- 1Environmental Physiology and Medicine Laboratory, Department of Biomedical Sciences, Università degli Studi di Padova, Padua, Italy
- 2DAN Europe Research Division, DAN Europe Foundation, Roseto degli Abruzzi, Italy
- 3Apnea Academy Research, Padua, Italy
- 4Department of Health Sciences, Università degli Studi di Milano, Milan, Italy
- 5Cardiothoracic and Vascular Department, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
Introduction: Nitric oxide (NO) is an essential signaling molecule modulating the endothelial adaptation during breath-hold diving (BH-diving). This study aimed to investigate changes in NO derivatives (NOx) and total antioxidant capacity (TAC), searching for correlations with different environmental and hyperbaric exposure.
Materials and methods: Blood samples were obtained from 50 breath-hold divers (BH-divers) before, and 30 and 60 min after the end of training sessions performed both in a swimming pool or the sea. Samples were tested for NOx and TAC differences in different groups related to their hyperbaric exposure, experience, and additional genetic polymorphism.
Results: We found statistically significant differences in NOx plasma concentration during the follow-up (decrease at T30 and increase at T60) compared with the pre-dive values. At T30, we found a significantly lower decrease of NOx in subjects with a higher diving experience, but no difference was detected between the swimming pool and Sea. No significant difference was found in TAC levels, as well as between NOx and TAC levels and the genetic variants.
Conclusion: These data showed how NO consumption in BH-diving is significantly lower in the expert group, indicating a possible training-related adaptation process. Data confirm a significant NO use during BH-diving, compatible with the well-known BH-diving related circulatory adaptation suggesting that the reduction in NOx 30 min after diving can be ascribed to the lower NO availability in the first few minutes after the dives. Expert BH-divers suffered higher oxidative stress. A preliminary genetic investigation seems to indicate a less significant influence of genetic predisposition.
Introduction
Nitric oxide (NO) is considered as one of the most important molecules regulating vascular adaptations during breath-hold diving (BH-diving; Lundberg et al., 2015) because of its action in the control of the cardiovascular system, blood flow, and blood pressure.
NO is involved in many physiological and pathological processes (Rand, 1992; Hou et al., 1999). Due to its short half-life (0.05–1.8 ms) (Liu et al., 1998), NO availability is ensured by a family of NO synthases (NOS), composed of at least three different isoforms (Moncada and Higgs, 1993).
The endothelial isoform (e-NOS) is mainly involved in modulating the vasodilator tone, vascular integrity preservation, and regulation of arterial blood pressure (Rand, 1992). eNOS also inhibits platelet aggregation and adhesion and enhances vascular permeability (Behrendt and Ganz, 2002). e-NOS levels are regulated by many factors, such as hypoxia and local substrate availability (Ostergaard et al., 2007), vascular shear stress (Chatterjee et al., 2008), and, most important, by different genetic variants (polymorphisms) (Ahsan et al., 2006; Wang et al., 2010). Indeed, functional variants in the endothelial NOS3 gene might alter the expression of the enzyme (Senthil et al., 2005).
Since NO is a radical, its levels are difficult to quantify (Liu et al., 1998), and it is preferable to measure stable NO derivates such as Nitrate and nitrites (NOx) (van Vliet et al., 1997) and byproducts of NO oxidation in blood and tissues (Moncada and Higgs, 1993). Specifically, NO is oxidated to nitrite (NO2) or, when oxyhemoglobin is available, to nitrate (NO3), with NO3 being predominant in blood circulation (Lundberg et al., 2008). Healthy individuals produce approximately 1 mmol of NO3 daily due to the oxidation of endogenously synthesized NO (Macallan et al., 1997). If necessary, NO3 can be reduced to NO2 by several enzymes, such as xanthine oxidase (Li et al., 2003) and xanthine oxidoreductase (Jansson et al., 2008). NO2 is further reduced to NO by different pathways, including hemoglobin (Cosby et al., 2003), myoglobin (Rassaf et al., 2007; Shiva et al., 2007a), xanthine oxidoreductase (Godber et al., 2000), and ascorbic acid (Carlsson et al., 2001). These pathways are significantly enhanced during hypoxia and acidosis to ensure NO production when the oxygen-dependent NOS enzyme activities are compromised (Giraldez et al., 1997; Ostergaard et al., 2007). In addition, NO2 reduction to NO during physiological hypoxia seems to contribute to physiological hypoxic signaling, vasodilation, and modulation of cellular respiration (Modin et al., 2001; Cosby et al., 2003; Shiva et al., 2007a,b).
Some studies have demonstrated an increase in circulating NO in breath-hold divers (BH-divers) after repetitive diving up to 20 m over 25 min in a pool and suggested possible correlations with physical exercise (Theunissen et al., 2013a). As BH-divers, marine mammals (Elsner et al., 1998) are subjected to post-diving ischemia-reperfusion when arterial and tissue oxygen levels are restored after reaching the surface, thus leading to an increase in reactive oxygen species (ROS) production (Dhaliwal et al., 1991; Bosco et al., 2010, 2020). Higher ROS levels can be harmful (Forkner et al., 2007; Diringer, 2008) by exacerbating the redox imbalance, increasing oxidative stress, and depleting acutely the antioxidant defenses of the body (Terraneo and Samaja, 2017).
Endothelial dysfunction has also been demonstrated in BH-diving (Brubakk et al., 2005; Obad et al., 2010) and can be explained by two hypotheses. First, the BH-diving-related transient hypoxia and accumulation of CO2 induce an increase in ROS levels, causing higher oxidative stress (Theunissen et al., 2013b; Mrakic-Sposta et al., 2019) and NO-related endothelial changes (Theunissen et al., 2013a). Second, the development of venous gas embolism, frequently observed in self-contained underwater breathing apparatus diving (SCUBA) despite correct decompression procedures (Valic et al., 2005), has also been recently demonstrated in BH-divers (Cialoni et al., 2016) and could play a role in the pathogenesis of BH-diving-related endothelial dysfunction.
Since NO is primarily released from arterial endothelium along with many other regulatory substances (Heitzer et al., 2001), any condition causing endothelial dysfunction inevitably affects NO levels and leads to cardiovascular diseases, such as coronary artery disease, peripheral arteriopathy (Drexler, 1997; Pepine, 1998), and atherosclerosis (Keaney and Vita, 1995; Tousoulis et al., 2010). Some studies have confirmed a primary role in increased oxidative stress caused by endothelium dysfunction in cardiovascular disease (Cai and Harrison, 2000). Thus, the investigation of hidden mechanisms predisposing BH-divers to the development of cardiovascular diseases is of paramount importance.
On the other hand, recent observations seem to indicate the existence of a genetic predisposition in developing BH-diving-related injuries (Cialoni et al., 2015) or diving reflex related adjustments (Baranova et al., 2017), but there is no clear evidence in the published literature whether NO availability and oxidative stress occurring in BH-diving are related to extreme environmental conditions (such as ambient pressure, salinity, and water temperature) rather than genetic predisposition. However, we also need to take into account that in niche sectors, such as diving, in which it is very difficult to plan genetic protocols in higher numbers of subjects, the recommendation in these conditions is to use biallelic markers [such as single nucleotide polymorphisms (SNPs)] to obtain indicative data even in lower numbers of subjects (Chandrika, 2001).
