
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 27 September 2021
Sec. Reproduction
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.732709
This article is part of the Research Topic Endocrinology and COVID-19: A Cross-Disciplinary Topic, volume II View all 17 articles
Objectives: To explore the appropriate controlled ovarian hyperstimulation (COH) protocols in infertility patients who received the in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) treatments during the COVID-19 pandemic.
Materials and Methods: This retrospective cohort study evaluated the efficiency of the early follicular-phase long-acting GnRH-agonist long (EFLL) protocol (a new protocol developed by Chinese clinicians), prolonged pituitary down-regulation of EFLL protocol (Pro-EFLL), and the GnRH-ant protocol for couples meeting the study criteria between February 2020 and June 2020 who were treated by the First Affiliated Hospital of Zhengzhou University during the COVID-19 pandemic, and compared the pregnancy rates and miscarriage rates per fresh transfer cycle, number of retrieved oocytes, endometrial thickness on the day of hCG injection and the number of fertilized oocytes, mature oocytes, fertilized oocytes, and transferable embryos among the three protocols.
Results: We found that the prolonged pituitary down-regulation during the COVID-19 pandemic by utilizing a full-dose of GnRH-a administrated in infertility patients were no differences in clinical outcomes than other protocols, The prolonged pituitary down-regulation protocol and EFLL protocol were associated with a higher Endometrial thickness on the day of hCG injection (12.67 ± 2.21 vs. 12.09 ± 2.35 vs. 10.79 ± 2.38, P < 0.001), retrieved oocytes (14.49 ± 6.30 vs. 15.02 ± 7.93 vs. 10.06 ± 7.63, P < 0.001), mature oocytes (11.60 ± 5.71 vs. 11.96 ± 6.00 vs. 7.63 ± 6.50, P < 0.001), fertilized oocytes (9.14 ± 5.43 vs. 8.44 ± 5.34 vs. 5.42 ± 5.20, P < 0.001), and transferable embryos (4.87 ± 2.96 vs. 6.47 ± 5.12 vs. 3.00 ± 3.28 vs. P < 0.001) in the GnRH-antagonist protocol.
Conclusion: We recommend that patients start Gn injections 33–42 days after a pituitary downregulated full dose (3.75 mg) of gonadotropin-releasing hormone agonist during the COVID-19 pandemic, even a delay of 2–4 weeks does not affect the implantation rate. The study can provide a more detailed estimate and clinical management strategies for infertile couples during the COVID-19 pandemic.
The coronavirus disease-19 (COVID-19) pandemic started in late December 2019 in Wuhan, Hubei Province, China, and has since spread rapidly around the globe, with many countries being severely affected (Lai et al., 2020; Vermeulen et al., 2020). The disease as an acute respiratory infectious disease has been managed according to A class infectious diseases as stipulated in the Law of China. The Chinese government began enforcing social distancing, including restrictions on gatherings, public transportation and school closures limitations, including reproductive medicine procedures (Xia et al., 2020). During the special period of the epidemic, the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) have come together to jointly affirm the importance of continued reproductive research during the COVID-19 pandemic (Gianaroli et al., 2020; Veiga et al., 2020), committed to continuous monitoring of the effects of COVID-19 on reproduction, collecting data on infertility patients during the pandemic, and helping the majority of patients who seek treatment to ultimately become parents. However, there is no uniform standard on how to deal with infertile people and how to arrange medical treatment during this difficult time (Lupia et al., 2020; Pasquale et al., 2020; Veiga et al., 2020).
In the special period of the COVID-19 pandemic, it is necessary to develop or refine robust controlled ovarian hyperstimulation (COH) protocols to minimize exposure risks, to reduce the rate of cycle cancelations and to alleviate the financial and emotional burden of interrupting treatment for infertile couples due to the epidemic. To meet current needs, one full-dose depot of long-acting gonadotropin-releasing hormone agonist (GnRH-a) per COH cycle would be more suitable and convenient for women than short-acting GnRH-a injections or the GnRH antagonist protocol during the COVID-19 pandemic (Ben-Kimhy et al., 2020; Li F. et al., 2020), because there are fewer incidences of potential exposure. The early follicular-phase long-acting GnRH-agonist long (EFLL) protocol (a new protocol developed by Chinese clinicians) applies a pituitary downregulated full dose (3.75 mg) of dipherelin on days 2–4 of menstruation, and Gn starts 30–42 days later along with confirmation of the pituitary downregulation (Ying et al., 2019; Li F. et al., 2020). A series of studies has suggested its advantages in improving endometrial receptivity, embryo implantation and clinical pregnancy rates (Ren et al., 2014; Schisterman et al., 2020). It is worth emphasizing that the EFLL protocol was initially applied in a Chinese in vitro fertilization (IVF) center in 2016, and it has become the mainstream protocol in most reproductive medicine centers now in China (Li F. et al., 2020).
