- Department of Medicine, The University of Chicago, Chicago, IL, United States
Anoctamin-1 (ANO1), also known as TMEM16A, is the most studied member of anoctamin family of calcium-activated chloride channels with diverse cellular functions. ANO1 controls multiple cell functions including cell proliferation, survival, migration, contraction, secretion, and neuronal excitation. This review summarizes the current knowledge of the cellular mechanisms governing the regulation of ANO1 expression and of ANO1-mediated intracellular signaling. This includes the stimuli and mechanisms controlling ANO1 expression, agonists and processes that activate ANO1, and signal transduction mediated by ANO1. The major conclusion is that this field is poorly understood, remains highly controversial, and requires extensive and rigorous further investigation.
Introduction
Anoctamin-1 (ANO1), also known as TMEM16A, DOG1 (Discovered On Gastrointestinal stromal tumors 1), ORAOV2 (Oral cancer Overexpressed), and TAOS-2 (Tumor Amplified and Overexpressed) was initially found to be overexpressed in a number of cancer tissues and is thought to contribute to cancer cell growth and tumorigenesis (Bill and Alex Gaither, 2017; Crottes and Jan, 2019). Subsequently, ANO1 was identified as calcium-activated chloride channel (Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008). Mutagenesis and structural studies revealed that the ANO1 channel pore is formed by transmembrane helices 3-8; and the residues responsible for Ca2+ binding, channel gating and ion selectivity have been identified, providing mechanistic insights on ANO1 activation (Tien et al., 2014; Peters et al., 2015, 2018; Dang et al., 2017; Paulino et al., 2017). Molecular mechanisms of ANO1 activation and regulation are comprehensively reviewed elsewhere (Pedemonte and Galietta, 2014; Contreras-Vite et al., 2016; Jin et al., 2016; Ji et al., 2019). The most recognized cellular functions of ANO1 include the control of cancer cell proliferation, survival and migration by ANO1 (Bill and Alex Gaither, 2017; Crottes and Jan, 2019), secretory function of ANO1 in the epithelia, such as airways, intestines, salivary glands, renal tubules and sweat glands (Jang and Oh, 2014), induction of electrical pacemaker activity of interstitial cells of Cajal in gastrointestinal smooth muscles (Sanders et al., 2012), control of acute pain sensation, chronic pain and anxiety-related behaviors (Oh and Jung, 2016; Cho et al., 2020), and contribution
to contraction of airway and vascular smooth muscles (Oh and Jung, 2016). While the cellular functions of ANO1 are well established, the signaling mechanisms providing these functions appear highly controversial. This review summarizes the current knowledge of mechanisms governing the regulation of ANO1 expression and ANO1-induced intracellular signaling.
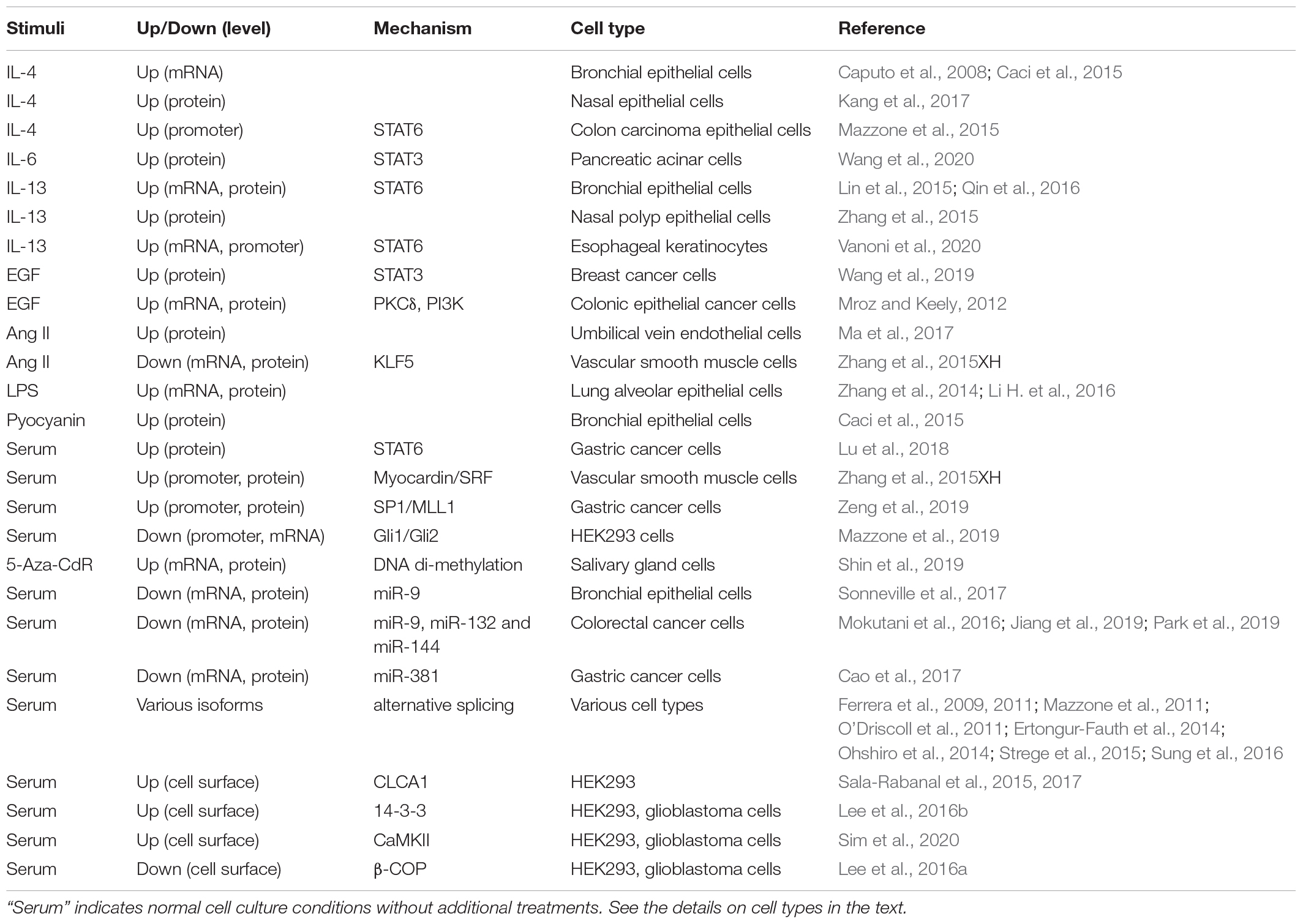
Regulation of ANO1 Expression (Table 1)
Most notably, ANO1 expression is increased in response to several interleukins including IL-4 in bronchial epithelial cells (Caputo et al., 2008; Caci et al., 2015) and human nasal epithelial cells (Kang et al., 2017), IL-6 in pancreatic acinar cells (Wang et al., 2020), and IL-13 in human bronchial epithelial cells (Lin et al., 2015; Qin et al., 2016), human nasal polyp epithelial cells (Zhang et al., 2015) and human esophageal keratinocytes (Vanoni et al., 2020). Epidermal growth factor (EGF) promotes ANO1 expression in colonic epithelial cancer cells (Mroz and Keely, 2012) and in breast cancer cells (Wang et al., 2019). ANO1 expression was increased by angiotensin II in human umbilical vein endothelial cells (HUVECs) (Ma et al., 2017); however, angiotensin II inhibited ANO1 expression in vascular smooth muscle cells (Zhang et al., 2015), demonstrating cell-specific effects. Among the pathological stimuli, ANO1 expression was transiently induced by the bacterial toxin lipopolysaccharide (LPS) in human lung adenocarcinoma A549 cells (Zhang et al., 2014), in cultured mouse lung cancer cell line LA795, and in mouse alveolar epithelial cells in vivo after intraperitoneal injection of LPS (Li H. et al., 2016). Bacterial pyocyanin and supernatants from Pseudomonas aeruginosa culture stimulated ANO1 expression in human bronchial epithelial cells (Caci et al., 2015).
