- 1Guizhou Provincial Key Laboratory for Agricultural Pest Management of Mountainous Region, Institute of Entomology, Guizhou University, Guiyang, China
- 2Guizhou Provincial Key Laboratory for Rare Animal and Economic Insect of the Mountainous Region, College of Biology and Environmental Engineering, Guiyang University, Guiyang, China
- 3Enshi Tobacco Company of Hubei Province, Enshi, China
- 4College of Tobacco Science, Guizhou University, Guiyang, China
Serine/threonine kinase Akt, an important component of the insulin signaling pathway, plays an essential role in many physiological processes. In this study, we identified and characterized an Akt gene (designated LsAkt) from the cigarette beetle, Lasioderma serricorne. LsAkt contains a 1614 bp open reading frame encoding a 537 amino acid protein that possesses a conserved pleckstrin homology domain and a serine/threonine kinase domain. The expression of LsAkt was high in pupal stages and peaked in day-4 female pupae. In adult tissues, LsAkt was highly expressed in the thorax, ovary, and midgut. The expression of LsAkt was induced by methoprene or bovine insulin in vivo, but significantly decreased by 20-hydroxyecdysone. RNA interference-mediated knockdown of LsAkt resulted in severely blocked ovarian development and reduced fecundity and hatchability. The vitellogenin (Vg) content and juvenile hormone (JH) titers of LsAkt-depletion beetles were decreased, and expressions of Vg and four JH signaling and biosynthetic genes were significantly decreased. Silencing of LsAkt reduced the amounts of glucose, glycogen, and trehalose in female adults and affected the expressions of seven key carbohydrate metabolic genes. Taken together, it is inferred that Akt implicates in L. serricorne reproduction by modification of Vg synthesis, juvenile hormone production and carbohydrate metabolism.
Introduction
Insulins are multifunctional peptide hormones and consist of insulin-like peptide (ILP), insulin-like growth factor (IGF), and relaxin (Wu and Brown, 2006). Insulin structure, function, and signal transduction are evolutionary conserved in both vertebrates and invertebrates (Taniguchi et al., 2006; Das and Dobens, 2015). In insects, ILPs specifically activate the insulin receptors, which in turn, transmit a signal via the phosphoinostide 3-kinase (PI3K)-serine/threonine kinase (Akt) pathway or the mitogen-activated protein kinase (MAPK) pathway. This mediates diverse physiological events including growth, metabolism, longevity, and reproduction (Zhang et al., 2009; Das and Arur, 2017; Xu et al., 2021).
Serine/threonine kinase Akt (also known as protein kinase B, PKB), transduces the insulin signal through the phosphorylation of several downstream proteins such as other kinases, signaling proteins, and transcription factors (Verdu et al., 1999; Teleman, 2010). In insects, Akt helps regulate development, behavior, reproduction, lifespan, and stress resistance. For example, Akt functions in the regulation of apoptosis and cell size during Drosophila development (Staveley et al., 1998; Scanga et al., 2000). In Haemaphysalis longicornis, RNA interference (RNAi)-mediated knockdown of HlAkt inhibited blood feeding and arrested internal organ growth (Umemiya-Shirafuji et al., 2012). Akt phophorylation is associated with the embryonic diapause process of Bombyx mori (Gu et al., 2019). In Maruca vitrata, silencing of Akt significantly decreased larval growth rate and pupal weight (Al Baki et al., 2019). Bioassay analysis by sparing with a mixture of transformed Escherichia coli expressing dsAkt and Bacillus thuringiensis (Bt) in fourth-instar larvae of M. vitrata produced higher control mortality than a single treatment with Bt alone or dsAkt alone (Al Baki et al., 2020). In Chrysopa pallens, knockdown of Akt suppressed the expression of the vitellogenin (Vg) gene, hampered ovarian development, and reduced egg mass and hatching rate (Han et al., 2020). By contrast, over-expression of active Akt in the fat body of Anopheles stephensi extended the lifespan and increased fecundity of females (Hun et al., 2019). In Aedes aegypti, depletion of Akt significantly decreased the 4E-binding protein (4E-BP) phosphorylation and reduced the lifespan of adult females (Roy and Raikhel, 2012). Increased Akt signaling in the midguts of A. stephensi females significantly reduced their longevity (Arik et al., 2015). Akt signal transduction also is involved in the cold hardiness of Epiblema scudderiana larvae (Zhang and Storey, 2017).
Juvenile hormone (JH) and 20-hydroxyecdysone (20E), as well as insulin-like peptides, are components of an endocrine network in insects that coordinate to regulate development, molting, and reproduction. For example, insulin signaling regulates Drosophila larval molting by controlling the synthesis of ecdysone in the prothoracic glands (Walkiewicz and Stern, 2009). In Tribolium castaneum, JH functions in the regulation of Vg synthesis in the fat body via an insulin signaling cascade (Sheng et al., 2011). In the prothoracic glands of B. mori, bovine insulin can enhance the phosphorylation of Akt and stimulate ecdysteriod secretion (Gu et al., 2011). In Manduca sexta, Akt phosphorylation level stimulation by insulin was a non-requisite step in ecdysone secretion (Smith et al., 2014). However, the interaction among the JH, 20E, and insulin signaling remains poorly understood.
The cigarette beetle, Lasioderma serricorne (Coleoptera: Anobiidae), is a destructive stored pest in the tobacco and food industry and occurs worldwide (Ashworth, 1993). L. serricorne larvae cause economic damage to stored materials by direct damage and production of fecal material (Riudavets et al., 2007). This species has high reproductive potential. A single female beetle can lay 10–100 eggs during its 9–11 d oviposition period (Sivik et al., 1957). Previous studies have focused on the biology and ecology of L. serricorne and its control (Edde, 2019; Yang et al., 2020). However, knowledge of the reproductive physiology of L. serricorne remains limited, and the molecular mechanism of L. serricorne reproduction is unknown. Here we report (1) the full-length open reading frame (ORF) sequence of Akt (LsAkt) in L. serricorne, (2) the expression profiles of LsAkt in different developmental stages and tissues, as well as in response to exogenous hormone treatments, (3) functional analysis of LsAkt by RNAi in ovarian development and female fecundity, and (4) the effects of LsAkt RNAi on Vg synthesis, JH production, and carbohydrate metabolism.
Materials and Methods
Insects
The L. serricorne strain was originally collected in 2014 from a tobacco warehouse in Guizhou province, China. Stock colonies were maintained in the laboratory at 28°C with a relative humidity of 40% and constant (24 h) darkness. The rearing method was described in a previous report (Chen et al., 2018). Under laboratory-reared conditions, they stay at the pupal stage for 5 days, and the ovaries of female adults mature 5 days post eclosion.
Gene Cloning and Sequence Analysis
Total RNA was extracted from L. serricorne adults using a MiniBEST Universal Extraction Kit (TaKaRa, Dalian, China). One microgram of RNA was used to synthesize the first-strand cDNA by TransScript Synthesis Supermix (TransGen Biotech, Beijing, China). One unigene cDNA encoding serine/threonine kinase Akt was obtained from a L. serricorne transcriptomic database (SRR13065789). The full-length cDNA sequence of LsAkt was verified by reverse transcription PCR using gene-specific primers (Supplementary Table 1). Amplified PCR product was inserted into pEASY® –T1 vector (TransGen Biotech) and then sequenced (Tsingke Bio, Chengdu, China).
The nucleotide sequence similarities were identified by using the National Center for Biotechnology Information (NCBI) basic local alignment search tool.1 The coding sequence was predicted by NCBI ORF Finder,2 and the putative amino acid sequences were deduced by using DNAMAN7 (Lynnon Biosoft, Vaudreuil, Quebec, Canada). Molecular weight and isoelectric point were determined using ExPASy Proteomics Server.3 Conserved domains were determined by using the Simple Modular Architecture Research Tool.4 Multiple amino acid sequence alignment was performed by Clustal X (Larkin et al., 2007). A neighbor-joining phylogenetic tree was constructed using MEGA7 (Kumar et al., 2016) with 1000 bootstrap replications, and evolutionary relationship of LsAkt was determined based on insect Akt sequences available in the NCBI GenBank database (Supplementary Table 2).
Spatio-Temporal Expression Pattern of LsAkt
For the temporal expression profile of LsAkt, whole bodies of L. serricorne at various developmental stages, including pupae (1–5 d old) and adults (1–5 d old) were collected. For tissue-specific expression analysis, seven samples dissected from day-5 adult females were prepared, including head, thorax, epidermis, midgut, fat body, and ovary. Each sample included 10–50 individuals, and three biological replicates were performed. Total RNA extraction and cDNA synthesis were performed as described above. The mRNA levels of LsAkt were determined by Quantitative real-time PCR (qPCR) using TransStart® Top Green qPCR SuperMix (TransGen) with the CFX-96 real-time PCR system (Bio-Rad, Hercules, CA, United States). The reaction conditions were as follows: denaturation for 3 min at 94°C, followed by 40 cycles at 94°C for 5 s and 60°C for 30 s. A melting curve was used to further assess the qPCR primer specificity. Relative mRNA levels of target genes were normalized by the stable reference genes elongation factor 1-alpha (EF1α) and 18S ribosomal RNA (18S) using the 2−ΔΔCt method (Livak and Schmittgen, 2001; Yang et al., 2020).