However, due to the interruption of medical treatment by COVID-19, many patients are affected by unexpected clinic closures (Sadeghi, 2020), and Gonadotrophins (Gns) would start > 42 days later along with confirmation of prolonged pituitary downregulation (pro-EFLL). Do these changes affect the outcome of assisted pregnancies in these infertile couples? Which controlled ovarian stimulation management strategies are most appropriate during the COVID-19 epidemic? In view of this, the aim of our study was to evaluate the appropriate COH protocol for infertility patients who received IVF/ICSI treatments during the COVID-19 pandemic. We gathered data comparing the clinical efficacy of the EFLL protocol, prolonged pituitary downregulation of the EFLL protocol and GnRH antagonist protocol before COH through collecting pregnancy rates and miscarriage rates per fresh transfer cycle, which are vital indicators for infertile couples (Vaegter et al., 2017; Veiga et al., 2020). To our knowledge, this is the first study to evaluate prolonged pituitary downregulation in infertile patients during the COVID-19 pandemic to provide a more detailed estimate and clinical management strategies for infertile couples.
This retrospective cohort study evaluated the efficiency of the EFLL protocol, the pro-EFLL protocol, and the GnRH-ant protocol for couples meeting the study criteria between February 2020 and June 2020 who were treated by the First Affiliated Hospital of Zhengzhou University during the COVID-19 pandemic. We screened eligible subjects and removed 433 patients who had abandoned treatment because of the COVID-19 pandemic, other ovarian hyperstimulation protocols and women who received transplantation genetic screening or transplantation genetic diagnosis or did not have complete laboratory data (e.g., Baseline data, Endocrine data, and Embryo data). Finally, the study analyzed clinical data from 199 cycles with IVF/ICSI in our reproductive medical center. The experimental materials in this study did not include identifiable part icipants data for the purpose of safeguarding patient privacy. This study was approved by the Ethics Committee of Reproductive Medicine Center, the First Affiliated Hospital of Zhengzhou University, China. Informed consent was waived, with approval from the ethics committee. A flow chart and the data processing procedure are listed in Figure 1.
For patients undergoing the standard EFLL protocol, we administered 3.75 mg long-acting GnRH agonist (Diphereline, Ipsen, France) on days 2–3 of menstruation. Patients were monitored by sex hormones level and ultrasound measurements. The following criteria were used for down-regulation standard: No functional cysts and follicle sizes larger than 3–5 mm by ultrasound; LH < 5 IU/L, FSH < 5 IU/L, and P < 1 ng/mL. The initial dose of gonadotropin was administered on the basis of the woman‘s age, AFC, BMI, and ovarian response to stimulation. The trigger was administered with 2000 IU u-HCG (Livzon Pharmaceuticals) in combination with 250 μg r-human chorionic gonadotropin (hCG) (Merck Serono) when most dominant follicles are mature, the Oocytes were then retrieved 36–37 h after the trigger (Figure 2).
The pro-EFLL protocol is analogous to the standard EFLL protocol as well, on days 2–3 of menstruation, we also administered 3.75 mg long-acting GnRH agonist (Diphereline, Ipsen, France). And all infertile patients were monitored by sex hormones level and ultrasound measurements, however, Gns would start >42 days later along with confirmation of prolonged pituitary down-regulation due to the COVID-19 interrupt medical treatment. The following process is the same as standard EFLL protocol (Figure 3).
For the GnRH-ant protocol, COH was started with 72.5–300 IU gonadotropin (Puregon, Organon, Netherlands) on day 2–3 of the menstrual cycle. The initial dose of gonadotropin was administered on the basis of the woman’s age, AFC, BMI, and ovarian response to stimulation. A daily dose of 0.25–0.75 mg GnRH antagonist (Cetrotide, Pierre Fabre, Aquitaine Pharm International) was initiated on the sixth day of rFSH stimulation or when the lead follicle reached a mean diameter of 12–14 mm, and the gonadotropin was continued until the day of the trigger administration (5000 IU u-HCG, Livzon Pharmaceuticals or 250 μg r-hCG, Merck Serono in combination with 2000 IU u-HCG). The Oocytes were then retrieved 35–37 h after the trigger (Li F. et al., 2020; Figure 4).
