
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 26 January 2022
Sec. Striated Muscle Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.804188
Cerebral palsy results from an upper motor neuron lesion and significantly affects skeletal muscle stiffness. The increased stiffness that occurs is partly a result of changes in the microstructural components of muscle. In particular, alterations in extracellular matrix, sarcomere length, fibre diameter, and fat content have been reported; however, experimental studies have shown wide variability in the degree of alteration. Many studies have reported changes in the extracellular matrix, while others have reported no differences. A consistent finding is increased sarcomere length in cerebral palsy affected muscle. Often many components are altered simultaneously, making it difficult to determine the individual effects on muscle stiffness. In this study, we use a three dimensional modelling approach to isolate individual effects of microstructural alterations typically occurring due to cerebral palsy on whole muscle behaviour; in particular, the effects of extracellular matrix volume fraction, stiffness, and sarcomere length. Causation between the changes to the microstructure and the overall muscle response is difficult to determine experimentally, since components of muscle cannot be manipulated individually; however, utilising a modelling approach allows greater control over each factor. We find that extracellular matrix volume fraction has the largest effect on whole muscle stiffness and mitigates effects from sarcomere length.
Cerebral palsy (CP) results from an upper motor neuron lesion and has a significant effect on the musculoskeletal system. It develops during early childhood and leads to muscle alterations including contracture, which is the chronic shortening of a muscle. Contracture results in muscle that cannot be stretched through its typical range of motion due to an increase in stiffness, and this has substantial effects on the ability of muscle to generate force and reduces daily functioning. Typically, this is observed in the upper and lower limb flexor muscles. CP will affect individuals differently, and the changes that can occur will vary depending on the location of the muscle and disease severity (de Bruin et al., 2014; Handsfield et al., 2016; Lieber and Fridén, 2019). This variability increases the difficulty in quantifying the amount and types of changes that occur as a result of CP. However, despite the variability, alterations in the microstructural properties of skeletal muscle are commonly observed (Tisha et al., 2019), which will have a significant effect on whole muscle behaviour, including force production and movement.
There are many structural differences comparing CP muscle to typically developed (TD) muscle, including changes in fat content (Ohata et al., 2009; D'Souza et al., 2020), extracellular matrix (ECM) stiffness (Lieber et al., 2003; Smith et al., 2011), amount of ECM (Lieber et al., 2003; Smith et al., 2011), fascicle length (Mohagheghi et al., 2008), fibre diameter (Mathewson et al., 2014a), fibre geometry (Barrett and Lichtwark, 2010), and sarcomere length (Lieber and Fridén, 2002; Smith et al., 2011; Mathewson et al., 2014a). Experimental studies have investigated CP muscle stiffness in vivo and have found stiffer tissue compared to TD muscle using shear wave elastography (Brandenburg et al., 2016; Lee et al., 2016) and through measuring joint movement (Barber et al., 2011; van der Krogt et al., 2016). However, these methods are unable to capture the underlying causes of this increased stiffness.
The exact microstructural changes that alter whole muscle stiffness have yet to be fully understood, as the extent of measured changes varies between studies (Lieber and Fridén, 2019). For example, Smith et al. (2011) performed passive mechanical experiments on both muscle fibre bundles and single fibres extracted from CP and TD muscle. They found that CP muscle had longer in vivo sarcomere lengths and increased fibre bundle, but not fibre, stiffness, which suggests that the changes in muscle stiffness are due to alterations in the ECM. Another study by Mathewson et al. (2014a), which used a similar experimental protocol as Smith et al. (2011), also showed increased in vivo sarcomere lengths. However, the authors demonstrated a difference in the single fibre stiffness and not the fibre bundles, suggesting that there is not a significant effect from the ECM, and that any alterations to passive stiffness occur on the muscle fibre level. Smith et al. (2011) performed studies on the Gracilis and Semitendinosus muscles, whereas, Mathewson et al. (2014a) looked at the Gastrocnemii and the Soleus muscles. Mathewson et al. (2014a) mention that the difference in the results is possibly due to the locations of the muscles or different mechanical properties of ECM between TD and CP muscle. Another possible explanation for the differences between the two studies is the that the TD groups in the study by Mathewson et al. (2014a) had a much older average age (47.7±15.3) compared to Smith et al. (2011) (15.8±1.8). Other studies have reported that CP muscle has a greater accumulation of fibrotic tissue, and potentially even results in an ECM with a larger volume fraction but compromised stiffness (Lieber et al., 2003). Additionally, Booth et al. (2001) suggested that collagen plays a role in the increased muscle stiffness that is observed in CP. However, it has been observed that fibrosis does not always alter the stiffness of muscle (Smith and Barton, 2014), and so there may be an effect from the stiffness and structure of the ECM. In TD muscle the ECM has been shown to be a major contributor to passive whole muscle mechanics due its composition of stiff collagen fibres (Gillies and Lieber, 2011; Meyer and Lieber, 2018). In particular, work has shown that in mammalian muscle, the ECM is responsible for about half of load bearing in passive tension (Meyer and Lieber, 2018). Sarcomere length is a commonly observed alteration in CP muscle, and has been said to have a large effect on active muscle mechanics (Lieber and Fridén, 2002; Smith et al., 2011; Mathewson et al., 2014b). While many changes have been observed in CP muscle, the changes that are most common between studies are changes in ECM volume fraction, ECM stiffness, and sarcomere length. However, the individual roles of the ECM properties and sarcomere length in passive whole muscle stiffness have yet to be fully understood.
The purpose of this study was to determine which microstructural change occurring with CP has the largest contribution to whole muscle stiffness. In particular, whether the ECM, through changes in volume fraction or stiffness, or the sarcomere, through increases in length that result in an increased passive response from the titin, will have the greatest influence on passive whole muscle behaviour. During experimental studies, it is not possible to change a component of muscle, while keeping all other components constant; therefore, causation cannot be determined. This is especially difficult in CP where many components of muscle are known to vary between individuals (Lieber and Fridén, 2019; Tisha et al., 2019). In this study, we try not to determine the cause of changes to the microstructure, but the relationship between changes to the microstucture and the overall muscle stiffness. Using a modelling approach, we investigated the influence of the microstructural components on passive muscle stiffness. We utilised a three dimensional continuum model of skeletal muscle, developed in previous studies (Rahemi et al., 2014; Ross et al., 2018b; Wakeling et al., 2020; Konno et al., 2021), which can be modified to incorporate the effects of ECM and passive fibre properties on whole muscle mechanics. Here we do not explicitly model the process of contracture, but instead the resulting changes to the microstructure. Any mechanical response we observe in this study will also be relevant to muscle without clear contracture, but with similar changes to the material properties. By modifying the material properties in the muscle, we investigated changes that occur with CP to understand how each component contributes to whole muscle stiffness.
In this study, we utilised a continuum mechanical model of muscle as a fibre-reinforced composite biomaterial. The model uses a three field formulation in terms of the velocity, u, pressure, p, and dilation, J, and the balance of strain-energy potentials based on work by Simo and Taylor (1991) and Weiss et al. (1996). In particular, we want to minimise the total strain-energy
where Uint is the internal strain-energy potential and Wext is the total external work done on the muscle. We characterised the passive mechanical behaviour of muscle in terms of its stress-strain behaviour, which is the relationship between the stress applied to the muscle and the strain experienced. Here we use the full Cauchy stress tensors, so the shear stress response is built into this tensor implicitly and has been investigated in Konno et al. (2021). The relationship between the stress in the material, σ, and the strain-energy potential of a material, U, is given by the constitutive law
where B is a strain tensor measuring the deformation. To capture muscle, we split the model into a three dimensional isotropic base material with one dimensional fibres running along the length of the muscle, making the composite material anisotropic. The total stress response from the muscle (σmuscle) is then additive contributions from the base material (σbase) and fibre (σfibre) components
Our model is a homogenized muscle material that included contributions from the fibres and base material at every element in the mesh. A more precise formulation of the model is described in the appendix of Wakeling et al. (2020) and in Konno et al. (2021). We used a finite element method to solve the continuum model that was implemented using an open source finite element library deal.II (Arndt et al., 2017).