Mechanistically, ANO1 expression is regulated at transcriptional, translational and post-translational levels. The signal transducer and activator of transcription (STAT) transcription factors were shown to control ANO1 gene transcription in several studies. STAT3 is important for ANO1 transcription in response to IL-6 in pancreatic acinar cells (Wang et al., 2020) and in response to EGF in breast cancer cells (Wang et al., 2019). EGF-induced ANO1 expression in colonic epithelial cancer cells was also dependent on the activity of protein kinase C-δ (PKCδ) and phosphatidylinositol 3-kinase (PI3K), but the link to STAT3 was not investigated (Mroz and Keely, 2012). ANO1 expression was reported to be dependent on STAT6 in human bronchial epithelial cells (Qin et al., 2016), in esophageal keratinocytes (Vanoni et al., 2020) and in gastric cancer cells (Lu et al., 2018). Others have identified STAT6-binding site on ANO1 promoter and demonstrated its role in IL-4-induced promoter activation by mutagenesis (Mazzone et al., 2015). In vascular smooth muscle cells, ANO1 transcription may be driven by myocardin / serum response factor (SRF) transcription factors (Zhang et al., 2015XH). In gastric cancer cells, the specificity protein 1 (SP1) was shown to bind ANO1 promoter and recruit the mixed lineage leukemia MLL1 protein to promoter region, facilitating histone H3K4 trimethylation, and subsequently promoting ANO1transcription (Zeng et al., 2019). On the other hand, glioma-associated oncogene proteins Gli1/2 were shown to bind and repress ANO1 promoter activation and ANO1 expression in HEK293 model (Mazzone et al., 2019). DNA methylation was also shown to regulate ANO1 expression. An increased expression of ANO1 during salivary gland organogenesis correlated with decreased DNA methylation of ANO1 gene; and demethylating agent 5-aza-2’-deoxycytidine (5-Aza-CdR) drove expression of ANO1 in cultured salivary gland cells (Shin et al., 2019). Conversely, analysis of squamous cell carcinoma [including human papillomavirus (HPV)-positive] samples from two datasets suggested a positive correlation of ANO1 expression with methylation of two CpG islands on ANO1 gene (Finegersh et al., 2017). However, forced hypermethylation of the positively correlated CpG island in normal oral keratinocytes by transfection with HPV oncoprotein E7 that recruits DNA methyl transferases (Sen et al., 2018) had no effect on ANO1 expression, demonstrating a disconnect between the in vivo and in vitro findings (Finegersh et al., 2017).
ANO1 translation is controlled by a number of microRNAs, including miR-9 in human bronchial epithelial cells (Sonneville et al., 2017), miR-9, miR-132 and miR-144 in colorectal cancer cells (Mokutani et al., 2016; Jiang et al., 2019; Park et al., 2019), and miR-381 in gastric cancer cells (Cao et al., 2017). In addition, alternative splicing of ANO1 in a tissue specific manner affects electrophysiological properties of the channel (Ferrera et al., 2009, 2011; Mazzone et al., 2011; O’Driscoll et al., 2011; Ertongur-Fauth et al., 2014; Ohshiro et al., 2014; Strege et al., 2015; Sung et al., 2016). Post-translationally, for appropriate channel function, ANO1 has to be expressed at the cell surface, which is controlled by other proteins. Secreted calcium-activated chloride channel regulator 1 (CLCA1) stabilizes ANO1 at the cell surface in HEK293 cell model possibly through inhibition of ANO1 internalization (Sala-Rabanal et al., 2015, 2017). Phospho-serine binding protein 14-3-3 directly interacts with ANO1 and also stabilizes ANO1 on cell surface by unknown mechanism, both in heterologous models and in glioblastoma U251 cells (Lee et al., 2016b). Cell surface expression of ANO1 is also promoted by Ca2+/Calmodulin-dependent protein kinase II (CaMKII) in heterologous models and in glioblastoma U251 cells (Sim et al., 2020), potentially (but not proven) through phosphorylation of ANO1 by CAMKII (Ayon et al., 2019). In contrast, β-COP, a subunit of Coat Protein Complex I (COPI), directly interacts with ANO1 and reduces its surface expression by promoting the retrograde transport, which was also shown at the endogenous levels of ANO1 and β-COP in U251 cells (Lee et al., 2016a). Importantly, in all these studies the regulated surface expression of ANO1 functionally translated to ANO1 activity.
Physiological and Pathological Activators of ANO1-Mediated Chloride Currents
Given that ANO1 is a calcium-activated chloride channel, agonists that promote intracellular accumulation of free Ca2+ are expected to stimulate ANO1 activity. This was initially demonstrated for G protein-coupled receptor (GPCR) agonists including endothelin-1, angiotensin II, extracellular ATP, histamine and carbachol in human embryonic kidney HEK293 cells co-transfected with cDNAs for ANO1 together with corresponding GPCRs (Yang et al., 2008). Similar finding was made for carbachol in Axolotl oocytes co-injected with Xenopus TMEM16A/ANO1 and human M1 muscarinic cRNA (Schroeder et al., 2008). Initial studies also demonstrated ANO1-dependent activation of calcium-dependent chloride currents (CaCC) by extracellular UTP in pancreatic CFPAC-1 cells endogenously expressing ANO1 and purinergic receptors for UTP (Caputo et al., 2008). Subsequently, multiple studies showed the induction of ANO1-mediated CaCC by GPCR agonists in cells with endogenous expression of ANO1 and corresponding GPCRs. Some examples include angiotensin II in cardiac fibroblasts (El Chemaly et al., 2014), basilar artery smooth muscle cells (Li R. S. et al., 2016), and human umbilical vein endothelial cells HUVEC (Ma et al., 2017); endothelin-1 in dorsal root ganglion (DRG) neurons (Cho et al., 2012); epinephrine in Calu-3 human bronchial serous cell model (Banga et al., 2014); carbachol in intestinal epithelial cells (Lee et al., 2019) and in dorsal root ganglion (DRG) neurons (Cho et al., 2012); bradykinin and histamine in DRG neurons (Liu et al., 2010; Cho et al., 2012); and extracellular ATP in FRTL-5 thyroid cells (Iosco et al., 2014). The contribution of ANO1 to CaCC in these studies was determined using pharmacological inhibitors of ANO1 (i.e., T16Ainh-A01) (Namkung et al., 2011a), siRNA-mediated ANO1 knockdown, or ablation of ANO1 gene. ANO1-mediated CaCC can be also induced by non-GPCR agonists. For example, the receptor tyrosine kinase (RTK) ligand EGF stimulated a transient calcium response and activated CaCC in the pancreatic cancer cell line AsPC-1, which was inhibited by ANO1 knockdown (Crottes et al., 2019). Likewise, brain-derived neurotrophic factor (BDNF), acting through tropomyosin-related kinase (Trk) receptors (Binder and Scharfman, 2004), induced robust Cl– currents, and these effects were blocked by pharmacological inhibition of ANO1 or by ANO1 knockout (Hong et al., 2019). Interestingly, LPS increased currents in whole-cell patch recordings in cultured mouse lung cancer cell line LA795, and this was reduced by ANO1 knockdown; however, the role of calcium in these currents was not demonstrated (Li H. et al., 2016).