The study aims to investigate the changes in NOx and antioxidant response [plasma total antioxidant capacity (TAC)] after a series of BH-dives in different environmental and hyperbaric exposure conditions. In the Supplementary Material, we also show the preliminary results related to the NO and oxidative stress response in BH-divers with different genetic variants of 10 selected polymorphisms.
Materials and Methods
Subjects and Dives
50 Expert healthy BH-Divers were studied in two settings: the first group during a series of deep dives at the swimming pool Y-40 “The Deep Joy®” (Montegrotto Terme, Italy) (42-m-deep); the second group during an open water training session at the Elba Island (Italy).
All the divers were informed about the risks and benefits of this study and read and signed a specific, informed consent form before the experiment. All the participants also signed a dedicated genetic informed consent allowing the genetic analysis. The study was conducted as per the Helsinki Declaration and was approved by the Ethical Committee of Università degli Studi di Milano, Italy (Aut. No. 37/17).
Subjects aged >18 years and non-pregnant women were included in the study. None of the subjects had previous or clinical evidence of arterial hypertension, cardio-pulmonary diseases, Taravana episodes (BH-diving-related loss of consciousness or seizure), or any other significant disease.
Subjects were asked to avoid food rich in NO3, such as red meat (Lundberg, 2009) and leafy green vegetables (Lundberg and Govoni, 2004). None of them took prescription drugs, suffered from any acute disease during the 15 days before the experiment, or reported assumption of anti-inflammatory medications, exposure to high altitude in the 7 days, or intense exercise during the 48 h before the investigation. None of the BH-divers performed any compressed-gas diving during the 30 days before the experiment.
All the subjects were affiliated to the “Apnea Academy” training agency as instructors or high-level divers; however, all the divers could easily reach a minimum of the following criteria:
– 20 m depth in constant weight;
– 3 min of static breath-hold (at the surface); and
– 75 m of dynamic BH-diving in a swimming pool (distance).
All the divers performed their usual training with a freely determined number and time of warm-up dives, bottom time, and surface intervals.
As per the “Apnea Academy” standard procedures, the training session involved a gradual approach to the maximum daily personal depth and an unrestricted number of deep dives, at the end of which all the divers returned to the laboratory for the post diving test protocol.
Diving profiles were recorded using a UP-X1 free-diving computer (Omersub S.p.a., Sovico, Italy), including mean depth, maximum depth (MD), and number of dives (ND). The free-diving computers measured and recorded data every 2 s. The included divers were stratified into several groups to be analyzed. First, divers were interviewed and divided by diving level (LD) in medium or high experience (ME vs. HE) considering their BH-diving skills, defined by years of activity, personal depth record, number of weekly training sessions, and certification level. Another stratification considered three parameters achieved on the day of the experiment, namely: average depth (AD), an average of MD reached, and an average ND; subjects were then divided into those who dived above (AD-above, MD-above, and ND-above) and those who dived below the calculated averages (AD-below, MD-below, and ND-below).
Experimental Protocol
The protocol was the same in both the swimming pool and the sea tests.
Venous peripheral access was obtained from the antecubital vein of each subject, and blood samples were collected 30 min before the start of the diving series (basal). Blood samples were then collected 30 min (T30) and 60 min (T60) after the end of the BH-diving session, after discarding 5 ml of the blood to remove any clots. Plasma was obtained by centrifugation (3,000 rpm for 10 min) and was refrigerated at −20°C. Plasma samples were then delivered to the Laboratory of Biochemistry of the Department of Health Sciences (DISS) of the Università degli Studi di Milano for analysis.
Epithelial oral cells were also obtained using two buccal swabs from each volunteer. DNA was isolated using the ChargeSwitch kit (Invitrogen Corp., Carlsbad, CA, United States), following the instructions of the manufacturer, and both buccal swabs were suspended in 100 μl of elution buffer.
We investigated for the following:
✓ Differences in plasma concentration of NOx and TAC for the following diving risk factors:
• BH-LD (ME vs. HE)
• Environmental (swimming pool vs. sea)
✓ Differences in plasma concentration of NOx and TAC in the following recorded diving risk factors, between those above and those below the calculated average:
• AD (AD-above vs. AD-below)
• MD (MD-above vs. MD-below)
• ND (ND-above vs. ND below)
✓ Differences in plasma concentration of NOx and TAC (before and after the dives) in genetic variants of 10 investigated polymorphisms (as explained in the following sections).
Plasma NOx Measurement
All 50 subjects were investigated for the NOx plasma level repeated for the three times specified in the protocol. Before the analysis, plasma was deproteinized. Briefly, 400 μl of the sample was treated with 400 μl of acetonitrile (Romitelli et al., 2007) to precipitate the proteins and centrifuged at 12,000 rpm for 10 min. NOx was measured in the deproteinized plasma using a method based on Griess’s reaction as an index of NO concentration (Green et al., 1982), according to Cialoni et al. (2019). Plasma NOx levels were obtained by interpolation of standard NaNO3 curves (Tsikas, 2005). All the samples were analyzed two times. Results were expressed as a percentage of difference of the control value (basal).
Plasma TAC
A total of 38 subjects out of 50 were also investigated for TAC, using the ferric reducing antioxidant capacity (FRAP) assay (Benzie and Strain, 1996), with some modifications. Briefly, 45 μl of plasma was added to 1.5 ml of freshly prepared FRAP reactive in plastic tubes. After 5 min of incubation at 37°C, absorbance was read at 593 nm in a Uvikon 931 UV-VIS spectrophotometer (Northstar Scientific, Bardsey, United Kingdom). Aqueous solutions of FeSO4 7H2O (100–1,000 μM) were used for the calibration curve. TAC values were obtained by interpolation of the FeSO4 calibration curve. All the samples were analyzed two times, and the results were expressed as FRAP value [μM Fe (II)] of the samples (Zarban et al., 2009).
DNA Polymorphisms
About, 10 different genetic polymorphisms related to the investigated risk factors (available NO and oxidative stress) were analyzed, especially, 2 involved with NO availability, 4 with the anti-inflammatory activity, and 3 with the antioxidant capacity. Also, angiotensin-converting enzyme polymorphisms were analyzed.
The polymorphisms were analyzed using a real-time PCR (RT-PCR) technique. Specific primers and probes for the SNP rs1799983 were designed according to the TaqMan genotyping assay (Applied Biosystems, Foster City, CA, United States), while SNP rs2070744 was analyzed using primers and probes designed according to the Kaspar genotype assay (KBIoscience [B]). Both SNPs were analyzed on ABI 7900 following the instructions of the manufacturer.
In all the investigated polymorphisms, the NOx and TAC levels before the diving and at follow-up (T30 and T60) for the different genetic variants were analyzed.
