
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 17 December 2020
Sec. Clinical and Translational Physiology
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.568812
This article is part of the Research TopicKidney and Distant Organ Crosstalk in Health and DiseaseView all 16 articles
 Ying Zhang1
Ying Zhang1 Xiao-Han Ding2
Xiao-Han Ding2 Fang Pang1
Fang Pang1 Laiping Zhang3,4
Laiping Zhang3,4 Yiqin Wang1
Yiqin Wang1 Weili Wang1
Weili Wang1 Rongsheng Rao5
Rongsheng Rao5 Shi-Zhu Bian3,4*
Shi-Zhu Bian3,4*Background and Aim: Tricuspid regurgitation (TR) is a frequent complication in various cardiovascular diseases. However, few studies have reported the prevalence of TR especially the moderate to severe or significant TR (ms-TR) maintenance dialysis patients. Thus, we aimed to identify the prevalence of ms-TR and its associated factors.
Methods: A total of 491 maintenance dialysis patients underwent echocardiographic examinations, while a subgroup (n = 283) also received routine blood tests, renal function examinations, and electrolyte analysis. We first compared the differences in abovementioned parameters among groups with various TR areas (TRAs). Finally, univariate and adjusted regression were also used to identify factors that were independently associated with ms-TR.
Results: The incidence of TR jets was 62.6%, which included a mildly increased TRA (47.8%), moderately increased TRA (10.4%), and severely increased TRA (3.5%). Most of the cardiac structures and functional parameters, such as the end-diastolic internal diameters of the left atrium (LA), left ventricle (LVDD), right atrium (RA), right ventricle (RV), left ventricular ejection fraction (LVEF), and fractional shortening (FS), were significantly associated with ms-TR. Among serum ions, only total CO2 (TCO2; r = −0.141, p = 0.047) was negatively correlated with TRA. After adjusted, only Na+ [odds ratio (OR): 0.871 0.888, p = 0.048], RA (OR: 1.370, p < 0.001), and FS (OR: 0.887, p < 0.001) were independently associated with ms-TR.
Conclusion: Tricuspid regurgitation occurs in maintenance hemodialysis patients with ESRD. Na+ FS and RA were independently associated with ms-TR, and these parameters may be potential risk factors/predictors for ms-TR.
End-stage renal disease (ESRD) has been demonstrated to be accompanied by various cardiovascular complications, which usually have poor outcomes (Kim and Parekh, 2015; Ramesh et al., 2016; Samanta et al., 2019). It has been reported that cardiovascular events, including heart failure, are the leading causes of death in ESRD patients (Kooman and van der Sande, 2019; Sugiura et al., 2019; Yogasundaram et al., 2019). The progressively deteriorating structure and function of the heart may be the consequence of renal failure (Cutshall et al., 2018; Unlu et al., 2018). On other hand, the insufficient perfusion of the kidney from the heart may aggravate renal dysfunction. Previous studies mainly focused on structural and functional cardiac changes in the left heart in ESRD patients and identified several biochemical markers, clinical factors, and hemodynamic predictors (Mahfouz et al., 2013; Minami et al., 2016; Lemor et al., 2018; Khan et al., 2019; Yogasundaram et al., 2019).
However, the structure and function of the right heart in ESRD patients, especially those who underwent dialysis treatment, have not been extensively studied. Tricuspid regurgitation (TR), which includes functional or mild TR, moderate TR and significant severe TR, is one of the most important functions of the right heart and is highly prevalent in various populations (Agricola et al., 2017; Al-Hijji et al., 2017; Sun and O’Gara, 2017). However, the prevalence and associated factors have not been identified in ESRD patients who received maintenance dialysis. TR may contribute to renal dysfunction in heart failure patients (Maeder et al., 2008).
Many previous studies have been studied the roles of tricuspid regurgitation in right heart failure in hemodialysis patients (Gumus and Saricaoglu, 2020). Part of the associated factors have also been found. However, interaction and relationship between TR especially ms-TR or significant TR and renal dysfunction attached less attentions. Furthermore, the prevalence of ms-TR in the special subgroup populations of dialysis patients has not been studied deeply (Cakici et al., 2020). Thus, we performed this retrospective study to identify the prevalence of ms-TR and potentially associated factors in a large sample size of a special population: ESRD patients who underwent maintenance dialysis.
From March 2011 to June 2019, ESRD patients who received maintenance dialysis (n = 491) at the Nephrology of Chongqing and Kidney Center of PLA, Xinqiao Hospital, and Army Medical University (Third Military Medical University) were included in this retrospective study. These hemodialysis patients underwent echocardiographic examinations, while a subgroup (n = 283) of this population also received routine blood tests, renal function examinations, and electrolyte analysis, which are shown in Figure 1.
