
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol., 06 October 2020
Sec. Cardiac Electrophysiology
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.01027
Objective: Fragmented QRS (fQRS) have been reported as a predictor of major adverse cardiac events (MACE) and mortality in several studies on cardiovascular disease. However, most studies have yielded discrepant results. This study aimed to explore the correlation between fQRS and cardiovascular events in patients with acute myocardial infarction (AMI) during their hospital stay and follow-up period, and the predictive value of fQRS in the prognosis of AMI.
Methods: We searched for relevant studies in four databases, Medline, Embase, PubMed, and the Cochrane Library from January 2010 to March 2020. Our initial search yielded 585 articles. Of these, we screened 19 studies, and finally included a total of 6,914 patients in this analysis, comparing death events or MACE in AMI patients with or without fQRS.
Results: Fragmented QRS was significantly associated with a higher risk of in-hospital mortality (OR, 3.97; 95% CI, 2.45–6.44; p < 0.00001), long-term mortality (OR, 2.93; 95% CI, 1.76–4.88; p < 0.0001), in-hospital MACE (OR, 2.48; 95% CI, 1.62–3.80; p < 0.0001), and long-term MACE (OR, 3.81; 95% CI, 2.21–6.57; p < 0.00001). In particular, it demonstrated a higher predictive value for in-hospital cardiovascular mortality and long-term all-cause mortality in AMI patients and in-hospital mortality in patients with ST-segment elevation myocardial infarction (STEMI). Moreover, fQRS was also associated with an increased risk of ventricular arrhythmias (OR, 2.76; 95% CI, 1.72–4.43; p < 0.0001) and heart failure (OR, 1.65; 95% CI, 1.02–2.66; p = 0.04). Fragmented QRS was negatively associated with left ventricular ejection function (LVEF) (MD, −5.47; CI, [−7.03, −3.91]; p < 0.00001) and positively associated with a high incidence of coronary artery triple vessel lesions (OR, 2.14; 95% CI, 1.31–3.51; p = 0.002) in AMI patients.
Conclusion: Fragmented QRS is significantly associated with in-hospital and long-term mortality and MACE in patients with AMI, as well as ventricular arrhythmias and heart failure. Furthermore, it may be a marker of mortality and MACE risk. Moreover, fQRS also indicates a reduced LVEF and a high incidence of coronary artery triple vessel lesions in AMI patients.
Meta-analysis Registration: https://www.crd.york.ac.uk/prospero; ID: CRD42020171668.
Acute myocardial infarction (AMI) is a significant cause of death in patients with coronary heart disease (CHD). Although reperfusion therapy is effective in improving the short- and long-term cardiac prognosis of patients with myocardial infarction(MI), risk stratification after a percutaneous coronary intervention (PCI) is still challenging (Wright et al., 2011; Mozaffarian et al., 2015). Moreover, the comorbidities of AMI are associated with an increased incidence of adverse events. For example, the co-existence of diabetes increases the risk of 1-year mortality and adverse cardiovascular events after infarction (Marfella et al., 2018a,b). Furthermore, myocardial function after infarction is a critical indicator for predicting future cardiovascular events. However, the imaging methods used to evaluate myocardial function are expensive and not easy to acquire (Eitel et al., 2014; Stone et al., 2016).
The purpose of established risk stratification tools is to identify individuals at high risk and require more intensive therapy (Vignoli et al., 2019). However, these tools have some limitations in the assessment of emergency admissions for AMI because more clinical and laboratory data are needed. Moreover, these tools do not incorporate the use of new electrocardiographic parameters that represent disorders in the electrical activity of the myocardial cells and provide accurate prognostic information, thereby promoting risk stratification and the evaluation of prognosis beyond traditionally used variables.