There are two main components of our muscle model: the fibre components and the three base material. The base material encompasses effects from extracellular matrix and cellular components, including satellite cells and capillaries, while the fibre component runs along the length of the muscle and contains the passive effects from titin and active effects from the contractile elements. To investigate the role of CP on whole muscle stiffness, considering the individual effects from the ECM and cellular components is necessary. To do this, we let α be the volume fraction of the ECM, which includes effects from the collagen fibre matrix (Figure 1). Meanwhile, 1 − α is the volume fraction of the cellular component, which includes effects from any other material in the muscle aside from the contractile units. In particular the cellular material includes the myofibres, satellite cells, and other cellular materials. We also introduced a parameter sECM (Figure 1), which is a stiffness factor multiplying the stiffness of ECM. A larger sECM corresponds to a stiffer ECM, which can occur as a result of structural changes while the ECM volume fraction remains the same (Gillies et al., 2016). Meanwhile, a smaller value of sECM results in an ECM with decreased stiffness, which can occur as a result of an ECM with compromised structure (Lieber et al., 2003). The total stress response from the base material is a homogenization of the ECM and cellular components given by
σECM(B) corresponds to the stress response from the ECM, while σCELL(B) is the stress response from the cellular component. To ensure the volume of the muscle remains nearly constant, the bulk modulus for the cellular component is chosen to be 1 × 107 Pa, while the bulk modulus of the ECM component was set to 1 × 106 Pa (Konno et al., 2021). While the cellular component of the muscle contributes less to the overall stress response, it consists largely of water, so its bulk modulus is set to be larger than the ECM component. The exact form of the stress-strain response for the microstructural components are given in Konno et al. (2021) along with more detail on the homogenized base material.
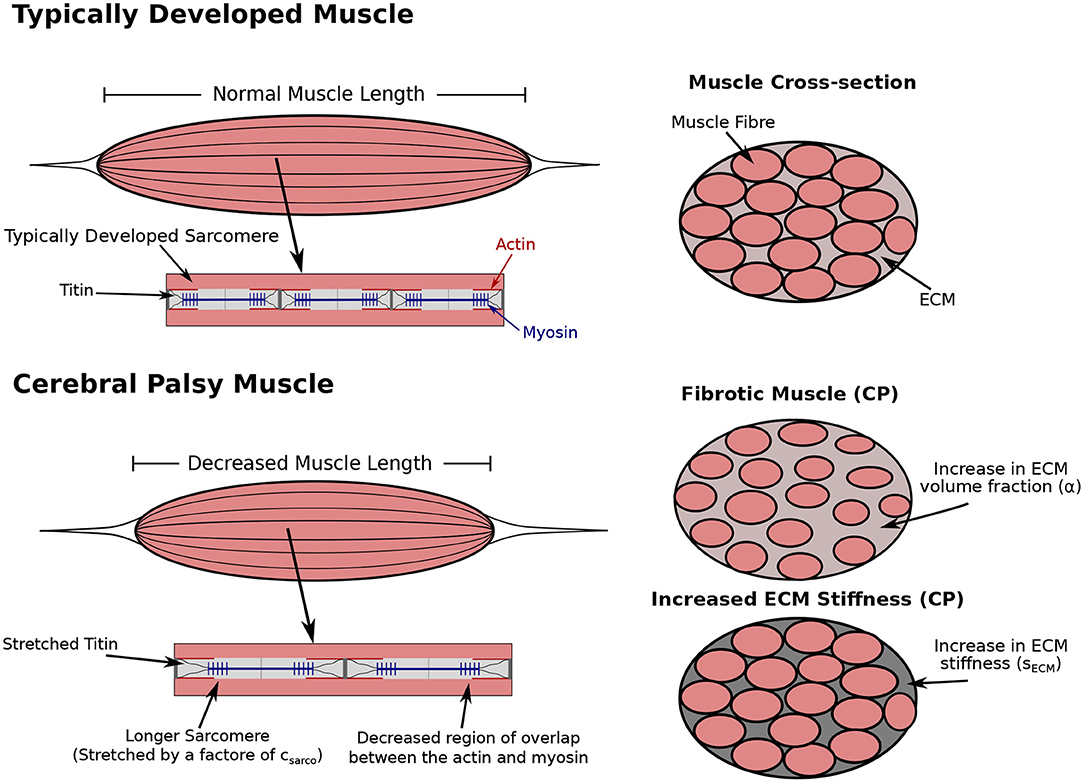
Figure 1. Comparison of typically developed (TD) and cerebral palsy (CP) muscle. CP results in contracture, which is the chronic shortening of muscle, decreasing muscle length relative to TD muscle (not investigated in this study). Longer sarcomere lengths relative to the rest of the muscle have also been observed compared to TD muscle (Smith et al., 2011; Mathewson et al., 2014b). There is an increase in passive forces due to the increased stretch in the titin proteins. The longer sarcomeres lengths in muscle affected by CP reduces the regions of overlap of the actin and myosin filaments, which results in decreased contractile forces. Additionally, there is an increase in extracellular matrix (ECM) volume fraction, and a possible increase or decrease in ECM stiffness. Any combination of changes to the sarcomere length or ECM properties could occur with CP.
The other alteration typically observed in CP is an increase in in vivo sarcomere length (Smith et al., 2011; Mathewson et al., 2014b), which alters the passive muscle stiffness by stretching the titin protein. Experimentally, the sarcomere lengths for muscles in the lower extremities were measured with 90 degrees hip and knee flexion (Smith et al., 2011; Mathewson et al., 2014a), and with the ankle in full dorsiflexion for the lower leg muscles (Mathewson et al., 2014a). Our model does not have a joint angle, and so we define the in vivo length of the sarcomeres to be the length of the sarcomeres when the whole muscle is at its resting length. This change in length also decreases the contractile force produced when the muscle is active by reducing the number of attached actin-myosin crossbridges (Figure 1). We modelled this using a dimensionless parameter, csarco, which corresponds to a shift in the passive force-length curve of the sarcomeres
where is the total average stretch of the fibres over the muscle volume. This parameter acts as an additive contribution to the intrinsic stress-stretch relationship, resulting in larger stresses in the fibres at a given stretch (Figure 2). It is important to note that, while the fibre component of the muscle depends on csarco, the intrinsic stress response from the base material, σbase(B), only depends on the deformation and stretch of the muscle, and not csarco. At a value of csarco = 0.0, the behaviour of the sarcomere is the same as that of TD muscle. Increasing values of csarco results in longer lengths of the sarcomeres given by
where lsarco is the new length of the sarcomere and we assume l0 = 2.2μm is the optimal length of a sarcomere (Burkholder and Lieber, 2001). This will vary depending on the value of csarco. The fibres in the model are based off the one dimensional Hill type model (Hill, 1938; Zajac, 1989) and are described in Wakeling et al. (2020). It is possible the behaviour of the fibre will vary in more than a shift of its force-length curve, such as a different force-length relationship, which may be due to factors in the myofibres other than the sarcomeres. However, as this is not observed frequently and data are limited, we focus on the effects from the sarcomere length.
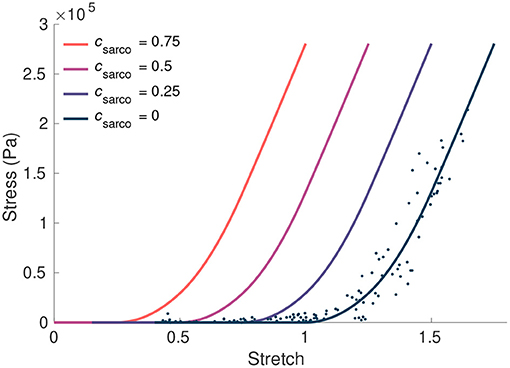
Figure 2. The influence of csarco on the intrinsic fibre stress-stretch relationship. Here we plot the stress from the fibre component of our model against the stretch in the fibres. The curve corresponding to typically developed muscle (csarco = 0) was obtained based on curves and data in Ross et al. (2018a). This was done through trigonometric polynomial and second-order piecewise polynomial fits to experimental data from Winters et al. (2011) (denoted by the dots).
