
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 05 February 2021
Sec. Exercise Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.625950
The study aimed to examine sex-specific associations between objectively measured sedentary patterns and pro- and anti-inflammatory biomarkers in older adults when considering the moderating impact of physical activity (PA). Accelerometer-based monitoring of sedentary patterns and PA was conducted in a population of older men (n = 83; age: 67.4 ± 1.5; height: 178.7 ± 6.6 cm; weight: 80.9 ± 10.6 kg) and women (n = 146; age: 67.4 ± 1.6; height: 164.2 ± 6.1 cm; weight: 64.6 ± 10.1 kg) aged 65–70. Blood samples were collected for the assessment of the inflammatory biomarkers C-reactive protein (CRP), fibrinogen, interleukin-6 (IL-6), IL-10, IL-18, and monocyte chemoattractant protein-1 (MCP-1). Data were analyzed using multiple linear regression models. Total and bouts of ≥10 min of sedentary time were inversely associated with the anti-inflammatory marker IL-10 in older men (accumulated sedentary time: β = −0.116; bouts: β = −0.099; all p < 0.05). Associations were independent of moderate-to-vigorous physical activity (MVPA) and total PA volume. In women, total and bouts of ≥10 min of sedentary time were detrimentally associated with the pro-inflammatory marker fibrinogen (accumulated sedentary time: β = −0.130; bouts: β = −0.085; all p < 0.05). Associations remained between accumulated sedentary time and fibrinogen when adjusting for MVPA and total PA volume. This study highlights sex-specific routes by which sedentary patterns impact on pro- and anti-inflammatory biomarkers in older adults. The findings support efforts to promote accumulation of time spent in PA at the expense of time in sedentary pursuits on low-grade inflammation in older men and women.
Leading a sedentary lifestyle is implicated with increased risk of several chronic conditions, including cardiovascular and metabolic diseases (Dunstan et al., 2011). In this respect, excessive amounts of time in a sedentary state have been linked to obesity, hypertension, dyslipidemia, and hyperglycemia, collectively known as the metabolic syndrome (Edwardson et al., 2012). While the pathophysiological mechanisms underlying progression of metabolic disorders are not fully clarified, alterations in the systemic inflammatory environment, have been highlighted as an important mediator (Franceschi and Campisi, 2014). Elevations in circulating levels of several pro-inflammatory mediators by advancing age promote as state of low-grade systemic inflammation (inflammaging). The two hepatocyte-derived acute-phase proteins; C-reactive protein (CRP) and fibrinogen, together with the monocyte chemoattractant protein (MCP)-1, a member of the C-C chemokine family and a potent chemotactic factor for monocytes, and the cytokines interleukin-6 (IL-6) and IL-18, represent biomarkers contributing to a pro-inflammatory systemic environment (Stec et al., 2000; Koenig et al., 2004; Zirlik et al., 2007; Jung and Choi, 2014). Interestingly, systemic inflammation in older adults is further influenced by a declined level of IL-10 (Meador et al., 2008), an anti-inflammatory cytokine mainly produced by macrophages and responsible for suppressing inflammatory responses (Asadullah et al., 2003; Dorneles et al., 2016).
Associations have been evidenced between daily sitting time, including TV viewing time, and the two common acute-phase reactants CRP and fibrinogen, indicating a potential detrimental influence of a sedentary lifestyle on systemic inflammation (Rudnicka et al., 2011; Yates et al., 2012; Gennuso et al., 2013; Henson et al., 2013; Hamer et al., 2015; Howard et al., 2015). However, previous studies have shed light on sex-specific differences in levels of pro- and anti-inflammatory biomarkers, where CRP and fibrinogen levels have been found to be significantly higher in women than men (Cartier et al., 2009; Rudnicka et al., 2011; Yates et al., 2012; Howard et al., 2015). Further, stronger relationships between inflammation and sedentary behavior have been indicated in women compared to men (Pereira et al., 2012; Yates et al., 2012; Howard et al., 2015). It is currently proposed that genetic, hormonal, and environmental factors represent underlying mediators behind sex-differences in the production of cytokines and chemokines by innate immune cells (Klein and Flanagan, 2016). For example, following menopause women experience a significant reduction in estrogen levels, which is associated with adverse inflammatory events (Campesi et al., 2016; Razmjou et al., 2016). Therefore, it may be hypothesized that sex-specific links between sedentary lifestyle and different pro- and anti-inflammatory factors exist and need to be further clarified. Furthermore, systemic inflammation is generally assessed by a few common inflammatory biomarkers (typically CRP, fibrinogen, and IL-6), which does not necessarily reflect the full spectrum of the inflammatory process in older adults. We have previously reported adverse associations between single and composite inflammatory score with adiposity in older adults suggesting at the importance of investigating the implications of several cytokines in regard to the inflammatory load in older adults (Bergens et al., 2019). Therefore, a better understanding of the relationship between sedentary behaviors and systemic inflammation would require investigation of inflammatory biomarkers encompassing clinically established ones, as well as novel inflammatory mediators. Importantly, when investigating these links attention should be paid to the pattern of sedentary time accumulation over the day, i.e., whether characterized by uninterrupted prolonged time periods or by shorter periods with frequent breaks in sedentary time. This includes analysis of different time bout lengths and number of breaks in sedentary time.