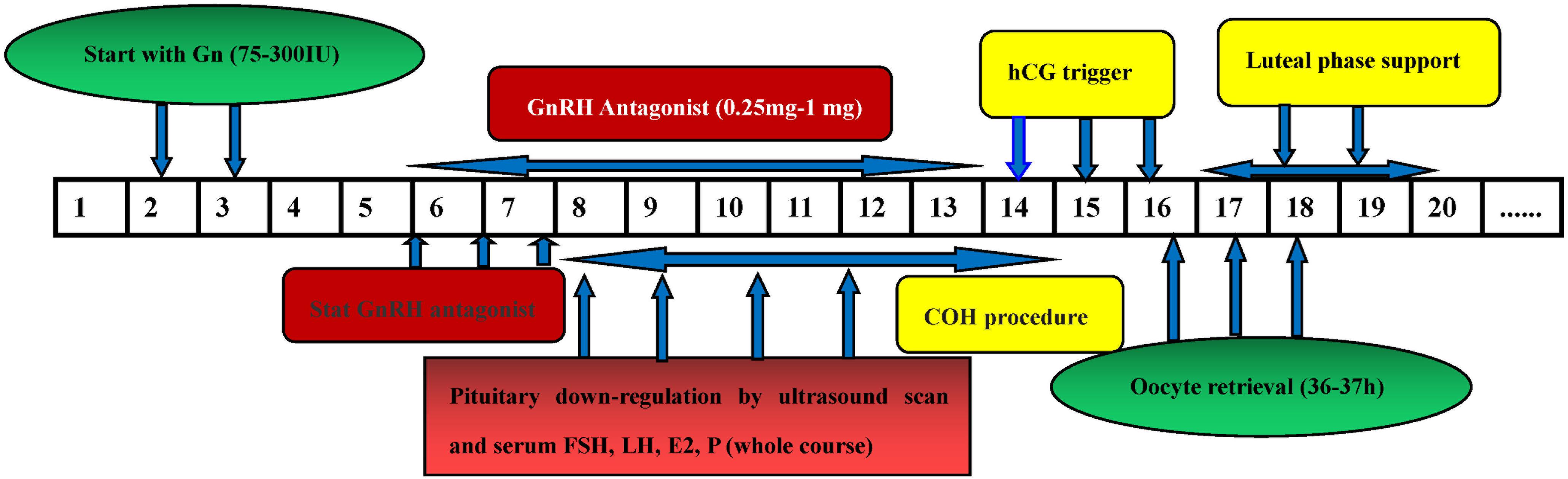
Figure 4. The flow chart of GnRH antagonist protocol. Figures 5, 6 comparison of treatment results among the three groups.
We performed the follow-up through outpatient visits. The follow-up time began from their first clinical encounter and continued until the pregnancy outcome and miscarriage outcome occurred or the last date of this study, whichever occurred first.
All Data was analyzed using the software R (version 3.6.1) and the Statistical Package for Social Sciences (Version 22.0). The primary outcomes in this retrospective comparative study were pregnancy rates and miscarriage rates per fresh transfer cycle. The secondary outcomes included endometrial thickness, retrieved oocytes, mature oocytes, fertilized oocytes, and transferable embryos. Continuous variables were compared using one-way analysis of variance (ANOVA). Categorical variables were compared using the chi-square test or Fisher’s exact test. P < 0.05 was considered statistical significance.
Patients who met the study criteria between February 2020 and June 2020 and were treated at the First Affiliated Hospital of Zhengzhou University during the COVID-19 pandemic included 85 patients given the EFLL protocol, 43 patients given the pro-EFLL protocol, and 71 patients given the GnRH antagonist protocol. In the pro-EFLL protocol, the continuous pituitary downregulation time was between 43 and 63 days, 27 patients had a continuous pituitary downregulation time of 43–56 days, and the remaining patients it was between 57 and 63 days. There were no significant differences in the basic characteristics (Age, BMI, FSH, LH, E2, P PRL, AMH, AFC, TSH, FT3, FT4, Blood glucose) among the three groups. The EFLL protocol was associated with a shorter duration of pituitary downregulation (35.73 ± 2.87 vs. 56.56 ± 11.00, P < 0.001) and lower FSH levels on the Gn commencing day (3.40 ± 1.87 vs. 4.72 ± 1.90, P < 0.001) than the Pro-EFLL protocol. However, there were no significant differences in the pregnancy or miscarriage rates between the two groups (Table 1).
Comparison of stimulation variables among the three groups revealed that the EFLL protocol was associated with a lower FSH level (3.40 ± 1.87 vs. 4.72 ± 1.90, P < 0.001) than the Pro-EFLL protocol on the Gn commencing day. We found that the EFLL protocol and the Pro-EFLL protocol were associated with a greater endometrial thickness (12.09 ± 2.35 vs. 12.67 ± 2.21 vs. 10.79 ± 2.38, P < 0.001), longer duration of Gn use (13.79 ± 1.96 vs. 13.02 ± 2.68 vs. 10.58 ± 2.51, P < 0.001), and a lower LH value (0.82 ± 0.81 vs. 1.08 ± 0.93 vs. 5.83 ± 0.99, P < 0.001) than the GnRH-ant protocol on the day of the hCG injection (Table 2).
We found that the GnRH-ant protocol was associated with a lower number of retrieved oocytes (10.06 ± 7.63 vs. 15.02 ± 7.93 vs. 14.49 ± 6.30, P < 0.001), mature oocytes (7.63 ± 6.50 vs. 11.96 ± 6.00 vs. 11.60 ± 5.71, P < 0.001), fertilized oocytes (5.42 ± 5.20 vs. 8.44 ± 5.34 vs. 9.14 ± 5.43, P < 0.001), and transferable embryos (3.00 ± 3.28 vs. 4.87 ± 2.96 vs. 6.47 ± 5.12, P < 0.001) than the EFLL protocol and the Pro-EFLL protocol; however, no statistically significant differences were seen for pregnancy rates (49.4 (42/85) vs. 34.9 (15/43) vs. 39.4 (28/71), P < 0.001) or miscarriage rates (11.9 (5/42) vs. 20.0 (3/15/34.9 (15/43) vs. 28.4 (28/71) per transfer cycle (Table 3). A comparison of treatment results among the three groups is shown in Figures 5, 6.
