
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 25 February 2021
Sec. Membrane Physiology and Membrane Biophysics
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.588664
This article is part of the Research TopicEmerging Roles of Monovalent Ion Transporters in Intracellular SignalingView all 7 articles
Sodium (Na+) electrochemical gradients established by Na+/K+ ATPase activity drives the transport of ions, minerals, and sugars in both excitable and non-excitable cells. Na+-dependent transporters can move these solutes in the same direction (cotransport) or in opposite directions (exchanger) across both the apical and basolateral plasma membranes of polarized epithelia. In addition to maintaining physiological homeostasis of these solutes, increases and decreases in sodium may also initiate, directly or indirectly, signaling cascades that regulate a variety of intracellular post-translational events. In this review, we will describe how the Na+/K+ ATPase maintains a Na+ gradient utilized by multiple sodium-dependent transport mechanisms to regulate glucose uptake, excitatory neurotransmitters, calcium signaling, acid-base balance, salt-wasting disorders, fluid volume, and magnesium transport. We will discuss how several Na+-dependent cotransporters and Na+-dependent exchangers have significant roles in human health and disease. Finally, we will discuss how each of these Na+-dependent transport mechanisms have either been shown or have the potential to use Na+ in a secondary role as a signaling molecule.
During the 1962 America’s cup, President John F. Kennedy stated “It is an interesting biological fact that all of us have, in our veins, the exact same percentage of salt in our blood that exists in the ocean, and, therefore, we have salt in our blood, in our sweat, in our tears. We are tied to the ocean. And when we go back to the sea, whether it is to sail or to watch it, we are going back from whence we came” (from the John F. Kennedy Presidential Library and Museum). While the salt (sodium chloride or NaCl) concentration in our blood is four times lower than in the sea (140 mM vs 599 mM), the 35th President of the United States was right to contemplate the fact that we all carry a bit of the ocean with us (Delpire and Gagnon, 2018b). The concentration of sodium (Na+) in our blood, interstitial fluids, and extracellular spaces, if not as high as sea water, is however elevated (140 mM) compared to the concentration of Na+ inside our cells (10–30 mM), thereby establishing an inward concentration gradient across the membrane of the cell. This gradient is opposite to the outward potassium (K+) gradient created by low extracellular K+ (3.5–5 mM) and high intracellular K+ concentrations (130–140 mM). These opposing Na+ and K+ gradients, maintained by the energy-(ATP)-consuming Na+/K+ ATPase, generate a membrane potential across the plasma membrane, and create electrical signals in the form of action potentials that sustain cardiac muscle contraction and promote long distance neuronal communication. John F. Kennedy was also correct regarding salt being in our sweat and tears, as the production and secretion of these fluids involve the transport of Na+ and Cl–, and obligatory water. For more than 30 years, the study of monovalent Na+ ions have been focused on epithelial transcellular transport and propagation of action potentials in central and peripheral neurons of the nervous system. However, intracellular Na+ in epithelial cells may also initiate a cascade of signaling events that elicit a “mitogen-like” response (Stanton and Kaissling, 1989). Indeed, Na+ may regulate intracellular post-translational events such as glycosylation, phosphorylation, and even exocytosis (Stanton and Kaissling, 1989).
In this review, we will discuss the importance of Na+ transport, including how the Na+/K+ ATPase maintains a Na+ gradient utilized by multiple Na+-dependent transport mechanisms (see Figure 1) to regulate glucose uptake, excitatory neurotransmitters, calcium signaling, acid-base balance, renal salt reabsorption, and fluid and volume homeostasis, and discuss the role of each transporter/exchanger in human health and disease. Importantly, we will also cover evidence that the cation, in some well-establish cases, serves as a “signal” to affect metabolism, and promote cell division and cell growth. The Merriam-Webster dictionary defines a signal as “a detectable physical quantity or impulse by which messages or information can be transmitted” [“signal.” Merriam-Webster.com. 2020. https://www.merriam-webster.com (12 Sept, 2020)]. In the human body, signaling molecules, ranging from single ions to small proteins, are transmitted between different organ systems, within individual organs, amongst distinct cell populations, and within single cells. This intracellular and intercellular communication governs and coordinates basic cellular activity. In the spirit of this special series on the emerging role of monovalent ion transporters in intracellular signaling, we will address the limited but growing evidence that Na+ participates in signaling.
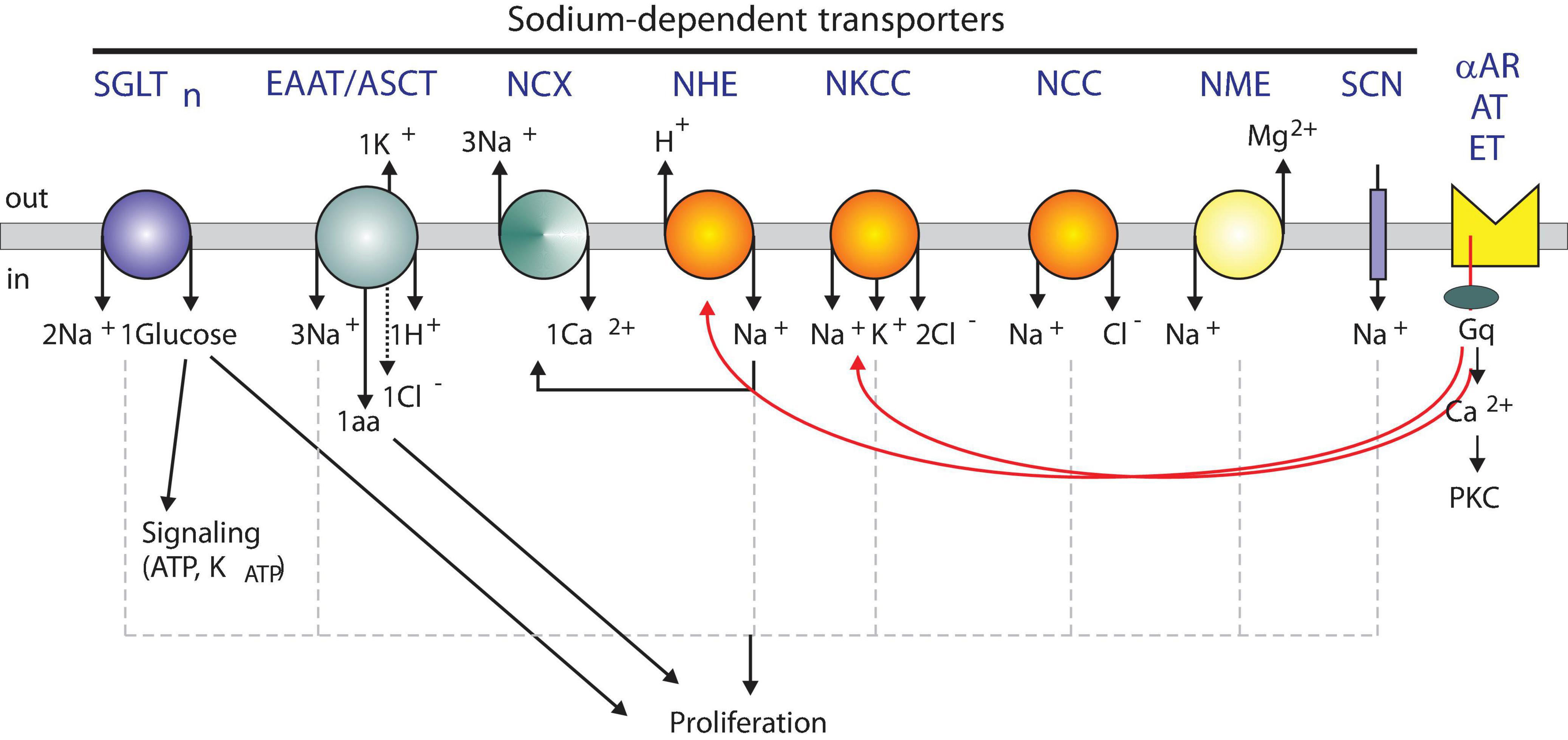
Figure 1. Model showing sodium-dependent transporters and possible signaling roles. Several Na+-dependent solute carriers with their individual stoichiometry of ions, sugars, and amino acids are depicted. SGLT, Na+-dependent glucose cotransporter; EAAT, Na+-dependent excitatory amino acid transporter; NCX, Na+-dependent Ca2+ exchanger; NHE, Na+-dependent hydrogen exchanger; NKCC, Na+-dependent potassium chloride cotransporter; NCC, Na+-dependent chloride cotransporter; NME, Na+-dependent magnesium exchanger; SCN, Sodium Channel. Transporters are regulated by alpha adrenergic (αAR), angiotensin II (AT), and endothelin (ET) receptors.
The Na+/K+-ATPase is a plasma membrane “ion pump” that utilizes the energy produced from hydrolyzing the terminal phosphate bond of 1 ATP molecule to transport 3 Na+ out of the cell and 2 K+ into the cell. Cation transport against their concentration gradients requires cation binding sites with “locking gates” to occlude ion movement in the opposite, energetically ideal direction following conformational changes in the enzyme. Jens Christian Skou, awarded the Nobel Prize in Chemistry (1997) for the discovery of the Na+/K+-ATPase, outlined six requirements for this ‘ion pump’. It needed to: (i) be located in the cell membrane, (ii) have a higher intracellular affinity for Na+ versus K+, (iii) have a higher extracellular affinity for K+ versus Na+, (iv) have an enzyme capacity to hydrolyze ATP, (v) be able to hydrolyze ATP at a rate dependent on intracellular [Na+] and extracellular [K+], and (vi) be found in cells with active, linked transport of Na+ and K+ (Skou, 1957, Skou, 1965).
The Na+/K+-ATPase (see Figure 2) consists of an alpha (α) subunit (∼100 kDa) containing the catalytic site for ATP hydrolysis, a beta (β) subunit (∼45 kDa) critical for stability and trafficking to the membrane, and sometime, in specific tissues, a gamma (γ) subunit (∼10 kDa) that regulates Na+ and K+ affinity and cation uptake (Shinoda et al., 2009). There are four catalytic alpha subunits encoded on chromosomes 1p13.1 (ATPA1), 1q23.2 (ATPA2), 19q13.2 (ATPA3), and 1q23.2 (ATP1A4). There are three chaperone beta subunits encoded on chromosomes 1q24.2 (ATPB1), 17p13.1 (ATPB2), and 3q23 (ATPB3). Heterodimerization of one alpha subunit and one beta subunit allows for the possibility of 12 different Na+/K+-ATPase isozymes with tissue-specific distinct functional activities (Blanco and Mercer, 1998). There are also seven ‘FXYD’ gamma subunits that influence tissue-specific and isozyme-specific Na+/K+-ATPase activity and cellular function (Crambert and Geering, 2003). For an exceptional review on the physiological roles and tissue expression patterns of each of these Na+/K+-ATPase subunit isoforms, see (Clausen et al., 2017).
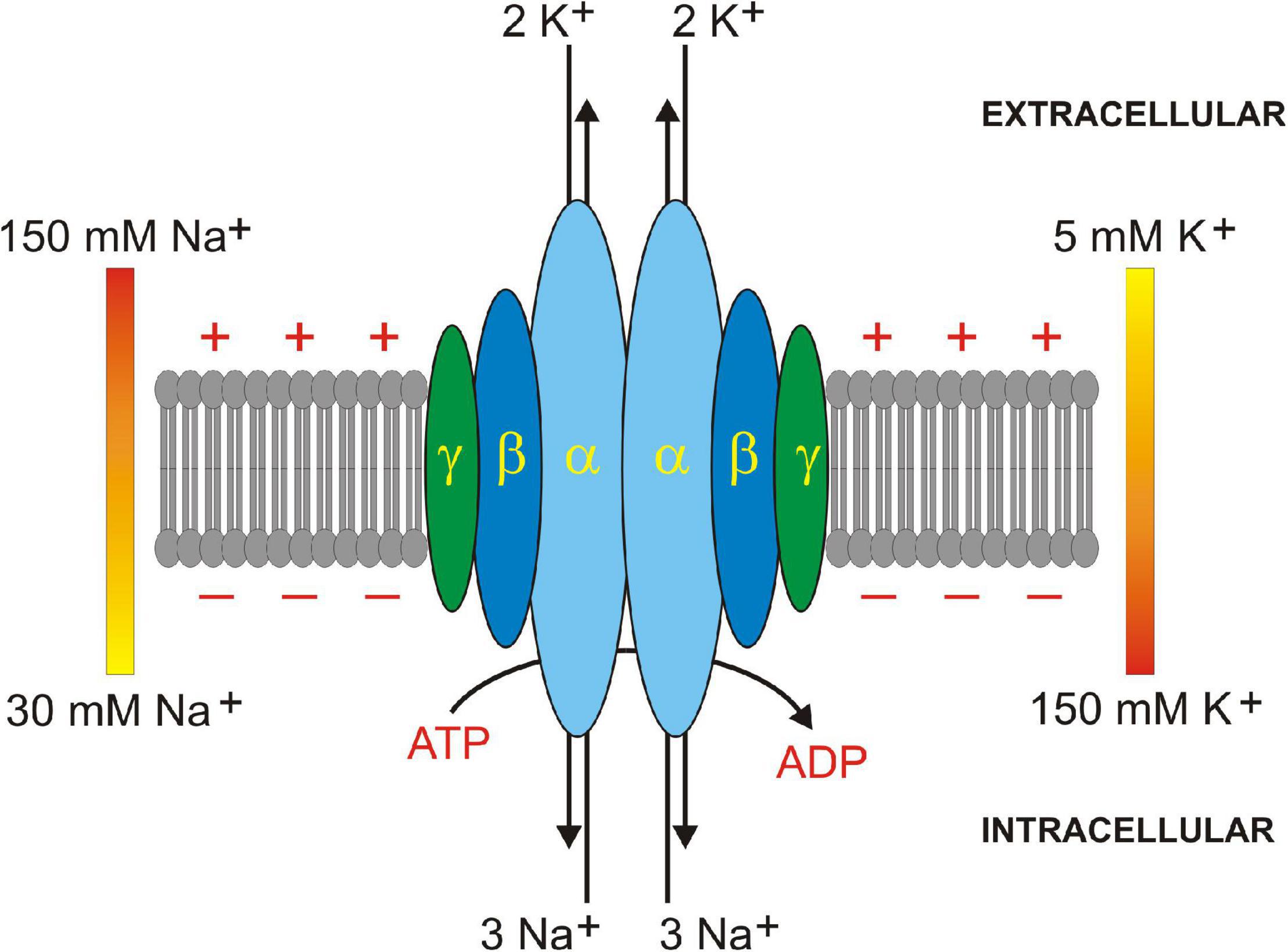
Figure 2. Na+/K+ ATPase subunit assembly in plasma membrane. Na+/K+ ATPase is a heterodimer of 1 alpha (α, light blue) and 1 beta (β, dark blue) subunit. A regulatory gamma (γ, dark green) subunit sometimes oligomerizes in some tissues. 3 Na+ and 2 K+ ions are translocated across the plasma membrane by hydrolysis of 1 ATP to 1 ADP molecule. Extracellular to intracellular ionic gradients for Na+ (150–30 mM) and K+ (5–150 mM) are shown. Positive and negative signs depict membrane potential created by Na+ and K+ ion translocation.
Alpha and beta subunits exhibit differential cation affinities and sensitivity to ouabain inhibition. Nearly every tissue expresses the α1 subunit, whereas, the α2 subunit is predominantly expressed in muscle, heart, brain, and adipocyte tissue. The α3 and α4 subunits are specifically expressed in nervous tissues and testes, respectively. Similarly, the β1 subunit is found in nearly every tissue, and accordingly, the α1β1 heterodimer is the most commonly expressed isozyme. The β2 subunit is found in muscle, pineal gland, and nervous tissue, whereas, the β3 subunit is expressed in the testis, retina, liver, and lung (Blanco and Mercer, 1998). Two recently published reviews from the Nissen group in Denmark characterize the structure and dynamics of these P-type ATPase ion pump isozymes (Dyla et al., 2019, 2020).
The ubiquitous expression of the various Na+/K+-ATPase isoforms in mammalian cells underlies its importance in maintaining Na+ and K+ electrochemical gradients across the plasma membrane. Multiple ion transporters and channels utilize these chemical gradients to drive ion, mineral, and sugar transport, and potentially downstream signaling pathways. As such, enzymatic dysfunction of the Na+/K+-ATPase, either through mutation, abnormal subunit dimerization, or abnormal membrane trafficking, has been linked to several human disorders. Multiple studies have associated Na+/K+-ATPase dysfunction with cancer initiation, growth, development, and metastasis (Babula et al., 2013). Cardiac glycosides have exhibited some anticancer effects via induction of apoptosis and autophagy, ROS production, and cell cycle arrest (Prassas and Diamandis, 2008). For more than 45 years, investigators have demonstrated a diabetic-induced decrease in Na+/K+-ATPase activity in the brain, heart, intestine, kidney, liver, and skeletal muscle (Vague et al., 2004).