Hormone Treatment
To examine the effect of exogenous hormones on LsAkt expression in pupae, the JH analog methoprene (purity: 95%) (Sigma-Aldrich, St. Louis, MO, United States), 20E (HPLC: ≥ 95% purity), and bovine insulin (purity: ≥ 27 USP units/mg) (Sigma-Aldrich) were used in vivo. In brief, a 10 μg/μL stock solution of methoprene or 20E dissolved in 95% ethanol were diluted to 1.0 mg/mL with distilled water. Either methoprene (200 ng/pupa) or 20E solution (120 ng/pupa) was then injected into day-3 pupae using a Nanoliter 2010 injector (World Precision Instruments, Sarasota, FL, United States). Pupae treated with an equivalent amount of ethanol were used as a control. For the insulin treatment, bovine insulin was solubilized in 25 mM HEPES (pH 8.2) and then diluted with distilled water to a final concentration of 3.0 mg/mL. Each pupa was injected with 100 nL insulin solution (300 ng/pupa), and controls were treated with an equal volume of HEPES buffer. Thirty individuals were randomly selected from each group at 3, 6, 12, and 24 h after injection, and the expression of LsAkt was determined by qPCR. Three biological replicates were used for each treatment.
RNAi and Fecundity Assay
RNA interference (RNAi) was performed to investigate the potential function of LsAkt in L. serricorne. The dsRNAs against LsAkt and green fluorescent protein (GFP, as control) were synthesized using a TranscriptAid T7 High Yield Transcription Kit (Thermo Scientific, Wilmington, DE, United States). The dsRNA was purified by phenol/chloroform solution, precipitated by ethanol, and dissolved in nuclease-free water. Day-3 female pupae were used in the RNAi experiments. Approximately 0.2 μL dsRNAs of LsAkt or GFP (1.5 μg/μL) were injected into each individual. All the dsRNA-treated insects were reared under the conditions stated above. At 72, 96, and 120 h after injection, female individuals (n = 20 each of three replicates) were collected to examine the efficiency of RNAi by qPCR. Day-3 female pupae were challenged with dsRNAs targeting LsAkt or GFP and emerged into adults. New emerged females at 0–6 h eclosion from the dsRNA injected pupae were collected for the paired mating assay. Each dsRNA-treated female was paired with one non-treated same age male in a petri dish for mating. Insects treated with dsGFP served as a parallel control. Each of the adult pairs was transferred daily into a new Petri dish containing artificial diet. The number of eggs laid was recorded each day using a stereomicroscope (Olympus SZX12, Tokyo, Japan). The hatching rate was computed every 12 h for 5 d until the unhatched eggs started to shrink. Thirty pairs of L. serricorne were used to analyze fecundity and hatchability for each treatment. To verify the effects of LsAkt on the oogenesis and ovary development of L. serricorne, the day-5 female adults from each treatment group were dissected. The ovaries were dissected in 1 × phosphate buffer saline (PBS) and photographed with a stereomicroscope VHX-6000 (Keyence Corporation, Osaka, Japan). The lengths of ovarian tube and lateral oviduct were measured using the Keyence application suite software.
Effect of LsAkt on Vitellogenin Content, Juvenile Hormone Titers, and Expression of Reproductive Genes
To study the effects of LsAkt RNAi on reproduction, samples were collected from pupae injected with dsLsAkt and dsGFP for 5 d. About 20 individuals were pooled as one sample, and three replications were performed. Each sample was weighed and then homogenized with a corresponding volume of PBS at the ratio of 1 g: 9 mL and centrifuged at 2500 g, 4°C for 20 min. The supernatants were collected for measurement of vitellogenin content according to the instructions of the Insect Vitellogenin Enzyme-linked immunosorbent assay (ELISA) Kit (Shanghai Meilian Biotechnology Co., Ltd). For JH titer determination, the supernatants were colleted for measurement of JH titers using the Insect JH ELISA Kit (Shanghai Meilian Biotechnology Co., Ltd). The relative expression profiles of Vg, VgR, and ten JH signaling and metabolic genes (Supplementary Table 1), were determined by qPCR as noted above. Thirty insects were treated as one replication, and three replications were performed.
Effect of LsAkt on Carbohydrate Metabolism
To explore the effects of LsAkt RNAi on carbohydrate metabolism, the glucose, glycogen, and trehalose content assay was performed using a previously reported method (Xu et al., 2020). The contents of glucose, glycogen, and trehalose in whole insect bodies were measured by the SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA, United States) at 5 d after dsRNA treatment. Each sample contained 50 individuals, and three biological replications were prepared. The insect samples were homogenized in 0.25 M Na2CO3 and then incubated at 70°C for 10 min. By adding 0.2 M Na-acetate and 1 M acetic acid, the mixture was adjusted to pH 5.2. To measure the glycogen content, one half of the mixture was incubated with amyloglucosidase (Sigma-Aldrich). For trehalose measurement, the other half of the mixture was incubated with trehalase (Sigma-Aldrich) at 37°C, and the treated insects were homogenized in PBS (pH 7.4, 137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, and 2 mM KH2PO4). The glucose level was determined using a glucose (GO) assay kit (Sigma-Aldrich) according to the manufacturer’s instructions. After LsAkt was knocked down in L. serricorne, the transcript levels of eleven carbohydrate metabolic genes (Supplementary Table 1) were detected by qPCR at 5 d after injection.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 6.01 software (GraphPad software, La Jolla, CA, United States). Significant differences between two samples and among multiple samples were determined by one-tailed Student’s t-test and a one-way analysis of variance followed by a least significant difference test, respectively.
Results
Identification and Sequence Analysis of LsAkt
The ORF of LsAkt is 1614 bp encoding a putative protein of 537 amino acids (GenBank accession number: MZ695806). The predicted molecular weight of LsAkt was 61.33 kDa and the isoelectric point was 5.98. Multiple sequence alignment revealed that LsAkt shares high conservation with other insects in the typical motifs of the Akts, harboring a pleckstrin homology (PH) domain, low complexity region, catalytic domain (S_TKc), and the extension to serine/threonine kinases (S_TK_X) (Figure 1). LsAkt protein contains putative phosphorylation sites, including 28, 26, and 8 sites for serine, threonine, and tyrosine, respectively (Supplementary Figure 1). LsAkt shared a high amino acid identity with other Coleoptera; 85.14% identity with Nicrophorus vespilloides (XP_017772201.1), 84.64% with Leptinotarsa decemlineata (XP_023017655.1), 84.14% with Anoplophora glabripennis (XP_018571651.1), and 81.43% with T. castaneum (XP_008191524.1). Phylogenetic analysis of Akt from different insect species showed that LsAkt has a close relationship to other Coleoptera and is clustered with N. vespilloides (Figure 1).