Another important question to address is whether potential links between sedentary patterns and pro- and anti-inflammatory biomarkers are independent of physical activity level. Some studies have previously reported independent associations between sedentary time and a few clinical markers of inflammation, indicating that moderate-to-vigorous physical activity (MVPA) time may not mitigate the detrimental impact of sedentary time (Healy et al., 2011b; Yates et al., 2012; Gennuso et al., 2013; Hamer et al., 2015; Howard et al., 2015). However, as time in MVPA represents a small part of the daily time, residual confounding from remaining time spent in lower intensity physical activity (PA) may still be present. Therefore, daily PA volume, incorporating both low and high intensity PA, should be considered in new investigations aiming to clarify the unique impact of sedentariness on systemic inflammation in older adults. Moreover, given the potential impact of dietary habits on inflammatory profiles, indicated in previous research (Nilsson et al., 2019), risk of confounding by diet when elucidating links between sedentariness and inflammation needs to be addressed.
Taken together, to what extent sedentary patterns are associated to systemic inflammation independent of PA level in older adults, and whether these links are sex-specific, remains to be clarified. Such knowledge is warranted in order to tailor sex-specific guidelines on health-related PA behaviors in order to dampen age-related systemic inflammation. Therefore, the aim of the present study was to explore relationships between objectively assessed sedentary patterns and pro- and anti-inflammatory biomarkers in older men and women when considering potential moderation by PA level.
The present study included 252 community-dwelling older men and women, 65–70 years old, recruited within the frame of the EURODIET project. Inclusion criteria: age between 65 and 70 years. Exclusion criteria: overt disease such as diabetes mellitus or diagnosed coronary heart disease, disability with respect to mobility, smoking, and anti-inflammatory treatment. All clinical investigations were conducted in accordance with the Declaration of Helsinki and all participants provided written informed consent. The study was approved by the regional ethics committee of Uppsala, Sweden.
Accelerometer-based assessment of PA was monitored with the Actigraph GT3x activity monitor (Actigraph, Pensacola, FL, United States) worn around the waist for 7 days. Briefly, participants were instructed to wear the monitor during all waking hours (except water-based activities) and note non-wear time. Inclusion criteria were that the activity monitor was worn for a minimum of 4 days with at least 10 h of wear time per day. Non-wear time was defined as a minimum of 60 min of continuous zero counts. Sedentary wear time was defined as all <100 counts, and MVPA as >2,020 in accordance with previous research (Troiano et al., 2008; Lynch et al., 2011). Breaks in sedentary behavior were defined as any 60 s of ≥100 counts. Time spent in sedentary patterns was retrieved as three separate outcomes: total accumulated sedentary time, amount of time occurring in bouts of ≥10 and ≥30 min. Breaks in sedentary behavior were expressed in relation to occurrences per sedentary hour (Break rate). Total accelerometer counts were retrieved as a marker of total volume of PA.
Information on dietary habits was collected using a validated food frequency questionnaire (Johansson et al., 2010). A dietary inflammatory index (DII) score was derived from 30 food items following calculation procedures presented elsewhere (Shivappa et al., 2014).
Blood samples were collected by venipuncture between 8.00 and 10.00 am after an overnight fast. For this study, six inflammatory biomarkers previously linked to metabolic syndrome were selected based on previous research (Nilsson et al., 2018; Bergens et al., 2019). High-sensitivity CRP was measured using fully automated immunoturbidimetric assay. Fibrinogen was determined using an automated immunoassay method with a polyclonal rabbit anti-human antibody (Dako, Glostrup, Denmark). IL-6, IL-10, IL-18, and MCP-1 were analyzed using the Olink Proseek Multiplex Inflammation panel (Olink, Uppsala, Sweden). Briefly, a pair of oligonucleotide-labeled antibodies are pairwise bound to the target protein present in the sample and a new PCR target sequence is formed by a proximity dependent DNA polymerization event. The resulting sequence is subsequently detected and quantified using standard RT-PCR. All OLINK biomarkers had complete Normalized Protein eXpression (NPX) detectability (0% missing data frequency) and intra-assay variations were as follows: MCP-1 (6%), IL-6 (6%), IL-10 (7%), and IL-18 (6%).
Metabolic risk outcome variables included in the International Diabetes Federation (IDF) definition of the metabolic syndrome were assessed (Alberti et al., 2006). Waist circumference was measured to the nearest 0.1 cm with a steel tape at the midpoint between iliac crest and lower costal margin. Systolic and diastolic blood pressure were measured manually after a 10-min rest in the supine position. Fasting blood triglycerides and HDL-cholesterol were assessed on plasma samples using chemistry kits from Ortho-Clinical Diagnostics on a Vitros-5.1 analyzer platform (Clinical Diagnostics, Raritan, NJ, United States) and plasma blood glucose was assessed using the Reflotron Plus® system (Roche Diagnostics Limited, Rotkreuz, Switzerland). A clustered metabolic risk score was created based on the assessment of measured metabolic risk outcome variables (level of triglycerides, HDL-cholesterol, glucose, waist circumference, and mean blood pressure). First, standardized values (z-scores) of each outcome variable were expressed. Z-scores on HDL-cholesterol were inverted before calculating the average z-score based on all standardized outcomes.
Data are presented as means ± SD. Differences between men and women were determined by independent sample t-test. To retrieve comparable effect outcomes from variables with different original units of measurement, all inflammatory biomarkers were standardized (z-score) before regression modeling. All variables were checked and transformed when necessary to fit a normal distribution. Prior to analysis, sedentary behaviors and MVPA were modeled in 30-min periods. All models were adjusted by accelerometer wear time. First, before adjusting for PA behaviors, linear associations between each inflammatory marker and time spent in sedentary behavior and break rate were assessed (Model 1). Second, linear regression models were conducted to determine the strength of associations between measured inflammatory biomarkers and sedentary behavior when adjusting for MVPA (Model 2) or total accelerometer counts (Model 3) and metabolic risk score (Model 4). All models were stratified by sex. Potential significant influences of age and dietary inflammatory score on inflammatory outcomes were first checked using simple linear regressions. As no significant associations were detected, age and dietary inflammatory score were omitted in final regression models in order to retain statistical power. Assumptions for regression models including linearity, homoscedasticity, and multicollinearity between independent variables were checked. Level of significance was set to p < 0.05. All statistical analyses were performed using SPSS version 26. A priori power calculation revealed a small to moderate effect size to be detected with a power of ≥80% given the current sample size and alpha set to 0.05.