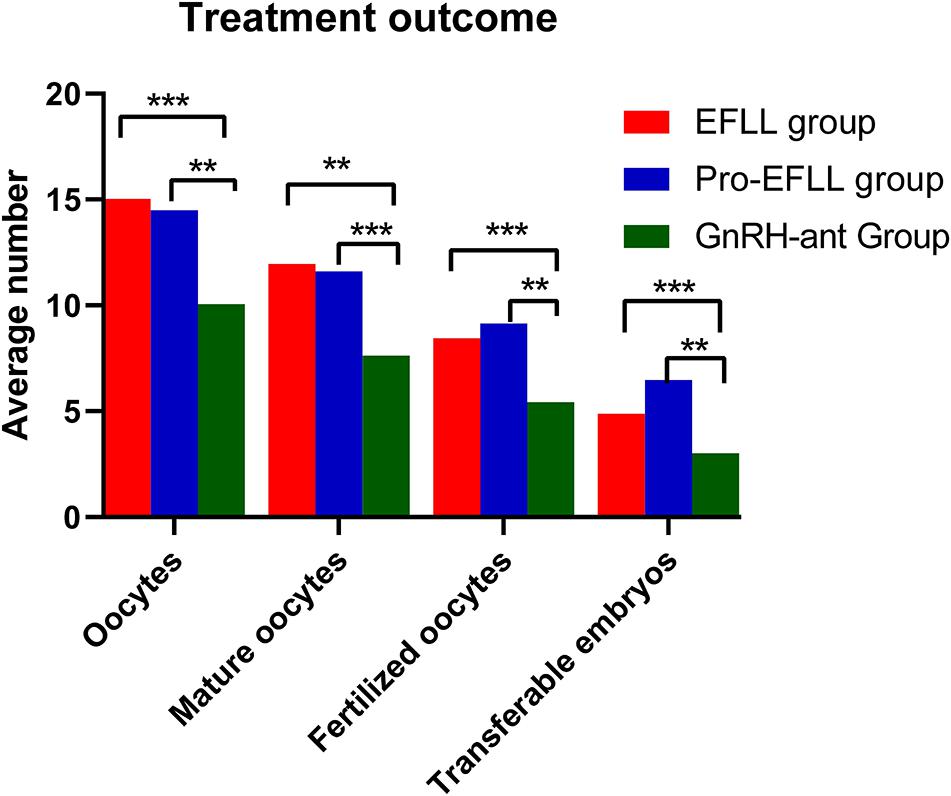
Figure 5. Comparison of oocytes retrieved, mature oocytes, fertilized oocytes, and transferable embryos among EFLL, Pro-EFLL, and GnRH-ant protocol. Values are presented as mean ± standard deviation. **Represents significant difference at P < 0.01, ***represents significant difference at P < 0.001.
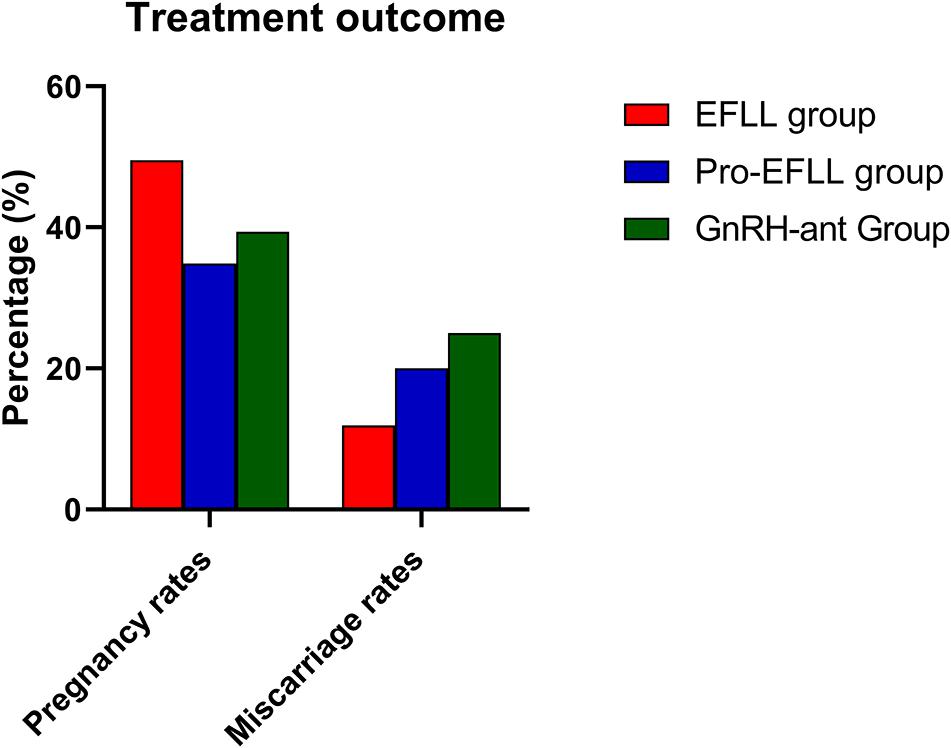
Figure 6. Comparison of pregnancy rates and miscarriage rates among EFLL, Pro-EFLL, and GnRH-ant protocol. Values are presented as frequencies.