ANO1 appears to be a mechano-sensitive channel. As mentioned above, ANO1 is activated by shear stress in biliary cells, likely through a release of extracellular ATP acting through purinergic receptors and an increase in intracellular Ca2+ levels (Dutta et al., 2013). In cerebral artery smooth muscle cells, ANO1 is activated by cell swelling and by pressure induced cell stretch through non-selective cation channels and local intracellular Ca2+ signaling (Bulley et al., 2012). ANO1 is also a heat sensor, being directly activated by heat over 44 °C; and heat has a synergistic effect on the Ca2+ response of ANO1 (Cho et al., 2012). Activation of ANO1 by heat causes depolarization of nociceptive dorsal root ganglion (DRG) neurons, which could be one of the mechanisms for pain sensation (Cho et al., 2012). Importantly, heat induced Cl– currents persisted in DRG neurons isolated from mice deficient for TRPV1 channel—the established heat sensor (Caterina et al., 1997). Finally, ANO1 also senses extracellular proton concentrations. In HEK293 cells transfected with ANO1 cDNA, extracellular acidification enables ANO1 activation in a voltage-independent manner without altering the apparent Ca2+ sensitivity of ANO1 (Cruz-Rangel et al., 2017). A number of physiological and pathological processes such as ischemia and cancer progression involve alterations in extracellular pH (Chiche et al., 2010), hence pH sensing of ANO1 could be of high relevance. Furthermore, given that acidification can cause pain in humans (Jones et al., 2004), regulation of ANO1 by protons could be an additional mechanism for pain sensation through ANO1.
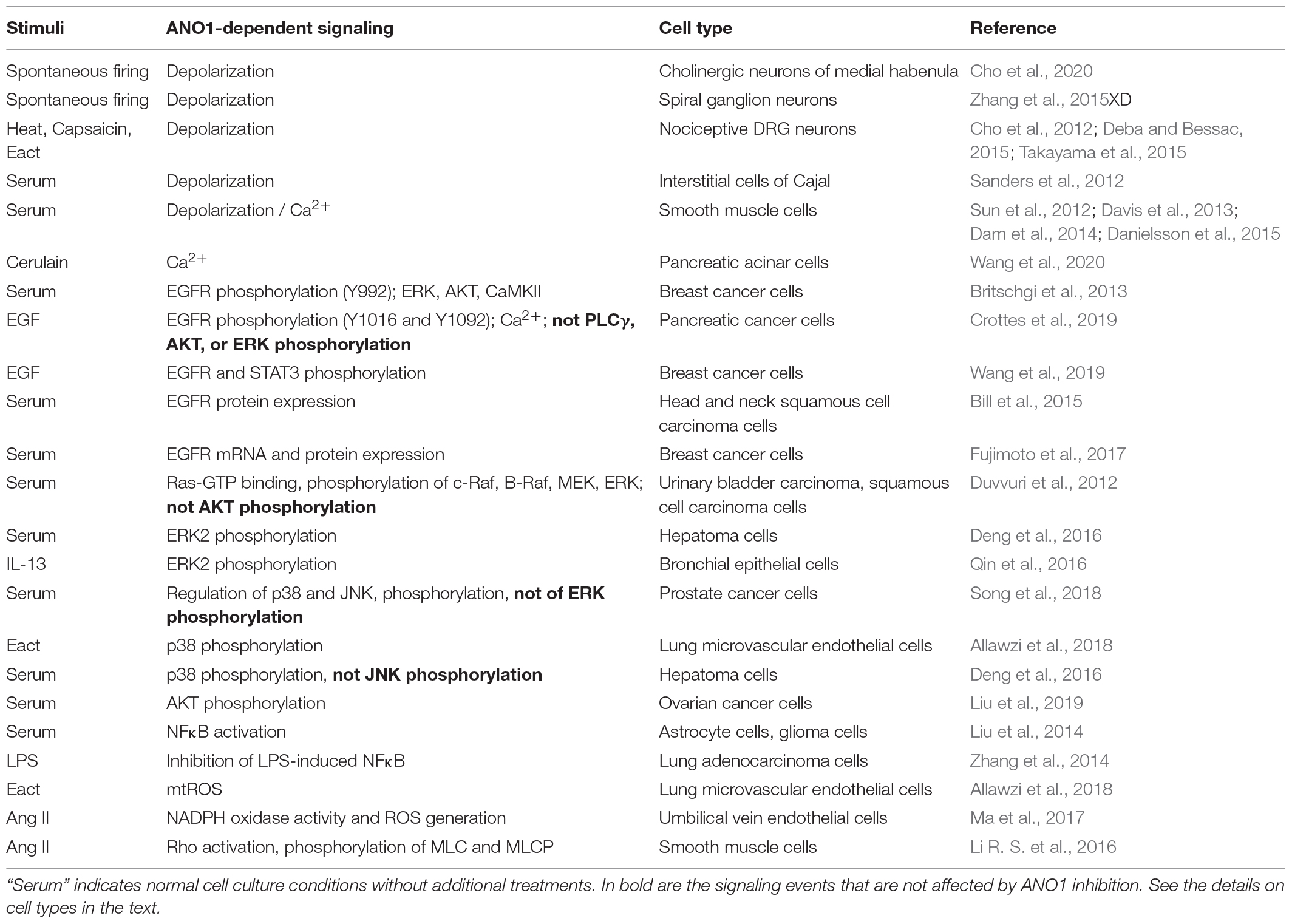
Cell Signaling Mediated by ANO1 (Table 2)
The direction of Cl– currents is determined by membrane potential and the intracellular concentration of Cl– ([Cl–]i). Most of mature brain neurons contain low [Cl–]i; and Cl– channel opening would result in Cl– influx and hyperpolarization of the cell, as widely exemplified by the inhibitory action of γ-aminobutyric acid (GABA) that opens Cl– channel GABAA receptors (Fukuda, 2020). In contrast, immature neurons of developing brain contain high [Cl–]i resulting in Cl– efflux and cell depolarization upon Cl– channel opening (Fukuda, 2020). Interestingly, in mature cholinergic neurons of medial habenula, ANO1 activity plays excitatory role by contributing to increased frequency of spontaneous firing, presumably due to high [Cl–]i in these neurons, although this was not measured in that study (Cho et al., 2020). Noteworthy, activation of the GABAA receptors in these neurons may also increase the firing of action potential (Kim and Chung, 2007). Outside the brain, ANO1 was shown to mediate depolarization and facilitate generation of action potential in spiral ganglion neurons (Zhang et al., 2015XD) and nociceptive DRG neurons (Cho et al., 2012; Deba and Bessac, 2015; Takayama et al., 2015); however, again, the [Cl–]i was not measured in these studies.