Statistical Analysis
Data are presented as mean and SD for parametric data and median and range for non-parametric data. To minimize the subject-to-subject variability, data were normalized against the basal value. The Shapiro–Wilk normality test was used to verify a Gaussian distribution. Genetic data were compared using a one-way ANOVA for multiple comparisons or the Friedman test for multiple comparisons for parametric and non-parametric data, respectively. NOx and TAC were compared using a Mann-Whitney test or an unpaired t-test.
A probability lower than 5% was assumed as the threshold to reject the null hypothesis (p < 0.05).
Results
A total of 50 healthy BH-Divers (40 male and 10 female; mean age 43.24 ± 9.8; mean height 176.3 cm ± 7.1; mean weight 74.4 kg ± 10.4; and BMI 23.8 ± 2.4) were studied in two different environmental conditions: 22 at the swimming pool and 28 at sea (Supplementary Table 1A).
The overall diving profiles showed an AD of 22.2 ± 8.5 m, n = 23 AD-above, and n = 27 AD-below; MD of 33.2 ± 8.2 m, n = 24 AM-above, and n = 26 AM-below; and an ND of 16.5 ± 5.8, (n = 26 ND-above vs. n = 24 ND-below) (Supplementary Table 1B).
Subjects were also classified as HE of n = 28 and ME of n = 22 (Supplementary Table 1A).
The groups obtained by dividing the sample in above and below the average (AD, MD, ND-above vs. AD, MD, ND-below, respectively) showed significant differences between the more performant subjects as compared with the less performing ones in terms of AD(AD-above vs. AD-below); MD (MD-above vs. MD-below) and ND (ND-above vs. ND-below) confirming a different diving exposure in the two groups (above vs. below).
Similar significant differences were also found between more experienced and less experienced divers [ME vs. HE and (Supplementary Table 1B)].
We did not find any statistical differences in BMI and age in the groups selected (Supplementary Table 1C) as the MD and ND were not statistically different in the two investigated environments (swimming pool vs. sea). We only found a higher mean of depth in the swimming pool group as compared with the sea group (Supplementary Table 1C).
Regarding overall plasma NOx concentration, a significant decrease of –27.6% at T30 (73.5% of the control value, p < 0.0001) and a significant increase of +24.1 at T60 (124.1%, of the control value, p < 0.012) were found. All these differences (decrease at T30 and increase at T60) were statistically significant in terms of the percent of the pre-diving control value (Figure 1).
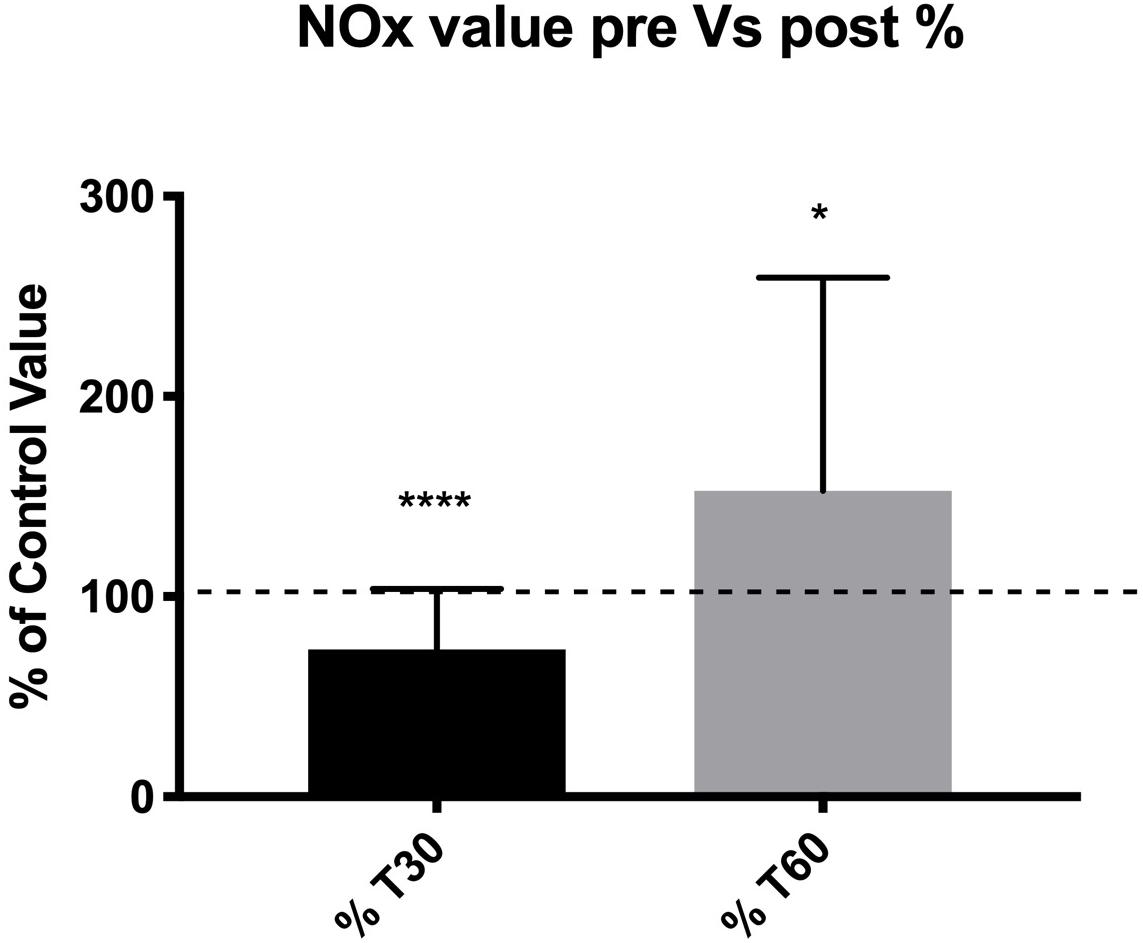
Figure 1. Subjects showed a lower NOx concentration at T30 suggesting a lower NO availability after dives, and a higher NOx concentration at T60 suggesting a NO availability re-balance effect. *p < 0.05, ***p < 0.001.
Regarding the differences in blood NOx concentration among the groups, a significantly lower decrease was found at T30 in experts (AD, p = 0.002; MD, p = 0.01; ND, p = 0.01; DL, p = 0.03) (Figure 2).
