
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 10 December 2021
Sec. Integrative Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.747144
This article is part of the Research TopicAdvances on Pathogenesis and Treatment of Lower Urinary Tract Symptoms and Pelvic Floor Dysfunction DiseasesView all 22 articles
Overactive bladder (OAB) is a common debilitating condition characterized by urgency symptoms with detrimental effects on the quality of life and survival. The exact etiology of OAB is still enigmatic, and none of therapeutic approaches seems curative. OAB is generally regarded as a separate syndrome, whereas in clinic, OAB symptoms could be found in numerous diseases of other non-urogenital systems, particularly nervous system. The OAB symptoms in neurological diseases are often poorly recognized and inadequately treated. This review provided a comprehensive overview of recent findings related to the neurogenic OAB symptoms. Relevant neurological diseases could be mainly divided into seven kinds as follows: multiple sclerosis and related neuroinflammatory disorders, Parkinson’s diseases, multiple system atrophy, spinal cord injury, dementia, peripheral neuropathy, and others. Concurrently, we also summarized the hypothetical reasonings and available animal models to elucidate the underlying mechanism of neurogenic OAB symptoms. This review highlighted the close association between OAB symptoms and neurological diseases and expanded the current knowledge of pathophysiological basis of OAB. This may increase the awareness of urological complaints in neurological disorders and inspire robust therapies with better outcomes.
Overactive bladder (OAB) was defined as a storage symptom syndrome characterized by “urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology” (Abrams et al., 2002). The prevalence of OAB increases with advancing age and is greatly varied across studies. A population-based survey included over 19,000 participants and demonstrated an overall prevalence of OAB to be 11.8% (10.8% in men and 12.8% in women). Other studies have reported the prevalence of up to 30–40% (Coyne et al., 2009). Despite great strides made in the past decades, the exact etiology of OAB is still enigmatic and none of therapeutic approaches seems curative. As OAB is a separate syndrome, its symptoms could also be found in numerous diseases of other non-urogenital systems, such as diabetes, cardiovascular diseases, and sleep disorders. Given the basis of the condition relying on a subjective symptom of urgency, available animal models with indirect or surrogate markers of urgency have been applied for basic science research into OAB. Therefore, exploration of the highly observed comorbidity between OAB symptoms and other diseases could potentially shed light on the pathophysiology of OAB and address the confusing situation hampering research and management.
An accumulating evidence has demonstrated that OAB symptoms are regarded as significant features in numerous neurological diseases, such as multiple sclerosis (MS), spinal cord injury (SCI), Parkinson’s disease (PD), stroke, and spina bifida (Panicker et al., 2015; Yamada et al., 2018). The OAB symptoms are highly prevalent among neurogenic patients, as shown by a recent study that over 50% of these patients reported OAB symptoms (Przydacz et al., 2021). This can be largely anticipated from the crucial regulatory effect of nervous system on the micturition reflex. Also, the severity of OAB symptoms varies with the type and degree of damage to the nervous system. However, the OAB symptoms in neurological diseases are often poorly recognized and relatively few of individuals with these symptoms seek care (Dmochowski and Newman, 2007; Przydacz et al., 2021). Moreover, since OAB symptoms in neurogenic patients often have their own particularities, respected clinical efficacy may not be achieved by referring to the conventional treatment programs of OAB. Even worse, older adults with neurocognitive dysfunction are at higher risk of taking multiple medications with anticholinergic properties for OAB symptoms (Duong et al., 2021). As yet, up to now only a scarcity of studies could totally present OAB symptoms in these neurological diseases and clearly summarize the possible mechanisms underlying this tight connection (Chapple et al., 2017; Cornu, 2017).
The aim of this review was to provide a comprehensive and state-of-the-art overview of the close correlation between OAB symptoms and neurological diseases. The summary from over 2,000 published papers showed the complexity and diversity of neurological diseases related to OAB symptoms (Table 1). This study was largely focused on the potential mechanisms underlying the cause of neurogenic OAB symptoms (Figures 1, 2). We aimed to increase the awareness of urological complaints in neurological diseases, inspire robust suitable therapies, and improve the quality of life.
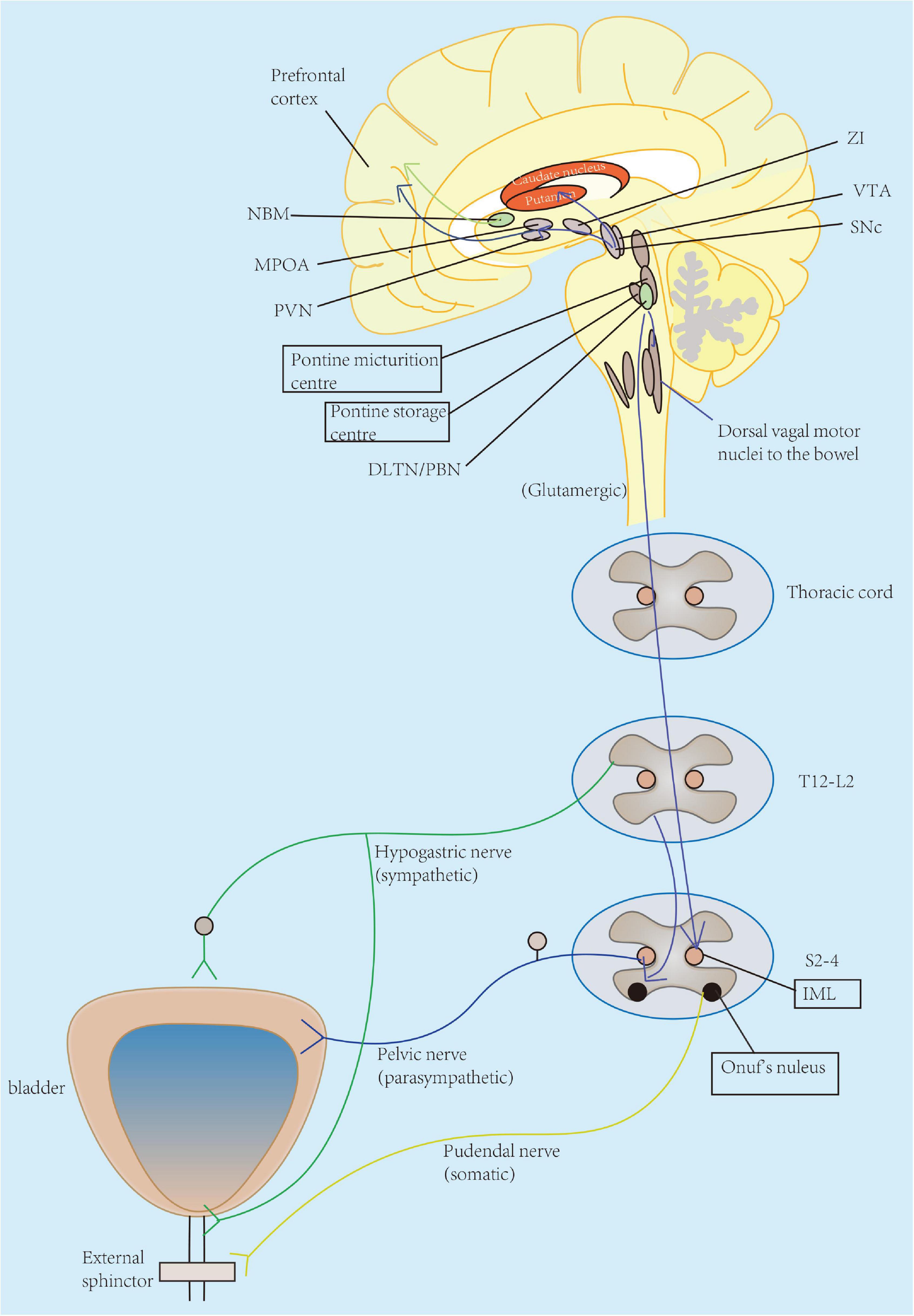
Figure 1. Pathophysiology of OAB symptoms in neurological diseases appears to be multifactorial across all levels of neural control of micturition, from cerebral cortex, brain stem, spinal cord, to the peripheral nerves. The lower urinary tract consists of two major components: the bladder and the urethra. The bladder receives innervation from the parasympathetic pelvic nerve. Parasympathetic (pelvic) nerves could excite the bladder (primarily through activation of muscarinic-3 receptors) and relax the urethra, while sympathetic (hypogastric) nerve enables to inhibit the bladder body (primarily through β-3 adrenergic receptors) and excite the urethra. Pudendal nerve originates from S2 to S4 motor neurons in Onuf’s nucleus and allows to the excitation of the external urethral sphincter. Particularly, urinary storage functions are primarily maintained by the spinal cord reflex with enhanced activity of hypogastric and pudendal nerves innervating the urethra. This function is also under-controlled by the pontine storage center located closely to the pontine micturition center (PMC), hypothalamus, cerebellum, basal ganglia, and frontal cortex. The prefrontal cortex is regarded as the center of planning of complex cognitive behaviors. Other regions also serve as active participants for awareness of visceral sensations, such as the periaqueductal gray (PAG), insula, and anterior cingulate gyrus. ZI, zona incerta; VTA, ventral tegmental area; SNc, substantia nigra pars compacta; NBM, nucleus basalis of Meynert; MPOA, medial preoptic area; PVN, paraventricular nucleus; DLTN, dorsolateral tegmental nucleus; PBN, parabrachial nucleus; IML, intermediolateral cell column; L, lumbar; S, sacral; T, thoracic.

