- 1Department of Cardio-thoracic Surgery, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China
- 2Department of Nephrology, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, China
- 3Department of Cardio-thoracic Surgery, Nanjing Drum Tower Hospital Clinical College of Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, China
Background: Serum lactate is commonly measured in the perioperative period in patients who have undergone surgery for an acute type A aortic dissection (ATAAD). However, conflicting data has been reported as to whether lactate elevation is associated with short-term prognosis. The aim of the current study was to determine the association between perioperative arterial lactate levels and postoperative 30-day mortality.
Methods: Patients who underwent repair of a ATAAD at our institution were retrospectively screened and those with comprehensive measurements of serum lactate before surgery and at 0, 1, 3, 6, 12, and 24 h after surgery in the intensive care unit (ICU) were selected for the analysis. Patients’ demographic features and outcomes were reviewed to determine risk factors associated with 30-day mortality using logistic regression modeling. The association between serum lactate levels at different time points and 30-day mortality were analyzed by receiver-operating characteristic curves.
Results: 513 patients were identified and retrospectively analyzed for this study including 66 patients (12.9%) who died within 30 days after surgery. Patients who died within 30 days after surgery had elevated lactate levels measured before surgery and at 0, 1, 3, 6, 12, and 24 h after their ICU stay. Lactate measured at 24 h post ICU admission (odds ratio, 2.131; 95% confidence interval, 1.346–3.374; p = 0.001) was a predictor of 30-day mortality. The area under the curve (AUC) for 30-day mortality with lactate levels at 12 h and 24 h post ICU stay were 0.820 and 0.805, respectively.
Conclusion: Early elevation of lactate level is correlated with increased 30-day mortality in patients who received ATAAD surgical repair.
Introduction
Acute type A aortic dissection (ATAAD) is a lethal disease with an in-hospital mortality ranging from 16% to 83% (Kilic et al., 2013). Despite recent advances in endovascular interventions and postoperative management, open surgery remains the gold standard for the treatment of ATAAD. A variety of risk factors for in-hospital mortality in ATAAD have been reported (Pansini et al., 1998; Santini et al., 2007). However, many other factors may contribute to this devastating outcome.
Serum lactate has now been shown to be associated with inflammation, autoimmunity, and cancer (Pucino et al., 2017; Certo et al., 2021; Manoharan et al., 2021). Recent evidence suggests that lactate accumulates at sites of chronic inflammation and that these levels can increase via metabolic reprogramming (Pucino et al., 2019). Clinically, it has been known that lactate levels can be used as a surrogate to measure tissue perfusion; and its elevation has been shown to be associated with an increased risk of death in infection, sepsis, trauma, and patients undergoing open surgical procedures (Nguyen et al., 2004; Ding et al., 2018; Ilias et al., 2018).
The association between elevated lactate levels and increased mortality has been well studied in cardiac surgery (Kogan et al., 2012; Hajjar et al., 2013; Renew et al., 2016; Bennett et al., 2017). However, conflicting results have been reported for its prognostic value to predict mortality in patients undergoing surgical repair of an ATAAD (Bennett et al., 2017; Zindovic et al., 2018; Gemelli et al., 2022). In addition, the lactate levels used in most studies were obtained at a single time point; while recent studies have indicated that serial lactate measurements, rather than one-time measurements, are more reliable for assessing risk and outcomes (Lazzeri et al., 2015; Li et al., 2015). Therefore, this study sought to examine the correlation between serial levels of serum lactate and 30-day mortality in patients who underwent ATAAD surgical repair.
Materials and methods
Study design
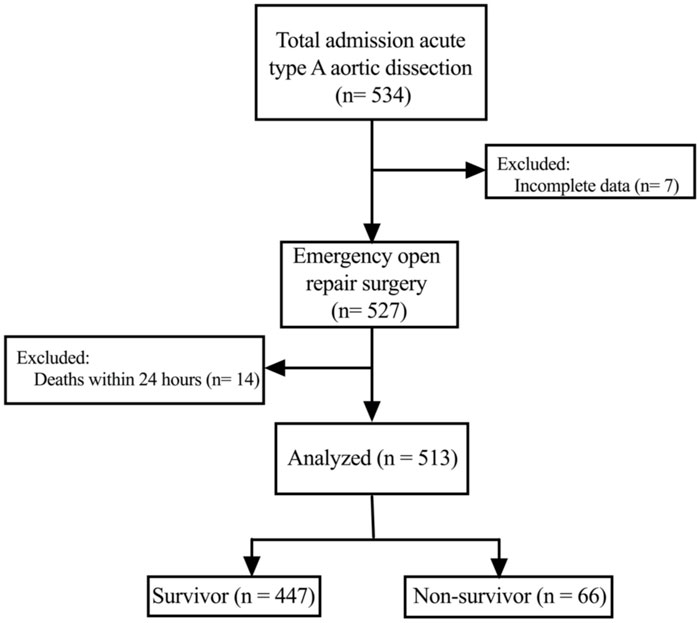
This study was a single-center, retrospective cohort study enrolling consecutive patients who received emergency surgical repair of an ATAAD between January 2019 and December 2020 at the Nanjing Drum Tower Hospital. Patients who died within 24 h after surgery and with incomplete medical records were excluded. All patients were divided into a non-survivor group and a survivor group based on their 30-day mortality. This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital with a waiver of informed consent considering the retrospective nature of this study (Approved number: 2022-084-01).