To investigate the passive effects of α, sECM, and csarco on skeletal muscle, we constructed a rectangular block of muscle with dimensions 3 cm × 1 cm × 1 cm. These dimensions, while not the same as muscle affected by CP, sufficiently capture the behaviour of muscle on a macroscopic scale. Using a block geometry reduces the need to consider the additional effects from architecture, aponeurosis, and pennation angle, which affects muscle behaviour (Wakeling et al., 2020), and instead allowed us to isolate the effects due to CP independent of a specific architecture. Additionally, this geometry has been previously validated to capture the general qualitative behaviour of muscle when compared to the mechanics within a MRI derived whole muscle geometry (Wakeling et al., 2020) and has the benefit of computational simplicity. To compare the passive behaviour of the model to experimental data, we performed stress-strain tests. This involved constraining one end face of the model from movement in all directions, while a normal stress was applied to the opposite face stretching the muscle. In addition to the stress-strain tests, we investigated the stiffness of the muscle to compare with experimental studies (e.g., Smith et al. (2011); Mathewson et al. (2014b)). α, sECM, and csarco each have an individual contribution to the overall stiffness of muscle. To investigate the stiffness in the model, the modulus (in Pa) of the muscle material was calculated during the stress-strain experiments using the slope of the tangent line to the overall stress-stretch relationship. This was done by performing a nonlinear least-squares fit of a cubic polynomial to the overall stress-strain data in the longitudinal direction. This method for calculating the modulus is only representative of the stiffness at the given stretch value, since the stress-stretch curves are nonlinear; however, we do this to compare with experimental studies.
TD muscle has been observed to have a value for α between 0.02 and 0.10 (Binder-Markey et al., 2020), while larger volume fractions (α≈0.6) have been observed for fibrotic tissue (Lieber et al., 2003; Smith and Barton, 2014). Experimental studies have only found an increase in in vivo sarcomere lengths (Lieber and Fridén, 2002; Smith et al., 2011; Mathewson et al., 2014a), so we varied csarco from 0 to 0.75. This corresponded to a 0–75 % increase in the sarcomeres relative to the sarcomeres in typically developed muscle, which has been observed in the literature (Lieber and Fridén, 2002; Smith et al., 2011; Mathewson et al., 2014b). Experiments are often performed on severe cases of CP, so larger sarcomere lengths have been reported (csarco > 0.75); however, in this study, we considered less severely stretched sarcomeres to represent less severe cases of CP. It is possible that muscle altered by CP does not always have such a substantial increase in sarcomere length, since measurements are typically taken from children with severe CP undergoing surgery (Lieber and Fridén, 2019). The final parameter that was manipulated in the model is sECM. While the stiffness of muscle can vary depending on the type of muscle, the properties of the base material, including the effects from the ECM, represent and have been validated for TD muscle (Konno et al., 2021), so we set sECM = 1 for TD muscle (this corresponds to a value of 150 in Konno et al., 2021). During our stress-strain tests, we then considered the possibility of the ECM component of muscle being stiffer (sECM = 1.33) and less stiff (sECM = 0.66). Note that a sECM value of 1.33 corresponds to a stiffness of 133 % compared to TD muscle, while a value 0.66 corresponds to a stiffness of 66 % relative to TD muscle. Data for the changes in stiffness of the ECM are not available experimentally, so these values of sECM were chosen to investigate the effects of altering this component. In summary, to investigate the effects of CP, our parameter ranges were α = 0.02 to 0.6, csarco = 0.0 to 0.75, and sECM = 0.66 to 1.33, while TD muscle had parameters α = 0.05, csarco = 0.0, and sECM = 1.0.
For TD muscle (csarco = 0.0, sECM = 1.0, and α = 0.05), we observed typical overall stress-stretch behaviour for passive skeletal muscle: as the stress increased, the muscle stretch and sarcomere lengths also increased (Figure 3). The muscle block in its resting and stretched states is shown in Figures 3C,D, respectively. The shift in the intrinsic passive sarcomere stress-stretch relationship, csarco, affected the muscle behaviour in both the stress-length for the sarcomeres and overall stress-stretch for the whole muscle relationships of the model (Figure 3). At in vivo lengths, we found that there is no longer zero stress for csarco > 0.0 (Figure 3A). This indicates that larger forces are required to stretch muscles with increased sarcomere lengths, as well as to hold it at the resting length of the muscle. We also see that there is a nonlinear relationship between the effect of csarco on the fibre component (Figure 2) and the overall muscle response which is influenced by the base material (Figure 3). Additionally, optimal length of the sarcomeres no longer occurred at the same resting length of the whole muscle. For the same range of stress values (0–3 × 105 Pa), we saw a larger range of whole muscle stretches with larger csarco (Figure 3). This is likely due to effects from the base material and, therefore, the ECM, which acts to deform the muscle back to optimal length. At stretch values less than 1.0, the base material works to extend the muscle to optimal length, while the sarcomeres are still working to shorten the muscle for csarco > 0.0.
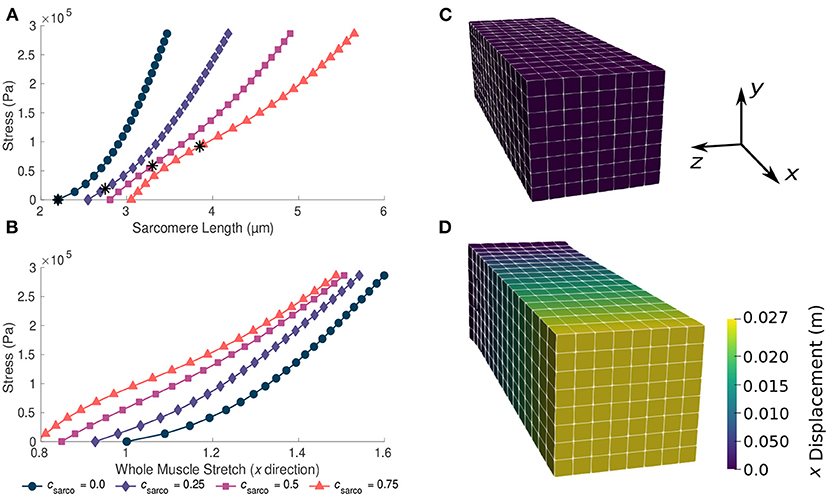
Figure 3. Plots of whole muscle stress in the along fibre direction against sarcomere length (A) and whole muscle stretch (B). Plots are from the computational model during passive lengthening with ECM volume fraction, α, of 0.05 and ECM stiffness factor, sECM, of 1.0. Each curve represents a shift in the sarcomere stretch by a factor of csarco. * represents in vivo sarcomere length for corresponding csarco. (C,D) show the mesh at resting length and at a deformed state after the stress has been applied to the model.
In addition to the behaviour in the along-fibre direction of the muscle, csarco also affects the behaviour transverse to the muscle fibres (Figure 4). We observed a similar change in concavity of the stress-stretch curves in both the stress-stretch relationships in the longitudinal (Figure 3B) and transverse (Figure 4) directions. This demonstrates similar effects from the muscle ECM component in both directions. For stretch values in the y direction less than 0.85, the influence of the sarcomeres on the stress-stretch relationship decreased, and there was larger influence from the ECM. For smaller normal stresses in the longitudinal directions, there were larger effects from sarcomere length relative to larger stresses (Figure 4). The sarcomeres, acting only in the along-fibre direction, altered three dimensional deformation, which could affect muscle force production (Wakeling et al., 2020).