Figure 2. Mechanism of overactive bladder (OAB) symptoms caused by various nervous system diseases. Multiple sclerosis (MS) cause detrusor overactivity (DO) due to the suprapontine lesions, axonal loss, novel C-fiber-mediated voiding reflex, and reductions in central serotonergic activity and stress hormones. Neuromyelitis optica spectrum disorder (NMOSD) causes DO due to spinal cord injury (SCI). Parkinson’s disease (PD) causes DO due to the decline in nigrostriatal dopaminergic function, frontal lobe executive impairment and REM sleep behavior disorder, and the reduction of several inhibitory neurotransmitters in the brain. Multiple system atrophy (MSA) causes lesions in locus coeruleus, prefrontal-basal ganglia D1 dopaminergic pathway, cerebellum, raphe serotonergic pathway, and frontal cortex. SCI leads to DO due to the impaired communication between the cerebral and spinal circuits that coordinate bladder and urethra activities, suprasacral spinal cord lesions, and emergence of a capsaicin-sensitive C-fiber-mediated spinal micturition reflex caused by a reorganization of synaptic connections in the spinal cord. Peripheral neuropathy can also lead to DO. Dementia affecting the prefrontal cortex might also lead to altered central micturition circuit. Other nervous system diseases such as cerebral palsy, pituitary adenoma compressing the hypothalamus, skull base chordoma, traumatic brain injury, and cerebrovascular accident may. Both frontal cortex and hypothalamus are involved.
A comprehensive electronic literature search was conducted using the PubMed database to identify publications related to the neurological diseases with OAB symptoms. The keywords included the following terms: “overactive bladder,” “nervous system,” “detrusor overactivity,” “detrusor instability,” “unstable bladder,” and “etiology,” used either alone or in combination. The search was restricted to studies published between January 1990 and December 2020. The title and abstract of each article were reviewed for their appropriateness and relevance to the symptoms of OAB in diseases of nervous system. Relevant articles published in English were fully reviewed subsequently.
The pathophysiology of OAB symptoms in neurological diseases appears to be multifactorial across all levels of neural control of micturition, from cerebral cortex, brain stem, spinal cord, to the peripheral nerves. The site and nature of the neurological lesions may affect the appearing time, progression, and severity of OAB symptoms. A better knowledge of the neural component of normal micturition appears to be necessary to the role of neurological diseases in the etiology of OAB symptoms.
In human body, the coordinated activity of the urinary bladder and its outlet is controlled by a complex neural network distributed across parasympathetic, sympathetic, and somatic pathways. Several literature reviews have demonstrated this neural control of micturition reflex (de Groat et al., 2015; Griffiths, 2015; Rahnama’i, 2019). Briefly, parasympathetic (pelvic) nerves could excite the bladder (primarily through activation of muscarinic-3 receptors) and relax the urethra, while sympathetic (hypogastric) nerve enables to inhibit the bladder body (primarily through β-3 adrenergic receptors) and excite the urethra. Pudendal nerve originates from S2 to S4 motor neurons in Onuf’s nucleus and allows to the excitation of external urethral sphincter. Particularly, urinary storage function is primarily maintained by the spinal cord reflex with enhanced activity of hypogastric and pudendal nerves innervating the urethra. This function is also controlled by the pontine storage center located closely to the pontine micturition center (PMC), hypothalamus, cerebellum, basal ganglia, and frontal cortex. PMC is thought to initiate the micturition cycle and receive afferent input from the lumbosacral spinal cord due to bladder distention as well as the prefrontal cortex, which gives social acceptability of voiding (de Groat et al., 2015). The prefrontal cortex is regarded as the center of planning of complex cognitive behaviors. Other regions also serve as active participants for awareness of visceral sensations, such as the periaqueductal gray (PAG), insula, and anterior cingulate gyrus. Furthermore, PAG is considered to mediate switching from storage to voiding, possibly regulated by higher brain regions such as the hypothalamus and prefrontal cortex (Kakizaki et al., 2011). Therefore, any lesion of the central or peripheral nervous system could possibly disrupt the voluntary control of micturition, resulting subsequent in the occurrence of voiding urgency, frequency, incontinence, and nocturia (namely, OAB symptoms).
The OAB symptoms among these neurogenic patients possibly derive from neurogenic detrusor overactivity (DO), which is characterized by involuntary detrusor contractions during bladder filling (Abrams et al., 2003; Defreitas et al., 2003; Karsenty et al., 2008). Although not synonymous of OAB, DO is traditionally thought to be the major causes of urinary urgency/frequency and incontinence (Abrams et al., 2002). Several potential OAB phenotypes have been identified according to urodynamic demonstration of DO, and multiple neurogenic factors could contribute to the development of DO via different pathophysiological mechanisms (Peyronnet et al., 2019). First, classic neurogenic DO is thought to be resulting from the loss of supraspinal inhibition control on the micturition reflex or the decreased capacity to functionally integrate afferent information due to the brain damages such as MS, stroke, and PD (Peyronnet et al., 2019). Other increasing evidence supports the idea that deep white matter disease (WMD), mostly in the prefrontal area of the brain, might be the anatomical substrate for brain-related DO (Sakakibara et al., 1999; Griffiths and Tadic, 2008; Tadic et al., 2012). Behavioral therapies (Griffiths et al., 2015), sacral neuromodulation (SNM) (Blok et al., 2006), and posterior tibial nerve stimulation (PTNS) (Finazzi-Agrò et al., 2009) seem appropriate to treat OAB symptoms due to supraspinal lesions.
Second, spinal cord damages such as MS and SCI could induce the occurrence of primitive spinal bladder reflexes mediated by C-fibers afferents, leading to the development of subsequent DO (de Groat, 1997). The C-fibers can be recruited under neuropathic conditions to form a new functional afferent pathway, which can be suppressed by certain drugs such as resiniferatoxin and capsaicin. These findings promote the application of intravesical administration of capsaicin for the treatment of DO in patients with SCI (Chancellor and de Groat, 1999). Of note, spinal lesions below the PMC and above the sacral cord could interrupt spinobulbar pathway and bring about detrusor external sphincter dyssynergia (DESD), such as simultaneous contractions of detrusor and the urethral and/or periurethral striated muscle (Suzuki Bellucci et al., 2012). Voiding features due to spinal cord damages comprise neurogenic DO, DESD, and different types of urinary incontinence.
Third, a growing body of evidence has indicated the coexistence of DO and detrusor underactivity (DU) (Peyronnet et al., 2019; Mancini et al., 2020). Urgency was considered the most common symptom in patients with urodynamically identified DU (Uren et al., 2017). Both DO and DU appear to, at least in part, share the common basis under neurogenic conditions, such as PD, MS, myelitis, and peripheral neuropathy (Balzarro et al., 2019; Mancini et al., 2020). Both DO and DU may be attributed to the impairment of afferents signaling function, central nerve control mechanism, or efferent innervation (Aldamanhori et al., 2018). Currently, no clinically effective drug treatments have been reported for restoring detrusor contractility. Clean intermittent self-catheterization and sacral nerve stimulation (SNM) seem to be helpful to those patients with DO and DU (Gani and Hennessey, 2017).
Fourth, the urethra is likely to play a key role in sensation and continuance, and the activation of urethral afferent signaling system could modulate the micturition reflex, the so-called urethral-vesical reflex (Shafik et al., 2003). Urethrogenic factors, such as the deterioration of urethral tone, are postulated to induce OAB symptoms (Peyronnet et al., 2019). Similarly, a lack of pudendal or central neurological control may also lead to urethral sphincter instability and subsequent urethra-driven OAB (Kirschner-Hermanns et al., 2016). Duloxetine and SNM are likely to be potential treatment options for urethra-driven OAB (Groenendijk et al., 2007; Steers et al., 2007).
Fifth, sensitization of the afferent nerve fibers by the urothelium/suburothelium is implicated in the pathophysiology of neurogenic DO. P2X purinoceptor 3 (P2 × 3) receptor is a type of sensory receptors, and the number of P2 × 3 immunoreactive nerve fibers has been reported an increase in the suburothelium of patients with neurogenic DO (Brady et al., 2004). The expression of other type of sensory receptor, e.g., transient receptor potential vanilloid type 1, also increased in neurogenic patients with DO (Apostolidis et al., 2005).
Lastly, neurogenic-myogenic mechanisms may contribute to the development of neurogenic DO. An early study has demonstrated that partial denervation is attributable to the alternation in smooth muscle properties, leading to enhanced excitability, coordinated myogenic contractions, and enlarged bladder pressure (Turner and Brading, 1997). Due to the complexity of the neural control of micturition, OAB symptoms and DO can be seen as a result of a variety of neurological disorders, including MS, PD, SCI, dementia, and other neurological diseases.
