
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 28 June 2021
Sec. Aquatic Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.696737
This article is part of the Research Topic Sperm Physiology in Fish: From Biochemistry to Biotechnology - Volume I. View all 4 articles
The marbled flounder (Pseudopleuronectes yokohamae) is a commercial flatfish in East Asia. The aim of this study was to improve its sperm cryopreservation protocol based on the vitality assessment of 7-day and 1-year cryopreserved sperm. Four extenders (extender-1: sucrose solution; extender-2: glucose solution; extender-3: fish Ringer's solution; and extender-4: modified fish Ringer's solution) were tested with a combination of five cryoprotectants (CPAs) (dimethyl sulfoxide: Me2SO; glycerol: GLY; ethylene glycol: EG; propylene glycol: PG; and methanol: MeOH) at four different concentrations (5, 10, 12, and 15%). Fluorescent technique was applied to detect the plasma membrane integrity (PMI), mitochondrial membrane potential (MMP), and DNA integrity of fresh and cryopreserved sperm specimens. Fresh sperm was diluted at a ratio of 1:2 (sperm:extender). Post-thaw motility of sperm cryopreserved using 15% Me2SO along with either extender-1 (86.0 ± 5.2%) or extender-2 (85.7 ± 7.1%) was similar (p > 0.05) to that of fresh sperm. Sperm cryopreserved using 12% GLY combined with extender-1 (83.67 ± 6.7%) or extender-2 (83.3 ± 4.7%) showed a similar motility to those cryopreserved with 15% Me2SO, but significantly lower from fresh sperm. The type of straw (0.25 or 0.50 mL) did not show any significant difference (p > 0.05) in post-thaw sperm motility. The highest values of PMI and MMP were observed for 7-day cryopreserved sperm using extender-1 in combination with 15% Me2SO (91.0 ± 2.9% and 90.0 ± 2.0%, respectively) or 12% GLY (90.0 ± 1.3% and 90.0 ± 4.6%, respectively). These results were similar to those of fresh sperm (95.3 ± 2.1% and 92.9 ± 2.5%, respectively). PMI and MMP of 1-year cryopreserved sperm using extender-1 in combination with 15% Me2SO (90.3 ± 2.5% and 89.3 ± 2.1%, respectively) or 12% GLY (90.0 ± 4.4% and 88.7 ± 2.2%, respectively) were significantly similar (p > 0.05) to those of fresh sperm. Sperm DNA integrity did not reveal any significant difference (p > 0.05) between fresh and cryopreserved (7-day and 1-year) sperm. Based on the assessed sperm vitality indicators, a cryopreservation protocol using extender-1 in combination with 15% Me2SO or 12% GLY has potential for hatchery as well as to create a germplasm bank.
The marbled flounder (Pseudopleuronectes yokohamae) is a commercially important species in Korea (Park et al., 2016), China (Liu et al., 2015), and Japan (Kusakabe et al., 2017). It is considered a valuable flatfish for coastal fisheries in Korea and Japan (Kusakabe et al., 2017). Although aquaculture technology for this species has been developed in Japan (Lucas et al., 2019), it has not been reported from other countries yet. Gonadal maturity occurs earlier in males than in females of this species (Kim et al., 2016), making its hatchery production difficult. Cryopreserved sperm can solve this problem by supplying sperm during hatchery production (Hassan et al., 2017; Gheller et al., 2019).
Sperm cryopreservation is an important method of storing genetic materials (Nahiduzzaman et al., 2011) and providing a constant supply of sperm (Kim et al., 2020a). This technique provides a stable source of sperm for artificial fertilization, selective breeding (Viveiros et al., 2012), and rapid sperm transfer among hatcheries (Xin et al., 2018a; Cejko et al., 2020). The cryopreservation protocol should optimize several factors, including sperm collection, sperm dilution (sperm:extenders), selection of cryoprotectants (CPAs), equilibration time, freezing in liquid nitrogen (LN), and thawing (Yang et al., 2017; Judycka et al., 2019). An extender can act as an artificial seminal plasma for sperm cryopreservation and reduce the metabolic activity of sperm (Sarosiek et al., 2016; Cejko et al., 2020). The extender is necessary to dilute sperm, and a CPA is required to protect sperm against damage during the freezing step (Suquet et al., 2000; Immerman and Goetz, 2014). The success of cryopreservation depends on both CPA type and concentration (Fernández-Santos et al., 2006; Soni et al., 2019). Recently, marine fish sperm cryopreservation has been accomplished using intracellular CPAs such as glycerol (GLY), dimethyl sulfoxide (Me2SO), methanol (MeOH), ethylene glycol (EG), and propylene glycol (PG). GLY (10–20%), Me2SO (10–20%), and PG (10%) are considered the most effective CPAs for the cryopreservation of teleost sperm (Cabrita et al., 2010). Currently, sperm cryopreservation techniques exist for over 200 fish species (Nahiduzzaman et al., 2011). Cryopreservation of sperm of several flounder species has been reported (Zhang et al., 2003; Lanes et al., 2008; Tian et al., 2008; Brown et al., 2012; Liu et al., 2015). However, very little is known about the cryopreservation of marbled flounder sperm. In addition, previous studies did not address the effects of the short-term and long-term cryopreservation on the post-thaw vitality of marbled flounder sperm (Song et al., 2016). Evaluation of the post-thaw vitality of the long-term cryopreserved sperm is needed for storage and commercial applications (Kim et al., 2020a).
Cryopreservation and thawing can decrease the integrity of the acrosome and plasma membrane. They can also reduce the motility and viability of sperm of all species (Xin et al., 2018a; Soni et al., 2019). Fluorescent technique is an important method of evaluating the sperm viability (He and Woods, 2004; Pereira et al., 2010). Recently, this technique has been used to assess the plasma membrane integrity (PMI), mitochondrial membrane potential (MMP), and acrosome integrity (AI) of sperm (Celeghini et al., 2007; Pereira et al., 2010; Hossen et al., 2021a,b). Post-thaw sperm motility (Agnihotri et al., 2016; Balamurugan et al., 2019) and MMP (Agnihotri et al., 2016) are the indicators of cryopreserved sperm quality. MMP indicates the mitochondrial energy status. It directly regulates the motility of sperm (Agnihotri et al., 2016). DNA integrity suggests the success of cryopreservation (Balamurugan et al., 2019). It can be detected using a comet assay (single-cell gel electrophoresis). The aim of this study was to improve the sperm cryopreservation technique and evaluate the post-thaw vitality of 7-day and 1-year cryopreserved marbled flounder sperm. The effects of (i) different concentrations of CPAs, (ii) different extenders, (iii) different types of straws, and (iv) different CPAs on PMI, MMP, and DNA integrity of cryopreserved sperm were observed to optimize the cryopreservation protocol of marbled flounder, P. yokohamae.
Experimental protocols used were approved by Chonnam National University Animal Care and Use Committee (Approval No.: CNU IACUC-YS-2019-10; Approval date: December 10, 2019). All fish experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Five sexually mature male marbled flounder were collected from Yeosu special fisheries market (Yeosu, Korea) in January and February of 2020 during the natural peak of its breeding season. Three to five individuals were previously used for the successful sperm cryopreservation of Atlantic halibut, Hippoglossus hippoglossus (Babiak et al., 2006); sterlet, Acipenser ruthenus (Xin et al., 2018b); striped catfish, Pseudoplatystoma magdaleniatum (Herrera-Cruz et al., 2019); and brown Trout (Rusco et al., 2020). Before the sperm collection, the area surrounding the genital pore was dried with a paper towel. Sperm was collected into 5-mL macro-tubes by gently stripping sperm ducts of fish. Special care was taken to prevent the contamination with blood, mucus, and urine. The abdomen of each fish was stripped to collect all possible sperm. These macro-tubes were immediately placed on ice after the sperm collection.
Sperm quality was assessed based on the sperm motility, PMI, and MMP to ensure the quality of fresh sperm. Sperm motility was observed according to the method described by Ding et al. (2009), Ahn et al. (2018), and Muchlisin et al. (2020). Briefly, an aliquot of 10 μL sperm was diluted with 100 μL of filtered seawater in a microtube. Subsequently, 2 μL of the diluted sperm was added to 100 μL of filtered seawater on a glass slide. Immediately, sperm were video-recorded using a microscope (Nikon Eclipse E600) with a 40x objective lens. Motility was calculated three times using three different samples (n = 3). Sperm survival rate was immediately assessed using a LIVE/DED® sperm viability kit. MMP was examined using the Rh123/PI® method. The density of fresh sperm was calculated using a hemocytometer (Laboratory Glassware, Germany).
Styrofoam boxes (25.0 ×25.0 ×21.0 cm) with a rack height of 6 cm were used to conduct the cryopreservation experiments. LN with a height of 5 cm was placed into Styrofoam boxes. The rack height was maintained from the surface of the LN. A digital water bath (JISICO Lab and Scientific Instruments) was used to thaw cryopreserved straws.