Data collection and definition
Patients’ clinical data were extracted from the institutional electronic medical record system. All patients were transferred to the intensive care unit (ICU) after completion of surgery. Acute kidney injury was diagnosed according to the Kidney Disease Improving Global Outcomes criteria (Kellum et al., 2013). Prolonged mechanical ventilation was defined as total ventilation time exceeded 48 h in the postoperative period. Malperfusion was defined as the presence of the following conditions before surgery: cardiac malperfusion with ST changes, echocardiographic signs of myocardial ischemia, or CK-MB level >75 mmol/L; cerebral malperfusion with loss of lateralized central neurological function; gastrointestinal malperfusion with mesenteric or liver ischemia; peripheral malperfusion with absent pulses or loss of sensory or motor function of the upper or lower extremities; spinal malperfusion with transient or permanent paraplegia (Zindovic et al., 2018). Shock was defined as systolic blood pressure less than 90 mmHg, regardless of the etiology.
Lactate
Arterial lactate levels were measured as part of a comprehensive blood gas analysis preoperatively and at 0, 1, 3, 6, 12, and 24 h after ICU admission. Addition measurements were taken for patients with unstable conditions. All lactate measurements were examined with the ABL Flex 800 system (Radiometer Medical, Copenhagen, Denmark) with a normal reference of 1.0–1.8 mmol/L.
Surgical procedures
All procedures were performed via a median sternotomy. Briefly, the femoral or right axillary artery was dissected for arterial inflow for cardiopulmonary bypass (CPB). Surgical repair techniques were chosen depending on the location of the intimal tear and the extent of the dissection. A root reinforcement reconstruction was routinely performed for the proximal segment. An aortic valve replacement or Bentall procedure was performed when the dissection involved the coronary ostia or aortic valve, or in the presence of an aortic root aneurysm. Coronary artery bypass grafting procedure was performed in the following scenarios: cardiac malperfusion upon admission or the surgeons’ choice according to the patients’ coronary artery status during the operation.
Statistical analysis
Categorical variables were presented as the counts and percentages and were compared using the χ2 test or the Fisher’s exact test, as appropriate. Continuous variables were compared with the student’s t-test or the Mann–Whitney U-test and were presented as mean ± standard deviation for normal distributed variables or medians (interquartile ranges, 25–75th percentile) for variables not following normal distribution. The discriminatory