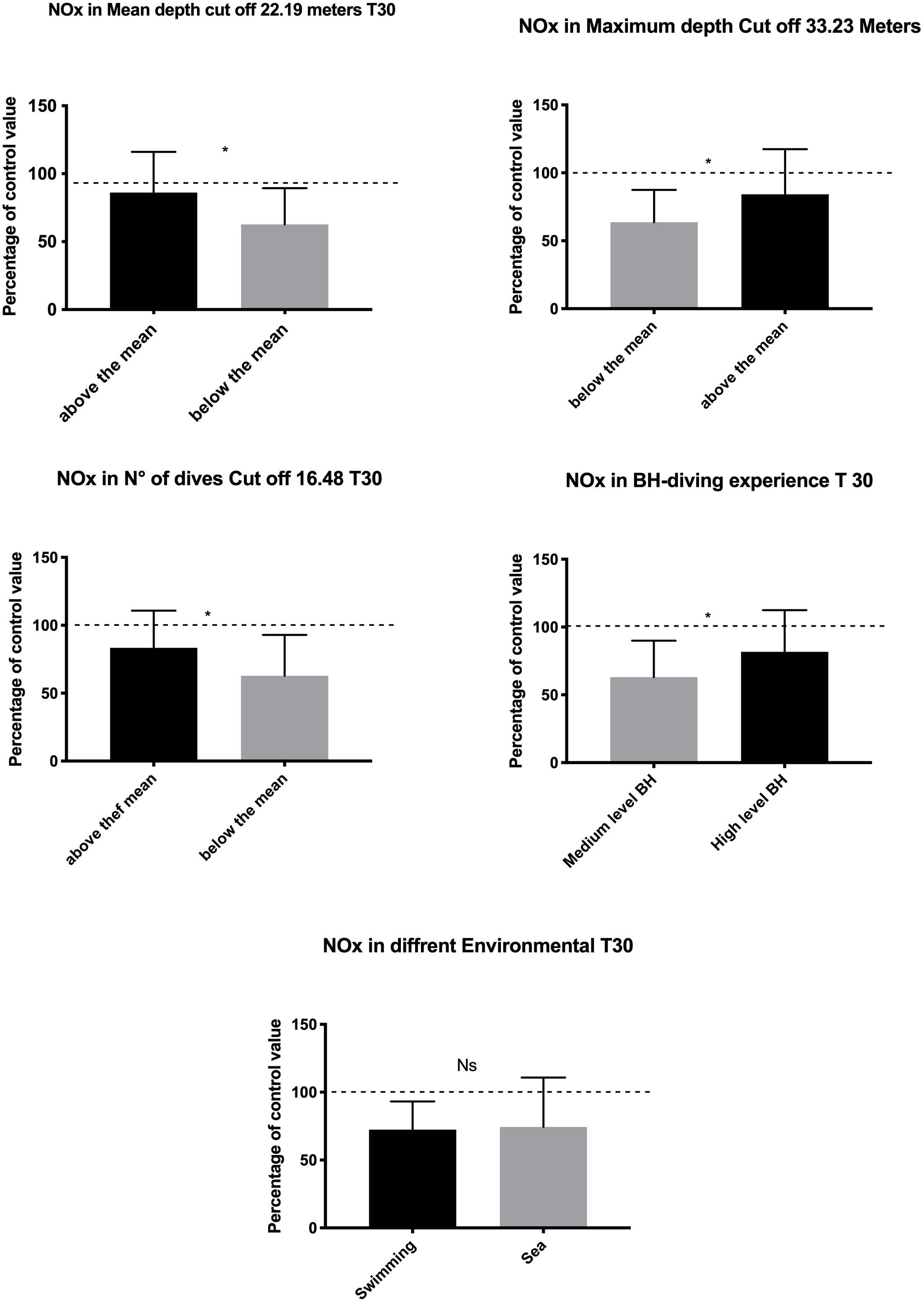
Figure 2. Data at T30 show lower NO usage in highly exposed and experienced subjects indicating adaptation in the management of hyperbaric-related vascular changes. Swimming Pool vs. sea BH-diving data showed no significant differences. *p < 0.05.
The difference, if any, in NOx plasma concentration was detected comparing the swimming pool vs. the sea setting (p = 0.81) (Figure 2).
At T60, a higher increase of NOx value was found in subjects with higher diving exposure in terms of MD (p = 0.018) and LD (p = 0.006). In contrast, the mean depth and the ND were not associated with significant changes in NOx levels. Finally, a higher NOx increase at T60 was found in the sea group than in the swimming pool group (p = 0.014) (Figure 3).
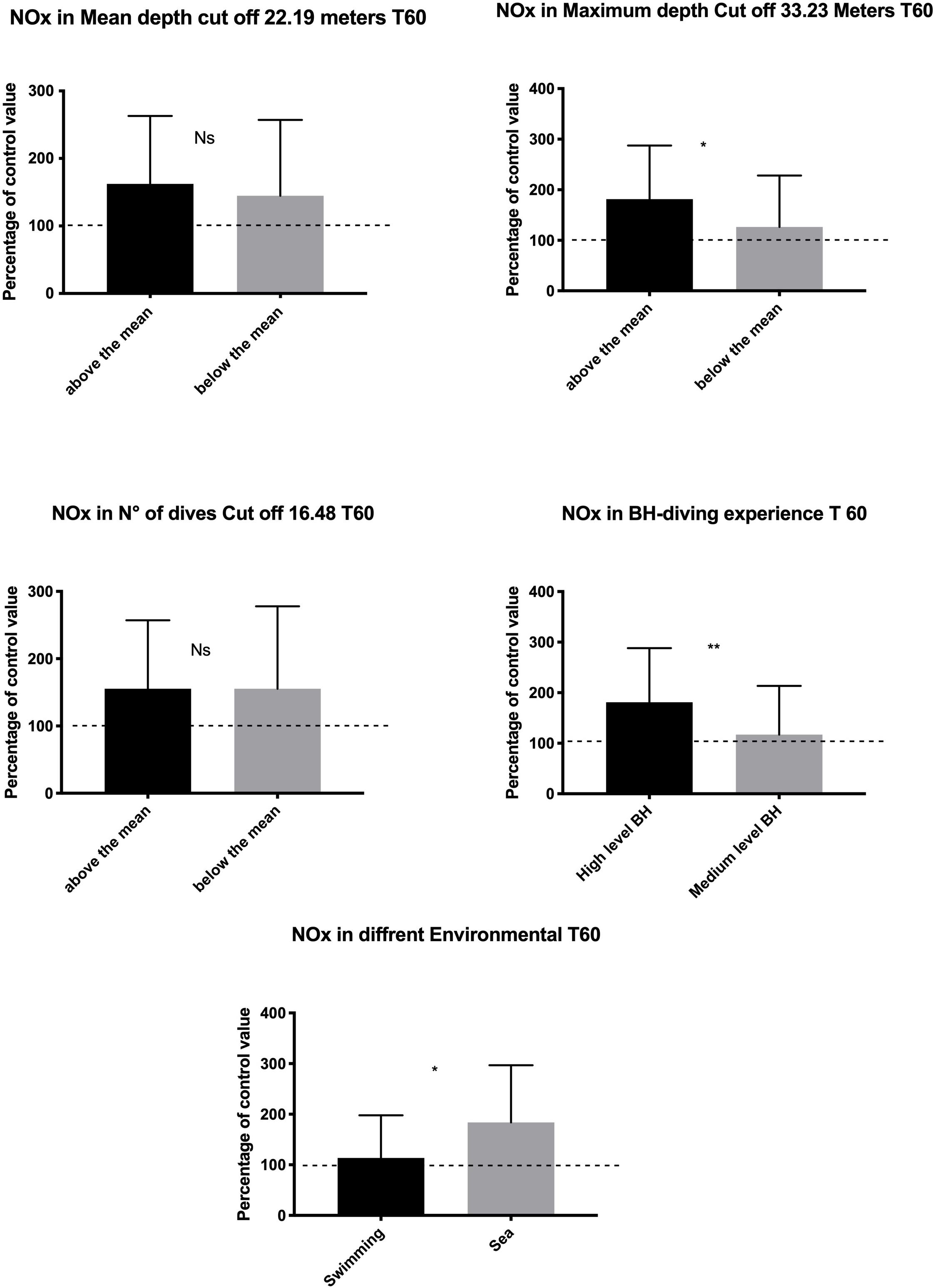
Figure 3. Data at T60 show a rebound effect with an increase of NO available probably to compensate for the higher underwater consumption. This rebound effect was larger in the subject who shows a higher diving exposure and experience and in sea diving as compared with the swimming pool diving. *p < 0.05, **p < 0.01.
