- College of Plant Protection, Shandong Agricultural University, Key Laboratory of Pesticide Toxicology and Application Technique, Taian, China
The diamondback moth (DBM), Plutella xylostella L., is an important pest of cruciferous vegetables, and population control mainly depends on chemical pesticides. Emamectin benzoate is a highly effective insecticide used for controlling DBM. However, it is unknown how the sublethal effects of low concentration residues of emamectin benzoate on DBM. So the population development sublethal effects of emamectin benzoate, at LC5, LC10, and LC20 with concentrations of 0.014 mg/L, 0.024 mg/L and 0.047 mg/L, respectively, on adult DBM and their progeny were investigated in this study. The pupal weight, pupal period, female fecundity, and vitellin content of the F0 DBM generation increased significantly compared to the control. And the single female oviposition number of DBM was increased by 20.21% with LC20 treatment. The pupation rate, adult longevity and ovariole length of the treatment groups decreased significantly. The fecundity of DBM in the treatment groups increased, and this increased the population by a presumptive 13.84%. Treatment also led to the shortening of ovarioles and the reduction of egg hatching, and increased pupal weight in the F1 generation. We concluded that the effects of sublethal/low concentration emamectin benzoate on the different life stages of DBM were variable, and the reproductive hormesis on DBM adults were attractive findings.
Introduction
The diamondback moth (DBM), Plutella xylostella L., is a widely distributed lepidopteran pest that causes serious damage to cruciferous vegetables. It has strong adaptability of host, long-distance migration, and overlapping generations (Furlong et al., 2013). DBM control relies on chemical insecticides. However, excessive pesticide use has selected for DBM resistance to more than 90 pesticides (Whalon et al., 2019).
Studies have found that pesticide applications usually induce strong lethal effects in most of arthropods. It primarily through direct mortality of exposed arthropods and a variety of sublethal physiological biological, and/or behavioral effects on arthropod individuals (Desneux et al., 2007; Shan et al., 2020). Arthropods may experience exposure to sublethal doses because of suboptimal spray coverage during applications and owing to a decrease in residue concentrations after the initial application (Guedes et al., 2016). Pesticide applications can significantly impact the effectiveness of biocontrol agents in most agroecosystems (Lu et al., 2012; Huang et al., 2020). It may also influence habitat shifts, induces hormesis in insect pests, resistance development, and direct and indirect interactions between species within food webs. Some insecticides may also disrupt biological control of secondary pests, leading to a secondary pest outbreak (Wang et al., 2017; Liang et al., 2021; Zhang et al., 2021a). Pesticide exposure can stimulate reproductive hormesis of Nilaparvata lugens and lead to N. lugens population outbreaks (Wu et al., 2019). All of the cases bring great challenges to the rational use of pesticides and pest control, which require extensive attention from entomologist and agrochemical scientists all of world.
Emamectin benzoate (4″-epi-methylamino-4″-deoxyavermectin B1) is a highly efficient, broad-spectrum, semi-synthetic insecticide used for control of agricultural and forestry insect pests. It is especially useful for controlling lepidoptera including Spodoptera exigua, Helicoverpa zea, P. xylostella, and Spodoptera littoralis (López et al., 2010; El-Sheikh, 2015). Field residues of emamectin benzoate gradually decrease to sublethal concentrations due to chemical, biological and/or natural degradation in the environment (Biondi et al., 2012; Khan et al., 2018). The half-life of emamectin benzoate on cabbage was determined to be 3.81 days (Wang et al., 2009). The LC5 and LC15 of emamectin benzoate prolonged the development time and longevity of Spodoptera littoralis and reduced the population (Mokbel and Huesien, 2020). The LC30 of emamectin benzoate had a significant negative impact on egg laying, ovarian development, mating rate, and survival of Conopomorpha sinensis (Yao et al., 2018).