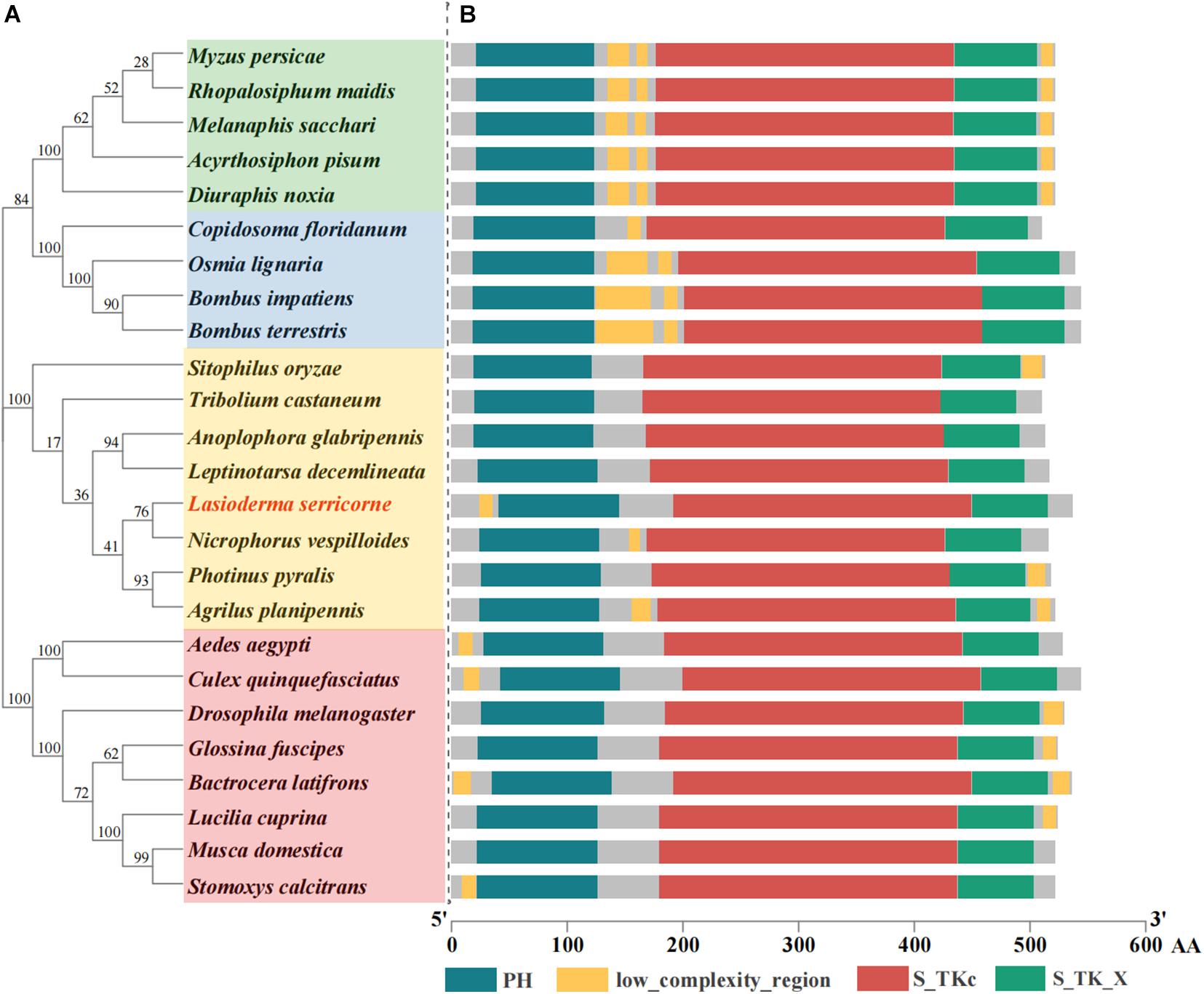
Figure 1. Phylogenetic analysis and protein structure comparison of insect Akts. (A) Phylogenetic tree generated with MEGA 7 by using neighbor-joining method with 1000 bootstrap replications. The GenBank accession numbers are listed in Supplementary Table 2. (B) Schematic alignment and comparison of domain architecture of insect Akts. AA, amino acids; PH, pleckstrin homology; S_TKc, catalytic domain; S_TK_X, the extension to serine/threonine-protein kinase.
Developmental and Tissue-Specific Expression of LsAkt
The qPCR was used to analyze the expression profiles of LsAkt on different developmental days and in different tissues. LsAkt was continuously expressed during the life stages tested. The expression of LsAkt remained at high levels in the pupal stages and had the highest expression levels in the day-4 female pupae. LsAkt had lower expression in the adult stages (Figure 2A). Tissue examination showed that LsAkt was expressed in all the selected tissues of day-5 adults, with relatively higher expression in the thorax, ovary, and midgut (Figure 2B).
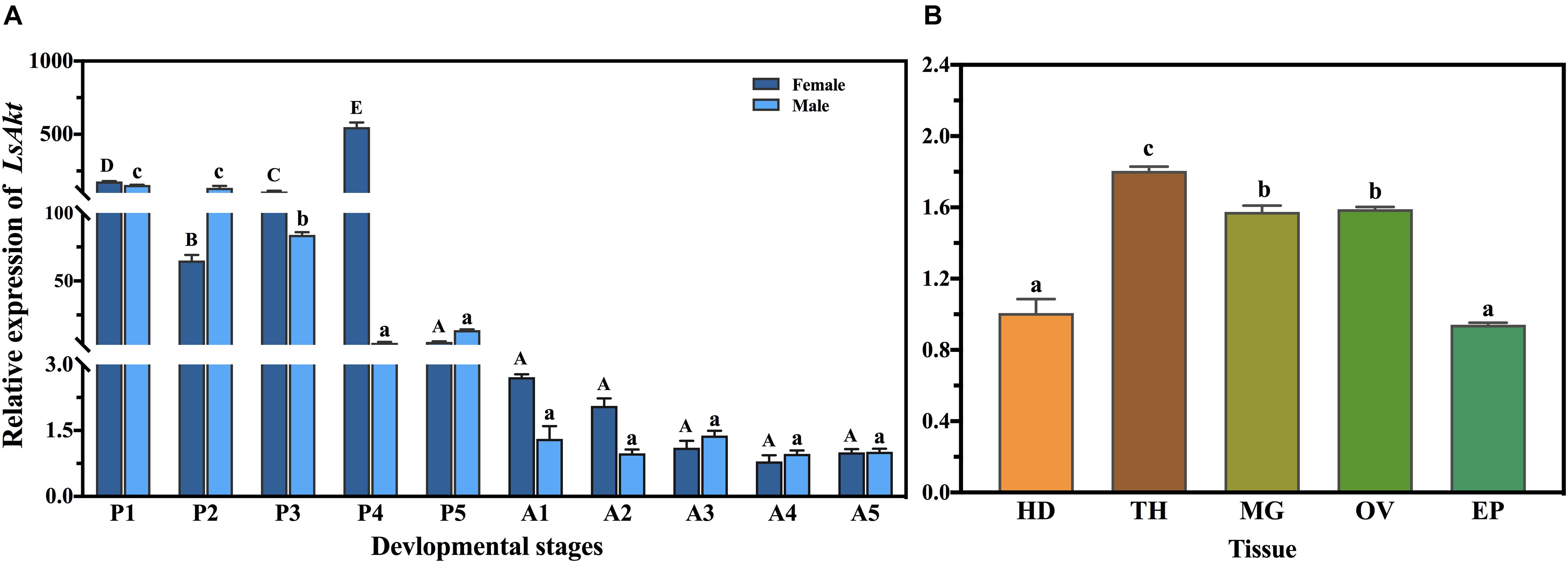
Figure 2. Developmental and tissue-specific expression patterns of LsAkt. (A) Temporal expression of LsAkt at different developmental stages. P1–P5, days 1–5 of the pupae; A1–5, days 1–5 of the adults. (B) Tissue distribution of LsAkt transcript in day-5 adults. HD, head; TH, thorax; MG, midgut; OV, ovary; EP, epidermis. Different uppercase letters above bars indicate significant differences of female insects, lowercase letters indicate significant differences of male insects or tissues based on one-way ANOVA followed by a least significant difference test (P < 0.05).
Effect of Exogenous Hormones on LsAkt Expression
To test whether LsAkt could be induced by exogenous hormones, the expression profiles of LsAkt were determined by qPCR at the same time points. The expression of LsAkt was significantly upregulated by the JH analog methoprene compared with the control group with 18. 5-, 29. 1-, and 23.6-fold increase at 3, 6, and 24 h, respectively. The transcript level of LsAkt reached a peak (62.0-fold) at 12 h after methoprene exposure. Conversely, the expression of LsAkt was significantly downregulated at 6 and 24 h after 20E injection compared with the control (Figure 3A). The expression of LsAkt was significantly increased up to 14. 6-, 20. 9-, 35. 4-, and 24.2-fold at 3, 6, 12, and 24 h after insulin treatment compared with the control (Figure 3B).