Our findings indicate that prolonged pituitary downregulation during the COVID-19 pandemic by utilizing a full dose of GnRH-a administered to infertile patients was not associated with differences in pregnancy outcomes, such as pregnancy rates and miscarriage rates per fresh transfer cycle, among the three protocols. In addition, we also found that prolonged downregulation protocols and EFLL protocols can acquire more mature oocytes and transplantable embryos than GnRH-ant protocols. Furthermore, we found that these two protocols were associated with a greater endometrial thickness, longer duration of Gn use, and lower LH value than GnRH-ant protocols on the day of hCG injection. These strategies warrant further investigation. Considering that the ASRM and the ESHRE have no uniform standards on how to treat infertile people and how to arrange medical treatment during these difficult times (Esposito et al., 2020; Simopoulou et al., 2020; Veiga et al., 2020), to meet the current needs, our research results can provide a more detailed view of clinical management strategies for infertile couples during the COVID-19 pandemic.
In the special period of the epidemic, it is necessary to develop or refine robust COH protocols to minimize possible exposure. Our study showed that one full-dose depot of long-acting GnRH-a per COH cycle would be more suitable and convenient for infertile couples than GnRH antagonist and short-acting GnRH-a injections during the COVID-19 pandemic because there are fewer possible incidences of potential exposure (Ren et al., 2014; Li F. et al., 2020). Some studies previously observed that a pituitary downregulated full-dose may require a higher dose of gonadotropins for ovarian stimulation (Pan et al., 2019; Xu et al., 2020); however, our study showed that the total dosage of Gns used in our study was not statistically different among the treatment groups, which means a full dose of gonadotropins or prolonged pituitary downregulation would not drastically increase the economic burden for Infertile couples.
We recommend that patients start Gn injections 33–42 days after a pituitary downregulated full dose (3.75 mg) of gonadotropin-releasing hormone agonist during the COVID-19 pandemic. Even a delay of 2–4 weeks does not affect the implantation rate. This would significantly reduce the rate of cycle cancelations and greatly alleviate the financial and emotional burden of interrupting treatment for infertile couples due to the epidemic. Our study can provide a more detailed view of the clinical management strategies for infertile couples during the COVID-19 pandemic; however, these strategies warrant large-scale, prospective and multicenter clinical trials for confirmation in the future.
Our study showed that although the Pro-EFLL protocol was associated with a greater endometrial thickness than GnRH-ant protocols on the day of the hCG injection, the pregnancy rates were not significantly different among the groups. Similar findings were also reported in the study of Wang et al. (2017), Huang et al. (2018), and Liu Y. et al. (2019), and their conclusions are consistent with our findings. However, some studies in the past have shown that endometrial thickness is closely related to pregnancy rates (Haas et al., 2019; Liu W. et al., 2019). Gallos et al. (2018) analyzed 25,767 IVF cycles from the CARE Fertility Group in the United Kingdom and found that when the endometrial thickness was less than 5 mm, the live birth rate was 15.6%, and when the endometrial thickness of 10 mm, the live birth rate was gradually increased to 33.1%. It seems that the thicker the endometrium, the higher the pregnancy rates. We analyzed the reasons of these studies are inconsistent with our results may be caused by the following factors. First, endometrial thickness is not the only factor that affects the pregnancy rate since it is influenced by many factors, such as age, endometrial receptivity, and embryo quality (Zhao et al., 2014; Bu and Sun, 2015). Second, these studies did not take into account the effect of basal FSH, AMH, AFC, TSH, FT3, blood glucose or the E2 value on the day of the hCG injection per fresh transfer cycle when adjusting for covariates compared with our work, and previous studies have reported that these variables are related to pregnancy rates and miscarriage rates per fresh transfer cycle (Vaegter et al., 2017; Song et al., 2020). Third, the research populations are different, and there are physiological differences between ethnic Chinese and ethnic Europeans, the physiological difference between the two ethnic groups are reflected in many aspects, such as body mass index difference, altered ovarian morphology and functional changes, Genes associated with reproduction and fertility changes, which might cause different pregnancy outcomes (Mura et al., 1991; Lachance and Tishkoff, 2013; Dumesic et al., 2015).