It was found that the third-instar larvae of DBM were treated by LC10 and LC25 of chlorantraniliprole. Which of the insecticide could reduce pupation, pupal weight, adult emergence rates, increase the duration of female preoviposition period, decrease fecundity and egg hatch, and decrease survival rates of the offspring. And, the mean values of the net reproductive rate (R0), intrinsic rate of increase (rm), and finite rate of increase (λ) were also significantly decreased in LC10 and LC25 treatment (Han et al., 2012). The negative effects by indoxacarb, metaflumizone, methylthio-diafenthiuron, spinetoram, broflanilide and fluxametamide with sublethal dose/concentration were also studied (Wang et al., 2011; Zhang et al., 2012; Su and Xia, 2020; Tamilselvan et al., 2021; Gope et al., 2022; Sun et al., 2022). However, a few of insecticides, e.g. fenvalerate, chlorpyrifos, chlorfenapyr (LC1) and abamectin with sublethal dose/concentration (Fujiwara et al., 2002; Deng et al., 2016; Rodríguez-Rodríguez et al., 2021; Jia et al., 2022) could stimulate or lead to hormesis effect on DBM. The LC1 (0.274 mg/L) of chlorfenapyr significantly increased female pupa weight of F0 and F1 generations, and F0 fecundity as well as F1 gross reproduction rate of DBM. And, the LC1-elicited rise in emergency rate and fecundity was significantly greater in F0 than in F1 (Jia et al., 2022). How does the sublethal effects of emamectin benzoate on DBM remains unknown until now. In this paper, we studied the effects of sublethal/low concentrations of emamectin benzoate, at LC5, LC10, and LC20 with concentrations of 0.014 mg/L, 0.024 mg/L and 0.047 mg/L, respectively, on the population development of DBM, and hope carry out a science assessment of its application on DBM, avoid or delay resurgence of DBM in the field.
Materials and methods
Insect
P. xylostella was collected from the experimental station of the South campus of Shandong Agricultural University in 2006 and cultured indoors on radish seedlings and cabbage leaves without pesticide exposure. The insect rearing room was maintained at 25 ± 2°C, relative humidity 60%–70%, and a photoperiod of 14:10 h (L:D). The adults were fed on 10% honey: water.
Insecticides and reagents
Emamectin benzoate (95.0%) was provided by Qingdao Dingfeng Biotechnology Co., Ltd. Shandong Province, China. Acetone, ether, and other solvents were analytical grade and were purchased from Tianjin Damao Chemical Reagent Factory, Tianjin, China.
Bioassay of acute toxicity
Acute toxicity was determined by the leaf dipping method (Liang et al., 2003). Emamectin benzoate was dissolved in acetone to prepare a concentrate of 200 mg/L. The concentrate was serially diluted with 1% Tween 80 aqueous solution to obtain emamectin benzoate solution concentrations of 0.05, 0.10, 0.20, 0.40 and 0.80 mg/L. Fresh cabbage leaves were cut into 5.0 ± 0.5 cm disks, dipped in the solution for 10 s and held vertically to allow excess solution to drip off, and placed on a rack to dry. Twenty third-instar DBM larvae were added to each culture dish containing a treated leaf disk. An aqueous solution without emamectin benzoate used as the negative control. After 72 h exposure on the cabbage leaves treated with the different concentration of emamectin benzoate, counted the surviving larvae that moved when touched slightly, and transferred to fresh leaves for subsequent experiments.
Bioassay of sublethal effects on DBM
Thirty third-instar DBM larvae were treated with emamectin benzoate for 72 h use the same leaf dip method as described above at sublethal/low concentrations of LC5, LC10, and LC20, respectively. The effects of the sublethal/low concentrations of emamectin benzoate on DBM development were determined. These effects included pupation rate, pupal weight, pupal period, adult emergence rate, adult survival number, single female oviposition number, adult longevity, eggs hatching rate, larval survival rate, and larval development duration. Both F0 and F1 generations were studied. An aqueous solution without emamectin benzoate was used as the control.
After the emergence of the treated insects, select one couple of male and female adults randomly eclosing on the same day, and put the couple into a can bottle with fresh cabbage leaves, providing with 10% honey solution. When the female adult begins to lay, count the number of eggs laid and the number of eggs hatched every 24 h until the adult died. Each treatment was 5 couple of adults and repeated 3 times independently.
Ovary anatomy and vitellin content determination
Third-instar DBM larvae were exposed to emamectin benzoate for 72 h use the leaf dip method mentioned above at sublethal/low concentrations of LC5, LC10, and LC20 and surviving female adults were collected. The female adults were anesthetized with CO2 and the thorax/abdomen was removed with ophthalmic surgical scissors. The abdomen was placed on a glass slide coated with physiological saline. Then, the end of the abdomen was squeezed gently with an insect pin and the ovaries were removed. The fat particles adhering to the ovarioles were removed with dissecting forceps and the ovarioles were stained with safranine dye solution for 5 min. Excess dye solution was then washed off the ovarioles. The ovarioles were observed and photographed with a continuously variable magnification stereomicroscope (SZX 10). The length or width diameter of mature eggs and ovarioles lengths were measured with ImageJ image processing software.