All of the ESRD patients were informed about the purpose and procedures of our study and provided written informed consent. Our present study complied with the Declaration of Helsinki.
In addition, the ethics committee of the Second Affiliated Clinic Hospital (Xinqiao Hospital) and Army Medical University (Third Military Medical University) approved the study.
The echocardiographic examinations of each patient were carried out by a skilled echocardiographer, Dr. Rongsheng Rao, according to the American Society of Echocardiography recommendations (Mansour et al., 2010). Echocardiographic examinations were performed in a supine position after resting for 10 min after hemodialysis. Two-dimensional echocardiographically guided motion mode (M-mode) images were obtained from standardized views by using an ultrasonography system (CX50, Philips, United States) with an S5 probe.
Among the echocardiographic parameters, our trained physicians recorded the left atrial end-diastolic internal diameter (LA), left ventricular end-diastolic internal diameter (LVDD), right atrial end-diastolic internal diameter (RA), right ventricular end-diastolic internal diameter (RV), and pulmonary arterial end-diastolic internal diameter (PA).
Additionally, the mobility and size of the left ventricular posterior wall (LVPW), the mobility and the size of the interventricular septum (IVS), fractional shortening (FS) and left ventricular ejection fraction (LVEF) and stroke volume (SV) were also recorded. Finally, TR-related parameters, such as TR area (TRA), TR velocity (TRV), and TR pressure, were also measured and recorded.
After admission to the hospital, we collected venous blood samples early in the morning after patients had fasted for more than 12 h before dialysis. The blood samples have also been obtained after dialysis in order to assess the effect of dialysis.
First, renal function, such as eGFR, plasma creatinine (Cr) and the blood urea nitrogen (BUN) concentration, was examined. Cr was also tested with Roche Diagnostics GmbH products (enzyme assay, Abbott, i2000, United States). BUN was also measured with ferene methods (Beckman AU5821, GA, United States).
Then, we performed routine blood examinations. An automated hematology chemistry analyzer was used to examine the hemoglobin concentration (Hb), red blood cell (RBC) count (RBC), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red blood cell distribution width (RDW) (type: AU400; Olympus Optical, Co., Tokyo, Japan). Furthermore, we also examined the white blood cell (WBC) and platelet (PLT) counts. The lymphocyte ratio (LYM), basophil ratio (BAS), neutrophil ratio (NEU), eosinophil ratio (EO), and monocyte ratio (MONO) were also tested and recorded.
Finally, we also performed an electrolyte examination, which included the sodium concentration (Na+, indirect ion selective electrode assay with EX-Z, JOKOH, Japan), serum calcium concentration (Ca2+, tri-azo methods), and phosphate concentration (P, phosphomolybdate ultraviolet assay, Roche Diagnostics GmbH, United States). In addition, we also measured the serum potassium concentration (K+, ferene methods, Beckman AU5821).
First, patients were divided into the non-TR group (without a TR jet), the mildly increased TRA group (0 < TRA < 5 cm2) and the moderately to severely increased TRA group (ms-TR, TRA ≥ 5 cm2) which is also called significant TR (ms-TR in this study).
We calculated the estimated glomerular filtration rate (eGFR) by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula: eGFR (ml/min/1.73 m2) = 1.41 × min(Cr/k,1)α × max(Cr/,1)–1.209 × 0.993age × 1.018 [k = 0.7 (female) and 0.9 (male); α = −0.329 (female) and −0.411 (male)].
If a continuous variable was normally distributed, it is presented as the mean ± standard deviation (SD). We employed one-way ANOVA to compare differences among the non-TR, mildly increased TRA and moderately to severely increased TRA groups. Further analysis comparing two groups was performed using least significant difference (LSD) methods after one-way ANOVA.
If one variable was non-normally distributed, it is presented as the median (25–75%). Comparisons among the non-TR, mildly increased TRA and moderately to severely increased TRA groups of variables with non-normal distributions were performed by one-way ANOVA after being transformed to achieve normality.
We performed univariate logistic regression analyses with each variable to screen for factors associated with moderate to severe TR. Variables with a p value less than 0.1 in the univariate logistic regression were included in multivariate (adjusted) logistic regression analyses to identify factors that were independently associated with moderate to severe TR. Our statistical analyses were performed by using the statistical software SPSS 22.0 (CA, United States) for Mac.