Furthermore, the EFLL protocol can acquire more mature oocytes and transplantable embryos than the GnRH-ant protocol; however, no statistically significant effects were seen for pregnancy and miscarriage rates per fresh transfer cycle. Some reports suggest that it seemed more strongly impaired to endometrial receptivity by GnRH-a than GnRH-ant treatments, a study revealed that the gene expression profiles of endometrial cells following GnRH-ant treatment are more similar to those during natural cycles using microarray data (Chen et al., 2019), this finding may explain the phenomenon overall. However, some reports on endometrial receptivity have been inconsistent for the GnRH-ant and GnRH-a protocols, and studies have suggested that a full-dose dipherelin injection can be used to achieve long-term suppression of the GnRH agonist, and it can increase endometrial receptivity in patients, although the exact mechanism remains unclear (Lambalk et al., 2017; Wu et al., 2019), so further analysis is required in the future.
It is worth emphasizing that if patients require more COH cycles to achieve better cumulative live birth rates, our research shows that GnRH-a protocols is significantly superior to the GnRH-ant protocol. An animal model study suggested that follicles and embryos quality was significantly enhanced by increasing concentrations of GnRH-a and its receptor (Liu M. et al., 2018). One study showed that the optimal serum LH concentration on the commencing day of ovarian stimulation after downregulation with GnRH-a was 0.1 IU/L∼1 IU/L, and when serum LH levels are less than 0.1 IU/L, exogenous LH is required to increase the number of follicles and embryos. When the serum LH is greater than 1 IU/L, it has been proven to have adverse effect on embryo quality. Our study showed that serum LH levels on the commencing day of ovarian stimulation after downregulation were 0.63 ± 0.40 IU/L (EFLL Group) and 0.64 ± 1.20 IU/L (prolonged Group), which are similar to those in the study of Li G. et al. (2020). In view of these findings, we think that it is better to give patients a pituitary downregulated full dose (3.75 mg) of dipherelin on days 2–4 of menstruation during the COVID-19 pandemic.
The clinical value of this study is that it is the first to observe the effect of prolonged GnRH agonist downregulation on pregnancy and miscarriage rates per fresh transfer cycle in Chinese patients with infertility during the COVID-19 pandemic. The findings of this study should be helpful for developing clinical management strategies for infertile couples. However, there are some limitations in the present study (Jansen et al., 2020; Niehus et al., 2020): (1) Retrospective cohort studies are always associated with selection bias issues, with selection bias being introduced, one might expect the result to be skewed in some ways. (2) In this study, our research subjects were all Chinese patients with infertility being given IVF/ICSI treatments during the COVID-19 pandemic. Therefore, there is a certain deficiency in the universality and generalizability of the research. (3) Because we excluded women who received transplantation genetic screening or other ovarian hyperstimulation protocols, the findings of this study cannot be used for these groups of people.
In conclusion, in the special period of the COVID-19 pandemic, prolonged pituitary downregulation by utilizing a full dose of GnRH-a administered to infertility patients showed no differences in clinical outcomes, such as pregnancy or miscarriage rates per fresh transfer cycle, among the different protocols. The prolonged downregulation protocol and the EFLL protocol can acquire more mature oocytes and transplantable embryos than the GnRH-ant protocol. We recommend that patients start Gn injections 33–42 days after a pituitary downregulated full dose (3.75 mg) of gonadotropin-releasing hormone agonist during the COVID-19 pandemic. Even a delay of 2–4 weeks does not affect the implantation rate. Our study can provide a more detailed estimate and clinical management strategies for infertile couples during the COVID-19 pandemic; however, these strategies warrant large-scale, prospective and multicenter clinical trials for confirmation in the future.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
This study was followed the Declaration of Helsinki Guideline and approved by the Ethics Committee of Reproductive Medicine Center, the First Affiliated Hospital of Zhengzhou University, China. Ethical code: ZZDX-2020-T260-16.
GL, HJ, and FL conceived of and designed the experiments. GL, YT, HZ, and WS selected and supervised suitable patients. GL, YW, and FL obtained basic clinical data including age, body mass index, FSH, LH, estradiol, progesterone, and AMH levels, total dosage of gonadotropin used, duration of gonadotropin use, oocyte number, and live birth rate per transfer. GL provided overall supervision. GL, HZ, and FL drafted the manuscript. All authors reviewed this manuscript.
This work was supported by the National Natural Science Foundation of China (81771534 to GL), the Key Science and Technology Foundation of Henan Province (212102310049 to FL), the Key Science and Technology Foundation of Henan Province (172102310082 to GL), the Key Research Projects of Henan Higher Education Institutions (18A320057 to GL), and the Research and Development Project of Young Doctors in Reproductive Medicine of the Chinese Medical Association (18010240753 to GL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the women who participated in this study and all the physicians and nurses at the Reproductive Medicine Center, the First Affiliated Hospital of Zhengzhou University, China, for their support in collecting the data.