performance of lactate as a predictor of mortality was determined using a receiver operating characteristic (ROC) curve. The area under the ROC curve was calculated to quantify the accuracy of lactate levels in predicting 30-day mortality. To estimate cut off values, Youden-Index J was calculated by the following equitation: J = sensitivity + (specificity-1). Cut off values were determined by the highest Youden-Index.
Univariable and multivariable logistic regression models were used to identify risk factors for 30-day mortality (stepwise forward method for multivariable regression analysis). Covariates that were either statistically significant on univariable analysis or considered clinically important were included in multivariable models. All probability values were two-tailed, and p values <0.05 were considered statistically significant. Statistical analyses were performed using the SPSS software, version 26 (IBM, Armonk, NY, United states).
Results
Demographic characteristics
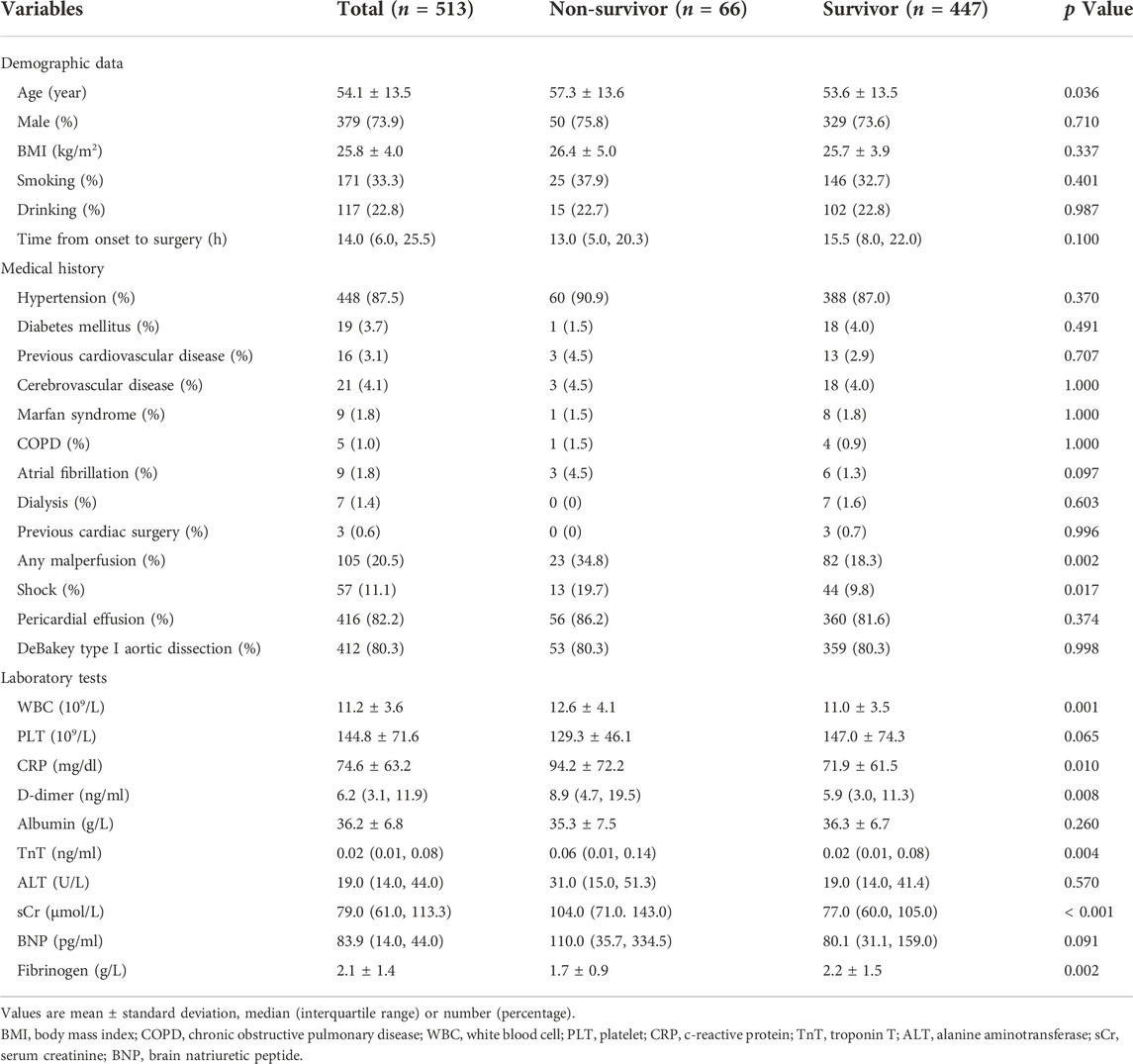
As shown in Figure 1, a total of 513 patients were included in this analysis. The mean age of the patients was 54.1 ± 13.5 years, and most were males. The 30-day mortality of all patients was 12.9% (66/513). The preoperative characteristics are presented in Table 1. There was no significant difference in sex, body mass index, alcohol consumption and smoking history between the two groups. However, compared with the survivor group, the average age of patients in the non-survivor group was older (57.3 ± 13.6 vs. 53.6 ± 13.5, p = 0.036). In addition, more patients in the non-survivor group had preoperative shock (19.7% vs. 9.8%; p = 0.017) and organ malperfusion (34.8% vs. 18.3%; p = 0.002). The average fibrinogen level was decreased and the levels of white blood cells, c-reactive protein, D-dimer, troponin T, and brain natriuretic peptide were increased in the non-survivor group compared to the survivor group.
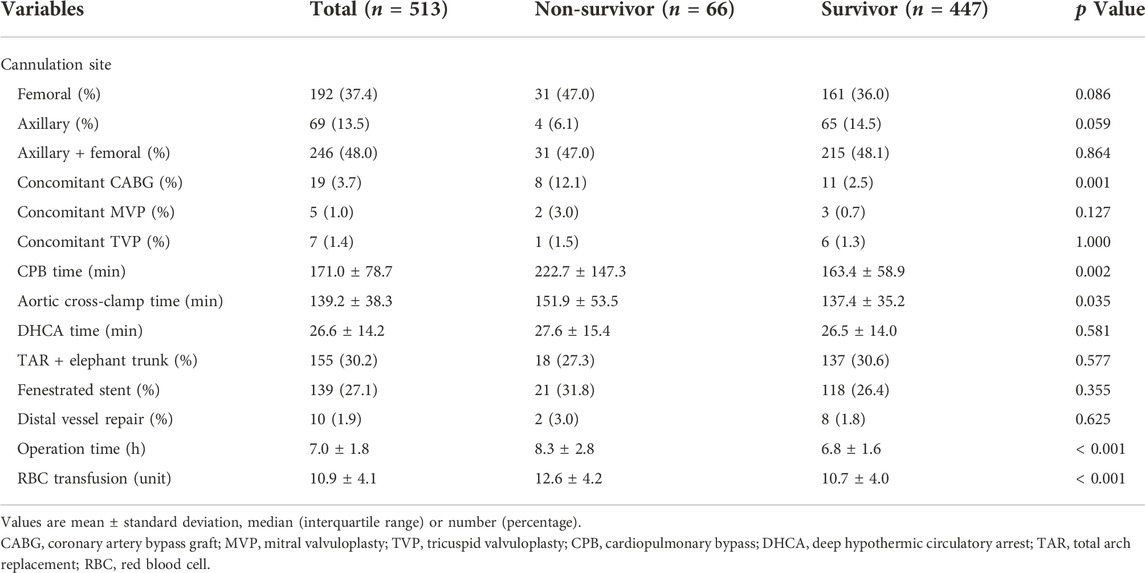
As shown in Table 2, patients in the non-survivor group had a longer duration of CPB (222.7 ± 147.3 vs. 163.4 ± 58.9 min, p = 0.002), aortic cross-clamp time (151.9 ± 53.5 vs. 137.4 ± 35.2 min, p = 0.035), and operation time (8.3 ± 2.8 vs. 6.8 ± 1.6 h, p < 0.001) compared to patients in the survivor group. In addition, a significantly increased number of patients required concomitant coronary artery bypass grafting after aortic central repair in the non-survivor group compared to the survivor group (12.1% vs. 2.5%; p = 0.001). Furthermore, an increased amount of red blood cell units was transfused perioperatively to patients in the non-survivor group compared to the survivor group (12.6 ± 4.2 vs. 10.7 ± 4.0, p < 0.001).
