract the larval parasitoid Microplitis mediator. Plants 11, 863. doi:10.3390/plants11070863
Lu Y., Wu K., Jiang Y., Guo Y., Desneux N. (2012). Wide spread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362–365. doi:10.1038/nature11153
Lu Y., Wu K., Jiang Y., Xia B., Li P., Feng H., et al. (2010). Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328, 1151–1154. doi:10.1126/science.1187881
Lu Y., Wu K. (2011). Mirid bugs in China: Pest status and management strategies. outlook. pest Man. 22, 248–252. doi:10.1564/22dec02
Mitra P., Mobarak S. H., Debnath R., Barik A. (2020). The role of Lathyrus sativus flower surface wax in short-range attraction and stimulant for nymph laying by an adult viviparous aphid. Bull. Entomol. Res. 110, 231–241. doi:10.1017/S0007485319000531
Mu D., Cui L., Ge J., Wang M. X., Liu L. F., Yu X. P., et al. (2012). Behavioral responses for evaluating the attractiveness of specific tea shoot volatiles to the tea green leafhopper, Empoaca vitis. Insect Sci. 19, 229–238. doi:10.1111/j.1744-7917.2011.01476.x
Nurkomar I., Buchori D., Taylor D., Kainoh Y. (2017). Innate olfactory responses of female and male parasitoid Apanteles taragamae Viereck (Hymenoptera: Braconidae) toward host plant infested by the cucumber moth Diaphania indica Saunders (Lepidoptera: Crambidae). Biocontrol Sci. Technol. 27, 1373–1382. doi:10.1080/09583157.2017.1401977
Pan H., Liu B., Lu Y., Wyckhuys K. A. (2015a). Seasonal alterations in host range and fidelity in the polyphagous mirid bug, Apolygus lucorum (heteroptera: Miridae). PloS one 10, e0117153. doi:10.1371/journal.pone.0117153
Pan H., Lu Y., Xiu C., Geng H., Cai X., Sun X., et al. (2015b). Volatile fragrances associated with flowers mediate host plant alternation of a polyphagous mirid bug. Sci. Rep. 5, 14805–14811. doi:10.1038/srep14805
Pan H., Xiu C., Lu Y. (2015c). A combination of olfactory and visual cues enhance the behavioral responses of Apolygus lucorum. J. Insect Behav. 28, 525–534. doi:10.1007/s10905-015-9521-5
Pan Y., Wang Z., Zhao S. W., Wang X., Li Y. S., Liu J. N., et al. (2022). The herbivore-induced plant volatile tetradecane enhances plant resistance to Holotrichia parallela larvae in maize roots. Pest Manag. Sci. 78, 550–560. doi:10.1002/ps.6660
Rizvi S., George J., Reddy G., Zeng X., Guerrero A. (2021). Latest developments in insect sexpheromone research and its application in agricultural pest management. Insects 12 (6), 484. doi:10.3390/insects12060484
Robert K. M., Iain M. (2010). Lure-and-kill as reduced-risk strategy for managing Helicoverpa spp. on conventional cotton crops within transgenic cotton fields. J. Biol. Control 24 (2), 91–103.
Shiojiri K., Karban R. (2008). Vascular systemic induced resistance for Artemisia cana and volatile communication for Artemisia douglasiana. Am. Midl. Nat. 159, 468–477. doi:10.1674/0003-0031(2008)159[468:vsirfa]2.0.co;2
Snodgrass G., Gore J., Abel C., Jackson R. (2009). Acephate resistance in populations of the tarnished plant bug (heteroptera: Miridae) from the Mississippi river delta. J. Econ. Entomol. 102, 699–707. doi:10.1603/029.102.0231
Sun Y., Yu H., Zhou J. J., Pickett J. A., Wu K. (2014). Plant volatile analogues strengthen attractiveness to insect. PLoS One 9, e99142. doi:10.1371/journal.pone.0099142
Sun Y. L., Huang L. Q., Pelosi P., Wang C. Z. (2012). Expression in antennae and reproductive organs suggests a dual role of an odorant-binding protein in two sibling Helicoverpa species. PloS one 7, e30040. doi:10.1371/journal.pone.0030040
Tasin M., Larsson Herrera S., Knight A. L., Barros-Parada W., Fuentes Contreras E., Pertot I. (2018). Volatiles of grape inoculated with microorganisms: Modulation of grapevine moth oviposition and field attraction. Microb. Ecol. 76, 751–761. doi:10.1007/s00248-018-1164-6
Weiss L. A., Dahanukar A., Kwon J. Y., Banerjee D., Carlson J. R. (2011). The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, 258–272. doi:10.1016/j.neuron.2011.01.001
Yin H., Li W., Xu M., Xu D., Wan P. (2021). The olfactory responses of mirid bugs to six plant extracts and their volatiles. J. Appl. Entomol. 145, 125–133. doi:10.1111/jen.12829
Zhang P., Zhao Y., Zhang X., Song Y., Zhang Z., Liu F. (2015). Field resistance monitoring of Apolygus lucorum (Hemiptera: Miridae) in shandong, China to seven commonly used insecticides. Crop Prot. 76, 127–133. doi:10.1016/j.cropro.2015.03.013
Zhang Y., van Loon J., Wang C. (2010). Tarsal taste neuron activity and proboscis extension reflex in response to sugars and amino acids in Helicoverpa armigera (Hübner). J. Exp. Biol. 213, 2889–2895. doi:10.1242/jeb.042705
Zhen C., Miao L., Liang P., Gao X. (2016). Survey of organophosphate resistance and an Ala216Ser substitution of acetylcholinesterase-1 gene associated with chlorpyrifos resistance in Apolygus lucorum (Meyer-Dür) collected from the transgenic Bt cotton fields in China. Pestic. Biochem. Physiol. 132, 29–37. doi:10.1016/j.pestbp.2016.04.008
Keywords: Adelphocoris suturalis, Apolygus lucorum, miridae, tetradecane, olfactory responses, pest management
Citation: Yin H, Li W, Xu M, Xu D and Wan P (2022) The role of tetradecane in the identification of host plants by the mirid bugs Apolygus lucorum and Adelphocoris suturalis and potential application in pest management . Front. Physiol. 13:1061817. doi: 10.3389/fphys.2022.1061817
Received: 05 October 2022; Accepted: 24 November 2022;
Published: 06 December 2022.
Edited by:
Xun Zhu, Institute of Plant Protection (CAAS), ChinaReviewed by:
Muhammad Musa Khan, Zhejiang University, ChinaCopyright © 2022 Yin, Li, Xu, Xu and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Wan, d2FucGVuZ2hiQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.