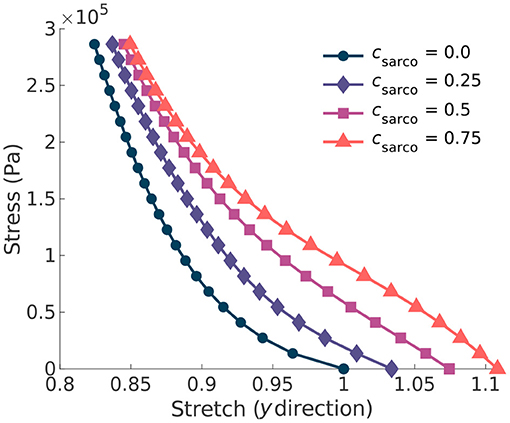
Figure 4. Plot of the normal stress applied in the along-fibre direction (x) against the stretch in the muscle transverse to the fibres. Given the symmetry in the muscle geometry transverse to the fibres (y), the stress-stretch response shown is the same in the z direction. Each line represents a shift in the sarcomere stretch by a factor of csarco.
The ECM properties also had a substantial effect on the overall stress-stretch relationships (Figure 5). Given the range of possible values for the ECM volume fraction, α, (0.02–0.6), it had a larger effect on the muscle stiffness compared to the ECM stiffness parameter, sECM. As sECM was increased, with fixed α = 0.05 or 0.6, the overall stress-stretch relationship became more linear and covered a smaller range of stretch values (Figure 5). Increases in both α and sECM reduced the effect from the sarcomere length on the stress-stretch relationship (Figure 5). Due to the lack of available data for the stiffness of the ECM, the sECM was only varied between 0.66 and 1.33, which is a relatively small range compared to the volume fraction of the ECM. Similar effects were observed from changing sECM and α, but α had more effect on the overall stress-stretch behaviour. This is expected given the change in composition of the base material; however, due to the limited data for sECM, it is possible that real muscle has a larger range of values than investigated in this study, but to ensure that we remain within realistic physiological ranges we chose this limited range. Our results showed that sECM had very little effect on the overall stress-stress relationship for smaller values of α and it has the most effect when α is large. The main effects from the sECM are in altering the influence of csarco on the overall muscle stress-stretch relationship (Figure 5). Varying sECM over a larger range of values would likely only influence the effect of the sarcomere length on the overall stress-stretch response.
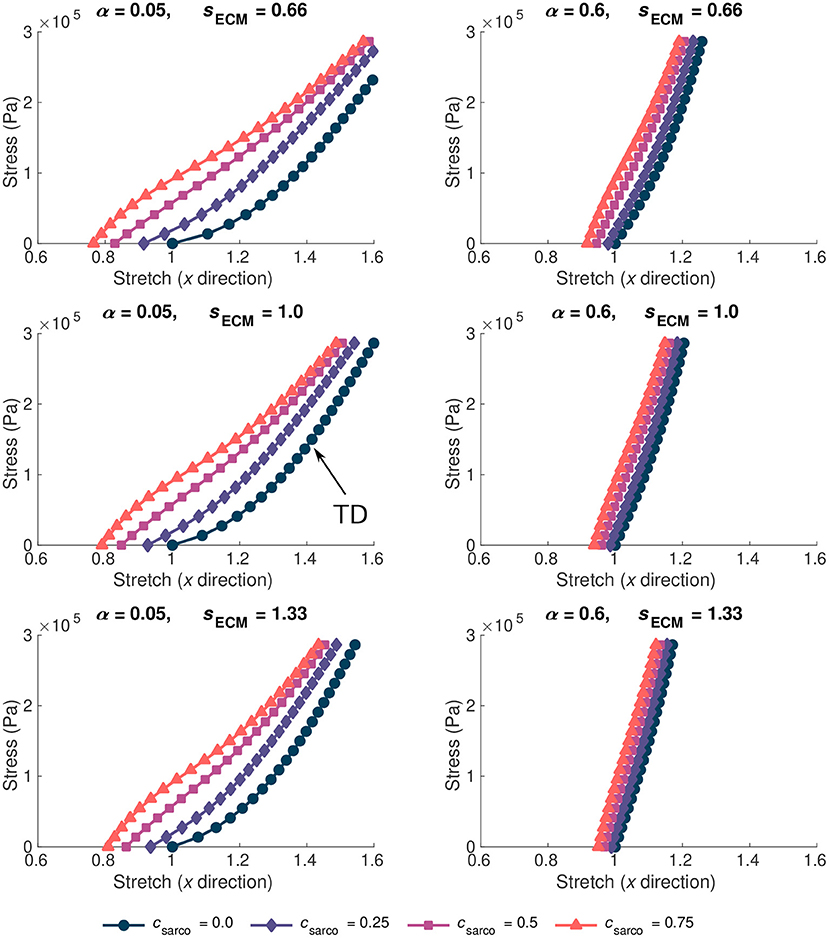
Figure 5. Stress-stretch plot during passive lengthening of the muscle model for various values of ECM volume fraction, α, and stiffness, sECM. The traction was linearly increased on the +x face of the muscle to 3 × 105 Pa, while the −x face was constrained in all directions. Individual lines on each plot represent a shift in sarcomere stretch by csarco. Typically developed (TD) muscle has values of α = 0.05, sECM = 1.0, and csarco = 0.0, while cerebral palsy muscle could have a combination of α > 0.1, sECM = 0.66 or 1.33, and csarco > 0.
As csarco was increased up to a value of 0.5, the modulus of the muscle in the x direction increased at optimal length of the muscle (Figures 6A,B). However, after a value of 0.5, the muscle modulus decreased, and this was observed when looking at the variations in csarco with constant α and sECM (Figures 6A,B). The modulus at optimal length was dominated by α. For changes in csarco and sECM, the change in modulus (at most 4 × 105 Pa) was less than the possible variation in modulus with changes in α (up to 15 × 105 Pa for highly fibrotic tissue). While holding csarco constant, we found a linear increase in the modulus when increasing α and sECM (Figures 6C,D).
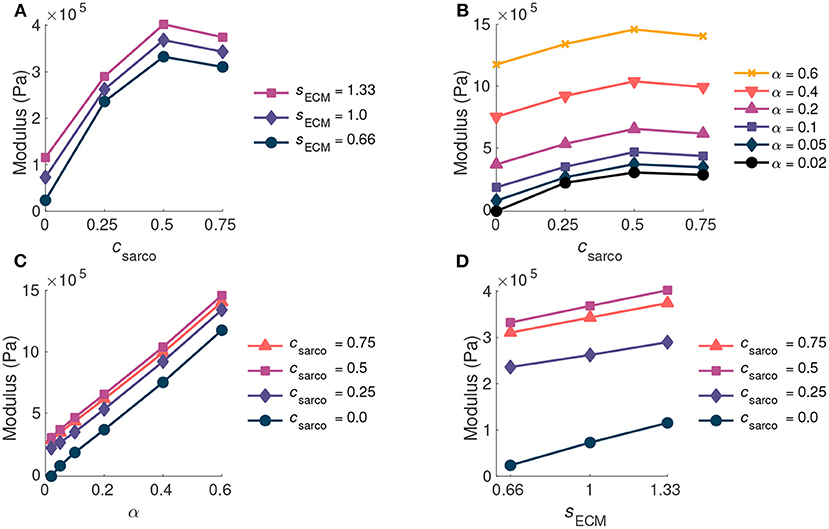
Figure 6. Plot of whole muscle modulus vs. csarco (A,B), α (C), and sECM (D) at optimal length (). Where α is the ECM volume fraction, sECM is the ECM stiffness factor, and csarco is the shift in the sarcomere stretch. In (A,D) α is held constant at 0.05, and in (B,C) sECM is held constant at 1.0.