A total of 229 community-dwelling older adults (146 women and 83 men) with complete data on all variables were included in the final analysis. Eight women and 15 men had either incomplete accelerometer recordings, missing biological data, were current smokers or used prescribed anti-inflammatory medication. On average, participants wore the activity monitor for 6.1 ± 0.9 days with 14 ± 1.2 h per day, without differences between the sexes. Data on body composition, CRP and fibrinogen and sedentary patterns are shown in Table 1. Women spent significantly less daily time in sedentary activities compared to men, both in terms of total accumulated time (p < 0.05) and in continuous time bouts of at least 10 min (p < 0.05) and 30 min (p < 0.05), respectively. Women also had significantly more breaks per sedentary hour (i.e., higher break rate) compared to their male counterparts (p < 0.05) (Table 1). While average PA level (women: 375.8 ± 136.9 counts per min; Men: 376.0 ± 111.8 counts per min) and time spent in MVPA (women: 42.3 ± 25.0 min; men: 40.9 ± 21.2 min) were similar between sexes, women spent significantly more time in LPA (women: 125.4 ± 51.1 min; men: 275.2 ± 69.6 min, p < 0.05). There were no significant differences between men and women in levels of the clinically well-established inflammatory biomarkers CRP and fibrinogen.
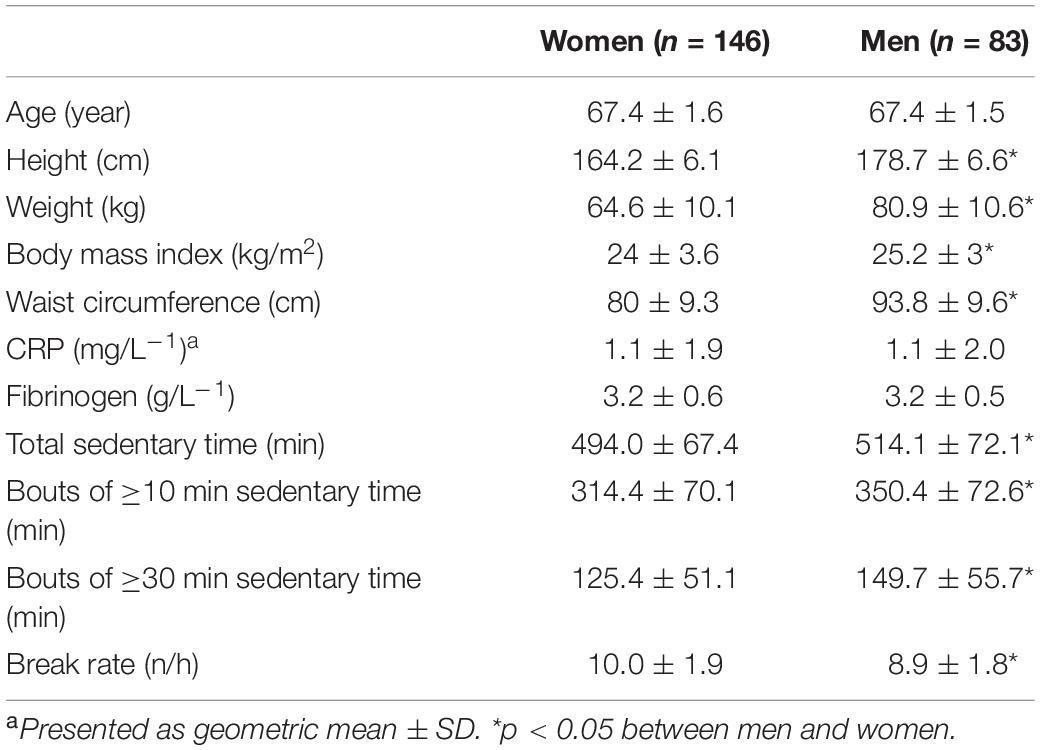
Table 1. Body composition (mean ± SD), levels of CRP and fibrinogen, and sedentary behavior variables in older men and women.
We sought to determine the influence of different sedentary behaviors on levels of inflammatory biomarkers. First, influences of daily amounts of sedentary time, either derived in uninterrupted time bouts of ≥10 or ≥30 min or accumulated over a day, were explored in sex-stratified models. In women, a higher amount of accumulated sedentary time was significantly related to higher levels of the pro-inflammatory biomarkers IL-6 (p < 0.05) and fibrinogen (p < 0.05) (Table 2). A positive relationship with fibrinogen was also evident when analyzing amount of sedentary time derived in continuous bouts of at least 10 min (p < 0.05) (Table 2). Besides links to sedentary time, an inverse significant relationship was further observed between break rate and fibrinogen level (p < 0.05). Importantly, all observed associations remained significant after further adjustment for time spent in MVPA. However, only the link between total accumulated sedentary time and fibrinogen remained after adjusting for total accelerometer counts instead of MVPA time (p < 0.05) (Table 2). Moreover, the detrimental impact of sedentary time on fibrinogen level remained after further adjustment by metabolic risk score (model 2: β = 0.105, 95% CI: 0.023–0.186; model 3: β = 0.099, 95% CI: 0.002–0.195, all p < 0.05). Finally, no relationships with other inflammatory biomarkers were observed (Table 2).