The vitellin content at 0–96 h female emergence was determined using an insect vitellin linked immunoassay (ELISA) kit (Shanghai Meilian Biotechnology Co., Ltd., Shanghai China) according to the directions of the kit.
Data analysis
Three independent replicates were used for each test. Probit analysis was used to determine the value of lethal concentration. All biological traits data were processed using SPSS V16.0 (SPSS, Inc., Chicago, IL, USA), and the results are shown as the mean ± standard deviation (SD, n = 3). All biological traits data were subjected to the analysis of variance (ANOVA) test, and mean differences were evaluated by Tukey’s multiple comparison test (p = 0.05). Significance was indicated at p < 0.05.
Results
Toxicity
The toxicity of emamectin benzoate to the third-instar DBM larvae was determined by the leaf dip method. After 72 h exposure the LC50 was 0.173 mg/L, and the LC5, LC10, and LC10 values were also obtained (Table 1).
Sublethal effects of emamectin benzoate on the F0 generation of DBM
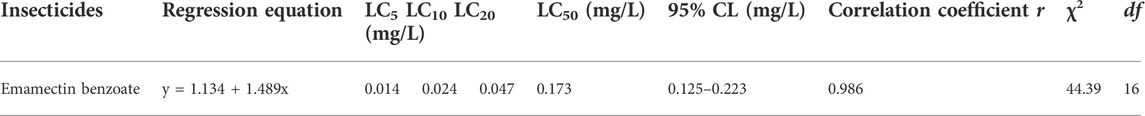
Third-instar DBM larvae were treated with emamectin benzoate at the sublethal/low concentrations of LC5, LC10, and LC20. The corrected larvae survival rate was 93.3%, 88.7% and 81.3%, respectively. The pupation rate of DBM larvae decreased with increased treatment concentration. The pupation rate of the LC10 and LC20 treatments was significantly (F = 7.47, df = 11, p = 0.010) lower than the control group (Figure 1A). The pupa weight of LC10 and LC20 treatment groups increased significantly (F = 16.49, df = 79, p = 0.0001) by 10.53% and 14.63%, respectively (Figure 1B), compared to the control. The pupal period of DBM in the LC10 treatment was significantly (F = 3.70, df = 79, p = 0.015) longer than the control group (Figure 1C). However, the three treatment groups had no significant (F = 2.60, df = 11, p = 0.125) effect on the adult emergence rate (Figure 1D). After pupation and adult emergence, single female oviposition number in the LC20 treatment group was 192.7 ± 3.37. It was increased 20.21% compared with the control (Figure 1E). The eggs laying peak in the LC20 group was the same as that of the control, but the daily oviposition number increased and the egg laying hours was prolonged by 7.31% (Figure 1F). When compared to the control, the egg hatching rate decreased by 4.08%; however, the average number of larvae in the F1 generation increased by 22.19, which ultimately led to DBM quantity presumptive increase of 13.84%.
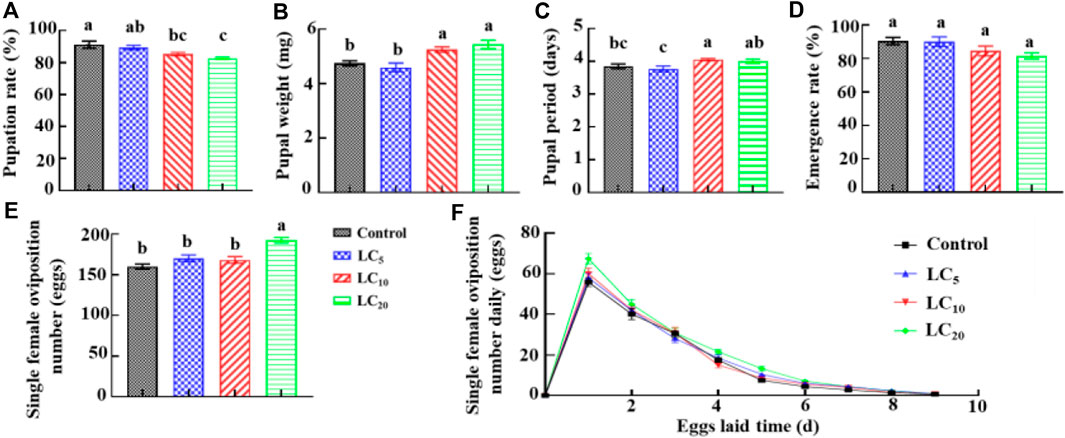
FIGURE 1. Effects of emamectin benzoate sublethal/low concentrations on pupation rate (A), pupal weight (B), pupal period (C), emergence rate (D), single female oviposition number (E), and single female oviposition number daily (F) of P. xylostella F0 generation.