In our study, 491 ESRD patients were included. The mean age was 52.95 ± 14.21 years, and the mean body-mass index (BMI) was 21.89 ± 4.89 kg/m2. The incidence of a TR jet was 62.6%, which included a mildly increased TRA (47.8%), a moderately increased TRA (10.4%), and a severely increased TRA (3.5%). Thus, the incidence of ms-TR was 13.9%. These results are shown in Figure 2.
First, we did not find any differences in the demographic data (age and BMI, p > 0.05) among the non-TR, mildly increased TRA and ms-TR groups.
Second, in the echocardiographic parameters, both left heart and right heart structures and functions showed significant differences among these three groups. LA (43.93 ± 6.58 vs. 36.74 ± 4.75) and LVDD (54.11 ± 7.18 vs. 48.80 ± 5.25) were significantly higher in the ms-TR group than in the non-TR group (all p values < 0.001). These parameters were also significantly higher in the ms-TR group than in the mildly increased TRA group (all p values < 0.001). However, there were no differences in LA and LVDD between the mildly increased TRA and non-TR groups (all p values > 0.05). Though there were no differences in IVS and LVPW thickness among the three subpopulations, and their mobility was significantly different among the various TRA groups. ms-TR patients had worse IVS mobility [3.00 (6.00–6.00)] and a more dispersive LVPW mobility [10.00 (7.00–10.00)] than the other two groups (all p values < 0.001, Table 1). FS was also significantly lower in the ms-TR population [29.00 (18.75–33.25)] than in the non-TR [35.00 (32.00–37.00), p < 0.001] and mildly increased TRA [34.00 (32.00–37.00), p < 0.001] populations. The left heart functions, including LVEF, were significantly different among the three groups. The ms-TR group was characterized by a lower LVEF (50.28 ± 13.93%) than the other two subgroups (62.55 ± 8.01 and 62.28 ± 7.78, p < 0.001). However, SV was similar in the three groups (all p values > 0.05). In addition, PA was also significantly higher in the ms-TR group than in the other groups (25.14 ± 2.58 vs. 23.65 ± 2.44 and 23.68 ± 2.49 mm, p = 0.001, Table 1).
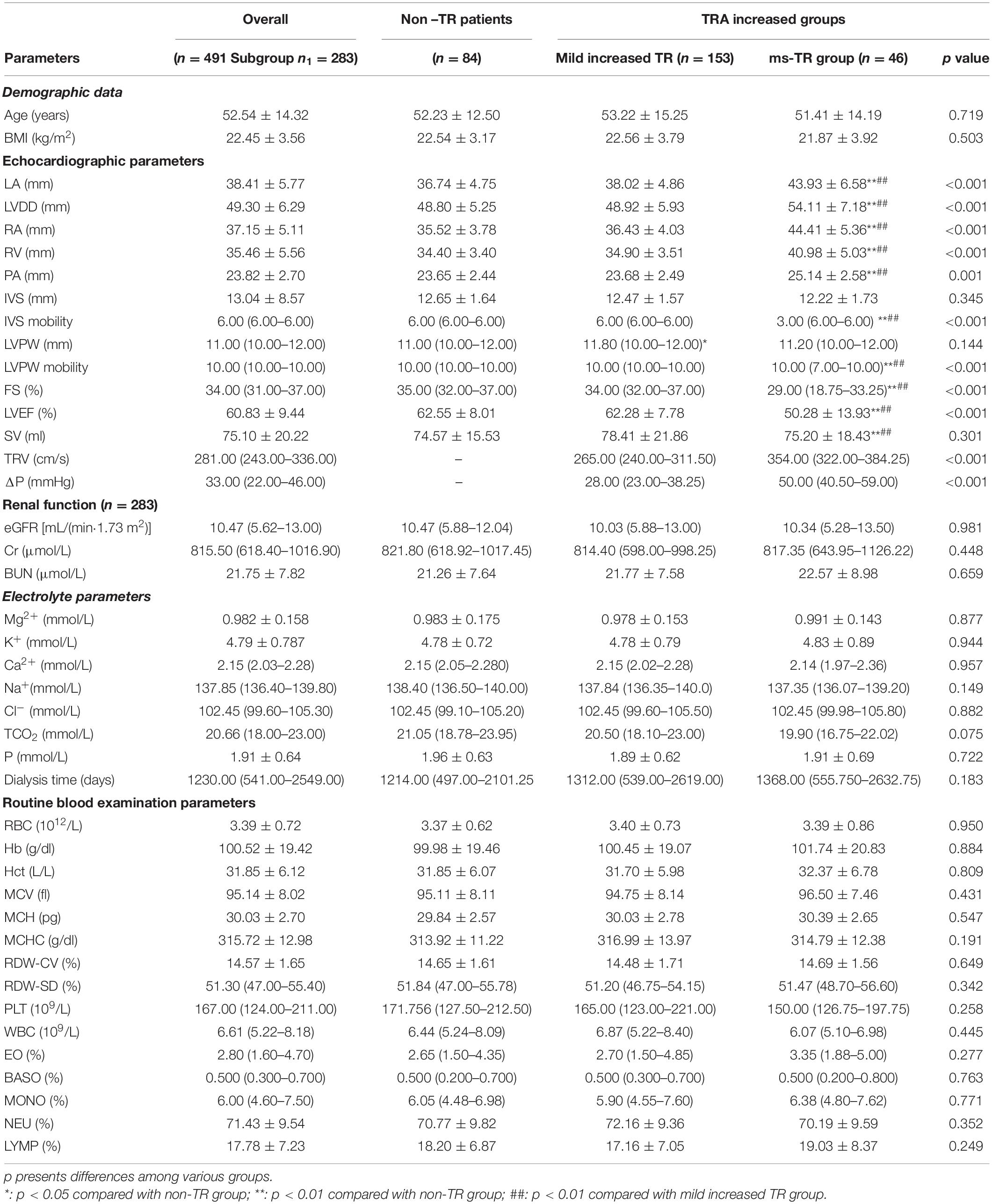
Table 1. Differences between of the hemodynamics, demographic data, renal function, electrolyte and routine blood examination parameters among various TRA groups.
In regard to renal function, none of the parameters (eGFR, Cr, and BUN) were significantly different among the three groups of patients (all p values were greater than 0.05, Table 1).