The following basic cryopreservation protocol was used in the present study (Figure 1). Sperm were diluted with extender solution and CPAs. The working solution (sperm with extender solution) was mixed gently in microtubes and placed into straws (0.25 and 0.50 mL). Straws (sealed with straw powder) were placed into Styrofoam boxes for the initial freezing. These boxes were covered for 10 min. Subsequently, straws were immediately submerged into LN for at least 3 h. For 1-year preservation, straws were stored in a 38-L LN tank (model: 38VHC-11M, serial: 80907, Worthington Industries, United States). It was observed that the height of LN in the tank decreased 5.5 cm in every week, and the tank was refilled with LN. Straws were thawed at 37°C for 5 s in seawater (SW) within 4 s. Thawed sperm were transferred to microtubes and observed under a microscope to assess the sperm motility. Sperm samples were activated with the filtered SW to assess the sperm motility as described in section Quality Evaluation of Fresh Sperm.
Four extenders were evaluated in this study: (1) extender-1 (sucrose solution), 110 mM sucrose, 100 mM KHCO3, and 10 mM HEPES, pH 8.2; (2) extender-2 (glucose solution), 83.25 mM glucose, 136.89 mM NaCl, and 8.72 mM KCl, pH 7.9; (3) extender-3 (fish Ringer's solution), 154 mM NaCl, 5.64 mM KCl, 2.2 mM CaCl2·6H2O, and 3.8 mM NaHCO3; (4) extender-4 (modified fish Ringer's solution), 65 mM sucrose, 154 mM NaCl, 5.64 mM KCl, 2.2 mM CaCl2·6H2O, and 3.8 mM NaHCO3. The following five CPAs were used to evaluate their effects on the post-thaw sperm motility: Me2SO, EG, PG, GLY, and MeOH. These CPAs were used at the final concentrations of 5, 10, 12, and 15% with each extender based on the published results for other flounder species (Zhang et al., 2003; Lanes et al., 2008; Ding et al., 2011; Brown et al., 2012; Hu et al., 2016). Working solutions were prepared by mixing different CPA concentrations with extenders and chilled at 4°C for ~24 h prior to use.
Two dilution ratios (1:2 and 1:4) were used to establish the optimal cryopreservation technique based on the published results for other flounder species (Zhang et al., 2003; Tian et al., 2008; Brown et al., 2012). Two concentrations (10 and 12%) of CPAs (Me2SO and GLY) with extender-1 and extender-2 were used to select the best dilution ratio for the effective cryopreservation.
Effects of straw size were evaluated by comparing the post-thaw sperm motility of sperm cryopreserved with 0.25 or 0.50 mL straws. This experiment was designed using the best concentration of CPAs and extenders selected from Experiment 1 and the best dilution ratio from Experiment 2.
Plasma membrane integrity values of fresh and cryopreserved sperm (7 days and 1 year) were assessed using a LIVE/DEAD® sperm viability kit (Invitrogen Molecular Probes, Eugene, OR, United States), which contained two fluorescent dyes, SYBR-14® and propidium iodide (PI), to facilitate the accurate and rapid assessment of PMI (Horváth et al., 2008; Merino et al., 2012; Hossen et al., 2021a,b). Briefly, 10 μL of post-thaw sperm was mixed with 990 μL of 1X PBS (phosphate-buffered saline) and tapped slightly to ensure proper dilution. An aliquot of 5 μL SYBR-14® was mixed with a sample and incubated at 37°C for 10 min in the dark followed by mixing with 10 μL PI. After slight tapping and incubating at 37°C in the dark again for 10 min, a 2 μL aliquot of the sample was then placed on a glass slide and covered with a cover slip. Samples were immediately observed under a fluorescent microscope (Nikon Eclipse E600). A green filter (excitation filter 450–490 nm) was used to capture images of green-stained live sperm (SYBR-14+/PI−), and a red filter (emission filter 510–560 nm) was used to capture images of red-stained dead sperm (SYBR-14−/PI+). Images captured with these green and red filters were merged with images taken without a filter to assess the sperm viability and PMI. Three replications (n = 3) were considered to analyze the PMI values, and a minimum of 200 sperm cells were considered in each replication.
Mitochondrial membrane potentials of cryopreserved sperm (7 days and 1 year) were evaluated using Rh123/PI® (Sigma-Aldrich Pty Ltd.) using a protocol described in the study by Kim et al. (2020b) with slight modifications. Briefly, 25 μL of post-thaw sperm was mixed with 975 μL of 1 × PBS and slightly tapped for appropriate dilution. Then 1 μL aliquot of Rh123 was mixed with the sample and incubated at 20°C in the dark for 10 min. Next, 5 μL PI was added followed by the slight tapping. The sample was incubated at 20°C in the dark again for 10 min. Then 2 μL aliquot of the sample was placed on a glass slide and covered with a cover slip. Samples were immediately observed under a fluorescent microscope (Nikon Eclipse E600). A green filter (excitation filter 450–490 nm) was used to capture images of green-stained intact mitochondrial membranes (Rh123+/PI−), and a red filter (emission filter 510–560 nm) was used to capture images of red-stained damaged mitochondrial membranes (Rh123−/PI+). Images captured with these green and red filters were merged with images taken without a filter to assess MMPs of cryopreserved sperm. Three replications (n = 3) were considered to analyze the PMI values, and a minimum of 200 sperm cells were considered in each replication.
Comet assay (single-cell gel electrophoresis) of fresh and cryopreserved sperm (7 days and 1 year) was performed using the protocol described in the study by Kim et al. (2020a) with slight modifications using a Comet Assay® kit (Trevigen Inc., Gaithersburg, MD, United States). Briefly, sperm were suspended (1 ×105 cells/mL) in pre-chilled 1 × PBS and immobilized in low-melting agarose gel on the comet slide. Slides were immersed in a lysis solution for 1 h and an alkaline unwinding solution for another 1 h at 4°C in the dark. These comet slides were electrophoresed at 21V for 30 min using a pre-chilled alkaline electrophoresis solution. Samples were stained with Vista green dye and then incubated in a dark chamber for 30 min. Slides were immediately visualized. Images were captured using a fluorescence microscope (excitation filter at 450–490 nm; Nikon Eclipse E600). A minimum of 100 cells were used to analyze the results of the comet assay. Comet Assay IV image analysis software (version 4.3.2, Perceptive Instruments Ltd., United Kingdom) was used to analyze the head length, tail length, percentage of DNA in the tail, tail moment, olive tail moment (OTM), and extent of tail moment.
All statistical analyses were performed using SPSS 16.00 software (SPSS Inc., Chicago, IL, United States). Statistical significance of results was determined using a non-parametric one-way ANOVA followed by Duncan's multiple range test. Differences were considered significant at p < 0.05. A GraphPad Prism software (GraphPad Prism version 5.00 for Windows, CA, United States) was used to generate graphs.
Male fish used for this study had an average (mean ±SD) body weight of 351.7 ± 53.5 g, a total length of 31.0 ± 1.4 cm, and a standard length of 26.1 ± 1.4 cm. Total milt volume was 8.3 ± 0.3 mL with a density of 18.0 ± 2.3 (×109) cells/mL. The total number of counted cells was 15.0 ± 1.6 ( ×1010).
Effects of extenders and CPAs on the post-thaw sperm motility are presented in Figure 2, Supplementary Table 1. The predominant post-thaw sperm motility was recorded using 15% Me2SO with extender-1 (86.0 ± 5.2%) or extender-2 (85.7 ± 7.1%). Post-thaw motility of sperm cryopreserved using extender-1 or extender-2 in combination with 15% Me2SO was similar to that of fresh sperm (p > 0.05) (Figure 2A).