Third-instar DBM larvae were exposed to deposits of emamectin benzoate applied at LC5, LC10, and LC20. After survivors developed into adults, the longevity of female adults in the LC20 group was determined to be significantly (F = 7.05, df = 39, p = 0.0008) shorter than control longevity. The male adult longevity in the three treatments was shortened to various degrees (Figure 2A). The vitellin content in adult females was determined by ELISA. With extension of the time after eclosion, the vitellin content in the LC20 treatment group was significantly (72 h: F = 24.17, df = 11, p = 0.0002) higher than in the control, and the maximum increase was 19.00%. The vitellin content of the other groups were significantly (LC10 24 h: F = 47.59, df = 11, p = 0.0001) changed with the eclosion time (Figure 2B).
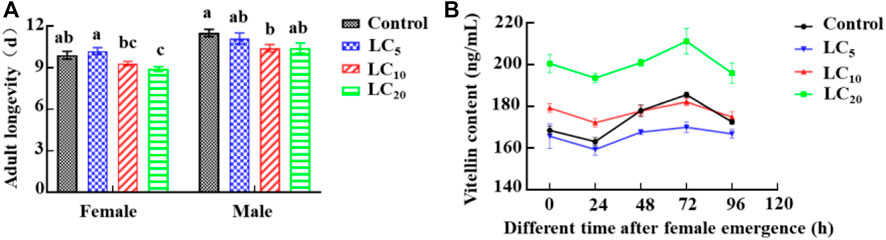
FIGURE 2. Effects of sublethal/low concentrations of emamectin benzoate on adult longevity (A) and vitellin content (B) in females of the P. xylostella F0 generation.
Sublethal effects of emamectin benzoate on the F1 generation of DBM
Emamectin benzoate treatment affected the length or width diameter of adult mature eggs. Compared to the control, the length of mature ovarioles in the LC20 group was shortened by 15.60% (Figure 3). Emamectin benzoate treatment did not significantly (F = 0.16, df = 39, p = 0.925) affect the mature egg ratio of the F1 generation, but it significantly (F = 8.611, df = 11, p = 0.006) decreased egg hatchability. The treatments had no significant (F = 0.353, df = 11, p = 0.789) effects on larval survival (Table 2).
FIGURE 3. Effect of sublethal/low concentrations of emamectin benzoate on the ovary of adult female P. xylostella. (A–D) are the Control, LC5, LC10, and LC20 treatment group.
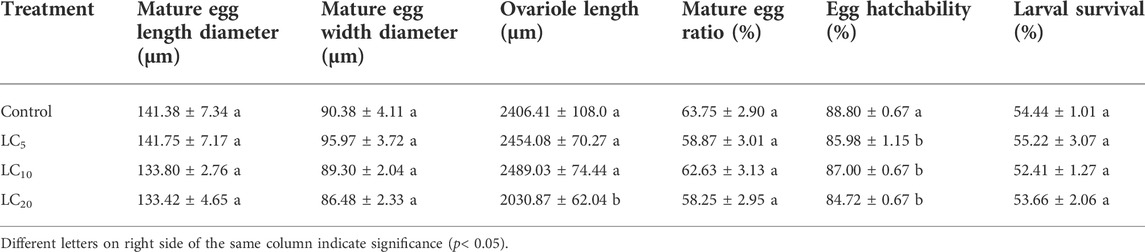
TABLE 2. Effects of sublethal/low concentrations of emamectin benzoate on mature eggs, ovarian canal, and larvae of F1 generation of P. xylostella.
The pupa weight of the F1 offspring in LC20 group was significantly (F = 3.187, df = 79, p = 0.028) increased. However, there was no significant effect on the pupation rate, pupal period, pupal emergence rate, single female oviposition number, female oviposition period, and adult longevity of the F1 offspring (Table 3).