We further compared the differences in serum ions [Mg2+, K+, Ca2+, Na+, Cl–, total CO2 (TCO2), and P]. However, none of these ions were significantly different among the non-TR, mildly increased TRA and ms-TR groups (Table 1).
Finally, we also investigated routine blood examinations. However, we did not find any differences among the three groups (Table 1).
To identify associations between TRA and other parameters, we performed Spearman’s correlation analysis. We found that LA (r = 0.510, p < 0.001), LVDD (r = 0.389, p < 0.001), RA (r = 0.615, p < 0.001), RV (r = 0.586, p < 0.001), and PA (r = 0.322, p < 0.001) were closely positively related to TRA. Meanwhile, the IVS mobility (r = −0.338, p < 0.001), LVPW mobility (r = −0.344, p < 0.001), LVEF (r = −0.401, p < 0.001), and FS (r = −0.403, p < 0.001) as well as TCO2 (r = −0.141, p = 0.047) were negatively correlated with TRA. We did not find other associations between TRA and the rest of the parameters (Table 2).
In univariate regression analysis, demographic data (age and BMI) were not associated with ms-TR (all p values > 0.05) (Table 3).
Among the echocardiographic parameters, we identified several factors that were potentially associated with ms-TR. First, the diameter of the left and right heart as well as the PA, LA [odds ratio (OR): 1.281, 95% confidence interval (CI): 1.166–1.408, p < 0.001], LVDD (OR: 1.149, 95% CI: 1.076–1.227, p < 0.001), RA (OR: 1.437, 95% CI: 1.281–1.612, p < 0.001), RV (OR: 1.410, 95% CI: 1.251–1.589, p < 0.001), and PA (OR: 1.262, 95% CI: 1.083–1.471, p = 0.003) were associated with ms-TR (Table 3).
Furthermore, it was also found that IVS mobility (OR: 0.440, 95% CI: 0.295–0.655, p < 0.001), LVPW mobility (OR: 0.588, 95% CI: 0.449–0.770, p < 0.001), FS (OR: 0.853, 95% CI: 0.801–0.909, p < 0.001), and LVEF (OR: 0.905, 95% CI: 0.869–0.943, p < 0.001) may also have been associated with ms-TR (Table 3).
However, none of the renal function parameters showed significant associations with ms-TR (Cr, BUN, and eGFR, Table 3).
Among the serum ions, we found that only Na+ (OR: 0.864, 95% CI: 0.749–0.996, p = 0.044) and TCO2 (OR: 0.890, 95% CI: 0.809–0.980, p = 0.017) were potentially related to ms-TR (Table 3).
Finally, in the routine blood examination, RBC- and WBC-related parameters showed no potential associations with ms-TR. In addition, the other parameters, such as the ratios of monocytes, lymphocytes, eosinophils, and basophilic granulocytes, were not associated with ms-TR (Table 3).