While holding csarco constant, there was a larger effect from volume fraction of the ECM, α, than the stiffness of the ECM, sECM, on the overall muscle stiffness (Figures 7A,B). However, as α was increased, there was a greater effect of sECM. The nonlinear behaviour, which showed increasing muscle modulus with increasing α and sECM was more pronounced at larger stretches (Figures 7B,D). At a stretch of 1.20 in the x direction, the stiffness appeared to be more nonlinear when moving along the lines of constant sECM and when moving along the lines of constant α for α > 0.2 (Figures 7C,D). When holding sECM constant, there was a larger effect α on the modulus of the muscle compared to csarco, particularly at larger stretch values (Figures 7C,D). As the stretch increased there was an increase in modulus from the ECM parameters; however, there was a decrease in the effects of csarco (Figures 7E,F). The reduced influence of csarco was due to more pronounced behaviour from the base material at larger stretches (Figures 5, 7).
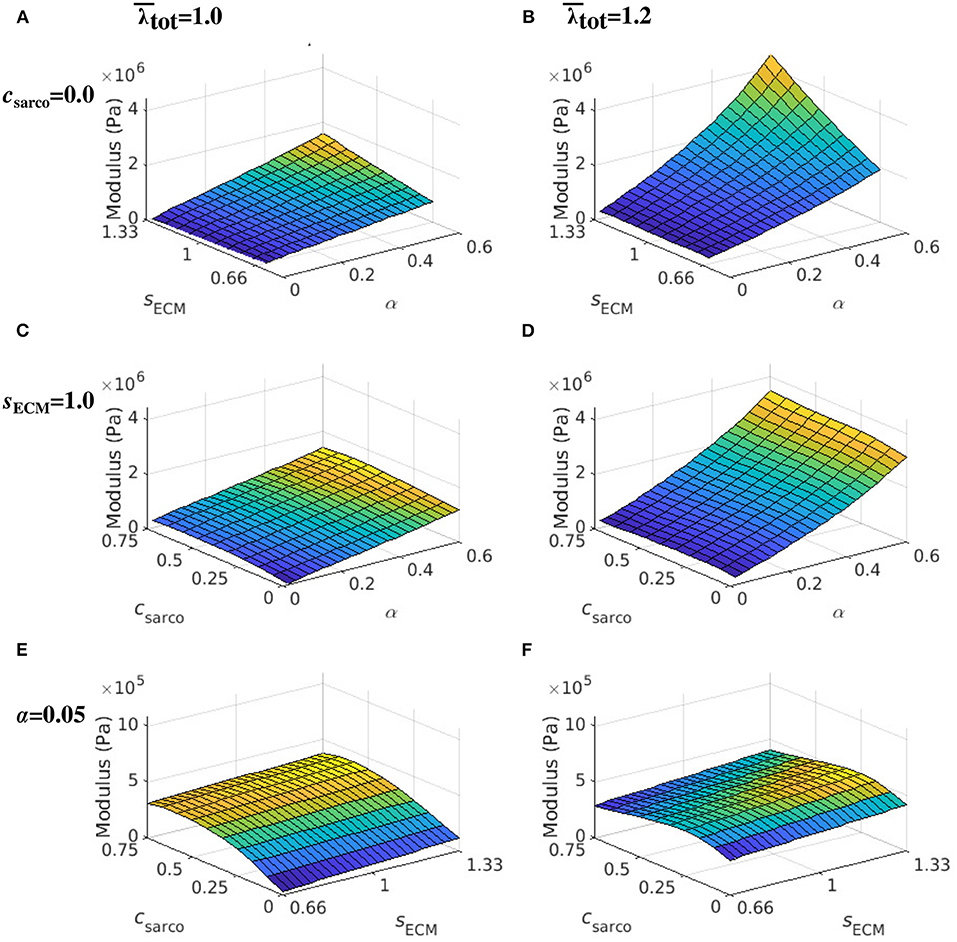
Figure 7. Surface plot of the whole muscle modulus at an average muscle stretch, , of 1.0 (A,C,E) and 1.2 (B,D,F). The ECM stiffness factor, sECM, was varied between values of 0.66 and 1.33; ECM volume fraction, α, from 0.02 to 0.6; and shift in sarcomere stretch, csarco, from 0.0 to 0.75. Modulus values were extracted from passive lengthening simulations with csarco = 0.0 in (A,B); sECM = 1.0 in (C,D); and α = 0.05 in (E,F).
In CP, alterations occur on the microstructural level that can influence whole muscle stiffness and reduce function. In particular, alterations to ECM properties and sarcomere length can occur; however, their relative contributions to muscle stiffness in CP is unknown. Isolating individual effects on passive muscle stiffness is difficult to do in experimental studies as there is large variability between subjects and individual muscles (Calvo et al., 2010; Takaza et al., 2013; Mohammadkhah et al., 2016). Therefore, to determine whether the ECM properties or sarcomere lengths have more effect on the passive muscle behaviour, we used a three dimensional continuum model (Rahemi et al., 2014; Ross et al., 2018b; Wakeling et al., 2020; Konno et al., 2021). This model does not actually develop joint contractures; however, it allows us to isolate the effects from individual microscopic components, and investigate the relative contributions to whole muscle function.
The ECM is composed of a highly structured arrangement of collagen fibres and plays a substantial role in skeletal muscle mechanics (Gillies and Lieber, 2011). In this study, we investigated the effects of changes to ECM volume fraction and stiffness on the whole muscle stiffness. Changes in ECM volume fraction have been observed in previous studies, particularly as a result of fibrosis (eg. Smith et al. (2011)). This corresponds to an increase in ECM material, while the contributions from the cellular components in muscle, such as the contractile fibres and other cells, decreases. In addition, fibrosis creates a physical barrier that can impact muscle regeneration (Chen and Li, 2009), which will reduce the ability for muscle to grow and add sarcomeres in the muscle fibres, further decreasing the compliance of the muscle. Additionally, it is possible that while the volume fraction stays constant, changes in the structure or composition of collagen types varies. However, studies have found that the ratio of collagen isoforms is the same in both TD and CP muscle (Smith et al., 2019), and so it is unlikely that a difference in collagen isoforms in muscle accounts for the increase in whole muscle stiffness with CP. It is possible that there are alterations in ECM structure, such as the organisation of collagen fibres, that occur with CP (Lieber et al., 2003), and this could increase or decrease ECM stiffness depending on the change. Therefore, both effects were considered in the model to investigate the relative contributions to stiffness on a whole muscle level.
It has also been well documented that increases in the sarcomere length occur with CP (Lieber and Fridén, 2019; Tisha et al., 2019). Stiffness changes have been reported on the fibre level by looking at the stress-strain relationship for TD and CP muscle fibres (Mathewson et al., 2014a). It is possible that these effects are not only a result of increased sarcomere lengths, but due to different titin isoforms, as they could result in a different stress-strain relationship for the individual sarcomeres (Prado et al., 2005). However, Smith et al. (2011) found that there is no change in the composition of titin isoforms between TD and CP muscle. Therefore, changes in stiffness due to the sarcomeres are not likely due to changes in titin isoforms. While it is possible that an increased stretch of the titin is responsible for the increased passive stiffness of the fibres, this could also be a result of changes to other mechanical properties in the myofibres. More investigation is required to confirm the main cause of the increased stiffness at a fibre level.
It has been demonstrated experimentally that the ECM has a significant contribution to muscle passive stiffness (Gillies and Lieber, 2011), and that fibrosis has been observed in CP (Lieber et al., 2003). We found that the volume fraction of the ECM had a larger influence on whole muscle stiffness compared to ECM stiffness and sarcomere length. The contribution from the ECM increased as stretch increased (Figure 7), demonstrating a nonlinear relationship between the ECM volume fraction and muscle stretch. At larger stretch values, the ECM contributes more to the whole muscle stiffness; these nonlinear effects imply that fibrosis will substantially reduce the ability of a muscle to deform at larger stretch values. The ECM is composed of crimped collagen fibres, which likely do not contribute as much to the stress initially (Gillies and Lieber, 2011), and this is reflected in a smaller effect from the ECM volume fraction at optimal length. Currently, experimental data for the variation in stiffness of the ECM due to structural changes are not available; however, in the ranges tested in this study, the stiffness of the ECM did not alter whole muscle stiffness as much as the volume fraction. At larger volume fractions of the ECM there was a more substantial contribution from the ECM stiffness (Figure 7B), and since larger volume fractions are typically seen in CP, this could play a larger role.