Overactive Bladder (OAB) is a symptom-based diagnosis in which urgency is the key symptom. The subjective nature of urgency hampers the development of animal models for OAB. Neurological animal models are not directly related to OAB, but they enable to provide a platform for seeking the mechanism of OAB and for assessing novel therapeutic options. Given the high prevalence of OAB symptoms among neurological diseases, a broad spectrum of neurological animal models has been applied to study the OAB symptoms and other lower urinary tract symptoms (LUTS). The commonly used neurological animal models mainly include suprapontine models, spinal cord transection/injury models, and experimental autoimmune encephalomyelitis model.
Suprapontine models are conducted to assess the voiding dysfunctions caused by various central nervous system (CNS) disorders such as PD, dementia, and cerebrovascular events. For instance, several available PD animal models are roughly divided into two groups, namely, toxin-based and genetic models (Kitta et al., 2020). Parkinsonism can be induced by administering the neurotoxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA), which are selective for the rapid degeneration of the nigrostriatal dopaminergic neurons (Schapira et al., 1989; Simola et al., 2007). Other transgenic PD models are developed, including the SNCA (α-synuclein) transgenic models (Matsuoka et al., 2001), DJ-1 KO models (Park et al., 2005), PINK1 KO models (Kitada et al., 2009), and LRRK2 models (Lee et al., 2010). Cerebral infarction animal models could be produced by occlusion of the middle cerebral artery (Yokoyama et al., 1998) or by the induction of midbrain ischemia (Yotsuyanagi et al., 2006), exhibiting voiding dysfunction including bladder overactivity.
Spinal cord transection/injury models are commonly utilized to study DO, DESD, and other types of lower urinary tract (LUT) dysfunction after any injury to the spinal cord, just as traumatic, developmental, infectious, vascular, degenerative injuries, and so on (Ethans et al., 2014; Panicker, 2020). Typically, the SCI model could be achieved by complete transection at different levels of spinal cord in different animals, particularly cats (de Groat et al., 1990) and rats (Shaker et al., 2003; Behr-Roussel et al., 2011). Besides, fetal rats with retinoic acid-induced myelomeningocele could be used to model spina bifida for bladder dysfunction investigation (Danzer et al., 2005).
Experimental autoimmune encephalomyelitis model is capable of mimicking MS-produced bladder dysfunction such as DO. This model can be induced by the activation of immunization with CNS immunogenic compounds, or transfer of encephalogenic T-cell lines from the affected animals (Petry et al., 2000) or infection with Semliki Forest virus (Moss et al., 1989) or coronavirus (McMillan et al., 2014).
Multiple sclerosis is described as an immune-mediated neuroinflammatory and neurodegenerative disease of the CNS with heterogeneous clinical presentations. According to previous reports, MS is the leading non-traumatic neurological cause of disability in young and middle-aged people in the developed world (Altmann, 2005; Çetinel et al., 2013). Bladder dysfunction is commonly seen in MS, affecting 80–100% of patients during the course of the disease (Minardi and Muzzonigro, 2005). Among them, OAB symptoms are the most common ones, reported by 60–80% of patients with MS. Moreover, a recent study has revealed that the OAB symptoms in MS could highly reach up to 96% (Declemy et al., 2021). Therefore, OAB symptoms remain a considerable clinical challenge to treat.
The mechanism underlying MS-related OAB symptoms is mainly due to the DO induced by suprapontine lesions, with disruption or lack of descending inhibitory impulses from the brain to the spinal cord (Ethans et al., 2014). Many studies about urodynamics in MS cases have demonstrated detrusor hyperreflexia or hyporeflexia (Akkoç et al., 2016; Wang et al., 2016). The primary target of the immune cells in MS is the myelin-producing oligodendrocytes of the CNS, characterized by demyelinated plaques on the brain, brain stem, cerebellum, and/or spinal cord. The myelinated nerve tracts innervating the LUT function would be eventually affected by these demyelinated lesions. Recently, a new concept has emerged that axonal loss, rather than demyelination, is the cause of progressive neurological deficits and much correlated with clinical disability (Dutta and Trapp, 2007). Neuroimaging and pathology studies have proved that in MS, the commonly affected regions relevant to micturition are the medial/prefrontal/insular cortex (Charil et al., 2003), cerebellum (Charil et al., 2003), brain stem (midbrain, Pozzilli et al., 1992; Charil et al., 2003, and pons, Charil et al., 2003; Weissbart et al., 2017), and cervicothoracic spinal cord (Weissbart et al., 2017). Moreover, the predominant cause of DO is thought to be from brain lesions. DO may also be induced by a novel C-fiber-mediated voiding reflex after spinal cord lesions in MS (Sakakibara, 2019). In addition, the reduction in central serotonergic activity and stress hormones in patients with MS may contribute to the occurrence of OAB symptoms (Koutsis et al., 2016).
Other neuroinflammatory disorders of CNS, such as neuromyelitis optica spectrum disorder (NMOSD) (Sakakibara, 2019), acute disseminated encephalomyelitis (Panicker et al., 2009), and Sjögren syndrome (Tarhan, 2013; Lee et al., 2019), can also lead to OAB symptoms. NMOSD is regarded as a type of neuroinflammatory disorders distinct from MS with respect to immunopathogenesis and suitable treatment. NMOSD usually causes more severe longitudinal myelitis or transverse myelitis than MS. Due to the major site of lesions in the spinal cord, NMOSD could cause disturbance of controllable voiding and lead to neurogenic LUTS. De Carvalho and colleagues reported that DO, DESD, and combination of DO and DESD could be urodynamically proven in 20.0%, 23.3%, and 36.6% of patients with NMOSD, respectively (de Carvalho et al., 2016). In Sjögren syndrome, the autoantibodies binding to the M3 muscarinic receptor could result in exocrine dysfunction or cholinergic hyperresponsiveness, subsequently leading to bladder detrusor, smooth muscles contraction, and OAB symptoms (Wang et al., 2004).
Parkinson’s disease is one of the major diseases characterized pathologically by abnormal α-synuclein aggregation (Ogawa et al., 2017). Lower urinary tract symptoms are one of the main non-motor features in PD, which could be presented during the course of the disease (Defreitas et al., 2003; Winge et al., 2005; Winge and Nielsen, 2012). The epidemiologic data about LUTS in PD are widely scattered. It was recently reported that LUTS occurred in 27–63.9% of patients with PD and increased with the severity of PD (Barone et al., 2009; Ogawa et al., 2017). Another previous report estimated that highly up to 80% of patients with PD may suffer from LUTS (McDonald et al., 2017). Overactive bladder symptoms are the most common type of LUTS (Shah and Weiss, 2012; McDonald et al., 2017) and could be as early symptoms than motor-related ones among patients with PD (Roy et al., 2020). Overactive bladder symptoms can be treated as a warning of progression to PD dementia (Xu et al., 2019). Additionally, patients with PD frequently have LUTS, such as nocturia, increased urinary frequency, and urinary incontinence, overlapping with those of OAB symptoms (Ogawa et al., 2017). During urodynamic testing, about 36–93% of patients with PD showed uninhibited contractions or DO (Ogawa et al., 2017).
In patients with PD, the etiology of OAB symptoms is centrally mediated and modulated in complex ways that are not fully understood. Strong evidence indicated that a decline in nigrostriatal dopaminergic function plays a crucial role in this progress (Sakakibara et al., 2001; Winge et al., 2005). Overactive bladder symptoms may arise from the disruption of the complex control loops within the context of neurodegeneration, instead of a focal lesion (Winge and Fowler, 2006; Campeau et al., 2011). For instance, basal ganglia could interfere with the function of the PMC. In PD, altered signaling in the nigrostriatal dopaminergic system results in a partial or total disconnection of the micturition reflex from voluntary control and subsequent uninhibited bladder contractions (Blackett et al., 2009). Besides, several studies confirmed and expanded the views above. Functional neuroimaging studies revealed a significant decline of dopamine transporter imaging in the brain of patients with PD with LUTS (Sakakibara et al., 2001; Winge et al., 2005). In animal studies, the disruption of nigrostriatal dopaminergic system was also proved to produced DO (Albanese et al., 1988; Yamamoto et al., 2005a), which could be inhibited by stimulating D1-like dopamine receptor with agonists or pergolide (Yoshimura et al., 2003). Additionally, enhanced activity of the adenosine A2A system in the brain may contribute to DO in PD, which could be suppressed by A2A antagonist ZM 241385 (Kitta et al., 2012). Clinical application of A2A receptor antagonists such as istradefylline may also be a promising candidate for the treatment of LUTS in patients with PD (Kitta et al., 2016). Moreover, several specific non-motor symptoms are also known to be correlated with LTUS in PD (Sakakibara et al., 2013; Rana et al., 2015). A cross-sectional study suggested that frontal lobe executive impairment and rapid eye movement (REM) sleep behavior disorder accompanied with a higher prevalence of OAB symptoms in patients with PD, possibly due to the overlap of locus coeruleus and pontine nucleus with some regions that control micturition (Xu et al., 2019). Other postulated mechanisms underlying the relationship between OAB symptoms and PD are the reduction of several inhibitory neurotransmitters in the brain related to micturition, such as γ-aminobutyric acid, serotonin, and norepinephrine (Boeve et al., 2007; Buddhala et al., 2015).