Complications
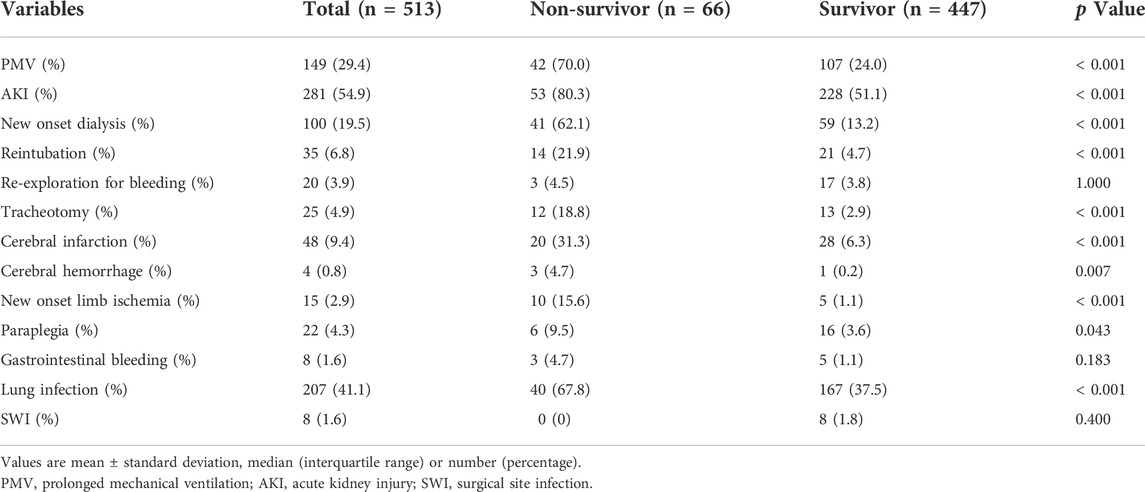
Table 3 shows that more complications occurred in the non-survivor group compared to the survivor group including prolonged mechanical ventilation (70.0% vs. 24.0%; p < 0.001), reintubation (21.9% vs. 4.7%; p < 0.001), tracheotomy (18.8% vs. 2.9%; p < 0.001), acute kidney injury (80.3% vs. 51.1%; p < 0.001), new onset dialysis (62.1% vs. 13.2%; p < 0.001), cerebral hemorrhage (4.7% vs. 0.2%; p = 0.007), cerebral infarction (31.3% vs. 6.3%; p < 0.001), new onset limb ischemia (15.6% vs. 1.1%; p < 0.001), and lung infection (67.8% vs. 37.5%; p < 0.001).
Blood lactate
Figure 2 depicts the change of lactate levels between the two groups. Preoperative lactate levels were significantly higher in the non-survivor group compared to the survivor group (5.4 ± 3.8 mmol/L vs. 3.3 ± 2.2 mmol/L vs; p = 0.007). After surgery, the difference between two groups persisted and peaked at 1 h after ICU admission.
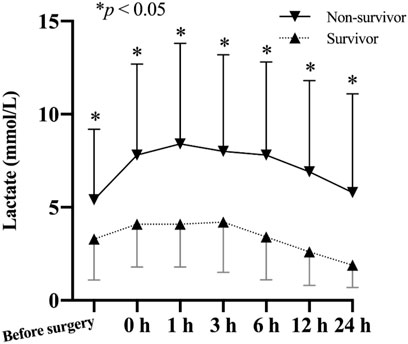
FIGURE 2. Comparison of lactate levels (mean and standard deviation) of patients from the non-survivor group and the survivor group based on their 30-day mortality.
Risk factors for 30-day mortality
Multivariable logistic risk analysis revealed that preoperative c-reactive protein (odds ratio [OR], 1.815; 95% confidence interval [CI], 1.202–3.028; p = 0.028), preoperative serum creatinine (OR, 1.406; 95% CI, 1.110–2.011; p = 0.046), CPB time (OR, 1.710; 95% CI, 1.202–2.517; p = 0.008), and lactate levels at 24 h after ICU admission (OR, 2.131; 95% CI, 1.346–3.374; p =0.001) were independent risk factors for 30-day mortality (Figure 3).
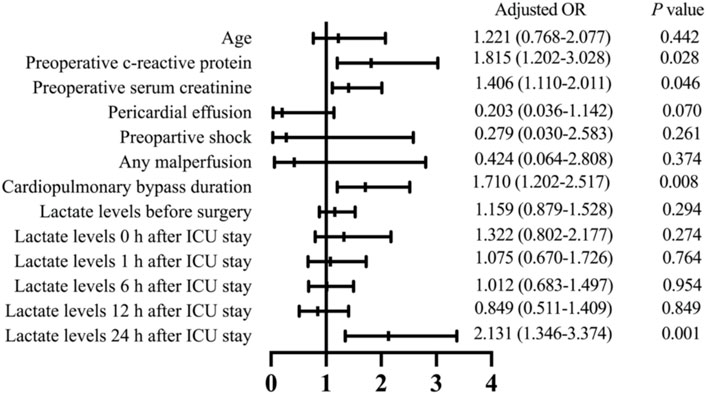
FIGURE 3. Multivariate analysis results to identify risk factors associated with 30-day mortality in patients who received ATAAD repair surgery. OR, odds ratio; ICU, intensive care unit.