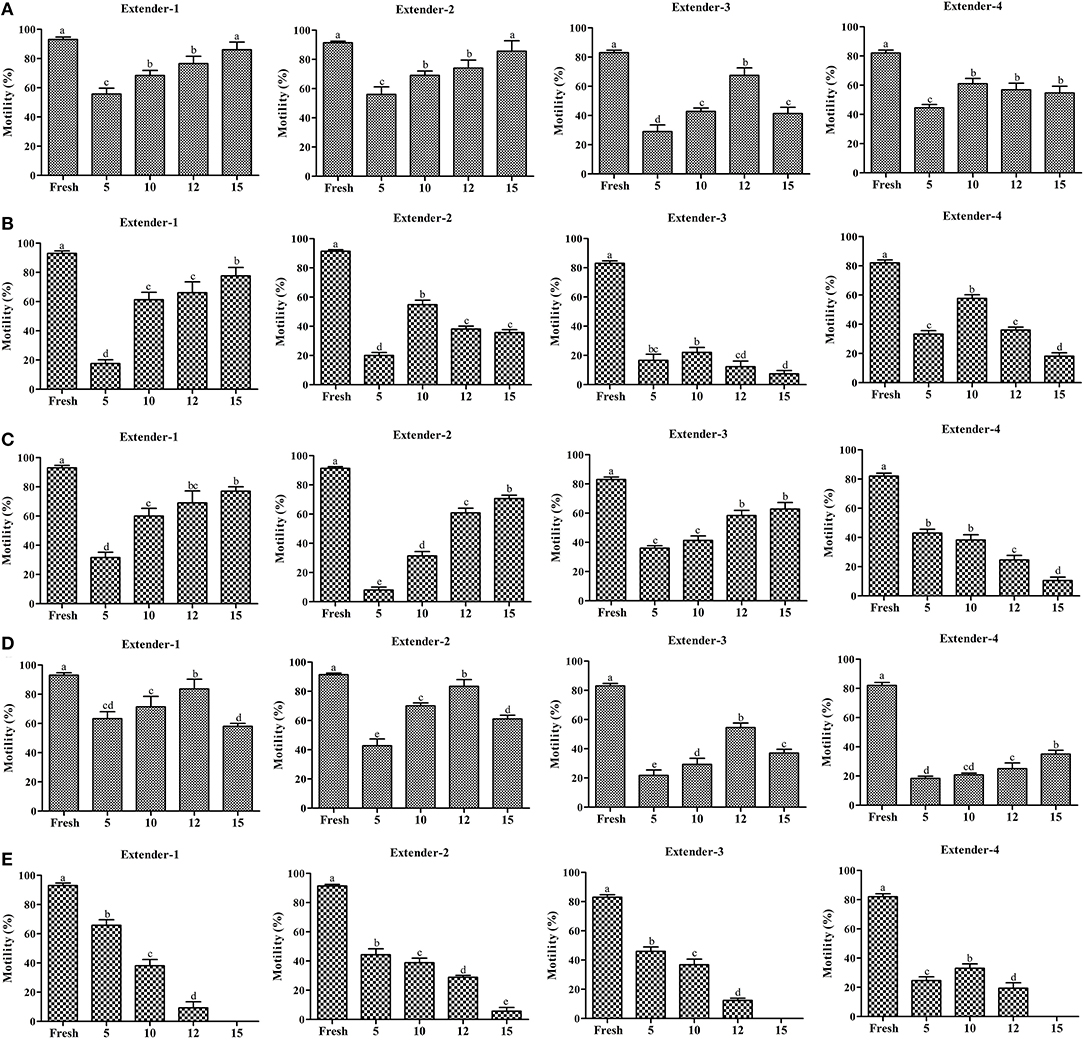
Figure 2. Post-thaw motility (n = 3) of sperm cryopreserved using different concentrations (5, 10, 12, and 15%) of cryoprotectants (Me2SO, EG, PG, GLY, and MeOH) combined with extenders (extenders-1,−2,−3, and −4). (A) Post-thaw motility of sperm cryopreserved using different concentration of Me2SO combined with different extenders; (B) post-thaw motility of sperm cryopreserved using different concentration of EG combined with different extenders; (C) post-thaw motility of sperm cryopreserved using different concentration of PG combined with different extenders; (D) post-thaw motility of sperm cryopreserved using different concentration of GLY combined with different extenders; (E) post-thaw motility of sperm cryopreserved using different concentration of MeOH combined with different extenders. Significant different levels (p < 0.05) are denoted by different letters.
Sperm cryopreserved using extender-1 combined with 15% EG (77.7 ± 5.7%) exhibited significantly higher (p < 0.05) post-thaw motility than other types of extender (Figure 2B). Sperm cryopreserved using EG exhibited lower post-thaw motility than those cryopreserved with Me2SO or GLY (Supplementary Table 1).
Post-thaw motility of sperm cryopreserved using extender-1 combined with 15% PG (77.0 ± 3.0%) exhibited significantly higher (p < 0.05) post-thaw motility than other types of extender in different concentrations of PG (Figure 2C).
Post-thaw motility of sperm cryopreserved using extender-1 (83.67 ± 6.7%) or extender-2 (83.3 ± 4.7%) in combination with 12% GLY was similar (p > 0.05) to that of sperm cryopreserved with 15% Me2SO, but lower from that of fresh sperm (Figure 2D; Supplementary Table 1).
Sperm cryopreserved using extender-1 combined with 5% MeOH (65.8 ± 3.9%) exhibited significantly higher (p < 0.05) post-thaw motility than other types of extender in different concentration of MeOH (Figure 2E). On the other hand, post-thaw motility was not observed when sperm were cryopreserved with 15% MeOH in combination with extender-1,−3, or−4 (Figure 2E).
In this experiment, all CPAs in combination with extender-1 or extender-2 showed significant differences between dilution ratios of 1:2 and 1:4 (Figure 3). Sperm diluted with extenders at a ratio of 1:2 showed significantly (p < 0.05) higher motility than those with extenders at a ratio of 1:4.
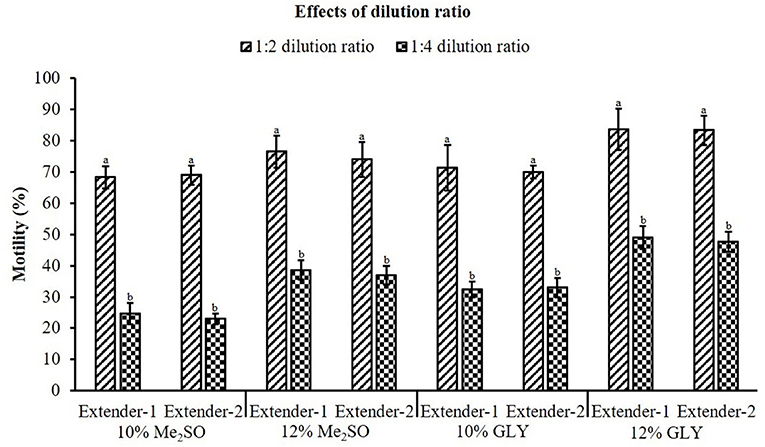
Figure 3. Effects of dilution ratio on motility of cryopreserved marbled flounder sperm (n = 3). Significant different levels (p < 0.05) are denoted by different letters.
Optimal combination of CPA and extender was selected based on the post-thaw motility results from Experiments 1 and 2. Sperm packaged in 0.25 mL straws and those packaged in 0.50 mL straws did not show a significant (p > 0.05) difference in post-thaw motility (Figure 4).
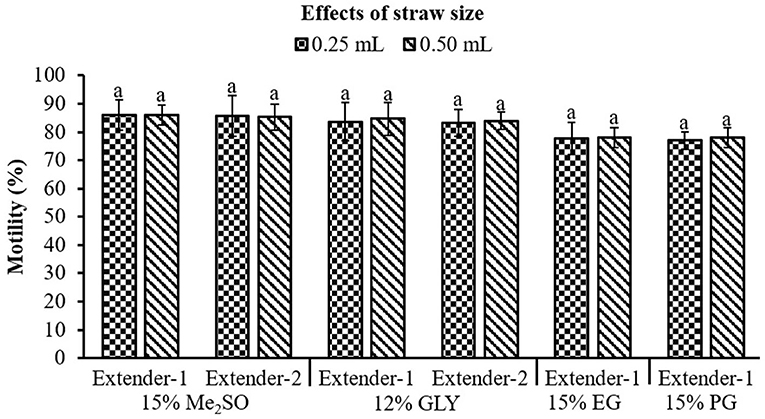
Figure 4. Effects of straw size (0.25 mL and 0.50 mL) on post-thaw motility of marbled flounder sperm (n = 3). Significant different levels (p < 0.05) are denoted by different letters.
Six combinations of CPAs and extenders were used to assess the PMI values of 7-day cryopreserved sperm based on the results of Experiment 1. PMI values (Figure 5A) of sperm cryopreserved with extender-1 in combination with 15% Me2SO (91.0 ± 2.9%), those of sperm cryopreserved with extender-1 in combination with 12% GLY (90 ± 1.3%), and those of fresh sperm (95.3 ± 2.1%) were not significantly different (p > 0.05). The morphology of cryopreserved sperm was similar to that of fresh sperm (Figure 6).
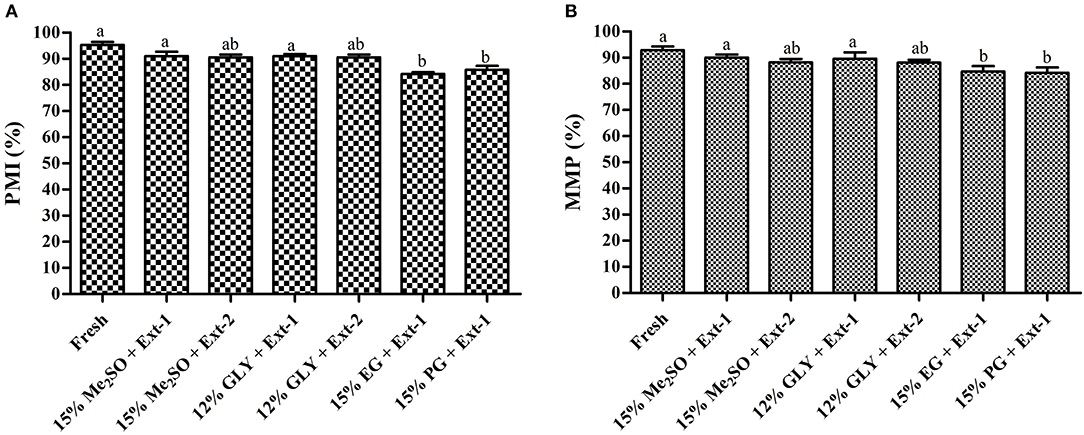
Figure 5. Plasma membrane integrity (%) and mitochondrial membrane potentials (%) of post-thaw marbled flounder sperm (n = 3). Significant different levels (p < 0.05) are denoted by different letters. (A) Plasma membrane integrity of cryopreserved sperm, (B) Mitochondrial membrane potentials of cryopreserved sperm.