In non-neuronal cells, presumably with high [Cl–]i levels, ANO1 promotes depolarization, and facilitates pacemaker activity by interstitial cells of Cajal in intestinal smooth muscle (Sanders et al., 2012), and also contributes to contraction of vascular and airway smooth muscle cells (Sun et al., 2012; Davis et al., 2013; Dam et al., 2014). The latter effect is likely mediated by activation of voltage gated Ca2+ channels, as it was inhibited by L-type calcium channel blocker, nifedipine (Sun et al., 2012; Danielsson et al., 2015). Thus, while being activated by Ca2+, ANO1 may further promote accumulation of cytosolic free Ca2+. There are other mechanisms by which ANO1 activation facilitates an increase in the intracellular Ca2+ concentration. ANO1 co-immunoprecipitated with the inositol 1,4,5-trisphosphate receptor (IP3R), was activated by IP3R-mediated Ca2+ release, but also contributed to Ca2+ release induced by cholecystokinin analog, cerulain, in pancreatic acinar AR42J cells (Wang et al., 2020). ANO1 is also required for EGF-induced store-operated calcium entry (SOCE) in pancreatic cancer cells (Crottes et al., 2019); however, in both latter studies, the precise mechanism by which ANO controls Ca2+ signaling was not determined.
Most of the findings on ANO1-dependent cell signaling have emerged from the cancer field; hence, the focus has been on mitogenic/survival signaling. Several reports point to a direct control of EGF receptor (EGFR) activation by ANO1. Knockdown or pharmacological inhibition of ANO1 in ZR75-1 and HCC1954 breast cancer cell lines resulted in a decreased phosphorylation of EGFR at Y992 as well as of phosphorylation of downstream signaling molecules - ERK1/2, AKT and calmodulin kinase II (CaMKII) under normal growth conditions (Britschgi et al., 2013). Knockdown of ANO1 in human pancreatic cancer cells resulted in a significantly inhibited EGF-induced phosphorylation of tyrosine residues Y1016 and Y1092 of EGFR and of Ca2+ response to EGF; however, signaling consequences of this are unclear, as EGF-induced phosphorylation of phospholipase Cγ, AKT, and ERK was not affected in that study (Crottes et al., 2019). Knockdown of ANO1 in MCF-7 or T47D breast cancer cells resulted in a reduced tyrosine phosphorylation of EGFR (the site is not reported) and of STAT3 transcription factor in response to EGF, whereas overexpression of ANO1 in T47D cells increased phosphorylation EGFR and STAT3 (Wang et al., 2019). It was reported that ANO1 forms a direct complex with EGFR through the trans/juxtamembrane domain of EGFR in head and neck squamous cell carcinoma (HNSCC) cells; knockdown of ANO1 resulted in greatly reduced EGFR protein levels, which functionally translated to decreased cell proliferation (Bill et al., 2015). Further, knockdown of ANO1 in breast cancer YMB-1 cells also resulted in reduced mRNA levels of HER2 EGFR, suggesting the regulation at a transcriptional level (Fujimoto et al., 2017).
ANO1-induced phosphorylation of ERK1/2 has been demonstrated in urinary bladder carcinoma T24 cells and squamous cell carcinoma SCC-1 cells under normal cell culture growth conditions; and this effect was dependent on ANO1 activity, as the expression of ANO1 K610A mutant (with greatly reduced chloride conductance) had no such effect (Duvvuri et al., 2012). Interestingly, overexpression of ANO1 resulted in activation of upstream signaling molecules of ERK-MAP kinase pathway, including Ras-GTP binding and phosphorylation of c-Raf, B-Raf, and MEK (Duvvuri et al., 2012). In human bronchial epithelial HBE16 cell line, ANO1 expression, elevated either by ANO1 cDNA transfection or by IL-13 treatment, resulted in an increased ERK1/2 phosphorylation; and the effect of IL-13 was abolished by knockdown or pharmacological inhibition of ANO1 (Qin et al., 2016). Knockdown of ANO1 greatly reduced ERK1/2 phosphorylation in human hepatoma cell line SMMC-7721 under standard growth conditions (Deng et al., 2016). However, some studies reported no role of ANO1 in a control of ERK1/2 activation. As such, ANO1 knockdown had no effect on EGF-induced ERK1/2 phosphorylation in human pancreatic cancer cells (Crottes et al., 2019). Silencing ANO1 in prostate cancer PC-3 cells also had no effect on ERK1/2 phosphorylation, but resulted in profound phosphorylation of other MAP kinases, p38, and Jun kinase (JNK), which was associated with increased expression of tumor necrosis factor TNFα and apoptosis (Song et al., 2018). While pharmacologic inhibition of p38 and JNK suggested their role in TNFα induction by ANO1 silencing, TNFα has been known as a powerful inducer of these MAP kinase pathways, which may also explain the effect of ANO1 on p38 and JNK phosphorylation; however, this was not investigated in that study (Song et al., 2018). In contrast, phosphorylation of p38 and apoptosis were induced by pharmacological activator of ANO1, Eact (Namkung et al., 2011b), in rat lung microvascular endothelial cells, which was inhibited by ANO1 knockdown (Allawzi et al., 2018). Similarly, silencing ANO1 in SMMC-7721 hepatoma cell line reduced phosphorylation of p38 (but not JNK) under growth conditions (Deng et al., 2016). The data on ANO1-mediated control of another protein kinase, AKT, relevant to growth and survival, is also controversial. Silencing ANO1 had no effect on AKT phosphorylation induced by EGF in human pancreatic cancer cells (Crottes et al., 2019), or in urinary bladder carcinoma T24 cells and squamous cell carcinoma SCC-1 cells (Duvvuri et al., 2012) under normal cell culture growth conditions. In contrast, ANO1 knockdown resulted in a profound inhibition of AKT phosphorylation in ovarian cancer cell lines SKOV3 and Caov-3 (Liu et al., 2019). Given that ANO1 can mediate CAMKII activation (Britschgi et al., 2013) and that CAMK pathway can activate AKT (Yano et al., 1998), authors postulate that this could be a mechanism for AKT activation by ANO1, but this was not experimentally addressed (Liu et al., 2019). Finally, the data on regulation of the relevant to cell growth and survival nuclear factor-κB (NFκB) transcription factor by ANO1 is also controversial. Overexpression of ANO1 in human astrocyte SVGp12 cells resulted in a decreased phosphorylation of NFκB inhibitor, IκB, accumulation of NFκB in the nucleus and activation of NFκB-dependent gene transcription; whereas knockdown of ANO1 in glioma U87MG cells inhibited NFκB activation (Liu et al., 2014). Similar effect of ANO1 expression on NFκB activation was observed in human bronchial epithelial HBE16 cells (Lin et al., 2015). In contrast, ANO1 overexpression in A549 cells inhibited LPS-induced NF-κB activation and cytokine release (Zhang et al., 2014).