Cardiac glycosides (e.g., digitalis) are routinely used to effectively treat patients experiencing heart failure. Studies have shown enzymatic inhibition leads to increased intracellular [Na+] (Erdmann et al., 1980; Grupp et al., 1985), resulting in lower driving forces for the Na+/Ca2+ exchanger, less Ca2+ extrusion from the cell, and ultimately more Ca2+ available to increase contractile force (Lee et al., 1985).
In addition to being an “ion pump,” several studies over the past 20 years have shown the Na+/K+-ATPase to function as a receptor, signal transducer, and multi-protein scaffold (Liu et al., 2018). Sub-inhibitory concentrations of cardiotonic steroid binding has been shown to activate mitogen-activated protein kinase signal cascades, mitochondrial reactive oxygen species production, and the phospholipase C signaling pathway (see Figure 3) (Yuan et al., 2005). Binding of sub-inhibitory concentrations of ouabain have also been shown to induce activation of a signaling cascade leading to VSMC proliferation (Aydemir-Koksoy et al., 2001). As a protein scaffold, the Na+/K+-ATPase directly interacts and inhibits the non-receptor tyrosine kinase, Src. Following ouabain binding, the Src kinase domain releases from Na+/K+-ATPase and activates the EGFR forming a cardiotonic steroid receptor intracellular signaling pathway (Tian et al., 2006; Li et al., 2009). Other intracellular signaling proteins that have been shown to scaffold to Na+/K+-ATPase are ankyrin, cofilin, phospholipase Cγ, inositol triphosphate receptor, and phosphoinositide 3-kinase (Lee et al., 2001).
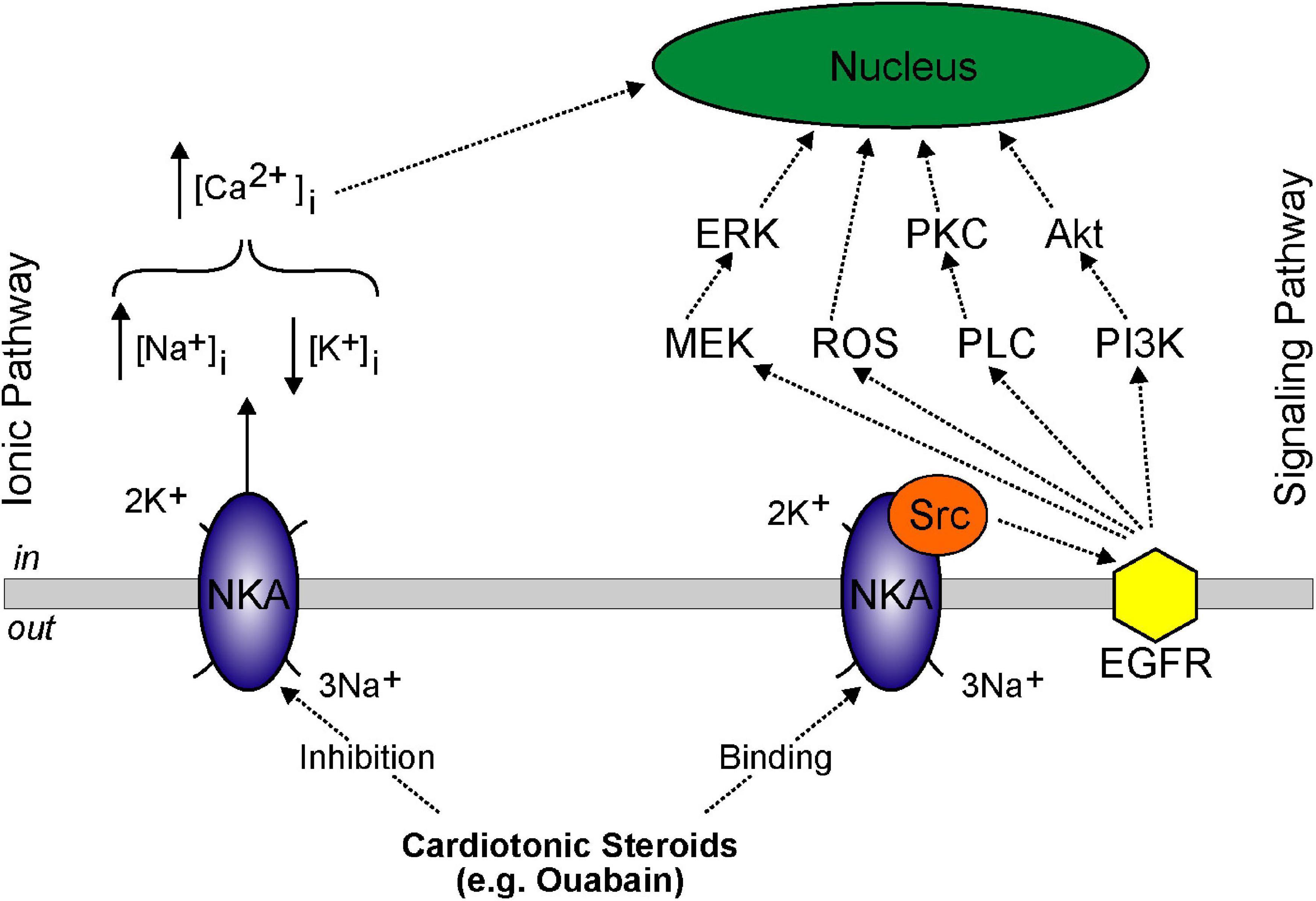
Figure 3. Model showing basolateral NKA ionic and signaling pathways. Ionic pathway involves: (i) high dose cardiotonic steroids inhibiting NKA activity, (ii) increased intracellular Na+ concentration, (iii) decreased intracellular K+ concentration, and (iv) increased intracellular Ca2+ concentration inducing genomic effects in the nucleus. Signaling pathway involves: (i) low dose cardiotonic steroid binding, (ii) release of Src, (iii) activation of EGFR, and (iv) signaling cascade activation of mitogen-activated protein kinase kinase (MEK/ERK), ROS generation, PLC/PKC activation, and PI3K/Akt activation.
The first mammalian Na+/glucose cotransporter (SGLT) was cloned from rabbit intestine in 1987 (Hediger et al., 1987). Subsequent studies identified and cloned human SGLT1 and SGLT2 from kidney, thyroid gland, spinal cord, heart, muscle, and lung (Wright et al., 2011). Four isoforms (SGLT1, SGLT2, SGLT4, and SGLT5) of the SLC5 gene family transport glucose and/or fructose in a Na+-dependent fashion. Human SGLT1 is encoded on chromosome 22q13.1 and expressed in multiple tissues (small intestine, trachea, BBM late PT of kidney, heart, brain, testis, and prostate). Human SGLT2 is encoded on chromosome 16p12-p11 and initially was found only in the brush-border membrane of the early proximal tubule of the kidney. Interestingly, recent studies have identified expression of SGLT2 in brain, pancreatic, and prostrate tumors (Scafoglio et al., 2015). Human SGLT4 is encoded on chromosome 1p32 and expressed in the small intestine, kidney, liver, brain, and lung. Human SGLT5 is encoded on chromosome 17p11.2 and, to date, only found expressed in the kidney (Wright and Turk, 2004).
Functional studies have determined that SGLT2 is a low-affinity, high-capacity cotransporter with a one-to-one stoichiometry for Na+ and glucose. SGLT1 is a high-affinity, low-capacity cotransporter with a two-to-one stoichiometry for Na+ and glucose (Wright, 2001). The combination of internal and external Na+ and glucose concentration gradients and the cell membrane potential determine the direction and rate of Na+-dependent transport. As such, the transport cycle of SGLTs is completely reversible (Wright et al., 2017).
SGLT2, expressed in the S1 and S2 segments of the proximal tubule, reabsorbs 90% of the glomerular filtered glucose. SGLT1, expressed in the S3 segment of the proximal tubule, reabsorbs the remainder of glomerular filtered glucose resulting in negligible levels of glucose in the urine. Basolateral GLUT2 expression in all three segments completes transcellular transport of glucose across the tubule (Ghezzi et al., 2018). Several FDA-approved SGLT2 inhibitors (Farxiga, Invokana, and Jardiance) reduce blood glucose levels in patients with Type II diabetes (Gallo et al., 2015; Koepsell, 2017). However, patients with pharmacological inhibition or non-functional genetic mutations of SGLT2 have been found with only 50% glucosuria indicating that SGLT1 has a reserve capacity for glucose reabsorption in the proximal tubule S3 segment (Santer et al., 2003). Along with promoting glycosuria and improving glycemic control in Type II diabetics, pre-clinical and clinical studies have demonstrated multiple beneficial effects of SGLT2 inhibitors on cardiovascular and renal health, as well as treating certain cancers. Unfortunately, SGLT2 inhibitors can also lead to diabetic ketoacidosis, a metabolic condition where the body breaks down fat too fast and produces excess ketones causing the blood to become acidic (Perry and Shulman, 2020).
Autosomal recessive mutations in SGLT1 cause glucose-galactose malabsorption due to abnormal trafficking of the cotransporter in the intestine. Familial renal glucosuria is a result of autosomal recessive mutations that result in a premature stop mutation in SGLT2. Lastly, autosomal recessive mutations in GLUT2 result in Fanconi-Bickel syndrome (Santer et al., 2002; Santer and Calado, 2010; Wright et al., 2011). Although Na+ gradients established by Na+/K+ ATPase activity is essential to transcellular glucose transport in the kidney and intestine, SGLTs and GLUT2 cotransport of Na+ doesn’t meet the definition of a signaling molecule.
Na+-dependent transport of glucose is a key component of glucose blood homeostasis in all tissues (Berger and Zdzieblo, 2020). In pancreatic β-cells, transport of glucose through SGLT2 is an essential first step in a glucose-mediated signaling cascade that affects production of ATP and setting of an ATP/ADP ratio that ultimately controls the ATP-sensitive K+ channel (KATP channels), membrane depolarization, and release of insulin (Demirbilek et al., 2019). The SGLT2-mediated signaling uses glucose instead of Na+ as a mediator, but again – this is only possible because of the primary activity of the Na+/K+ ATPase that sets a highly favorable gradient for Na+-dependent glucose uptake.
There are seven members of the mammalian solute carrier family 1 (SLC1) consisting of five high-affinity excitatory amino acid (glutamate) transporters (EAATs) and two neutral amino acid (alanine, serine, and cysteine) transporters (ASCTs). Along with L-glutamate, the five EAATs also transport L-aspartic acid and are expressed in neurons, astrocytes, intestine, kidney, liver, heart, retina, and retinal cells (Kanai et al., 2013). As the primary excitatory neurotransmitter in the mammalian central nervous system, glutamate is involved in cellular migration, nervous system development, cognition, learning, memory, cellular differentiation, and neuronal death (Magi et al., 2019). Glutamate release into the synaptic cleft triggers AMPA glutamate receptors that are permeable to Na+, leading to membrane depolarization and action potential propagation. Na+-dependent glutamate transporters expressed in glia and neuronal postsynaptic boutons are essential to removing excitatory neurotransmitter from the synaptic cleft and preventing neurotoxicity. The two ASCTs mediate Na+-dependent exchange of the neutral amino acids L-alanine, L-serine, L-cysteine, L-threonine, L-glutamine, and L-asparagine. Both ASC transporters have widespread non-neuronal tissue expression including lung, skeletal muscle, intestine, kidney, testes, and adipose tissue (Kanai et al., 2013). As a result, dysfunction of these transporters are linked to metabolic reprogramming issues, renal pathologies, autophagy, tumor cell proliferation, and diabetes (Kandasamy et al., 2018).
Calcium (Ca2+) is an essential mineral necessary for life. Total human body Ca2+ ranges from 1,000 to 1,200 g with only 1% present in extracellular and intracellular spaces (i.e., 99% sequestered in the human skeleton) (Blaine et al., 2015). Despite the enormous disparity between free and sequestered Ca2+, free Ca2+ serves a vital role, including, but not limited to, hormone secretion, muscular contraction, blood coagulation, nerve impulse transmission, and intracellular adhesion (Hebert and Brown, 1996). Cytoplasmic concentrations of Ca2+ (∼100 nM) are maintained 20,000-fold lower than extracellular concentrations through plasma membrane Ca2+ ATPase-dependent (PMCA) and sarco(endo)plasmic reticulum Ca2+ ATPase-dependent (SERCA) mechanisms (Brini and Carafoli, 2009). Sudden and deliberate increases in cytoplasmic Ca2+ levels, either through internal release from ER/SR stores or external Ca2+ entry through plasma membrane ion channels, drives intracellular processes by exerting direct and indirect regulatory effects on a multitude of enzymes and proteins (Clapham, 2007). As a ubiquitous second messenger, Ca2+ mediates a wide-range of physiological events in nearly every cell of the human body. The speed, amplitude, spatial, and temporal patterns of intracellular [Ca2+] changes permits extensive versatility to Ca2+ signaling (Berridge et al., 2000).
The Na+/Ca2+ exchanger (NCX) directly couples the electrogenic transport of three Na+ and one Ca2+ ion across the cell membrane. Depending on ionic concentrations, net flux can either result in Ca2+ extrusion/Na+ entry (forward mode) or Ca2+ entry/Na+ extrusion (reverse mode) (Bers, 2002; Khananshvili, 2014). NCX1 was originally characterized from heart tissue and subsequently found to be highly expressed in brain, kidney, liver, pancreas, lung, placenta, and skeletal muscle (Kofuji et al., 1992). NCX2 and NCX3 protein expression appears to be unique to the brain and skeletal muscle (Li et al., 1994; Nicoll et al., 1996). Human SLC8A1 (NCX1) is encoded on chromosome 2p22.1 (Shieh et al., 1992), SLC8A2 (NCX2) is encoded on chromosome 19q13.3 (Li et al., 1994), and SLC8A3 (NCX3) is encoded on chromosome 14q24.1 (Nicoll et al., 1996). A fourth mammalian Na+/Ca2+ exchanger (NCLX) expressed in mitochondrial cristae of skeletal and cardiac muscle, pancreatic β-cells, and lymphocyte B-cells, and the brain plays a significant role in mitochondrial Ca2+ homeostasis (Palty et al., 2004, 2006, 2010; Lytton, 2007; Kim et al., 2012). Oxidative phosphorylation, synaptic transmission, cellular Ca2+ regulation, SR/ER Ca2+ content, hormonal secretion, and release of apoptotic factors are all fundamental processes dependent on mitochondrial Ca2+ homeostasis (Contreras et al., 2010; Drago et al., 2011; Mammucari et al., 2011).
NCX activity plays a pivotal role in Ca2+ balance during excitation (neuronal action potential) -contraction (muscle tissue) coupling. Overexpression/increased activity of cardiac NCX1 has been reported to contribute to (i) arrhythmias, (ii) heart failure, and (iii) myocardial ischemia-reperfusion injury (Egger and Niggli, 1999; Sipido et al., 2002; Pott et al., 2011). Several studies have implicated NCX1 upregulation in human vascular tissue to primary pulmonary hypertension (Nakasaki et al., 1993; Iwamoto et al., 2004; Zhang et al., 2010). In skeletal muscle, increases in Ca2+ lead to cross bridging of myosin-actin and contraction of muscle fibers (Baylor and Hollingworth, 2011). Similarly, control of myosin’s interaction with cytoskeletal actin by Ca2+/calmodulin regulates smooth muscle contraction/relaxation (Ledoux et al., 2006). Reduced expression of NCX1 and NCX3 results in intracellular Ca2+ excess and muscle degeneration (Kuyumcu-Martinez and Cooper, 2006). In neurons, cytosolic Ca2+ increases are important for electrical activity synchronization in the excitatory synapse (Franks and Sejnowski, 2002), neurogenesis (Rash et al., 2016; Toth et al., 2016), and synaptic plasticity (Hosseinian et al., 2020). Multiple studies suggest disruption of NCX expression/activity alters intracellular Na+ and Ca2+ homeostasis and may lead to several pathophysiological conditions (e.g., stroke and ischemia) in the brain (Pignataro et al., 2004; Jeffs et al., 2008; Molinaro et al., 2008). Increases in cytoplasmic [Ca2+] reduce and/or halt immune system cell motility (Gallo et al., 2006). NCX activity has been demonstrated to play a role in modulating Ca2+ signaling and cytokine production in human mast cells, macrophages, and monocytes (Aneiros et al., 2005; Staiano et al., 2009). Intracellular oscillations of Ca2+ in pancreatic β-cells is partially regulated by two NCX1 isoforms (NCX1.3 and NCX1.7) contributing to controlling [Ca2+] and release of insulin (Van Eylen et al., 1997; Ximenes et al., 2003). As a result, NCX1 expression/activity may have a possible involvement in the pathophysiology of type-1 diabetes and therefore may represent novel therapeutic targets (Herchuelz et al., 2007). NCX operating in reverse mode is a necessary step for vascular endothelial growth factor-induced ERK1/2 phosphorylation and angiogenesis in human endothelial cells, left ventricular fibrosis in cardiac fibroblasts, and nitric oxide-induced neuronal apoptosis in neuroblastoma cells (Andrikopoulos et al., 2011; Nashida et al., 2011; Kamimura et al., 2012).
