- 1The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Liver Disease Center, Qinhuangdao Third Hospital, Qinhuangdao, China
Non-alcoholic Fatty Liver Disease (NAFLD) is a chronic liver disease that is strongly related to insulin resistance and metabolic syndrome, and it has become the most common liver disorder in developed countries. NAFLD embraces the full pathological process of three conditions: steatosis, non-alcoholic steatohepatitis, and finally, cirrhosis. As NAFLD progresses, symptoms will become increasingly severe as fibrosis develops. Therefore, evaluating the fibrosis stage is crucial for patients with NAFLD. A liver biopsy is currently considered the gold standard for staging fibrosis. However, due to the limitations of liver biopsy, non-invasive alternatives were extensively studied and validated in patients with NAFLD. The advantages of non-invasive methods include their high safety and convenience compared with other invasive approaches. This review introduces the non-invasive methods, summarizes their benefits and limitations, and assesses their diagnostic performance for NAFLD-induced fibrosis.
Introduction
Non-alcoholic Fatty Liver Disease (NAFLD) has become a significant cause of chronic liver disease worldwide (Loomba and Sanyal, 2013). For those patients with NAFLD, the critical issue is how to evaluate the stage of their diseases, as they face a significant risk of developing chronic liver diseases such as hepatocellular carcinoma or cirrhosis (Marignani and Angeletti, 2002; Byrne and Targher, 2015; Fan et al., 2017). The prognosis and management of NAFLD greatly depend on the progression of non-alcoholic steatohepatitis and liver fibrosis. Early-stage fibrosis is reversible, and patients will recover better if they get treatments in time.
Liver biopsy remains the most appropriate method for differentiating non-alcoholic fatty liver (NAFL) from non-alcoholic steatohepatitis and staging liver fibrosis (Byrne and Targher, 2015). However, its accuracy has been questioned, as liver biopsy presents some limitations, including sampling errors, variability, and invasiveness (Ratziu et al., 2005; Merriman et al., 2006; Bonekamp et al., 2014). These limitations contribute to the search for new non-invasive approaches to detect clinically significant samples and help patients get treatments ahead of time. Recent research is performed from two perspectives: serum biomarkers and imaging techniques (ultrasound, CT, MRI, etc.) for evaluating liver stiffness (Castera and Pinzani, 2010). We review non-invasive diagnostic methods for liver fibrosis and assess their advantages, limitations, and diagnostic performance in patients with NAFLD.
Histologic stages of hepatic fibrosis
Patients with NAFLD suffer from a continuous spectrum of steatosis, inflammation, and fibrosis. Therefore, it would be difficult to assess the stage and progression of the disease. The SAF scoring system, developed by the European consortium for Fatty Liver Inhibition of Progression, was specifically designed to evaluate NALFD. For each case, a SAF score was created based on the semiquantitative scoring of steatoses (S), activity (A), and fibrosis (F). The stage of fibrosis (F) was assessed using the score described by non-alcoholic steatohepatitis-CRN as follows: stage 0 (F0) none; stage 1 (F1): 1a or 1b perisinusoidal zone 3 or 1c portal fibrosis; stage 2 (F2): perisinusoidal and periportal fibrosis without bridging; stage 3 (F3): bridging fibrosis; and stage 4 (F4): cirrhosis (Bedossa and Consortium, 2014).
Diagnosis and staging of hepatic fibrosis
Hepatic fibrosis can be non-invasively measured through two complementary approaches, including a “biological” approach (quantifying serum biomarkers) or a “physical” approach (measuring liver stiffness with the use of image technology). These two approaches can perform their unique functions according to different rationales. Imaging-based liver stiffness is consistent with an intrinsic physical property of liver tissue (European Association for Study of, 2015).
Serum biomarkers
Serum biomarkers can be divided into direct markers and indirect markers. Direct markers can reflect the deposition or removal of fibrotic tissue in the liver. Indirect markers are markers of comprehensive liver function.
Routine laboratory tests
Indirect markers, such as serum bilirubin and albumin levels, are often abnormal in patients with cirrhosis, and prothrombin time will increase (Schuppan and Afdhal, 2008). The platelet count will be low because of the hypersplenism related to portal hypertension. When these laboratory markers become abnormal, liver fibrosis is often already clinically apparent and irreversible. Although these biomarkers might help evaluate the stage of advanced liver diseases, they are often not able to detect early-stage fibrosis. Other indirect markers include the ratio of aspartate aminotransferase to alanine aminotransferase (AST/ALT), AST: platelet ratio index (APRI), α2-macroglobulin (A2M), apolipoprotein A1, glutamyl transpeptidase (GGT). The connection between AST/ALT ratio and liver fibrosis has been confirmed (Sheth et al., 1998). The ratio of AST/ALT is often <1 in patients with early-stage fibrosis (F1-F2). However, it will increase with the stage of fibrosis evolving into cirrhosis (Angulo et al., 1999). The APRI score (AST/platelet ratio) is recommended as another marker for advanced liver fibrosis. The accuracy of the APRI score in assessing the stage of liver fibrosis in patients with NAFLD has been confirmed in many studies (Kruger et al., 2011). A study involving 111 patients reported an AUROC value of APRI of 0.85, a Se of 75%, an Sp of 86%, a PPV of 54%, and an NPV of 93%. Elevated serum ferritin levels have been found in patients with NAFLD (Chitturi et al., 2002; Bugianesi et al., 2004). Most researchers believe high serum iron indices are related to liver damage and inflammation (Chitturi et al., 2002; Bugianesi et al., 2004; Manousou et al., 2011; Feldman et al., 2016). A recent cross-sectional descriptive study involving 284 patients confirmed a significant connection between serum ferritin levels and liver stiffness. Therefore, a low serum ferritin level may be one cost-effective option to exclude patients with advanced fibrosis from liver biopsy and elastography (Seyedian et al., 2017).
In recent years, novel serum biomarkers have been discovered, including Mac-2 binding protein glycan isomer (M2BPGi), Wisteria floribunda agglutinin-positive Mac-2 binding protein (WF), soluble Axl (sAxl), osteopontin, angiotensin-converting enzyme (ACE) (Pereira et al., 2016; Miranda and Simoes, 2017; Staufer et al., 2017; Ogawa et al., 2018; Shirabe et al., 2018), and cytokeratin 18 (M30 and M65) (Lee et al., 2020). However, the accuracy of these biomarkers as a marker of fibrosis is still unknown. More research is needed before they can be recommended as appropriate fibrosis severity markers.