Ben-Kimhy, R., Youngster, M., Medina-Artom, T., Avraham, S., Gat, I., Haham, L., et al. (2020). Fertility patients under COVID-19: attitudes, perceptions, and psychological reactions. Hum. Reprod. 35, 2774–2783. doi: 10.1093/humrep/deaa248
Bu, Z., and Sun, Y. (2015). The impact of endometrial thickness on the day of Human Chorionic Gonadotrophin (hCG) administration on ongoing pregnancy rate in patients with different ovarian response. PloS One 10:e0145703. doi: 10.1371/journal.pone.0145703
Chen, Q., Yu, F., Li, Y., Zhang, A., and Zhu, X. (2019). Comparative proteomics reveal negative effects of gonadotropin-releasing hormone agonist and antagonist on human endometrium. Drug Design Dev. Ther. 13, 1855–1863. doi: 10.2147/dddt.s201871
Dumesic, D., Oberfield, S., Stener-Victorin, E., Marshall, J., Laven, J., and Legro, R. (2015). Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocrine Rev. 36, 487–525. doi: 10.1210/er.2015-1018
Esposito, V., Rania, E., Lico, D., Pedri, S., Fiorenza, A., Strati, M., et al. (2020). Influence of COVID-19 pandemic on the psychological status of infertile couples. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 148–153. doi: 10.1016/j.ejogrb.2020.08.025
Gallos, I., Khairy, M., Chu, J., Rajkhowa, M., Tobias, A., Campbell, A., et al. (2018). Optimal endometrial thickness to maximize live births and minimize pregnancy losses: Analysis of 25,767 fresh embryo transfers. Reprod. Biomed. Online 37, 542–548. doi: 10.1016/j.rbmo.2018.08.025
Gianaroli, L., Ata, B., Lundin, K., Rautakallio-Hokkanen, S., Tapanainen, J., Vermeulen, N., et al. (2020). The calm after the storm: re-starting ART treatments safely in the wake of the COVID-19 pandemic. Hum. Reprod. 36, 275–282. doi: 10.1093/humrep/deaa285
Haas, J., Smith, R., Zilberberg, E., Nayot, D., Meriano, J., Barzilay, E., et al. (2019). Endometrial compaction (decreased thickness) in response to progesterone results in optimal pregnancy outcome in frozen-thawed embryo transfers. Fertil. Steril. 112, 503–509. doi: 10.1016/j.fertnstert.2019.05.001
Huang, M., Tzeng, S., Lee, C., Chen, H., Huang, C., Lee, T., et al. (2018). GnRH agonist long protocol versus GnRH antagonist protocol for various aged patients with diminished ovarian reserve: A retrospective study. PLoS One 13:e0207081. doi: 10.1371/journal.pone.0207081
Jansen, M., Angelos, P., Schrantz, S., Donington, J., Madariaga, M., and Zakrison, T. (2020). Fair and equitable subject selection in concurrent COVID-19 clinical trials. J. Med. Ethics 47, 7–11. doi: 10.1136/medethics-2020-106590
Lachance, J., and Tishkoff, S. (2013). Population genomics of human adaptation. Annu. Rev. Ecol. Evol. Syst. 44, 123–143.
Lai, C., Shih, T., Ko, W., Tang, H., and Hsueh, P. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 55:105924.
Lambalk, C., Banga, F., Huirne, J., Toftager, M., Pinborg, A., Homburg, R., et al. (2017). GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum. Reprod. Update. 23, 560–579. doi: 10.1093/humupd/dmx017
Li, F., Ye, T., Kong, H., Li, J., Hu, L., Jin, H., et al. (2020). Efficacies of different ovarian hyperstimulation protocols in poor ovarian responders classified by the POSEIDON criteria. Aging 12, 9354–9364. doi: 10.18632/aging.103210
Li, G., Wu, Y., Niu, W., Xu, J., Hu, L., Shi, H., et al. (2020). Analysis of the number of euploid embryos in preimplantation genetic testing cycles with early-follicular phase long-acting gonadotropin-releasing hormone agonist long protocol. Front. Endocrinol. 11:424. doi: 10.3389/fendo.2020.00424
Liu, M., Sun, A., Zhao, S., Liu, H., Ma, S., Li, M., et al. (2018). Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril. 109, 900–907. doi: 10.1016/j.fertnstert.2018.01.020
Liu, W., Nong, Y., Ruan, J., Chen, Y., Fan, L., Huang, Q., et al. (2019). Impact of endometrial thickness during menstruation and endometrial scratching on the pregnancy in frozen-thawed embryo transfer. J. Obstet. Gynaecol. Res. 45, 619–625. doi: 10.1111/jog.13872
Liu, Y., Ye, X., and Chan, C. (2019). The association between endometrial thickness and pregnancy outcome in gonadotropin-stimulated intrauterine insemination cycles. Reprod. Biol. Endocrinol. 17:14.
Lupia, T., Scabini, S., Mornese Pinna, S., Di Perri, G., De Rosa, F., and Corcione, S. (2020). 2019 novel coronavirus (2019-nCoV) outbreak: A new challenge. J. Glob. Antimicrob. Resist. 21, 22–27.