To identify factors that were independently associated with ms-TR, we performed an adjusted (multivariate) logistic regression. We found that only Na+ (OR: 0.871 95% CI: 0.760–0.999, p = 0.048), RA (OR: 1.370, 95% CI: 1.234–1.520, p < 0.001), and FS (OR: 0.887, 95% CI: 0.822–0.957, p < 0.001) were independently associated with ms-TR (Table 4).
In our present study, we found that TR was prevalent (62.6%) in 491 ESRD patients who received maintenance dialysis treatment. Though TCO2 and other renal functions were potentially associated with TR, adjusted regression showed that only Na+, FS, and RA were independently associated with ms-TR; thus, further cohort studies are warranted to confirm their causality.
As discussed above, though TR is common in normal populations or populations with other cardiovascular diseases (Arsalan et al., 2017), TR was more prevalent and had a relatively higher incidence in a special subgroup, maintenance dialysis patients with ESRD (Walsh et al., 1998; Maeder et al., 2008; Al-Hijji et al., 2017; Del Forno et al., 2018; Dietz et al., 2019). Furthermore, we also found that ms-TR showed a higher incidence than other degrees of TRA. In particular, a few individuals had a TRA that was larger than 15 cm2; these patients needed further examination or treatment by the cardiovascular disease department. In the past, few studies had investigated the roles of TR, as well as the function and structure of the left heart, in renal function in heart failure patients (Minami et al., 2016; Cutshall et al., 2018; Konigsbrugge and Ay, 2019). However, no papers have reported the prevalence of TR in a large population of maintenance dialysis patients with ESRD. Our study was the first to describe the prevalence and TRA degree in patients who received dialysis at our center. Our data revealed that a TR jet occurred in maintenance dialysis patients with ESRD. Some patients had a TR jet with an extremely large area and may have needed cardiac surgery interventions. Thus, echocardiographic examinations are a highly valuable non-invasive method to evaluate the cardiovascular complications of ESRD, providing guidelines for the treatment of patients.
It has been indicated that kidney dysfunction may contribute to cardiovascular complications (Yogasundaram et al., 2019). We investigated renal function in ESRD patients, but the eGFR, Cr and BUN were not significantly different among the various TRA groups and were not associated with TRA. Though few previous studies have indicated that renal dysfunction affects cardiac structure and function (Minami et al., 2016; Cutshall et al., 2018; Lemor et al., 2018), we did not find a potential association of eGFR, BUN and Cr with ms-TR. Thus, these parameters were not included in the final adjusted logistic regression. Though our present study did not find an association of renal functions with ms-TR, the causal relationship between renal function and TR jets may need further cohort studies or basic studies to be confirmed.
Ions are known to play numerous key roles in signal transduction, protein synthesis, cell proliferation and differentiation. In ESRD patients, serum ion disturbances are the most common clinical manifestations. Thus, we also investigated the associations between serum ions (Mg2+, Ca2+, K+, Cl–, TCO2, Na+, and P) and TR jets. First, there were no significant differences in the abovementioned ions in the various TRA groups. Then, we found that TCO2 was closely negatively correlated with TRA. In the past several decades, researchers have found that TCO2 levels changed in uremia, indicating that renal dysfunction may alter TCO2 levels, resulting in changes in respiration and oxygen usage. If this situation is persistent, it may cause hypoxia-induced cardiac injury or changes in cardiac structure and function. Through further univariate regression analysis, we found that TCO2 and Na+ were potentially associated with an ms-TR jet. However, in the adjusted regression analysis, these factors were excluded by other variables and did not show independent associations with ms-TR.
Anemia, which is the consequence of the insufficient production of EPO and a higher demand for EPO, is another critical complication of ESRD and leads to insufficient Hb and RBC production (Santoro and Canova, 2005; van der Meer et al., 2008; Yokoro et al., 2017). It proposed that the anemia may contribute to TR jet. Thus, we investigated the association between RBC-related parameters and WBC-related parameters. However, we did not find any associations between RBC/WBC-related parameters and TR. This result may have been caused by the effect of anemia on cardiovascular diseases; this may involve a long-term process that requires cohort studies to identify their associations.
Our present study found that Na+ FS and RA were independently associated with ms-TR. The structure and function of the left heart showed an association with ms-TR, which may have been caused by the interactions between the left and right heart. Furthermore, changes in the right heart may result in relative or absolute tricuspid insufficiency, which may aggravate changes in structure and function (enlargement and right heart failure) (Al-Hijji et al., 2017; Sun and O’Gara, 2017; Hahn, 2019).