ANO1 appears to control reactive oxygen species (ROS) signaling. Pharmacological activation of ANO1 by Eact in rat lung microvascular endothelial cells resulted in an increased mitochondrial mtROS production (Allawzi et al., 2018), whereas in HUVEC cells, angiotensin II stimulated NADPH oxidase activity and ROS generation, which were significantly reduced by silencing ANO1 (Ma et al., 2017). Mechanistically, ANO1 interacted with Nox2 protein (a key component of NADPH oxidase complex) and reduced the proteasome-dependent degradation of Nox2; however, the role of ANO-mediated chloride conduction was not investigated in this study (Ma et al., 2017). ANO1 was reported to control Rho signaling as related to smooth muscle cell contraction. In basilar artery smooth muscle cells, ANO1 knockdown abolished angiotensin II-induced RhoA activation and phosphorylation of downstream targets, myosin light chain (MLC) and MLC phosphatase, whereas overexpression of ANO1 resulted in opposite effects (Li R. S. et al., 2016). Interestingly, proteomics approach suggested that ANO1 can be in a complex with RhoA, although this was not experimentally confirmed (Perez-Cornejo et al., 2012).
Discussion
The major message of this review is that the current knowledge of the mechanisms governing the regulation of ANO1 expression and signaling remains poorly understood and is highly controversial. The following major challenges relevant to the topics of this review that could be addressed in future studies are pointed below. (i) The inconstancies between the reports on regulation of ANO1 expression and signaling should be resolved in terms of cell-specificity, laboratory-specific approaches, robustness of the data, and the rigor of the studies. (ii) The proximal consequences of ANO1 activation, such as changes in cell membrane potential and Ca2+ signaling are suggested by only a few studies, and this needs to be explored broader. (iii) Activation of ANO1 should logically lead to a change in [Cl–]i, which has not been (but should be) measured in a majority of studies on ANO1 function. Recently, With-No-Lysine kinase (WNK1) has emerged as a protein whose activity is controlled by Cl– concentration (Piala et al., 2014). WNK1 is expressed in many tissues; hence, ANO1 should control the activity of WNK1 through changes in the concentration of intracellular Cl–, which should be investigated, together with the downstream consequences. (iv) The ANO1-mediated signaling should be explored globally through such approaches as transcriptomics, proteomics, metabolomics, etc. (v) Finally, it is crucial to examine how the knowledge of signaling mechanisms obtained in cell culture models translates to pathophysiological processes related to ANO1 function in vivo.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
This study was supported by the National Institute of Health Grant R01 HL149993 (ND).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer OL declared a past co-authorship with the author ND to the handling editor.
References
Allawzi, A. M., Vang, A., Clements, R. T., Jhun, B. S., Kue, N. R., Mancini, T. J., et al. (2018). Activation of anoctamin-1 limits pulmonary endothelial cell proliferation via p38-mitogen-activated protein kinase-dependent apoptosis. Am. J. Respir. Cell Mol. Biol. 58, 658–667. doi: 10.1165/rcmb.2016-0344oc
Ayon, R. J., Hawn, M. B., Aoun, J., Wiwchar, M., Forrest, A. S., Cunningham, F., et al. (2019). Molecular mechanism of TMEM16A regulation: role of CaMKII and PP1/PP2A. Am. J. Physiol. Cell Physiol. 317, C1093–C1106.
Banga, A., Flaig, S., Lewis, S., Winfree, S., and Blazer-Yost, B. L. (2014). Epinephrine stimulation of anion secretion in the Calu-3 serous cell model. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L937–L946.
Bill, A., and Alex Gaither, L. (2017). The mechanistic role of the calcium-activated chloride channel ANO1 in tumor growth and signaling. Adv. Exp. Med. Biol. 966, 1–14. doi: 10.1007/5584_2016_201
Bill, A., Gutierrez, A., Kulkarni, S., Kemp, C., Bonenfant, D., Voshol, H., et al. (2015). ANO1/TMEM16A interacts with EGFR and correlates with sensitivity to EGFR-targeting therapy in head and neck cancer. Oncotarget 6, 9173–9188. doi: 10.18632/oncotarget.3277
Binder, D. K., and Scharfman, H. E. (2004). Brain-derived neurotrophic factor. Growth Factors 22, 123–131.
Britschgi, A., Bill, A., Brinkhaus, H., Rothwell, C., Clay, I., Duss, S., et al. (2013). Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc. Natl. Acad. Sci. U.S.A. 110, E1026–E1034.
Bulley, S., Neeb, Z. P., Burris, S. K., Bannister, J. P., Thomas-Gatewood, C. M., Jangsangthong, W., et al. (2012). TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ. Res. 111, 1027–1036. doi: 10.1161/circresaha.112.277145
Caci, E., Scudieri, P., Di Carlo, E., Morelli, P., Bruno, S., De Fino, I., et al. (2015). Upregulation of TMEM16A protein in bronchial epithelial cells by bacterial pyocyanin. PLoS One 10:e0131775. doi: 10.1371/journal.pone.0131775
Cao, Q., Liu, F., Ji, K., Liu, N., He, Y., Zhang, W., et al. (2017). MicroRNA-381 inhibits the metastasis of gastric cancer by targeting TMEM16A expression. J. Exp. Clin. Cancer Res. 36:29.
Caputo, A., Caci, E., Ferrera, L., Pedemonte, N., Barsanti, C., Sondo, E., et al. (2008). TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594. doi: 10.1126/science.1163518
Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. doi: 10.1038/39807
Chiche, J., Brahimi-Horn, M. C., and Pouyssegur, J. (2010). Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J. Cell. Mol. Med. 14, 771–794. doi: 10.1111/j.1582-4934.2009.00994.x
Cho, C. H., Lee, S., Kim, A., Yarishkin, O., Ryoo, K., Lee, Y. S., et al. (2020). TMEM16A expression in cholinergic neurons of the medial habenula mediates anxiety-related behaviors. EMBO Rep. 21:e48097.
Cho, H., Yang, Y. D., Lee, J., Lee, B., Kim, T., Jang, Y., et al. (2012). The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 15, 1015–1021. doi: 10.1038/nn.3111
Contreras-Vite, J. A., Cruz-Rangel, S., De Jesus-Perez, J. J., Figueroa, I. A. A., Rodriguez-Menchaca, A. A., Pérez-Cornejo, P., et al. (2016). Revealing the activation pathway for TMEM16A chloride channels from macroscopic currents and kinetic models. Pflugers Arch. 468, 1241–1257. doi: 10.1007/s00424-016-1830-9
Crottes, D., and Jan, L. Y. (2019). The multifaceted role of TMEM16A in cancer. Cell Calcium 82:102050. doi: 10.1016/j.ceca.2019.06.004
Crottes, D., Lin, Y. T., Peters, C. J., Gilchrist, J. M., Wiita, A. P., Jan, Y. N., et al. (2019). TMEM16A controls EGF-induced calcium signaling implicated in pancreatic cancer prognosis. Proc. Natl. Acad. Sci. U.S.A. 116, 13026–13035. doi: 10.1073/pnas.1900703116
Cruz-Rangel, S., De Jesus-Perez, J. J., Arechiga-Figueroa, I. A., Rodriguez-Menchaca, A. A., Perez-Cornejo, P., Hartzell, H. C., et al. (2017). Extracellular protons enable activation of the calcium-dependent chloride channel TMEM16A. J. Physiol. 595, 1515–1531. doi: 10.1113/jp273111
Dam, V. S., Boedtkjer, D. M., Nyvad, J., Aalkjaer, C., and Matchkov, V. (2014). TMEM16A knockdown abrogates two different Ca(2+)-activated Cl (-) currents and contractility of smooth muscle in rat mesenteric small arteries. Pflugers Arch. 466, 1391–1409. doi: 10.1007/s00424-013-1382-1
Dang, S., Feng, S., Tien, J., Peters, C. J., Bulkley, D., Lolicato, M., et al. (2017). Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552, 426–429. doi: 10.1038/nature25024
Danielsson, J., Perez-Zoghbi, J., Bernstein, K., Barajas, M. B., Zhang, Y., Kumar, S., et al. (2015). Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium. Anesthesiology 123, 569–581. doi: 10.1097/aln.0000000000000769
Davis, A. J., Shi, J., Pritchard, H. A., Chadha, P. S., Leblanc, N., Vasilikostas, G., et al. (2013). Potent vasorelaxant activity of the TMEM16A inhibitor T16A(inh) -A01. Br. J. Pharmacol. 168, 773–784. doi: 10.1111/j.1476-5381.2012.02199.x
Deba, F., and Bessac, B. F. (2015). Anoctamin-1 Cl(-) channels in nociception: activation by an N-aroylaminothiazole and capsaicin and inhibition by T16A[inh]-A01. Mol. Pain 11:55.