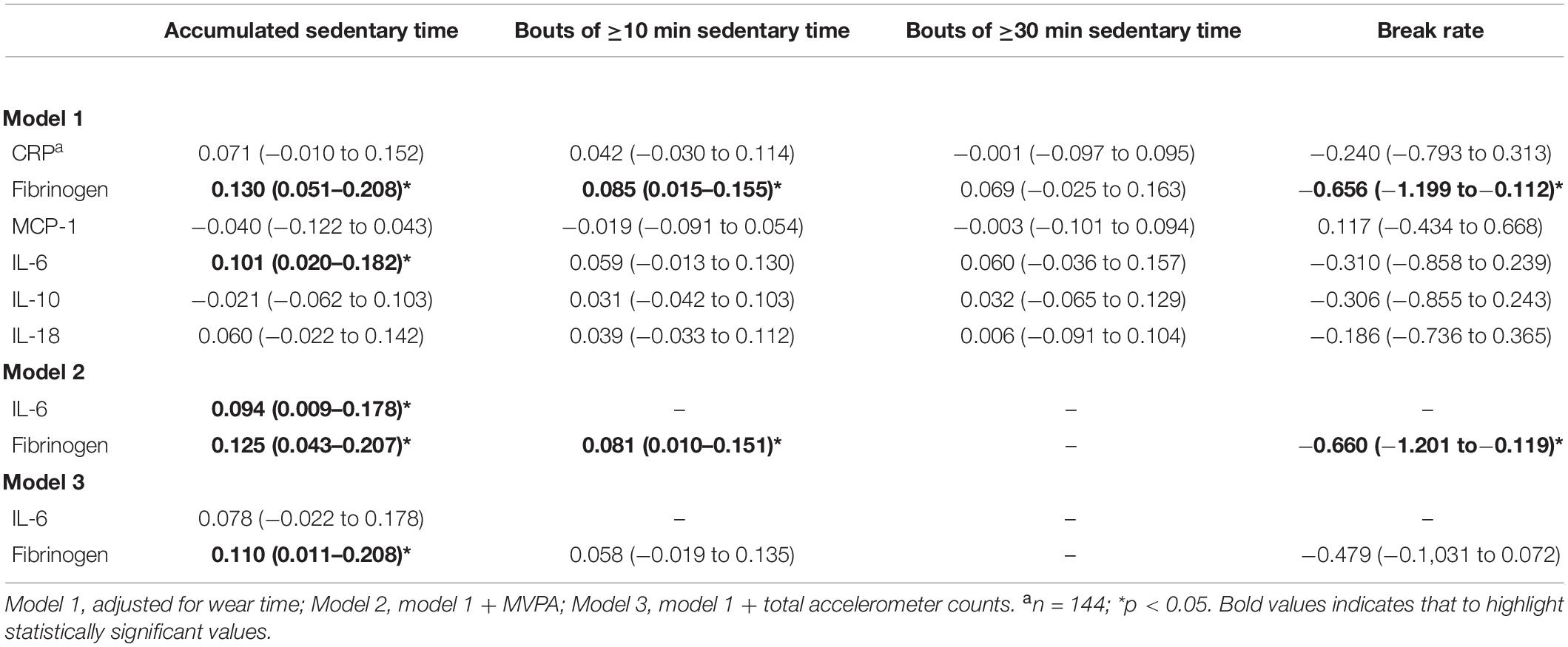
Table 2. Associations (β-coefficients, 95% CI) between dimensions of sedentary time and inflammatory biomarkers in older women.
In men, higher amounts of sedentary time derived either accumulated or in continuous bouts of at least 10 min, were significantly related to lower levels of the anti-inflammatory biomarker IL-10 (p < 0.05) (Table 3). Further, a significant positive relationship between IL-10 and break rate was observed (p < 0.05), indicating a beneficial impact of breaking up sedentary activities on levels of this anti-inflammatory agent. Interestingly, adjustment for PA either by time in MVPA or total accelerometer counts did not attenuate the significant influence of sedentary behavior on IL-10 level (Table 3). Importantly, we further adjusted our models by metabolic risk score, which left significant associations unchanged (accumulated sedentary time: model 2, β = −0.124, 95% CI: −0.241 to −0.007; model 3, β = −0.146, 95% CI: −0.272 to −0.020; bouts of ≥10 min: β = −0.101, 95% CI: −0.199 to −0.004; model 3, β = −0.110, 95% CI: −0.210 to −0.011; break rates: β = 0.140, 95% CI: 0.011–0.269; model 3, β = 0.139, 95% CI: 0.016–0.261; all p < 0.05). Notably, no other biomarkers of inflammation were significantly associated with sedentary behaviors in the male participants (Table 3).