No statistical difference was detected in TAC levels between pre- and post-dive values (Figure 4).
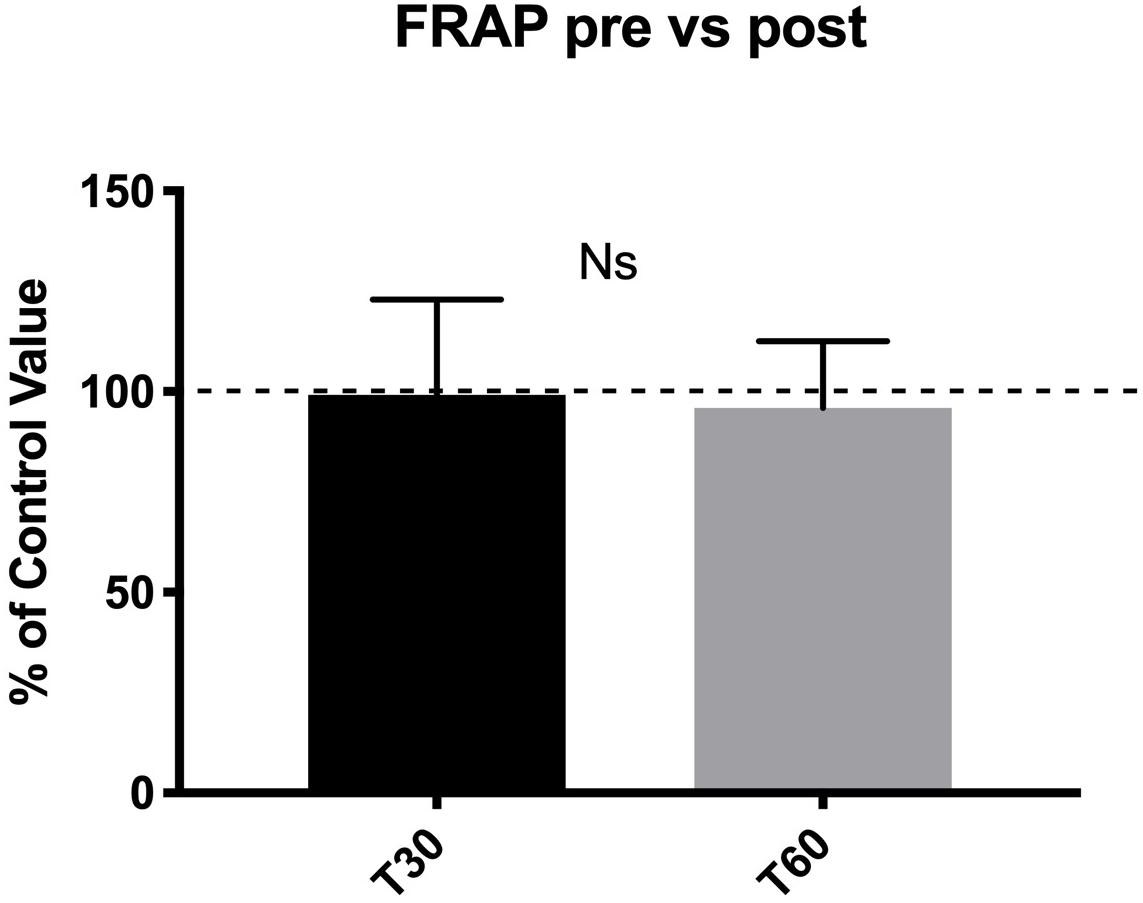
Figure 4. No statistical difference in TAC levels were found between pre- and post-dive values at T30 and T60.
Similarly, no differences were found at T30 in the groups above vs. below average for the four-diving risk levels (AD, MD, ND, and LD) and in the two different environmental conditions (sea vs. swimming pool). Only the follow-up at T60 demonstrated higher oxidative stress levels in divers who performed BH-diving above the mean of MD and in HE divers HE (p = 0.01 and p = 0.03, respectively).
No significant difference was found as well between the swimming pool and the sea BH-dives.
Finally, no significant relationship was found in the NOx and TAC levels in the different genetic variant (Supplementary Table 2).
Discussion
The NOx (Tsukiyama et al., 2017) and the TAC changes (Marciniak et al., 2009) after a BH-diving training session were investigated in a group of 50 BH-divers divided into two groups with different hyperbaric exposure and different experiences.
In the appendix to this primary aim, we also observed the behavior of NOx and TAC in the same divers sorted by their genetic variant of 10 anti-inflammatory, vascular, and antioxidant related polymorphisms.
Nitric oxide (Koshland, 1992) plays an essential and complex role as a signaling molecule in several human physiological and pathological responses, including diving and hyperbaric-related adaptations (Theunissen et al., 2013a; Sureda et al., 2014). NO behavior is a complex molecule to be studied, for these multifaceted actions and the short half-life (Liu et al., 1998), especially, when adding the challenging test conditions of an extreme environment. A standard method to investigate plasma NO changes is by looking at the variations of its oxidation products, NOx, because the half-lives of NO3 and NO2 in blood circulation are 5–8 h and 20–40 min, respectively (Dejam et al., 2007; Lundberg et al., 2008). Physical exercise increases eNOS activity and resulting in a higher level of circulating NOx (Jungersten et al., 1997; Green et al., 2004).
Under particular conditions, such as hypoxia, NOx can be reconverted to NO through different pathways involving proteins (hemoglobin and myoglobin), enzymes (xanthine oxidase and xanthine oxidoreductase), and ascorbate to ensure NO production when O2 supply is reduced. In blood vessels, NO2 generates vasodilatory NO by reacting with deoxygenated hemoglobin (deoxy-Hb) and contributes to physiological hypoxic blood flow regulation. When hemoglobin O2-saturation drops to 50%, the reduction of NO2 to NO is enhanced. This effect results from two mechanisms: the availability of deoxyhemes (reaction substrate) to bind NO2, which is maximal in deoxygenated hemoglobin, and the amount of oxygenated hemoglobin tetramer, which increases the intrinsic reactivity of the heme with NO2 (Lundberg et al., 2008).