Mura, C., Broyard, J., Jacqz-Aigrain, E., and Krishnamoorthy, R. (1991). Distinct phenotypes and genotypes of debrisoquine hydroxylation among Europeans and Chinese. Br. J. Clin. Pharmacol. 32, 135–136. doi: 10.1111/j.1365-2125.1991.tb05629.x
Niehus, R., De Salazar, P., Taylor, A., and Lipsitch, M. (2020). Using observational data to quantify bias of traveller-derived COVID-19 prevalence estimates in Wuhan, China. Lancet Infect. Dis. 20, 803–808. doi: 10.1016/s1473-3099(20)30229-2
Pan, W., Tu, H., Jin, L., Hu, C., Li, Y., Wang, R., et al. (2019). Decision analysis about the cost-effectiveness of different in vitro fertilization-embryo transfer protocol under considering governments, hospitals, and patient. Medicine 98:e15492. doi: 10.1097/md.0000000000015492
Pasquale, C., Montanino Oliva, M., Benedetti, M., Berlinghieri, V., Bielli, W., Buonomo, G., et al. (2020). Assisted reproduction and coronavirus in Italy. Eur. Rev. Med. Pharmacol. Sci. 24, 7512–7515.
Ren, J., Sha, A., Han, D., Li, P., Geng, J., and Ma, C. (2014). Does prolonged pituitary down-regulation with gonadotropin-releasing hormone agonist improve the live-birth rate in in vitro fertilization treatment? Fertil. Steril. 102, 75–81. doi: 10.1016/j.fertnstert.2014.03.030
Sadeghi, M. (2020). Implications of assisted human reproduction during coronavirus disease 2019 (COVID-19) pandemic. J. Reprod. Infertil. 21, 155–156.
Schisterman, E., Sjaarda, L., Clemons, T., Carrell, D., Perkins, N., Johnstone, E., et al. (2020). Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: A randomized clinical trial. JAMA 323, 35–48. doi: 10.1001/jama.2019.18714
Simopoulou, M., Sfakianoudis, K., Giannelou, P., Rapani, A., Siristatidis, C., Bakas, P., et al. (2020). Navigating assisted reproduction treatment in the time of COVID-19: concerns and considerations. J. Assisted Reprod. Genet. 37, 2663–2668. doi: 10.1007/s10815-020-01942-z
Song, H., Hu, K., Du, X., Zhang, J., and Zhao, S. (2020). Risk factors, changes in serum inflammatory factors, and clinical prevention and control measures for puerperal infection. J. Clin. Lab. Anal. 34:e23047.
Vaegter, K., Lakic, T., Olovsson, M., Berglund, L., Brodin, T., and Holte, J. (2017). Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil. Steril. 107, 641–648. doi: 10.1016/j.fertnstert.2016.12.005
Veiga, A., Gianaroli, L., Ory, S., Horton, M., Feinberg, E., and Penzias, A. (2020). Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS. Hum. Reprod. Open 2020:hoaa033.
Vermeulen, N., Ata, B., Gianaroli, L., Lundin, K., Mocanu, E., Rautakallio-Hokkanen, S., et al. (2020). A picture of medically assisted reproduction activities during the COVID-19 pandemic in Europe. Hum. Reprod. Open 2020:hoaa035.
Wang, R., Lin, S., Wang, Y., Qian, W., and Zhou, L. (2017). Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: A systematic review and meta-analysis. PLoS One 12:e0175985. doi: 10.1371/journal.pone.0175985
Wu, L., Ren, X., Chen, W., Huang, B., Zhou, Y., and Jin, L. (2019). Influence of different gonadotropin-releasing hormone agonist administration methods on pregnancy outcomes of patients undergoing in-vitro fertilization-embryo transfer. Curr. Med. Sci. 39, 437–441. doi: 10.1007/s11596-019-2056-9
Xia, S., Liu, M., Wang, C., Xu, W., Lan, Q., Feng, S., et al. (2020). Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 30, 343–355. doi: 10.1038/s41422-020-0305-x
Xu, B., Geerts, D., Hu, S., Yue, J., Li, Z., Zhu, G., et al. (2020). The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum. Reprod. 35, 1306–1318. doi: 10.1093/humrep/deaa086
Ying, Y., Yang, T., Zhang, H., Liu, C., and Zhao, J. (2019). Prolonged pituitary down-regulation with full-dose of gonadotropin-releasing hormone agonist in different menstrual cycles: a retrospective cohort study. PeerJ. 7:e6837. doi: 10.7717/peerj.6837
Keywords: COVID-19 pandemic, controlled ovarian hyperstimulation, pituitary down-regulation, infertility, IVF/ICSI
Citation: Li F, Zhang H, Shi W, Wu Y, Tian Y, Guo Y, Jin H and Li G (2021) Controlled Ovarian Hyperstimulation Protocol in Infertile Patients During the COVID-19 Pandemic. Front. Physiol. 12:732709. doi: 10.3389/fphys.2021.732709
Received: 29 June 2021; Accepted: 25 August 2021;
Published: 27 September 2021.
Edited by:
Jeff M. P. Holly, University of Bristol, United KingdomReviewed by:
Kajal Khodamoradi, University of Miami, United StatesCopyright © 2021 Li, Zhang, Shi, Wu, Tian, Guo, Jin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: HaiXia Jin, amh4Z2xAMTI2LmNvbQ==; Gang Li, bGd2aWdvckAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.