Deng, L., Yang, J., Chen, H., Ma, B., Pan, K., Su, C., et al. (2016). Knockdown of TMEM16A suppressed MAPK and inhibited cell proliferation and migration in hepatocellular carcinoma. Onco Targets Ther. 9, 325–333. doi: 10.2147/ott.s95985
Dutta, A. K., Woo, K., Khimji, A. K., Kresge, C., and Feranchak, A. P. (2013). Mechanosensitive Cl- secretion in biliary epithelium mediated through TMEM16A. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G87–G98.
Duvvuri, U., Shiwarski, D. J., Xiao, D., Bertrand, C., Huang, X., Edinger, R. S., et al. (2012). TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 72, 3270–3281. doi: 10.1158/0008-5472.can-12-0475-t
El Chemaly, A., Norez, C., Magaud, C., Bescond, J., Chatelier, A., Fares, N., et al. (2014). ANO1 contributes to angiotensin-II-activated Ca2+-dependent Cl- current in human atrial fibroblasts. J. Mol. Cell. Cardiol. 68, 12–19. doi: 10.1016/j.yjmcc.2013.12.027
Ertongur-Fauth, T., Hochheimer, A., Buescher, J. M., Rapprich, S., and Krohn, M. (2014). A novel TMEM16A splice variant lacking the dimerization domain contributes to calcium-activated chloride secretion in human sweat gland epithelial cells. Exp. Dermatol. 23, 825–831. doi: 10.1111/exd.12543
Ferrera, L., Caputo, A., Ubby, I., Bussani, E., Zegarra-Moran, O., Ravazzolo, R., et al. (2009). Regulation of TMEM16A chloride channel properties by alternative splicing. J. Biol. Chem. 284, 33360–33368. doi: 10.1074/jbc.m109.046607
Ferrera, L., Scudieri, P., Sondo, E., Caputo, A., Caci, E., Zegarra-Moran, O., et al. (2011). A minimal isoform of the TMEM16A protein associated with chloride channel activity. Biochim. Biophys. Acta 1808, 2214–2223. doi: 10.1016/j.bbamem.2011.05.017
Finegersh, A., Kulich, S., Guo, T., Favorov, A. V., Fertig, E. J., Danilova, L. V., et al. (2017). DNA methylation regulates TMEM16A/ANO1 expression through multiple CpG islands in head and neck squamous cell carcinoma. Sci. Rep. 7:15173.
Fujimoto, M., Inoue, T., Kito, H., Niwa, S., Suzuki, T., Muraki, K., et al. (2017). Transcriptional repression of HER2 by ANO1 Cl(-) channel inhibition in human breast cancer cells with resistance to trastuzumab. Biochem. Biophys. Res. Commun. 482, 188–194. doi: 10.1016/j.bbrc.2016.11.033
Fukuda, A. (2020). Chloride homeodynamics underlying modal shifts in cellular and network oscillations. Neurosci. Res. 156, 14–23. doi: 10.1016/j.neures.2020.02.010
Hong, G. S., Lee, S. H., Lee, B., Choi, J. H., Oh, S. J., Jang, Y., et al. (2019). ANO1/TMEM16A regulates process maturation in radial glial cells in the developing brain. Proc. Natl. Acad. Sci. U.S.A. 116, 12494–12499. doi: 10.1073/pnas.1901067116
Iosco, C., Cosentino, C., Sirna, L., Romano, R., Cursano, S., Mongia, A., et al. (2014). Anoctamin 1 is apically expressed on thyroid follicular cells and contributes to ATP- and calcium-activated iodide efflux. Cell. Physiol. Biochem. 34, 966–980. doi: 10.1159/000366313
Jang, Y., and Oh, U. (2014). Anoctamin 1 in secretory epithelia. Cell Calcium 55, 355–361. doi: 10.1016/j.ceca.2014.02.006
Ji, Q., Guo, S., Wang, X., Pang, C., Zhan, Y., Chen, Y., et al. (2019). Recent advances in TMEM16A: structure, function, and disease. J. Cell. Physiol. 234, 7856–7873. doi: 10.1002/jcp.27865
Jiang, Y., Cai, Y., Shao, W., Li, F., Guan, Z., Zhou, Y., et al. (2019). MicroRNA144 suppresses aggressive phenotypes of tumor cells by targeting ANO1 in colorectal cancer. Oncol. Rep. 41, 2361–2370.
Jin, X., Shah, S., Du, X., Zhang, H., and Gamper, N. (2016). Activation of Ca(2+) -activated Cl(-) channel ANO1 by localized Ca(2+) signals. J. Physiol. 594, 19–30. doi: 10.1113/jphysiol.2014.275107
Jones, N. G., Slater, R., Cadiou, H., McNaughton, P., and McMahon, S. B. (2004). Acid-induced pain and its modulation in humans. J. Neurosci. 24, 10974–10979. doi: 10.1523/jneurosci.2619-04.2004
Kang, J. W., Lee, Y. H., Kang, M. J., Lee, H. J., Oh, R., Min, H. J., et al. (2017). Synergistic mucus secretion by histamine and IL-4 through TMEM16A in airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L466–L476.
Kim, U., and Chung, L. Y. (2007). Dual GABAergic synaptic response of fast excitation and slow inhibition in the medial habenula of rat epithalamus. J. Neurophysiol. 98, 1323–1332. doi: 10.1152/jn.00575.2007
Lee, B., Hong, G. S., Lee, S. H., Kim, H., Kim, A., Hwang, E. M., et al. (2019). Anoctamin 1/TMEM16A controls intestinal Cl(-) secretion induced by carbachol and cholera toxin. Exp. Mol. Med. 51:91.
Lee, Y. S., Bae, Y., Park, N., Yoo, J. C., Cho, C. H., Ryoo, K., et al. (2016a). Surface expression of the Anoctamin-1 (ANO1) channel is suppressed by protein-protein interactions with beta-COP. Biochem. Biophys. Res. Commun. 475, 216–222. doi: 10.1016/j.bbrc.2016.05.077
Lee, Y. S., Lee, J. K., Bae, Y., Lee, B. S., Kim, E., Cho, C. H., et al. (2016b). Suppression of 14-3-3gamma-mediated surface expression of ANO1 inhibits cancer progression of glioblastoma cells. Sci. Rep. 6:26413.