Receiver operating characteristic analysis
ROC curves were plotted to identify the proper cut-off value for applying serum lactate level to predict 30-day mortality (Figure 4). The area under the curve (AUC) in relation to lactate level at 12 h after ICU stay was 0.820. A Youden’s index value of 3.35 mmol/L was derived from this curve, giving a sensitivity of 75.8% and a specificity of 80.1%. For lactic acid levels measured at 24 h after ICU stay, the AUC for 30-day mortality was 0.805 with corresponding Youden’s index value of 2.95 mmol/L and a sensitivity of 59.4% while a specificity of 91.8%. For lactate levels before surgery, the AUC for 30-day mortality was 0.690, and the Youden’s index value was 4.15 mmol/L. To test the preoperative lactate value as a categorical dichotomic variable against mortality, we divided the patients into two groups: group A (lactate level >4.15 mmol/L) and group B (lactate level ≤4.15 mmol/L). Data showed that the lactate levels at all postoperative timepoints were significantly higher in group A compared to group B (Figure 5).
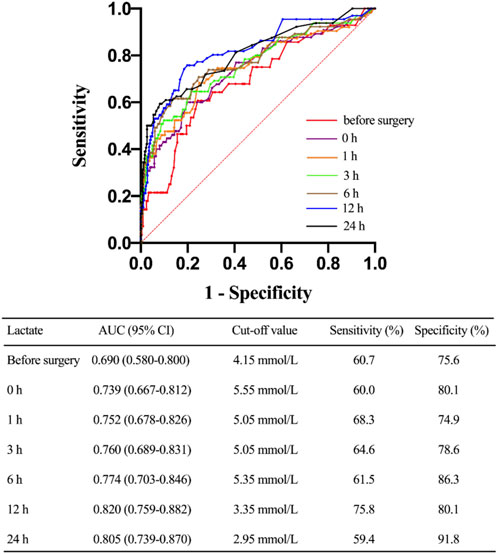
FIGURE 4. Characteristic of receiving operating curves evaluating the predictive power of different timepoints of lactate levels for 30-day mortality. CI, confidence interval.
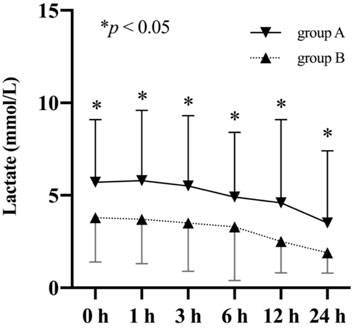
FIGURE 5. Comparison of lactate levels (mean and standard deviation) of patients stratified based on their preoperative lactate levels.
Discussion
Our study suggests that the increasing and dynamic change of lactate levels can be used to predict poor survival with high sensitivity and specificity in patients undergoing ATAAD surgery. The data demonstrated that lactate levels higher than 3.35 mmol/L at 12 h after ICU admission or 2.95 mmol/L at 24 h were associated with an AUC of 0.820 and 0.805, respectively. In addition, an elevated lactate level at 24 h after ICU admission was independently associated with a 2.131-fold increased risk of 30-day mortality in these patients. The results of our study not only proved this association but also sheds light on new perspectives for patient management after ATAAD surgery.
Many risk factors such as hypotension, ischemic events and neurologic abnormalities were identified to be associated with in-hospital mortality in patients who underwent ATAAD surgery (Spirito et al., 2001; Mehta et al., 2002; Czerny et al., 2003; Bekkers et al., 2013). Based on the Penn classification criteria, the risk of having lethal outcomes in patients with ATAAD can be evaluated based on patients’ ischemic events (Olsson et al., 2011; Kimura et al., 2014; Tien et al., 2020). However, many of these risk factors are subjective and require imaging scans that might not be applicable in emergent situations.
It has been shown that serum lactate level is a promising surrogate to evaluate perfusion and has already been used to identify patients with increased risk after undergoing ATAAD surgery. However, conflicting results have been reported regarding its association with disease prognosis. Bennett et al. (Bennett et al., 2017) showed that a cut-off preoperative lactate of 6 mmol/L could predict in-hospital death with an AUC of 0.88. However, this observation was challenged by another study conducted by Zindovic et al. (Zindovic et al., 2018) which demonstrated that preoperative hyperlactemia could not be used to predict disease prognosis. In this study, they found that the AUC for in-hospital mortality in relation to preoperative lactate levels was 0.684, which was similar to our results. However, for lactate levels measured upon ICU admission, Zindovic and others showed the AUC for their association with in-hospital death was only 0.582, which was lower than the value reported in our study. We found that preoperative hyperlactemia was transient in a large portion of their patients, as the levels rapidly declined upon ICU admission. On the other hand, our study showed that the average serum lactate level continued to increase and peaked after ICU admission, which was similar to the dynamic changes reported in a recent study conducted by Gemelli et al. (2022). The discrepancy of dynamic patterns of lactate levels between the two studies might explain these conflicting conclusions. Additional studies are needed to determine how sustained hyperlactatemia after surgery can affect clinical outcomes.