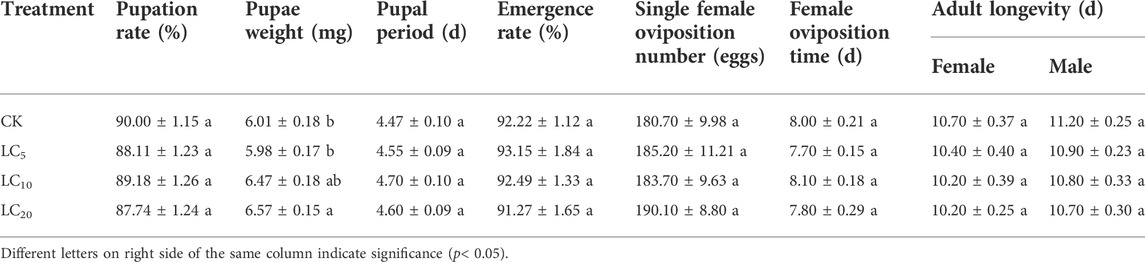
TABLE 3. Effects of sublethal/low concentrations of emamectin benzoate on pupae and adults of F1 generation of P. xylostella.
Discussion
Only a small proportion of chemical pesticide applied directly kills target pests. Most pesticide residue remains in the environment and it may exert sublethal effect on surviving insects. A sublethal/low dose of residual insecticide can effect insect development, morphology, pupa weight, longevity, and fecundity (Zhang et al., 2022). The sublethal effects of pesticides influence the biological characteristics and population development of insects and can provide insight into optimal pesticide use.
Treatment of second-instar Spodoptera litura larvae with sublethal doses of chlorantraniliprole or indoxacarb increased the pupal period and increased pupal weight (Moustafa et al., 2021). The fecundity of Laodelphax striatellus was significantly decreased by imidacloprid LC30 treatment. However, the fecundity was significantly increased when the test insects were treated with an LC10 dose of imidacloprid (Zhang et al., 2021b). When the third-instar DBM larvae were treated with a LC20 dose of emamectin benzoate in this study the development time of larvae was prolonged by 17.0 ± 3.0 h, the pupation rate of F0 larvae was decreased by 9.40%, the pupa weight was increased by 14.63%, the average single female oviposition number increased by 30.9 eggs, and the longevity of female adults was shortened by 10.10%. The egg hatch rate decreased by 4.08%, but the number of larvae in the F1 generation increased. The survival rate, pupation rate and pupae emergence rate of the F1 generation were similar to the control, and this could ultimately lead to a presumptive population increase of 13.84%. Sublethal concentration (LC10 and LC30) exposures of emamectin benzoate had a significant negative impact on the larval, protonymph, and deutonymph developmental periods on Panonychus citri (Khan et al., 2021). Female fecundity of P. citri was decreased, and the adult pre-oviposition period and total pre-oviposition periodwere increased in the sublethal treatments. The age-stage specific survival rates (Sxj), age-specific fecundity (Mx), net reproductive rate (R0), age-stage specific life expectancy (Exj), and age-stage reproductive value (Vxj) was reduced by LC10 and LC30 exposure (Khan et al., 2021), that of the results were different from this study. The reason need to be further study.
A low concentration nitenpyram induced transgenerational hormesis effects in terms of fitness-related traits and insecticide tolerance in Nilaparvata lugens after exposure to the LC20 concentrations for six generations (Gong et al., 2022), but, we did not find transgenerational hormesis in DBM by emamectin benzoate treated. It was found that insecticide-induced hormesis in life history traits may augment the development of insecticide tolerance or resistance in pest insects, allowing fitter individuals to survive and reproduce, with significant management and environmental implications (Guedes et al., 2016). The reproduction hormesis of parental generation maybe one of reasons of DBM resurgence. Based on this case, a few of control policies conform to IPM strategies could be used, e.g., combination use of compatible insecticides and biological control agents, ‘attract-and-kill’ control strategies, development of new safer, environmentally friendly and target-specific insecticides or cultivation safety transgenic crops.