The contribution of the sarcomere length to whole muscle stiffness varied depending on the ECM properties. There was minimal effect of the sarcomeres on the passive stiffness in the fibrotic tissue (Figure 6), which corresponds to volume fractions of ECM greater than 10%, and this larger effect from the ECM has been observed during experiments (Smith et al., 2011). Furthermore, the sarcomere effects are mitigated at larger stretches as the ECM begins to dominate the muscle stiffness. At whole muscle stretches near 1.0, we found a larger effect of sarcomere length (Figure 6), which agrees with the results from Mathewson et al. (2014a) for fibre bundles. The work by Mathewson et al. (2014a) indicates that there was no increase in the stiffness of the ECM during CP in the muscles investigated. They found that there was no difference in the stiffness of fibre bundles at larger stretches, which could be explained by the dominating behaviour of the ECM. This agrees with our findings that demonstrate that if a muscle is operating near optimal length, then there might be a noticeable effect of sarcomere length. However, if the muscle has a larger range of motion, then the ECM would likely have a larger contribution to muscle passive stiffness. It is likely that the lengthening of the sarcomeres during CP has a larger effect on the active properties of the muscle (which we have not evaluated in this study) compared to the passive properties as noted by Lieber and Fridén (2019).
Using this model we can obtain a deeper understanding of the three-dimensional effects that occur in muscle altered by CP. As shown in previous modelling (Ryan et al., 2020; Wakeling et al., 2020) and experimental studies (Randhawa and Wakeling, 2018), the ability of a muscle to deform both in the along and transverse fibre directions can alter muscle function. Additionally, our results agree with experimental evidence that the whole muscle response is not the same as the response from individual fibres (Ward et al., 2020). In our model, the three-dimensional behaviour is captured in part by the base material, which works to return the muscle to its original state. At longer muscle lengths, the base material will work in the same direction as the sarcomeres, which are trying to shorten the muscle. Meanwhile, when the whole muscle stretch is less than one, the ECM will be working to return the muscle back to the original muscle length. We have observed in the model that the stiffness of the muscle decreases after sarcomere lengths greater than 3.3 μm (Figure 6), and this is due to the three dimensional behaviour of the model we are using. In a one dimensional model, there are no effects from the volume conserving nature of the base material or other effects transverse to the fibres. This is a nearly incompressible and nonlinear model, and so the effects from the volumetric component of the model contribute more with larger shifts in the sarcomere force-length curve. While these effects have been observed based on our assumptions for the model (see Wakeling et al., 2020; Konno et al., 2021), which are typical of many finite element models (Blemker and Delp, 2005; Sharafi and Blemker, 2011; Spyrou et al., 2017), these effects have not been reported experimentally. Experimentally, the decrease in muscle stiffness may not be as substantial as the changes observed in this study; however it is likely a similar trend would appear. Another important consequence of the three-dimensional behaviour is that changes occurring strictly in the along-fibre direction, such as changes in the sarcomere length, affect the stretch transverse to the fibres (Figure 4). In particular, the bulging and stretching in the transverse direction is decreased by increased in vivo sarcomere lengths, which increases the passive stiffness of the muscle fibres. Given this reduced movement in the transverse direction, it is likely that there would be a decreased contractile force produced given the significant effect of three dimensional deformation (Ryan et al., 2020). This demonstrates that to accurately capture all of the effects from CP, investigating three dimensional behaviour is required to completely understand the mechanical behaviour of the muscle.
Experimental studies are key to understanding the mechanical changes that occur with CP; however, many of the procedures are invasive and unable to determine the exact role each change due to CP plays in altering muscle stiffness (Smith et al., 2011; Lieber and Fridén, 2019; Tisha et al., 2019). Additionally, there is contradicting data as to whether fibres, ECM, or both have a substantial contribution to passive stiffness (Smith et al., 2011; Mathewson et al., 2014a), which likely depends on the severity of the disease (Tisha et al., 2019). There are less invasive procedures that have been developed to investigate the relationship between muscle stiffness and CP (Lee et al., 2016); however, they are still unable to isolate the role of individual factors. For example, experimental studies have found that stiffness of CP muscle is twice as high as TD muscle (von Walden et al., 2017); however, they were not able to determine which microstructural changes led to this increase in stiffness. While this model cannot directly determine which microstructural changes will cause this experimental increase in stiffness, it can provide insight into how various changes on the microscopic level could lead to these effects on muscle stiffness. We have observed that there is approximately double the increase in stiffness when the volume fraction of ECM in our model increases from 5 to 20%. Another possible way to achieve this increase in muscle stiffness is through increasing the stiffness of the ECM, or some combination of the two. The possible changes that cause increased stiffness can be investigated through our modelling approach and can indicate which factors may have the most impact on muscle behaviour. It is difficult to perform experimental tests on whole muscles affected by CP, although tests have been done on mice with spasticity or fibrosis (Ziv et al., 1984; Smith and Barton, 2014), as muscle can only be dissected during surgery making it difficult to obtain data for an accurate comparison to similar TD muscle tissue. Muscle is a three-dimensional material, so applying a continuum model to CP muscle allows us to understand the underlying muscle mechanics. In particular, developing an understanding of the complete behaviour of muscle will give insight into the role each microstructural alteration that occurs in CP will play in whole muscle mechanical behaviour.
While the model has the ability to investigate behaviour of muscle that is difficult to examine experimentally, it relies on accurate experimental data for its intrinsic properties. Unfortunately, mechanical data for the effects of stiffness of the ECM are not available, so the value for the ECM stiffness parameter was chosen to vary by 33% from healthy muscle. It is possible that changes in the structure of the ECM would change by more than this value; however, these values were chosen to probe the behaviour of the ECM stiffness. Given the derivation of the whole muscle stress in the model (Equation 4) it is likely that the volume fraction of the ECM would still have the largest contribution to whole muscle stiffness. Both the ECM volume fraction and stiffness multiply the ECM stress response, so they have similar contributions to whole muscle behaviour for small variations in their values. However, only the ECM volume fraction decreases the contributions from the cellular components. This aspect of the model is realistic, since it is not likely changes in the structure of the ECM will decrease the contribution of the fibres to whole muscle stiffness.
A limitation of this model is the lack of current experimental certainty on changes that occur with CP. Many changes to individual components have been observed in CP affected muscle; however, the extent to which microstructural changes occur are varied (Tisha et al., 2019). Therefore, the effectiveness of the model in providing a comparison to CP muscle will depend on the specific muscle. Additionally, there are very little data available for the changes in stiffness of the ECM, and so it is possible that this could vary more than investigated in this study. This would result in a large influence of the stiffness of the ECM component. Work by Brashear et al. Brashear et al. (2021) found that stiffness and orientation of the ECM component may have more effect than the amount of the ECM, so this lack of experimental data for ECM stiffness is a limitation of our model. Additionally, it is possible that a focalized accumulation of collagen de Bruin et al. (2014); Von Walden et al. (2018) could occur in contrast to the even distribution investigated in this study. This would likely influence the mechanical response of muscle; however, this was not investigated in this study. The response of the base material likely changes in response to compression as opposed to tension. Currently, the available data for the ECM is limited to tension, and so a different response for compression was not implemented in the model. However, we expect the effect on the output from our model would be minimal.