Combination with clinical features
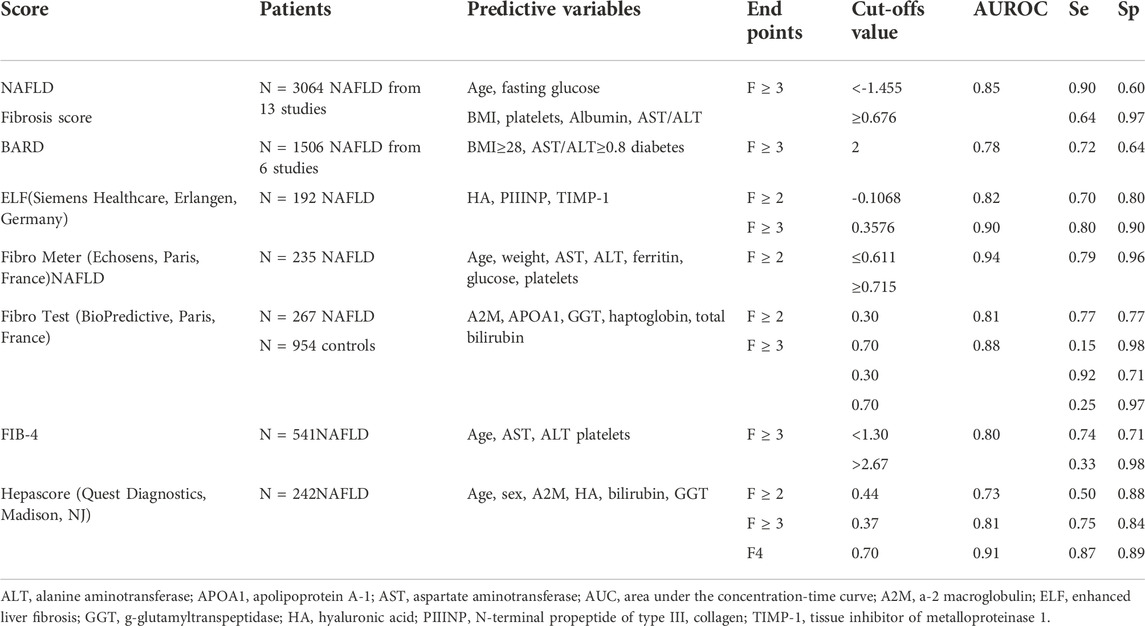
To improve the sensitivity and predictive effect of simple laboratory tests for liver fibrosis, serum biomarkers (most direct markers and several indirect markers) and clinical features have been combined to create several multivariate analyses for liver fibrosis: the FIB-4 index; the NAFLD fibrosis score; the ELF (Siemens Healthcare, Erlangen, Germany); the BARD score; the FibroTest (BioPredictive, Paris, France); the FibroMeter™ NAFLD (BioPredictive, Paris, France); and the Hepascore (Quest Diagnostics, Madison, NJ) (Table 1) (Ratziu et al., 2006; Guha et al., 2008; Cales et al., 2009; Nobili et al., 2009; Shah et al., 2009; Adams et al., 2011; Musso et al., 2011; Sumida et al., 2012; Dincses and Yilmaz, 2015).
A simple scoring system such as the NAFLD fibrosis score could accurately separate patients with NAFLD with and without advanced fibrosis and allow a substantial proportion of patients to avoid liver biopsies (Angulo et al., 2007). In a meta-analysis of 3064 people with NAFLD, the value of the Area Under the Receiver Operating Characteristic (AUROC) for patients with stage 3–4 fibrosis was 0.8526The NAFLD practice guidelines emphasized its effectiveness in identifying patients with advanced NAFLD (Chalasani et al., 2012). The American Association for the Study of Liver Disease has endorsed its use to routinely determine the need for liver biopsy for fibrosis staging in patients with NAFLD.
FIB-4 has been validated independently by three NAFLD cohorts in the United States, Europe, and Asia. Although these studies have used different cutoff values, they were successful in excluding advanced fibrosis (Shah et al., 2009; McPherson et al., 2010; Sumida et al., 2012). The ALT/AST ratio, FIB-4, and NAFLD fibrosis scores can reliably exclude advanced fibrosis in a high proportion of patients with NAFLD, allowing liver biopsy to be used in a more directed manner.
Complex fibrosis models such as Hepascore, FibroTest, and FIB4 have been reported to be more accurate in detecting fibrosis than simple fibrosis models (BARD or APRI) (Adams et al., 2011; Musso et al., 2011). When compared with the NAFLD fibrosis score, these biomarker panels generally have comparable accuracy in diagnosing advanced liver fibrosis in patients with NAFLD (Cales et al., 2009).
The FibroMeter NAFLD, which estimates the stage of fibrosis based on age, weight, AST, ALT, ferritin, glucose, and platelets values, has been validated in a NAFLD cohort with 235 patients. The AUROC to detect advanced fibrosis (F3-F4) was excellent and markedly better than the APRI (Cales et al., 2009).
The Enhanced Liver Fibrosis (ELF) test is a commercially available algorithm that includes three serum biomarkers: hyaluronic acid (HA), the N-terminal pro-peptide of collagen type III (PIIINP), and tissue inhibitor of metalloproteinase-1 (TIMP1) (Rosenberg et al., 2004; Guha et al., 2008). The ELF (the cutoff value is 10.5) was recently recommended to detect advanced fibrosis in patients with NAFLD (Glen et al., 2016). However, this recommendation is contentious because it is based on a pediatric NAFLD study (Nobili et al., 2009). A recent prospective, direct comparison of tests that included 289 patients showed that the AUROC of ELF-identified patients with advanced liver fibrosis is 0.92 (95% confidence interval 0.89–0.96). This study also compared ELF with FibroTest and Elastography. ELF had a generally high diagnostic accuracy (AUROC values of 0.90 or higher) in identifying advanced liver fibrosis (Thiele et al., 2018
All these algorithms and systems, which combine serum biomarkers and clinical features, seem helpful in identifying patients with a low risk of advanced liver fibrosis, so a liver biopsy for staging purposes can be avoided. However, none of these algorithms were designed to predict disease progression. Moreover, these models are not sufficiently accurate for patients with suspected advanced fibrosis to replace a liver biopsy.
Imaging
Transient elastography
Transient Elastography (TE; FibroScan, Echosens, Paris, France) is the imaging technique used most frequently to assess fibrosis in patients with NAFLD in clinical practice. The stage of liver fibrosis can be assessed using TE (Sandrin et al., 2003).
TE is a technique based on ultrasound (United Kingdom) (5 MHz) and low-frequency (50 Hz) elastic waves, whose propagation velocity through the liver is directly related to liver tissue stiffness. Elastic modulus is the terminology used to describe tissue stiffness and is expressed as E = 3 ρv (Marignani and Angeletti, 2002), where v is the shear velocity and ? is the density of tissue as an invariant. In brief, the faster propagation of the shear wave indicates stiffer tissue. TE measures liver stiffness in a volume that is approximately a 1 cm wide per 4 cm long cylinder, with the M probe measuring 25–65 mm and the XL probe measuring 35–75 mm below the skin surface (Roulot et al., 2008). The values are expressed in kilopascals (kPa) which range between 2.4 and 75.4 kPa. The optimal LSM cutoff for maximum specificity and sensitivity ranges from 7.2 to 11.4 kpa (Wong et al., 2012; Imajo et al., 2016).