Ca2+ surges released from internal stores during mammalian egg fertilization stimulates a signaling cascade resulting in the completion of meiosis and triggers specific mitotic events in the development of the embryo (Miyazaki et al., 1993; Swanson et al., 1997; Jones et al., 2000). Primary saliva production in parotid acinar cells is dependent on the coordination of intracellular Ca2+ signaling (Imbery et al., 2019). Finally, disruption of Ca2+ signaling can induce apoptosis through a complex interplay involving Ca2+ activated proteases, phospholipases, and endonucleases (Kass and Orrenius, 1999). The reversible role of Na+/Ca2+ exchange in multiple cell types and tissues underlies its importance in human health and disease. The question of whether Na+ or Ca2+ ions transported by NCXs participates as signaling molecules requires more investigation.
The Na+/H+ exchanger belongs to the Slc9 gene family. Members SLC9A1–SLC9A5 (or NHE1-5) are targeted and expressed at the plasma membrane where they are activated by intracellular acidification and they function to exchange extracellular Na+ for intracellular protons (Fuster and Alexander, 2014). The first member, NHE1, was functionally identified by Pouysségur (Paris and Pouysségur, 1984). It is expressed in most mammalian cells. Intracellular acidification can occur in a variety of conditions, including ischemia (Karmazyn, 1996; Karmazyn et al., 2001) and cancer (Hulikova et al., 2013). The stoichiometry of exchange is strictly one for one and when the transmembrane concentration gradient for Na+ is balanced by an equivalent and opposite gradient for H+, the exchanger is at equilibrium and mediates no net ion flux (Grinstein and Rothstein, 1986).
While ischemic conditions lead to an intracellular acidification that is mitigated by activation of NHE1, the Na+/H+ exchanger paradoxically participates to cell injury (Karmazyn et al., 2001). The mechanism of injury involves activation of the Na+/Ca2+ exchanger due to Na+ accumulation in cells that have reduced Na+/K+ ATPase activity. Activation of the Na+/Ca2+ exchanger leads to Ca2+ overload and cell death (see previous section). Under normal conditions, the Na+ that enters the cell through the Na+/H+ exchanger is extruded by the Na+/K+ ATPase, but under ischemic conditions, ATP levels are low and consequently function of the Na+/K+ ATPase is reduced. In addition, some have speculated that the exchanger might also regulate the pH microenvironment of gap junction proteins and possibly of the sarcoplasmic reticulum Ca2+ release channel, i.e., components that likewise participate to the injury. Aside from intracellular acidification, the Na+/H+ exchanger is also activated by several hypertrophic factors such as α1-adrenergic stimulation, endothelin-1, and angiotensin II (Khandoudi et al., 1994; Matsui et al., 1995; Yokoyama et al., 1998). These receptors mediate activation of the heterotrimeric G protein, rise in calcium, and activation of PKC. While the mechanism of NHE1 activation is not fully understood, under these receptor agonist conditions, an increase in intracellular Na+ occurs, leading to an increase in cell volume, cell size, protein content, and ultimately tissue hypertrophy (Gu et al., 1998). Importantly, Na+/H+ exchanger inhibitors reduce this hypertrophic response (Kusumoto et al., 2001). In addition, absence of NHE1 expression provides some resistance to cardiac-ischemia reperfusion injury (Wang et al., 2003).
The Na+/H+ exchanger, like the Na-K-2Cl cotransporter, is also involved in cell volume regulation. When cells are exposed to hypertonic conditions, they first rapidly lose water and shrink to equilibrate inside/outside osmolarities. This shrinkage is often followed by a return to baseline volume through a process called regulatory volume increase (RVI). The Na+/H+ exchanger is a mechanism of RVI in lymphocytes (Grinstein et al., 1983), red blood cells (Cala, 1980; Parker, 1983) and other cells (Grinstein and Rothstein, 1986). An unexpected outcome came with the generation of the NHE1 knockout mouse. Mice lacking the exchanger exhibit diminished growth, ataxia, and epileptic-like seizures (Bell et al., 1999). The exchanger was shown to be critical in the response of neurons to acid load (Yao et al., 1999). Loss of either NHE1 or NHE2 also leads to reduced saliva secretion (Park et al., 2001). In colonic crypt epithelial cells, it is NHE2 that regulates intracellular pH and volume homeostasis (Bachmann et al., 2004).
There are also several forms of the Na+/H+ exchanger expressed in intracellular organelles. NHE6 is expressed in mitochondria (Numata et al., 1998), and endosomes (Brett et al., 2002), while members NHE7 and NHE9 are expressed in the Golgi. In these compartments, the exchanger regulates intra organelle pH to maximize their individual function. NHE9 is expressed in the inner ear and seems to prefer exchanging K+ instead of Na+ for protons (Hill et al., 2006).
The Na-K-2Cl cotransporter (NKCC) mediates the coupled electroneutral movement of 1Na+, 1K+, and 2Cl– ions across the plasma membrane. SLC12A1, located on the long (q) arm of human chromosome 15 (15q21.1) encodes NKCC2 – a cotransporter predominantly located in the thick ascending limb of Henle (hTAL), a portion of the kidney nephron involved in active trans-epithelial movement of Na+ (and Cl–), and secondarily of Ca2+ and Mg2+. Expressed at the apical membrane of the hTAL cells, NKCC2 facilitates the movement of Na+, K+, and Cl– from the pro-urine into the cell, while the Na+/K+ pump on the opposite membrane moves Na+ into the interstitial space. This pathway contributes to 10–15% of the filtered Na+ being reabsorbed. By transporting K+ and Cl–, NKCC2 also actively participates in the creation of a driving force for the movement of Ca2+ and Mg2+ through the paracellular pathway. At the apical membrane, K+ ions entering the cell through NKCC2 leak back through K+ channels (ROMK) thereby creating an electropositive lumen. In addition, Cl– ions co-transported with Na+ and K+ at the apical membrane, leak at the basolateral membrane through Cl– channels (CLCK) creating an electronegative serosal side. The result of these two conductance is a trans-epithelial electrical potential that favors the movement of divalent cations through the narrow spaces existing between the epithelial cells and the tight junction proteins that form a significant barrier but are permeable to the divalent cations (Hou and Goodenough, 2010). Thus, each of the ions transported by this unique protein play a critical role in the overall function of this nephron segment. Loss-of-function mutations in NKCC2 results in a salt wasting disorder called Bartter syndrome type I (Simon et al., 1996), characterized by severe salt wasting, hypokalemic metabolic alkalosis and hypercalciuria (Fremont and Chan, 2012). The NKCC2 knockout mouse model dies before weaning due to failure to thrive and is non-viable unless treated with indomethacin from day 1 (Takahashi et al., 2000).
Note that while renal physiologists focus on Na+ reabsorption, they in the process mostly ignore Cl–, the accompanied anion. It is interesting that the opposite situation exists for NKCC1, the other isoform of the NKCC. NKCC1, encoded by SLC12A2 a gene located on the long (q) arm of human chromosome 5 (5q23.3), is a widespread (but not ubiquitous) transporter and is mostly considered in terms of Cl– transport. However, as a protein carrying Na+, K+, and Cl– ions, NKCC1 participates in multiple functions ranging from Cl– homeostasis (neurons, muscle) to trans-epithelial Na+ and Cl– secretion (airway, fluid secreting glands, intestine), and even K+ secretion (inner ear). The role of NKCC1 in each of these functions will be discussed briefly.
Chloride (Cl–) is the major anion in the human body. Its concentration in the blood and extracellular fluid (95–110 mM) is relatively high. Inside cells, due to a large number of fixed anionic charges on macromolecules (Donnan effect) and a luminal-positive membrane potential generated by the leak of K+, the Cl– concentration is generally much lower. It ranges from 5 to 10 mM in mature CNS neurons (Delpire and Staley, 2014) to 90–160 mM in red blood cells (Gunn et al., 1973). Most cells have an intracellular [Cl–] around 30–40 mM. NKCC1 actively participates in intracellular Cl– homeostasis in both neurons and muscle cells. Indeed, NKCC1 is highly expressed in immature central neurons (Plotkin et al., 1997; Dzhala et al., 2005) as well as immature and mature peripheral neurons (Sung et al., 2000; Granados-Soto et al., 2005). By using the Na+ gradient generated by the Na+/K+ pump, NKCC1 can drive Cl– uphill and accumulate the anion to levels that are far above its electrical potential equilibrium. In the young brain, because NKCC1 is expressed and KCC is not yet expressed, the Cl– concentration is high, and opening of GABAA receptors leads to giant depolarizing potentials (Ben-Ari et al., 1989; Ben-Ari, 2012). As the neurons mature, NKCC1 expression decreases and KCC2 (a neuronal-specific K-Cl cotransporter) expression increases, leading to much lower intracellular Cl– concentrations that facilitate hyperpolarizing GABA responses and inhibition (Delpire, 2000; Delpire and Staley, 2014). Note that in the brain Cl– ions might also participate in reciprocal signaling between neurons and astrocytes (Wilson and Mongin, 2019).
The situation is a quite different with peripheral neurons, as KCC2 is never expressed in the periphery and NKCC1 expression remains high in these cells throughout adulthood. As a consequence, the intracellular Cl– concentration in sensory neurons is high (Alvarez-Leefmans et al., 1988), facilitating depolarizing GABA responses at the terminal of sensory fibers and presynaptic inhibition (Willis, 1999; Sung et al., 2000; Delpire and Austin, 2010).
NKCC1 is also expressed in skeletal (Gosmanov et al., 2003) and smooth muscle cells (Kaplan et al., 1996; Akar et al., 2001; Bulley and Jaggar, 2014) where it accumulates intracellular Cl–. High Cl–, in turn, allows for Cl– channel-mediated membrane depolarization, thereby facilitating the opening of Ca2+ channels, the entry of Ca2+ into the cell, and muscle contraction. Thus, NKCC1 affects neurons and muscle cells function by regulating the level of intracellular Cl–. We will see below that these functions are affected in the NKCC1 knockout mouse and in the few individuals with mutations in the cotransporter.
NKCC1 plays an important role in Cl– secreting epithelia, e.g., in shark rectal gland (Burger and Hess, 1960), mammalian salivary, sweat, lacrimal, pancreatic, gastric glands, but also in airway and intestinal epithelia (Grubb et al., 2001; McDaniel et al., 2005; Kidokoro et al., 2014; Delpire and Gagnon, 2016, 2018a). Located on the basolateral membrane, the cotransporter facilitates the trans-epithelial movement of Cl– by bringing Cl– into the cells for Cl– channels (e.g., CFTR) which in turn transport it across the apical membrane. Thus, the combination of NKCC1 and CFTR function provides a pathway for transepithelial transport of Cl–. The Cl– conductance at the apical surface is stimulated by cyclic adenosine monophosphate (cAMP), leading to a drop in intracellular Cl–. This, in turn, leads to the activation of With No lysine Kinases (WNK) which through SPAK activates NKCC1 to replenish intracellular Cl– (Piala et al., 2014).
In the inner ear, NKCC1 fulfills a different function, namely participating in K+ secretion and the creation of a K+-rich endolymph. In the cochlea, the cotransporter is expressed in the stria vascularis (Crouch et al., 1997; Delpire et al., 1999), a multi-layer epithelium secreting K+. As it is the case for Cl– secretion, NKCC1 on the basal side of stria vascularis epithelial cells replenish K+ as it is transported in the endolymphatic cavity by K+ channels formed by both KCNQ1 + KCNE1 subunits (Hibino et al., 2004; Rivas and Francis, 2005).
Importance of NKCC1 as a widespread protein was demonstrated with the development of knockout mouse models (Delpire et al., 1999; Flagella et al., 1999). NKCC1 knockout mice are severely deaf and suffer from inner ear balance deficit (Delpire et al., 1999; Flagella et al., 1999), have low blood pressure and decreased vascular tone (Meyer et al., 2002). They have deficits in saliva production (Evans et al., 2000), intestinal transit (Wouters et al., 2006; Zhu et al., 2016), intestinal hydration (Flagella et al., 1999; Koumangoye et al., 2020), deficit in goblet cell mucus release (Koumangoye et al., 2020). They also have alterations in airway ion transport (Grubb et al., 2001), display a pain perception phenotype (Sung et al., 2000; Laird et al., 2004), and the males are sterile (Pace et al., 2000).
Interestingly, to date, there are only three individuals with complete loss of function in NKCC1 (Macnamara et al., 2019; Stodberg et al., 2020). The case of a 5-year old boy who inherited, through uniparental disomy, two copies of chromosome 5 from his father (an asymptomatic carrier) was described. The boy suffers from hearing loss, developmental delay, intellectual disability, as well as deficits in sweat production, saliva production, lung and intestinal fluid production (Macnamara et al., 2019). Then there is the case of a compound heterozygous 8-year old girl having inherited from her mother a single base pair mutation of a splicing donor site leading to the skipping of exon 13, and from her father a 1 base deletion in exon 8 leading to an open reading frameshift. She suffers from severe neurodevelopmental disorder, hearing impairment, gastrointestinal problems, and deficit in sweat, tear, and saliva production (Stodberg et al., 2020). Note that this proband had an older sister that died 22 days after birth of cardiac arrest during assisted ventilation. Because her fibroblasts were collected, genetic analysis revealed that she carried both her parent’s mutations. Evidence is now mounting that de novo (single allele) mutations lead to neurodevelopmental deficits and cochlea-vestibular defects (McNeill et al., 2020; Mutai et al., 2020). Another patient with NKCC1 mutation deserves some attention in the context of this review article. A 17-year-old girl in California carries a de novo mutation in a single allele of exon 22 of SLC12A2, leading to premature termination of the open reading frame and truncation of the carboxyl-terminal tail of the protein (Delpire et al., 2016). For the longest time, her physicians and parents thought that she was suffering from some type of mitochondrial disease. As a baby, she was difficult to wake-up and otherwise would be in constant need of energy, as if her cells were in constant state of starvation. A muscle biopsy demonstrated increased mitochondrial DNA content, a sign of possible mitochondrial dysfunction (Delpire et al., 2016). From this description, it seems clear that she has a metabolic phenotype. Part of the issue might have been poor intestinal function, limiting the amount of nutrients absorbed from her diet. In fact, her treatment regiment included additional nutrition through a gastric tube, followed by a gastrostomy-jejunostomy tube, followed by a complete intravenous (parenteral) route. We were successful in creating a mouse model that recapitulated the patient mutation (Koumangoye et al., 2018). The mouse exhibited mis-trafficking of the cotransporter to the apical membrane of epithelial cells, decreased intestinal function due to deficit in luminal hydration, deficit in release and expansion of mucus layers (Koumangoye et al., 2020). As surprising, was our demonstration that cells from the mouse had increased mitochondrial respiration, and signs of starvation (Omer et al., 2020).
Is NKCC1 involved in metabolism? A recent paper provides compelling evidence that NKCC1 is indeed involved in cellular metabolism (Demian et al., 2019). The authors demonstrated that NKCC1 function provides a break to the uptake of leucine through the LAT1 transporter and that reduction in NKCC1 function therefore facilitated leucine uptake leading to mTORC1 activation (Figure 4). This was shown through ShRNA-mediated downregulation of transporter expression, as well as through the use of the cotransporter-specific inhibitor, bumetanide. Activation of mTORC1 was blocked by PI3K and Akt inhibitors, as well as the insulin receptor inhibitor, indicating that signal leading to mTORC1 activation was mediated by both the receptor and the two kinases. Thus, NKCC1 activity affects mTORC1 independently through amino acid uptake and through the insulin receptor. Activation of mTORC1 by amino acid uptake ultimately led to the translocation of the complex to the lysosomal membrane. Inhibition of NKCC1 also led to enhanced cell proliferation and a redistribution of cells within the cell cycle. More cells were in the S phase, whereas fewer cells were in the G1 phase (see Figure 5).