In the model, we have assumed for simplicity that with changes in the volume fraction of the ECM, there is no effect on the amount of force produced by the fibres. Any reduction in contribution from the muscle fibres is assumed to be included in the decrease in cellular component contribution to the base material response. In addition to changes in the microstucture, it is possible that changes occur to the geometry of the muscle in CP. The results of this study only demonstrate the effects of the changes to the material properties, and the effect of changes to the geometry could be investigated in future work. We have not investigated the active behaviour of muscle in this study, although it is fundamental in muscle function. In CP, the contractile force produced has been seen to decrease (Stackhouse et al., 2005), so using this model to investigate the influence of ECM and sarcomere properties on active force would be valuable and would give additional insight into how the structural alterations that occur with CP individually impact muscle contraction. In this model, the properties of our TD muscle may not be representative of all muscles as the material properties vary both across and within studies (Calvo et al., 2010; Takaza et al., 2013; Mohammadkhah et al., 2016). So, while the qualitative passive behaviour is captured in this model, the exact values could vary between muscles. However, we would expect the general trends observed during this study to hold.
The purpose of this study was to determine the effects of the microstructural changes that are normally observed during experimental studies of CP muscle, including variation in ECM volume fraction, stiffness, and sarcomere length, on whole muscle stiffness. To do this, a three dimensional computational model of skeletal muscle was used, and overall stress-stretch relationships and muscle stiffness were calculated by measuring the passive stress of the whole muscle. We found that the volume fraction of the ECM had a larger effect on overall muscle stiffness compared to the ECM stiffness and sarcomere length, and that the effects of the sarcomere length were mitigated at larger ECM volume fractions. Investigating these effects provides a causal relationship between changes to microstructural properties and the overall response of the muscle. Experimental research is currently unable to vary independent components of muscle, and so this work can be used to help direct future experimental research. In this study, we were able to determine the crucial role that the microstructural alterations observed in CP have on whole skeletal muscle behaviour.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
RK carried out experimental design, performed simulations, analyses of results, and drafted and edited the manuscript. NN, JW, and SR carried out experimental design, analyses of results, and editing of the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
We would like to acknowledge funding from Natural Sciences and Engineering Research Council of Canada for Discovery Grants to NN and JW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arndt, D., Bangerth, W., Davydov, D., Heister, T., Heltai, L., Kronbichler, M., et al. (2017). The deal.II Library, version 8.5. J. Num. Math. 25, 137–146. doi: 10.1515/jnma-2017-0058
Barber, L., Barrett, R., and Lichtwark, G. (2011). Passive muscle mechanical properties of the medial gastrocnemius in young adults with spastic cerebral palsy. J. Biomech. 44, 2496–2500. doi: 10.1016/j.jbiomech.2011.06.008
Barrett, R. S., and Lichtwark, G. A. (2010). Gross muscle morphology and structure in spastic cerebral palsy: a systematic review. Dev. Med. Child Neurol. 52, 794–804. doi: 10.1111/j.1469-8749.2010.03686.x
Binder-Markey, B. I., Broda, N. M., and Lieber, R. L. (2020). Intramuscular anatomy drives collagen content variation within and between muscles. Front. Physiol. 11:293. doi: 10.3389/fphys.2020.00293
Blemker, S. S., and Delp, S. L. (2005). Three-dimensional representation of complex muscle architectures and geometries. Ann. Biomed. Eng. 33, 661–673. doi: 10.1007/s10439-005-1433-7
Booth, C. M., Cortina-Borja, M. J., and Theologis, T. N. (2001). Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev. Med. Child Neurol. 43, 314–320. doi: 10.1017/S0012162201000597
Brandenburg, J. E., Eby, S. F., Song, P., Kingsley-Berg, S., Bamlet, W., Sieck, G. C., et al. (2016). Quantifying passive muscle stiffness in children with and without cerebral palsy using ultrasound shear wave elastography. Dev. Med. Child Neurol. 58, 1288–1294. doi: 10.1111/dmcn.13179
Brashear, S. E., Wohlgemuth, R. P., Gonzalez, G., and Smith, L. R. (2021). Passive stiffness of fibrotic skeletal muscle in mdx mice relates to collagen architecture. J. Physiol. 599, 943–962. doi: 10.1113/JP280656
Burkholder, T. J., and Lieber, R. L. (2001). Sarcomere length operating range of vertebrate muscles during movement. J. Exp. Biol. 204, 1529–1536. doi: 10.1242/jeb.204.9.1529
Calvo, B., Ramírez, A., Alonso, A., Grasa, J., Soteras, F., Osta, R., et al. (2010). Passive nonlinear elastic behaviour of skeletal muscle: Experimental results and model formulation. J. Biomech. 43, 318–325. doi: 10.1016/j.jbiomech.2009.08.032
Chen, X., and Li, Y. (2009). Role of matrix metalloproteinases in skeletal muscle. Cell. Adh. Mig. 3, 337–341. doi: 10.4161/cam.3.4.9338
de Bruin, M., Smeulders, M. J., Kreulen, M., Huijing, P. A., and Jaspers, R. T. (2014). Intramuscular connective tissue differences in spastic and control muscle: a mechanical and histological study. PLoS ONE 9:e101038. doi: 10.1371/journal.pone.0101038
D'Souza, A., Bolsterlee, B., Lancaster, A., and Herbert, R. D. (2020). Intramuscular fat in children with unilateral cerebral palsy. Clin. Biomech. 80, 105183. doi: 10.1016/j.clinbiomech.2020.105183
Gillies, A. R., Chapman, M. A., Bushong, E. A., Deerinck, T. J., Ellisman, M. H., and Lieber, R. L. (2016). High resolution three-dimensional reconstruction of fibrotic skeletal muscle extracellular matrix. J. Physiol. 595, 1159–1171. doi: 10.1113/JP273376
Gillies, A. R., and Lieber, R. L. (2011). Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44, 318–331. doi: 10.1002/mus.22094
Handsfield, G. G., Meyer, C. H., Abel, M. F., and Blemker, S. S. (2016). Heterogeneity of muscle sizes in the lower limbs of children with cerebral palsy: Cp muscle size heterogeneity. Muscle Nerve 53, 933–945. doi: 10.1002/mus.24972
Hill, A. (1938). The Heat of Shortening and the Dynamic Constants of Muscle. Proc. R. Soc. B. 126, 136–195. doi: 10.1098/rspb.1938.0050
Konno, R. N., Nigam, N., and Wakeling, J. M. (2021). Modelling extracellular matrix and cellular contributions to whole muscle mechanics. PLoS ONE 16:e0249601. doi: 10.1371/journal.pone.0249601
Lee, S. S., Gaebler-Spira, D., Zhang, L.-Q., Rymer, W. Z., and Steele, K. M. (2016). Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin. Biomech. 31, 20–28. doi: 10.1016/j.clinbiomech.2015.10.006
Lieber, R. L., and Fridén, J. (2002). Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve 25, 265–270. doi: 10.1002/mus.10036
Lieber, R. L., and Fridén, J. (2019). Muscle contracture and passive mechanics in cerebral palsy. J. Appl. Physiol. 126, 1492–1501. doi: 10.1152/japplphysiol.00278.2018
Lieber, R. L., Runesson, E., Einarsson, F., and Fridén, J. (2003). Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve 28, 464–471. doi: 10.1002/mus.10446