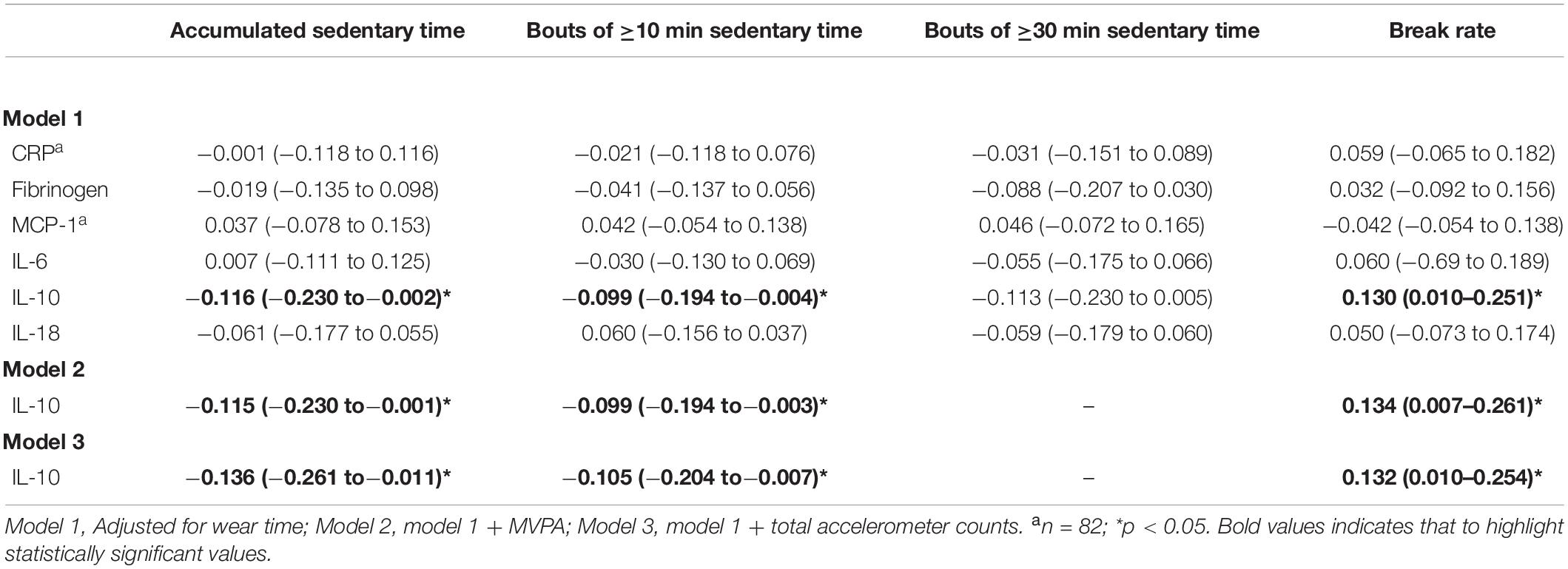
Table 3. Associations (β-coefficients, 95% CI) between dimensions of sedentary time and inflammatory biomarkers in older men.
The present study revealed sex-specific links between sedentary time patterns and biomarkers of low-grade systemic inflammation. Higher accumulated amounts of sedentary time were related to higher levels of the pro-inflammatory biomarkers IL-6 and fibrinogen in women, whereas an inverse relationship with the anti-inflammatory biomarker IL-10 was observed in men. Interestingly, time spent in MVPA did not offset the detrimental impacts of accumulating excessive amounts of sedentary time in both sexes. However, adjustment for total PA volume attenuated observed links with IL-6 in women.
It has been hypothesized that biological sex interacts with regulation of systemic inflammation, which should be considered when elucidating impacts of sedentariness on inflammatory status. Here we show detrimental sex-specific links for levels of IL-6, fibrinogen, and IL-10, which were generally confirmed by the association between a higher break rate and a more beneficial inflammatory profile. These findings are in accordance with previous observations highlighting stronger associations between sedentary time and levels of fibrinogen and IL-6 in women compared to men (Pereira et al., 2012; Yates et al., 2012; Howard et al., 2015). This is also in line with previous clinical and experimental findings showing compelling evidence of marked sex differences in inflammatory responses, where women exhibit a more pronounced pro-inflammatory response during acute infections (Van Eijk et al., 2007; Klein and Flanagan, 2016). For example, women show significantly higher increases in the pro-inflammatory biomarkers TNF-α and IL-6 compared to men, whereas the anti-inflammatory cytokine IL-10 was significantly higher in men (Engler et al., 2016). The significant difference in inflammatory response was in young men and women after administrating a low-dose bacterial endotoxin. It was observed that women mounted a stronger pro-inflammatory response to the endotoxin, while IL-10 was significantly raised in men. It is currently proposed that differences in hormonal levels, including estrogen, partly explain sex-specific modulations of the pro- and anti-inflammatory systemic environment (Rettew et al., 2009). Altogether, these findings suggest that biological sex needs to be considered when depicting factors involved in the regulation of systemic inflammatory environment and its relationship to PA behaviors in older adults.
An important finding of the present study was that total daily PA rather than time in MVPA attenuates links between sedentary patterns and inflammation in older women. Generally, time spent in MVPA has been considered when exploring the impact of sedentary patterns on systemic inflammation (Allison et al., 2012; Gennuso et al., 2013; Hamer et al., 2015; Howard et al., 2016; Parsons et al., 2017). For instance, Gennuso et al. (2013) observed that sufficient time spent in MVPA (≥ 150 min/week) derived from accelerometers did not significantly attenuate associations between sedentary time and CRP. However, as time in MVPA represents a small part of the daily time, residual confounding from remaining time spent in lower intensity PA may still be present. Thus, our findings are in favor of the hypothesis that detrimental links between sedentary patterns and systemic inflammation are partly explained by lower amounts of daily PA rather than by prolonged sedentariness itself.