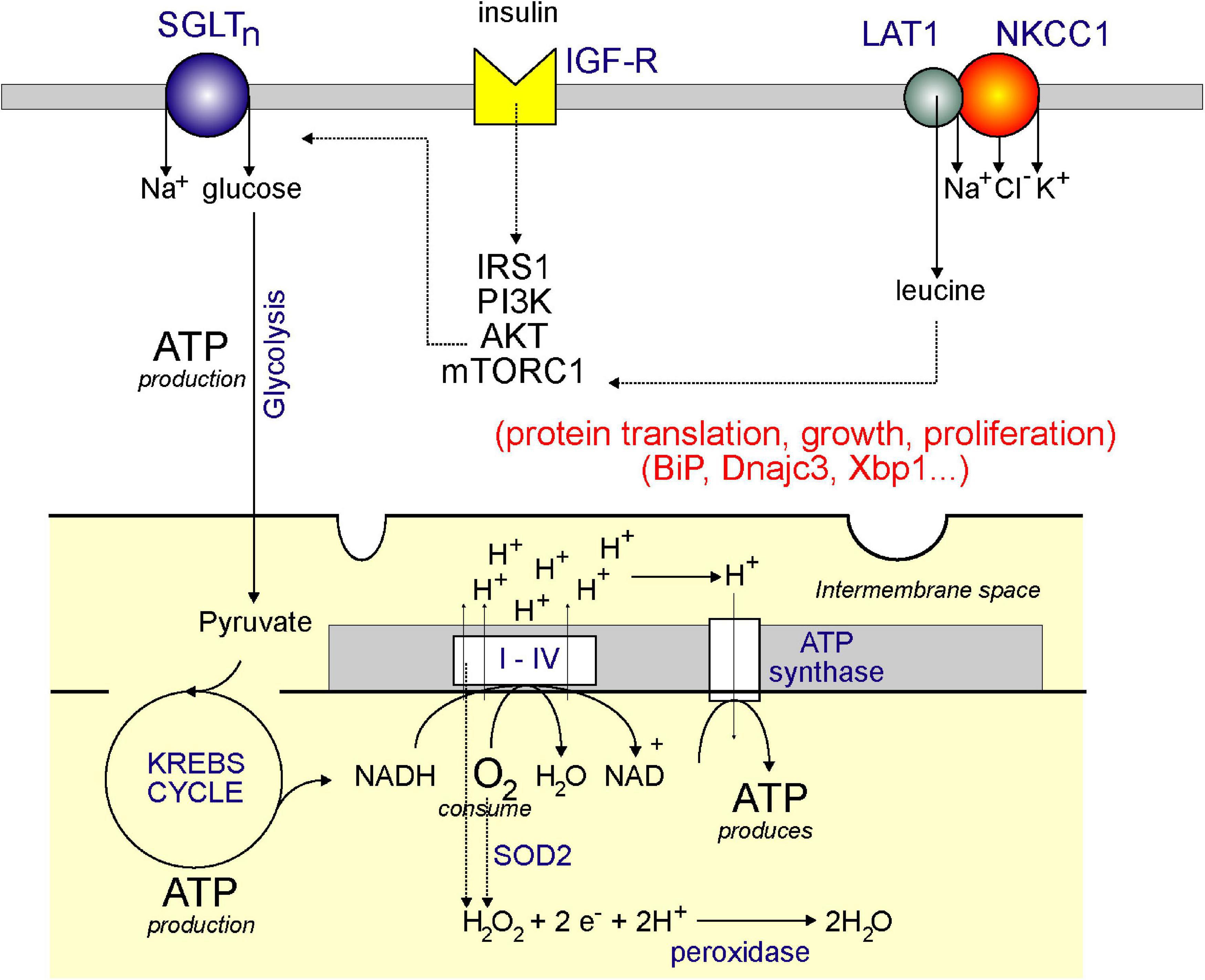
Figure 4. Model showing plasma membrane NKCC1 and mitochondrial respiration: a link through leucine? Aerobic respiration utilizes the KREBS cycle and the electron transport chain. Through the consumption of oxygen (O2) and NADH (produced by the KREBS/TCA cycle), complexes I–IV produces a proton (H+) gradient in the inner membrane of the mitochondria, which is then used by ATP synthase to produce ATP. The KREBS or TCA cycle is powered by pyruvate which comes from glucose through the process of glycolysis. The glucose comes from uptake at the plasma membrane through a Na+-driven glucose transporter. Glucose transport is facilitated by activation of the IGF-R receptor by insulin and stimulation of the IRS1/PI3K/AKT pathway. AKT also stimulates mTORC1, which stimulates protein translation, thereby facilitating cell growth and proliferation. mTORC1 is also stimulated by leucine which enters cells through the LAT1 transporter – which requires interaction with NKCC1. Note that mitochondrial respiration also produces hydrogen peroxide (through complexes I–IV and through SOD2), which can be converted to water by peroxidases.
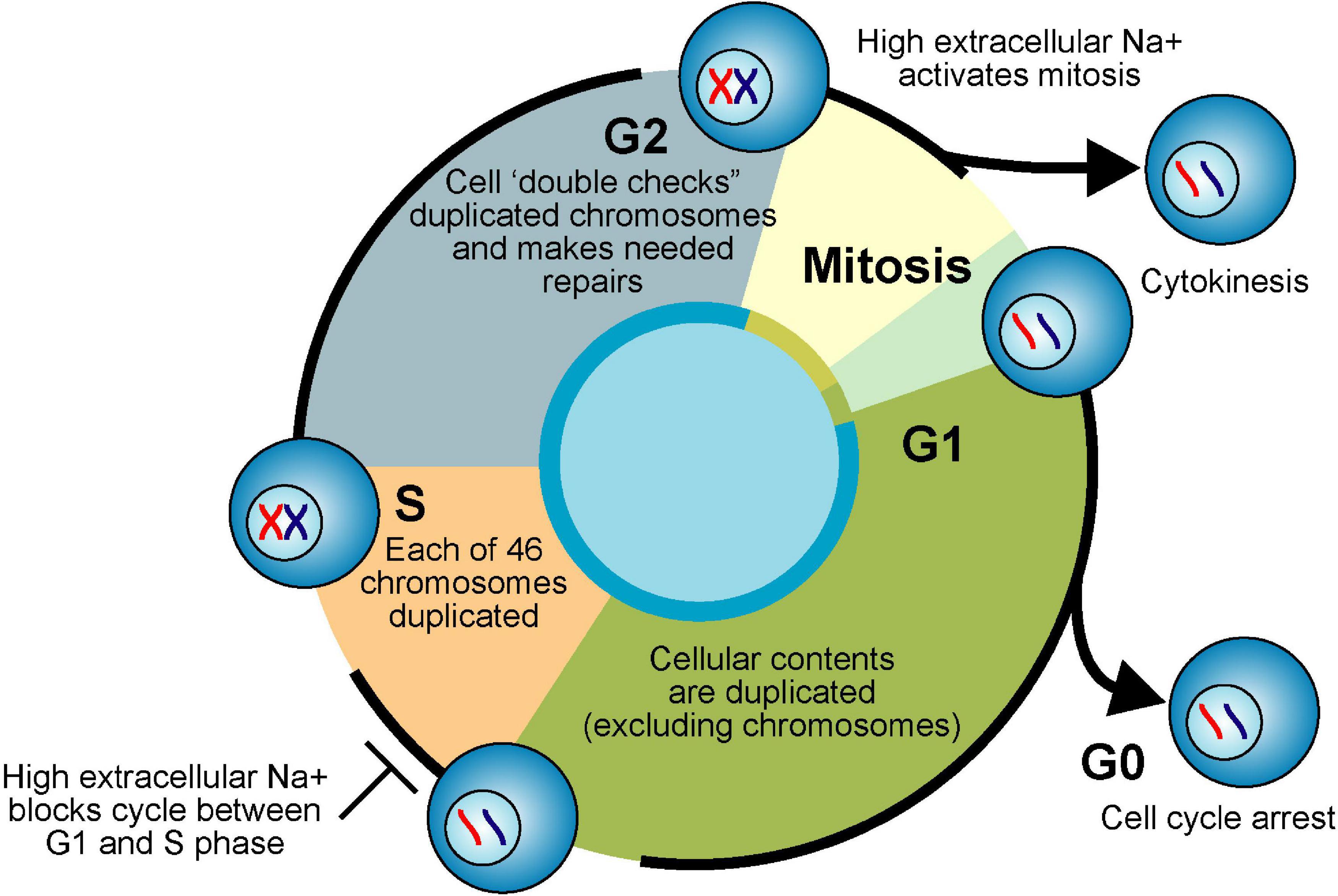
Figure 5. Cell cycle and Na+. Actively proliferating eukaryote cells pass through four phases of the cell cycle. Cellular contents (excluding chromosomes) are duplicated in first gap phase (G1). Each chromosome is duplicated in synthesis phase (S). Duplicated chromosomes are “error checked” during second gap phase (G2). G1, S, and G2 are collectively called interphase. Equal division of duplicated nuclear material occurs in mitosis phase (M). Division of parent cell into two daughter cells (cytokinesis) completes the cell cycle. If nutrients are limiting or the cells are fully differentiated due to internal genetic programming, cells exit interphase and enter a “resting phase” (G0).
Increased activation of the nutrient/energy sensor, mTORC1 is consistent with the increased need for mitochondrial respiration that we observed in cells isolated from the NKCC1-DFX patient and in cells from mice expressing the same mutant transporter (Omer et al., 2020). If NKCC1 functions as a break to the amino acid transporter LAT1 and to the insulin receptor, in macrophages, it also provides a break to the function of phagocytosis (Perry et al., 2019). In this case, however, it seems that intracellular Cl– is a key component, as the process is slowed down by inactivation of KCC1 (SLC12A4) (Perry et al., 2019).
In the kidney, Cl– plays an important role in the regulation of glomerular filtration. When filtration increases, the amount of Cl– that is filtered increases and when the anion flows at the macula densa, it is “sensed” by a mechanism that involves NKCC2 on the apical membrane of macula densa cells which uptake NaCl and swell, thereby releasing ATP. The release of ATP activates A1 receptor on the afferent arteriole leading to contraction. Cl– is also affecting the synthesis and secretion of renin through NKCC1 (Castrop et al., 2005), a cotransporter highly expressed in the renin-containing cells of the afferent arteriole (Kaplan et al., 1996). Under normal conditions, NKCC1 function keeps Cl– high in renin cells, thereby tonically suppressing renin release and secretion. A drop in urinary Cl– through NKCC2 will translate into a drop in interstitial Cl– which then translates through NKCC1 into a decrease in intracellular Cl–, leading to increased renin secretion. Accordingly, in NKCC1 knockout mice, the basal levels of renin mRNA were elevated compared to wild-type mice (Castrop et al., 2005).
The distal convoluted tubule (DCT) of the kidney reabsorbs a small, but critical, portion of the filtered body Na+ prior to being lost in the urine (Moes et al., 2014). Na+ reabsorption in the DCT is highly regulated and affects blood volume and blood pressure. It occurs mainly through the combined actions of the apical Na-Cl cotransporter (NCC) and the basolateral Na+/K+ ATPase (Gamba et al., 1994; Plotkin et al., 1996). Thiazide diuretics, for instance, which inhibit NCC function, are widely used in the clinic to treat volume expansion disorders such as congestive heart failure, kidney disease (Sica, 2003; Listed, 2014). Compared to other nephron segments, the DCT segment is relatively short. Its size, however, varies widely depending on the activity of NCC. To illustrate the tight relationship that exists between NCC function and morphology of the DCT, we will discuss analyses done in mice with manipulation of the Ste20p-related Proline-Alanine rich Kinase (SPAK, also known as STK39). SPAK is the major terminal kinase in the DCT that binds, phosphorylates, and activate the cotransporter (Vitari et al., 2005; Pacheco-Alvarez et al., 2006). Consistently, mice lacking SPAK display a Gitelman-like phenotype (similar to patients with loss-of-function mutations in NCC): being highly sensitive to dietary salt restriction and displaying prolonged negative sodium balance and hypotension (Grimm et al., 2012). As quantified by morphometric analysis using parvalbumin as a marker for DCT1, Grimm and colleagues showed a significantly smaller DCT1 in SPAK knockout mice (Grimm et al., 2012). In accordance with these observations, abundance of NCC and parvalbumin was significantly decreased, whereas abundance of calbindin (DCT2) and NKCC2 (TAL) was unchanged. The authors stated “these observations provide unambiguous evidence that the mass of the DCT, specifically the DCT1, is reduced in SPAK null mice”. Similar observations and conclusions were made in an independent study that used the same SPAK knockout mouse model (McCormick et al., 2011). Note that the same phenotype is observed in thiazide diuretic treated rats (Loffing et al., 1996) and in global NCC and parvalbumin-tissue specific NCC knockout mice (Loffing et al., 2004; Belge et al., 2007). In each case, NCC function is reduced or eliminated and the intracellular Na+ concentration is decreased. Importantly, the reverse situation also provides compelling evidence for the relationship between NCC, Na+, and tissue mass. Indeed, mice expressing a constitutively active SPAK display high blood pressure with hyperkalemia and metabolic acidosis without changes in plasma aldosterone or creatinine clearance. In this mouse model, NCC abundance and phosphorylation was enhanced and hypertrophy of the DCT was observed (Grimm et al., 2017). Both parvalbumin-positive DCT1 tubule length and cross-sectional area were expanded in these mice, compared to controls, while the mass of the more distal CNT (connecting tubule) significantly decreased.
The Cl– regulation of WNK has been best studied in the DCT which constitutes a sensor for plasma K+ (Hoorn et al., 2020). A change in plasma [K+], through Kir4.1/1.1 channels, will lead to parallel changes in intracellular K+ and Cl– concentrations in the DCT (Su et al., 2019). A decrease in plasma K+ results in a decrease in Cl– in DCT leading to stimulation of the WNK4/SPAK cascade and to the activation of NCC and Na+ reabsorption. Conversely, an increase in plasma K+ leads to an increase in intracellular Cl–, inhibition of WNK4/SPAK and NCC, and to decreased Na+ reabsorption (Terker et al., 2015; Ellison et al., 2016).
Similar data were obtained by manipulating WNK4, a kinase that binds (Piechotta et al., 2003), phosphorylates and activates SPAK (Vitari et al., 2005). Studies have shown that mice over-expressing a WNK responsible for the activation of NCC, also demonstrate larger NCC-stained tubules in the transgenic mouse than the wild-type mouse (Lalioti et al., 2006). Thus, all these studies clearly indicate that the mass of the DCT is highly dynamic, following changes in NCC function. Even though the cotransporter utilizes the energy of the Na+ gradient to move Cl– into the cell, it is possible that the movement of the cation may also serve a secondary role as a signal for tissue growth and expansion (Figure 6).
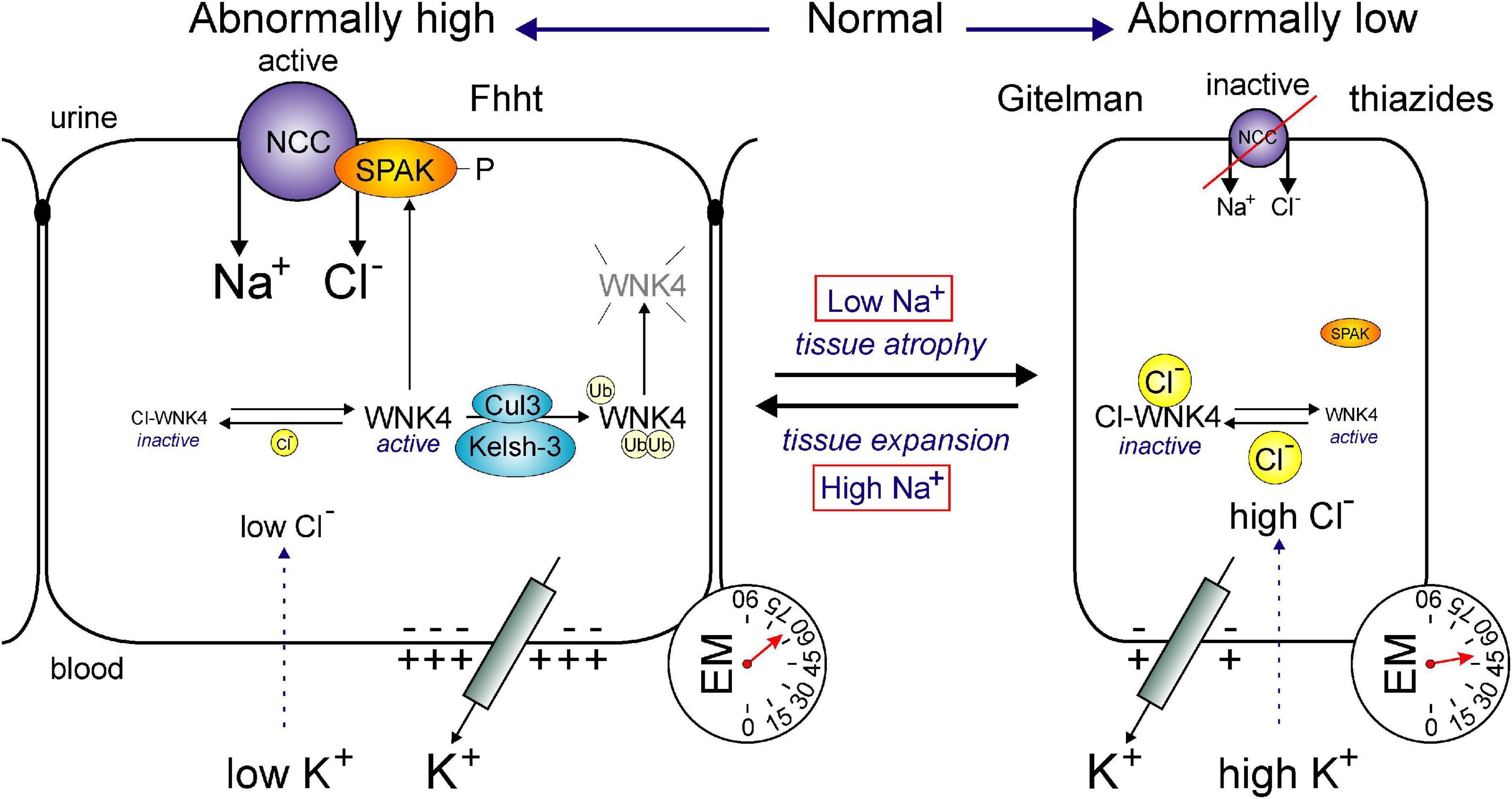
Figure 6. Hypertrophy or atrophy of DCT segment as a function of Na+ transport. Function of the NCC affects the size of the DCT segment. In conditions of abnormally high transport (left), such as Familial hyperkalemia and hypertension (Fhht, or Gordon syndrome) or low plasma K+, the DCT segment hypertrophies. Whereas, in conditions of low transport (right), such as Gitelman syndrome or chronic use of thiazides or high plasma K+, the DCT segment atrophies. The transport of Na+ through NCC is regulated by the WNK4/SPAK cascade. WNK4 activity is modulated by intracellular Cl– levels which are sensitive to plasma K+. Low intracellular Cl– activates WNK4, SPAK and NCC, whereas high intracellular Cl– inhibits WNK4, SPAK, and NCC. DCT-mediated Fhht is due to either mutations in WNK4 or mutations in ubiquitin ligase protein complex Cullin-3 and Kelsh-3. In each case, expression of WNK4 is enhanced leading to increased SPAK and NCC phosphorylation.