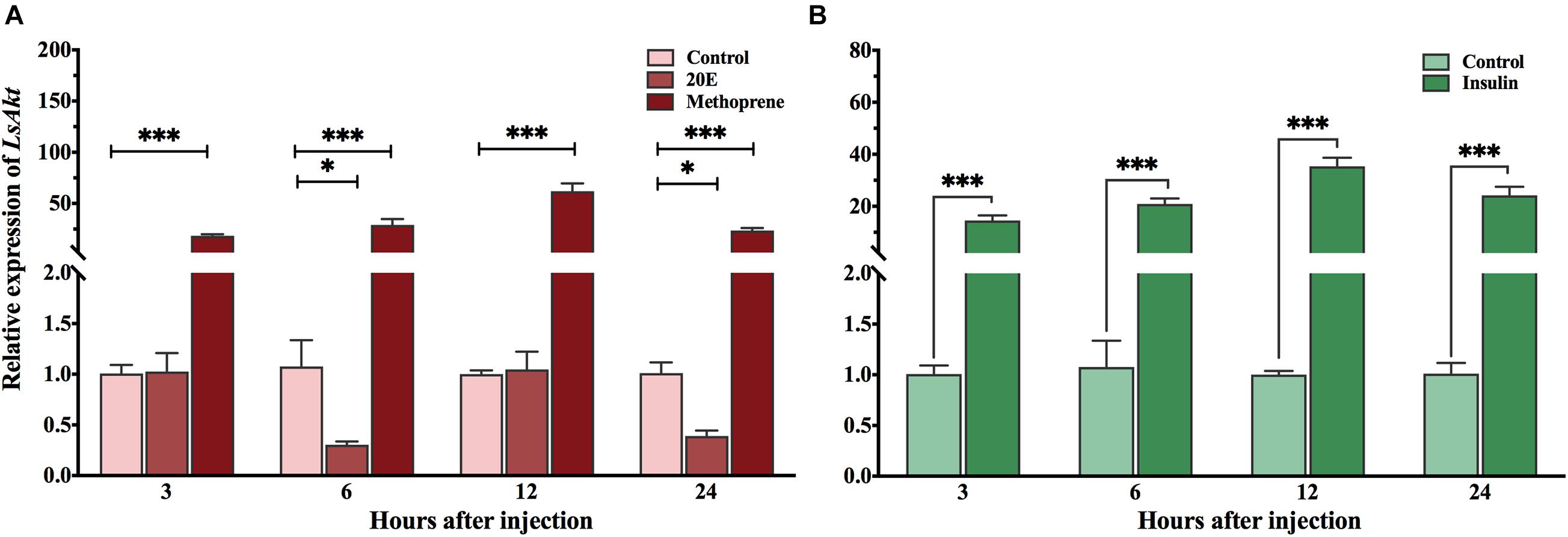
Figure 3. Expression profiles of LsAkt after exogenous hormone treatments. (A) Effect of 20E and methoprene on the expression of LsAkt. Control: insects injected with distilled water containing 0.1% ethanol; 20E: insects injected with 20E (120 ng/pupa); Methoprene; insects injected with methoprene (200 ng/pupa). (B) Effect of insulin on the expression of LsAkt. Control: insects injected with HEPES buffer; insulin, insects injected with bovine insulin (300 ng/pupa). Significant differences between treatment group and control group at the same point were determined using Student’s t-test (∗ P < 0.05, ∗∗∗ P < 0.001).
Knockdown of LsAkt Impairs Female Fecundity and Ovarian Development
To characterize the role of LsAkt in the reproductive process of L. serricorne, dsRNAs targeting LsAkt and GFP were injected into day-3 female pupae. Compared with the control, the expression level of LsAkt significantly decreased by 80.2%, 62.7%, and 48.7% at 72, 96, and 120 h, respectively, after dsLsAkt injection (Figure 4A). Notably, depletion of LsAkt had no negative effect on the pupa-adult transition. Female pupae were alive and successfully molted to adults at day 3 after injection with dsLsAkt or dsGFP. Newly emerged female adults were collected and allowed to pair with one wild-type male. Each dsLsAkt-treated female laid an average of 5.9 eggs, whereas the dsGFP-injected controls laid an average of 25.6 eggs per female. The egg hatchability in LsAkt-deficient females was significantly reduced by 42.5% compared with dsGFP-treated controls (P < 0.01) (Figure 4B).
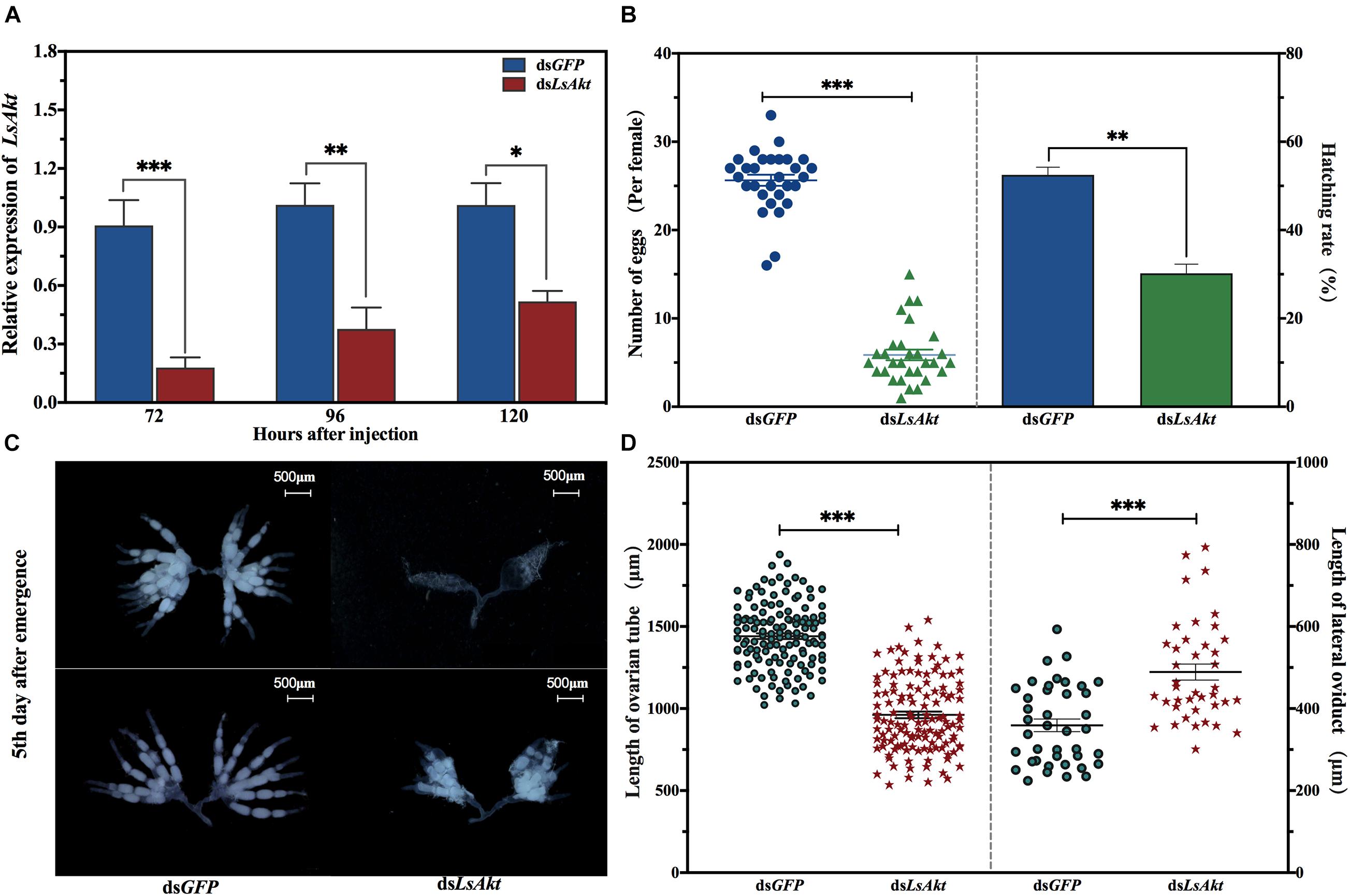
Figure 4. Effect of LsAkt RNAi on female reproduction in L. serricorne. (A) Relative expression levels of LsAkt at 72, 96, and 120 h after LsAkt or GFP dsRNA injection. (B) Determination of female fecundity after LsAkt knockdown. (C) Ovary morphology after LsAkt knockdown. Day-3 female pupae were injected with LsAkt or GFP dsRNA. Ovaries were dissected and photographed on the fifth day after adult eclosion. (D) Effects of LsAkt knockdown on the lengths of ovarian tube and lateral oviduct. Significant differences between the RNAi group and control group were determined using Student’s t-test (∗ P < 0.05, ∗∗ P < 0.01, ∗∗∗ P < 0.001).
Because LsAkt knockdown caused female reproductive deficiency, we examined ovarian development after dsRNA treatment. Females injected with dsLsAkt showed severely blocked in ovarian development. The abnormal ovaries had many non-vitellogenic oocytes and fewer mature eggs with less yolk protein deposition. In contrast, the ovaries of dsGFP-injected females were completely filled with regular banana-shaped oocytes, which were closely arranged in the ovarioles (Figure 4C). The lengths of ovarian tubes of the dsLsAkt group were significantly shorter than those of the dsGFP group, while the lengths of the lateral oviducts were longer than the controls (Figure 4D).
Knockdown of LsAkt Disturbs Vitellogenin Synthesis and Juvenile Hormone Signal
The vitellogenin content was significantly decreased by 32.3% at 120 h after injection with dsLsAkt (P < 0.01) compared with the dsGFP group (Figure 5A). The expression of LsVg was significantly reduced by 81.8% in the LsAkt-depleted beetles. However, the expression of LsVgR did not vary significantly between the dsLsAkt and dsGFP females (Figure 5B). The JH titers of LsAkt knockdown individuals were significantly lower than those injected with dsGFP (P < 0.05) (Figure 5C). The mRNA levels of four JH synthesis and signaling genes, including JH methyltransferase (LsJHAMT), farnesoic acid O-methyltransferase (LsFAmet), methoprene-tolerant (LsMET), and krüppel homolog 1 (LsKr-h1) were significantly decreased after knockdown of LsAkt. In contrast, the expressions of JH esterase (LsJHE) and JH epoxide hydrolase (LsJHEH) were increased (Figure 5D).