Multiple system atrophy (MSA) is a rare type of neurodegenerative disorders characterized by varied combinations of autonomic (orthostatic or bladder) with motor (parkinsonian or cerebellar dysfunction; Tsuchiya et al., 2020). Patients with MSA could present Parkinson-like motor symptoms and some similar LUTS. MSA may initially present with bladder dysfunction, particularly urinary retention. Over 90% of patients with MSA could have LUTS, which are more prevalent and severe than those with PD (Sakakibara et al., 2000; Yamamoto et al., 2011). About 50% of patients with MSA could also develop OAB symptoms (Roy et al., 2020). Moreover, DO could be confirmed in 33–100% patients with MSA during urodynamic investigations (Ogawa et al., 2017). The occurrence of OAB symptoms and other LUTS may arise from lesions in the area relevant to micturition, including locus coeruleus, prefrontal-basal ganglia D1 dopaminergic pathway, cerebellum, raphe serotonergic pathway, and frontal cortex (Gilman et al., 2008; Cykowski et al., 2015). Tsuchiya et al. (2020) also elucidated that DO occurred independently from motor disorder in MSA.
Spinal cord injury (SCI) arises from traumatic and non-traumatic events, with an annual incidence of up to 40 cases per million people. The prevalence of LUTS ranges from 20 to 88.3% in patients with traumatic SCI and 5.9% to 90% in patients with non-traumatic SCI (Ruffion et al., 2013; Fergany et al., 2017). The most common finding during urodynamic studies is DO in patients with SCI, with a prevalence ranging from 11 to 85% (Ruffion et al., 2013). Detrusor overactivity usually emerges when spinal reflexes return.
The appearance of LUTS, including OAB symptoms, in patients with SCI possibly derive from the impaired communication between the cerebral and spinal circuits that coordinate bladder and urethra activities (de Groat et al., 2015). The degree of urinary symptoms is related to the disease process itself, site of affected, and severity of neurological impairment. Suprapontine or suprasacral spinal cord lesions could induce storage dysfunctions, leading to DO. Lesions in the spinal cord may cause DESD and incomplete bladder emptying. Sacral or infrasacral lesions result in denervation of bladder and/or sphincters with incompetent sphincter and poorly sustained/absent detrusor contractions (Vichayanrat et al., 2021). As aforementioned, DO may be correlated with the emergence of a capsaicin-sensitive C-fiber-mediated spinal micturition reflex caused by a reorganization of synaptic connections in the spinal cord (Banakhar et al., 2012). As shown in chronic spinalized cats, administration of capsaicin could desensitize TRPV1-expressing C-fiber afferent pathways and completely block DO (de Groat et al., 1990; Cheng et al., 1999). Similarly, desensitization of C-fiber afferents by capsaicin pretreatment could also inhibit DO and DESD in chronic SCI rats (Cheng et al., 1995; Seki et al., 2004). In addition, cold-sensitive C-fiber afferents likely promote the occurrence of SCI-related DO and DESD mediated by transient receptor potential melastatin 8 (TRPM8) (Fall et al., 1990).
The hyperexcitability of C-fiber afferent pathway may be attributed to a wide variety of peripheral-to-central mechanisms. For instance, transient receptor potential ankyrin 1 (TRPA1) in the suburothelial nerve fibers may play a role in the C-fiber-mediated DO in SCI (Andrade et al., 2012). Increased activation of P2 × 2/3 receptors in the bladder is shown to stimulate bladder afferents and induce DO in rats following SCI (Smith et al., 2008; Munoz et al., 2012). After SCI, bladder afferent neurons seem to be more sensitive to the bladder stimuli due to pathophysiological alternations, such as ion channel transformations (Yoshimura and de Groat, 1997; Takahashi et al., 2013). Other changes in lumbosacral spinal cord, including increased expression of pituitary adenylate cyclase-activating polypeptide, could also contribute to the emergence of DO in SCI (Zvarova et al., 2005).
In addition, various other alternations in LUT, peripheral nerve system, and CNS also contribute to the development of DO after SCI, such as aberrant expressions/activation of M2 muscarinic receptor (Pontari et al., 2004), neurotrophic factors (Lamb et al., 2004), glutamate system (Yoshiyama et al., 1999), glycine (Miyazato et al., 2003), and γ-aminobutyric acid (Miyazato et al., 2008).
Overactive bladder (OAB) symptoms can also occur in other spinal cord diseases, including spina bifida (Dorsher and McIntosh, 2012), cauda equina lesions (Deffontaines Rufin et al., 2010), myelomeningocele (Dean and Long, 2011), spinal cord ischemia (Grosse et al., 2005), and spinal stenosis (Semins and Chancellor, 2004).
Dementia diseases, such as Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), and subacute combined degeneration, are also known as independent-risk factors for OAB symptoms (Takahashi et al., 2012). Previous studies reported a much higher prevalence of OAB in patients with AD aged 56–92 years (72.6%) than that in the general population, and almost twice as high as that in the general population above 75 years of age (Jung et al., 2017). Dementia with Lewy bodies is the second most common degenerative cause of dementia and OAB symptoms are more prevalent in DLB than in AD and PD (Ransmayr et al., 2008). Detrusor overactivity on urodynamic studies could be identified in 71.4–92% patients with DLB (Sakakibara et al., 2005; Ransmayr et al., 2008; Tateno et al., 2015). White matter disease is a chronic, bilateral form of cerebrovascular disease, leading to a high prevalence of OAB (up to 90%) (Sakakibara et al., 2014). The pathological mechanisms for OAB symptoms in dementia diseases remain unclear, but experimental and neuroimaging studies have suggested that the prefrontal cortex is critical for the higher control of voiding and enhanced bladder sensation might also result from altered central micturition circuit (Tsunoyama et al., 2011).
Peripheral neuropathy refers to a broad range of disorders causing damage and dysfunction of the nerves of the peripheral nervous system (Barrell and Smith, 2019). The damage to the peripheral nerves related to micturition reflex could bring about various types of LUTS including OAB symptoms. For example, about 75% of patients with Guillain-Barré syndrome developed micturition problems and both DO and DU were commonly seen in urodynamic analysis (Sakakibara et al., 2009). Patients with chronic inflammatory demyelinating polyneuropathy (CIDP) reported less LUTS in comparison to Guillain-Barré syndrome and the rate of LUTS ranged from 2 to 25%. Voiding difficulty and urinary urgency are the major urinary symptoms in CIDP. Charcot-Marie-Tooth (CMT), a type of hereditary peripheral neuropathy, may also occur with OAB symptoms, possibly due to the elevated transient receptor potential vanilloid 4 activity (Nilius and Owsianik, 2010). Thus, LUTS are usually rare in patients with CMT and CIDP. A careful exclusion of urological comorbidities and other neurological conditions, such as stroke and spinal stenosis, is required in patients with CMT and CIDP who present with LUTS.
Other brain lesions are also relevant to OAB symptoms, including cerebral palsy, pituitary adenoma compressing the hypothalamus (Yamamoto et al., 2005b), skull base chordoma (Akhavan-Sigari et al., 2014), traumatic brain injury (Sakakibara, 2015), and cerebrovascular accident (Gupta et al., 2009). The incidence of LUTS in these patients ranges from 14 to 53%, mostly OAB symptoms, and is much higher when the frontal cortex is involved (Sakakibara, 2015). As mentioned above, supraspinal lesions can lead to neurogenic OAB symptoms due to the loss of inhibition control on the micturition reflex or the decreased capacity to functionally integrate afferent information. Besides, hypothalamic lesions can lead to severe LUT dysfunction in both the storage and voiding phases of micturition, suggesting the crucial role of the hypothalamus in regulating micturition in humans (Yamamoto et al., 2005b).
Additionally, OAB symptoms could occur in patients with infections in the nervous system, including meningitis-retention syndrome (Tateno et al., 2011), human T-lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis (Santos et al., 2012; Rahnama’i, 2019), and human immunodeficiency virus (HIV)-associated neuropathy (Hermieu et al., 1996). Individuals with Machado-Joseph disease (Musegante et al., 2011) could also report OAB symptoms.
The etiology of OAB is multifactorial and may arise from a broad spectrum of medical conditions, in particular neurogenic ones. This study provided a comprehensive overview of OAB-related neurogenic disorders and summarized the compensatory mechanisms underlying the emergence of OAB symptoms in these neurogenic disorders. This may provide a robust rationale for therapy, as it could tackle several mechanisms increasing the chance of therapeutic success. However, given limited publications could present neurogenic OAB symptoms totally and clearly; further studies including etiology, epidemiology, and treatment are required.