Serum lactate was a rapid and cost-effective alternative clinical marker for aortic dissection severity or malperfusion/ischemia, regardless of timing of presentation. Although the origin of the hyperlactatemia in ATAAD is numerous and diverse, lactate reducing therapy raise the possibility that targeting therapy to reduce or prevent the initial increase in this variable may prevent complications and improve postoperative outcomes. Firstly, when considering hyperlactemia was induced by organ malperfusion, particularly if mesenteric, coronary ischemia or cerebral is present, adjunctive techniques including catheter-based endovascular and even open surgical revascularization may be appropriate (Stewart and Chikwe, 2015; Li et al., 2016). Secondly, the inflammatory response also played a vital role in the postoperative hyperlactemia development. Mini-CPB could lead to fewer alterations of microperfusion and improve outcomes in patients with longer procedures (Donndorf et al., 2012). In addition, reducing the use of catecholamines during cardiac surgery could lead to decreased lactate levels owing to their action on oxidative glucose metabolism (Bellomo, 2002).
Using a single lactate level measured at a single time point to evaluate a patient’s condition could be misleading, as mild or moderate elevations are often difficult to interpret. Alternatively, serial measurements during the first 24 h after ATAAD surgery may be a more reliable predictor of 30-day mortality as seen in our study. It is important to note that only lactate levels measured at 24 h after ICU admission, but not other earlier time points, were identified as a risk factor for mortality. There may be several explanations for this observation. One hypothesis is that the elevated lactate levels at earlier time points might be due to inflammatory responses rather than reflecting systemic perfusion. Furthermore, many other conditions can contribute to early elevation of lactate levels after surgery including hyperglycemia, prolonged CPB, inotropic and vasopressor effects of epinephrine (which is often required in the post-surgery period), and elevated secretion of endogenous catecholamines (O'Connor and Fraser, 2012).
Our study showed that increased CPB time was another independent risk factor for short-term mortality. Recent studies indicated that increased CPB time could induce postoperative hyperlactemia in patients undergoing ATAAD surgery (Wang et al., 2021). It has been well known that microcirculation can affect the oxygen supply to cells. Greenwood et al. (2021) demonstrated that microcirculation was often impaired in cardiac surgery with CPB and was associated with postoperative hyperlactemia. In addition, liver clearance of lactate can be reduced secondary to the use of anesthetics and lower temperatures during CPB which might reduce the rate of cellular metabolism (Takala et al., 1996; Raper et al., 1997).
Malperfusion syndromes identified upon hospital admission was identified in a previous study to be associated with poor outcomes after surgery for ATAAD (Czerny et al., 2015). Our data showed the organ malperfusion rate and the preoperative lactate levels were higher in the non-survivor group. However, the association between preoperative malperfusion syndromes and poor outcomes was not observed in our logistic model. This discrepancy could be explained by the fact that prompt surgery was often performed for these patients in our center. Under such a policy, the prognosis of these patients following emergent surgery can be similar to those who did not present with malperfusion syndromes upon admission (Xue et al., 2021). With recent advances of surgical techniques and equipment, the overall perioperative mortality and long‐term survival rate of ATAAD patients with organ malperfusion has been greatly improved.
Limitations
Our study has several limitations. First, all patients enrolled in this study were retrospectively collected from a single center, and therefore might not be representative of all AATD patients undergoing emergent surgery. Second, the causes of elevated lactate levels were not further stratified due to limitations of our database. Furthermore, the mortality rate reported in our study was relatively low compared to previous studies which might underestimate the statistical power of other potential risk factors.
Conclusion
In conclusion, our data indicates that dynamic lactate levels after ATAAD surgery provides important prognostic data and is associated with increased postoperative mortality.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Nanjing Drum Tower Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
DJW and ZGW: research idea and study design. ZGW, KL, JFX, and XFC: data acquisition. KL, JFX, and XFC: statistical analysis. DJW and ZGW: supervision or mentorship. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the staff and participants from the Nanjing Drum Tower Hospital, Nanjing, China, for providing the clinical data for this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ATAAD, acute type A aortic dissection; ICU, intensive care unit; AUC, the area under the curve; ROC, receiver operating characteristic; CPB, cardiopulmonary bypass; OR, odds ratio; CI, confidence interval.