Insect fecundity is mainly regulated by the synthesis of vitellogenin (Vg) and vitellin (Vn) (Jing et al., 2021). Vitellogenin is synthesized in the fat body of adult females and released into the hemolymph. It is then absorbed by oocytes through special channels to synthesize vitellin to provide essential nutrients for egg development (Tufail et al., 2014). The expression of vitellogenin and vitellogenin receptor genes were significantly increased in a flubendiamide resistant DBM strain compared to a susceptible strain, and vitellin content also increased in the resistant strain (Sun et al., 2020). The LC30 of emamectin benzoate can reduce the transcription of CsVg and CsVgR at 24-h, 48-h, and 72-h exposure and decrease the egg production of C. sinensis (Yao et al., 2018). After injecting 20-hydroxyecdysone into silkworm larvae, the Vg content in the hemolymph increased, and the Vn content in the ovary also increased. This led to a significant increase in the total egg weight (Shen et al., 2014), this is similar to the results of the present study. When DBM larvae were treated with the LC20 of emamectin benzoate, the number of eggs laid by single adults was significantly increased compared to the control. The Vn content of the LC20 DBM treatment after adult eclosion was significantly higher than that of the control. This indicated a direct correlation between the Vn content and egg production. Similar results have been elaborated in many other insecticide-exposed insects (Zhou et al., 2020).
In conclusion, the effects of sublethal/low concentration emamectin benzoate on the different life stages of DBM were variable, and the reproductive hormesis on DBM adults were attractive. However, the reason of hormesis deserve further detailed study on insect biology and genetics in combination with DBM resistance. This case makes it necessary for us to re-understand the population development of P. xylostella. In addition, combination use of compatible insecticides and biological control agents, ‘attract-and-kill’ control strategies, development of new safer, environmentally friendly and target-specific insecticides, one or more these policies can be selected for the control of DBM in fields.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, C-BX; methodology, C-BX and K-XL; validation, K-XL and YG; formal analysis, C-BX, C-XZ, and K-XL; writing—original draft preparation, YG, C-XZ, and K-XL; writing—review and editing, C-BX, YG, C-XZ, and K-XL; supervision, C-BX. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31972295).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Biondi A., Desneux N., Siscaro G., Zappalà L. (2012). Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87 (7), 803–812. doi:10.1016/j.chemosphere.2011.12.082
Deng Z., Zhang F., Wu Z., Yu Z., Wu G. (2016). Chlorpyrifos-induced hormesis in insecticide-resistant and -susceptible Plutella xylostella under normal and high temperatures. Bull. Entomol. Res. 106 (3), 378–386. doi:10.1017/S000748531600002X
Desneux N., Decourtye A., Delpuech J. M. (2007). The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106. doi:10.1146/annurev.ento.52.110405.091440
El-Sheikh E. A. (2015). Comparative toxicity and sublethal effects of emamectin benzoate, lufenuron and spinosad on Spodoptera littoralis Boisd. (Lepidoptera: Noctuidae). Crop Prot. 67, 228–234. doi:10.1016/j.cropro.2014.10.022
Fujiwara Y., Takahashi T., Yoshioka T., Nakasuji F. (2002). Changes in egg size of the diamondback moth Plutella xylostella (Lepidoptera: Yponomeutidae) treated with fenvalerate at sublethal doses and viability of the eggs. Appl. Entomol. Zool. 37, 103–109. doi:10.1303/aez.2002.103
Furlong M. J., Wright D. J., Dosdall L. M. (2013). Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 58 (1), 517–541. doi:10.1146/annurev-ento-120811-153605
Gong Y., Cheng S., Desneux N., Gao X., Xiu X., Wang F., et al. (2022). Transgenerational hormesis effects of nitenpyram on fitness and insecticide tolerance/resistance of Nilaparvata lugens. J. Pest Sci. doi:10.1007/s10340-022-01494-4