YG conceived the idea for the manuscript. CQ drafted the manuscript with significant contribution from YG. YW revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China (grant no. 81800669) and from the Natural Science Foundation of Hunan Province (grant no. 2021JJ40829) to YG.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abrams, P., Cardozo, L., Fall, M., Griffiths, D., Rosier, P., Ulmsten, U., et al. (2002). The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol. Urodyn. 21, 167–178. doi: 10.1002/nau.10052
Abrams, P., Cardozo, L., Fall, M., Griffiths, D., Rosier, P., Ulmsten, U., et al. (2003). The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61, 37–49. doi: 10.1016/s0090-4295(02)02243-4
Akhavan-Sigari, R., Abili, M., Rohde, V., and Tezval, H. (2014). The influence of skull base chordoma on lower urinary tract symptoms. Urology 83, 756–761. doi: 10.1016/j.urology.2013.12.018
Akkoç, Y., Ersöz, M., Yüceyar, N., Tunç, H., Köklü, K., Yoldaş, T. K., et al. (2016). Overactive bladder symptoms in patients with multiple sclerosis: frequency, severity, diagnosis and treatment. J. Spinal Cord Med. 39, 229–233. doi: 10.1179/2045772315y.0000000021
Albanese, A., Jenner, P., Marsden, C. D., and Stephenson, J. D. (1988). Bladder hyperreflexia induced in marmosets by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurosci. Lett. 87, 46–50. doi: 10.1016/0304-3940(88)90143-7
Aldamanhori, R., Osman, N. I., and Chapple, C. R. (2018). Underactive bladder: pathophysiology and clinical significance. Asian J. Urol. 5, 17–21. doi: 10.1016/j.ajur.2017.02.003
Altmann, D. (2005). Evaluating the evidence for multiple sclerosis as an autoimmune disease. Arch. Neurol. 62, 688–689. doi: 10.1001/archneur.62.4.688-a
Andrade, E. L., Meotti, F. C., and Calixto, J. B. (2012). TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 133, 189–204. doi: 10.1016/j.pharmthera.2011.10.008
Apostolidis, A., Popat, R., Yiangou, Y., Cockayne, D., Ford, A. P., Davis, J. B., et al. (2005). Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J. Urol. 174, 977–982. doi: 10.1097/01.ju.0000169481.42259.54
Balzarro, M., Rubilotta, E., Mancini, V., Trabacchin, N., Oppezzi, L., Li Marzi, V., et al. (2019). Impact of overactive bladder-wet syndrome on female sexual function: a systematic review and meta-analysis. Sex. Med. Rev. 7, 565–574. doi: 10.1016/j.sxmr.2019.05.002
Banakhar, M. A., Al-Shaiji, T. F., and Hassouna, M. M. (2012). Pathophysiology of overactive bladder. Int. Urogynecol. J. 23, 975–982. doi: 10.1007/s00192-012-1682-6
Barone, P., Antonini, A., Colosimo, C., Marconi, R., Morgante, L., Avarello, T. P., et al. (2009). The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 24, 1641–1649. doi: 10.1002/mds.22643
Barrell, K., and Smith, A. G. (2019). Peripheral neuropathy. Med. Clin. North Am. 103, 383–397. doi: 10.1016/j.mcna.2018.10.006
Behr-Roussel, D., Oger, S., Caisey, S., Sandner, P., Bernabé, J., Alexandre, L., et al. (2011). Vardenafil decreases bladder afferent nerve activity in unanesthetized, decerebrate, spinal cord-injured rats. Eur. Urol. 59, 272–279. doi: 10.1016/j.eururo.2010.10.037
Blackett, H., Walker, R., and Wood, B. (2009). Urinary dysfunction in Parkinson’s disease: a review. Parkinson. Relat. Disord. 15, 81–87. doi: 10.1016/j.parkreldis.2007.10.016
Blok, B., Groen, J., Bosch, J., Veltman, D., and Lammertsma, A. (2006). Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int. 98, 1238–1243. doi: 10.1111/j.1464-410X.2006.06521.x
Boeve, B. F., Silber, M. H., Saper, C. B., Ferman, T. J., Dickson, D. W., Parisi, J. E., et al. (2007). Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130(Pt 11), 2770–2788. doi: 10.1093/brain/awm056
Brady, C. M., Apostolidis, A., Yiangou, Y., Baecker, P. A., Ford, A. P., Freeman, A., et al. (2004). P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur. Urol. 46, 247–253. doi: 10.1016/j.eururo.2003.12.017
Buddhala, C., Loftin, S. K., Kuley, B. M., Cairns, N. J., Campbell, M. C., Perlmutter, J. S., et al. (2015). Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann. Clin. Transl. Neurol. 2, 949–959. doi: 10.1002/acn3.246
Campeau, L., Soler, R., and Andersson, K. (2011). Bladder dysfunction and parkinsonism: current pathophysiological understanding and management strategies. Curr. Urol. Rep. 12, 396–403. doi: 10.1007/s11934-011-0219-8
Çetinel, B., Tarcan, T., Demirkesen, O., Özyurt, C., Şen, Ý, Erdoğan, S., et al. (2013). Management of lower urinary tract dysfunction in multiple sclerosis: a systematic review and Turkish consensus report. Neurourol. Urodyn. 32, 1047–1057. doi: 10.1002/nau.22374
Chancellor, M., and de Groat, W. (1999). Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J. Urol. 162, 3–11. doi: 10.1097/00005392-199907000-00002
Chapple, C. R., Nazir, J., Hakimi, Z., Bowditch, S., Fatoye, F., Guelfucci, F., et al. (2017). Persistence and adherence with mirabegron versus antimuscarinic agents in patients with overactive bladder: a retrospective observational Study in UK Clinical Practice. Eur. Urol. 72, 389–399. doi: 10.1016/j.eururo.2017.01.037
Charil, A., Zijdenbos, A., Taylor, J., Boelman, C., Worsley, K., Evans, A., et al. (2003). Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: application to 452 patient data sets. NeuroImage 19, 532–544. doi: 10.1016/s1053-8119(03)00117-4
Cheng, C. L., Liu, J. C., Chang, S. Y., Ma, C. P., and de Groat, W. C. (1999). Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am. J. Physiol. 277, R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786
Cheng, C.-L., Ma, C.-P., and de Groat, W. C. (1995). Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 678, 40–48. doi: 10.1016/0006-8993(95)00212-9
Cornu, J. N. (2017). Re: onabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: a randomized clinical trial. Eur. Urol. 71, 988–989. doi: 10.1016/j.eururo.2017.02.013
Coyne, K. S., Sexton, C. C., Thompson, C. L., Milsom, I., Irwin, D., Kopp, Z. S., et al. (2009). The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the epidemiology of LUTS (EpiLUTS) study. BJU Int. 104, 352–360. doi: 10.1111/j.1464-410X.2009.08427.x
Cykowski, M. D., Coon, E. A., Powell, S. Z., Jenkins, S. M., Benarroch, E. E., Low, P. A., et al. (2015). Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138(Pt 8), 2293–2309. doi: 10.1093/brain/awv114
Danzer, E., Schwarz, U., Wehrli, S., Radu, A., Adzick, N. S., and Flake, A. W. (2005). Retinoic acid induced myelomeningocele in fetal rats: characterization by histopathological analysis and magnetic resonance imaging. Exp. Neurol. 194, 467–475. doi: 10.1016/j.expneurol.2005.03.011
de Carvalho, F. L., Gomes, C. M., Apostolos-Pereira, S. L., Bessa, J. Jr., Pinheiro, M., Marchiori, P. E., et al. (2016). Voiding dysfunction in patients with neuromyelitis optica spectrum disorders. Neurourol. Urodyn. 35, 39–43. doi: 10.1002/nau.22667
de Groat, W. C. (1997). A neurologic basis for the overactive bladder. Urology 50(6A Suppl.), 36–52. doi: 10.1016/s0090-4295(97)00587-6
de Groat, W. C., Griffiths, D., and Yoshimura, N. (2015). Neural control of the lower urinary tract. Compr. Physiol. 5, 327–396. doi: 10.1002/cphy.c130056
de Groat, W. C., Kawatani, M., Hisamitsu, T., Cheng, C. L., Ma, C. P., Thor, K., et al. (1990). Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Autonom. Nervous Syst. 30, S71–S77. doi: 10.1016/0165-1838(90)90105-R
Dean, G. E., and Long, C. (2011). Updates in the management of the overactive bladder in patients with myelomeningocele. Curr. Urol. Rep. 12, 413–418. doi: 10.1007/s11934-011-0218-9
Declemy, A., Haddad, R., Chesnel, C., Charlanes, A., Le Breton, F., Sheikh Ismael, S., et al. (2021). Prevalence of comorbidities in multiple sclerosis patients with neurogenic bladder. Prog. Urol. 31, 732–738. doi: 10.1016/j.purol.2020.10.011
Deffontaines Rufin, S., Jousse, M., Verollet, D., Guinet, A., Ismael, S. S., and Amarenco, G. (2010). Cold perception of the bladder during ice water test. Study on 120 patients. Ann. Phys. Rehabil. Med. 53, 559–567. doi: 10.1016/j.rehab.2010.08.031
Defreitas, G. A., Lemack, G. E., Zimmern, P. E., Dewey, R. B., Roehrborn, C. G., and O’Suilleabhain, P. E. (2003). Distinguishing neurogenic from non-neurogenic detrusor overactivity: a urodynamic assessment of lower urinary tract symptoms in patients with and without Parkinson’s disease. Urology 62, 651–655. doi: 10.1016/s0090-4295(03)00507-7
Dmochowski, R. R., and Newman, D. K. (2007). Impact of overactive bladder on women in the United States: results of a national survey. Curr. Med. Res. Opin. 23, 65–76. doi: 10.1185/030079907x159533
Dorsher, P. T., and McIntosh, P. M. (2012). Neurogenic bladder. Adv. Urol. 2012:816274. doi: 10.1155/2012/816274
Duong, V., Iwamoto, A., Pennycuff, J., Kudish, B., and Iglesia, C. (2021). A systematic review of neurocognitive dysfunction with overactive bladder medications. Int. Urogynecol. J. 32, 2693–2702. doi: 10.1007/s00192-021-04909-5
Dutta, R., and Trapp, B. D. (2007). Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 68(22 Suppl. 3), S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32