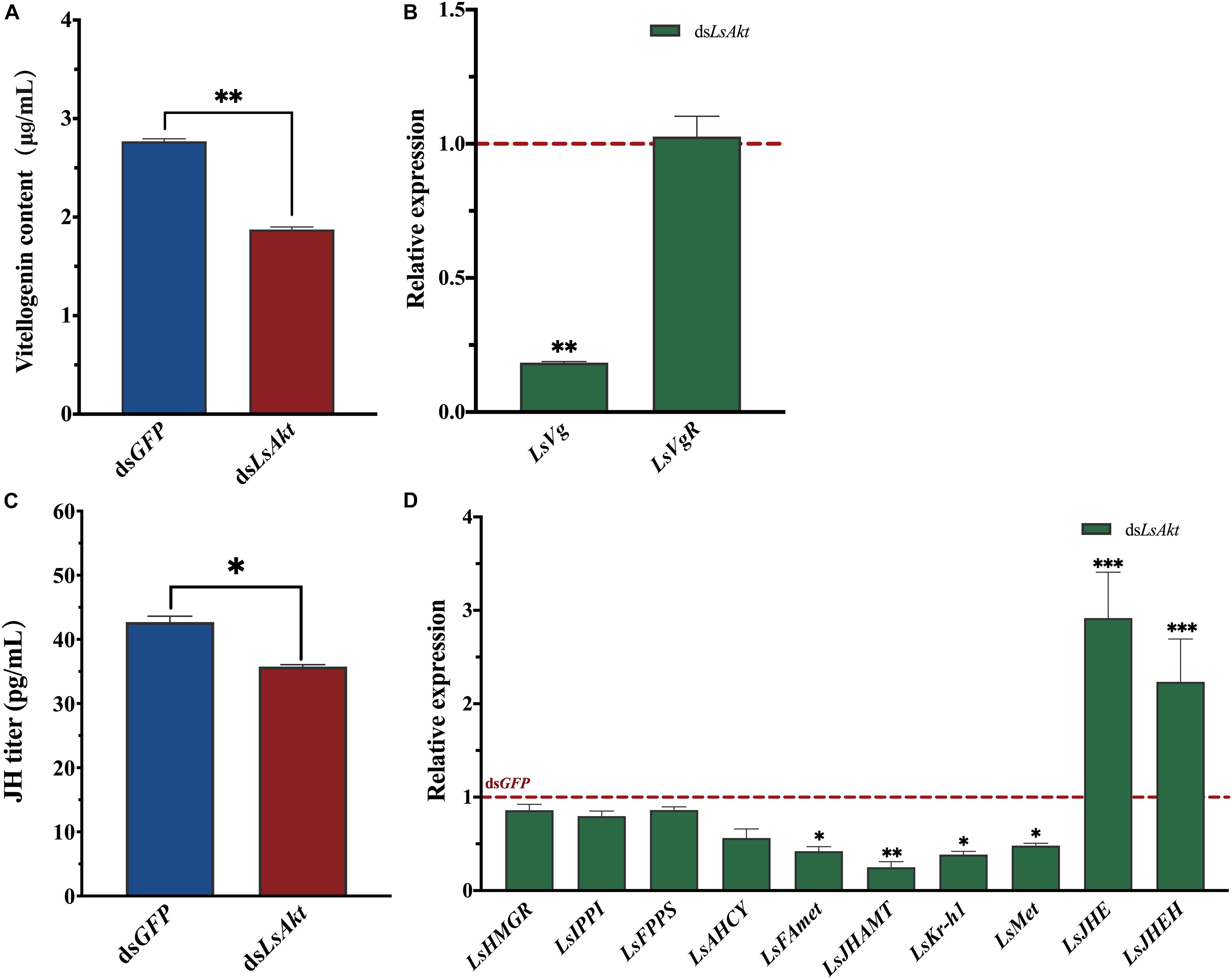
Figure 5. Effect of LsAkt RNAi on vitellogenin synthesis and juvenile hormone signaling. (A) Vitellogenin content in female adults after LsAkt knockdown. (B) Relative expression of Vg and VgR after RNAi. (C) JH tiers in female adults after LsAkt knockdown. (D) Relative expression of juvenile hormone signaling pathway genes after RNAi. The expression values were calculated by comparison to the dsGFP group, which was normalized at 1. Significant differences between the RNAi group and control group were determined using Student’s t-test (∗ P < 0.05, ∗∗ P < 0.01, ∗∗∗ P < 0.001).
Knockdown of LsAkt Affects Carbohydrate Metabolism
We also used RNAi to investigate the roles of LsAkt in carbohydrate metabolism of L. serricorne. The contents of glucose, glycogen, and trehalose were significantly decreased in LsAkt-depleted beetles (P < 0.01). Also, the expression levels of glucose transporter (LsGLUT), glucose-6-phosphate dehydrogenase (LsG6PDH), glycogen synthase (LsGlys), and a trehalose synthase (LsTPS) were significantly decreased after knockdown of LsAkt. In contrast, the expression levels of hexokinase (LsHK), phosphofructokinase (LsPFK), and trehalase (LsTRE) were significantly upregulated compared with those in control insects (Figure 6).
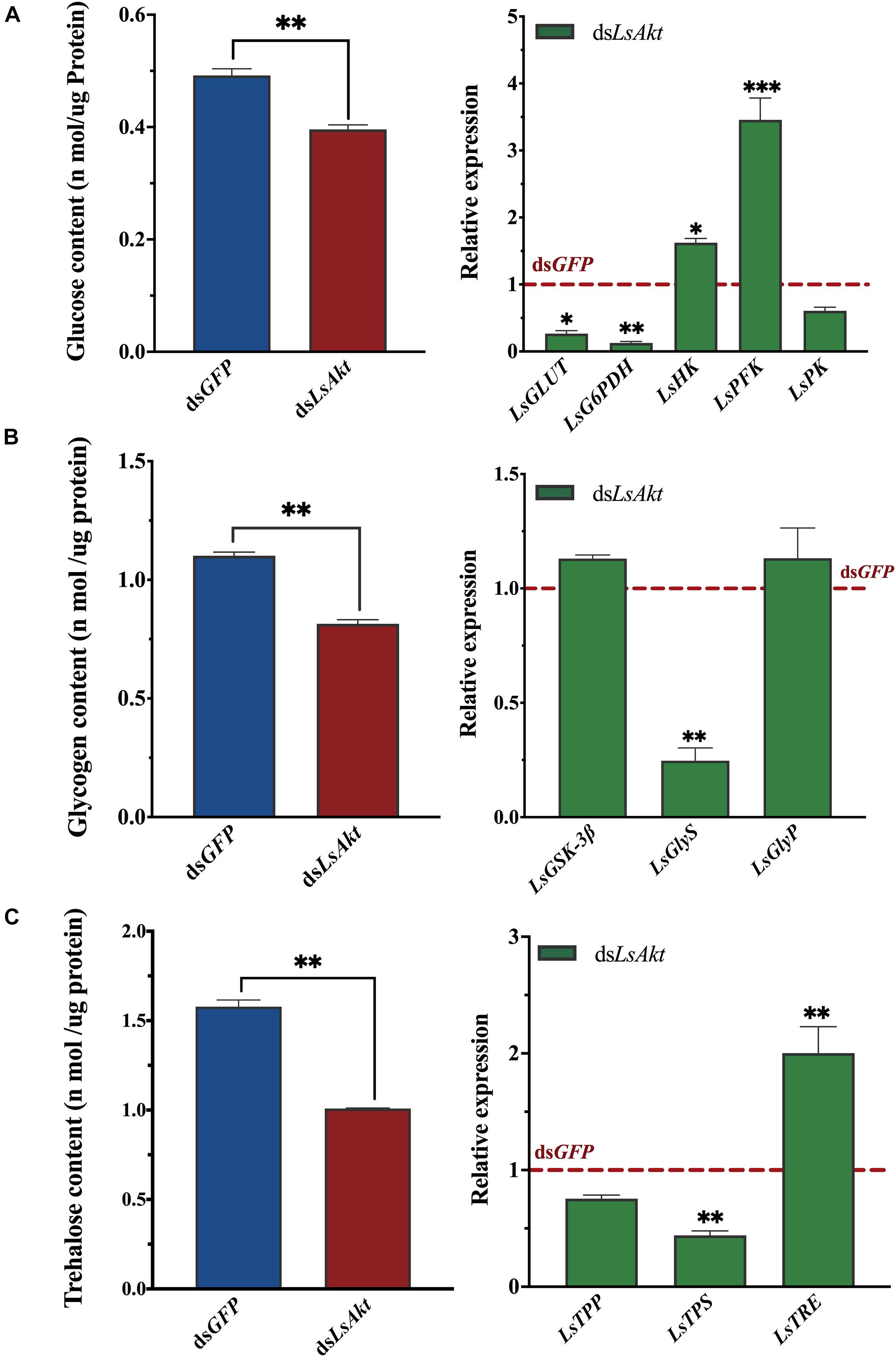
Figure 6. Effect of LsAkt RNAi on carbohydrate metabolism of L. serricorne. (A) Effects of LsAkt knockdown on glucose content and expression of four glucose-related genes. (B) Effects of LsAkt knockdown on glycogen content and expression of glycolytic pathway genes. (C) Effects of LsAkt knockdown on trehalose content and expression of three trehalose metabolic genes. The expression values were calculated by comparison to the dsGFP group, which was normalized at 1. Significant differences between the RNAi group and control group were determined using Student’s t-test (∗ P < 0.05, ∗∗ P < 0.01, ∗∗∗ P < 0.001).