Magnesium (Mg2+) is an essential element and the second most abundant intracellular divalent cation in biological systems (Blaine et al., 2015). Mg2+ is a key component of many enzymes, present in every cell type in every organism, and therefore critical in essentially every metabolic pathway (Romani, 2013). Adenosine triphosphate (ATP) must bind magnesium to be biologically active, and as such, Mg2+ has a significant role in the stability of all polyphosphate compounds (e.g., RNA and DNA) in cells (Allen, 2013). The divalent cation is also critical for the function of protein kinases, including receptor tyrosine kinases (e.g., EFGR, VEGFR, PDGFR, and FGFR), non-receptor tyrosine kinases such as Src, and serine/threonine protein kinases, all of which propagate metabolic signals within cells (Cowan, 2002; Zou et al., 2019). Although abundant and readily bioavailable, Mg2+ cannot cross biological membranes, so transport proteins must facilitate Mg2+ movement into and out of cells and intracellular compartments (Romani and Maguire, 2002).
Transcripts of the human Na+/Mg2+ exchangers SLC41A1 (1q31-32), SLC41A2 (12q23.3), and SLC41A3 (3q21.2) mediates the electrogenic transport of 1 Na+ ion and 1 Mg2+ ion across the plasma membrane (Wabakken et al., 2003; Sahni et al., 2007; Kolisek et al., 2008; Quamme, 2010). cAMP signaling is an important second messenger that regulates processes such as neurotransmitter synthesis (Guseva et al., 2014), ganglion synaptic transmission (Patterson et al., 1996), inflammatory response (Park et al., 2008; Chang et al., 2013), myocardial atrophy (Brown et al., 2012), and transcription factor regulation (Metz and Ziff, 1991). Hormones like glucagon (liver) and adrenaline (muscle) are two examples of extracellular first messengers that bind to G-protein coupled receptors in the plasma membrane and signal transmembrane adenylyl cyclases, anchored to the inner leaflet of the plasma membrane, to convert ATP into cAMP (Rahman et al., 2013). Stimulation of cAMP-dependent protein kinase A (PKA) induces phosphorylation of multiple proteins to evoke specific cellular reactions (Brown et al., 2012). Several functional studies have linked cAMP-activated PKA and PKC to the phosphorylation mediated regulation of the Na+/Mg2+ exchanger isoform 1 (NME1) (Wabakken et al., 2003; Kolisek et al., 2008; Quamme, 2010; Kolisek et al., 2012).
In the DCT of the kidney, transcellular Mg2+ reabsorption is mediated by an apical cation channel (TRPM6) and a basolateral sodium-dependent exchanger (NME1). The expression of an apical K+ channel establishes a favorable luminal potential for Mg2+ absorption across the apical cytoplasmic membrane (Blaine et al., 2015). Basolateral Na+/K+ ATPase activity provides the driving force for NME1 extrusion of Mg2+ (Goytain and Quamme, 2005; Kolisek et al., 2008). Hypomagnesemia (acute Mg2+ deficiency) has been associated with diabetes, anxiety disorders, migraines, osteoporosis, cardiovascular disease, hypertension, and stroke (Larsson et al., 2008; Romani, 2013). While Mg2+ is clearly essential to human health, it could more accurately described as a physiological cofactor rather than a signaling factor. As a result, Na+ movement through NMEs doesn’t necessarily represent a signaling cascade as much as a driving force for Mg2+ homeostasis.
The interphase component of the pluripotent eukaryotic cell cycle is subdivided into a growth phase (G1), amassing of energy and DNA replication phase (S), and division preparation phase (G2) (see Figure 5). During the mitotic phase (M) of the cell cycle, the parent cell’s chromosomes are first divided equally between the sister cells (mitosis) and then the two distinct daughter cells are formed by the division of the parent cell’s cytoplasm (cytokinesis) (Kapinas et al., 2013). In 1960, Stubblefield and Mueller demonstrated that increases in extracellular NaCl concentrations from 120 to 220 mM shifted HeLa cells in culture from rapid proliferation (hyperplasia) to increased size (hypertrophy). Interestingly, despite increased cellular size, the DNA per cell remained constant against various extracellular NaCl concentrations, suggesting that high-salt treatment blocks the cell cycle between G1 and S phase (Stubblefield and Mueller, 1960). In the early 1970s, it was shown that mitosis is activated by changes in the electrical membrane potential and increases in the intracellular Na+ concentration (Cone and Tongier, 1971, 1973). For instance, DNA synthesis was induced in spinal cord neurons isolated from chick embryos by exposure to ouabain, veratridine, or the ionophore gramicidin (Cone and Cone, 1976). Increased intracellular Na+ (1.3 to 2-fold) was also demonstrated in CHO cells during the late S phase and mitosis (Marakhova et al., 1987). As far back as 1926, Dr. Charles Packard observed that the [Na+] in the blood of tumor-bearing rats was 25% higher than normal when the tumor was actively growing and 60% higher still when the tumor was receding (Packard, 1926). Amiloride, a compound that inhibits sodium influx and proliferation of normal cells (Koch and Leffert, 1979; Villereal, 1981) reduced tumor growth, tumor cell proliferation, and intra-nuclear Na+ content (Sparks et al., 1983).
Every cell in the body requires at some point in its life cycle the capacity to move either prior to terminal differentiation or to maintain tissue homeostasis (Schwab et al., 2012). Angiogenesis (Stupack and Cheresh, 2004), wound healing (Martin and Leibovich, 2005; Blikslager et al., 2007; Dignass, 2001), immune response (Friedl and Weigelin, 2008; Nourshargh et al., 2010; Silva, 2010), gastro-intestinal barrier preservation (Yen and Wright, 2006), and neuronal development (Komuro and Rakic, 1998; Komuro and Kumada, 2005; Valiente and Marín, 2010) are all cellular processes dependent on cell motility. Cellular migration machinery (Mogilner and Oster, 2003; Insall and Machesky, 2009; Cramer, 2010), cell adhesion receptors (Jones et al., 2006), and chemokine receptors (Mañes et al., 1999), are just some of the components that need to be asymmetrically distributed to the front and rear poles of the cell for persistent cell migration. Several ionic mechanisms also have significant roles in cell motility. Ca2+-sensitive calpain proteases contribute to disassembly of focal adhesion components at the rear pole of migrating cells (Chan et al., 2010). Integrin adhesion has also been shown to be regulated by the activity of various K+ channels (Artym and Petty, 2002; Arcangeli and Becchetti, 2006). Coordinated attachment and release of the focal adhesion contacts between cells and the extracellular matrix involves integrins and the Na+/H+ exchanger (NHE1) (Plopper et al., 1995; Hynes, 2002). Polarization and directed movement of migrating cells requires both cytoskeletal anchoring and ion transport activity of NHE1. Wound healing assays demonstrated that mutations impairing either function resulted in slower fibroblast cell migration than wild type cells (Denker and Barber, 2002). Regulation of intracellular pH and efficient chemotaxis are dependent on NHE1 expression to the leading edge of polarized cells. Adhesion of these migrating cells to the extracellular matrix is also dependent on the extracellular pH (Lehenkari and Horton, 1999; Eble and Tuckwell, 2003; Patel and Barber, 2005; Krähling et al., 2009; Paradise et al., 2011). Taken together, these studies suggest that Na+ likely has an indirect role in cell migration as the activity of NHE1 more likely contributes to the extracellular pH microenvironment.
In order to overcome the Donnan forces associated with macromolecules and organic metabolites unable to diffuse across the plasma membrane, mammalian cells transport Na+ (and osmotically obligatory water) out of the cell primarily by the action of the Na+/K+ ATPase (Chatton et al., 2016). The energy produced by the 10-fold Na+ gradient created by the hydrolysis of ATP is utilized by astrocytes to clear neurotransmitters and recycle metabolic waste products (Weber and Barros, 2015). Another study has demonstrated that increased Na+ uptake in adipose tissue promotes increased plasma adiponectin via stimulation of peroxisome proliferator-activated receptors. Enhanced adiponectin, in turn, inhibits renal SGLT2 function and ultimately proximal tubule reabsorption of Na+ and glucose. Interestingly, this metabolic pathway is impaired by diabetes and thereby results in hyperglycemia-induced Na+ retention (Zhao et al., 2016). Chronic hyponatremia, a common disorder in elderly people, has been linked to increased skeletal fractures (Gankam Kengne et al., 2008; Sandhu et al., 2009). In vitro experiments demonstrated that decreased extracellular Na+ in the medium of cultured bone marrow monocytes dose-dependently increased osteoclastogenesis and osteoclastic resorptive activity. One possible mechanism through which extracellular [Na+] might inversely regulate this resorptive activity is through the Na+-dependent vitamin C transporter (Barsony et al., 2011). It is well documented that vitamin C protects osteoblasts (Wilson and Dixon, 1989) and osteoclasts (Xiao et al., 2005) from reactive oxygen species accumulation and oxidative stress, and that decreased extracellular [Na+] inhibits vitamin C transporter activity. Indeed, bone mineral density studies in humans have inversely associated the oxidative stress response as a mechanism for chronic hyponatremia-induced pathologies (Basu et al., 2001).
The extracellular to intracellular Na+ gradient maintained by the action of the Na+/K+ ATPase provides an electrochemical driving force for multiple Na+-dependent transport mechanisms. Overall, it is clear that President John F. Kennedy was correct in stating that “we are all tied to the sea” and that Na+ is critical to all aspects of human physiology. In this review we have endeavored to both highlight the functional role of these transporters and exchangers in human health and disease and consider their potential role as components of intracellular signaling pathways using Na+ ions.
KG and ED have contributed to the writing, editing, creation of Figures, and have approved the final version of the manuscript.
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney diseases RO1 DK093501 and by the Leducq Transatlantic Network of Excellence grant 17CVDO5.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akar, F., Jiang, G., Paul, R. J., and O’neill, W. C. (2001). Contractile regulation of the Na(+)-K(+)-2Cl(-) cotransporter in vascular smooth muscle. Am. J. Physiol. Cell Physiol. 281, C579–C584.
Alvarez-Leefmans, F. J., Gamiño, S. M., Giraldez, F., and Nogueron, I. (1988). Intracellular chloride regulation in amphibian dorsal root ganglion neurons studied with ion-selective microelectrodes. J. Physiol. 406, 225–246. doi: 10.1113/jphysiol.1988.sp017378
Andrikopoulos, P., Baba, A., Matsuda, T., Djamgoz, M. B., Yaqoob, M. M., and Eccles, S. A. (2011). Ca2 + influx through reverse mode Na+/Ca2 + exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J. Biol. Chem. 286, 37919–37931. doi: 10.1074/jbc.m111.251777
Aneiros, E., Philipp, S., Lis, A., Freichel, M., and Cavalié, A. (2005). Modulation of Ca2 + signaling by Na+/Ca2 + exchangers in mast cells. J. Immunol. 174, 119–130. doi: 10.4049/jimmunol.174.1.119
Arcangeli, A., and Becchetti, A. (2006). Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol. 16, 631–639. doi: 10.1016/j.tcb.2006.10.003
Artym, V. V., and Petty, H. R. (2002). Molecular proximity of Kv1.3 voltage-gated potassium channels and beta(1)-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J. Gen. Physiol. 120, 29–37. doi: 10.1085/jgp.20028607
Aydemir-Koksoy, A., Abramowitz, J., and Allen, J. C. (2001). Ouabain-induced signaling and vascular smooth muscle cell proliferation. J. Biol. Chem. 276, 46605–46611. doi: 10.1074/jbc.m106178200
Babula, P., Masarik, M., Adam, V., Provaznik, I., and Kizek, R. (2013). From Na+/K+-ATPase and cardiac glycosides to cytotoxicity and cancer treatment. Anticancer Agents Med. Chem. 13, 1069–1087. doi: 10.2174/18715206113139990304
Bachmann, O., Riederer, B., Rossmann, H., Groos, S., Schultheis, P. J., Shull, G. E., et al. (2004). The Na+/H+ exchanger isoform 2 is the predominant NHE isoform in murine colonic crypts and its lack causes NHE3 upregulation. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G125–G133.
Barsony, J., Sugimura, Y., and Verbalis, J. G. (2011). Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J. Biol. Chem. 286, 10864–10875. doi: 10.1074/jbc.m110.155002
Basu, S., Michaëlsson, K., Olofsson, H., Johansson, S., and Melhus, H. (2001). Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 288, 275–279. doi: 10.1006/bbrc.2001.5747
Baylor, S. M., and Hollingworth, S. (2011). Calcium indicators and calcium signalling in skeletal muscle fibres during excitation-contraction coupling. Prog. Biophys. Mol. Biol. 105, 162–179. doi: 10.1016/j.pbiomolbio.2010.06.001
Belge, H., Gailly, P., Schwaller, B., Loffing, J., Debaix, H., Riveira-Munoz, E., et al. (2007). Renal expression of parvalbumin is critical for NaCl handling and response to diuretics. Proc. Natl. Acad. Sci. U.S.A. 104, 14849–14854. doi: 10.1073/pnas.0702810104
Bell, S. M., Schreiner, C. M., Schultheis, P. J., Miller, M. L., Evans, R. L., Vorhees, C. V., et al. (1999). Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am. J. Physiol. 276, C788–C795.
Ben-Ari, Y. (2012). The Yin and Yen of GABA in brain development and operation in health and disease. Front. Cell Neurosci. 6:45. doi: 10.3389/fncel.2012.00045
Ben-Ari, Y., Cherubini, E., Corradetti, R., and Gaiarsa, J. L. (1989). Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 416, 303–325. doi: 10.1113/jphysiol.1989.sp017762
Berger, C., and Zdzieblo, D. (2020). Glucose transporters in pancreatic islets. Pflugers Arch. 472, 1249–1272. doi: 10.1007/s00424-020-02383-4
Berridge, M. J., Lipp, P., and Bootman, M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21. doi: 10.1038/35036035
Blaine, J., Chonchol, M., and Levi, M. (2015). Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 10, 1257–1272. doi: 10.2215/cjn.09750913
Blanco, G., and Mercer, R. W. (1998). Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275, F633–F650.
Blikslager, A. T., Moeser, A. J., Gookin, J. L., Jones, S. L., and Odle, J. (2007). Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 87, 545–564. doi: 10.1152/physrev.00012.2006
Brett, C. L., Wei, Y., Donowitz, M., and Rao, R. (2002). Human Na(+)/H(+) exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am. J. Physiol. Cell Physiol. 282, C1031–C1041.
Brini, M., and Carafoli, E. (2009). Calcium pumps in health and disease. Physiol Rev 89, 1341–1378. doi: 10.1152/physrev.00032.2008
Brown, K. M., Lee, L. C., Findlay, J. E., Day, J. P., and Baillie, G. S. (2012). Cyclic AMP-specific phosphodiesterase, PDE8A1, is activated by protein kinase A-mediated phosphorylation. FEBS Lett. 586, 1631–1637. doi: 10.1016/j.febslet.2012.04.033
Bulley, S., and Jaggar, J. H. (2014). Cl- channels in smooth muscle cells. Pflügers Arch. Eur. J. Physiol. 466, 5861–5872.
Burger, J. W., and Hess, W. N. (1960). Function of the rectal gland in the spiny dogfish. Science 131, 670–671. doi: 10.1126/science.131.3401.670
Cala, P. M. (1980). Volume regulation by Amphiuma red blood cells: the membrane potential and its implications regarding the nature of the ion-flux pathways. J. Gen. Physiol. 76, 683–708. doi: 10.1085/jgp.76.6.683
Castrop, H., Lorenz, J. N., Hansen, P. B., Friis, U., Mizel, D., Oppermann, M., et al. (2005). Contribution of the basolateral isoform of the Na-K-2Cl– cotransporter (NKCC1/BSC2) to renin secretion. Am. J. Physiol. Renal Physiol. 289, F1185–F1192.