Ethans, K. D., Casey, A. R., Bard, R. J., and Namaka, M. P. (2014). Neurogenic overactive bladder in spinal cord injury and multiple sclerosis: role of onabotulinumtoxinA. Degener. Neurol. Neuromuscul. Dis. 4, 65–75. doi: 10.2147/dnnd.S40349
Fall, M., Lindström, S., and Mazières, L. (1990). A bladder-to-bladder cooling reflex in the cat. J. Physiol. 427, 281–300. doi: 10.1113/jphysiol.1990.sp018172
Fergany, L. A., Shaker, H., Arafa, M., and Elbadry, M. S. (2017). Does sacral pulsed electromagnetic field therapy have a better effect than transcutaneous electrical nerve stimulation in patients with neurogenic overactive bladder? Arab. J. Urol. 15, 148–152. doi: 10.1016/j.aju.2017.01.007
Finazzi-Agrò, E., Rocchi, C., Pachatz, C., Petta, F., Spera, E., Mori, F., et al. (2009). Percutaneous tibial nerve stimulation produces effects on brain activity: study on the modifications of the long latency somatosensory evoked potentials. Neurourol. Urodyn. 28, 320–324. doi: 10.1002/nau.20651
Gani, J., and Hennessey, D. (2017). The underactive bladder: diagnosis and surgical treatment options. Transl. Androl. Urol. 6, (Suppl. 2), S186–S195. doi: 10.21037/tau.2017.04.07
Gilman, S., Wenning, G. K., Low, P. A., Brooks, D. J., Mathias, C. J., Trojanowski, J. Q., et al. (2008). Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71, 670–676. doi: 10.1212/01.wnl.0000324625.00404.15
Griffiths, D. (2015). Neural control of micturition in humans: a working model. Nat. Rev. Urol. 12, 695–705. doi: 10.1038/nrurol.2015.266
Griffiths, D., Clarkson, B., Tadic, S., and Resnick, N. (2015). Brain mechanisms underlying urge incontinence and its response to pelvic floor muscle training. J. Urol. 194, 708–715. doi: 10.1016/j.juro.2015.03.102
Griffiths, D., and Tadic, S. D. (2008). Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol. Urodyn. 27, 466–474. doi: 10.1002/nau.20549
Groenendijk, P. M., Heesakkers, J. P., and Lycklama, A. N. A. A. (2007). Urethral instability and sacral nerve stimulation-a better parameter to predict efficacy? J. Urol. 178, 568–572. doi: 10.1016/j.juro.2007.03.120
Grosse, J., Kramer, G., and Stöhrer, M. (2005). Success of repeat detrusor injections of botulinum a toxin in patients with severe neurogenic detrusor overactivity and incontinence. Eur. Urol. 47, 653–659. doi: 10.1016/j.eururo.2004.11.009
Gupta, A., Taly, A. B., Srivastava, A., and Thyloth, M. (2009). Urodynamics post stroke in patients with urinary incontinence: is there correlation between bladder type and site of lesion? Ann. Indian Acad. Neurol. 12, 104–107. doi: 10.4103/0972-2327.53078
Hermieu, J., Delmas, V., and Boccon-Gibod, L. (1996). Micturition disturbances and human immunodeficiency virus infection. J. Urol. 156, 157–159. doi: 10.1097/00005392-199607000-00050
Jung, H., Choi, D., Lee, S., Cho, S., Na, H., and Park, M. (2017). Correlation between overactive bladder symptom score and neuropsychological parameters in Alzheimer’s disease patients with lower urinary tract symptom. Int. Braz. J. Urol. 43, 256–263. doi: 10.1590/s1677-5538.Ibju.2015.0664
Kakizaki, H., Kita, M., and Wada, N. (2011). Models for sensory neurons of dorsal root ganglia and stress urinary incontinence. Neurourol. Urodyn. 30, 653–657. doi: 10.1002/nau.21138
Karsenty, G., Denys, P., Amarenco, G., De Seze, M., Gamé, X., Haab, F., et al. (2008). Botulinum toxin A (Botox) intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic literature review. Eur. Urol. 53, 275–287. doi: 10.1016/j.eururo.2007.10.013
Kirschner-Hermanns, R., Anding, R., Rosier, P., Birder, L., Andersson, K. E., and Djurhuus, J. C. (2016). Fundamentals and clinical perspective of urethral sphincter instability as a contributing factor in patients with lower urinary tract dysfunction—ICI-RS 2014. Neurourol. Urodyn. 35, 318–323. doi: 10.1002/nau.22815
Kitada, T., Tong, Y., Gautier, C. A., and Shen, J. (2009). Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J. Neurochem. 111, 696–702. doi: 10.1111/j.1471-4159.2009.06350.x
Kitta, T., Chancellor, M., de Groat, W., Kuno, S., Nonomura, K., and Yoshimura, N. (2012). Suppression of bladder overactivity by adenosine A2A receptor antagonist in a rat model of Parkinson disease. J. Urol. 187, 1890–1897. doi: 10.1016/j.juro.2011.12.062
Kitta, T., Ouchi, M., Chiba, H., Higuchi, M., Togo, M., Abe-Takahashi, Y., et al. (2020). Animal model for lower urinary tract dysfunction in Parkinson’s disease. Int. J. Mol. Sci. 21:6520. doi: 10.3390/ijms21186520
Kitta, T., Yabe, I., Takahashi, I., Matsushima, M., Sasaki, H., and Shinohara, N. (2016). Clinical efficacy of istradefylline on lower urinary tract symptoms in Parkinson’s disease. Int. J. Urol. 23, 893–894. doi: 10.1111/iju.13160
Koutsis, G., Evangelopoulos, M. E., Sfagos, C., and Markianos, M. (2016). Neurochemical and neuroendocrine correlates of overactive bladder at first demyelinating episode. Neurourol. Urodyn. 35, 955–958. doi: 10.1002/nau.22834
Lamb, K., Gebhart, G. F., and Bielefeldt, K. (2004). Increased nerve growth factor expression triggers bladder overactivity. J. Pain 5, 150–156. doi: 10.1016/j.jpain.2004.01.001
Lee, B. D., Shin, J. H., VanKampen, J., Petrucelli, L., West, A. B., Ko, H. S., et al. (2010). Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat. Med. 16, 998–1000. doi: 10.1038/nm.2199
Lee, C. K., Tsai, C. P., Liao, T. L., Huang, W. N., Chen, Y. H., Lin, C. H., et al. (2019). Overactive bladder and bladder pain syndrome/interstitial cystitis in primary Sjogren’s syndrome patients: a nationwide population-based study. PLoS One 14:e0225455. doi: 10.1371/journal.pone.0225455
Mancini, V., Tarcan, T., Serati, M., Wyndaele, M., Carrieri, G., and Abrams, P. (2020). Is coexistent overactive-underactive bladder (with or without detrusor overactivity and underactivity) a real clinical syndrome? ICI-RS 2019. Neurourol. Urodyn. 39(Suppl. 3), S50–S59. doi: 10.1002/nau.24311
Matsuoka, Y., Vila, M., Lincoln, S., McCormack, A., Picciano, M., LaFrancois, J., et al. (2001). Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol. Dis. 8, 535–539. doi: 10.1006/nbdi.2001.0392
McDonald, C., Winge, K., and Burn, D. J. (2017). Lower urinary tract symptoms in Parkinson’s disease: prevalence, aetiology and management. Parkinson. Relat. Disord. 35, 8–16. doi: 10.1016/j.parkreldis.2016.10.024
McMillan, M. T., Pan, X.-Q., Smith, A. L., Newman, D. K., Weiss, S. R., Michael, R., et al. (2014). Coronavirus-induced demyelination of neural pathways triggers neurogenic bladder overactivity in a mouse model of multiple sclerosis. Am. J. Physiol. Renal Physiol. 307, F612–F622. doi: 10.1152/ajprenal.00151.2014
Minardi, D., and Muzzonigro, G. (2005). Lower urinary tract and bowel disorders and multiple sclerosis: role of sacral neuromodulation: a preliminary report. Neuromodulation 8, 176–181. doi: 10.1111/j.1525-1403.2005.05236.x
Miyazato, M., Sasatomi, K., Hiragata, S., Sugaya, K., Chancellor, M. B., de Groat, W. C., et al. (2008). GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J. Urol. 179, 1178–1183. doi: 10.1016/j.juro.2007.10.030
Miyazato, M., Sugaya, K., Nishijima, S., Ashitomi, K., Hatano, T., and Ogawa, Y. (2003). Inhibitory effect of intrathecal glycine on the micturition reflex in normal and spinal cord injury rats. Exp. Neurol. 183, 232–240. doi: 10.1016/s0014-4886(03)00175-4
Moss, H. E., Tansey, E. M., and Burnstock, G. (1989). Abnormalities of responses to autonomic stimulation in the mouse urinary bladder associated with Semliki Forest virus-induced demyelination. J. Urol. 142, 850–854. doi: 10.1016/s0022-5347(17)38929-2
Munoz, A., Somogyi, G., Boone, T., Ford, A., and Smith, C. (2012). Modulation of bladder afferent signals in normal and spinal cord-injured rats by purinergic P2X3 and P2X2/3 receptors. BJU Int. 110, E409–E414. doi: 10.1111/j.1464-410X.2012.11189.x
Musegante, A. F., Almeida, P. N., Barboza, A. L., and Barroso, U. Jr. (2011). Urinary symptoms and urodynamic findings in patients with Machado-Joseph disease. J. Neurol. 258, 623–626. doi: 10.1007/s00415-010-5810-2
Nilius, B., and Owsianik, G. (2010). Channelopathies converge on TRPV4. Nat. Genet. 42, 98–100. doi: 10.1038/ng0210-98
Ogawa, T., Sakakibara, R., Kuno, S., Ishizuka, O., Kitta, T., and Yoshimura, N. (2017). Prevalence and treatment of LUTS in patients with Parkinson disease or multiple system atrophy. Nat. Rev. Urol. 14, 79–89. doi: 10.1038/nrurol.2016.254
Panicker, J., Nagaraja, D., Kovoor, J., Nair, K., and Subbakrishna, D. (2009). Lower urinary tract dysfunction in acute disseminated encephalomyelitis. Multiple Sclerosis 15, 1118–1122. doi: 10.1177/1352458509106614
Panicker, J. N. (2020). Neurogenic bladder: epidemiology, diagnosis, and management. Semin. Neurol. 40, 569–579. doi: 10.1055/s-0040-1713876
Panicker, J. N., Fowler, C. J., and Kessler, T. M. (2015). Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol. 14, 720–732. doi: 10.1016/s1474-4422(15)00070-8
Park, J., Kim, S. Y., Cha, G. H., Lee, S. B., Kim, S., and Chung, J. (2005). Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene 361, 133–139. doi: 10.1016/j.gene.2005.06.040
Petry, K. G., Boullerne, A. I., Pousset, F., Brochet, B., Caillé, J. M., and Dousset, V. (2000). Experimental allergic encephalomyelitis animal models for analyzing features of multiple sclerosis. Pathol. Biol. 48, 47–53.