Gope A., Chakraborty G., Ghosh S. M., Sau S., Mondal K., Biswas A., et al. (2022). Toxicity and sublethal effects of fluxametamide on the key biological parameters and life history traits of diamondback moth Plutella xylostella (L.). Agronomy 12, 1656. doi:10.3390/agronomy12071656
Guedes R. N. C., Smagghe G., Stark J. D., Desneux N. (2016). Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 61, 43–62. doi:10.1146/annurev-ento-010715-023646
Han W., Zhang S., Shen F., Liu M., Ren C., Gao X.-W. (2012). Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 68, 1184–1190. doi:10.1002/ps.3282
Huang N. X., Jaworski C. C., Desneux N., Zhang F., Yang P. Y., Wang S. (2020). Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 40, 331–335. doi:10.1127/entomologia/2020/0994
Jia B., Zhang J., Hong S., Chang X., Li X. (2022). Sublethal effects of chlorfenapyr on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. doi:10.1002/ps.7175
Jing Y.-P., Wen X.-P., Li L.-J., Zhang S.-J., Zhang C., Zhou S.-T. (2021). The vitellogenin receptor functionality of the migratory locust depends on its phosphorylation by juvenile hormone. Proc. Natl. Acad. Sci. U. S. A. 118 (37), e2106908118. doi:10.1073/PNAS.2106908118
Khan M. M., Ali M. W., Hafeez M., Fan Z.-Y., Ali S., Qiu B.-L. (2021). Lethal and sublethal effects of emamectin benzoate on life-table and physiological parameters of citrus red mite, Panonychus citri. Exp. Appl. Acarol. 85, 173–190. doi:10.1007/s10493-021-00667-7
Khan M. M., Nawaz M., Hua H., Cai W., Zhao J. (2018). Lethal and sublethal effects of emamectin benzoate on the rove beetle, Paederus fuscipes, a non-target predator of rice Brown planthopper. Ecotoxicol. Environ. Saf. 165, 19–24. doi:10.1016/j.ecoenv.2018.08.047
Liang H.-Y., Yang X.-M., Sun L.-J., Zhao C.-D., Chi H., Zheng C.-Y. (2021). Sublethal effect of spirotetramat on the life table and population growth of Frankliniella occidentalis (Thysanoptera: Thripidae). Entomol. Gen. 41 (3), 219–231. doi:10.1127/entomologia/2020/0902
Liang P., Gao X. W., Zheng B. Z. (2003). Genetic basis of resistance and studies on cross-resistance in a population of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 59 (11), 1232–1236. doi:10.1002/ps.760
López J. D., Latheef M. A., Hoffmann W. C. (2010). Effect of emamectin benzoate on mortality, proboscis extension, gustation and reproduction of the corn earworm, Helicoverpa zea. J. Insect Sci. 10 (89), 89–16. doi:10.1673/031.010.8901
Lu Y., Wu K., Jiang Y., Guo Y., Desneux N. (2012). Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362–365. doi:10.1038/nature11153
Mokbel E., Huesien A. (2020). Sublethal effects of emamectin benzoate on life table parameters of the cotton leafworm, Spodoptera littoralis (Boisd). Bull. Natl. Res. Cent. 44 (1), 155–158. doi:10.1186/s42269-020-00412-x
Moustafa M. A. M., Fouad E. A., Yasmin A. M., Hamow K. Á. A., Mikó Z., Molnár B. P., et al. (2021). Toxicity and sublethal effects of chlorantraniliprole and indoxacarb on Spodoptera littoralis (Lepidoptera:Noctuidae). Appl. Entomol. Zool. 56, 115–124. doi:10.1007/S13355-020-00721-7
Rodríguez-Rodríguez J. F., Cerna-Chávez E., Ochoa-Fuentes Y. M., Landeros-Flores J., Guevara-Acevedo L. P., Cisneros-López H. C. (2021). Sublethal effects and costs of resistance to abamectin in diamondback moth (Plutella xylostella) (Lepidoptera: Plutellidae). Rev. Colomb. Entomol. 47 (2), e10657. doi:10.25100/socolen.v47i2.10657
Shan Y.-X., Zhu Y., Li J.-J., Wang N.-M., Yu Q.-T., Xue C.-B. (2020). Acute lethal and sublethal effects of four insecticides on the lacewing (Chrysoperla sinica Tjeder). Chemosphere 250, 126321. doi:10.1016/j.chemosphere.2020.126321
Shen G. W., Lin Y., Lv Y. H., Wang J. Y., Xing R. M., Xia Q. Y. (2014). Regulation of ecdysone on vitellogenin gene expression in silkworm eggs. Chin. J. Biochem. Mol. Biol. 30 (11), 1106–1112. doi:10.13865/j.cnki.cjbmb.2014.11.08
Su C.-Y., Xia X.-M. (2020). Sublethal effects of methylthio-diafenthiuron on the life table parameters and enzymatic properties of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Pestic. Biochem. Physiol. 162, 43–51. doi:10.1016/j.pestbp.2019.08.011