Discussion
Spatio-temporal expression analysis of LsAkt revealed ubiquitous expression in all developmental stages and tissues. The highest expression levels of LsAkt occurred in the pupal stage, which is the key preparation period for L. serricorne reproductive development. Expression of the Akt gene varies among insect species. In A. aegypti, Akt was only expressed in the early stages of embryos and in adult females (Riehle and Brown, 2003). Dramatically increased expression of Akt occurred in fifth-instar larvae and pre-pupae of B. mori, indicating that Akt may be related to metamorphosis in this species (Liu et al., 2010). Insect Akt expression also exhibits tissue specificity. In B. dorsalis, Akt was highly expressed in the midgut and Malpighian tubules (Xu K. K. et al., 2015). In A. aegypti, Akt was specifically expressed in the ovary of adult females (Riehle and Brown, 2003). In situ hybridization of Diacamma sp. ovaries revealed that DiaAkt was expressed in nurse cells, oocytes, and upper germarial regions of mated egglaying workers (Okada et al., 2010). Similar results were observed in this study showing that LsAkt was expressed primarily in the ovary of L. serricorne. However, there was non-negligible expression of LsAkt in other tissues, especially in the thorax and midgut, and its functions in these tissues are not known.
There is a functional relationship between hormones and the insulin signaling pathway. Dramatically increased expression of Akt by JH occurred in newly molted fourth instar larvae of B. mori (Cheng et al., 2014), while 20E-treated B. dorsalis larvae had suppressed transcript levels of Akt (Xu K. K. et al., 2015). In the fat body of B. mori larvae, application of insulin and methoprene increased Akt expression at the active growth period, while 20E decreased Akt expression in starved larvae during the terminal growth period (Thounaojam and Keshan, 2017). In Helicoverpa armigera, the expression of phosphoinositide-dependent kinase-1 (PDK1, another insulin signaling pathway component) was induced by insulin, but repressed by 20E (Pan et al., 2018). In the present study, the transcript levels of LsAkt were upregulated substantially by injection of methoprene or bovine insulin in vivo, whereas expression was downregulated by 20E treatment. These results suggest that crosstalk exists among JH, 20E, and insulin signal transduction, but the details are unclear.
The insulin signaling pathway plays crucial roles in insect reproduction. In D. melanogaster, insulin signaling directly regulates oocyte growth. Interfering with the insulin cascade blocked the uptake of yolk protein precursors and disrupted oocyte maturation (Das and Arur, 2017). In M. vitrata, knockdown of four insulin signaling components (InR, Akt, FOXO, and TOR) suppressed Vg and VgR expression and blocked ovarian development (Al Baki et al., 2019). In N. lugens, silencing of NlInR1 or four insulin-like peptides (Nlilp1-4) significantly reduced female fecundity (Xue et al., 2020). By comparing the phenotypes of T. castaneum after knockdown of different insulin-signaling genes, the decrease in egg production after the depletion of InR, IRS, and TOR was more severe than after suppression of PI3K, Akt, and PTEN (Parthasarathy and Palli, 2011). In adult females of Rhodnius prolixus, depletion of InR1, IGF, and ILP1 disrupted the development of ovarian follicles and reduced the numbers of eggs laid (Leyria et al., 2021). In this study, silencing of LsAkt significantly decreased Vg expression and vitellogenin amount and resulted in atrophied ovaries with less yolk protein deposition. The fecundity and egg hatchability were significantly reduced after knockdown of LsAkt expression. Similar defective phenotypes were observed after RNAi of Akt in C. pallens (Han et al., 2020). Depletion of Akt may have inhibited Vg synthesis and ovarian growth, thereby reducing the fecundity of L. serricorne.
Insulin signaling principal function in JH metabolism, and its roles have been elucidated in many insects. In D. melanogaster, mutations in InR or IRS caused significant reduction in JH titers (Tatar et al., 2001; Tu et al., 2005). In Blattella germanica, silencing of InR reduced the mRNA levels of JH biosynthetic enzymes and JH synthesis in corpora allata and affected vitellogensis in adult females (Abrisqueta et al., 2014). In L. decemlineata, RNAi of ILP2 substantially suppressed the expression levels of JHAMT and Kr-h1, and decreased JH titers (Fu et al., 2016). Depletion of FOXO, a transcription factor in the insulin signaling pathway in B. mori, induced the expression of three JH degradation pathway genes (Zeng et al., 2017). In this study, we found that the expressions of two JH signaling genes (LsMet and LsKr-h1) and two JH biosynthesis genes (LsFAmet and LsJHAMT) were significantly decreased in LsAkt-depleted beetles, and expressions of two JH degradation genes (LsJHE and LsJHEH) were dramatically increased. This indicates that LsAkt depletion inhibited JH signaling and activated JH degradation. Accordingly, knockdown of LsAkt led to a significant decrease in JH titers. Since JH is a vital hormone promoting Vg uptake in the ovaries, it is possible that decreasing LsAkt expression could affect ovarian development.
Carbohydrate metabolism is critical for supplying the energy needed for insect development and reproduction (Hou et al., 2015; Mattila and Hietakangas, 2017). Insulin signaling is functionally related to carbohydrate metabolism. In D. melanogaster, ablation of ILPs increased carbohydrate levels in the hemolymph, increased lipid storage in the fat body, retarded growth, reduced fecundity, and increased resistance to stress (Rulifson et al., 2002). In L. decemlineata, RNAi of IRS or PI3K92E inhibited the expression of four trehalose metabolic genes (LdTPS, LdTRE1a, LdTRE1B, and LdTRE2) and a glycogen synthase gene (LdGS), caused a decrease of glycogen accumulation and an increase of glucose and trehalose concentrations, and decreased food consumption (Deng et al., 2018). In N. lugens, suppression of Nlipl1, Nlipl2, Nlipl3 or NlInR1 disrupted carbohydrate metabolism and nymph development. However, knockdown of Nlipl1-3 resulted in increased contents of glucose, trehalose, and glycogen, which contrasts with the effect derived from NlInR1 knockdown (Xu H. J. et al., 2015). In this study, silencing of LsAkt inhibited the expression of TPS, increased the transcription level of TRE, and caused a dramatic reduction in trehalose content. Trehalose is the energy fuel for Vg formation and oocyte maturation (Lu et al., 2019). This indicates that repression of Akt may affect trehalose metabolism during the reproductive process of L. serricorne. We also found that the glycogen content was significantly reduced in the LsAkt RNAi beetles and the expression level of LsGlys was considerably decreased. In Culex pipiens, RNAi depletion of Glys reduced glycogen and lipid levels and increased the mortality of the diapause females (Olademehin et al., 2020). Studies in D. melanogaster revealed that glycogen accumulation is involved in development competence of the oocyte (Sieber et al., 2016). LsAkt knockdown severely reduced the expression of a glucose transporter gene (GLUT), decreased the expression of a pentose phosphate pathway (PPP) gene (G6PDH), and inhibited glucose uptake. G6PDH mediates the rate-limiting and committed step of PPP, which provides energy for insect growth and reproduction (Smolinski et al., 2019). Silencing LsAkt increased the transcription activation of two glycolytic pathway genes (LsHK and LsPFK) and lowered the glucose content. These results were not consistent with previous studies. In B. mori, downregulated expression of HK and PFK genes induced by the suppressed estrogen-related receptor gene increased glucose levels and influenced embryonic development (Long et al., 2020). In T. castaneum and N. lugens, silencing of the hexokinase gene increased the glucose amounts and reduced fecundity (Fraga et al., 2013; Ge et al., 2019). The underlying mechanism by which insulin signaling regulates carbohydrate metabolism appears to be complicated and in need of further investigation.