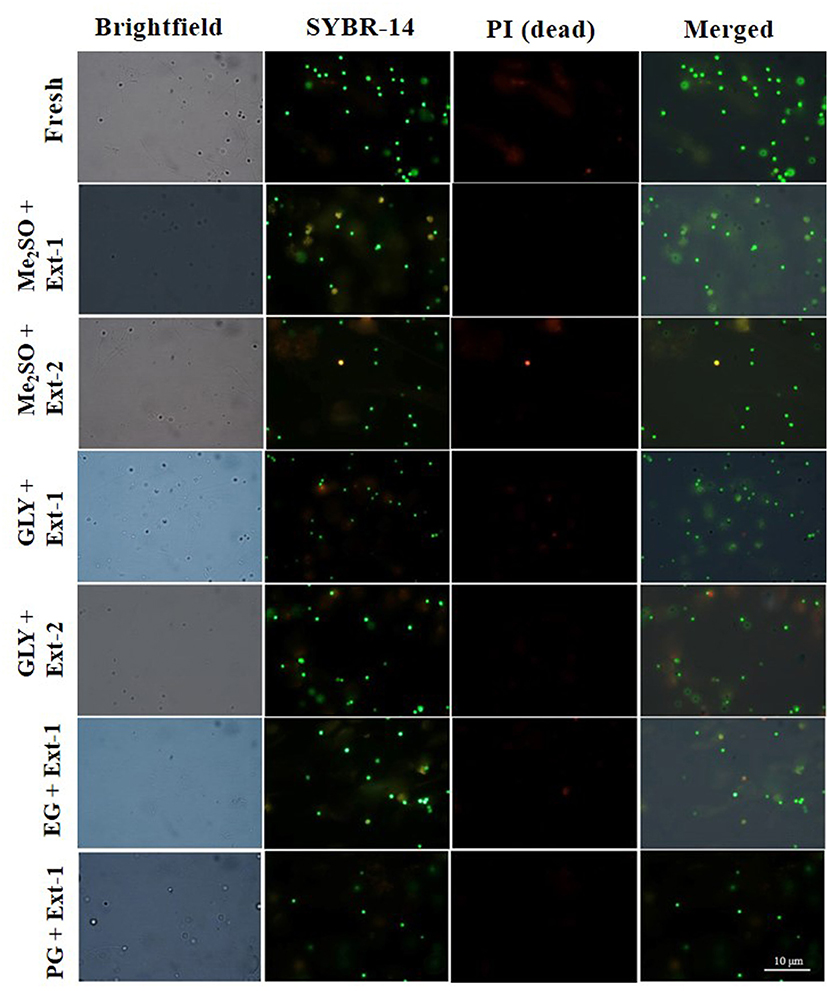
Figure 6. Fluorescent-stained photographs for detecting the plasma membrane integrity of cryopreserved marbled flounder sperm ( ×100 magnification).
Based on the post-thaw motility index, six combinations of CPAs and extenders were used to assess MMPs of 7-day cryopreserved sperm. MMPs (Figure 5B) of sperm cryopreserved using extender-1 in combination with 15% Me2SO (90.0 ± 2.0%), those of sperm cryopreserved with extender-1 in combination with 12% GLY (90.0 ± 4.6%), and those of fresh sperm (92.9 ± 2.5%) did not show a significant difference (p > 0.05). The mitochondrial membrane of cryopreserved sperm was similar to that of fresh sperm (Figure 7).
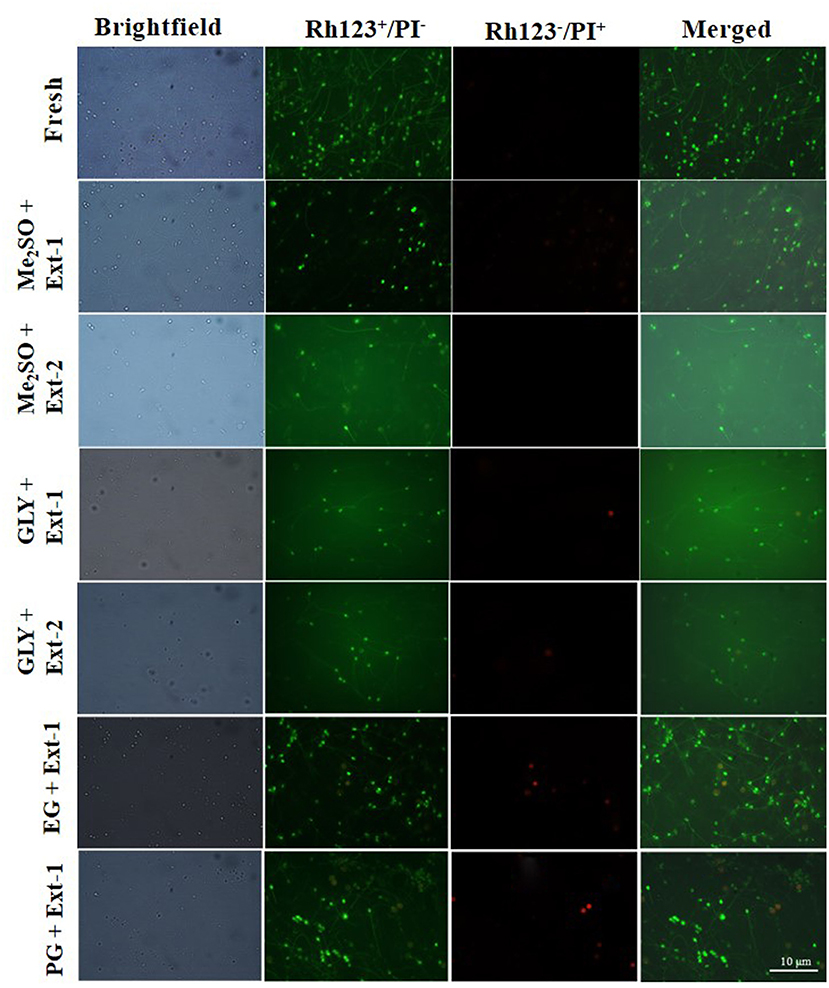
Figure 7. Fluorescent-stained photographs for detecting mitochondrial membrane potential of cryopreserved marbled flounder sperm ( ×100 magnification).
Two combinations of CPAs and extenders (15% Me2SO + extender-1 and 12% GLY + extender-1) were used to evaluate the post-thaw vitality of 1-year cryopreserved sperm based on the results of Experiments 1 and 4. Post-thaw motility of 1-year cryopreserved sperm using 15% Me2SO + extender-1 (85.0 ± 3.6%) or 12% GLY + extender-1 (82.3± 2.5%) was significantly similar (p > 0.05) to that of immediate (3 h) cryopreserved sperm motility (Figure 8A). PMI values of 1-year cryopreserved sperm with extender-1 in combination with 15% Me2SO (90.3 ± 2.5%) or 12% GLY (90.0 ± 4.4%) did not show differences (p > 0.05) with those cryopreserved 7 days and those of fresh sperm (Figures 8B,D). MMP values of 1-year cryopreserved sperm with extender-1 in combination with 15% Me2SO (89.3 ± 2.1%) or 12% GLY (88.7 ± 2.2%) did not show differences (p > 0.05) with those cryopreserved 7 days and those of fresh sperm (Figures 8C,E).
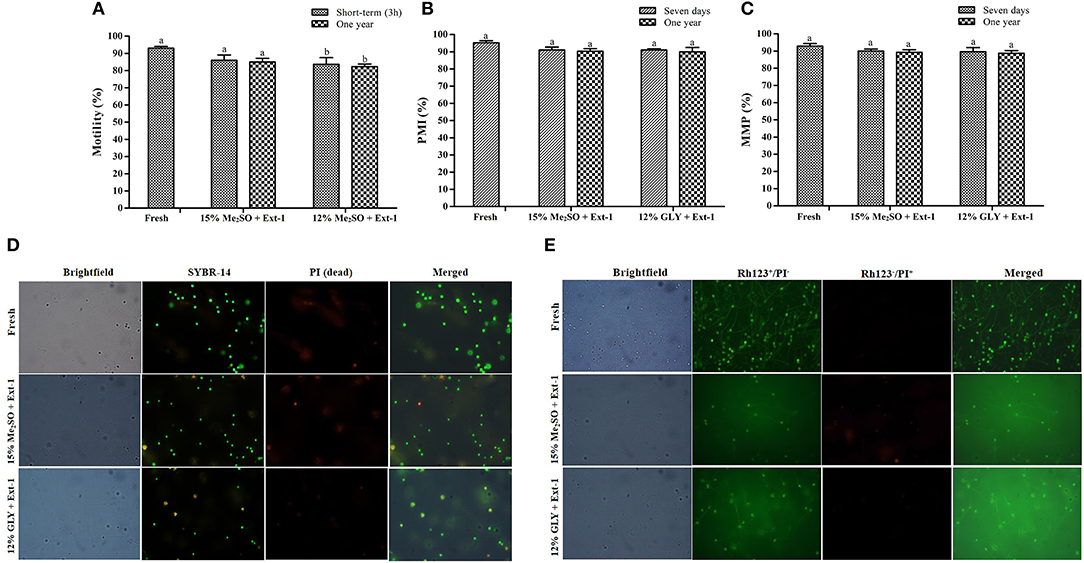
Figure 8. Effects of 1-year cryopreservation on post-thaw sperm vitality of marbled flounder. (A) Post-thaw sperm motility of 1-year cryopreserved sperm (n = 3); (B) plasma membrane integrity of 1-year cryopreserved sperm (n = 3); (C) mitochondrial membrane potential of 1-year cryopreserved sperm (n = 3); (D) fluorescent-stained photographs for detecting the plasma membrane integrity of 1-year cryopreserved marbled flounder sperm ( ×100 magnification). (E) Fluorescent-stained photographs for detecting the mitochondrial membrane potential of 1-year cryopreserved marbled flounder sperm ( ×100 magnification). Significant different levels (p < 0.05) are denoted by different letters.