Peyronnet, B., Mironska, E., Chapple, C., Cardozo, L., Oelke, M., Dmochowski, R., et al. (2019). A comprehensive review of overactive bladder pathophysiology: on the way to tailored treatment. Eur. Urol. 75, 988–1000. doi: 10.1016/j.eururo.2019.02.038
Pontari, M. A., Braverman, A. S., and Ruggieri, M. R. Sr. (2004). The M2 muscarinic receptor mediates in vitro bladder contractions from patients with neurogenic bladder dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R874–R880. doi: 10.1152/ajpregu.00391.2003
Pozzilli, C., Grasso, M., Bastianello, S., Anzini, A., Salvetti, M., Bozzao, L., et al. (1992). Structural brain correlates of neurologic abnormalities in multiple sclerosis. Eur. Neurol. 32, 228–230. doi: 10.1159/000116829
Przydacz, M., Chlosta, M., Golabek, T., and Chlosta, P. (2021). Population-based study of prevalence, bother and behavior related to treatment for lower urinary tract symptoms and overactive bladder among polish neurogenic patients. Brain Sci. 11:712. doi: 10.3390/brainsci11060712
Rahnama’i, M. S. (2019). Neuromodulation for functional bladder disorders in patients with multiple sclerosis. Mult. Scler. 26, 1274–1280. doi: 10.1177/1352458519894714
Rana, A. Q., Paul, D. A., Qureshi, A. M., Ghazi, A., Alenezi, S., Rana, M. A., et al. (2015). Association between nocturia and anxiety in Parkinson’s disease. Neurol. Res. 37, 563–567. doi: 10.1179/1743132815y.0000000010
Ransmayr, G. N., Holliger, S., Schletterer, K., Heidler, H., Deibl, M., Poewe, W., et al. (2008). Lower urinary tract symptoms in dementia with Lewy bodies, Parkinson disease, and Alzheimer disease. Neurology 70, 299–303. doi: 10.1212/01.wnl.0000296826.61499.26
Roy, H. A., Nettleton, J., Blain, C., Dalton, C., Farhan, B., Fernandes, A., et al. (2020). Assessment of patients with lower urinary tract symptoms where an undiagnosed neurological disease is suspected: a report from an International Continence Society consensus working group. Neurourol. Urodyn. 39, 2535–2543. doi: 10.1002/nau.24469
Ruffion, A., Castro-Diaz, D., Patel, H., Khalaf, K., Onyenwenyi, A., Globe, D., et al. (2013). Systematic review of the epidemiology of urinary incontinence and detrusor overactivity among patients with neurogenic overactive bladder. Neuroepidemiology 41, 146–155. doi: 10.1159/000353274
Sakakibara, R. (2015). Lower urinary tract dysfunction in patients with brain lesions. Handb. Clin. Neurol. 130, 269–287. doi: 10.1016/b978-0-444-63247-0.00015-8
Sakakibara, R. (2019). Neurogenic lower urinary tract dysfunction in multiple sclerosis, neuromyelitis optica, and related disorders. Clin. Autonomic Res. 29, 313–320. doi: 10.1007/s10286-018-0551-x
Sakakibara, R., Hattori, T., Uchiyama, T., Kita, K., Asahina, M., Suzuki, A., et al. (2000). Urinary dysfunction and orthostatic hypotension in multiple system atrophy: which is the more common and earlier manifestation? J. Neurol. Neurosurg. Psychiatry 68, 65–69. doi: 10.1136/jnnp.68.1.65
Sakakibara, R., Hattori, T., Uchiyama, T., and Yamanishi, T. (1999). Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J. Neurol. Neurosurg. Psychiatry 67, 658–660. doi: 10.1136/jnnp.67.5.658
Sakakibara, R., Ito, T., Uchiyama, T., Asahina, M., Liu, Z., Yamamoto, T., et al. (2005). Lower urinary tract function in dementia of Lewy body type. J. Neurol. Neurosurg. Psychiatry 76, 729–732. doi: 10.1136/jnnp.2004.046243
Sakakibara, R., Ito, T., Yamamoto, T., Uchiyama, T., Yamanishi, T., Kishi, M., et al. (2013). Depression, anxiety and the bladder. Low Urin. Tract. Symptoms 5, 109–120. doi: 10.1111/luts.12018
Sakakibara, R., Panicker, J., Fowler, C. J., Tateno, F., Kishi, M., Tsuyusaki, Y., et al. (2014). Is overactive bladder a brain disease? The pathophysiological role of cerebral white matter in the elderly. Int. J. Urol. 21, 33–38. doi: 10.1111/iju.12288
Sakakibara, R., Shinotoh, H., Uchiyama, T., Yoshiyama, M., Hattori, T., and Yamanishi, T. (2001). SPECT imaging of the dopamine transporter with [(123)I]-beta-CIT reveals marked decline of nigrostriatal dopaminergic function in Parkinson’s disease with urinary dysfunction. J. Neurol. Sci. 187, 55–59. doi: 10.1016/s0022-510x(01)00521-4
Sakakibara, R., Uchiyama, T., Kuwabara, S., Mori, M., Ito, T., Yamamoto, T., et al. (2009). Prevalence and mechanism of bladder dysfunction in Guillain-Barré Syndrome. Neurourol. Urodyn. 28, 432–437. doi: 10.1002/nau.20663
Santos, S. B., Oliveira, P., Luna, T., Souza, A., Nascimento, M., Siqueira, I., et al. (2012). Immunological and viral features in patients with overactive bladder associated with human T-cell lymphotropic virus type 1 infection. J. Med. Virol. 84, 1809–1817. doi: 10.1002/jmv.23341
Schapira, A., Cooper, J., Dexter, D., Jenner, P., Clark, J., and Marsden, C. (1989). Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1:1269. doi: 10.1016/s0140-6736(89)92366-0
Seki, S., Sasaki, K., Igawa, Y., Nishizawa, O., Chancellor, M. B., de Groat, W. C., et al. (2004). Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J. Urol. 171, 478–482. doi: 10.1097/01.ju.0000088340.26588.74
Semins, M. J., and Chancellor, M. B. (2004). Diagnosis and management of patients with overactive bladder syndrome and abnormal detrusor activity. Nat. Clin. Pract. Urol. 1, 78–84. doi: 10.1038/ncpuro0054
Shafik, A., Shafik, A. A., El-Sibai, O., and Ahmed, I. (2003). Role of positive urethrovesical feedback in vesical evacuation. The concept of a second micturition reflex: the urethrovesical reflex. World J. Urol. 21, 167–170. doi: 10.1007/s00345-003-0340-5
Shah, M. B., and Weiss, J. P. (2012). Medical causes of overactive bladder. Curr. Bladder Dysfunct. Rep. 8, 51–56. doi: 10.1007/s11884-012-0168-1
Shaker, H., Mourad, M. S., Elbialy, M. H., and Elhilali, M. (2003). Urinary bladder hyperreflexia: a rat animal model. Neurourol. Urodyn. 22, 693–698. doi: 10.1002/nau.10147
Simola, N., Morelli, M., and Carta, A. R. (2007). The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 11, 151–167. doi: 10.1007/bf03033565
Smith, C., Gangitano, D., Munoz, A., Salas, N., Boone, T., Aoki, K., et al. (2008). Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem. Int. 52, 1068–1075. doi: 10.1016/j.neuint.2007.11.006
Steers, W. D., Herschorn, S., Kreder, K. J., Moore, K., Strohbehn, K., Yalcin, I., et al. (2007). Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int. 100, 337–345. doi: 10.1111/j.1464-410X.2007.06980.x
Suzuki Bellucci, C., Wöllner, J., Gregorini, F., Birnböck, D., Kozomara, M., Mehnert, U., et al. (2012). External urethral sphincter pressure measurement: an accurate method for the diagnosis of detrusor external sphincter dyssynergia? PLoS One 7:e37996. doi: 10.1371/journal.pone.0037996