Another important finding was the independent inverse relationship between sedentary patterns and IL-10 levels in men. This is in line with previous findings showing higher levels of IL-10 gene expression in active compared to sedentary older men (Ferrer et al., 2018). While assessment of activity status was achieved through questionnaires, Ferrer et al. (2018) demonstrated significant anthropometric differences between active and sedentary older adults and suggests that elevations in IL-10 is associated with a reduction in adiposity by PA. Given that IL-10 is a potent anti-inflammatory factor, this finding proposes that reducing sedentary behaviors could promote anti-inflammatory processes in older men across different levels of PA and metabolic risk. Although exact mechanisms underpinning the regulation of IL-10 levels remain unclear, sedentary behaviors characterized by low muscle activity may contribute to lower levels of IL-10 and their main cellular source (Treg cells) (Wang et al., 2012; Dorneles et al., 2016).
Interestingly, unlike previous studies, we did not observe a significant association between sedentary behavior and the pro-inflammatory biomarkers CRP, IL-18, and MCP-1 (Healy et al., 2011b; Pereira et al., 2012; Howard et al., 2015). There are several possible reasons for the discrepant findings. Firstly, there are differences in age, with Healy et al. (2011b) and Howard et al. (2015) also including younger and middle-aged adults, and Pereira et al. (2012) including only middle-aged adults. Secondly, time spent in sedentary behavior was assessed by interview-administered questionnaires of overall sitting behavior (Howard et al., 2015) or questionnaires on television-viewing and work-sitting (Pereira et al., 2012). Notably, CRP was significantly related to time in MVPA in both sexes, confirming previous observations that PA above light intensity is required to influence on this clinical marker of inflammation (Nilsson et al., 2018). The lack of associations with sedentary time denotes the complex inter-relationships between systemic inflammation and PA behaviors, where different dimensions of PA behaviors, including total amounts and time spent in different intensities, may have separate influences on inflammatory biomarkers depending on their pro- and anti-inflammatory actions.
Although sedentary behaviors lasting at least 30 min or longer are thought to be particularly detrimental to health, most of previous research supporting this hypothesis have been based on self-report measures, which is limited to cruder estimations of sedentary behavior compared to objective methods. Based on our objective assessment of sedentary patterns, significant associations were reported when analyzing time bouts lasting at least 10 min, while no corresponding links were observed for the 30-min bouts. A previous study investigating how to best capture prolonged sedentary behaviors when using accelerometers, including both ≥10- and ≥30-min bouts, concluded the ≥10-min bout to be an appropriate threshold to capture the prolonged nature of sedentary behavior and its relation to health outcomes (Kim et al., 2015). Thus, the contrasting result depending on whether a 10- or 30-min time window is employed, is likely due to the natural occurrence of sporadic breaks (i.e., ≥100 counts) when extending the time bout from ≥10- to ≥30-min, which would prevent detection of prolonged sedentary behaviors. Noteworthy, those who break-up their sedentary behavior more frequently (i.e., higher break rate) showed a more favorable inflammatory profile than those with a lower break rate, which highlights the beneficial health impact of avoiding prolonged sedentary behaviors. Interestingly, our results further indicate that the amount of daily sedentary time, regardless if occurring in uninterrupted time bouts or simply accumulated throughout the day, has a detrimental association to systemic inflammation. This further emphasizes the health-related potential of reducing daily amounts of sedentary time in favor of increasing PA level.
While our cross-sectional analysis highlights important associations between sedentariness and systemic inflammation, direction of the relationships cannot be determined. The older men and women included in this study may not be representative of broader populations of older adults, including differences in ethnicity and health status. Nonetheless, reported time spent in sedentary behavior are similar to what others have reported in older adults (Hagströmer et al., 2010). Further, PA behaviors were objectively assessed by accelerometers, which allowed capturing ambulatory activities but not postural data. However, the Actigraph activity monitor has been shown to accurately capture more than 80% of sedentary activities in a sitting or lying position (Carr and Mahar, 2012) and is frequently used to assess sedentary behaviors (Healy et al., 2011a).
Although our study revealed sedentary pattern-related impacts on different pro- and anti-inflammatory biomarkers, the systemic inflammatory environment is likely influenced by additional mediators not included in the present work. Further research is warranted to uncover molecular mechanism underpinning the systemic inflammatory profiles in aging populations.
In conclusion, our findings confirm the hypothesis of sex-specific routes by which sedentary patterns impact on pro- and anti-inflammatory biomarkers in older adults. The detrimental impact of sedentary patterns is partly explained by total amounts of daily PA rather than by sedentariness itself. These findings hold clinical and public health implications, by reinforcing efforts aiming to promote daily PA at the expense of less sedentary pursuits in older men and women. Future efforts should be put on elucidating molecular mechanisms underlying observed links revealed in this study.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Regional ethics committee of Uppsala, Sweden. The patients/participants provided their written informed consent to participate in this study.
OB and AN: conception of the work, acquisition, analysis, and interpretation of data, drafting and revision of the work, and approval of final draft including agreement for accountability. FK: conception of the work, acquisition, analysis, and interpretation of data, revising and approval of final draft including agreement for accountability K-GP: acquisition of data, revision of manuscript, approval of final draft including agreement for accountability. All authors contributed to the article and approved the submitted version.
This research protocol is funded by the EU HORIZON 2020 Research and Innovation Program (European Joint Programming Initiative “A healthy diet for a healthy life” “JPI HDHL” and the ERA-NET co-fund HDHL-INTIMIC, “GA no. 727565”).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the European Commission through the Marie Skłodowska-Curie Actions, Cofunding of Regional, National and International Programs (MSCA COFUND) for supporting K-GP.