Parameters of the comet assay for fresh and cryopreserved (7 days and 1 year) sperm are listed in Table 1. The percentage of DNA in the tail of 7-day cryopreserved sperm using extender-1 in combination with 12% GLY or 15% Me2SO was 0.7 ± 0.1% or 0.9 ± 0.1%, respectively. The percentage of DNA in the tail of 1-year cryopreserved sperm using extender-1 in combination with 12% GLY (0.9 ± 1.2%) or 15% Me2SO (0.9 ± 1.2%) did not show significant differences with those 7-day cryopreserved or fresh sperm. Intact DNA was seen for cryopreserved sperm and fresh sperm (Table 1). Comet assay results did not reveal any significant (p > 0.05) difference in DNA integrity between fresh and cryopreserved (7-day and 1-year) marbled flounder sperm (Figure 9).
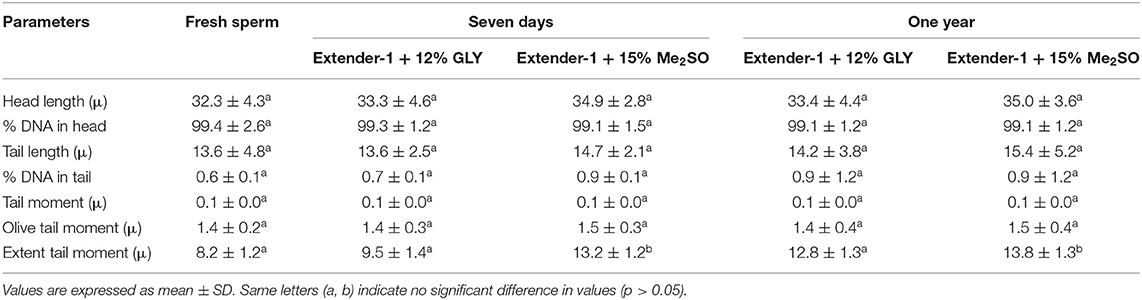
Table 1. Results of comet assay parameters (mean ± standard deviation) of fresh and cryopreserved sperm (7-day and 1-year) samples.
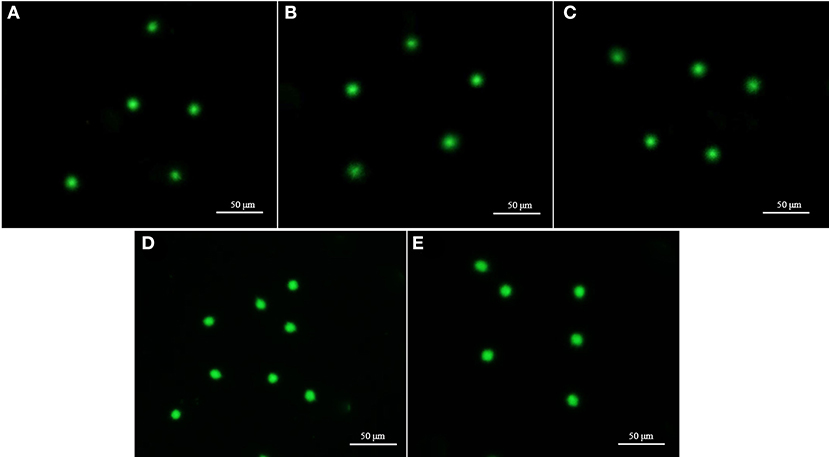
Figure 9. Fluorescent images of fresh and cryopreserved sperm after comet assay. Each comet represents the undamaged DNA in sperm. (A) Intact nuclei of fresh sperm. (B) Intact nuclei of 7-day cryopreserved sperm using extender-1 in combination with 12% GLY. (C) Intact nuclei of 7-day cryopreserved sperm using extender-1 in combination with 15% Me2SO. (D) Intact nuclei of 1-year cryopreserved sperm using extender-1 in combination with 12% GLY. (E) Intact nuclei of 1-year cryopreserved sperm using extender-1 in combination with 15% Me2SO. The comet gel was stained with Vista green dye.
The development of an effective sperm cryopreservation technique is a vital goal because of its significance for the conservation of biodiversity, reduced inbreeding, and minimization of domestic selection. Cryopreservation of fish sperm is affected by factors such as extenders, CPAs, dilution ratio, freezing process, and thawing process (Ahn et al., 2018). Present study improved a cryopreservation technique for marbled flounder sperm by optimizing these factors. In the present study, a sucrose base solution was used as extender-1. It had a composition similar to the extender used for the cryopreservation of sperm of summer flounder, Paralichthys dentatus (Brown et al., 2012); Brazilian flounder, Paralichthys orbignyanus (Lanes et al., 2008); and spotted halibut, Verasper variegatus (Tian et al., 2008). Glucose solution was used as extender-2. It was similar to the solution used for the cryopreservation of sperm of P. yokohamae (Song et al., 2016). Ringer's solution (extender-3) and modified Ringer's solution (extender-4) were also used in this study to compare their effects on the post-thaw sperm motility. The optimal dilution ratio of extender to sperm is species-specific (Ding et al., 2011). The present study showed a significant difference in the post-thaw sperm motility between two dilution ratios (1:2 and 1:4), similar to the results reported for sperm of H. hippoglossus (Ding et al., 2011) and Paralichthys dentatus (Brown et al., 2012).
In the present study, five common types of CPAs at four different concentrations were used for the cryopreservation of marbled flounder sperm. These CPAs used in the present study are categorized as penetrating CPAs (Cabrita et al., 2010; Iorio et al., 2019). All of them act with a similar principle (Iorio et al., 2019). CPAs can prevent ice crystallization during the freeze–thaw process (Fuller, 2004; Yang et al., 2010).
The present study showed the significant effects of extenders and CPAs on the motility of post-thaw sperm. In the present study, 15% Me2SO and 12% GLY had similar (p > 0.05) effects on the post-thaw sperm motility when they were combined with extender-1 and extender-2, respectively. Cryopreservation using Me2SO or GLY also resulted in the highest post-thaw motility of sperm of Paralichthys olivaceus (Zhang et al., 2003) and Paralichthys dentatus (Brown et al., 2012; Liu et al., 2015). This is likely because sugars such as sucrose and glucose can stabilize phospholipids in the cell membrane during the freezing step (Ahn et al., 2018). Sugars may supply energy, reduce ice crystallization, and decrease the toxicity of CPA during the sperm cryopreservation process (Tian et al., 2015; Ahn et al., 2018). The current study did not reveal any effects of straw size on the post-thaw sperm motility when sperm were preserved with extender-1 or −2. Velasco-Santamaría et al. (2006) have also reported that the straw size has no effect on cryopreservation. In the present study, the post-thaw motility of 1-year cryopreserved sperm showed a significant similarity with the immediate cryopreserved (3-h) sperm and those of fresh sperm motility. Tanaka et al. (2002) reported that different storage periods showed a significantly similar post-thaw motility of Japanese eel. Similar phenomena were also reported from the cryopreserved turbot sperm (Suquet et al., 1998).
Cryopreservation with MeOH resulted in a decreased post-thaw sperm motility compared to that with Me2SO, GLY, EG, or PG. It also produced the lowest post-thaw motility for sperm of other flounder species such as Paralichthys olivaceus (Zhang et al., 2003), Paralichthys dentatus (Brown et al., 2012), and V. variegatus (Tian et al., 2008). Although it is associated with a low post-thaw motility of sperm of marine flounders, it has been reported as an effective CPA for cryopreserving sperm of freshwater fish (Lahnsteiner et al., 1997). Cryopreservation with Ringer's solution (extender-3) resulted in a lower post-thaw motility of sperm than cryopreservation with other types of the extender.
The present study revealed that extender-3 was not suitable for the cryopreservation of marbled flounder sperm. However, it has been previously reported that extender-3 is suitable for cryopreserving sperm of Rasbora tawarensis (Muchlisin et al., 2020) and yellow catfish, Pelteobagrus fulvidraco (Pan et al., 2008). The present findings suggest that the absence of sugar in extender-3 might be responsible for the reduced motility of post-thaw sperm, although a positive impact of sugar-based extenders (extender-1 and extender-2) on the sperm cryopreservation has been described in the previous section because such extender can additionally act as a non-penetrating CPA (Rusco et al., 2019). Similarly, extender-4 was not appropriate for the cryopreservation of marbled flounder sperm, although it resulted in improved motility than extender-3. This improvement might be due to a slight addition of sugar in the composition of extender-4. However, it has been previously reported that extender-4 is appropriate for the cryopreservation of sperm of filefish, Thamnaconus modestus (Le et al., 2008). These differences shed some light on the variation in the effectiveness of CPAs for sperm cryopreservation. Species-specific effectiveness of extender might also be responsible for such kinds of variation. Species-specific effects of extenders on cryopreservation have been previously reported for fish sperm (Le et al., 2011).