Chan, K. T., Bennin, D. A., and Huttenlocher, A. (2010). Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK). J. Biol. Chem. 285, 11418–11426. doi: 10.1074/jbc.m109.090746
Chang, S. Y., Kim, D. B., Ryu, G. R., Ko, S. H., Jeong, I. K., Ahn, Y. B., et al. (2013). Exendin-4 inhibits iNOS expression at the protein level in LPS-stimulated Raw264.7 macrophage by the activation of cAMP/PKA pathway. J. Cell Biochem. 114, 844–853. doi: 10.1002/jcb.24425
Chatton, J. Y., Magistretti, P. J., and Barros, L. F. (2016). Sodium signaling and astrocyte energy metabolism. Glia 64, 1667–1676. doi: 10.1002/glia.22971
Clausen, M. V., Hilbers, F., and Poulsen, H. (2017). The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 8:371. doi: 10.3389/fphys.2017.00371
Cone, C. D. Jr., and Cone, C. M. (1976). Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science 192, 155–158. doi: 10.1126/science.56781
Cone, C. D. Jr., and Tongier, M. Jr. (1971). Control of somatic cell mitosis by simulated changes in the transmembrane potential level. Oncology 25, 168–182. doi: 10.1159/000224567
Cone, C. D. Jr., and Tongier, M. Jr. (1973). Contact inhibition of division: involvement of the electrical transmembrane potential. J. Cell Physiol. 82, 373–386. doi: 10.1002/jcp.1040820307
Contreras, L., Drago, I., Zampese, E., and Pozzan, T. (2010). Mitochondria: the calcium connection. Biochim. Biophys. Acta 1797, 607–618. doi: 10.1016/j.bbabio.2010.05.005
Cowan, J. A. (2002). Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals 15, 225–235.
Crambert, G., and Geering, K. (2003). FXYD proteins: new tissue-specific regulators of the ubiquitous Na,K-ATPase. Sci. STKE 2003:RE1.
Cramer, L. P. (2010). Forming the cell rear first: breaking cell symmetry to trigger directed cell migration. Nat. Cell Biol. 12, 628–632. doi: 10.1038/ncb0710-628
Crouch, J. J., Sakaguchi, N., Lytle, C., and Schulte, B. A. (1997). Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J. Histochem. 45, 773–778. doi: 10.1177/002215549704500601
Delpire, E. (2000). Cation-chloride cotransporters in neuronal communication. NIPS 15, 309–312. doi: 10.1152/physiologyonline.2000.15.6.309
Delpire, E., and Austin, T. M. (2010). Kinase regulation of Na+-K+-2Cl– cotransport in primary afferent neurons. J. Physiol. 588, 3365–3373. doi: 10.1113/jphysiol.2010.190769
Delpire, E., and Gagnon, K. B. (2016). “Na-K-2Cl cotransporter,” in Ion Channels and Transporters in Epithelia in Health and Disease, eds K. L. Hamilton and D. C. Trevor (Rockville, MD: American Physiological Society), 375–400. doi: 10.1007/978-1-4939-3366-2_11
Delpire, E., and Gagnon, K. B. (2018a). Na+-K+-2Cl– cotransporter (NKCC) physiological function in nonpolarized cells and transporting epithelia. Comprehens. Physiol. 8, 871–901. doi: 10.1002/cphy.c170018
Delpire, E., and Gagnon, K. B. (2018b). Water homeostasis and cell volume maintenance and regulation. Curr. Top. Membr. 81, 3–52. doi: 10.1016/bs.ctm.2018.08.001
Delpire, E., Lu, J., England, R., Dull, C., and Thorne, T. (1999). Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat. Genet. 22, 192–195. doi: 10.1038/9713
Delpire, E., and Staley, K. J. (2014). Novel determinants of the neuronal Cl- concentration. J. Physiol. 592, 4099–4114. doi: 10.1113/jphysiol.2014.275529
Delpire, E., Wolfe, L., Flores, B., Koumangoye, R., Schornak, C. C., Omer, S., et al. (2016). A patient with multisystem dysfunction carries a truncation mutation in human SLC12A2, the gene encoding the Na-K-2Cl cotransporter. NKCC1. Cold Spring Harb. Mol. Case Stud. 2:a001289. doi: 10.1101/mcs.a001289
Demian, W. L., Persaud, A., Jiang, C., Coyaud, É, Liu, S., Kapus, A., et al. (2019). The ion transporter NKCC1 links cell volume to cell mass regulation by suppressing mTORC1. Cell Rep. 27, 1886–1896. doi: 10.1016/j.celrep.2019.04.034
Demirbilek, H., Galcheva, S., Vuralli, D., Al-Khawaga, S., and Hussain, K. (2019). Ion transporters, channelopathies, and glucose disorders. Int. J. Mol. Sci. 20:2590. doi: 10.3390/ijms20102590
Denker, S. P., and Barber, D. L. (2002). Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J. Cell Biol. 159, 1087–1096. doi: 10.1083/jcb.200208050
Dignass, A. U. (2001). Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel Dis. 7, 68–77. doi: 10.1097/00054725-200102000-00014
Drago, I., Pizzo, P., and Pozzan, T. (2011). After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J. 30, 4119–4125. doi: 10.1038/emboj.2011.337
Dyla, M., Basse Hansen, S., Nissen, P., and Kjaergaard, M. (2019). Structural dynamics of P-type ATPase ion pumps. Biochem. Soc. Trans. 47, 1247–1257. doi: 10.1042/bst20190124
Dyla, M., Kjærgaard, M., Poulsen, H., and Nissen, P. (2020). Structure and mechanism of P-Type ATPase ion pumps. Annu. Rev. Biochem. 89, 583–603. doi: 10.1146/annurev-biochem-010611-112801
Dzhala, V. I., Talos, D. M., Sdrulla, D. A., Brumback, A. C., Mathews, G. C., Benke, T. A., et al. (2005). NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 11, 1205–1213. doi: 10.1038/nm1301
Eble, J. A., and Tuckwell, D. S. (2003). The alpha2beta1 integrin inhibitor rhodocetin binds to the A-domain of the integrin alpha2 subunit proximal to the collagen-binding site. Biochem. J. 376, 77–85. doi: 10.1042/bj20030373
Egger, M., and Niggli, E. (1999). Regulatory function of Na-Ca exchange in the heart: milestones and outlook. J. Membr. Biol. 168, 107–130. doi: 10.1007/s002329900502
Ellison, D. H., Terker, A. S., and Gamba, G. (2016). Potassium and its discontents: new insight, new treatments. J. Am. Soc. Nephrol. 27, 981–984. doi: 10.1681/asn.2015070751
Erdmann, E., Philipp, G., and Scholz, H. (1980). Cardiac glycoside receptor, (Na+ + K+)-ATPase activity and force of contraction in rat heart. Biochem. Pharmacol. 29, 3219–3229. doi: 10.1016/0006-2952(80)90295-6
Evans, R. L., Park, K., Turner, R. J., Watson, G. E., Nguyen, H.-V., Dennett, M. R., et al. (2000). Severe impairment of salivation in Na+/K+/2Cl– cotransporter (NKCC1)-deficient mice. J. Biol. Chem. 275, 26720–26726. doi: 10.1016/s0021-9258(19)61435-3
Flagella, M., Clarke, L. L., Miller, M. L., Erway, L. C., Giannella, R. A., Andringa, A., et al. (1999). Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J. Biol. Chem. 274, 26946–26955. doi: 10.1074/jbc.274.38.26946
Franks, K. M., and Sejnowski, T. J. (2002). Complexity of calcium signaling in synaptic spines. Bioessays 24, 1130–1144. doi: 10.1002/bies.10193
Fremont, O. T., and Chan, J. C. (2012). Understanding bartter syndrome and gitelman syndrome. World J. Pediatr. 8, 25–30. doi: 10.1007/s12519-012-0333-9
Friedl, P., and Weigelin, B. (2008). Interstitial leukocyte migration and immune function. Nat. Immunol. 9, 960–969. doi: 10.1038/ni.f.212
Fuster, D. G., and Alexander, R. T. (2014). Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 466, 61–76. doi: 10.1007/s00424-013-1408-8
Gallo, E. M., Canté-Barrett, K., and Crabtree, G. R. (2006). Lymphocyte calcium signaling from membrane to nucleus. Nat. Immunol. 7, 25–32. doi: 10.1038/ni1295
Gallo, L. A., Wright, E. M., and Vallon, V. (2015). Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab. Vasc. Dis. Res. 12, 78–89. doi: 10.1177/1479164114561992
Gamba, G., Miyanoshita, A., Lombardi, M., Lytton, J., Lee, W.-S., Hediger, M., et al. (1994). Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 269, 17713–17722. doi: 10.1016/s0021-9258(17)32499-7
Gankam Kengne, F., Andres, C., Sattar, L., Melot, C., and Decaux, G. (2008). Mild hyponatremia and risk of fracture in the ambulatory elderly. Qjm 101, 583–588. doi: 10.1093/qjmed/hcn061
Ghezzi, C., Loo, D. D. F., and Wright, E. M. (2018). Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 61, 2087–2097. doi: 10.1007/s00125-018-4656-5
Gosmanov, A. R., Lindinger, M. I., and Thomason, D. B. (2003). Riding the tides: K+ concentration and volume regulation by muscle Na+-K+-2Cl– cotransport activity. News Physiol. Sci. 18, 196–200. doi: 10.1152/nips.01446.2003
Goytain, A., and Quamme, G. A. (2005). Functional characterization of human SLC41A1, a Mg2 + transporter with similarity to prokaryotic MgtE Mg2 + transporters. Physiol. Genomics 21, 337–342. doi: 10.1152/physiolgenomics.00261.2004
Granados-Soto, V., Arguelles, C. F., and Alvarez-Leefmans, F. J. (2005). Peripheral and central antinociceptive action of Na–K–2Cl cotransporter blockers on formalin-induced nociception in rats. Pain 114, 231–238. doi: 10.1016/j.pain.2004.12.023
Grimm, P. R., Coleman, R., Delpire, E., and Welling, P. A. (2017). Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J. Am. Soc. Nephrol. 28, 2597–2606. doi: 10.1681/asn.2016090948
Grimm, P. R., Taneja, T. K., Liu, J., Coleman, R., Chen, Y. Y., Delpire, E., et al. (2012). SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J. Biol. Chem. 287, 37673–37690. doi: 10.1074/jbc.m112.402800
Grinstein, S., Clarke, C. A., and Rothstein, A. (1983). Activation of Na+/H+ exchange in lymphocytes by osmotically induced volume changes and by cytoplasmic acidification. J. Gen. Physiol. 82, 619–638. doi: 10.1085/jgp.82.5.619
Grinstein, S., and Rothstein, A. (1986). Mechanisms of regulation of the Na+/H+ exchanger. J. Membrane Biol. 90, 1–12. doi: 10.1007/bf01869680
Grubb, B. R., Pace, A. J., Lee, E., Koller, B. H., and Boucher, R. C. (2001). Alterations in airway ion transport in NKCC1-deficient mice. Am. J. Physiol. Cell Physiol. 281, C615–C623.
Grupp, I., Im, W. B., Lee, C. O., Lee, S. W., Pecker, M. S., and Schwartz, A. (1985). Relation of sodium pump inhibition to positive inotropy at low concentrations of ouabain in rat heart muscle. J. Physiol. 360, 149–160. doi: 10.1113/jphysiol.1985.sp015609
Gu, J. W., Anand, V., Shek, E. W., Moore, M. C., Brady, A. L., Kelly, W. C., et al. (1998). Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension 31, 1083–1087. doi: 10.1161/01.hyp.31.5.1083
Gunn, R. B., Dalmark, M., Tosteson, D. C., and Wieth, J. O. (1973). Characteristics of chloride transport in human red blood cells. J. Gen. Physiol. 61, 185–206. doi: 10.1085/jgp.61.2.185
Guseva, D., Wirth, A., and Ponimaskin, E. (2014). Cellular mechanisms of the 5-HT7 receptor-mediated signaling. Front. Behav. Neurosci. 8:306. doi: 10.3389/fnbeh.2014.00306
Hebert, S. C., and Brown, E. M. (1996). The scent of an ion: calcium-sensing and its roles in health and disease. Curr. Opin. Nephrol. Hypertens. 5, 45–53. doi: 10.1097/00041552-199601000-00009
Hediger, M. A., Coady, M. J., Ikeda, T. S., and Wright, E. M. (1987). Expression cloning and cDNA sequencing of the Na+/glucose co-transport. Nature 330, 379–381. doi: 10.1038/330379a0
Herchuelz, A., Kamagate, A., Ximenes, H., and Van Eylen, F. (2007). Role of Na/Ca exchange and the plasma membrane Ca2 +-ATPase in beta cell function and death. Ann. N. Y. Acad. Sci. 1099, 456–467. doi: 10.1196/annals.1387.048
Hibino, H., Higashi-Shingai, K., Fujita, A., Iwai, K., Ishii, M., and Kurachi, Y. (2004). Expression of an inwardly rectifying K+ channel, Kir5.1, in specific types of fibrocytes in the cochlear lateral wall suggests its functional importance in the establishment of endocochlear potential. Eur. J. Neurosci. 19, 76–84. doi: 10.1111/j.1460-9568.2004.03092.x
Hill, J. K., Brett, C. L., Chyou, A., Kallay, L. M., Sakaguchi, M., Rao, R., et al. (2006). Vestibular hair bundles control pH with (Na+, K+)/H+ exchangers NHE6 and NHE9. J. Neurosci. 26, 9944–9955. doi: 10.1523/jneurosci.2990-06.2006
Hoorn, E. J., Gritter, M., Cuevas, C. A., and Fenton, R. A. (2020). Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol. Rev. 100, 321–356. doi: 10.1152/physrev.00044.2018
Hosseinian, S., Arefian, E., Rakhsh-Khorshid, H., Eivani, M., Rezayof, A., Pezeshk, H., et al. (2020). A meta-analysis of gene expression data highlights synaptic dysfunction in the hippocampus of brains with Alzheimer’s disease. Sci. Rep. 10:8384.
Hou, J., and Goodenough, D. A. (2010). Claudin-16 and claudin-19 function in the thick ascending limb. Curr. Opin. Nephrol. Hypertens. 19, 483–488. doi: 10.1097/mnh.0b013e32833b7125
Hulikova, A., Harris, A. L., Vaughan-Jones, R. D., and Swietach, P. (2013). Regulation of intracellular pH in cancer cell lines under normoxia and hypoxia. J. Cell Physiol. 228, 743–752. doi: 10.1002/jcp.24221
Imbery, J. F., Iqbal, A. K., Desai, T., and Giovannucci, D. R. (2019). Role of NAADP for calcium signaling in the salivary gland. Cell Calc. 80, 29–37. doi: 10.1016/j.ceca.2019.03.001
Insall, R. H., and Machesky, L. M. (2009). Actin dynamics at the leading edge: from simple machinery to complex networks. Dev. Cell 17, 310–322. doi: 10.1016/j.devcel.2009.08.012
Iwamoto, T., Kita, S., Zhang, J., Blaustein, M. P., Arai, Y., Yoshida, S., et al. (2004). Salt-sensitive hypertension is triggered by Ca2 + entry via Na+/Ca2 + exchanger type-1 in vascular smooth muscle. Nat. Med. 10, 1193–1199. doi: 10.1038/nm1118
Jeffs, G. J., Meloni, B. P., Sokolow, S., Herchuelz, A., Schurmans, S., and Knuckey, N. W. (2008). NCX3 knockout mice exhibit increased hippocampal CA1 and CA2 neuronal damage compared to wild-type mice following global cerebral ischemia. Exp. Neurol. 210, 268–273. doi: 10.1016/j.expneurol.2007.10.013
Jones, K. T., Matsuda, M., Parrington, J., Katan, M., and Swann, K. (2000). Different Ca2 +-releasing abilities of sperm extracts compared with tissue extracts and phospholipase C isoforms in sea urchin egg homogenate and mouse eggs. Biochem. J. 346(Pt 3), 743–749. doi: 10.1042/bj3460743
Jones, M. C., Caswell, P. T., and Norman, J. C. (2006). Endocytic recycling pathways: emerging regulators of cell migration. Curr. Opin. Cell Biol. 18, 549–557. doi: 10.1016/j.ceb.2006.08.003
Kamimura, D., Ohtani, T., Sakata, Y., Mano, T., Takeda, Y., Tamaki, S., et al. (2012). Ca2 + entry mode of Na+/Ca2 + exchanger as a new therapeutic target for heart failure with preserved ejection fraction. Eur. Heart J. 33, 1408–1416. doi: 10.1093/eurheartj/ehr106
Kanai, Y., Clémençon, B., Simonin, A., Leuenberger, M., Lochner, M., Weisstanner, M., et al. (2013). The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Aspects Med. 34, 108–120. doi: 10.1016/j.mam.2013.01.001
Kandasamy, P., Gyimesi, G., Kanai, Y., and Hediger, M. A. (2018). Amino acid transporters revisited: new views in health and disease. Trends Biochem. Sci. 43, 752–789. doi: 10.1016/j.tibs.2018.05.003
Kapinas, K., Grandy, R., Ghule, P., Medina, R., Becker, K., Pardee, A., et al. (2013). The abbreviated pluripotent cell cycle. J. Cell Physiol. 228, 9–20. doi: 10.1002/jcp.24104
Kaplan, M. R., Plotkin, M. D., Brown, D., Hebert, S. C., and Delpire, E. (1996). Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal IMCD, the glomerular and extraglomerular mesangium and the glomerular afferent arteriole. J. Clin. Invest. 98, 723–730. doi: 10.1172/jci118844
Karmazyn, M. (1996). The sodium-hydrogen exchange system in the heart: its role in ischemic and reperfusion injury and therapeutic implications. Can. J. Cardiol. 12, 1074–1082.