TE-based liver stiffness measurements using the M probe have been shown to correlate with fibrosis stages, particularly in severe fibrosis and cirrhosis (Friedrich-Rust et al., 2008; de Ledinghen et al., 2012; Pavlov et al., 2016). The benefits of using TE to measure liver stiffness include a quick procedure (<5 min), immediate results, and the ability to conduct the test when the patient is in the hospital or an outpatient clinic. It is easy to learn the procedure of TE and can be performed by a medical intern or a nurse after a bit of training (Tsai and Lee, 2018). However, accurate TE results require careful interpretation of data based on at least ten validated measurements, limiting its simplicity, application, and stability (Castera et al., 2008). An important limitation of TE is the high failure rates in overweight or obese patients with a BMI >28 kg/m2, which limits the measurement of steatosis and liver stiffness in obese patients with NAFLD (Foucher et al., 2006; de Ledinghen et al., 2014). However, a new TE probe (XL) equipped with CAP has been proposed to reduce the failure rate of detecting fibrosis in patients who are overweight or obese (de Ledinghen et al., 2012; Wong et al., 2012).
Performance of TE for staging liver fibrosis
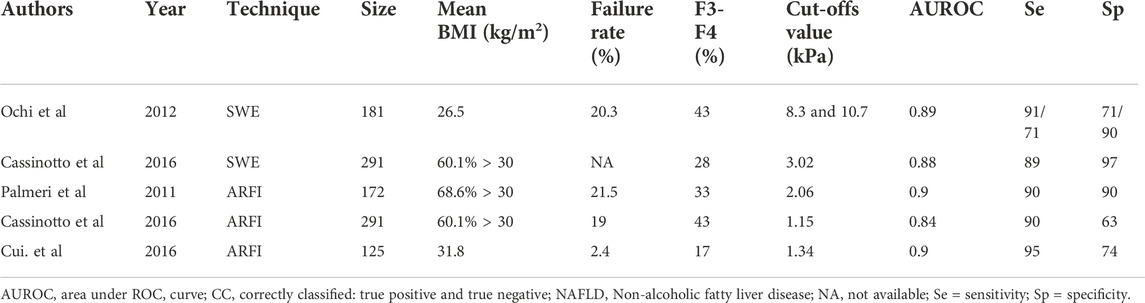
Although TE has been evaluated in many studies on patients with viral hepatitis, it has been used in fewer studies of NAFLD. TE estimated cirrhosis with higher AUROC values (0.95–0.97) than significant fibrosis (0.80) (Castera, 2015). A meta-analysis including nine studies and 1047 patients with NAFLD found that (vibration-controlled transient elastography) VCTE detected cirrhosis with an aggregate value of 92%, significant fibrosis with a 79% sensitivity, and cirrhosis with a 75% sensitivity (Kwok et al., 2014). Table 2 enumerates the diagnostic performance of TE for measuring advanced liver fibrosis in patients with NAFLD (Nobili et al., 2008; Yoneda et al., 2008; Lupsor et al., 2010; Wong et al., 2010; Gaia et al., 2011; Petta et al., 2011; Myers et al., 2012; Wong et al., 2012; Kumar et al., 2013; Mahadeva et al., 2013; Aykut et al., 2014; Naveau et al., 2014; Chan et al., 2015; Petta et al., 2015; Boursier et al., 2016; Cassinotto et al., 2016; Imajo et al., 2016; Pavlov et al., 2016; Tapper et al., 2016; Chen et al., 2017; Park et al., 2017).
These studies indicate that TE could be used to confidently exclude severe fibrosis in NAFLD. Still, the high rate of unreliable results with transient elastography remains a challenge, which is not entirely addressed using the XL probe. However, due to the high prevalence of NAFLD in the general population, using TE could be significant in helping to determine which patients still require liver biopsies.
Shear-wave elastography
Shear-Wave Elastography (SWE) is a novel, non-invasive method that the FDA has approved to assess liver stiffness. In SWE, the operator targets the liver using a 2D mode ultrasonography image to find a homogeneous area free of large vascular structures. The variable depth and diameter of the region of interest are defined in the visualized liver. The shear wave propagation speed in the area will be recorded by converting it into stiffness measurements, and a color map superimposed on the 2D-mode images can be constructed (Deffieux et al., 2015).
Performance of SWE for staging liver fibrosis
Compared with TE, fewer studies focus on the performance of SWE. The largest study, including 291 patients with NAFLD, found that the combined failure rates were 20.3% and the AUROC values for advanced fibrosis were 0.89. Cutoffs of 8.7 kPa provided 90% sensitivity and specificity (Ochi et al., 2012; Cassinotto et al., 2016).
Acoustic radiation force impulse imaging
With technological advances and clinical practice, Acoustic Radiation Force Impulse imaging (ARFI) is considered a standard ultrasound device. The ARFI operator, using a curved abdominal probe, defines a large area free of large vascular structures. Following that, short-duration (∼262 μs) acoustic pulses (with frequencies between 1.0 and 4.5 MHz) propagate shear waves and generate localized, µ-scale displacements in liver tissue. The ultrasound receiver tracks the shear-wave velocity of ARFI in a smaller volume (5 mm by 4 mm) than TE. The significant advantage of ARFI is that it can be easily implemented on a regular ultrasound machine. In addition, it has higher applicability than TE (Friedrich-Rust et al., 2009).
Performance of ARFI for staging liver fibrosis
ARFI performance for staging liver fibrosis has been evaluated in five studies of patients with NAFLD (Table 3) (Ebinuma et al., 2011; Palmeri et al., 2011; Cassinotto et al., 2016; Cui et al., 2016). The AUROC for advanced fibrosis is 0.90, similar to TE and SWE. However, it also suffers from the risks of technical failure or unreliable results. In addition, technical failure rates tend to increase in patients with higher BMI (>30 kg/m2). Although the optimal cutoff is hard to determine, a shear-wave speed (about 1.34 m/s) is closely associated with advanced fibrosis (Ebinuma et al., 2011; Yoon et al., 2012). The largest study up to date by Cassinoto et al. provides cutoffs of 1.15 m/s and 1.53 m/s, which yield 90% sensitivity and specificity, respectively (Cassinotto et al., 2016).
Magnetic resonance elastography
Magnetic Resonance (MR) elastography has been shown to accurately diagnose fibrosis and cirrhosis in patients with NAFLD. MR elastography can be implemented on a conventional magnetic resonance imaging (MRI) system with special adaptation software. The propagation characteristics of the shear wave in the liver can be imaged with a modified phase-contrast method. Elasticity is quantified by MR elastography (expressed in kPa) using a formula that calculates the shear modules. MR elastography has obvious advantages compared with other imaging methods, including the assessment of almost the entire liver and a lower technical failure rate (Venkatesh et al., 2013; Loomba et al., 2014; Loomba et al., 2016). The largest study of MR elastography found that the failure rate was 7.7% in patients with NAFLD (Wagner et al., 2017). In general, MR elastography performs better than all ultrasound-based methods. Moreover, it has a lower risk of failure in patients with severe obesity (Cui et al., 2015; Cui et al., 2016).