Plasma membrane integrity is the most investigated physiological parameter of cryopreserved fish sperm (Figueroa et al., 2016). The concept of sperm viability is associated with the intactness of the plasma membrane (Hernández-Avilés et al., 2019). In this study, 7-day cryopreserved sperm showed a similar morphology as fresh sperm under a fluorescent microscope. Six combinations of CPAs and extenders were used to assess their effects on PMI based on the post-thaw sperm motility. Among various combinations, extender-1 + 15% Me2SO and extender-1 + 12% GLY did not show any significant difference in PMI compared to PMI of fresh sperm (p > 0.05). However, slight differences in the integrity of the plasma membrane were observed between sperm cryopreserved with extender-2 + 15% Me2SO and those cryopreserved with extender-2 + 12% GLY. Notably, PMI values of summer flounder and Brazilian flounder showed significant differences (p < 0.05) between fresh and cryopreserved sperm (Lanes et al., 2008; Brown et al., 2012). In the present study, PMI of 1-year cryopreserved sperm using extender-1 combined with 15% Me2SO or 12% GLY showed a significant value similar to PMI values of 7-day cryopreserved sperm and those of fresh sperm. PMI values of rainbow trout cryopreserved sperm did not show significant differences when sperm were preserved in different storage periods (Pérez-Cerezales et al., 2010), which showed consistent results with the present findings. The present study suggests that a combination of 15% Me2SO or 12% GLY with extender-1 does not affect the integrity of the plasma membrane of the long-term cryopreserved sperm.
Six combinations of CPAs and extenders were also used to assess their effects on MMP as an index of the post-thaw sperm quality. There were no significant differences in MMP (p > 0.05) between fresh sperm and sperm cryopreserved (7 days) with extender-1 + 15% Me2SO or extender-1 + 12% GLY. However, 15% Me2SO + extender-2, 12% GLY + extender-2, 15% EG + extender-1, and 15% PG + extender-1 resulted in significant (p < 0.05) differences in MMP values compared to MMP values of fresh sperm. Therefore, cryopreservation may not affect the mitochondrial membrane of cryopreserved sperm. The MMP of cryopreserved sperm derived from Atlantic salmon (Salmo salar) was significantly (p < 0.05) lower than that of fresh sperm (Figueroa et al., 2019). Similar to MMP, the post-thaw sperm motility of Atlantic salmon was also significantly lower than that of fresh sperm. In the present study, MMP of 1-year cryopreserved sperm using extender-1 combined with 15% Me2SO or 12% GLY showed a significant value similar to MMP values of 7-day cryopreserved sperm and those of fresh sperm. Kim et al. (2016) reported the effects of long-term (3-year) cryopreservation on MMP of seven-band grouper. However, the present study demonstrated that post-thaw motility of cryopreserved sperm was similar to that of fresh sperm, which could explain the absence of difference in MMP between fresh and sperm cryopreserved (7 days and 1 year) using extender-1 + 15% Me2SO or extender-1 + 12% GLY.
In the present study, motility, PMI, and MMP were used as three indicators of sperm vitality. Results showed that 15% Me2SO or 12% GLY along with extender-1 was appropriate for the cryopreservation of marbled flounder sperm. DNA integrity is another important indicator of successful cryopreservation (Balamurugan et al., 2019; Kim et al., 2020a). The freeze–thaw process may damage the DNA integrity of cryopreserved sperm (Steele et al., 2000; Balamurugan et al., 2019). Thus, a comet assay was performed to assess the DNA integrity of 7-day and 1-year cryopreserved sperm in the present study. However, a previous study has reported that the sperm nucleus is stable and undamaged during a freeze–thaw process (Gwo et al., 2003). Based on PMI and MMP results, comet assay was performed using sperm cryopreserved (7 days and 1 year) with extender-1 in combination with 15% Me2SO or 12% GLY. Results of the comet assay showed that cryopreservation did not damage the integrity of sperm DNA. There was no fragmentation of DNA from the nucleus of 7-day and 1-year cryopreserved sperm. Similarly, it has been reported that gray mullet, Atlantic croaker, and rainbow trout, seven-band grouper sperm are not damaged by the freeze–thaw process after the immediate or long-term cryopreservation (Labbe et al., 2001; Balamurugan et al., 2019; Kim et al., 2020a). However, conflicting findings have been reported for the sperm of sea bream and sea bass, showing large quantities of fragmented DNAs in cryopreserved sperm (Zilli et al., 2003; Cabrita et al., 2005). Natural variations between species are responsible for the specific tolerance to cryo-damage, particular chromatin structure, and variation in extender applicability for sperm cryopreservation (Pérez-Cerezales et al., 2009).
Indicators of post-thaw sperm vitality such as motility, PMI, MMP, and DNA integrity of sperm cryopreserved (7 days and 1 year) with extender-1 along with 15% Me2SO or 12% GLY did not show any significant difference compared to those of fresh sperm. Based on the findings of these indicators, we recommend the use of lower concentrations of CPAs (12% GLY and 15% Me2SO) with extender-1 over the use of higher concentrations of CPAs with extender-2 (20% EG or PG) reported previously (Song et al., 2016).
The present study suggests that a sucrose-based extender in combination with 15% Me2SO or 12% GLY is optimal for the cryopreservation of marbled flounder sperm. This study analyzed several parameters associated with vitality indicators, including post-thaw sperm motility, PMI, MMP, and DNA integrity, to demonstrate the effectiveness of each combination of CPA and extender. This study also reported that post-thaw vitality of 1-year cryopreserved sperm using sucrose-based extender in combination with 15% Me2SO or 12% GLY is significant similar to fresh sperm. The suggested cryopreservation protocol may be helpful for hatchery production as improvement in P. yokohamae sperm cryopreservation techniques is required to create a germplasm bank.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Experimental protocols used were approved by Chonnam National University Animal Care and Use Committee (Approval No.: CNU IACUC-YS-2019-10; Approval date: December 10th, 2019). All fish experiments were performed according to Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
KK and SH conceptualized the study, wrote the original paper, and involved in the review and editing of the manuscript. KK, SH, SK, and YC performed the methodology and investigation. SH performed data curation, visualization, formal analysis, and software analysis. KK did supervision, validation, and fund acquisition. All authors have read and agreed to the published version of the manuscript.