Conclusion
We obtained a serine/threonine kinase Akt gene (LsAkt) from L. serricorne. The expression of LsAkt was stimulated by methoprene or insulin, while suppressed by 20E exposure. RNAi screening demonstrated that LsAkt plays a pivotal role in the ovarian development and fecundity of L. serricorne. Knockdown of LsAkt inhibited Vg synthesis, disturbed JH production, and disrupted carbohydrate metabolism, resulting in reproductive defects. These results provide fundamental evidence for clarifying regulatory mechanisms of Akt in L. serricorne reproduction.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GenBank, MZ695806.
Author Contributions
K-KX and HY conceived and designed the experiments and wrote the manuscript. YY, S-YY, and P-LX performed the experiments. YY and K-KX analyzed the data. W-JY, HY, and CL revised the manuscript. All authors gave final approval for the publication.
Funding
This work was supported by the National Natural Science Foundation of China (32160637), Program of Excellent Innovation Talents in Guizhou Province (20206003), Program for Science and Technology Youth Talents in Department of Education in Guizhou Province (2018298), Special Funding of Guiyang Science and Technology Bureau and Guiyang University (GYU-KY-2021), Major Special Project of China Tobacco Company [110201901038(LS-01)], and Science and Technology Program of Hubei Tabacco Company [027Y2018-025].
Conflict of Interest
P-LX was employed by company Enshi Tobacco Company of Hubei Province.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.765819/full#supplementary-material
Supplementary Figure 1 | Nucleotide and deduced amino acid sequences of LsAkt cDNA. The start codon is indicated in bold and the stop codon in bold with an asterisk. The predicted phosphorylation sites, including serine (S), threonine (T), and tyrosine (Y), are marked in red, yellow, and green, respectively. The low complexity region is shaded and PH domain is underlined. The S_TKc domain is boxed and S_TK_X domain is underlined with wavy lines.
Footnotes
- ^ https://blast.ncbi.nlm.nih.gov/Blast.cgi
- ^ https://www.ncbi.nlm.nih.gov/orffinder
- ^ http://www.expasy.ch
- ^ http://smart.embl-heidelberg.de
References
Abrisqueta, M., Süren-Castillo, S., and Maestro, J. L. (2014). Insulin receptor-mediated nutritional signaling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 49, 14–23. doi: 10.1016/j.ibmb.2014.03.005
Al Baki, A., Jung, J. K., and Kim, Y. (2020). Alteration of insulin signaling to control insect pest by using transformed bacteria expressing dsRNA. Pest Manag. Sci. 76, 1020–1030. doi: 10.1002/ps.5612
Al Baki, M. A., Lee, D. W., Jung, J. K., and Kim, Y. (2019). Insulin signaling mediates previtellogenic development and enhances juvenile hormone-mediated vitellogenesis in a lepidopteran insect, Maruca vitrata. BMC Dev. Biol. 19:14. doi: 10.1186/s12861-019-0194-8
Arik, A. J., Hun, L. V., Quicke, K., Piatt, M., Ziegler, R., Scaraffia, P. Y., et al. (2015). Increased Akt signaling in the mosquito fat body increases adult survivorship. FASEB J. 29, 1403–1413. doi: 10.1096/fj.14-261479
Ashworth, J. R. (1993). The biology of Lasioderma serricorne. J. Stored Prod. Res. 29, 291–303. doi: 10.1016/0022-474X(93)90044-5
Chen, X. Y. L., Xu, K. K., Yan, X., Chen, C. X., Cao, Y., Wang, Y. W., et al. (2018). Characterization of a β-N-acetylglucosaminidase gene and its involvement in the development of Lasioderma serricorne (Fabricius). J. Stored Prod. Res. 77, 156–165. doi: 10.1016/j.jspr.2018.04.012
Cheng, D. J., Peng, J., Meng, M., Wei, L., Kang, L. X., Qian, W. L., et al. (2014). Microarray analysis of the juvenile hormone response in larval integument of the silkworm, Bombyx mori. Int. J. Genomics 2014:426025. doi: 10.1155/2014/426025
Das, D., and Arur, S. (2017). Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 84, 444–459. doi: 10.1002/mrd.22806
Das, R., and Dobens, L. L. (2015). Conservation of gene and tissue networks regulating insulin signaling in flies and vertebrates. Biochem. Soc. Trans. 43, 1057–1062. doi: 10.1042/bst20150078
Deng, P., Xu, Q. Y., Fu, K. Y., Guo, W. C., and Li, G. Q. (2018). RNA interference against the putative insulin receptor substrate gene chico affects metamorphosis in Leptinotarsa decemlineata. Insect Biochem. Mol. Biol. 103, 1–11. doi: 10.1016/j.ibmb.2018.10.001
Edde, P. A. (2019). Biology, ecology, and control of Lasioderma serricorne (F.) (Coleoptera: anobiidae): a review. J. Econ. Entomol. 112, 1011–1031. doi: 10.1093/jee/toy428
Fraga, A., Ribeiro, L. R., Lobato, M., Santos, V., Silva, J. R., Gomes, H., et al. (2013). Glycogen and glucose metabolism are essential for early embryonic development of the red flour beetle Tribolium castaneum. PLoS One 8:e65125. doi: 10.1371/journal.pone.0065125
Fu, K. Y., Zhu, T. T., Guo, W. C., Ahmat, T., and Li, G. Q. (2016). Knockdown of a putative insulin-like peptide gene LdILP2 in Leptinotarsa decemlineata by RNA interference impairs pupation and adult emergence. Gene 581, 170–177. doi: 10.1016/j.gene.2016.01.037
Ge, L. Q., Gu, H. T., Li, X., Zheng, S., Zhou, Z., Miao, H., et al. (2019). Silencing of triazophos-induced hexokinase-1-like reduces fecundity in Nilaparvata lugens (Stål) (Hemiptera: delphacidae). Pestic. Biochem. Physiol. 153, 176–184. doi: 10.1016/j.pestbp.2018.11.016
Gu, S. H., Lin, P. L., and Hsieh, H. Y. (2019). Bombyxin/Akt signaling in relation to the embryonic diapause process of the silkworm, Bombyx mori. J. Insect Physiol. 116, 32–40. doi: 10.1016/j.jinsphys.2019.04.007
Gu, S. H., Young, S. C., Lin, J. L., and Lin, P. L. (2011). Involvement of PI3K/Akt signaling in PTTH-stimulated ecdysteroidogenesis by prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 41, 197–202. doi: 10.1016/j.ibmb.2010.12.004
Han, B. F., Zhang, T. T., Feng, Y. J., Liu, X. P., Zhang, L. S., Chen, H. Y., et al. (2020). Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens. J. Insect Physiol. 123:104049. doi: 10.1016/j.jinsphys.2020.104049
Hou, Y., Wang, X. L., Saha, T. T., Roy, S., Zhao, B., Raikhel, A. S., et al. (2015). Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction. PLoS Genet. 11:e1005309. doi: 10.1371/journal.pgen.1005309
Hun, L. V., Luckhart, S., and Riehle, M. A. (2019). Increased Akt signaling in the fat body of Anopheles stephensi extends lifespan and increases lifetime fecundity through modulation of insulin-like peptides. J. Insect Physiol. 118:103932. doi: 10.1016/j.jinsphys.2019.103932
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. doi: 10.1093/bioinformatics/btm404
Leyria, J., Orchard, I., and Lange, A. B. (2021). The involvement of insulin/ToR signaling pathway in reproductive performance of Rhodnius prolixus. Insect Biochem. Mol. Biol. 130:103526. doi: 10.1016/j.ibmb.2021.103526
Liu, Y., Zhou, S., Ma, L., Tian, L., Wang, S., Sheng, Z., et al. (2010). Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the Bombyx fat body. J. Insect Physiol. 56, 1436–1444. doi: 10.1016/j.jinsphys.2010.02.011
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Long, W., Wu, J., Shen, G., Zhang, H., Liu, H., Xu, Y., et al. (2020). Estrogen-related receptor participates in regulating glycolysis and influences embryonic development in silkworm Bombyx mori. Insect Mol. Biol. 29, 160–169. doi: 10.1111/imb.12619
Lu, K., Wang, Y., Chen, X., Zhang, X. Y., Li, W. R., Cheng, Y. B., et al. (2019). Adipokinetic hormone receptor mediates trehalose homeostasis to promote vitellogenin uptake by oocytes in Nilaparvata lugens. Front. Physiol. 9:1904. doi: 10.3389/fphys.2018.01904
Mattila, J., and Hietakangas, V. (2017). Regulation of Carbohydrate Energy Metabolism in Drosophila melanogaster. Genetics 207, 1231–1253. doi: 10.1534/genetics.117.199885