Performance of MRE for staging liver fibrosis
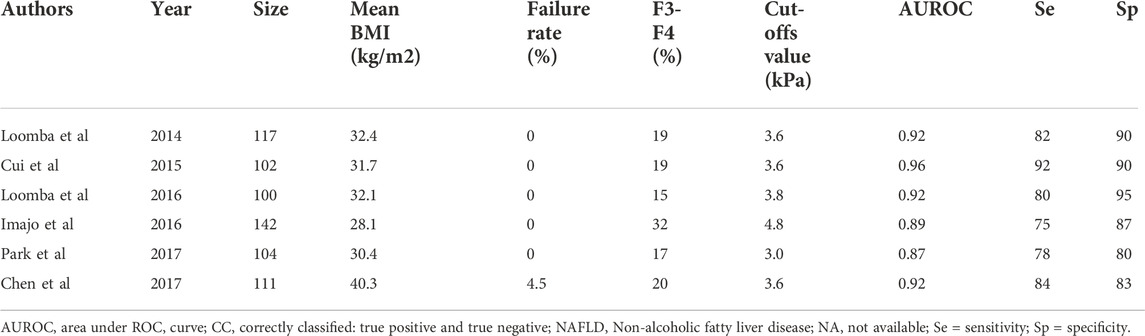
Six studies have focused on the performance of MR elastography for staging liver fibrosis (Table 4) (Huwart et al., 2008; Talwalkar, 2008; Loomba et al., 2014; Cui et al., 2015; Cui et al., 2016; Imajo et al., 2016; Loomba et al., 2016; Chen et al., 2017; Park et al., 2017). These studies found that MR elastography has a similar accuracy in diagnosing significant fibrosis and cirrhosis. In all studies, the AUROC values ranged between 0.92 and 0.94 for the diagnosis of significant fibrosis. However, the optimal cutoff values remained undetermined. In a study enrolling 100 patients with NAFLD (mean BMI of 32.4 kg/m2), the optimal cutoff for significant fibrosis and cirrhosis was 3.63 kPa. Another study, involving 104 patients, compared the performance of MR elastography and TE and found that the optimal cutoff value was 2.99 kPa (Park et al., 2017).
Comparison of approaches
Studies comparing TE and biomarkers, and other imaging methods, are limited. When patients had successful imaging-logic examinations, TE performed better than these simple laboratory serum biomarkers in detecting advanced fibrosis (Castera et al., 2005; Poynard et al., 2008; Adams, 2010; Wong et al., 2010; Kim et al., 2013a). However, signal-positive predictive results remain suspect because of some well-known confounders, including obesity and liver inflammation. This is the reason why strategies combining imaging approaches and serum biomarkers have shown increased diagnostic accuracy. Moreover, positive values need careful evaluation considering factors such as age, laboratory tests, and the scoring system (the NAFLD score, FIB-4).
A study of 291 patients comparing TE with ARFI and SWE found that no method could get more reliable results than others (Cassinotto et al., 2016). They also shared similar AUROC values for advanced fibrosis (VCTE 0.86, SWE 0.89, and ARFI 0.84). Three studies compared TE with MR elastography (Cui et al., 2016; Imajo et al., 2016; Park et al., 2017). An analysis of 143 patients has compared MR elastography with simple algorithms such as the NAFLD fibrosis score and FIB-4 score for assessing advanced fibrosis. The AUROC value for MRE, FIB-4 score, and NAFLD fibrosis score were 0.945, 0.88, and 0.86, respectively. In general, MR elastography is the best imaging modality for detecting liver fibrosis in patients with NAFLD because of its low risk of technical failure in overweight patients. However, TE and other ultrasound-based approaches may obtain unreliable results in a similar setting. The most significant limitation of MR elastography is its high cost and limited access. As a result, other imaging methods discussed here are typically attempted first. However, the optimal BMI cutoff value to decide which modality should be used first needs further investigation.
Prospects
Non-invasive diagnoses of liver fibrosis in patients with NAFLD have made significant progress over the past decade. Given the increasing prevalence of NAFLD, this improvement is effective and promising. Serum biomarkers and developing scoring systems (the FIB-4 score, the NAFLD fibrosis score) are of increasing diagnostic and screening value for patients with NAFLD (Ngo et al., 2006; Naveau et al., 2009; Nunes et al., 2010; Parkes et al., 2010; Robic et al., 2011; Vergniol et al., 2011) and advanced fibrosis (Kim et al., 2013b; Treeprasertsuk et al., 2013).
Future blood fibrosis biomarkers
The next-generation of functional genomic biomarkers is an emerging tool for evaluating fibrogenesis’s dynamic nature. However, validating these complex and relatively expensive methodologies is difficult, thereby limiting their clinical application. The sustained studies of the genome-wide association are a promising way to identify genomic factors and biomarkers of fibrosis (Anstee and Day, 2015). For instance, the PNPLA3 variant encoding I148M has been associated with NAFLD. However, further studies are needed to validate how these factors contribute to pathogenesis. Current proteomic research also provides several candidate serum biomarkers. Another example is represented by eicosanoid metabolites, which have been identified as potential fibrosis biomarkers (Dongiovanni et al., 2013; Anstee and Day, 2015; Dongiovanni et al., 2015).
MicroRNAs (miRNA) are small non-coding RNAs that regulate posttranscriptional gene expression and are associated with a diverse range of pathophysiologic processes (Panera et al., 2014). Several miRNAs have been proposed as potential biomarkers of progressive liver fibrosis. For instance, hepatic and serum concentrations of miRNA-122 have been associated with liver fibrosis (Miyaaki et al., 2014; Pirola et al., 2015). Identifying biomarkers based on miRNA transcripts detectable in blood or urine represents a novel approach to non-invasively diagnosing liver fibrosis and cirrhosis. However, quantifying miRNAs remains unreliable and it can produce different and varying results. Long non-coding RNAs (>200 nucleotides) and genomic and proteomic profiles of circulating extracellular vesicles may be associated with NAFLD pathogenesis. They could be used in biomarker analyses in the future. Unfortunately, compared to long non-coding RNAs (>200 nucleotides), miRNAs measured in blood or urine have not yet shown viable diagnostic or prognostic utility (Lemoinne et al., 2014; Takahashi et al., 2014).
Additionally, the application of liquid biopsy was also trialed to diagnose the presence and severity of NASH or liver fibrosis (Angelini et al., 2022). In a recent multicenter study involving 250 patients with NAFLD (Angelini et al., 2022), proteomics was performed in circulating monocytes and hepatic stellate cells, and perilipin-2 and RAB14 were measured in peripheral blood CD14+CD16− monocytes. Results suggested that the diagnostic method based on liquid biopsy was superior to FIB4 and NAFLD fibrosis scores and that it was comparable to two-dimensional shear wave elastography. More studies on liquid biopsies to evaluate liver fibrosis should be conducted in the future.
Future imaging methods
Elastography for detecting fibrosis in patients with NAFLD emerges as an effective method in contemporary clinical practice. However, the specific role of each test in both the clinic and investigative endeavors remains to be clarified. Moving forward, several aspects of study design should be considered.
Because of the mounting data on the effect of necroinflammatory activity and potentially hepatic steatosis on LSM, further research should define and operationalize how LSM cutoffs are interpreted in patients with variable inflammatory activity and steatosis. VCTE combined with CAP has found enough data. However, more research is needed for MRE, SWE, and ARFI to determine how to incorporate these methods into clinical practice (Petta et al., 2017). Algorithms that better calculate the effect of confounding factors could be better options.