References
Bekkers J. A., Raap G. B., Takkenberg J. J., Bogers A. J. (2013). Acute type a aortic dissection: Long-term results and reoperations. Eur. J. Cardiothorac. Surg. 43 (2), 389–396. doi:10.1093/ejcts/ezs342
Bellomo R. (2002). Bench-to-Bedside review: Lactate and the kidney. Crit. Care 6 (4), 322–326. doi:10.1186/cc1518
Bennett J. M., Wise E. S., Hocking K. M., Brophy C. M., Eagle S. S. (2017). Hyperlactemia predicts surgical mortality in patients presenting with acute Stanford type-a aortic dissection. J. Cardiothorac. Vasc. Anesth. 31 (1), 54–60. doi:10.1053/j.jvca.2016.03.133
Certo M., Tsai C. H., Pucino V., Ho P. C., Mauro C. (2021). Lactate Modulation of Immune responses in inflammatory versus Tumour Microenvironments. Nat. Rev. Immunol. 21 (3), 151–161. doi:10.1038/s41577-020-0406-2
Czerny M., Fleck T., Zimpfer D., Dworschak M., Hofmann W., Hutschala D., et al. (2003). Risk factors of mortality and permanent neurologic injury in patients undergoing Ascending aortic and arch repair. J. Thorac. Cardiovasc. Surg. 126 (5), 1296–1301. doi:10.1016/s0022-5223(03)01046-8
Czerny M., Schoenhoff F., Etz C., Englberger L., Khaladj N., Zierer A., et al. (2015). The Impact of Pre-operative malperfusion on outcome in acute type a aortic dissection: Results from the Geraada registry. J. Am. Coll. Cardiol. 65 (24), 2628–2635. doi:10.1016/j.jacc.2015.04.030
Ding X. F., Yang Z. Y., Xu Z. T., Li L. F., Yuan B., Guo L. N., et al. (2018). Early Goal-Directed and lactate-Guided therapy in Adult patients with Severe sepsis and Septic shock: A Meta-analysis of randomized Controlled Trials. J. Transl. Med. 16 (1), 331. doi:10.1186/s12967-018-1700-7
Donndorf P., Kuhn F., Vollmar B., Rosner J., Liebold A., Gierer P., et al. (2012). Comparing Microvascular alterations during Minimal Extracorporeal circulation and Conventional cardiopulmonary bypass in coronary artery bypass graft surgery: A Prospective, randomized study. J. Thorac. Cardiovasc. Surg. 144 (3), 677–683. doi:10.1016/j.jtcvs.2012.05.037
Gemelli M., Di Tommaso E., Chivasso P., Sinha S., Ahmed E. M., Rajakaruna C., et al. (2022). Blood lactate predicts mortality after surgical repair of type a acute aortic dissection. J. Card. Surg. 37, 1206–1211. doi:10.1111/jocs.16324
Greenwood J. C., Jang D. H., Spelde A. E., Gutsche J. T., Horak J., Acker M. A., et al. (2021). Low microcirculatory perfused Vessel Density and high Heterogeneity are associated with increased intensity and duration of lactic Acidosis after cardiac surgery with cardiopulmonary bypass. Shock 56 (2), 245–254. doi:10.1097/SHK.0000000000001713
Hajjar L. A., Almeida J. P., Fukushima J. T., Rhodes A., Vincent J. L., Osawa E. A., et al. (2013). High lactate levels are predictors of Major complications after cardiac surgery. J. Thorac. Cardiovasc. Surg. 146 (2), 455–460. doi:10.1016/j.jtcvs.2013.02.003
Ilias I., Apollonatou S., Vassiliadi D. A., Nikitas N., Theodorakopoulou M., Diamantakis A., et al. (2018). Adipose tissue lactate clearance but not blood lactate clearance is associated with clinical outcome in sepsis or Septic shock during the post-resuscitation period. Metabolites 8 (2), E28. doi:10.3390/metabo8020028
Kellum J. A., Lameire N., Group K. A. G. W. (2013). Diagnosis, evaluation, and management of acute kidney injury: A Kdigo summary (Part 1). Crit. Care 17 (1), 204. doi:10.1186/cc11454
Kilic A., Tang R., Whitson B. A., Sirak J. H., Sai-Sudhakar C. B., Crestanello J., et al. (2013). Outcomes in the current surgical Era following operative repair of acute type a aortic dissection in the Elderly: A single-institutional Experience. Interact. Cardiovasc. Thorac. Surg. 17 (1), 104–109. doi:10.1093/icvts/ivt155
Kimura N., Ohnuma T., Itoh S., Sasabuchi Y., Asaka K., Shiotsuka J., et al. (2014). Utility of the Penn classification in predicting outcomes of surgery for acute type a aortic dissection. Am. J. Cardiol. 113 (4), 724–730. doi:10.1016/j.amjcard.2013.11.017
Kogan A., Preisman S., Bar A., Sternik L., Lavee J., Malachy A., et al. (2012). The Impact of hyperlactatemia on postoperative outcome after Adult cardiac surgery. J. Anesth. 26 (2), 174–178. doi:10.1007/s00540-011-1287-0
Lazzeri C., Valente S., Chiostri M., Gensini G. F. (2015). Clinical significance of lactate in acute cardiac patients. World J. Cardiol. 7 (8), 483–489. doi:10.4330/wjc.v7.i8.483
Li C. L., Wang H., Jia M., Ma N., Meng X., Hou X. T. (2015). The early dynamic Behavior of lactate is Linked to mortality in Postcardiotomy patients with Extracorporeal Membrane Oxygenation Support: A retrospective observational study. J. Thorac. Cardiovasc. Surg. 149 (5), 1445–1450. doi:10.1016/j.jtcvs.2014.11.052
Li H. W., Shih Y. C., Liu H. H., Chen C. W., Chien T. M., Chen H. M., et al. (2016). Reconsidering the Impact of Pre-operative malperfusion on acute type a dissection: The Modified Penn classification. J. Am. Coll. Cardiol. 67 (1), 121–122. doi:10.1016/j.jacc.2015.09.102