In a recent study, data obtained from an underwater blood draw (−42 m) carried out on 12 expert BH-divers clearly showed the NOx kinetics in BH-diving. These data indicate a significant underwater increase in plasma NOx concentration (+410.5% compared with pre-dive value) and an immediate return to baseline values after reaching the surface (Cialoni et al., 2021). These data confirmed a significant use of NO during BH-diving, compatible with the well-known BH-diving-related circulatory adaptations, but unexpectedly showed a swift return of circulating NOx to basal levels at the surface. This last aspect confirms that the NOx measured after diving reflects the availability of NO in real-time, without any diving-related “accumulation” effect in tissues. This observation helps to understand the results reported in this new protocol performed after a BH-diving training session, where we found a decrease of NOx 30 min after the training session, followed by an increase at T60. These data partially confirm a previous study where a similar increase was found, although without any initial decrease (Theunissen et al., 2013a). This difference could be easily explained by the different diving protocols of the previous test compared with that proposed in this research, especially concerning the ND and the descent technique.
Therefore, the T30 post-diving reduction of NOx found in this experiment can be ascribed to the lower NO availability in the first few minutes after the dives caused by the higher use of this molecule during diving (Cialoni et al., 2021) to ensure the BH-diving related vascular adaptations. On the other hand, an increase at T60 could be a rebound of the efforts of the body to restore basal conditions after exceptional stress exposure (Figure 1).
Hyperbaric exposure-related oxidative stress is the second aspect taken into account due to the consequences potentially affecting BH-divers. Indeed, BH-diving results in higher ROS production and oxidative stress, as confirmed by several authors (Theunissen et al., 2013a; Mrakic-Sposta et al., 2019; Cialoni et al., 2021), along with the activation of endogenous antioxidant defenses (Bulmer et al., 2008; Sureda et al., 2015). As recently suggested (Cialoni et al., 2021), oxidative stress is transitory, increasing in the underwater phases but returning near pre-dive levels after reaching the surface.
Unlike the previous paper (decrease of TAC: −60% to pre-dive value) (Cialoni et al., 2021), TAC did not show any difference between pre- and post-diving in the present experiment. This fact suggests the absence of an oxidative stimulus at the end of the training session, despite the hyperbaric exposure.
The data, in this study, indicate significant differences in NO consumption only when stratifying the divers into the two groups of high or low hyperbaric exposure or in the two groups of more expert vs. less expert subjects.
It is also intriguing to note that the lower NO consumption was observed in expert divers (HE) and those with higher hyperbaric exposure on the day of the experiment. We can hypothesize that a possible adaptation effect was undergone in these subjects who trained more intensely or had higher performances on the day of the investigation, with respect to the BH-divers that dived below the average. This aspect can also be explained by the “relax and comfort” training and diving techniques adopted by expert BH-divers, most likely decreasing the NO necessary to support the BH-diving induced hyperbaric-related physiological changes. However, this variable was not explicitly investigated and is worthy of further assessment in the future.
Another important observation concerns the changes in NOx and TAC when comparing swimming pool and sea exposures. Data at T30 were similar in the two different environments indicating a low influence of variables such as temperature (34 vs. 24°C) and salinity (freshwater vs. seawater). Therefore, NOx level changes are probably more influenced by the magnitude of hyperbaric exposure. An in-depth analysis of this aspect showed that the rebound effect noted at T60 is significantly higher in the sea subjects than in the swimming pool subjects (183.9 ± 112.9 vs. 113.5 ± 84.3). This could be related to the characteristics of sea BH-diving, requiring complex logistics for the training sessions, the use of a boat and a diving suit, more time inside the water (even if the ND is similar in the two groups), and more demanding environmental conditions (e.g., colder temperatures, waves, and currents).
However with all the limits that our genetic investigation shows, it is intriguing to note that the data did not show any differences in NOx and TAC values in the single nucleotide variant in all the 10 polymorphisms investigated. If this data will be confirmed by future studies, more focus on the genetic aspect could be indicated to confirm that the genetic predisposition is less critical as compared with hyperbaric exposure when concerning BH-diving related NOx and oxidative stress.
Conclusion
This study showed the importance of hyperbaric exposure and expertise regarding NO availability and oxidative stress in BH-divers. NO consumption seemed to be significantly lower in high-performance BH-divers and the expert group indicating a possible training-related adaptation process. On the other hand, expert BH-divers demonstrated higher oxidative stress due to higher hyperbaric exposure in the sessions. A preliminary genetic investigation seems to indicate a lack of specific influence of genetic predisposition as compared with the increase of diving exposure.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of Università degli Studi di Milano, Italy (Aut. No. 37/17). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DC proposed the protocol and the search strategy, extracted and analyzed the data, and wrote the first draft. AB was involved in the conception and design of this work, reviewed the critical appraisal of selected articles, and assisted with the compilation of the systematic review. MP and NS extracted and analyzed the data and reviewed the manuscript. VL was involved in the test on the field and reviewed the manuscript. MS, AB, and AM supervised the entire process. All authors contributed to at least three of the four major components of a study and were involved in the conception and design of this work, contributed to the process of writing, and approval of the final manuscript.
Funding
This study was part of the DAN Research funded by the DAN Europe Foundation and the “E-heart” project, funded by the private donation of Marina Scardi, in memory of his father, the late Sabino Scardi, a valued cardiologist.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Omersub Spa, Monza Brianza, Italy for the supply of the free-diving computer UP-X1 and Aqua Lung Italia-Technisub SPA, Genova, Italy, for the diving equipment. We would like to thank the Y-40 “The Deep Joy” Montegrotto Terme Padova, Italy, for their friendly help and logistic support, and the NGB Genetics Srl, Bologna, Italy, for the genetic analysis. The authors also thank the BH-divers for participating in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.692204/full#supplementary-material
References
Ahsan, A., Mohd, G., Norboo, T., Baig, M. A., and Pasha, M. A. (2006). Heterozygotes of NOS3 polymorphisms contribute to reduced nitrogen oxides in high-altitude pulmonary edema. Chest 130, 1511–1519. doi: 10.1378/chest.130.5.1511
Baranova, T. I., Berlov, D. N., Glotov, O. S., Korf, E. A., Minigalin, A. D., Mitrofanova, A. V., et al. (2017). Genetic determination of the vascular reactions in humans in response to the diving reflex. Am. J. Physiol. Heart Circ. Physiol. 312, H622–H631.
Behrendt, D., and Ganz, P. (2002). Endothelial function. From vascular biology to clinical applications. Am. J. Cardiol. 90, 40L–48L.