For MRE, SWE, and ARFI, which have been increasingly applied to clinical practice in recent times, efforts must be done to provide consistent, reproducible quality criteria. MRE, SWE, and ARFI each require the operator to define the region of interest, which may cause human error and fault. As a result, research is required to determine this critical aspect of the test procedure and to formulate consensus-defined criteria that can be used to unify the quality of the selected region operator. If the regions of interest are not consistent among operators or undergo different imaging tests, the clinical meaning of any test result would be unclear (Dietrich et al., 2017).
Future strategies for staging liver fibrosis
The rapid development of non-invasive technology has brought excellent progress in diagnosing liver fibrosis. However, it challenges researchers and brings many problems to clinical trials. Unreliable results and undeliberate clinical approaches will be a disaster for patients; that’s why liver biopsy and non-invasive methods should be used as part of an integrated system to enable more efficient and convenient management of patients with NAFLD. In addition, the combination of serum biomarkers and TE seemed to be more effective than the combination of serum biomarkers for detecting significant fibrosis, probably because diagnostic models, including liver stiffness measurements, can describe fibrotic status more completely than models merely including serum biomarkers. Further studies, such as meta-analyses and cost-effectiveness analyses that compare the accuracy and cost-saving strategies of the several biomarkers of liver fibrosis in NAFLD, both alone and in combination with imaging methods, are encouraged. Besides, artificial intelligence has provided a powerful tool to evaluate liver fibrosis in patients with NAFLD (Li et al., 2022).
Finally, this study focused on advanced fibrosis because of its high risk for hepatocellular carcinoma. It should be noted that the differentiation of NASH from simple steatosis and the identification of advanced hepatic fibrosis are critical issues in NAFLD (Castera et al., 2019However, most studies focused on significant fibrosis, advanced fibrosis, and cirrhosis, and the research for early fibrosis remained limited. Therefore, future studies should investigate the non-invasive diagnosis of early liver fibrosis.
Author contributions
This work was supported by the National Natural Science Foundation of China (No. 81972283). JW, TQ, and JS wrote the manuscript. SL collected articles. LC and XL revised and edited the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81972283).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams L. (2010). Transient elastography in nonalcoholic fatty liver disease: Making sense of echoes. Hepatology 51 (2), 370–372. doi:10.1002/hep.23422
Adams L. A., George J., Bugianesi E., Rossi E., De Boer W. B., van der Poorten D., et al. (2011). Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 26 (10), 1536–1543. doi:10.1111/j.1440-1746.2011.06774.x
Angelini G., Panunzi S., Castagneto-Gissey L., Pellicano F., De Gaetano A., Pompili M., et al. (2022). Accurate liquid biopsy for the diagnosis of non-alcoholic steatohepatitis and liver fibrosis. Gut–2022. 327498. doi:10.1136/gutjnl-2022-327498
Angulo P., Hui J. M., Marchesini G., Bugianesi E., George J., Farrell G. C., et al. (2007). The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45 (4), 846–854. doi:10.1002/hep.21496
Angulo P., Keach J. C., Batts K. P., Lindor K. D. (1999). Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 30 (6), 1356–1362. doi:10.1002/hep.510300604
Anstee Q. M., Day C. P. (2015). The genetics of nonalcoholic fatty liver disease: Spotlight on PNPLA3 and TM6SF2. Semin. Liver Dis. 35 (3), 270–290. doi:10.1055/s-0035-1562947
Aykut U. E., Akyuz U., Yesil A., Eren F., Gerin F., Ergelen R., et al. (2014). A comparison of FibroMeter NAFLD Score, NAFLD fibrosis score, and transient elastography as noninvasive diagnostic tools for hepatic fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 49 (11), 1343–1348. doi:10.3109/00365521.2014.958099
Bedossa P., Consortium F. P. (2014). Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 60 (2), 565–575. doi:10.1002/hep.27173
Bonekamp S., Tang A., Mashhood A., Wolfson T., Changchien C., Middleton M. S., et al. (2014). Spatial distribution of MRI-Determined hepatic proton density fat fraction in adults with nonalcoholic fatty liver disease. J. Magn. Reson. Imaging 39 (6), 1525–1532. doi:10.1002/jmri.24321
Boursier J., Vergniol J., Guillet A., Hiriart J. B., Lannes A., Le Bail B., et al. (2016). Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J. Hepatol. 65 (3), 570–578. doi:10.1016/j.jhep.2016.04.023
Bugianesi E., Manzini P., D'Antico S., Vanni E., Longo F., Leone N., et al. (2004). Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology 39 (1), 179–187. doi:10.1002/hep.20023
Byrne C. D., Targher G. (2015). Nafld: A multisystem disease. J. Hepatol. 62, S47–S64. doi:10.1016/j.jhep.2014.12.012
Cales P., Laine F., Boursier J., Deugnier Y., Moal V., Oberti F., et al. (2009). Comparison of blood tests for liver fibrosis specific or not to NAFLD. J. Hepatol. 50 (1), 165–173. doi:10.1016/j.jhep.2008.07.035
Cassinotto C., Boursier J., de Ledinghen V., Lebigot J., Lapuyade B., Cales P., et al. (2016). Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 63 (6), 1817–1827. doi:10.1002/hep.28394
Castera L., Forns X., Alberti A. (2008). Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 48 (5), 835–847. doi:10.1016/j.jhep.2008.02.008
Castera L., Friedrich-Rust M., Loomba R. (2019). Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156 (5), 1264–1281. doi:10.1053/j.gastro.2018.12.036
Castera L. (2015). Noninvasive evaluation of nonalcoholic fatty liver disease. Semin. Liver Dis. 35 (3), 291–303. doi:10.1055/s-0035-1562948
Castera L., Pinzani M. (2010). Non-invasive assessment of liver fibrosis: Are we ready? Lancet 375 (9724), 1419–1420. doi:10.1016/S0140-6736(09)62195-4
Castera L., Vergniol J., Foucher J., Le Bail B., Chanteloup E., Haaser M., et al. (2005). Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128 (2), 343–350. doi:10.1053/j.gastro.2004.11.018
Chalasani N., Younossi Z., Lavine J. E., Diehl A. M., Brunt E. M., Cusi K., et al. (2012). The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American gastroenterological association, American association for the study of liver diseases, and American college of gastroenterology. Gastroenterology 142 (7), 1592–1609. doi:10.1053/j.gastro.2012.04.001
Chan W. K., Nik Mustapha N. R., Mahadeva S. (2015). A novel 2-step approach combining the NAFLD fibrosis score and liver stiffness measurement for predicting advanced fibrosis. Hepatol. Int. 9 (4), 594–602. doi:10.1007/s12072-014-9596-7
Chen J., Yin M., Talwalkar J. A., Oudry J., Glaser K. J., Smyrk T. C., et al. (2017). Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology 283 (2), 418–428. doi:10.1148/radiol.2016160685