Karmazyn, M., Sostaric, J. V., and Gan, X. T. (2001). The myocardial Na+/H+ exchanger: a potential therapeutic target for the prevention of myocardial ischaemic and reperfusion injury and attenuation of postinfarction heart failure. Drugs 61, 375–389. doi: 10.2165/00003495-200161030-00006
Kass, G. E., and Orrenius, S. (1999). Calcium signaling and cytotoxicity. Environ. Health Perspect. 107, (Suppl. 1), 25–35. doi: 10.2307/3434469
Khananshvili, D. (2014). Sodium-calcium exchangers (NCX): molecular hallmarks underlying the tissue-specific and systemic functions. Pflugers Arch. 466, 43–60. doi: 10.1007/s00424-013-1405-y
Khandoudi, N., Ho, J., and Karmazyn, M. (1994). Role of Na(+)-H+ exchange in mediating effects of endothelin-1 on normal and ischemic/reperfused hearts. Circ. Res. 75, 369–378. doi: 10.1161/01.res.75.2.369
Kidokoro, M., Nakamoto, T., Mukaibo, T., Kondo, Y., Munemasa, T., Imamura, A., et al. (2014). Na(+)-K(+)-2Cl(-) cotransporter-mediated fluid secretion increases under hypotonic osmolarity in the mouse submandibular salivary gland. Am. J. Physiol. Renal Physiol. 306, F1155–F1160.
Kim, B., Takeuchi, A., Koga, O., Hikida, M., and Matsuoka, S. (2012). Pivotal role of mitochondrial Na+-Ca2 + exchange in antigen receptor mediated Ca2 + signalling in DT40 and A20 B lymphocytes. J. Physiol. 590, 459–474.
Koch, K. S., and Leffert, H. L. (1979). Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell 18, 153–163. doi: 10.1016/0092-8674(79)90364-7
Koepsell, H. (2017). The Na(+)-D-glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer. Pharmacol. Ther. 170, 148–165. doi: 10.1016/j.pharmthera.2016.10.017
Kofuji, P., Hadley, R. W., Kieval, R. S., Lederer, W. J., and Schulze, D. H. (1992). Expression of the Na-Ca exchanger in diverse tissues: a study using the cloned human cardiac Na-Ca exchanger. Am. J. Physiol. 263, C1241–C1249.
Kolisek, M., Launay, P., Beck, A., Sponder, G., Serafini, N., Brenkus, M., et al. (2008). SLC41A1 is a novel mammalian Mg2 + carrier. J. Biol. Chem. 283, 16235–16247. doi: 10.1074/jbc.m707276200
Kolisek, M., Nestler, A., Vormann, J., and Schweigel-Röntgen, M. (2012). Human gene SLC41A1 encodes for the Na+/Mg2 + exchanger. Am. J. Physiol. Cell Physiol. 302, C318–C326.
Komuro, H., and Kumada, T. (2005). Ca2 + transients control CNS neuronal migration. Cell Calc. 37, 387–393. doi: 10.1016/j.ceca.2005.01.006
Komuro, H., and Rakic, P. (1998). Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2 + fluctuations. J. Neurobiol. 37, 110–130. doi: 10.1002/(sici)1097-4695(199810)37:1<110::aid-neu9>3.0.co;2-c
Koumangoye, R., Omer, S., and Delpire, E. (2018). Mistargeting of a truncated Na-K-2Cl cotransporter in epithelial cells. Am. J. Physiol. Cell Physiol. 315, C258–C276.
Koumangoye, R., Omer, S., Kabeer, M. H., and Delpire, E. (2020). Novel human NKCC1 mutations cause defects in goblet cells mucus secretion and chronic inflammation Cell. Mol. Gastroenterol. Hepatol. 9, 239–255. doi: 10.1016/j.jcmgh.2019.10.006
Krähling, H., Mally, S., Eble, J. A., Noël, J., Schwab, A., and Stock, C. (2009). The glycocalyx maintains a cell surface pH nanoenvironment crucial for integrin-mediated migration of human melanoma cells. Pflugers Arch. 458, 1069–1083. doi: 10.1007/s00424-009-0694-7
Kusumoto, K., Haist, J. V., and Karmazyn, M. (2001). Na(+)/H(+) exchange inhibition reduces hypertrophy and heart failure after myocardial infarction in rats. Am. J. Physiol. Heart Circ. Physiol. 280, H738–H745.
Kuyumcu-Martinez, N. M., and Cooper, T. A. (2006). Misregulation of alternative splicing causes pathogenesis in myotonic dystrophy. Prog. Mol. Subcell. Biol. 44, 133–159. doi: 10.1007/978-3-540-34449-0_7
Laird, J. M., Garcia-Nicas, E., Delpire, E. J., and Cervero, F. (2004). Presynaptic inhibition and spinal pain processing in mice: a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci. Lett. 361, 200–203. doi: 10.1016/j.neulet.2003.12.015
Lalioti, M. D., Zhang, J., Volkman, H. M., Kahle, K. T., Hoffmann, K. E., Toka, H. R., et al. (2006). Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat. Genet. 38, 1124–1132. doi: 10.1038/ng1877
Larsson, S. C., Virtanen, M. J., Mars, M., Männistö, S., Pietinen, P., Albanes, D., et al. (2008). Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch. Intern. Med. 168, 459–465. doi: 10.1001/archinte.168.5.459
Ledoux, J., Werner, M. E., Brayden, J. E., and Nelson, M. T. (2006). Calcium-activated potassium channels and the regulation of vascular tone. Physiology 21, 69–78. doi: 10.1152/physiol.00040.2005
Lee, C. O., Abete, P., Pecker, M., Sonn, J. K., and Vassalle, M. (1985). Strophanthidin inotropy: role of intracellular sodium ion activity and sodium-calcium exchange. J. Mol. Cell Cardiol. 17, 1043–1053. doi: 10.1016/s0022-2828(85)80120-6
Lee, K., Jung, J., Kim, M., and Guidotti, G. (2001). Interaction of the alpha subunit of Na,K-ATPase with cofilin. Biochem. J. 353, 377–385. doi: 10.1042/0264-6021:3530377
Lehenkari, P. P., and Horton, M. A. (1999). Single integrin molecule adhesion forces in intact cells measured by atomic force microscopy. Biochem. Biophys. Res. Commun. 259, 645–650. doi: 10.1006/bbrc.1999.0827
Li, Z., Cai, T., Tian, J., Xie, J. X., Zhao, X., Liu, L., et al. (2009). NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J. Biol. Chem. 284, 21066–21076. doi: 10.1074/jbc.m109.013821
Li, Z., Matsuoka, S., Hryshko, L. V., Nicoll, D. A., Bersohn, M. M., Burke, E. P., et al. (1994). Cloning of the NCX2 isoform of the plasma membrane Na(+)-Ca2 + exchanger. J. Biol. Chem. 269, 17434–17439. doi: 10.1016/s0021-9258(17)32458-4
Listed, N. A. (2014). Treating essential hypertension. The first choice is usually a thiazide diuretic. Prescrire Int. 23, 215–220.
Liu, J., Lilly, M. N., and Shapiro, J. I. (2018). Targeting Na/K-ATPase signaling: a new approach to control oxidative stress. Curr. Pharm. Des. 24, 359–364. doi: 10.2174/1381612824666180110101052
Loffing, J., Loffing-Cueni, D., Hegyi, I., Kaplan, M. R., Hebert, S. C., Le Hir, M., et al. (1996). Thiazide treatment of rats provokes apoptosis in distal tubule cells. Kidney Int. 50, 1180–1190. doi: 10.1038/ki.1996.426
Loffing, J., Vallon, V., Loffing-Cueni, D., Aregger, F., Richter, K., Pietri, L., et al. (2004). Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman’s syndrome. J. Am. Soc. Nephrol. 15, 2276–2288. doi: 10.1097/01.asn.0000138234.18569.63
Lytton, J. (2007). Na+/Ca2 + exchangers: three mammalian gene families control Ca2 + transport. Biochem. J. 406, 365–382. doi: 10.1042/bj20070619
Macnamara, E. F., Koehler, A. E., D’souza, P., Estwick, T., Lee, P., Vezina, G., et al. (2019). Kilquist syndrome: a novel syndromic hearing loss disorder caused by homozygous deletion of SLC12A2. Hum. Mut. 40, 532–538. doi: 10.1002/humu.23722
Magi, S., Piccirillo, S., and Amoroso, S. (2019). The dual face of glutamate: from a neurotoxin to a potential survival factor-metabolic implications in health and disease. Cell Mol. Life Sci. 76, 1473–1488. doi: 10.1007/s00018-018-3002-x
Mammucari, C., Patron, M., Granatiero, V., and Rizzuto, R. (2011). Molecules and roles of mitochondrial calcium signaling. Biofactors 37, 219–227. doi: 10.1002/biof.160
Mañes, S., Mira, E., Gómez-Moutón, C., Lacalle, R. A., Keller, P., Labrador, J. P., et al. (1999). Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 18, 6211–6220. doi: 10.1093/emboj/18.22.6211
Marakhova, T. A., and Efimova, E. V. (1987). [Cation transport and content in serum-stimulated CHO-773 cells. III. The intracellular content of sodium, calcium and magnesium]. Tsitologiia 29, 315–320.
Martin, P., and Leibovich, S. J. (2005). Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607. doi: 10.1016/j.tcb.2005.09.002
Matsui, H., Barry, W. H., Livsey, C., and Spitzer, K. W. (1995). Angiotensin II stimulates sodium-hydrogen exchange in adult rabbit ventricular myocytes. Cardiovasc. Res. 29, 215–221. doi: 10.1016/0008-6363(96)88573-7
McCormick, J. A., Mutig, K., Nelson, J. H., Saritas, T., Hoorn, E. J., Yang, C.-L., et al. (2011). A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab. 14, 352–364. doi: 10.1016/j.cmet.2011.07.009
McDaniel, N., Pace, A. J., Spiegel, S., Engelhardt, R., Koller, B. H., Seidler, U., et al. (2005). Role of Na-K-2Cl cotransporter-1 in gastric secretion of nonacidic fluid and pepsinogen. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G550–G560.
McNeill, A., Lovino, E., Bedoukian, E., Cox, H., Dean, J., Goudie, D., et al. (2020). SLC12A2 variants cause a neurodevelopmental disorder or cochleovestibular defect. Brain 143, 2380–2387. doi: 10.1093/brain/awaa176
Metz, R., and Ziff, E. (1991). cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to trans-locate to the nucleus and induce c-fos transcription. Genes Dev. 5, 1754–1766. doi: 10.1101/gad.5.10.1754
Meyer, J. W., Flagella, M., Sutliff, R. L., Lorenz, J. N., Nieman, M. L., Weber, C. S., et al. (2002). Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na(+)-K(+)-2Cl(-) cotransporter. Am. J. Physiol. Heart Circ. Physiol. 283, H1846–H1855.
Miyazaki, S., Shirakawa, H., Nakada, K., and Honda, Y. (1993). Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2 + release channel in Ca2 + waves and Ca2 + oscillations at fertilization of mammalian eggs. Dev. Biol. 158, 62–78. doi: 10.1006/dbio.1993.1168
Moes, A. D., Van Der Lubbe, N., Zietse, R., Loffing, J., and Hoorn, E. J. (2014). The sodium chloride cotransporter SLC12A3: new roles in sodium, potassium, and blood pressure regulation. Pflugers Arch. 466, 107–118. doi: 10.1007/s00424-013-1407-9
Mogilner, A., and Oster, G. (2003). Polymer motors: pushing out the front and pulling up the back. Curr. Biol. 13, R721–R733.
Molinaro, P., Cuomo, O., Pignataro, G., Boscia, F., Sirabella, R., Pannaccione, A., et al. (2008). Targeted disruption of Na+/Ca2 + exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J. Neurosci. 28, 1179–1184. doi: 10.1523/jneurosci.4671-07.2008
Mutai, H., Wasano, K., Momozawa, Y., Kamatani, Y., Miya, F., Masuda, S., et al. (2020). Variants encoding a restricted carboxy-terminal domain of SLC12A2 cause hereditary hearing loss in humans. PLoS Genet. 16:e1008643. doi: 10.1371/journal.pgen.1008643
Nakasaki, Y., Iwamoto, T., Hanada, H., Imagawa, T., and Shigekawa, M. (1993). Cloning of the rat aortic smooth muscle Na+/Ca2 + exchanger and tissue-specific expression of isoforms. J. Biochem. 114, 528–534. doi: 10.1093/oxfordjournals.jbchem.a124211
Nashida, T., Takuma, K., Fukuda, S., Kawasaki, T., Takahashi, T., Baba, A., et al. (2011). The specific Na(+)/Ca(2+) exchange inhibitor SEA0400 prevents nitric oxide-induced cytotoxicity in SH-SY5Y cells. Neurochem. Int. 59, 51–58. doi: 10.1016/j.neuint.2011.03.026
Nicoll, D. A., Quednau, B. D., Qui, Z., Xia, Y. R., Lusis, A. J., and Philipson, K. D. (1996). Cloning of a third mammalian Na+-Ca2 + exchanger, NCX3. J. Biol. Chem. 271, 24914–24921. doi: 10.1074/jbc.271.40.24914
Nourshargh, S., Hordijk, P. L., and Sixt, M. (2010). Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378. doi: 10.1038/nrm2889
Numata, M., Petrecca, K., Lake, N., and Orlowski, J. (1998). Identification of a mitochondrial Na+/H+ exchanger. J. Biol. Chem. 273, 6951–6959. doi: 10.1074/jbc.273.12.6951
Omer, S., Koumangoye, R., and Delpire, E. (2020). A mutation in the Na-K-2Cl cotransporter-1 leads to changes in cellular metabolism. J. Cell. Physiol. 235, 7239–7250. doi: 10.1002/jcp.29623
Pace, A. J., Lee, E., Athirakul, K., Coffman, T. M., O’brien, D. A., and Koller, B. H. (2000). Failure of spermatogenesis in mouse lines deficient in the Na+-K+-2Cl– cotransporter. J. Clin. Invest. 105, 441–450. doi: 10.1172/jci8553
Pacheco-Alvarez, D., Cristóbal, P. S., Meade, P., Moreno, E., Vazquez, N., Muñoz, E., et al. (2006). The Na+:Cl– cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J. Biol. Chem. 281, 28755–28763. doi: 10.1074/jbc.m603773200
Palty, R., Hershfinkel, M., Yagev, O., Saar, D., Barkalifa, R., Khananshvili, D., et al. (2006). Single alpha-domain constructs of the Na+/Ca2 + exchanger, NCLX, oligomerize to form a functional exchanger. Biochemistry 45, 11856–11866. doi: 10.1021/bi060633b
Palty, R., Ohana, E., Hershfinkel, M., Volokita, M., Elgazar, V., Beharier, O., et al. (2004). Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J. Biol. Chem. 279, 25234–25240. doi: 10.1074/jbc.m401229200
Palty, R., Silverman, W. F., Hershfinkel, M., Caporale, T., Sensi, S. L., Parnis, J., et al. (2010). NCLX is an essential component of mitochondrial Na+/Ca2 + exchange. Proc. Natl. Acad. Sci. U.S.A. 107, 436–441. doi: 10.1073/pnas.0908099107
Paradise, R. K., Lauffenburger, D. A., and Van Vliet, K. J. (2011). Acidic extracellular pH promotes activation of integrin α(v)β(3). PLoS One 6:e15746. doi: 10.1371/journal.pone.0015746
Paris, S., and Pouysségur, J. (1984). Growth factors activate the Na+/H+ antiporter in quiescent fibroblasts by increasing its affinity for intracellular H+. J. Biol. Chem. 259, 10989–10994. doi: 10.1016/s0021-9258(18)90611-3
Park, K., Evans, R. L., Watson, G. E., Nehrke, K., Richardson, L., Bell, S. M., et al. (2001). Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchanger-deficient mice. J. Biol. Chem. 276, 27042–27050. doi: 10.1074/jbc.m102901200
Park, P. H., Huang, H., Mcmullen, M. R., Bryan, K., and Nagy, L. E. (2008). Activation of cyclic-AMP response element binding protein contributes to adiponectin-stimulated interleukin-10 expression in RAW 264.7 macrophages. J. Leukoc. Biol. 83, 1258–1266. doi: 10.1189/jlb.0907631
Parker, J. C. (1983). Volume-responsive sodium movements in dog red cells. Am. J. Physiol. 244, C324–C330.