Benzie, I. F., and Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76. doi: 10.1006/abio.1996.0292
Bosco, G., Paganini, M., Rizzato, A., Martani, L., Garetto, G., Lion, J., et al. (2020). Arterial blood gases in divers at surface after prolonged breath-hold. Eur. J. Appl. Physiol. 120, 505–512. doi: 10.1007/s00421-019-04296-2
Bosco, G., Yang, Z. J., Di Tano, G., Camporesi, E. M., Faralli, F., Savini, F., et al. (2010). Effect of in-water oxygen prebreathing at different depths on decompression-induced bubble formation and platelet activation. J. Appl. Physiol. 108, 1077–1083. doi: 10.1152/japplphysiol.01058.2009
Brubakk, A. O., Duplancic, D., Valic, Z., Palada, I., Obad, A., Bakovic, D., et al. (2005). A single air dive reduces arterial endothelial function in man. J. Physiol. 566(Pt 3), 901–906. doi: 10.1113/jphysiol.2005.089862
Bulmer, A. C., Coombes, J. S., Sharman, J. E., and Stewart, I. B. (2008). Effects of maximal static apnea on antioxidant defenses in trained free divers. Med. Sci. Sports Exerc. 40, 1307–1313. doi: 10.1249/mss.0b013e31816a7188
Cai, H., and Harrison, D. G. (2000). Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840–844. doi: 10.1161/01.res.87.10.840
Carlsson, S., Wiklund, N. P., Engstrand, L., Weitzberg, E., and Lundberg, J. O. (2001). Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide 5, 580–586. doi: 10.1006/niox.2001.0371
Chandrika, B. (2001). Sample size considerations in genetic polymorphism studies. Hum. Hered. 52, 191–200. doi: 10.1159/000053376
Chatterjee, A., Black, S. M., and Catravas, J. D. (2008). Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul. Pharmacol. 49, 134–140. doi: 10.1016/j.vph.2008.06.008
Cialoni, D., Brizzolari, A., Samaja, M., Bosco, G., Paganini, M., Pieri, M., et al. (2021). Nitric oxide and oxidative stress changes at depth in breath-hold diving. Front. Physiol. 11:609642.
Cialoni, D., Brizzolari, A., Samaja, M., Pieri, M., and Marroni, A. (2019). Altered venous blood nitric oxide levels at depth and related bubble formation during scuba diving. Front. Physiol. 10:57.
Cialoni, D., Marabotti, C., Sponsiello, N., Pieri, M., Balestra, C., Lucchini, V., et al. (2015). Genetic predisposition to breath-hold diving-induced hemoptysis: preliminary study. Undersea Hyperb. Med. 42, 75–83.
Cialoni, D., Pieri, M., Giunchi, G., Sponsiello, N., Lanzone, A. M., Torcello, L., et al. (2016). Detection of venous gas emboli after repetitive breath-hold dives: case report. Undersea Hyperb. Med. 43, 449–455.
Cosby, K., Partovi, K. S., Crawford, J. H., Patel, R. P., Reiter, C. D., Martyr, S., et al. (2003). Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 9, 1498–1505. doi: 10.1038/nm954
Dejam, A., Hunter, C. J., Tremonti, C., Pluta, R. M., Hon, Y. Y., Grimes, G., et al. (2007). Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation 116, 1821–1831. doi: 10.1161/circulationaha.107.712133
Dhaliwal, H., Kirshenbaum, L. A., Randhawa, A. K., and Singal, P. K. (1991). Correlation between antioxidant changes during hypoxia and recovery on reoxygenation. Am. J. Physiol. 261(3 Pt 2), H632–H638.
Diringer, M. N. (2008). Hyperoxia: good or bad for the injured brain? Curr. Opin. Crit. Care 14, 167–171. doi: 10.1097/mcc.0b013e3282f57552
Drexler, H. (1997). Endothelial dysfunction: clinical implications. Prog. Cardiovasc. Dis. 39, 287–324. doi: 10.1016/s0033-0620(97)80030-8
Elsner, R., Oyasaeter, S., Almaas, R., and Saugstad, O. D. (1998). Diving seals, ischemia-reperfusion and oxygen radicals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 119, 975–980. doi: 10.1016/s1095-6433(98)00012-9
Forkner, I. F., Piantadosi, C. A., Scafetta, N., and Moon, R. E. (2007). Hyperoxia-induced tissue hypoxia: a danger? Anesthesiology 106, 1051–1055. doi: 10.1097/01.anes.0000265167.14383.44
Giraldez, R. R., Panda, A., Xia, Y., Sanders, S. P., and Zweier, J. L. (1997). Decreased nitric-oxide synthase activity causes impaired endothelium-dependent relaxation in the postischemic heart. J. Biol. Chem. 272, 21420–21426. doi: 10.1074/jbc.272.34.21420
Godber, B. L., Doel, J. J., Sapkota, G. P., Blake, D. R., Stevens, C. R., Eisenthal, R., et al. (2000). Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 275, 7757–7763.
Green, D. J., Maiorana, A., O’Driscoll, G., and Taylor, R. (2004). Effect of exercise training on endothelium-derived nitric oxide function in humans. J. Physiol. 561(Pt 1), 1–25. doi: 10.1113/jphysiol.2004.068197
Green, L. C., Wagner, D. A., Glogowski, J., Skipper, P. L., Wishnok, J. S., and Tannenbaum, S. R. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126, 131–138. doi: 10.1016/0003-2697(82)90118-x
Heitzer, T., Schlinzig, T., Krohn, K., Meinertz, T., and Münzel, T. (2001). Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104, 2673–2678. doi: 10.1161/hc4601.099485
Hou, Y. C., Janczuk, A., and Wang, P. G. (1999). Current trends in the development of nitric oxide donors. Curr. Pharm. Des. 5, 417–441.
Jansson, E. A., Huang, L., Malkey, R., Govoni, M., Nihlén, C., Olsson, A., et al. (2008). A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 4, 411–417. doi: 10.1038/nchembio.92
Jungersten, L., Ambring, A., Wall, B., and Wennmalm, A. (1997). Both physical fitness and acute exercise regulate nitric oxide formation in healthy humans. J. Appl. Physiol. 82, 760–764. doi: 10.1152/jappl.1997.82.3.760
Keaney, J. F., and Vita, J. A. (1995). Atherosclerosis, oxidative stress, and antioxidant protection in endothelium-derived relaxing factor action. Prog. Cardiovasc. Dis. 38, 129–154. doi: 10.1016/s0033-0620(05)80003-9
Li, H., Samouilov, A., Liu, X., and Zweier, J. L. (2003). Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry 42, 1150–1159. doi: 10.1021/bi026385a
Liu, X., Miller, M. J., Joshi, M. S., Sadowska-Krowicka, H., Clark, D. A., and Lancaster, J. R. (1998). Diffusion-limited reaction of free nitric oxide with erythrocytes. J. Biol. Chem. 273, 18709–18713. doi: 10.1074/jbc.273.30.18709
Lundberg, J. O. (2009). Cardiovascular prevention by dietary nitrate and nitrite. Am. J. Physiol. Heart Circ. Physiol. 296, H1221–H1223.
Lundberg, J. O., and Govoni, M. (2004). Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 37, 395–400. doi: 10.1016/j.freeradbiomed.2004.04.027
Lundberg, J. O., Gladwin, M. T., and Weitzberg, E. (2015). Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 14, 623–641. doi: 10.1038/nrd4623
Lundberg, J. O., Weitzberg, E., and Gladwin, M. T. (2008). The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167. doi: 10.1038/nrd2466
Macallan, D. C., Smith, L. M., Ferber, J., Milne, E., Griffin, G. E., Benjamin, N., et al. (1997). Measurement of NO synthesis in humans by L-[15N2]arginine: application to the response to vaccination. Am. J. Physiol. 272(6 Pt 2), R1888–R1896.