We have identified that Na+ was independently associated with TR which was in concordant with the previous studies that Na+ was correlated to the cardiovascular diseases in ERSD dialysis patients. This may be caused by the roles of Na+ in the vascular systems. In according to the previous studies, cardiovascular diseases are the common complications in ESRD patients especially in the maintenance dialysis subset populations (Cakici et al., 2020; Gumus and Saricaoglu, 2020). The precise mechanisms of Na+ in TR have not been widely investigated.
Our present results were consistent with previous studies on various cardiovascular diseases that are induced by a TR jet (Lee et al., 2010; Al-Hijji et al., 2017; Kopic et al., 2017; Mangieri et al., 2017). However, the roles of FS in TR jets have not been reported before. We found that FS was significantly associated with ms-TR, which was a novel finding that revealed that left heart functions may affect the right heart (due to preloading or other hemodynamic changes), which is partly in according with the previous studies (Badano et al., 2019; Dietz et al., 2019; Prihadi et al., 2019). However, the precise cause and mechanisms were not confirmed; thus, further cohort studies and research to uncover the basic mechanisms are warranted.
This was a retrospective study focusing on the prevalence of TR and its associated factors. However, there are several limitations that need to be improved in our future studies. First, our present study was a retrospective study that identified only the factors associated with ms-TR. The causality between clinical factors and ms-TR needs to be analyzed by further cohort studies. Another limitation is that we investigated only a few of the frequently used clinical parameters; however, there may be other valuable factors that should be examined. Finally, the parameters after dialysis were not included in this study, but these factors should be included in future research.
Tricuspid regurgitation is also prevalent in maintenance hemodialysis patients with ESRD. Na+, FS, and RA were independently associated with ms-TR; these may be potential risk factors/predictors for ms-TR and warrant further cohort studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Second Affiliated Clinic Hospital (Xinqiao Hospital) and Army Medical University (Third Military Medical University). The patients/participants provided their written informed consent to participate in this study.
S-ZB and YZ designed the study, drafted the manuscript, and performed the statistical analysis. S-ZB and X-HD critically reviewed and revised the manuscript for important intellectual content. RR performed the echocardiographic examinations and LZ recorded the measurements of TR-related parameters. YZ and X-HD collected the demographic data and performed routine blood examinations. YW, FP, and LZ performed the laboratory measurements. YW and WW obtained the dialysis-related data. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (grant no. 81901916), the Excellent Scientist Pool Program of Army Medical University (“Miaopu Program 2019R046”), and The Major Clinical Program of Xinqiao Hospital (2018JSLC0024).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all the individuals who participated in this study for their support. Finally, S-ZB thank his wife Meng Zhou for her help and care during the preparation of this manuscript.
BAS, basophil ratio; BUN, blood urea nitrogen concentration; Ca2+, serum calcium concentration; Cr, plasma creatinine; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; EO, eosinophil ratio; FS, fractional shortening; Hb, hemoglobin concentration; HCT, hematocrit; IVS, interventricular septum; K+, serum potassium concentration; LA, left atrial end-diastolic internal diameter; LVDD, left ventricular end-diastolic internal diameter; LVEF, left ventricular ejection fraction; LVPW, left ventricular posterior wall; LYM, lymphocyte ratio; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MONO, monocyte ratio; Na+, sodium concentration; NEU, neutrophil ratio; ms-TR, moderate to severe TRA; P, phosphate concentration; PA, pulmonary arterial end-diastolic internal diameter; PLT, platelet; RA, right atrial end-diastolic internal diameter; RBC, red blood cell count; RDW, red blood cell distribution width; RV, right ventricular end-diastolic internal diameter; SV, stroke volume; TR, tricuspid regurgitation; TRA, TR area; TRV, TR velocity; WBC, white blood cell count; Δ P, mitral transmitral pressure gradient.
Agricola, E., Marini, C., Stella, S., Monello, A., Fisicaro, A., Tufaro, V., et al. (2017). Effects of functional tricuspid regurgitation on renal function and long-term prognosis in patients with heart failure. J. Cardiovasc. Med. 18, 60–68. doi: 10.2459/jcm.0000000000000312
Al-Hijji, M., Fender, E. A., El Sabbagh, A., and Holmes, D. R. (2017). Current treatment strategies for tricuspid regurgitation. Curr. Cardiol. Rep. 19:106.
Arsalan, M., Walther, T., Smith, R. L. II, and Grayburn, P. A. (2017). Tricuspid regurgitation diagnosis and treatment. Eur. Heart J. 38, 634–638.