Li, H., Yan, X., Li, R., Zhang, A., Niu, Z., Cai, Z., et al. (2016). Increased TMEM16A involved in alveolar fluid clearance after lipopolysaccharide stimulation. Inflammation 39, 881–890. doi: 10.1007/s10753-016-0320-8
Li, R. S., Wang, Y., Chen, H. S., Jiang, F. Y., Tu, Q., Li, W. J., et al. (2016). TMEM16A contributes to angiotensin II-induced cerebral vasoconstriction via the RhoA/ROCK signaling pathway. Mol. Med. Rep. 13, 3691–3699. doi: 10.3892/mmr.2016.4979
Lin, J., Jiang, Y., Li, L., Liu, Y., Tang, H., and Jiang, D. (2015). TMEM16A mediates the hypersecretion of mucus induced by Interleukin-13. Exp. Cell Res. 334, 260–269. doi: 10.1016/j.yexcr.2015.02.026
Liu, B., Linley, J. E., Du, X., Zhang, X., Ooi, L., Zhang, H., et al. (2010). The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J. Clin. Invest. 120, 1240–1252. doi: 10.1172/jci41084
Liu, J., Liu, Y., Ren, Y., Kang, L., and Zhang, L. (2014). Transmembrane protein with unknown function 16A overexpression promotes glioma formation through the nuclear factor-kappaB signaling pathway. Mol. Med. Rep. 9, 1068–1074. doi: 10.3892/mmr.2014.1888
Liu, Z., Zhang, S., Hou, F., Zhang, C., Gao, J., and Wang, K. (2019). Inhibition of Ca(2+) -activated chloride channel ANO1 suppresses ovarian cancer through inactivating PI3K/Akt signaling. Int. J. Cancer 144, 2215–2226. doi: 10.1002/ijc.31887
Lu, G., Shi, W., and Zheng, H. (2018). Inhibition of STAT6/Anoctamin-1 activation suppresses proliferation and invasion of gastric cancer cells. Cancer Biother. Radiopharm. 33, 3–7. doi: 10.1089/cbr.2017.2287
Ma, M. M., Gao, M., Guo, K. M., Wang, M., Li, X. Y., Zeng, X. L., et al. (2017). TMEM16A contributes to endothelial dysfunction by facilitating Nox2 NADPH oxidase-derived reactive oxygen species generation in hypertension. Hypertension 69, 892–901. doi: 10.1161/hypertensionaha.116.08874
Mazzone, A., Bernard, C. E., Strege, P. R., Beyder, A., Galietta, L. J., Pasricha, P. J., et al. (2011). Altered expression of Ano1 variants in human diabetic gastroparesis. J. Biol. Chem. 286, 13393–13403. doi: 10.1074/jbc.m110.196089
Mazzone, A., Gibbons, S. J., Bernard, C. E., Nowsheen, S., Middha, S., Almada, L. L., et al. (2015). Identification and characterization of a novel promoter for the human ANO1 gene regulated by the transcription factor signal transducer and activator of transcription 6 (STAT6). FASEB J. 29, 152–163. doi: 10.1096/fj.14-258541
Mazzone, A., Gibbons, S. J., Eisenman, S. T., Strege, P. R., Zheng, T., D’Amato, M., et al. (2019). Direct repression of anoctamin 1 (ANO1) gene transcription by Gli proteins. FASEB J. 33, 6632–6642. doi: 10.1096/fj.201802373r
Mokutani, Y., Uemura, M., Munakata, K., Okuzaki, D., Haraguchi, N., Takahashi, H., et al. (2016). Down-regulation of microRNA-132 is associated with poor prognosis of colorectal cancer. Ann. Surg. Oncol. 23, 599–608. doi: 10.1245/s10434-016-5133-3
Mroz, M. S., and Keely, S. J. (2012). Epidermal growth factor chronically upregulates Ca(2+)-dependent Cl(-) conductance and TMEM16A expression in intestinal epithelial cells. J. Physiol. 590, 1907–1920. doi: 10.1113/jphysiol.2011.226126
Namkung, W., Phuan, P. W., and Verkman, A. S. (2011a). TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J. Biol. Chem. 286, 2365–2374. doi: 10.1074/jbc.m110.175109
Namkung, W., Yao, Z., Finkbeiner, W. E., and Verkman, A. S. (2011b). Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J. 25, 4048–4062. doi: 10.1096/fj.11-191627
O’Driscoll, K. E., Pipe, R. A., and Britton, F. C. (2011). Increased complexity of Tmem16a/Anoctamin 1 transcript alternative splicing. BMC Mol. Biol. 12:35. doi: 10.1186/1471-2199-12-35
Oh, U., and Jung, J. (2016). Cellular functions of TMEM16/anoctamin. Pflugers Arch. 468, 443–453. doi: 10.1007/s00424-016-1790-0
Ohshiro, J., Yamamura, H., Saeki, T., Suzuki, Y., and Imaizumi, Y. (2014). The multiple expression of Ca(2)(+)-activated Cl(-) channels via homo- and hetero-dimer formation of TMEM16A splicing variants in murine portal vein. Biochem. Biophys. Res. Commun. 443, 518–523. doi: 10.1016/j.bbrc.2013.11.117
Park, Y. R., Lee, S. T., Kim, S. L., Zhu, S. M., Lee, M. R., Kim, S. H., et al. (2019). Down-regulation of miR-9 promotes epithelial mesenchymal transition via regulating anoctamin-1 (ANO1) in CRC cells. Cancer Genet. 231–232, 22–31. doi: 10.1016/j.cancergen.2018.12.004
Paulino, C., Neldner, Y., Lam, A. K., Kalienkova, V., Brunner, J. D., Schenck, S., et al. (2017). Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. eLife 6:e26232.
Pedemonte, N., and Galietta, L. J. (2014). Structure and function of TMEM16 proteins (anoctamins). Physiol. Rev. 94, 419–459. doi: 10.1152/physrev.00039.2011
Perez-Cornejo, P., Gokhale, A., Duran, C., Cui, Y., Xiao, Q., Hartzell, H. C., et al. (2012). Anoctamin 1 (Tmem16A) Ca2+-activated chloride channel stoichiometrically interacts with an ezrin-radixin-moesin network. Proc. Natl. Acad. Sci. U.S.A. 109, 10376–10381. doi: 10.1073/pnas.1200174109
Peters, C. J., Gilchrist, J. M., Tien, J., Bethel, N. P., Qi, L., Chen, T., et al. (2018). The sixth transmembrane segment is a major gating component of the TMEM16A calcium-activated chloride channel. Neuron 97, 1063–1077.e4.
Peters, C. J., Yu, H., Tien, J., Jan, Y. N., Li, M., and Jan, L. Y. (2015). Four basic residues critical for the ion selectivity and pore blocker sensitivity of TMEM16A calcium-activated chloride channels. Proc. Natl. Acad. Sci. U.S.A. 112, 3547–3552. doi: 10.1073/pnas.1502291112
Piala, A. T., Moon, T. M., Akella, R., He, H., Cobb, M. H., and Goldsmith, E. J. (2014). Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci. Signal. 7:ra41. doi: 10.1126/scisignal.2005050
Qin, Y., Jiang, Y., Sheikh, A. S., Shen, S., Liu, J., and Jiang, D. (2016). Interleukin-13 stimulates MUC5AC expression via a STAT6-TMEM16A-ERK1/2 pathway in human airway epithelial cells. Int. Immunopharmacol. 40, 106–114. doi: 10.1016/j.intimp.2016.08.033
Sala-Rabanal, M., Yurtsever, Z., Berry, K. N., Nichols, C. G., and Brett, T. J. (2017). Modulation of TMEM16A channel activity by the von Willebrand factor type A (VWA) domain of the calcium-activated chloride channel regulator 1 (CLCA1). J. Biol. Chem. 292, 9164–9174. doi: 10.1074/jbc.m117.788232
Sala-Rabanal, M., Yurtsever, Z., Nichols, C. G., and Brett, T. J. (2015). Secreted CLCA1 modulates TMEM16A to activate Ca(2+)-dependent chloride currents in human cells. eLife 4:e05875.