This research was a part of the project entitled Jeonnam Sea Grant Program (Grant No. 20190401) funded by the Ministry of Ocean and Fisheries, Korea.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.696737/full#supplementary-material
Agnihotri, S. K., Agrawal, A. K., Hakim, B. A., Vishwakarma, A. L., Narender, T., Sachan, R., et al. (2016). Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell. Dev. Biol. Anim. 52, 953–960. doi: 10.1007/s11626-016-0061-x
Ahn, J. Y., Park, J. Y., and Lim, H. K. (2018). Effects of different diluents, cryoprotective agents, and freezing rates on sperm cryopreservation in Epinephelus akaara. Cryobiology 83, 60–64. doi: 10.1016/j.cryobiol.2018.06.003
Babiak, I., Ottesen, O., Rudolfsen, G., and Johnsen, S. (2006). Quantitative characteristics of Atlantic halibut, Hippoglossus hippoglossus L., semen throughout the reproductive season. Theriogenology 65, 1587–1604. doi: 10.1016/j.theriogenology.2005.09.004
Balamurugan, R., Prapaporn, W., and Munuswamy, N. (2019). Sperm activation and effects of cryopreservation on motility, ultrastructure and DNA integrity in Grey mullet Mugil cephalus. Aquac. Rep. 14:100204. doi: 10.1016/j.aqrep.2019.100204
Brown, R. T., Colburn, H. R., Nardi, G. C., and Berlinsky, D. L. (2012). Cryopreservation of summer flounder, Paralichthys dentatus L., sperm. Aquac. Res. 44, 1560–1567. doi: 10.1111/j.1365-2109.2012.03163.x
Cabrita, E., Robles, V., Rebordinos, L., Sarasquete, C., and Herráez, M. P. (2005). Evaluation of DNA damage in rainbow trout (Oncorhynchus mykiss) and gilthead sea bream (Sparus aurata) cryopreserved sperm. Cryobiology 50, 144–153. doi: 10.1016/j.cryobiol.2004.12.003
Cabrita, E., Sarasquete, C., Martínez-Páramo, S., Robles, V., Beirao, J., Pérez-Cerezales, S., et al. (2010). Cryopreservation of fish sperm: applications and perspectives. J. Appl. Ichthyol. 26, 623–635. doi: 10.1111/j.1439-0426.2010.01556.x
Cejko, B. I., Sarosiek, B., Dryl, K., Judycka, S., Szczepkowska, B., Szczepkowski, M., et al. (2020). The effect of cryopreservation extender on sperm motility and hatch success in northern pike (Esox lucius). Aquaculture 514:734482. doi: 10.1016/j.aquaculture.2019.734482
Celeghini, E. C. C., De Arruda, R. P., De Andrade, A. F. C., Nascimento, J., and Raphael, C. F. (2007). Practical techniques for bovine sperm simultaneous fluorimetric assessment of plasma, acrosomal, and mitochondrial membranes. Reprod. Domest. Anim. 42, 479–488. doi: 10.1111/j.1439-0531.2006.00810.x
Ding, F., Lall, S. P., Li, J., Lei, J., Rommens, M., and Milley, J. E. (2011). Cryopreservation of sperm from Atlantic halibut (Hippoglossus hippoglossus, L.) for commercial application. Cryobiology 63, 56–60. doi: 10.1016/j.cryobiol.2011.04.009
Ding, S., Ge, J., Hao, C., Zhang, M., Yan, W., Xu, Z., et al. (2009). Long-term cryopreservation of sperm from Mandarin fish Siniperca chuatsi. Anim. Reprod. Sci. 113, 229–235. doi: 10.1016/j.anireprosci.2008.08.003
Fernández-Santos, M. R., Esteso, M. C., Montoro, V., Soler, A. J., and Garde, J. J. (2006). Cryopreservation of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa: effects of egg yolk, glycerol, and cooling rate. Theriogenology 66, 1931–1942. doi: 10.1016/j.theriogenology.2006.05.012
Figueroa, E., Lee-Estevez, M., Valdebenito, I., Watanabe, I., Oliveira, R. P. S., Romero, J., et al. (2019). Effects of cryopreservation on mitochondrial function and sperm quality in fish. Aquaculture 511:634190. doi: 10.1016/j.aquaculture.2019.06.004
Figueroa, E., Valdebenito, I., and Farias, J. G. (2016). Technologies used in the study of sperm function in cryopreserved fish spermatozoa. Aquac. Res. 47, 1691–1705. doi: 10.1111/are.12630
Fuller, B. J. (2004). Cryoprotectants: the essential antifreezes to protect life in the frozen state. CryoLetters 25, 375–388. doi: 10.1201/9780203647073
Gheller, S. M. M., Corcini, C. D., de Brito, C. R., Acosta, I. B., Tavares, G. C., Soares, S. L., et al. (2019). Use of trehalose in the semen cryopreservation of Amazonian catfish Leiarius marmoratus. Cryobiology 87, 74–77. doi: 10.1016/j.cryobiol.2019.02.001
Gwo, J. C., Wu, C. Y., Chang, W. S. P., and Cheng, H. Y. (2003). Evaluation of damage in Pacific oyster (Crassostrea gigas) spermatozoa before and after cryopreservation using comet assay. Cryoletters 24, 171–180.
Hassan, M. M., Li, X., and Qin, J. G. (2017). Improvement of post-thaw sperm survivals using liquid nitrogen vapor in a spermcasting oyster Ostrea angasi. Cryobiology 78, 1–7. doi: 10.1016/j.cryobiol.2017.08.003
He, S., and Woods, L. C. (2004). Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology 48, 254–262. doi: 10.1016/j.cryobiol.2004.01.009
Hernández-Avilés, C., Zambrano-Varón, J., and Jiménez-Escobar, C. (2019). Current trends on stallion semen evaluation: what other methods can be used to improve our capacity for semen quality assessment? J. Vet. Androl. 4, 1–19.
Herrera-Cruz, E., Aristizabal-Regino, J., Yepes-Blandón, J., Estrada-Posada, A., Espinosa-Araujo, J., and Atencio-Garcia, V. (2019). Evaluation of three cryoprotectants to preserve striped catfish (Pseudoplatystoma magdaleniatum) semen. Rev. Colomb. Biotecnol. 21, 55–62. doi: 10.15446/rev.colomb.biote.v21n2.77847
Horváth, Á., Wayman, W. R., Dean, J. C., Urbányi, B., Tiersch, T. R., Mims, S. D., et al. (2008). Viability and fertilizing capacity of cryopreserved sperm from three North American acipenseriform species: a retrospective study. J. Appl. Ichthyol. 24, 443–449. doi: 10.1111/j.1439-0426.2008.01134.x
Hossen, S., Sharker, M., Cho, Y., Sukhan, Z. P., and Kho, K. H. (2021b). Effects of antifreeze protein III on sperm cryopreservation of Pacific abalone, Haliotis discus hannai. Int. J. Mol. Sci. 22:3917. doi: 10.3390/ijms22083917
Hossen, S., Sukhan, Z. P., Cho, Y., and Kho, K. H. (2021a). Effects of cryopreservation on gene expression and post thaw sperm quality of Pacific abalone, Haliotis discus hannai. Front. Mar. Sci. 8:399. doi: 10.3389/fmars.2021.652390
Hu, E., Cuevas-Uribe, R., Yang, H., Sanderson, R., Gill, A. O., Daniels, H., et al. (2016). High-throughput cryopreservation of sperm from sex-reversed Southern Flounder, Paralichthys lethostigma. J. World Aquac. Soc. 47, 555–565. doi: 10.1111/jwas.12293
Immerman, D. A., and Goetz, F. W. (2014). The activation and cryopreservation of sablefish (Anoplopoma fimbria) sperm. Aquaculture 430, 211–217. doi: 10.1016/j.aquaculture.2014.04.010
Iorio, M. D., Esposito, S., Rusco, G., Roncarati, A., Miranda, M., Gibertoni, P. P., et al. (2019). Semen cryopreservation for the Mediterranean brown trout of the Biferno River (Molise-Italy): comparative study on the effects of basic extenders and cryoprotectants. Sci. Rep. 9, 1–9. doi: 10.1038/s41598-019-45006-4
Judycka, S., Nynca, J., Liszewska, E., Mostek, A., and Ciereszko, A. (2019). Comparative analysis of sperm freezability of sex-reversed female brook trout and sex-reversed female rainbow trout semen. Aquaculture 498, 201–207. doi: 10.1016/j.aquaculture.2018.08.064
Kim, S. C., Hossen, S., and Kho, K. H. (2020a). Effects of 3 years of cryopreservation on sperm quality of seven-band grouper, Epinephelus septemfasciatus. Aquac. Res. 51, 3050–3053. doi: 10.1111/are.14615
Kim, S. C., Hossen, S., and Kho, K. H. (2020b). Cryopreservation of sperm from farmed Pacific abalone, Haliotis discus hannai. Cryobiology 94, 49–56. doi: 10.1016/j.cryobiol.2020.04.011
Kim, S. R., Cha, H. K., Lee, J. B., Lee, H. W., Yang, J. H., Baek, H. J., et al. (2016). Maturity and spawning of the marbled flounder Pseudopleuronectes yokohamae off the coast of Pohang, East Sea. Korean J. Fish. Aquat. Sci. 49, 367–375. doi: 10.5657/KFAS.2016.0367
Kusakabe, K., Hata, M., Shoji, J., Hori, M., and Tomiyama, T. (2017). Effects of water temperature on feeding and growth of juvenile marbled flounder Pseudopleuronectes yokohamae under laboratory conditions: evaluation by group-and individual-based methods. Fish. Sci. 83, 215–219. doi: 10.1007/s12562-016-1053-1
Labbe, C., Martoriati, A., Devaux, A., and Maisse, G. (2001). Effect of sperm cryopreservation on sperm DNA stability and progeny development in rainbow trout. Mol. Reprod. Dev. 60, 397–404. doi: 10.1002/mrd.1102
Lahnsteiner, F., Weismann, T., and Patzner, R. A. (1997). Methanol as cryoprotectant and the suitability of 1.2 ml and 5 ml straws for cryopreservation of semen from salmonid fishes. Aquac. Res. 28, 471–479. doi: 10.1111/j.1365-2109.1997.tb01065.x
Lanes, C. F. C., Okamoto, M., Cavalcanti, P. V., Collares, T., Campos, V. F., Deschamps, J. C., et al. (2008). Cryopreservation of Brazilian flounder (Paralichthys orbignyanus) sperm. Aquaculture 275, 361–365. doi: 10.1016/j.aquaculture.2007.12.025
Le, M. H., Lim, H. K., Min, B. H., Park, M. W., and Chang, Y. J. (2011). Semen cryopreservation of yellow croaker Larimichthys polyactis. Rev. Fish Biol. Fish. 21, 789–797. doi: 10.1007/s11160-011-9209-7
Le, M. H., Lim, H. K., Min, B. H., Son, M. H., Lee, J. U., and Chang, Y. J. (2008). Diluents and cryoprotectants for cryopreservation of filefish Thamnaconus modestus sperm. J. of Aquac. 21, 54–59.
Liu, Q. H., Ma, D. Y., Xu, S. H., Xiao, Z. Z., Xiao, Y. S., Song, Z. C., et al. (2015). Summer flounder (Paralichthys dentatus) sperm cryopreservation and application in interspecific hybridization with olive flounder (P olivaceus). Theriogenology 83, 703–710. doi: 10.1016/j.theriogenology.2014.11.004
Lucas, J. S., Southgate, P. C., and Tucker, C. S., (eds.). (2019). Aquaculture: Farming Aquatic Animals and Plants. Hoboken, NJ: John Wiley and Sons.