Mathewson, M. A., Chambers, H. G., Girard, P. J., Tenenhaus, M., Schwartz, A. K., and Lieber, R. L. (2014a). Stiff muscle fibers in calf muscles of patients with cerebral palsy lead to high passive muscle stiffness. J. Orthop. Res. 32, 1667–1674. doi: 10.1002/jor.22719
Mathewson, M. A., Ward, S. R., Chambers, H. G., and Lieber, R. L. (2014b). High resolution muscle measurements provide insights into equinus contractures in patients with cerebral palsy. J. Orth. Res. 33, 33–39. doi: 10.1002/jor.22728
Meyer, G., and Lieber, R. L. (2018). Muscle fibers bear a larger fraction of passive muscle tension in frogs compared with mice. J. Exp. Biol. 221:jeb182089. doi: 10.1242/jeb.182089
Mohagheghi, A., Khan, T., Meadows, T., Giannikas, K., Baltzopoulos, V., and Maganaris, C. (2008). In vivogastrocnemius muscle fascicle length in children with and without diplegic cerebral palsy. Dev. Med. Child Neurol. 50, 44–50. doi: 10.1111/j.1469-8749.2007.02008.x
Mohammadkhah, M., Murphy, P., and Simms, C. K. (2016). The in vitro passive elastic response of chicken pectoralis muscle to applied tensile and compressive deformation. J. Mech. Behav. Biomed. Mat. 62, 468–480. doi: 10.1016/j.jmbbm.2016.05.021
Ohata, K., Tsuboyama, T., Haruta, T., Ichihashi, N., and Nakamura, T. (2009). Longitudinal change in muscle and fat thickness in children and adolescents with cerebral palsy. Dev. Med. Child Neurol. 51, 943–948. doi: 10.1111/j.1469-8749.2009.03342.x
Prado, L. G., Makarenko, I., Andresen, C., Krüger, M., Opitz, C. A., and Linke, W. A. (2005). Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J. Gen. Physiol. 126, 461–480. doi: 10.1085/jgp.200509364
Rahemi, H., Nigam, N., and Wakeling, J. M. (2014). Regionalizing muscle activity causes changes to the magnitude and direction of the force from whole muscles - a modeling study. Front. Physiol. 5:298. doi: 10.3389/fphys.2014.00298
Randhawa, A., and Wakeling, J. M. (2018). Transverse anisotropy in the deformation of the muscle during dynamic contractions. J. Exp. Biol. 221:jeb175794. doi: 10.1242/jeb.175794
Ross, S. A., Nigam, N., and Wakeling, J. M. (2018a). A modelling approach for exploring muscle dynamics during cyclic contractions. PLoS Comp. Biol. 14:e1006123. doi: 10.1371/journal.pcbi.1006123
Ross, S. A., Ryan, D. S., Dominguez, S., Nigam, N., and Wakeling, J. M. (2018b). Size, history-dependent, activation and three-dimensional effects on the work and power produced during cyclic muscle contractions. Int. Comp. Biol. 58, 232–250. doi: 10.1093/icb/icy021
Ryan, D. S., Domínguez, S., Ross, S. A., Nigam, N., and Wakeling, J. M. (2020). The energy of muscle contraction. II. transverse compression and work. Front. Physiol. 11:538522. doi: 10.3389/fphys.2020.538522
Sharafi, B., and Blemker, S. S. (2011). A mathematical model of force transmission from intrafascicularly terminating muscle fibers. J. Biomech. 44, 2031–2039. doi: 10.1016/j.jbiomech.2011.04.038
Simo, J. C., and Taylor, R. L. (1991). Quasi-incompressible finite elasticity in principal stretches. continuum basis and numerical algorithms. Comput. Methods. Appl. Mech. Eng. 85, 273–310. doi: 10.1016/0045-7825(91)90100-K
Smith, L. R., and Barton, E. R. (2014). Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle inmdxmice. Am. J. Physiol. Cell Physiol. 306, C889–C898. doi: 10.1152/ajpcell.00383.2013
Smith, L. R., Lee, K. S., Ward, S. R., Chambers, H. G., and Lieber, R. L. (2011). Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J. Physiol. 589, 2625–2639. doi: 10.1113/jphysiol.2010.203364
Smith, L. R., Pichika, R., Meza, R. C., Gillies, A. R., Baliki, M. N., Chambers, H. G., et al. (2019). Contribution of extracellular matrix components to the stiffness of skeletal muscle contractures in patients with cerebral palsy. Connect. Tissue Res. 62, 287–298. doi: 10.1080/03008207.2019.1694011
Spyrou, L., Agoras, M., and Danas, K. (2017). A homogenization model of the voigt type for skeletal muscle. J. Theor. Biol. 414, 50–61. doi: 10.1016/j.jtbi.2016.11.018
Stackhouse, S. K., Binder-Macleod, S. A., and Lee, S. C. K. (2005). Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve 31, 594–601. doi: 10.1002/mus.20302
Takaza, M., Moerman, K. M., Gindre, J., Lyons, G., and Simms, C. K. (2013). The anisotropic mechanical behaviour of passive skeletal muscle tissue subjected to large tensile strain. J. Mech. Behav. Biomed. Mat. 17, 209–220. doi: 10.1016/j.jmbbm.2012.09.001
Tisha, A. L., Armstrong, A. A., Johnson, A. W., and López-Ortiz, C. (2019). Skeletal muscle adaptations and passive muscle stiffness in cerebral palsy: a literature review and conceptual model. J. Appl. Biomech. 35, 68–79. doi: 10.1123/jab.2018-0049
van der Krogt, M. M., Bar-On, L., Kindt, T., Desloovere, K., and Harlaar, J. (2016). Neuro-musculoskeletal simulation of instrumented contracture and spasticity assessment in children with cerebral palsy. J. NeuroEng. Rehabil. 13:64. doi: 10.1186/s12984-016-0170-5
Von Walden, F., Gantelius, S., Liu, C., Borgström, H., Björk, L., Gremark, O., et al. (2018). Muscle contractures in patients with cerebral palsy and acquired brain injury are associated with extracellular matrix expansion, pro-inflammatory gene expression, and reduced rRNA synthesis. Muscle and Nerve 58, 277–285. doi: 10.1002/mus.26130
von Walden, F., Jalaleddini, K., Evertsson, B., Friberg, J., Valero-Cuevas, F. J., and Pontén, E. (2017). Forearm flexor muscles in children with cerebral palsy are weak, thin and stiff. Front. Comp. Neurosci. 11:30. doi: 10.3389/fncom.2017.00030
Wakeling, J. M., Ross, S. A., Ryan, D. S., Bolsterlee, B., Konno, R., Domínguez, S., et al. (2020). The energy of muscle contraction. i. tissue force and deformation during fixed-end contractions. Front. Physiol. 11:813. doi: 10.3389/fphys.2020.00813
Ward, S. R., Winters, T. M., O'Connor, S. M., and Lieber, R. L. (2020). Non-linear scaling of passive mechanical properties in fibers, bundles, fascicles and whole rabbit muscles. Front. Physiol. 11:211. doi: 10.3389/fphys.2020.00211
Weiss, J. A., Maker, B. N., and Govindjee, S. (1996). Finite element implementation of incompressible, transversely isotropic hyperelasticity. Comput. Methods. Appl. Mech. Eng. 135, 107–128. doi: 10.1016/0045-7825(96)01035-3
Winters, T. M., Takahashi, M., Lieber, R. L., and Ward, S. R. (2011). Whole muscle length-tension relationships are accurately modeled as scaled sarcomeres in rabbit hindlimb muscles. J. Biomech. 44, 109–115. doi: 10.1016/j.jbiomech.2010.08.033
Zajac, F. (1989). Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit. Rev. Biomed. Eng. 17, 358–410.
Keywords: skeletal muscle, extracellular matrix, finite element method, cerebral palsy, muscle stiffness, continuum model, biomechanics
Citation: Konno RN, Nigam N, Wakeling JM and Ross SA (2022) The Contributions of Extracellular Matrix and Sarcomere Properties to Passive Muscle Stiffness in Cerebral Palsy. Front. Physiol. 12:804188. doi: 10.3389/fphys.2021.804188
Received: 29 October 2021; Accepted: 20 December 2021;
Published: 26 January 2022.
Edited by:
Kenneth Scott Campbell, University of Kentucky, United StatesReviewed by:
Geoffrey Handsfield, The University of Auckland, New ZealandCopyright © 2022 Konno, Nigam, Wakeling and Ross. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan N. Konno, cmtvbm5vQHNmdS5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.