Badano, L. P., Hahn, R., Rodriguez-Zanella, H., Araiza Garaygordobil, D., Ochoa-Jimenez, R. C., and Muraru, D. (2019). Morphological assessment of the tricuspid apparatus and grading regurgitation severity in patients with functional tricuspid regurgitation: thinking outside the box. JACC Cardiovasc. Imaging 12, 652–664. doi: 10.1016/j.jcmg.2018.09.029
Cakici, E. K., Cakici, M., Gumus, F., Tan Kurklu, T. S., Yazilitas, F., Orun, U. A., et al. (2020). Effects of hemodialysis access type on right heart geometry in adolescents. J. Vasc. Access. 21, 658–664. doi: 10.1177/1129729819897454
Cutshall, B. T., Duhart, B. T. Jr., Saikumar, J., Samarin, M., Hutchison, L., and Hudson, J. Q. (2018). Assessing guideline-directed medication therapy for heart failure in end-stage renal disease. Am. J. Med. Sci. 355, 247–251. doi: 10.1016/j.amjms.2017.11.008
Del Forno, B., Lapenna, E., Dalrymple-Hay, M., Taramasso, M., Castiglioni, A., Alfieri, O., et al. (2018). Recent advances in managing tricuspid regurgitation. F1000Res 7:355. doi: 10.12688/f1000research.13328.1
Dietz, M. F., Prihadi, E. A., van der Bijl, P., Goedemans, L., Mertens, B. J. A., Gursoy, E., et al. (2019). Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation 140, 836–845. doi: 10.1161/circulationaha.119.039630
Gumus, F., and Saricaoglu, M. C. (2020). Assessment of right heart functions in the patients with arteriovenous fistula for hemodialysis access: right ventricular free wall strain and tricuspid regurgitation jet velocity as the predictors of right heart failure. Vascular 28, 96–103. doi: 10.1177/1708538119866616
Hahn, R. T. (2019). Assessment and procedural guidance with echocardiography for transcatheter tricuspid regurgitation devices. Prog. Cardiovasc. Dis. 62, 452–458. doi: 10.1016/j.pcad.2019.09.003
Khan, R. A., Khan, N. A., Bauer, S. R., Li, M., Duggal, A., Wang, X., et al. (2019). Association between volume of fluid resuscitation and intubation in high-risk patients with sepsis, heart failure, end-stage renal disease, and cirrhosis. Chest 3692:7.
Kim, E. D., and Parekh, R. S. (2015). Calcium and sudden cardiac death in end-stage renal disease. Semin. Dial. 28, 624–635. doi: 10.1111/sdi.12419
Konigsbrugge, O., and Ay, C. (2019). Atrial fibrillation in patients with end-stage renal disease on hemodialysis: magnitude of the problem and new approach to oral anticoagulation. Res. Pract. Thromb. Haemost. 3, 578–588. doi: 10.1002/rth2.12250
Kooman, J. P., and van der Sande, F. M. (2019). Body fluids in end-stage renal disease: statics and dynamics. Blood Purif. 47, 223–229. doi: 10.1159/000494583
Kopic, S., Stephensen, S. S., Heiberg, E., Arheden, H., Bonhoeffer, P., Ersboll, M., et al. (2017). Isolated pulmonary regurgitation causes decreased right ventricular longitudinal function and compensatory increased septal pumping in a porcine model. Acta Physiol. 221, 163–173. doi: 10.1111/apha.12904
Lee, J. W., Song, J. M., Park, J. P., Lee, J. W., Kang, D. H., and Song, J. K. (2010). Long-term prognosis of isolated significant tricuspid regurgitation. Circ. J. 74, 375–380. doi: 10.1253/circj.cj-09-0679
Lemor, A., Hernandez, G. A., Lee, S., Patel, N., Blumer, V., Badiye, A., et al. (2018). Impact of end stage renal disease on in-hospital outcomes of patients with systolic and diastolic heart failure (insights from the Nationwide Inpatient Sample 2010 to 2014). Int. J. Cardiol. 266, 174–179. doi: 10.1016/j.ijcard.2018.02.117
Maeder, M. T., Holst, D. P., and Kaye, D. M. (2008). Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J. Card. Fail. 14, 824–830. doi: 10.1016/j.cardfail.2008.07.236
Mahfouz, R. A., Elawady, W., Hossein, E., and Yosri, A. (2013). Impact of atrioventricular compliance on clinical outcome of patients undergoing successful percutaneous balloon mitral valvuloplasty. Echocardiography 30, 1187–1193. doi: 10.1111/echo.12256
Mangieri, A., Montalto, C., Pagnesi, M., Jabbour, R. J., Rodes-Cabau, J., Moat, N., et al. (2017). Mechanism and implications of the tricuspid regurgitation: from the pathophysiology to the current and future therapeutic options. Circ. Cardiovasc. Interv. 10:12.