Sun S.-Q., Wang N.-M., Li J.-J., Jin M.-H., Xue C.-B. (2020). Reduced fecundity and regulation of reproductive factors in flubendiamide-resistant strains of Plutella xylostella. Pestic. Biochem. Physiol. 169, 104668. doi:10.1016/j.pestbp.2020.104668
Sun X., Wei R., Li L., Zhu B., Liang P., Gao X.-W. (2022). Resistance and fitness costs in diamondback moths after selection using broflanilide, a novel meta-diamide insecticide. Insect Sci. 29, 188–198. doi:10.1111/1744-7917.12917
Tamilselvan R., Kennedy J. S., Suganthi A. (2021). Sublethal and transgenerational effects of spinetoram on the biological traits of Plutella xylostella (L.) (Lepidoptera: Plutellidae). Ecotoxicology 30, 667–677. doi:10.1007/s10646-021-02385-7
Tufail M., Nagaba Y., Elgendy A. M., Takeda M. (2014). Regulation of vitellogenin genes in insects. Entomol. Sci. 17 (3), 269–282. doi:10.1111/ens.12086
Wang G., Huang X., Wei H., Fadamiro H. Y. (2011). Sublethal effects of larval exposure to indoxacarb on reproductive activities of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Pestic. Biochem. Physiol. 101, 227–231. doi:10.1016/j.pestbp.2011.09.010
Wang L., Ma X., Hua R. M., Tang F., Wu X. W., Li X. D., et al. (2009). Degradation dynamics and compound effect of chlorpyrifos and emamectin benzoate on cabbage and glass slides. J. Anhui. Agric. Univ. 36 (2), 309–314. doi:10.13610/j.cnki.1672-352x.2009.02.009
Wang S., Qi Y., Desneux N., Shi X., Biondi A., Gao X.-W. (2017). Sublethal and transgenerational effects of short-term and chronic exposures to the neonicotinoid nitenpyram on the cotton aphid Aphis gossypii. J. Pest Sci. 90, 389–396. doi:10.1007/s10340-016-0770-7
Whalon M. E., Mota-Sanchez D., Hollingworth R. M. (2019). The arthropod pesticide resistance database [DB/OL] East Lansing, MI: Michigan State University. Available at: http://www.pesticideresistance.org (Accessed 25 June 2019).
Wu J., Ge L., Liu F., Song Q., Stanley D. (2019). Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 65 (1), 409–429. doi:10.1146/annurev-ento-011019-025215
Yao Q., Xu S., Dong Y., Que Y., Quan L., Chen B. (2018). Characterization of vitellogenin and vitellogenin receptor of Conopomorpha sinensis Bradley and their responses to sublethal concentrations of insecticide. Front. Physiol. 9, 1250. doi:10.3389/fphys.2018.01250
Zhang C.-X., Wang Z.-J., Li J.-J., Wang N.-M., Xue C.-B. (2022). Sublethal effects of tolfenpyrad on the development, reproduction, and predatory ability of Chrysoperla sinica. Ecotoxicol. Environ. Saf. 236, 113482. doi:10.1016/J.ECOENV.2022.113482
Zhang Q., Liu Y., Wyckhuys K. A., Liang H., Desneux N., Lu Y. (2021a). Lethal and sublethal effects of chlorantraniliprole on Helicoverpa armigera adults enhance the potential for use in ‘attract-and-kill’ control strategies. Entomol. Gen. 41 (1), 111–120. doi:10.1127/entomologia/2020/1104
Zhang Y. Y., Xu G., Jiang Y., Ma C., Yang G. (2021b). Sublethal effects of imidacloprid on fecundity, apoptosis and virus transmission in the small Brown planthopper Laodelphax striatellus. Insects 12 (12), 1131. doi:10.3390/insects12121131
Zhang Z., Li J. H., Gao X.-W. (2012). Sublethal effects of metaflumizone on Plutella xylostella (Lepidoptera: Plutellidae). J. Integr. Agric. 11, 1145–1150. doi:10.1016/S2095-3119(12)60108-7
Keywords: fecundity, sublethal concentration, emamectin benzoate, hormesis, development
Citation: Liu K-X, Guo Y, Zhang C-X and Xue C-B (2022) Sublethal effects and reproductive hormesis of emamectin benzoate on Plutella xylostella. Front. Physiol. 13:1025959. doi: 10.3389/fphys.2022.1025959
Received: 23 August 2022; Accepted: 10 October 2022;
Published: 19 October 2022.
Edited by:
Youhui Gong, Institute of Plant Protection (CAAS), ChinaReviewed by:
Lei Guo, Qingdao Agricultural University, ChinaJianhong Li, Huazhong Agricultural University, China
Farman Ullah, China Agricultural University, China
Copyright © 2022 Liu, Guo, Zhang and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao-Bin Xue, Y2J4dWVAc2RhdS5lZHUuY24=
†These authors have contributed equally to this work
 Kong-Xing Liu†
Kong-Xing Liu† Chao-Bin Xue
Chao-Bin Xue