Sanders, K. M., Zhu, M. H., Britton, F., Koh, S. D., and Ward, S. M. (2012). Anoctamins and gastrointestinal smooth muscle excitability. Exp. Physiol. 97, 200–206. doi: 10.1113/expphysiol.2011.058248
Schroeder, B. C., Cheng, T., Jan, Y. N., and Jan, L. Y. (2008). Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029. doi: 10.1016/j.cell.2008.09.003
Sen, P., Ganguly, P., and Ganguly, N. (2018). Modulation of DNA methylation by human papillomavirus E6 and E7 oncoproteins in cervical cancer. Oncol. Lett. 15, 11–22.
Shin, Y., Lee, S. W., Namkoong, E., An, W., Lee, J. H., Brown, P. D., et al. (2019). Epigenetic modification as a regulatory mechanism for spatiotemporal dynamics of ANO1 expression in salivary glands. Int. J. Mol. Sci. 20:6298. doi: 10.3390/ijms20246298
Sim, K. M., Lee, Y. S., Kim, H. J., Cho, C. H., Yi, G. S., Park, M. J., et al. (2020). Suppression of CaMKIIbeta inhibits ANO1-mediated glioblastoma progression. Cells 9:1079. doi: 10.3390/cells9051079
Song, Y., Gao, J., Guan, L., Chen, X., Gao, J., and Wang, K. (2018). Inhibition of ANO1/TMEM16A induces apoptosis in human prostate carcinoma cells by activating TNF-alpha signaling. Cell Death Dis. 9:703.
Sonneville, F., Ruffin, M., Coraux, C., Rousselet, N., Le Rouzic, P., Blouquit-Laye, S., et al. (2017). MicroRNA-9 downregulates the ANO1 chloride channel and contributes to cystic fibrosis lung pathology. Nat. Commun. 8:710.
Strege, P. R., Bernard, C. E., Mazzone, A., Linden, D. R., Beyder, A., Gibbons, S. J., et al. (2015). A novel exon in the human Ca2+-activated Cl- channel Ano1 imparts greater sensitivity to intracellular Ca2. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G743–G749.
Sun, H., Xia, Y., Paudel, O., Yang, X. R., and Sham, J. S. (2012). Chronic hypoxia-induced upregulation of Ca2+-activated Cl- channel in pulmonary arterial myocytes: a mechanism contributing to enhanced vasoreactivity. J. Physiol. 590, 3507–3521. doi: 10.1113/jphysiol.2012.232520
Sung, T. S., O’Driscoll, K., Zheng, H., Yapp, N. J., Leblanc, N., Koh, S. D., et al. (2016). Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. Am. J. Physiol. Cell Physiol. 311, C437–C451.
Takayama, Y., Uta, D., Furue, H., and Tominaga, M. (2015). Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 112, 5213–5218. doi: 10.1073/pnas.1421507112
Tien, J., Peters, C. J., Wong, X. M., Cheng, T., Jan, Y. N., Jan, L. Y., et al. (2014). A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. eLife 3:e02772.
Vanoni, S., Zeng, C., Marella, S., Uddin, J., Wu, D., Arora, K., et al. (2020). Identification of anoctamin 1 (ANO1) as a key driver of esophageal epithelial proliferation in eosinophilic esophagitis. J. Allergy Clin. Immunol. 145, 239–254.e2.
Wang, H., Yao, F., Luo, S., Ma, K., Liu, M., Bai, L., et al. (2019). A mutual activation loop between the Ca(2+)-activated chloride channel TMEM16A and EGFR/STAT3 signaling promotes breast cancer tumorigenesis. Cancer Lett. 455, 48–59. doi: 10.1016/j.canlet.2019.04.027
Wang, Q., Bai, L., Luo, S., Wang, T., Yang, F., Xia, J., et al. (2020). TMEM16A Ca(2+)-activated Cl(-) channel inhibition ameliorates acute pancreatitis via the IP3R/Ca(2+)/NFkappaB/IL-6 signaling pathway. J. Adv. Res. 23, 25–35. doi: 10.1016/j.jare.2020.01.006
Yang, Y. D., Cho, H., Koo, J. Y., Tak, M. H., Cho, Y., Shim, W. S., et al. (2008). TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215. doi: 10.1038/nature07313
Yano, S., Tokumitsu, H., and Soderling, T. R. (1998). Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396, 584–587. doi: 10.1038/25147
Zeng, X., Pan, D., Wu, H., Chen, H., Yuan, W., Zhou, J., et al. (2019). Transcriptional activation of ANO1 promotes gastric cancer progression. Biochem. Biophys. Res. Commun. 512, 131–136. doi: 10.1016/j.bbrc.2019.03.001
Zhang, A., Yan, X., Li, H., Gu, Z., Zhang, P., Zhang, H., et al. (2014). TMEM16A protein attenuates lipopolysaccharide-mediated inflammatory response of human lung epithelial cell line A549. Exp. Lung Res. 40, 237–250. doi: 10.3109/01902148.2014.905655
Zhang, X. D., Lee, J. H., Lv, P., Chen, W. C., Kim, H. J., Wei, D., et al. (2015). Etiology of distinct membrane excitability in pre-, and posthearing auditory neurons relies on activity of Cl- channel TMEM16A. Proc. Natl. Acad. Sci. U.S.A. 112, 2575–2580. doi: 10.1073/pnas.1414741112
Zhang, X. H., Zheng, B., Yang, Z., He, M., Yue, L. Y., Zhang, R. N., et al. (2015). TMEM16A and myocardin form a positive feedback loop that is disrupted by KLF5 during Ang II-induced vascular remodeling. Hypertension 66, 412–421. doi: 10.1161/hypertensionaha.115.05280
Keywords: anoctamin-1, TMEM16A, chloride, calcium, signaling
Citation: Dulin NO (2020) Calcium-Activated Chloride Channel ANO1/TMEM16A: Regulation of Expression and Signaling. Front. Physiol. 11:590262. doi: 10.3389/fphys.2020.590262
Received: 31 July 2020; Accepted: 13 October 2020;
Published: 05 November 2020.
Edited by:
Alexander A. Mongin, Albany Medical College, United StatesReviewed by:
Olga Lopina, Lomonosov Moscow State University, RussiaJorge Arreola, Autonomous University of San Luis Potosí, Mexico
Jae-Yong Park, Korea University, South Korea
Copyright © 2020 Dulin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nickolai O. Dulin, bmR1bGluQG1lZGljaW5lLmJzZC51Y2hpY2Fnby5lZHU=
 Nickolai O. Dulin
Nickolai O. Dulin