Chitturi S., Weltman M., Farrell G. C., McDonald D., Kench J., Liddle C., et al. (2002). HFE mutations, hepatic iron, and fibrosis: Ethnic-specific association of NASH with C282Y but not with fibrotic severity. Hepatology 36 (1), 142–149. doi:10.1053/jhep.2002.33892
Cui J., Ang B., Haufe W., Hernandez C., Verna E. C., Sirlin C. B., et al. (2015). Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: A prospective study. Aliment. Pharmacol. Ther. 41 (12), 1271–1280. doi:10.1111/apt.13196
Cui J., Heba E., Hernandez C., Haufe W., Hooker J., Andre M. P., et al. (2016). Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology 63 (2), 453–461. doi:10.1002/hep.28337
de Ledinghen V., Vergniol J., Capdepont M., Chermak F., Hiriart J. B., Cassinotto C., et al. (2014). Controlled attenuation parameter (CAP) for the diagnosis of steatosis: A prospective study of 5323 examinations. J. Hepatol. 60 (5), 1026–1031. doi:10.1016/j.jhep.2013.12.018
de Ledinghen V., Wong V. W., Vergniol J., Wong G. L. H., Foucher J., Chu S. H. T., et al. (2012). Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan®. J. Hepatol. 56 (4), 833–839. doi:10.1016/j.jhep.2011.10.017
Deffieux T., Gennisson J. L., Bousquet L., Corouge M., Cosconea S., Amroun D., et al. (2015). Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J. Hepatol. 62 (2), 317–324. doi:10.1016/j.jhep.2014.09.020
Dietrich C. F., Bamber J., Berzigotti A., Bota S., Cantisani V., Castera L., et al. (2017). EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall Med. 38 (4), e16–e47. doi:10.1055/s-0043-103952
Dincses E., Yilmaz Y. (2015). Diagnostic usefulness of FibroMeter VCTE for hepatic fibrosis in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 27 (10), 1149–1153. doi:10.1097/MEG.0000000000000409
Dongiovanni P., Donati B., Fares R., Lombardi R., Mancina R. M., Romeo S., et al. (2013). PNPLA3 I148M polymorphism and progressive liver disease. World J. Gastroenterol. 19 (41), 6969–6978. doi:10.3748/wjg.v19.i41.6969
Dongiovanni P., Romeo S., Valenti L. (2015). Genetic factors in the pathogenesis of nonalcoholic fatty liver and steatohepatitis. Biomed. Res. Int. 2015, 460190. doi:10.1155/2015/460190
Ebinuma H., Saito H., Komuta M., Ojiro K., Wakabayashi K., Usui S., et al. (2011). Evaluation of liver fibrosis by transient elastography using acoustic radiation force impulse: Comparison with Fibroscan(®). J. Gastroenterol. 46 (10), 1238–1248. doi:10.1007/s00535-011-0437-3
European Association for Study of L. (2015). EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 63 (1), 237–264. doi:10.1016/j.jhep.2015.04.006
Fan J. G., Kim S. U., Wong V. W. (2017). New trends on obesity and NAFLD in Asia. J. Hepatol. 67 (4), 862–873. doi:10.1016/j.jhep.2017.06.003
Feldman M., Friedman L. S., Brandt L. J. (2016). Sleisenger and fordtran's gastrointestinal and liver disease : Pathophysiology/diagnosis/management. Tenth edition. Philadelphia, PA: Saunders/Elsevier.
Foucher J., Castera L., Bernard P. H., Adhoute X., Laharie D., Bertet J., et al. (2006). Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur. J. Gastroenterol. Hepatol. 18 (4), 411–412. doi:10.1097/00042737-200604000-00015
Friedrich-Rust M., Ong M. F., Martens S., Sarrazin C., Bojunga J., Zeuzem S., et al. (2008). Performance of transient elastography for the staging of liver fibrosis: A meta-analysis. Gastroenterology 134 (4), 960–974. doi:10.1053/j.gastro.2008.01.034
Friedrich-Rust M., Wunder K., Kriener S., Sotoudeh F., Richter S., Bojunga J., et al. (2009). Liver fibrosis in viral hepatitis: Noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 252 (2), 595–604. doi:10.1148/radiol.2523081928
Gaia S., Carenzi S., Barilli A. L., Bugianesi E., Smedile A., Brunello F., et al. (2011). Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J. Hepatol. 54 (1), 64–71. doi:10.1016/j.jhep.2010.06.022
Glen J., Floros L., Day C., Pryke R., Guideline Development G. (2016). Non-alcoholic fatty liver disease (NAFLD): Summary of NICE guidance. BMJ 354, i4428. doi:10.1136/bmj.i4428
Guha I. N., Parkes J., Roderick P., Chattopadhyay D., Cross R., Harris S., et al. (2008). Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 47 (2), 455–460. doi:10.1002/hep.21984
Huwart L., Sempoux C., Vicaut E., Salameh N., Annet L., Danse E., et al. (2008). Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 135 (1), 32–40. doi:10.1053/j.gastro.2008.03.076
Imajo K., Kessoku T., Honda Y., Tomeno W., Ogawa Y., Mawatari H., et al. (2016). Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 150 (3), 626–637. doi:10.1053/j.gastro.2015.11.048
Kim D., Kim W. R., Kim H. J., Therneau T. M. (2013). Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 57 (4), 1357–1365. doi:10.1002/hep.26156
Kim D., Kim W. R., Talwalkar J. A., Kim H. J., Ehman R. L. (2013). Advanced fibrosis in nonalcoholic fatty liver disease: Noninvasive assessment with MR elastography. Radiology 268 (2), 411–419. doi:10.1148/radiol.13121193
Kruger F. C., Daniels C. R., Kidd M., Swart G., Brundyn K., van Rensburg C., et al. (2011). Apri: A simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr. Med. J. 101 (7), 477–480.
Kumar R., Rastogi A., Sharma M. K., Bhatia V., Tyagi P., Sharma P., et al. (2013). Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: Diagnostic performance and clinicopathological correlation. Dig. Dis. Sci. 58 (1), 265–274. doi:10.1007/s10620-012-2306-1
Kwok R., Tse Y. K., Wong G. L., Ha Y., Lee A. U., Ngu M. C., et al. (2014). Systematic review with meta-analysis: Non-invasive assessment of non-alcoholic fatty liver disease-the role of transient elastography and plasma cytokeratin-18 fragments. Aliment. Pharmacol. Ther. 39 (3), 254–269. doi:10.1111/apt.12569
Lee J., Vali Y., Boursier J., Duffin K., Verheij J., Brosnan M. J., et al. (2020). Accuracy of cytokeratin 18 (M30 and M65) in detecting non-alcoholic steatohepatitis and fibrosis: A systematic review and meta-analysis. PLoS One 15 (9), e0238717. doi:10.1371/journal.pone.0238717
Lemoinne S., Thabut D., Housset C., Moreau R., Valla D., Boulanger C. M., et al. (2014). The emerging roles of microvesicles in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 11 (6), 350–361. doi:10.1038/nrgastro.2014.7
Li Y., Wang X., Zhang J., Zhang S., Jiao J. (2022). Applications of artificial intelligence (ai) in researches on non-alcoholic fatty liver disease(NAFLD) : A systematic review. Rev. Endocr. Metab. Disord. 23 (3), 387–400. doi:10.1007/s11154-021-09681-x
Loomba R., Cui J., Wolfson T., Haufe W., Hooker J., Szeverenyi N., et al. (2016). Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: A prospective study. Am. J. Gastroenterol. 111 (7), 986–994. doi:10.1038/ajg.2016.65
Loomba R., Sanyal A. J. (2013). The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10 (11), 686–690. doi:10.1038/nrgastro.2013.171
Loomba R., Wolfson T., Ang B., Hooker J., Behling C., Peterson M., et al. (2014). Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: A prospective study. Hepatology 60 (6), 1920–1928. doi:10.1002/hep.27362
Lupsor M., Badea R., Stefanescu H., Grigorescu M., Serban A., Radu C., et al. (2010). Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J. Gastrointestin. Liver Dis. 19 (1), 53–60.