Alberti, K. G. M. M., Zimmet, P., and Shaw, J. (2006). Metabolic syndrome-a new world-wide definition. a consensus statement from the international diabetes federation. Diabet. Med. 23, 469–480. doi: 10.1111/j.1464-5491.2006.01858.x
Allison, M. A., Jensky, N. E., Marshall, S. J., Bertoni, A. G., and Cushman, M. (2012). Sedentary behavior and adiposity-associated inflammation. Am. J. Prev. Med. 42, 8–13. doi: 10.1016/j.amepre.2011.09.023
Asadullah, K., Sterry, W., and Volk, H. D. (2003). Interleukin-10 therapy-review of a new approach. Pharmacol. Rev. 55, 241–269. doi: 10.1124/pr.55.2.4
Bergens, O., Nilsson, A., and Kadi, F. (2019). Cardiorespiratory fitness does not offset adiposity-related systemic inflammation in physically active older women. J. Clin. Endocrinol. Metab. 104, 4119–4126. doi: 10.1210/jc.2019-00067
Campesi, I., Occhioni, S., Tonolo, G., Cherchi, S., Basili, S., Carru, C., et al. (2016). Ageing/menopausal status in healthy women and ageing in healthy men differently affect cardiometabolic parameters. Int. J. Med. Sci. 13, 124–132. doi: 10.7150/ijms.14163
Carr, L. J., and Mahar, M. T. (2012). Accuracy of intensity and inclinometer output of three activity monitors for identification of sedentary behavior and light-intensity activity. J. Obes. 2012:460271. doi: 10.1155/2012/460271
Cartier, A., Côté, M., Lemieux, I., Pérusse, L., Tremblay, A., Bouchard, C., et al. (2009). Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am. J. Clin. Nutr. 89, 1307–1314. doi: 10.3945/ajcn.2008.27030
Dorneles, G. P., Haddad, D. O., Fagundes, V. O., Vargas, B. K., Kloecker, A., Romão, P. R. T., et al. (2016). High intensity interval exercise decreases IL-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight-obese individuals. Cytokine 77, 1–9. doi: 10.1016/j.cyto.2015.10.003
Dunstan, D. W., Thorp, A. A., and Healy, G. N. (2011). Prolonged sitting: is it a distinct coronary heart disease risk factor? Curr. Opin. Cardiol. 26, 412–419. doi: 10.1097/HCO.0b013e3283496605
Edwardson, C. L., Gorely, T., Davies, M. J., Gray, L. J., Khunti, K., Wilmot, E. G., et al. (2012). Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One 7:e34916. doi: 10.1371/journal.pone.0034916
Engler, H., Benson, S., Wegner, A., Spreitzer, I., Schedlowski, M., and Elsenbruch, S. (2016). Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav. Immun. 52, 18–26. doi: 10.1016/j.bbi.2015.08.013
Ferrer, M. D., Capó, X., Martorell, M., Busquets-Cortés, C., Bouzas, C., Carreres, S., et al. (2018). Regular practice of moderate physical activity by older adults ameliorates their anti-inflammatory status. Nutrients 10, 1–13. doi: 10.3390/nu10111780
Franceschi, C., and Campisi, J. (2014). Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69, S4–S9. doi: 10.1093/gerona/glu057
Gennuso, K. P., Gangnon, R. E., Matthews, C. E., Thraen-Borowski, K. M., and Colbert, L. H. (2013). Sedentary behavior, physical activity, and markers of health in older adults. Med. Sci. Sports Exerc. 45, 1493–1500. doi: 10.1249/MSS.0b013e318288a1e5
Hagströmer, M., Troiano, R. P., Sjöström, M., and Berrigan, D. (2010). Levels and patterns of objectively assessed physical activity-a comparison between Sweden and the United States. Am. J. Epidemiol. 171, 1055–1064. doi: 10.1093/aje/kwq069
Hamer, M., Smith, L., and Stamatakis, E. (2015). Prospective association of TV viewing with acute phase reactants and coagulation markers: english longitudinal study of ageing. Atherosclerosis 239, 322–327. doi: 10.1016/j.atherosclerosis.2015.02.009
Healy, G. N., Clark, B. K., Winkler, E. A. H., Gardiner, P. A., Brown, W. J., and Matthews, C. E. (2011a). Measurement of adults’ sedentary time in population-based studies. Am. J. Prev. Med. 41, 216–227. doi: 10.1016/j.amepre.2011.05.005
Healy, G. N., Matthews, C. E., Dunstan, D. W., Winkler, E. A. H., and Owen, N. (2011b). Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 200306. Eur. Heart J. 32, 590–597. doi: 10.1093/eurheartj/ehq451
Henson, J., Yates, T., Edwardson, C. L., Khunti, K., Talbot, D., Gray, L. J., et al. (2013). Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS One 8:e78350. doi: 10.1371/journal.pone.0078350
Howard, B. J., Balkau, B., Thorp, A. A., Magliano, D. J., Shaw, J. E., Owen, N., et al. (2015). Associations of overall sitting time and TV viewing time with fibrinogen and C reactive protein: the AusDiab study. Br. J. Sports Med. 49, 255–258. doi: 10.1136/bjsports-2013-093014
Howard, B. J., Hurtig-Wennlöf, A., Olsson, L. A., Nilsson, T. K., Dunstan, D. W., and Wennberg, P. (2016). Self-reported sitting time, physical activity and fibrinolytic and other novel cardio-metabolic biomarkers in active Swedish seniors. PLoS One 11:e0163409. doi: 10.1371/journal.pone.0163409
Johansson, I., Van Guelpen, B., Hultdin, J., Johansson, M., Hallmans, G., and Stattin, P. (2010). Validity of food frequency questionnaire estimated intakes of folate and other B vitamins in a region without folic acid fortification. Eur. J. Clin. Nutr. 64, 905–913. doi: 10.1038/ejcn.2010.80