Marciniak, A., Brzeszczyńska, J., Gwoździński, K., and Jegier, A. (2009). Antioxidant Capacity and physical exercise. Biol. Sport 26, 197–213. doi: 10.5604/20831862.894649
Modin, A., Björne, H., Herulf, M., Alving, K., Weitzberg, E., and Lundberg, J. O. (2001). Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol. Scand. 171, 9–16. doi: 10.1046/j.1365-201x.2001.00771.x
Moncada, S., and Higgs, A. (1993). The L-arginine-nitric oxide pathway. N. Engl. J. Med. 329, 2002–2012.
Mrakic-Sposta, S., Vezzoli, A., Rizzato, A., Della Noce, C., Malacrida, S., Montorsi, M., et al. (2019). Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 119, 2449–2456.
Obad, A., Marinovic, J., Ljubkovic, M., Breskovic, T., Modun, D., Boban, M., et al. (2010). Successive deep dives impair endothelial function and enhance oxidative stress in man. Clin. Physiol. Funct. Imaging 30, 432–438. doi: 10.1111/j.1475-097x.2010.00962.x
Ostergaard, L. S. E., Andersen, M. R., Eskildsen-Helmond, Y., Ledet, T., Mulvany, M. J., and Simonsen, U. (2007). Diminished NO release in chronic hypoxic human endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 293, H2894–H2903.
Pepine, C. J. (1998). Clinical implications of endothelial dysfunction. Clin. Cardiol. 21, 795–799. doi: 10.1002/clc.4960211103
Rand, M. J. (1992). Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin. Exp. Pharmacol. Physiol. 19, 147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x
Rassaf, T., Flögel, U., Drexhage, C., Hendgen-Cotta, U., Kelm, M., and Schrader, J. (2007). Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 100, 1749–1754. doi: 10.1161/circresaha.107.152488
Romitelli, F., Santini, S. A., Chierici, E., Pitocco, D., Tavazzi, B., Amorini, A. M., et al. (2007). Comparison of nitrite/nitrate concentration in human plasma and serum samples measured by the enzymatic batch Griess assay, ion-pairing HPLC and ion-trap GC-MS: the importance of a correct removal of proteins in the Griess assay. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 851, 257–267. doi: 10.1016/j.jchromb.2007.02.003
Senthil, D., Raveendran, M., Shen, Y. H., Utama, B., Dudley, D., Wang, J., et al. (2005). Genotype-dependent expression of endothelial nitric oxide synthase (eNOS) and its regulatory proteins in cultured endothelial cells. DNA Cell Biol. 24, 218–224. doi: 10.1089/dna.2005.24.218
Shiva, S., Huang, Z., Grubina, R., Sun, J., Ringwood, L. A., MacArthur, P. H., et al. (2007a). Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 100, 654–661. doi: 10.1161/01.res.0000260171.52224.6b
Shiva, S., Sack, M. N., Greer, J. J., Duranski, M., Ringwood, L. A., Burwell, L., et al. (2007b). Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 204, 2089–2102. doi: 10.1084/jem.20070198
Sureda, A., Batle, J. M., Capó, X., Martorell, M., Córdova, A., Tur, J. A., et al. (2014). Scuba diving induces nitric oxide synthesis and the expression of inflammatory and regulatory genes of the immune response in neutrophils. Physiol. Genomics 46, 647–654. doi: 10.1152/physiolgenomics.00028.2014
Sureda, A., Batle, J. M., Tur, J. A., and Pons, A. (2015). Competitive apnea diving sessions induces an adaptative antioxidant response in mononucleated blood cells. J. Physiol. Biochem. 71, 373–380. doi: 10.1007/s13105-015-0417-9
Terraneo, L., and Samaja, M. (2017). Comparative response of brain to chronic hypoxia and hyperoxia. Int. J. Mol. Sci. 18:1914. doi: 10.3390/ijms18091914
Theunissen, S., Guerrero, F., Sponsiello, N., Cialoni, D., Pieri, M., Germonpré, P., et al. (2013a). Nitric oxide-related endothelial changes in breath-hold and scuba divers. Undersea Hyperb. Med. 40, 135–144.
Theunissen, S., Sponsiello, N., Rozloznik, M., Germonpré, P., Guerrero, F., Cialoni, D., et al. (2013b). Oxidative stress in breath-hold divers after repetitive dives. Diving Hyperb. Med. 43, 63–66.
Tousoulis, D., Koutsogiannis, M., Papageorgiou, N., Siasos, G., Antoniades, C., Tsiamis, E., et al. (2010). Endothelial dysfunction: potential clinical implications. Minerva Med. 101, 271–284.
Tsikas, D. (2005). Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 39, 797–815. doi: 10.1080/10715760500053651
Tsukiyama, Y., Ito, T., Nagaoka, K., Eguchi, E., and Ogino, K. (2017). Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J. Clin. Biochem. Nutr. 60, 180–186. doi: 10.3164/jcbn.16-108
Valic, Z., Duplancić, D., Baković, D., Ivancev, V., Eterović, D., Wisløff, U., et al. (2005). Diving-induced venous gas emboli do not increase pulmonary artery pressure. Int. J. Sports Med. 26, 626–631. doi: 10.1055/s-2004-830379
van Vliet, J. J., Vaessen, H. A. M. G., van den Burg, G., and Schothorst, R. C. (1997). Twenty-four-hour duplicate diet study 1994; nitrate and nitrite: method development and intake per person per day. Cancer Lett. 114, 305–307. doi: 10.1016/s0304-3835(97)04688-0
Wang, P., Ha, A. Y., Kidd, K. K., Koehle, M. S., and Rupert, J. L. (2010). A variant of the endothelial nitric oxide synthase gene (NOS3) associated with AMS susceptibility is less common in the Quechua, a high altitude Native population. High Alt. Med. Biol. 11, 27–30. doi: 10.1089/ham.2009.1054
Keywords: nitric oxide, oxidative stress, breath hold diving, genetic prone, diving
Citation: Cialoni D, Brizzolari A, Samaja M, Bosco G, Paganini M, Sponsiello N, Lancellotti V and Marroni A (2021) Endothelial Nitric Oxide Production and Antioxidant Response in Breath-Hold Diving: Genetic Predisposition or Environment Related? Front. Physiol. 12:692204. doi: 10.3389/fphys.2021.692204
Received: 07 April 2021; Accepted: 08 June 2021;
Published: 09 July 2021.
Edited by:
Frederic Lemaitre, Université de Rouen, FranceReviewed by:
Pierre Louge, Geneva University Hospitals (HUG), SwitzerlandMelissa Ilardo, Maze Therapeutics, United States
Copyright © 2021 Cialoni, Brizzolari, Samaja, Bosco, Paganini, Sponsiello, Lancellotti and Marroni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danilo Cialoni, dcialoni@daneurope.org
 Danilo Cialoni
Danilo Cialoni