Merino, O., Sánchez, R., Risopatrón, J., Isachenko, E., Katkov, I. I., Figueroa, E., et al. (2012). Cryoprotectant-free vitrification of fish (Oncorhynchus mykiss) spermatozoa: first report. Andrologia 44, 390–395. doi: 10.1111/j.1439-0272.2011.01196.x
Muchlisin, Z. A., Sarah, P. I., Aldila, D. F., Eriani, K., Hasri, I., Batubara, A. S., et al. (2020). Effect of Dimethyl sulfoxide (DMSO) and egg yolk on sperm motility, fertility and hatching rates of depik Rasbora tawarensis (Pisces: Cyprinidae) eggs after short-term cryopreservation. Aquac. Res. 51, 1700–1705. doi: 10.1111/are.14516
Nahiduzzaman, M., Hassan, M. M., Khanam, U. H., Mamun, S. N. A., Hossain, M. A., and Tiersch, T. R. (2011). Sperm cryopreservation of the critically endangered olive barb (Sarpunti) Puntius sarana (Hamilton, 1822). Cryobiology 62, 62–67. doi: 10.1016/j.cryobiol.2010.12.004
Pan, J., Ding, S., Ge, J., Yan, W., Hao, C., Chen, J., et al. (2008). Development of cryopreservation for maintaining yellow catfish Pelteobagrus fulvidraco sperm. Aquaculture 279, 173–176. doi: 10.1016/j.aquaculture.2008.03.037
Park, J. M., Kwak, S. N., Choi, H. C., Jawad, L. A., and Riedel, R. (2016). Diet patterns of the marbled flounder, Pseudopleuronectes yokohamae, in the mid-western coast of Korea. Sci. Int. 4, 94–100. doi: 10.17311/sciintl.2016.94.100
Pereira, G. R., Becker, E. G., Siqueira, L. C., Ferreira, R., Severo, C. K., Truzzi, V. S., et al. (2010). Assessment of bovine spermatozoa viability using different cooling protocols prior to cryopreservation. Ital. J. Anim. Sci. 9, 465–470. doi: 10.4081/ijas.2010.e88
Pérez-Cerezales, S., Martínez-Páramo, S., Beirão, J., and Herráez, M. P. (2010). Evaluation of DNA damage as a quality marker for rainbow trout sperm cryopreservation and use of LDL as cryoprotectant. Theriogenology 74, 282–289. doi: 10.1016/j.theriogenology.2010.02.012
Pérez-Cerezales, S., Martínez-Páramo, S., Cabrita, E., Martínez-Pastor, F., De Paz, P., and Herráez, M. P. (2009). Evaluation of oxidative DNA damage promoted by storage in sperm from sex-reversed rainbow trout. Theriogenology 71, 605–613. doi: 10.1016/j.theriogenology.2008.09.057
Rusco, G., Di Iorio, M., Gibertoni, P. P., Esposito, S., Penserini, M., Roncarati, A., et al. (2019). Optimization of sperm cryopreservation protocol for mediterranean brown trout: a comparative study of non-permeating cryoprotectants and thawing rates in vitro and in vivo. Animals 9:304. doi: 10.3390/ani9060304
Rusco, G., Di Iorio, M., Iampietro, R., Esposito, S., Gibertoni, P. P., Penserini, M., et al. (2020). A simple and efficient semen cryopreservation method to increase the genetic variability of endangered mediterranean brown trout inhabiting molise rivers. Animals 10:403. doi: 10.3390/ani10030403
Sarosiek, B., Dryl, K., Judycka, S., Dobosz, S., Grudniewska, J., and Kowalski, R. K. (2016). Cryopreservation method for whitefish (Coregonus lavaretus) semen possible for use in large-scale fertilisation. Aquac. Res. 47, 4038–4042. doi: 10.1111/are.12810
Song, L., Tian, Y., Li, X., Li, H., Sun, Z., Chen, Z., et al. (2016). Cryopreservation of marbled flounder (Pseudopleuronectes yokohamae) sperm and analysis of its physiological characteristics. J. Agric. Biotechnol. 24, 584–592. doi: 10.3969/j.issn.1674-7968.2016.04.013
Soni, Y., Talluri, T. R., Kumar, A., Ravi, S. K., Mehta, J. S., and Tripathi, B. N. (2019). Effects of different concentration and combinations of cryoprotectants on sperm quality, functional integrity in three Indian horse breeds. Cryobiology 86, 52–57. doi: 10.1016/j.cryobiol.2018.12.005
Steele, E. K., McClure, N., and Lewis, S. E. (2000). Comparison of the effects of two methods of cryopreservation on testicular sperm DNA. Fertil. Steril. 74, 450–453. doi: 10.1016/S0015-0282(00)00680-4
Suquet, M., Dreanno, C., Fauvel, C., Cosson, J., and Billard, R. (2000). Cryopreservation of sperm in marine fish. Aquac. Res. 31, 231–243. doi: 10.1046/j.1365-2109.2000.00445.x
Suquet, M., Dreanno, C., Petton, B., Normant, Y., Omnes, M. H., and Billard, R. (1998). Long-term effects of the cryopreservation of turbot (Psetta maxima) spermatozoa. Aquat. Living Resour. 11, 45–48. doi: 10.1016/S0990-7440(99)80030-8
Tanaka, S., Zhang, H., Horie, N., Yamada, Y., Okamura, A., Utoh, T., et al. (2002). Long-term cryopreservation of sperm of Japanese eel. J. Fish Biol. 60, 139–146. doi: 10.1111/j.1095-8649.2002.tb02393.x
Tian, Y., Jiang, J., Wang, N., Qi, W., Zhai, J., Li, B., et al. (2015). Sperm of the giant grouper: cryopreservation, physiological, and morphological analysis and application in hybridizations with red-spotted grouper. J. Reprod. Dev. 64, 333–339. doi: 10.1262/jrd.2014-087
Tian, Y. S., Chen, S. L., Ji, X. S., Zhai, J. M., Sun, L. J., Chen, C., et al. (2008). Cryopreservation of spotted halibut (Verasper variegatus) sperm. Aquaculture 284, 268–271. doi: 10.1016/j.aquaculture.2008.07.047
Velasco-Santamaría, Y. M., Medina-Robles, V. M., and Cruz-Casallas, P. E. (2006). Cryopreservation of yamú (Brycon amazonicus) sperm for large scale fertilization. Aquaculture 256, 264–271. doi: 10.1016/j.aquaculture.2006.02.039
Viveiros, A. T. M., Isaú, Z. A., Caneppele, D., and Leal, M. C. (2012). Sperm cryopreservation affects postthaw motility, but not embryogenesis or larval growth in the Brazilian fish Brycon insignis (Characiformes). Theriogenology 78, 803–810. doi: 10.1016/j.theriogenology.2012.03.028
Xin, M., Shaliutina-Kolesova, A., Sterba, J., Konik, P., Boryshpolets, S., Rodina, M., et al. (2018a). Impact of cryopreservation on sterlet, Acipenser ruthenus sperm motility and proteome. Anim. Reprod. Sci. 192, 280–289. doi: 10.1016/j.anireprosci.2018.03.025
Xin, M., Tučková, V., Rodina, M., Kholodnyy, V., Dadras, H., Boryshpolets, S., et al. (2018b). Effects of antifreeze proteins on cryopreserved sterlet (Acipenser ruthenus) sperm motility variables and fertilization capacity. Anim. Reprod. Sci. 196, 143–149. doi: 10.1016/j.anireprosci.2018.07.007
Yang, H., Norris, M., Winn, R., and Tiersch, T. R. (2010). Evaluation of cryoprotectant and cooling rate for sperm cryopreservation in the euryhaline fish medaka Oryzias latipes. Cryobiology 61, 211–219. doi: 10.1016/j.cryobiol.2010.07.006
Yang, S., Han, L., Huang, R., Liufu, Y., Meng, Z., and Lin, H. (2017). Optimization of conditions for the cryopreservation of yellow catfish (Pelteobagrus fulvidraco) sperm. Cryobiology 76, 104–110. doi: 10.1016/j.cryobiol.2017.03.009
Zhang, Y. Z., Zhang, S. C., Liu, X. Z., Xu, Y. Y., Wang, C. L., Sawant, M. S., et al. (2003). Cryopreservation of flounder (Paralichthys olivaceus) sperm with a practical methodology. Theriogenology 60, 989–996. doi: 10.1016/S0093-691X(03)00097-9
Keywords: cryopreservation, comet assay, motility, mitochondrial membrane potential, plasma membrane integrity, sperm
Citation: Hossen S, Kim SC, Cho Y and Kho KH (2021) Vital Analysis of Cryopreserved Sperm of Marbled Flounder, Pseudopleuronectes yokohamae. Front. Physiol. 12:696737. doi: 10.3389/fphys.2021.696737
Received: 17 April 2021; Accepted: 04 June 2021;
Published: 28 June 2021.
Edited by:
Jorge G. Farias, University of La Frontera, ChileReviewed by:
John Quiñones Diaz, University of La Frontera, ChileCopyright © 2021 Hossen, Kim, Cho and Kho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang Hee Kho, a2toQGNob25uYW0uYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.