Jung, U. J., and Choi, M. S. (2014). Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15, 6184–6223. doi: 10.3390/ijms15046184
Kim, Y., Welk, G. J., Braun, S. I., and Kang, M. (2015). Extracting objective estimates of sedentary behavior from accelerometer data: measurement considerations for surveillance and research applications. PLoS One 10:e0118078. doi: 10.1371/journal.pone.0118078
Klein, S. L., and Flanagan, K. L. (2016). Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638. doi: 10.1038/nri.2016.90
Koenig, W., Löwel, H., Baumert, J., and Meisinger, C. (2004). C-reactive protein modulates risk prediction based on the Framingham score-implications for future risk assessment: results from a large cohort study in southern Germany. Circulation 109, 1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3
Lynch, B. M., Friedenreich, C. M., Winkler, E. A. H., Healy, G. N., Vallance, J. K., Eakin, E. G., et al. (2011). Associations of objectively assessed physical activity and sedentary time with biomarkers of breast cancer risk in postmenopausal women: findings from NHANES (2003-2006). Breast Cancer Res. Treat. 130, 183–194. doi: 10.1007/s10549-011-1559-2
Meador, B. M., Krzyszton, C. P., Johnson, R. W., and Huey, K. A. (2008). Effects of IL-10 and age on IL-6, IL-1β, and TNF-α responses in mouse skeletal and cardiac muscle to an acute inflammatory insult. J. Appl. Physiol. 104, 991–997. doi: 10.1152/japplphysiol.01079.2007
Nilsson, A., Bergens, O., and Kadi, F. (2018). Physical activity alters inflammation in older adults by different intensity levels. Med. Sci. Sports Exerc. 50, 1502–1507. doi: 10.1249/MSS.0000000000001582
Nilsson, A., Halvardsson, P., and Kadi, F. (2019). Adherence to dash-style dietary pattern impacts on adiponectin and clustered metabolic risk in older women. Nutrients 11, 1–9. doi: 10.3390/nu11040805
Parsons, T. J., Sartini, C., Welsh, P., Sattar, N., Ash, S., Lennon, L. T., et al. (2017). Physical activity, sedentary behavior, and inflammatory and hemostatic markers in men. Med. Sci. Sports Exerc. 49, 459–465. doi: 10.1249/MSS.0000000000001113
Pereira, S. M., Ki, M., and Power, C. (2012). Sedentary behaviour and biomarkers for cardiovascular disease and diabetes in mid-life: the role of television-viewing and sitting at work. PLoS One 7:e31132. doi: 10.1371/journal.pone.0031132
Razmjou, S., Bastard, J. P., Doucet, E., Rabasa-Lhoret, R., Fellahi, S., Lavoie, J. M., et al. (2016). Effect of the menopausal transition and physical activity energy expenditure on inflammatory markers: a MONET group study. Menopause 23, 1330–1338. doi: 10.1097/GME.0000000000000716
Rettew, J. A., Huet, Y. M., and Marriott, I. (2009). Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology 150, 3877–3884. doi: 10.1210/en.2009-0098
Rudnicka, A. R., Rumley, A., Whincup, P. H., Lowe, G. D., and Strachan, D. P. (2011). Sex differences in the relationship between inflammatory and hemostatic biomarkers and metabolic syndrome: British 1958 birth cohort. J. Thromb. Haemost. 9, 2337–2344. doi: 10.1111/j.1538-7836.2011.04517.x
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R., and Hébert, J. R. (2014). Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17, 1689–1696. doi: 10.1017/S1368980013002115
Stec, J. J., Silbershatz, H., Tofler, G. H., Matheney, T. H., Sutherland, P., Lipinska, I., et al. (2000). Association of fibrinogen with cardiovascular risk factors and cardiovascluar disease the Framingham offspring population. Circulation 102, 1634–1638. doi: 10.1161/01.CIR.102.14.1634
Troiano, R. P., Berrigan, D., Dodd, K. W., Mâsse, L. C., Tilert, T., and Mcdowell, M. (2008). Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 40, 181–188. doi: 10.1249/mss.0b013e31815a51b3
Van Eijk, L. T., Dorresteijn, M. J., Smits, P., Van Der Hoeven, J. G., Netea, M. G., and Pickkers, P. (2007). Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit. Care Med. 35, 1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8
Wang, J., Song, H., Tang, X., Yang, Y., Vieira, V. J., Niu, Y., et al. (2012). Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand. J. Med. Sci. Sport 22, 643–652. doi: 10.1111/j.1600-0838.2010.01288.x
Yates, T., Khunti, K., Wilmot, E. G., Brady, E., Webb, D., Srinivasan, B., et al. (2012). Self-reported sitting time and markers of inflammation, insulin resistance, and adiposity. Am. J. Prev. Med. 42, 1–7. doi: 10.1016/j.amepre.2011.09.022
Keywords: aging, physical activity, sedentary behaviors, inflammatory biomarkers, metabolic health
Citation: Bergens O, Nilsson A, Papaioannou K-G and Kadi F (2021) Sedentary Patterns and Systemic Inflammation: Sex-Specific Links in Older Adults. Front. Physiol. 12:625950. doi: 10.3389/fphys.2021.625950
Received: 11 November 2020; Accepted: 15 January 2021;
Published: 05 February 2021.
Edited by:
Katsuhiko Suzuki, Waseda University, JapanReviewed by:
Masataka Uchida, Ritsumeikan University, JapanCopyright © 2021 Bergens, Nilsson, Papaioannou and Kadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fawzi Kadi, RmF3emkua2FkaUBvcnUuc2U=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.