Mahadeva S., Mahfudz A. S., Vijayanathan A., Goh K. L., Kulenthran A., Cheah P. L. (2013). Performance of transient elastography (TE) and factors associated with discordance in non-alcoholic fatty liver disease. J. Dig. Dis. 14 (11), 604–610. doi:10.1111/1751-2980.12088
Manousou P., Kalambokis G., Grillo F., Watkins J., Xirouchakis E., Pleguezuelo M., et al. (2011). Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 31 (5), 730–739. doi:10.1111/j.1478-3231.2011.02488.x
Marignani M., Angeletti S. (2002). Nonalcoholic fatty liver disease. N. Engl. J. Med. 347 (10), 768–769.
McPherson S., Stewart S. F., Henderson E., Burt A. D., Day C. P. (2010). Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 59 (9), 1265–1269. doi:10.1136/gut.2010.216077
Merriman R. B., Ferrell L. D., Patti M. G., Weston S. R., Pabst M. S., Aouizerat B. E., et al. (2006). Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology 44 (4), 874–880. doi:10.1002/hep.21346
Miranda A. S., Simoes E. S. A. C. (2017). Serum levels of angiotensin converting enzyme as a biomarker of liver fibrosis. World J. Gastroenterol. 23 (48), 8439–8442. doi:10.3748/wjg.v23.i48.8439
Miyaaki H., Ichikawa T., Kamo Y., Taura N., Honda T., Shibata H., et al. (2014). Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver Int. 34 (7), e302–e307. doi:10.1111/liv.12429
Musso G., Gambino R., Cassader M., Pagano G. (2011). Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 43 (8), 617–649. doi:10.3109/07853890.2010.518623
Myers R. P., Pomier-Layrargues G., Kirsch R., Pollett A., Duarte-Rojo A., Wong D., et al. (2012). Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology 55 (1), 199–208. doi:10.1002/hep.24624
Naveau S., Gaude G., Asnacios A., Agostini H., Abella A., Barri-Ova N., et al. (2009). Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology 49 (1), 97–105. doi:10.1002/hep.22576
Naveau S., Lamouri K., Pourcher G., Njike-Nakseu M., Ferretti S., Courie R., et al. (2014). The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes. Surg. 24 (10), 1693–1701. doi:10.1007/s11695-014-1235-9
Ngo Y., Munteanu M., Messous D., Charlotte F., Imbert-Bismut F., Thabut D., et al. (2006). A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin. Chem. 52 (10), 1887–1896. doi:10.1373/clinchem.2006.070961
Nobili V., Parkes J., Bottazzo G., Marcellini M., Cross R., Newman D., et al. (2009). Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology 136 (1), 160–167. doi:10.1053/j.gastro.2008.09.013
Nobili V., Vizzutti F., Arena U., Abraldes J. G., Marra F., Pietrobattista A., et al. (2008). Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 48 (2), 442–448. doi:10.1002/hep.22376
Nunes D., Fleming C., Offner G., Craven D., Fix O., Heeren T., et al. (2010). Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am. J. Gastroenterol. 105 (6), 1346–1353. doi:10.1038/ajg.2009.746
Ochi H., Hirooka M., Koizumi Y., Miyake T., Tokumoto Y., Soga Y., et al. (2012). Real-time tissue elastography for evaluation of hepatic fibrosis and portal hypertension in nonalcoholic fatty liver diseases. Hepatology 56 (4), 1271–1278. doi:10.1002/hep.25756
Ogawa Y., Honda Y., Kessoku T., Tomeno W., Imajo K., Yoneda M., et al. (2018). Wisteria floribunda agglutinin-positive mac-2-binding protein and type 4 collagen 7S: Useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 33, 1795–1803. doi:10.1111/jgh.14156
Palmeri M. L., Wang M. H., Rouze N. C., Abdelmalek M. F., Guy C. D., Moser B., et al. (2011). Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J. Hepatol. 55 (3), 666–672. doi:10.1016/j.jhep.2010.12.019
Panera N., Gnani D., Crudele A., Ceccarelli S., Nobili V., Alisi A. (2014). MicroRNAs as controlled systems and controllers in non-alcoholic fatty liver disease. World J. Gastroenterol. 20 (41), 15079–15086. doi:10.3748/wjg.v20.i41.15079
Park C. C., Nguyen P., Hernandez C., Bettencourt R., Ramirez K., Fortney L., et al. (2017). Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 152 (3), 598–607. doi:10.1053/j.gastro.2016.10.026
Parkes J., Roderick P., Harris S., Day C., Mutimer D., Collier J., et al. (2010). Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 59 (9), 1245–1251. doi:10.1136/gut.2009.203166
Pavlov C. S., Casazza G., Nikolova D., Tsochatzis E., Gluud C. (2016). Systematic review with meta-analysis: Diagnostic accuracy of transient elastography for staging of fibrosis in people with alcoholic liver disease. Aliment. Pharmacol. Ther. 43 (5), 575–585. doi:10.1111/apt.13524
Pereira T. A., Syn W. K., Pereira F. E., Lambertucci J. R., Secor W. E., Diehl A. M. (2016). Serum osteopontin is a biomarker of severe fibrosis and portal hypertension in human and murine schistosomiasis mansoni. Int. J. Parasitol. 46 (13-14), 829–832. doi:10.1016/j.ijpara.2016.08.004
Petta S., Di Marco V., Camma C., Butera G., Cabibi D., Craxi A. (2011). Reliability of liver stiffness measurement in non-alcoholic fatty liver disease: The effects of body mass index. Aliment. Pharmacol. Ther. 33 (12), 1350–1360. doi:10.1111/j.1365-2036.2011.04668.x
Petta S., Maida M., Macaluso F. S., Di Marco V., Camma C., Cabibi D., et al. (2015). The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology 62 (4), 1101–1110. doi:10.1002/hep.27844
Petta S., Wong V. W., Camma C., Hiriart J. B., Wong G. L. H., Marra F., et al. (2017). Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology 65 (4), 1145–1155. doi:10.1002/hep.28843
Pirola C. J., Fernandez Gianotti T., Castano G. O., Mallardi P., San Martino J., Mora Gonzalez Lopez Ledesma M., et al. (2015). Circulating microRNA signature in non-alcoholic fatty liver disease: From serum non-coding RNAs to liver histology and disease pathogenesis. Gut 64 (5), 800–812. doi:10.1136/gutjnl-2014-306996
Poynard T., Ingiliz P., Elkrief L., Munteanu M., Lebray P., Morra R., et al. (2008). Concordance in a world without a gold standard: A new non-invasive methodology for improving accuracy of fibrosis markers. PLoS One 3 (12), e3857. doi:10.1371/journal.pone.0003857
Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E., et al. (2005). Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128 (7), 1898–1906. doi:10.1053/j.gastro.2005.03.084
Ratziu V., Massard J., Charlotte F., Messous D., Imbert-Bismut F., Bonyhay L., et al. (2006). Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 6, 6. doi:10.1186/1471-230X-6-6
Robic M. A., Procopet B., Metivier S., Peron J. M., Selves J., Vinel J. P., et al. (2011). Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: A prospective study. J. Hepatol. 55 (5), 1017–1024. doi:10.1016/j.jhep.2011.01.051
Rosenberg W. M., Voelker M., Thiel R., Becka M., Burt A., Schuppan D., et al. (2004). Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology 127 (6), 1704–1713. doi:10.1053/j.gastro.2004.08.052
Roulot D., Czernichow S., Le Clesiau H., Costes J. L., Vergnaud A. C., Beaugrand M. (2008). Liver stiffness values in apparently healthy subjects: Influence of gender and metabolic syndrome. J. Hepatol. 48 (4), 606–613. doi:10.1016/j.jhep.2007.11.020
Sandrin L., Fourquet B., Hasquenoph J. M., Yon S., Fournier C., Mal F., et al. (2003). Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29 (12), 1705–1713. doi:10.1016/j.ultrasmedbio.2003.07.001
Schuppan D., Afdhal N. H. (2008). Liver cirrhosis. Lancet 371 (9615), 838–851. doi:10.1016/S0140-6736(08)60383-9
Seyedian S. S., Hajiani E., Hashemi S. J., Masjedizadeh A., Shayesteh A. A., Alavinejad P., et al. (2017). Relationship between serum ferritin level and transient elastography findings among patients with nonalcoholic fatty liver disease. J. Fam. Med. Prim. Care 6 (4), 750–754. doi:10.4103/jfmpc.jfmpc_158_17
Shah A. G., Lydecker A., Murray K., Tetri B. N., Contos M. J., Sanyal A. J., et al. (2009). Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7 (10), 1104–1112. doi:10.1016/j.cgh.2009.05.033
Sheth S. G., Flamm S. L., Gordon F. D., Chopra S. (1998). AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 93 (1), 44–48. doi:10.1111/j.1572-0241.1998.044_c.x
Shirabe K., Bekki Y., Gantumur D., Araki K., Ishii N., Kuno A., et al. (2018). Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: More than a biomarker of liver fibrosis. J. Gastroenterol. 53 (7), 819–826. doi:10.1007/s00535-017-1425-z
Staufer K., Dengler M., Huber H., Marculescu R., Stauber R., Lackner C., et al. (2017). The non-invasive serum biomarker soluble Axl accurately detects advanced liver fibrosis and cirrhosis. Cell Death Dis. 8 (10), e3135. doi:10.1038/cddis.2017.554
Sumida Y., Yoneda M., Hyogo H., Itoh Y., Ono M., Fujii H., et al. (2012). Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 12, 2. doi:10.1186/1471-230X-12-2
Takahashi K., Yan I., Haga H., Patel T. (2014). Long noncoding RNA in liver diseases. Hepatology 60 (2), 744–753. doi:10.1002/hep.27043
Talwalkar J. A. (2008). Elastography for detecting hepatic fibrosis: Options and considerations. Gastroenterology 135 (1), 299–302. doi:10.1053/j.gastro.2008.05.038
Tapper E. B., Challies T., Nasser I., Afdhal N. H., Lai M. (2016). The performance of vibration controlled transient elastography in a US cohort of patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 111 (5), 677–684. doi:10.1038/ajg.2016.49
Thiele M., Madsen B. S., Hansen J. F., Detlefsen S., Antonsen S., Krag A. (2018). Accuracy of the enhanced liver fibrosis test vs FibroTest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology 154 (5), 1369–1379. doi:10.1053/j.gastro.2018.01.005
Treeprasertsuk S., Bjornsson E., Enders F., Suwanwalaikorn S., Lindor K. D. (2013). NAFLD fibrosis score: A prognostic predictor for mortality and liver complications among NAFLD patients. World J. Gastroenterol. 19 (8), 1219–1229. doi:10.3748/wjg.v19.i8.1219
Tsai E., Lee T. P. (2018). Diagnosis and evaluation of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, including noninvasive biomarkers and transient elastography. Clin. Liver Dis. 22 (1), 73–92. doi:10.1016/j.cld.2017.08.004
Venkatesh S. K., Yin M., Ehman R. L. (2013). Magnetic resonance elastography of liver: Technique, analysis, and clinical applications. J. Magn. Reson. Imaging 37 (3), 544–555. doi:10.1002/jmri.23731
Vergniol J., Foucher J., Terrebonne E., Bernard P. H., le Bail B., Merrouche W., et al. (2011). Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 140 (7), 1970–1979. doi:10.1053/j.gastro.2011.02.058
Wagner M., Corcuera-Solano I., Lo G., Esses S., Liao J., Besa C., et al. (2017). Technical failure of MR elastography examinations of the liver: Experience from a large single-center study. Radiology 284 (2), 401–412. doi:10.1148/radiol.2016160863
Wong V. W., Vergniol J., Wong G. L., Foucher J., Chan A. W. H., Chermak F., et al. (2012). Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 107 (12), 1862–1871. doi:10.1038/ajg.2012.331
Wong V. W., Vergniol J., Wong G. L., Foucher J., Chan H. L. Y., Le Bail B., et al. (2010). Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 51 (2), 454–462. doi:10.1002/hep.23312
Yoneda M., Yoneda M., Mawatari H., Fujita K., Endo H., Iida H., et al. (2008). Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Liver Dis. 40 (5), 371–378. doi:10.1016/j.dld.2007.10.019
Keywords: NAFLD, non-invasive diagnosis, liver fibrosis, biomarkers, prediction
Citation: Wang J, Qin T, Sun J, Li S, Cao L and Lu X (2022) Non-invasive methods to evaluate liver fibrosis in patients with non-alcoholic fatty liver disease. Front. Physiol. 13:1046497. doi: 10.3389/fphys.2022.1046497
Received: 16 September 2022; Accepted: 15 November 2022;
Published: 14 December 2022.
Edited by:
Gao Xuejuan, Jinan University, ChinaReviewed by:
Lingli He, Harvard University, United StatesMatthias J. Bahr, University Hospital Ruppin-Brandenburg, Brandenburg Medical School, Germany
Ka Zhang, Sun Yat-sen University, China
Copyright © 2022 Wang, Qin, Sun, Li, Cao and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojie Lu, MTg5QHdodS5lZHUuY24=; Lihua Cao, Y2xoMjc3N0AxNjMuY29t
†These authors share first authorship
 Jincheng Wang
Jincheng Wang Tao Qin
Tao Qin Jinyu Sun1†
Jinyu Sun1† Xiaojie Lu
Xiaojie Lu