Tadic, S. D., Griffiths, D., Schaefer, W., Murrin, A., Clarkson, B., and Resnick, N. M. (2012). Brain activity underlying impaired continence control in older women with overactive bladder. Neurourol. Urodyn. 31, 652–658. doi: 10.1002/nau.21240
Takahashi, O., Sakakibara, R., Panicker, J., Fowler, C. J., Tateno, F., Kishi, M., et al. (2012). White matter lesions or Alzheimer’s disease: which contributes more to overactive bladder and incontinence in elderly adults with dementia? J. Am. Geriatr. Soc. 60, 2370–2371. doi: 10.1111/jgs.12004
Takahashi, R., Yoshizawa, T., Yunoki, T., Tyagi, P., Naito, S., de Groat, W., et al. (2013). Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 α-subunit in rats with spinal cord injury. J. Urol. 190, 2296–2304. doi: 10.1016/j.juro.2013.07.058
Tarhan, F. (2013). Increased overactive bladder symptoms in primary sjogren’s syndrome. World J. Nephrol. Urol. 2, 10–14. doi: 10.4021/wjnu85w
Tateno, F., Sakakibara, R., Ogata, T., Kishi, M., Tsuyusaki, Y., Takahashi, O., et al. (2015). Lower urinary tract function in dementia with Lewy bodies (DLB). Mov. Disord. 30, 411–415. doi: 10.1002/mds.25985
Tateno, F., Sakakibara, R., Sugiyama, M., Takahashi, O., Kishi, M., Ogawa, E., et al. (2011). Meningitis-retention syndrome: first case of urodynamic follow-up. Intern. Med. 50, 1329–1332. doi: 10.2169/internalmedicine.50.4747
Tsuchiya, A., Terayama, K., Sakakibara, R., Panicker, J., Tateno, F., Aiba, Y., et al. (2020). Urodynamic and gait analyses in multiple system atrophy. J. Neurol. Sci. 411:116676. doi: 10.1016/j.jns.2020.116676
Tsunoyama, K., Sakakibara, R., Yamaguchi, C., Uchiyama, T., Yamamoto, T., Yamanishi, T., et al. (2011). Pathogenesis of reduced or increased bladder sensation. Neurourol. Urodyn. 30, 339–343. doi: 10.1002/nau.20953
Turner, W., and Brading, A. (1997). Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol. Ther. 75, 77–110. doi: 10.1016/s0163-7258(97)00038-7
Uren, A., Cotterill, N., Harding, C., Hillary, C., Chapple, C., Klaver, M., et al. (2017). Qualitative exploration of the patient experience of underactive bladder. Eur. Urol. 72, 402–407. doi: 10.1016/j.eururo.2017.03.045
Vichayanrat, E., Hentzen, C., Batla, A., Simeoni, S., Iodice, V., and Panicker, J. N. (2021). Lower urinary tract dysfunction in Parkinsonian syndromes. Neurol. Sci. 42, 4045–4054. doi: 10.1007/s10072-021-05411-y
Wang, F., Jackson, M. W., Maughan, V., Cavill, D., Smith, A. J., Waterman, S. A., et al. (2004). Passive transfer of Sjogren’s syndrome IgG produces the pathophysiology of overactive bladder. Arthr. Rheum. 50, 3637–3645. doi: 10.1002/art.20625
Wang, T., Huang, W., and Zhang, Y. (2016). Clinical characteristics and urodynamic analysis of urinary dysfunction in multiple sclerosis. Chin. Med. J. 129, 645–650. doi: 10.4103/0366-6999.177970
Weissbart, S., Pechersky, D., Malykhina, A., Bavaria, T., Parrillo, L., Arya, L., et al. (2017). The impact of pontine disease on lower urinary tract symptoms in patients with multiple sclerosis. Neurourol. Urodyn. 36, 453–456. doi: 10.1002/nau.22953
Winge, K., and Fowler, C. (2006). Bladder dysfunction in Parkinsonism: mechanisms, prevalence, symptoms, and management. Mov. Disord. 21, 737–745. doi: 10.1002/mds.20867
Winge, K., Friberg, L., Werdelin, L., Nielsen, K., and Stimpel, H. (2005). Relationship between nigrostriatal dopaminergic degeneration, urinary symptoms, and bladder control in Parkinson’s disease. Eur. J. Neurol. 12, 842–850. doi: 10.1111/j.1468-1331.2005.01087.x
Winge, K., and Nielsen, K. (2012). Bladder dysfunction in advanced Parkinson’s disease. Neurourol. Urodyn. 31, 1279–1283. doi: 10.1002/nau.22237
Xu, D., Han, S., Wang, J., and Feng, J. (2019). Relationship between lower urinary tract dysfunction and clinical features in chinese Parkinson’s disease patients. Parkinsons Dis. 2019:6820937. doi: 10.1155/2019/6820937
Yamada, S., Ito, Y., Nishijima, S., Kadekawa, K., and Sugaya, K. (2018). Basic and clinical aspects of antimuscarinic agents used to treat overactive bladder. Pharmacol. Ther. 189, 130–148. doi: 10.1016/j.pharmthera.2018.04.010
Yamamoto, T., Sakakibara, R., Hashimoto, K., Nakazawa, K., Uchiyama, T., Liu, Z., et al. (2005a). Striatal dopamine level increases in the urinary storage phase in cats: an in vivo microdialysis study. Neuroscience 135, 299–303. doi: 10.1016/j.neuroscience.2005.06.007
Yamamoto, T., Sakakibara, R., Uchiyama, T., Liu, Z., Ito, T., Yamanishi, T., et al. (2005b). Lower urinary tract function in patients with pituitary adenoma compressing hypothalamus. J. Neurol. Neurosurg. Psychiatry 76, 390–394. doi: 10.1136/jnnp.2004.044644
Yamamoto, T., Sakakibara, R., Uchiyama, T., Yamaguchi, C., Nomura, F., Ito, T., et al. (2011). Pelvic organ dysfunction is more prevalent and severe in MSA-P compared to Parkinson’s disease. Neurourol. Urodyn. 30, 102–107. doi: 10.1002/nau.20948
Yokoyama, O., Komatsu, K., Ishiura, Y., Nakamura, Y., Morikawa, K., and Namiki, M. (1998). Change in bladder contractility associated with bladder overactivity in rats with cerebral infarction. J. Urol. 159, 577–580. doi: 10.1016/S0022-5347(01)63987-9
Yoshimura, N., and de Groat, W. (1997). Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J. Physiol. 503(Pt 2), 269–276. doi: 10.1111/j.1469-7793.1997.269bh.x
Yoshimura, N., Kuno, S., Chancellor, M. B., De Groat, W. C., and Seki, S. (2003). Dopaminergic mechanisms underlying bladder hyperactivity in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal pathway. Br. J. Pharmacol. 139, 1425–1432. doi: 10.1038/sj.bjp.0705388
Yoshiyama, M., Nezu, F. M., Yokoyama, O., Chancellor, M. B., and de Groat, W. C. (1999). Influence of glutamate receptor antagonists on micturition in rats with spinal cord injury. Exp. Neurol. 159, 250–257. doi: 10.1006/exnr.1999.7124
Yotsuyanagi, S., Narimoto, K., and Namiki, M. (2006). Mild brain ischemia produces bladder hyperactivity without brain damage in rats. Urol. Int. 77, 57–63. doi: 10.1159/000092936
Keywords: overactive bladder, nerve system disease, detrusor overactivity, lower urinary tract symptoms, etiology
Citation: Qin C, Wang Y and Gao Y (2021) Overactive Bladder Symptoms Within Nervous System: A Focus on Etiology. Front. Physiol. 12:747144. doi: 10.3389/fphys.2021.747144
Received: 25 July 2021; Accepted: 16 November 2021;
Published: 10 December 2021.
Edited by:
Guiming Liu, Case Western Reserve University, United StatesReviewed by:
Tatsuya Yamamoto, Chiba Prefectural University of Health Sciences, JapanCopyright © 2021 Qin, Wang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunliang Gao, WXVubGlhbmcuZ2FvQGNzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.