Okada, Y., Miyazaki, S., Miyakawa, H., Ishikawa, A., Tsuji, K., and Miura, T. (2010). Ovarian development and insulin-signaling pathways during reproductive differentiation in the queenless ponerine ant Diacamma sp. J. Insect Physiol. 56, 288–295. doi: 10.1016/j.jinsphys.2009.10.013
Olademehin, O. P., Liu, C. Y., Rimal, B., Adegboyega, N. F., Chen, F., Sim, C., et al. (2020). DsiRNA knockdown of genes regulated by Foxo reduces glycogen and lipid accumulations in diapausing Culex pipiens. Sci. Rep. 10:17201. doi: 10.1038/s41598-020-74292-6
Pan, J., Di, Y. Q., Li, Y. B., Chen, C. H., Wang, J. X., and Zhao, X. F. (2018). Insulin and 20-hydroxyecdysone oppose each other in the regulation of phosphoinositide-dependent kinase-1 expression during insect pupation. J. Biol. Chem. 293, 18613–18623. doi: 10.1074/jbc.RA118.004891
Parthasarathy, R., and Palli, S. R. (2011). Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 41, 294–305. doi: 10.1016/j.ibmb.2011.01.006
Riehle, M. A., and Brown, M. R. (2003). Molecular analysis of the serine/threonine kinase Akt and its expression in the mosquito Aedes aegypti. Insect Mol. Biol. 12, 225–232. doi: 10.1046/j.1365-2583.2003.00405.x
Riudavets, J., Salas, I., and Pons, M. J. (2007). Damage characteristics produced by insect pests in packaging film. J. Stored Prod. Res. 43, 564–570. doi: 10.1016/j.jspr.2007.03.006
Roy, S. G., and Raikhel, A. S. (2012). Nutritional and hormonal regulation of the TOR effector 4E-binding protein (4E-BP) in the mosquito Aedes aegypti. FASEB J. 26, 1334–1342. doi: 10.1096/fj.11-189969
Rulifson, E. J., Kim, S. K., and Nusse, R. (2002). Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120. doi: 10.1126/science.1070058
Scanga, S. E., Ruel, L., Binari, R. C., Snow, B., Stambolic, V., Bouchard, D., et al. (2000). The conserved PI3’K/PTEN/Akt signaling pathway regulates both cell size and survival in Drosophila. Oncogene 19, 3971–3977. doi: 10.1038/sj.onc.1203739
Sheng, Z. T., Xu, J. J., Bai, H., Zhu, F., and Palli, S. R. (2011). Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J. Biol. Chem. 286, 41924–41936. doi: 10.1074/jbc.M111.269845
Sieber, M. H., Thomsen, M. B., and Spradling, A. C. (2016). Electron transport chain remodeling by GSK3 during oogenesis connects nutrient state to reproduction. Cell 164, 420–432. doi: 10.1016/j.cell.2015.12.020
Sivik, F. P., Tenhet, J. N., and Delamar, C. D. (1957). An ecological study of the cigarette beetle in tobacco storage warehouses. J. Econ. Entomol. 50, 310–316. doi: 10.1093/jee/50.3.310
Smith, W. A., Lamattina, A., and Collins, M. (2014). Insulin signaling pathways in lepidopteran ecdysone secretion. Front. Physiol. 5:19. doi: 10.3389/fphys.2014.00019
Smolinski, M. B., Green, S. R., and Storey, K. B. (2019). Glucose-6-phosphate dehydrogenase is posttranslationally regulated in the larvae of the freeze-tolerant gall fly, Eurosta solidaginis, in response to freezing. Arch. Insect Biochem. Physiol. 102:e21618. doi: 10.1002/arch.21618
Staveley, B. E., Ruel, L., Jin, J., Stambolic, V., Mastronardi, F. G., Heitzler, P., et al. (1998). Genetic analysis of protein kinase B (AKT) in Drosophila. Curr. Biol. 8, 599–602. doi: 10.1016/S0169-5150(96)01217-0
Taniguchi, C. M., Emanuelli, B., and Kahn, C. R. (2006). Critical nodes in signaling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96. doi: 10.1038/nrm1837
Tatar, M., Kopelman, A., Epstain, D., Tu, M. P., Yin, C. M., and Garofalo, R. S. (2001). A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110. doi: 10.1126/science.1057987
Teleman, A. A. (2010). Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 425, 13–26. doi: 10.1042/BJ20091181
Thounaojam, B., and Keshan, B. (2017). Modulation of gene expression by nutritional state and hormones in Bombyx larvae in relation to its growth period. Gene Expr. Patterns 25–26, 175–183. doi: 10.1016/j.gep.2017.08.003
Tu, M. P., Yin, C. M., and Tatar, M. (2005). Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen. Comp. Endocrinol. 142, 347–356. doi: 10.1016/j.ygcen.2005.02.009
Umemiya-Shirafuji, R., Tanaka, T., Boldbaatar, D., Tanaka, T., and Fujisaki, K. (2012). Akt is an essential player in regulating cell/organ growth at the adult stage in the hard tick Haemaphysalis longicornis. Insect Biochem. Mol. Biol. 42, 164–173. doi: 10.1016/j.ibmb.2011.12.001
Verdu, J., Buratovich, M. A., Wilder, E. L., and Birnbaum, M. J. (1999). Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell Biol. 1, 500–506. doi: 10.1038/70293
Walkiewicz, M. A., and Stern, M. (2009). Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS One 4:e5072. doi: 10.1371/journal.pone.0005072
Wu, Q., and Brown, M. R. (2006). Signaling and function of insulin like peptides in insects. Annu. Rev. Entomol. 51, 1–24. doi: 10.1146/annurev.ento.51.110104.151011
Xu, H. J., Xue, J., Lu, B., Zhang, X. C., Zhang, J. C., He, S. F., et al. (2015). Two insulin receptors determine alternative wing morphs in planthoppers. Nature 519, 464–467. doi: 10.1038/nature14286
Xu, K. K., Pan, B. Y., Wang, Y. Y., Ren, Q. Q., and Li, C. (2020). Roles of the PTP61F gene in regulating energy metabolism of Tribolium castaneum (Coleoptera: tenebrionidae). Front. Physiol. 11:1071. doi: 10.3389/fphys.2020.01071
Xu, K. K., Yang, W. J., Tian, Y., Wu, Y. B., and Wang, J. J. (2015). Insulin signaling pathway in the oriental fruit fly: the role of insulin receptor substrate in ovarian development. Gen. Comp. Endocrinol. 216, 125–133. doi: 10.1016/j.ygcen.2014.11.022
Xu, Y. Y., Wei, W., Lin, G. Z., Yan, S., Zhang, J. Z., Shen, J., et al. (2021). The Ras/MAPK pathway is required for regenerative growth of wing discs in the black cutworm Agrotis ypsilon. Insect Biochem. Mol. Biol. 131:103552. doi: 10.1016/j.ibmb.2021.103552
Xue, W. H., Liu, Y. L., Jiang, Y. Q., He, S. F., Wang, Q. Q., Yang, Z. N., et al. (2020). Molecular characterization of insulin-like peptides in the brown planthopper, Nilaparvata lugens (Hemiptera: delphacidae). Insect Mol. Biol. 29, 309–319. doi: 10.1111/imb.12636
Yang, W. J., Xu, K. K., Yan, Y., Li, C., and Jin, D. C. (2020). Role of chitin deacetylase 1 in the molting and metamorphosis of the cigarette beetle Lasioderma serricorne. Int. J. Mol. Sci. 21:2449. doi: 10.3390/ijms21072449
Zeng, B. S., Huang, Y. P., Xu, J., Shiotsuki, T., Bai, H., Palli, S. R., et al. (2017). The FOXO transcription factor controls insect growth and development by regulating juvenile hormone degradation in the silkworm, Bombyx mori. J. Biol. Chem. 292, 11659–11669. doi: 10.1074/jbc.M117.777797
Zhang, H., Liu, J. N., Li, C. R., Momen, B., Kohanski, R. A., and Pick, L. (2009). Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl. Acad. Sci. U. S. A. 106, 19617–19622. doi: 10.1073/pnas.0905083106
Keywords: serine/threonine kinase, cigarette beetle, insulin, reproduction, juvenile hormone, carbohydrate metabolism
Citation: Xu K-K, Yan Y, Yan S-Y, Xia P-L, Yang W-J, Li C and Yang H (2021) Disruption of the Serine/Threonine Kinase Akt Gene Affects Ovarian Development and Fecundity in the Cigarette Beetle, Lasioderma serricorne. Front. Physiol. 12:765819. doi: 10.3389/fphys.2021.765819
Received: 27 August 2021; Accepted: 16 September 2021;
Published: 07 October 2021.
Edited by:
Fernando Ariel Genta, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Wen Liu, Huazhong Agricultural University, ChinaKlaus H. Hoffmann, University of Bayreuth, Germany
Copyright © 2021 Xu, Yan, Yan, Xia, Yang, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Yang, YXh5cmlkaXNAMTYzLmNvbQ==
 Kang-Kang Xu
Kang-Kang Xu Yi Yan1,2
Yi Yan1,2 Wen-Jia Yang
Wen-Jia Yang Can Li
Can Li