Mansour, I. N., Lang, R. M., Aburuwaida, W. M., Bhave, N. M., and Ward, R. P. (2010). Evaluation of the clinical application of the ACCF/ASE appropriateness criteria for stress echocardiography. J. Am. Soc. Echocardiogr. 23, 1199–1204. doi: 10.1016/j.echo.2010.07.008
Minami, Y., Kajimoto, K., Sato, N., Hagiwara, N., Takano, T., and Attend study investigators. (2016). End-stage renal disease patients on chronic maintenance hemodialysis in a hospitalized acute heart failure cohort: prevalence, clinical characteristics, therapeutic options, and mortality. Int. J. Cardiol. 224, 267–270. doi: 10.1016/j.ijcard.2016.09.038
Prihadi, E. A., Delgado, V., Leon, M. B., Enriquez-Sarano, M., Topilsky, Y., and Bax, J. J. (2019). Morphologic types of tricuspid regurgitation: characteristics and prognostic implications. JACC Cardiovasc. Imaging 12, 491–499. doi: 10.1016/j.jcmg.2018.09.027
Ramesh, S., Zalucky, A., Hemmelgarn, B. R., Roberts, D. J., Ahmed, S. B., Wilton, S. B., et al. (2016). Incidence of sudden cardiac death in adults with end-stage renal disease: a systematic review and meta-analysis. BMC Nephrol. 17:78. doi: 10.1186/s12882-016-0293-8
Samanta, R., Chan, C., and Chauhan, V. S. (2019). Arrhythmias and sudden cardiac death in end stage renal disease: epidemiology, risk factors, and management. Can. J. Cardiol. 35, 1228–1240. doi: 10.1016/j.cjca.2019.05.005
Santoro, A., and Canova, C. (2005). Anemia and erythropoietin treatment in chronic kidney diseases. Minerva Urol. Nefrol. 57, 23–31.
Sugiura, E., Dohi, K., Tanimura, M., Kumagai, N., Ishikawa, E., and Ito, M. (2019). Successful peritoneal dialysis for the treatment of inotrope-dependent end-stage heart failure. Int. Heart J. 60, 1211–1218. doi: 10.1536/ihj.18-550
Sun, Y. P., and O’Gara, P. T. (2017). Epidemiology, anatomy, pathophysiology and clinical evaluation of functional tricuspid regurgitation. Minerva Cardioangiol. 65, 469–479.
Unlu, S., Sahinarslan, A., Gokalp, G., Seckin, O., Arinsoy, S. T., Boyaci, N. B., et al. (2018). The impact of volume overload on right heart function in end-stage renal disease patients on hemodialysis. Echocardiography 35, 314–321. doi: 10.1111/echo.13768
van der Meer, P., Lipsic, E., van Gilst, W. H., and van Veldhuisen, D. J. (2008). Anemia and erythropoietin in heart failure. Heart Fail. Monit. 6, 28–33.
Walsh, J. T., Glennon, P., and Schofield, P. M. (1998). Tricuspid regurgitation following central line insertion in a patient undergoing haemodialysis. Postgrad. Med. J. 74, 631–632. doi: 10.1136/pgmj.74.876.631
Yogasundaram, H., Chappell, M. C., Braam, B., and Oudit, G. Y. (2019). Cardiorenal syndrome and heart failure-challenges and opportunities. Can. J. Cardiol. 35, 1208–1219. doi: 10.1016/j.cjca.2019.04.002
Keywords: prevalence, tricuspid regurgitation, maintenance dialysis, end-stage renal disease, retrospective study
Citation: Zhang Y, Ding X-H, Pang F, Zhang L, Wang Y, Wang W, Rao R and Bian S-Z (2020) The Prevalence and Independent Risk Factors of Significant Tricuspid Regurgitation Jets in Maintenance Hemodialysis Patients With ESRD. Front. Physiol. 11:568812. doi: 10.3389/fphys.2020.568812
Received: 02 June 2020; Accepted: 30 November 2020;
Published: 17 December 2020.
Edited by:
Jonatan Barrera-Chimal, National Autonomous University of Mexico, MexicoReviewed by:
Vladyslav Chubuchny, Gabriele Monasterio Tuscany Foundation (CNR), ItalyCopyright © 2020 Zhang, Ding, Pang, Zhang, Wang, Wang, Rao and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Zhu Bian, YmlhbnNoaXpodUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.