Manoharan I., Prasad P. D., Thangaraju M., Manicassamy S. (2021). Lactate-dependent regulation of Immune responses by Dendritic cells and Macrophages. Front. Immunol. 12, 691134. doi:10.3389/fimmu.2021.691134
Mehta R. H., Suzuki T., Hagan P. G., Bossone E., Gilon D., Llovet A., et al. (2002). Predicting death in patients with acute type a aortic dissection. Circulation 105 (2), 200–206. doi:10.1161/hc0202.102246
Nguyen H. B., Rivers E. P., Knoblich B. P., Jacobsen G., Muzzin A., Ressler J. A., et al. (2004). Early lactate clearance is associated with improved outcome in Severe sepsis and Septic shock. Crit. Care Med. 32 (8), 1637–1642. doi:10.1097/01.ccm.0000132904.35713.a7
O'Connor E., Fraser J. F. (2012). The Interpretation of perioperative lactate abnormalities in patients undergoing cardiac surgery. Anaesth. Intensive Care 40 (4), 598–603. doi:10.1177/0310057X1204000404
Olsson C., Hillebrant C. G., Liska J., Lockowandt U., Eriksson P., Franco-Cereceda A. (2011). Mortality in acute type a aortic dissection: Validation of the Penn classification. Ann. Thorac. Surg. 92 (4), 1376–1382. doi:10.1016/j.athoracsur.2011.05.011
Pansini S., Gagliardotto P. V., Pompei E., Parisi F., Bardi G., Castenetto E., et al. (1998). Early and late risk factors in surgical treatment of acute type a aortic dissection. Ann. Thorac. Surg. 66 (3), 779–784. doi:10.1016/s0003-4975(98)00555-4
Pucino V., Bombardieri M., Pitzalis C., Mauro C. (2017). Lactate at the Crossroads of metabolism, inflammation, and autoimmunity. Eur. J. Immunol. 47 (1), 14–21. doi:10.1002/eji.201646477
Pucino V., Certo M., Bulusu V., Cucchi D., Goldmann K., Pontarini E., et al. (2019). Lactate Buildup at the site of chronic inflammation Promotes disease by inducing Cd4(+) T cell metabolic rewiring. Cell Metab. 30 (6), 1055–1074. e8. doi:10.1016/j.cmet.2019.10.004
Raper R. F., Cameron G., Walker D., Bowey C. J. (1997). Type B lactic Acidosis following cardiopulmonary bypass. Crit. Care Med. 25 (1), 46–51. doi:10.1097/00003246-199701000-00011
Renew J. R., Barbara D. W., Hyder J. A., Dearani J. A., Rivera M., Pulido J. N. (2016). Frequency and outcomes of Severe hyperlactatemia after elective cardiac surgery. J. Thorac. Cardiovasc. Surg. 151 (3), 825–830. doi:10.1016/j.jtcvs.2015.10.063
Santini F., Montalbano G., Casali G., Messina A., Iafrancesco M., Luciani G. B., et al. (2007). Clinical Presentation is the Main predictor of in-hospital death for patients with acute type a aortic dissection Admitted for surgical treatment: A 25 Years Experience. Int. J. Cardiol. 115 (3), 305–311. doi:10.1016/j.ijcard.2006.03.013
Spirito R., Pompilio G., Alamanni F., Agrifoglio M., Dainese L., Parolari A., et al. (2001). A preoperative index of mortality for patients undergoing surgery of type a aortic dissection. J. Cardiovasc. Surg. 42 (4), 517–524.
Stewart A., Chikwe J. (2015). Thinking beyond the Tube graft: Using malperfusion as a Guide to define treatment of type a dissection. J. Am. Coll. Cardiol. 65 (24), 2636–2637. doi:10.1016/j.jacc.2015.05.006
Takala J., Uusaro A., Parviainen I., Ruokonen E. (1996). Lactate metabolism and regional lactate Exchange after cardiac surgery. New Horiz. 4 (4), 483–492.
Tien M., Ku A., Martinez-Acero N., Zvara J., Sun E. C., Cheung A. T. (2020). The Penn classification predicts hospital mortality in acute Stanford type a and type B aortic dissections. J. Cardiothorac. Vasc. Anesth. 34 (4), 867–873. doi:10.1053/j.jvca.2019.08.036
Wang S., Wang D., Huang X., Wang H., Le S., Zhang J., et al. (2021). Risk factors and in-hospital mortality of postoperative hyperlactatemia in patients after acute type a aortic dissection surgery. BMC Cardiovasc. Disord. 21 (1), 431. doi:10.1186/s12872-021-02244-7
Xue Y., Tang X., Zhu X., Lu Y., Zhang H., Xie W., et al. (2021). Prompt surgery is effective for acute type a aortic dissection with cerebral ischemia. J. Thorac. Dis. 13 (3), 1403–1412. doi:10.21037/jtd-20-2349
Keywords: aortic dissection, 30-day mortality, lactate, intensive care unit, risk factor, outcome
Citation: Wang Z, Li K, Xu J, Cheng X and Wang D (2022) Construction of a lactate-related prognostic signature for predicting prognosis after surgical repair for acute type a aortic dissection. Front. Physiol. 13:1008869. doi: 10.3389/fphys.2022.1008869
Received: 01 August 2022; Accepted: 03 November 2022;
Published: 16 November 2022.
Edited by:
Zhen W. Zhuang, Yale University, United StatesReviewed by:
Stefano Schena, Medical College of Wisconsin, United StatesShu Cao, University of South Florida, United States
Copyright © 2022 Wang, Li, Xu, Cheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Wang, Z2x5eXd6Z0AxNjMuY29t; Dongjin Wang, Z2x5eXdkakAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Zhigang Wang1*†
Zhigang Wang1*† Dongjin Wang
Dongjin Wang