Patel, H., and Barber, D. L. (2005). A developmentally regulated Na-H exchanger in Dictyostelium discoideum is necessary for cell polarity during chemotaxis. J. Cell Biol. 169, 321–329. doi: 10.1083/jcb.200412145
Patterson, S. L., Abel, T., Deuel, T. A., Martin, K. C., Rose, J. C., and Kandel, E. R. (1996). Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16, 1137–1145. doi: 10.1016/s0896-6273(00)80140-3
Perry, J. S. A., Morioka, S., Medina, C. B., Barron, B., Raymond, M. H., Lucas, C. D., et al. (2019). Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nature Cell Biol. 21, 1532–1543. doi: 10.1038/s41556-019-0431-1
Perry, R. J., and Shulman, G. I. (2020). Sodium glucose cotransporter-2 inhibitors: understanding the mechanisms for therapeutic promise and persisting risks. J. Biol. Chem. 295, 14379–14390. doi: 10.1074/jbc.rev120.008387
Piala, A. T., Moon, T. M., Akella, R., He, H., Cobb, M. H., and Goldsmith, E. J. (2014). Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci. Signal. 7:ra41. doi: 10.1126/scisignal.2005050
Piechotta, K., Garbarini, N. J., England, R., and Delpire, E. (2003). Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl– cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J. Biol. Chem. 278, 52848–52856. doi: 10.1074/jbc.m309436200
Pignataro, G., Gala, R., Cuomo, O., Tortiglione, A., Giaccio, L., Castaldo, P., et al. (2004). Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke 35, 2566–2570. doi: 10.1161/01.str.0000143730.29964.93
Plopper, G. E., Mcnamee, H. P., Dike, L. E., Bojanowski, K., and Ingber, D. E. (1995). Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol. Biol. Cell 6, 1349–1365. doi: 10.1091/mbc.6.10.1349
Plotkin, M. D., Kaplan, M. R., Verlander, J. W., Lee, W.-S., Brown, D., Poch, E., et al. (1996). Localization of the thiazide sensitive Na-Cl cotransporter, rTSC, in the rat kidney. Kidney Int. 50, 174–183. doi: 10.1038/ki.1996.300
Plotkin, M. D., Snyder, E. Y., Hebert, S. C., and Delpire, E. (1997). Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J. Neurobiol. 33, 781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5
Pott, C., Eckardt, L., and Goldhaber, J. I. (2011). Triple threat: the Na+/Ca2 + exchanger in the pathophysiology of cardiac arrhythmia, ischemia and heart failure. Curr. Drug Targets 12, 737–747. doi: 10.2174/138945011795378559
Prassas, I., and Diamandis, E. P. (2008). Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 7, 926–935. doi: 10.1038/nrd2682
Quamme, G. A. (2010). Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 298, C407–C429.
Rahman, N., Buck, J., and Levin, L. R. (2013). pH sensing via bicarbonate-regulated “soluble” adenylyl cyclase (sAC). Front. Physiol. 4:343. doi: 10.3389/fphys.2013.00343
Rash, B. G., Ackman, J. B., and Pasko Rakic, P. (2016). Bidirectional radial Ca2 + activity regulates neurogenesis and migration during early cortical column formation. Sci. Adv. 2:e1501733. doi: 10.1126/sciadv.1501733
Rivas, A., and Francis, H. W. (2005). Inner ear abnormalities in a Kcnq1 (Kvlqt1) knockout mouse: a model of Jervell and Lange-Nielsen syndrome. Otol. Neurotol. 26, 415–424. doi: 10.1097/01.mao.0000169764.00798.84
Romani, A. M., and Maguire, M. E. (2002). Hormonal regulation of Mg2 + transport and homeostasis in eukaryotic cells. Biometals 15, 271–283.
Sahni, J., Nelson, B., and Scharenberg, A. M. (2007). SLC41A2 encodes a plasma-membrane Mg2 + transporter. Biochem. J. 401, 505–513. doi: 10.1042/bj20060673
Sandhu, H. S., Gilles, E., Devita, M. V., Panagopoulos, G., and Michelis, M. F. (2009). Hyponatremia associated with large-bone fracture in elderly patients. Int. Urol. Nephrol. 41, 733–737. doi: 10.1007/s11255-009-9585-2
Santer, R., and Calado, J. (2010). Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin. J. Am. Soc. Nephrol. 5, 133–141. doi: 10.2215/cjn.04010609
Santer, R., Groth, S., Kinner, M., Dombrowski, A., Berry, G. T., Brodehl, J., et al. (2002). The mutation spectrum of the facilitative glucose transporter gene SLC2A2 (GLUT2) in patients with Fanconi-Bickel syndrome. Hum. Genet. 110, 21–29. doi: 10.1007/s00439-001-0638-6
Santer, R., Kinner, M., Lassen, C. L., Schneppenheim, R., Eggert, P., Bald, M., et al. (2003). Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J. Am. Soc. Nephrol. 14, 2873–2882. doi: 10.1097/01.asn.0000092790.89332.d2
Scafoglio, C., Hirayama, B. A., Kepe, V., Liu, J., Ghezzi, C., Satyamurthy, N., et al. (2015). Functional expression of sodium-glucose transporters in cancer. Proc. Natl. Acad. Sci. U.S.A. 112, E4111–E4119.
Schwab, A., Fabian, A., Hanley, P. J., and Stock, C. (2012). Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913. doi: 10.1152/physrev.00018.2011
Shieh, B. H., Xia, Y., Sparkes, R. S., Klisak, I., Lusis, A. J., Nicoll, D. A., et al. (1992). Mapping of the gene for the cardiac sarcolemmal Na(+)-Ca2 + exchanger to human chromosome 2p21-p23. Genomics 12, 616–617. doi: 10.1016/0888-7543(92)90459-6
Shinoda, T., Ogawa, H., Cornelius, F., and Toyoshima, C. (2009). Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature 459, 446–450. doi: 10.1038/nature07939
Sica, D. A. (2003). Metolazone and its role in edema management. Congest. Heart Fail. 9, 100–105. doi: 10.1111/j.1527-5299.2003.01907.x
Silva, M. T. (2010). When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 87, 93–106. doi: 10.1189/jlb.0809549
Simon, D. B., Karet, F. E., Hamdan, J. M., Di Pietro, A., Sanjad, S. A., and Lifton, R. P. (1996). Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat. Gen. 13, 183–188. doi: 10.1038/ng0696-183
Sipido, K. R., Volders, P. G., Vos, M. A., and Verdonck, F. (2002). Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc. Res. 53, 782–805. doi: 10.1016/s0008-6363(01)00470-9
Skou, J. C. (1957). The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta 23, 494–401.
Skou, J. C. (1965). Enzymatic basis for active transport of Na+ and K+ across cell membrane. Physiol. Rev. 45, 596–617. doi: 10.1152/physrev.1965.45.3.596
Sparks, R. L., Pool, T. B., Smith, N. K., and Cameron, I. L. (1983). Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 43, 73–77.
Staiano, R. I., Granata, F., Secondo, A., Petraroli, A., Loffredo, S., Frattini, A., et al. (2009). Expression and function of Na+/Ca2 + exchangers 1 and 3 in human macrophages and monocytes. Eur. J. Immunol. 39, 1405–1418. doi: 10.1002/eji.200838792
Stanton, B. A., and Kaissling, B. (1989). Regulation of renal ion transport and cell growth by sodium. Am. J. Physiol. Renal Physiol. 257, F1–F10.
Stodberg, T., Magnusson, M., Lesko, N., Wredenberg, A., Martin Munoz, D., Stranneheim, H., et al. (2020). SLC12A2 mutations cause NKCC1 deficiency with encephalopathy and impaired secretory epithelia. Neurol. Genet. 6:e478. doi: 10.1212/nxg.0000000000000478
Stubblefield, E., and Mueller, G. C. (1960). Effects of sodium chloride concentration on growth, biochemical composition, and metabolism of HeLa cells. Cancer Res. 20, 1646–1655.
Stupack, D. G., and Cheresh, D. A. (2004). Integrins and angiogenesis. Curr. Top. Dev. Biol. 64, 207–238.
Su, X. T., Ellison, D. H., and Wang, W. H. (2019). Kir4.1/Kir5.1 in the DCT plays a role in the regulation of renal K(+) excretion. Am. J. Physiol. Renal Physiol. 316, F582–F586.
Sung, K.-W., Kirby, M., Mcdonald, M. P., Lovinger, D. M., and Delpire, E. (2000). Abnormal GABAA-receptor mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J. Neurosci. 20, 7531–7538. doi: 10.1523/jneurosci.20-20-07531.2000
Swanson, C. A., Arkin, A. P., and Ross, J. (1997). An endogenous calcium oscillator may control early embryonic division. Proc. Natl. Acad. Sci. U.S.A. 94, 1194–1199. doi: 10.1073/pnas.94.4.1194
Takahashi, N., Chernavvsky, D. R., Gomez, R. A., Igarashi, P., Gitelman, H. J., and Smithies, O. (2000). Uncompensated polyuria in a mouse model of Bartter’s syndrome. Proc. Natl. Acad. Sci. U.S.A. 97, 5434–5439. doi: 10.1073/pnas.090091297
Terker, A. S., Zhang, C., Mccormick, J. A., Lazelle, R. A., Zhang, C., Meermeier, N. P., et al. (2015). Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 21, 39–50. doi: 10.1016/j.cmet.2014.12.006
Tian, J., Cai, T., Yuan, Z., Wang, H., Liu, L., Haas, M., et al. (2006). Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell 17, 317–326. doi: 10.1091/mbc.e05-08-0735
Toth, A. B., Shum, A. K., and Prakriya, M. (2016). Regulation of neurogenesis by calcium signaling. Cell Calc. 59, 124–134. doi: 10.1016/j.ceca.2016.02.011
Vague, P., Coste, T. C., Jannot, M. F., Raccah, D., and Tsimaratos, M. (2004). C-peptide, Na+,#K(+)-ATPase, and diabetes. Exp. Diabes. Res. 5, 37–50. doi: 10.1080/15438600490424514
Valiente, M., and Marín, O. (2010). Neuronal migration mechanisms in development and disease. Curr. Opin. Neurobiol. 20, 68–78. doi: 10.1016/j.conb.2009.12.003
Van Eylen, F., Svoboda, M., and Herchuelz, A. (1997). Identification, expression pattern and potential activity of Na/Ca exchanger isoforms in rat pancreatic B-cells. Cell Calc. 21, 185–193. doi: 10.1016/s0143-4160(97)90043-9
Villereal, M. L. (1981). Sodium fluxes in human fibroblasts: kinetics of serum-dependent and serum-independent pathways. J. Cell Physiol. 108, 251–259. doi: 10.1002/jcp.1041080215
Vitari, A. C., Deak, M., Morrice, N. A., and Alessi, D. R. (2005). The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome, phosphorylate and active SPAK and OSR1 protein kinases. Biochem. J. 391, 17–24. doi: 10.1042/bj20051180
Wabakken, T., Rian, E., Kveine, M., and Aasheim, H. C. (2003). The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2 + transporters. Biochem. Biophys. Res. Commun. 306, 718–724. doi: 10.1016/s0006-291x(03)01030-1
Wang, Y., Meyer, J. W., Ashraf, M., and Shull, G. E. (2003). Mice with a null mutation in the NHE1 Na+-H+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circ. Res. 93, 776–782. doi: 10.1161/01.res.0000094746.24774.dc
Weber, B., and Barros, L. F. (2015). The astrocyte: powerhouse and recycling center. Cold Spring Harb. Perspect. Biol. 7:a020396. doi: 10.1101/cshperspect.a020396
Willis, W. D. (1999). Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp. Brain Res. 124, 395–421. doi: 10.1007/s002210050637
Wilson, C. S., and Mongin, A. A. (2019). The signaling role for chloride in the bidirectional communication between neurons and astrocytes. Neurosci. Lett. 689, 33–44. doi: 10.1016/j.neulet.2018.01.012
Wilson, J. X., and Dixon, S. J. (1989). High-affinity sodium-dependent uptake of ascorbic acid by rat osteoblasts. J. Membr. Biol. 111, 83–91. doi: 10.1007/bf01869211
Wouters, M., De Laet, A., Ver Donck, L., Delpire, E., Van Bogaert, P. P., Timmermans, J. P., et al. (2006). Subtractive hybridization unravels a role for the ion co-transporter NKCC1 in the murine intestinal pacemaker. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1219–G1227.
Wright, E. M. (2001). Renal Na(+)-glucose cotransporters. Am. J. Physiol. Renal Physiol. 280, F10–F18.
Wright, E. M., Ghezzi, C., and Loo, D. D. F. (2017). Novel and unexpected functions of SGLTs. Physiology 32, 435–443. doi: 10.1152/physiol.00021.2017
Wright, E. M., Loo, D. D., and Hirayama, B. A. (2011). Biology of human sodium glucose transporters. Physiol. Rev. 91, 733–794. doi: 10.1152/physrev.00055.2009
Wright, E. M., and Turk, E. (2004). The sodium/glucose cotransport family SLC5. Pflugers Arch. 447, 510–518. doi: 10.1007/s00424-003-1063-6
Xiao, X. H., Liao, E. Y., Zhou, H. D., Dai, R. C., Yuan, L. Q., and Wu, X. P. (2005). Ascorbic acid inhibits osteoclastogenesis of RAW264.7 cells induced by receptor activated nuclear factor kappaB ligand (RANKL) in vitro. J. Endocrinol. Invest. 28, 253–260. doi: 10.1007/bf03345382
Ximenes, H. M., Kamagate, A., Van Eylen, F., Carpinelli, A., and Herchuelz, A. (2003). Opposite effects of glucose on plasma membrane Ca2 + -ATPase and Na/Ca exchanger transcription, expression, and activity in rat pancreatic beta-cells. J. Biol. Chem. 278, 22956–22963. doi: 10.1074/jbc.m212339200
Yao, H., Ma, E., Gu, X. Q., and Haddad, G. G. (1999). Intracellular pH regulation of CA1 neurons in Na(+)/H(+) isoform 1 mutant mice. J. Clin. Invest. 104, 637–645. doi: 10.1172/jci6785
Yen, T. H., and Wright, N. A. (2006). The gastrointestinal tract stem cell niche. Stem Cell Rev. 2, 203–212. doi: 10.1007/s12015-006-0048-1
Yokoyama, H., Yasutake, M., and Avkiran, M. (1998). Alpha1-adrenergic stimulation of sarcolemmal Na+-H+ exchanger activity in rat ventricular myocytes: evidence for selective mediation by the alpha1A-adrenoceptor subtype. Circ. Res. 82, 1078–1085. doi: 10.1161/01.res.82.10.1078
Yuan, Z., Cai, T., Tian, J., Ivanov, A. V., Giovannucci, D. R., and Xie, Z. (2005). Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol. Biol. Cell 16, 4034–4045. doi: 10.1091/mbc.e05-04-0295
Zhang, J., Ren, C., Chen, L., Navedo, M. F., Antos, L. K., Kinsey, S. P., et al. (2010). Knockout of Na+/Ca2 + exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2 + channel current and lowers blood pressure. Am. J. Physiol. Heart Circ. Physiol. 298, H1472–H1483.
Zhao, Y., Gao, P., Sun, F., Li, Q., Chen, J., Yu, H., et al. (2016). Sodium intake regulates glucose homeostasis through the PPARδ/adiponectin-mediated SGLT2 pathway. Cell Metab. 23, 699–711. doi: 10.1016/j.cmet.2016.02.019
Zhu, M. H., Sung, T. S., Kurahashi, M., O’kane, L. E., O’driscoll, K., Koh, S. D., et al. (2016). Na+-K+-Cl- cotransporter (NKCC) maintains the chloride gradient to sustain pacemaker activity in interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G1037–G1046.
Keywords: Na+/K+ pump, Na/Glucose transporter, Na+/H+ exchanger, Na+/Ca2+ exchanger, Na+ channels, Na-K-2Cl cotransporter, Na-Cl cotransporter, cell growth
Citation: Gagnon KB and Delpire E (2021) Sodium Transporters in Human Health and Disease. Front. Physiol. 11:588664. doi: 10.3389/fphys.2020.588664
Received: 29 July 2020; Accepted: 06 October 2020;
Published: 25 February 2021.
Edited by:
Walter Sandtner, Medical University of Vienna, AustriaReviewed by:
Baruch Kanner, Hebrew University of Jerusalem, IsraelCopyright © 2021 Gagnon and Delpire. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric Delpire, ZXJpYy5kZWxwaXJlQHZhbmRlcmJpbHQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.