Several studies have evaluated the potential clinical usefulness of fragmented QRS (fQRS) to identify patients with poor outcomes. Das et al. found a correlation between fQRS and the presence of a myocardial scar. They reported that fQRS had a higher diagnostic sensitivity and negative predictive value for old MI than a pathological Q wave (Das et al., 2006). During the past decade, extensive clinical studies have demonstrated that fQRS has a predictive value for mortality and major adverse cardiac events (MACE) in patients with AMI. However, the predictive value of fQRS for in-hospital MACE and long-term mortality is still controversial. This systematic review and meta-analysis provide a framework for assessing the value of fQRS in predicting in-hospital and long-term cardiovascular events in AMI patients.
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020171668).
Two researchers independently searched the Embase, Cochrane-Library, Medline, and PubMed databases to retrieve published studies from January 2010 to March 2020, using a search strategy that included the terms “fragmented QRS,” “acute myocardial infarction,” “STEMI,” “NSTEMI,” “MACE,” “cardiovascular events,” and “mortality.”
The inclusion criteria were as follows: (a) cohort studies (prospective or retrospective), cross-sectional studies, or randomized control trials; (b) studies that enrolled patients with recent AMI; (c) studies allocating patients to an fQRS group (experimental group) and a non-fQRS group (control group); (d) the first electrocardiogram recorded on admission or within 48 h of admission; (e) studies reporting in-hospital or long-term mortality/MACE.
The exclusion criteria were as follows: (a) studies including populations with other diseases, such as hypertrophic cardiomyopathy, congenital heart disease, Brugada syndrome, Chagas' disease atrial fibrillation, and renal failure; (b) studies not published in English; (c) studies including patients with unstable angina; (d) publications without full texts or with an unknown date.
The following information was obtained from each study: title, first author, year of publication, country, the demographic data of participants, the method used to identify cases and controls, the prevalence of fQRS, the technique used to diagnose AMI, and main conclusions. The prespecified outcomes were mortality and MACE in-hospital or during follow-up.
fQRS was defined as the presence of a new R wave (R0) or notching in the nadir of the S wave, or the presence of >1 R0 (fragmentation) in 2 contiguous leads, corresponding to a major coronary artery territory. AMI was defined as acute STEMI and NSTEMI. Mortality was defined by all-cause mortality or cardiovascular death. MACE were defined as cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke (Wise et al., 2019). Heart failure was defined as Killip ≥ 2 (Cenko et al., 2019), or definitions from each trial were used (Table 3).
The internal validity of studies was assessed using the Quality in Prognosis Studies (QUIPS) tool (Hayden et al., 2013). A Funnel plot and the Egger test were used to test for any potential publication bias.
Statistical analysis was performed using Review Manager 5.1, State 16, and Meta-Disc 1.4 software. We extracted the baseline data and end events of the experimental and control groups in each study. For the baseline data, the counting data used the chi-square test, and the continuity data used the t-test after summarizing the data: the difference was statistically significant with p < 0.05. Meta-analysis was performed according to different endpoint events. We also conducted a subgroup analysis to evaluate NSTEMI and STEMI, inclusion criteria, QRS duration, previous MI, and, more importantly, cardiovascular mortality and all-cause mortality, respectively.
The effect size was presented as the odds ratio (OR), likelihood ratio (LR) indicating how many times more (or less) likely a patient experiencing an endpoint is to express fQRS. Since heterogeneity in endpoints from study to study was anticipated, a random effect model was used for the primary analysis, with study as a random variable. Heterogeneity was assessed the I2 statistic test and its 95% confidence intervals (CI) and the random-effects model was used to account for significant statistical variation. The sensitivity analysis was performed by calculating the number of patients in the trial as a percentage of the total number analyzed, to value the weight of the overall results of the meta-analysis for each study. Meta-regression analysis was used to assess the potential impact of baseline characteristics on heterogeneity.
Following the above search strategy, initial studies were identified from four online databases. After 188 duplicates were removed, we screened the remaining 395 articles by reading the title and abstract. We excluded 347 articles because they did not meet the inclusion criteria. Out of the remaining 48 articles, 29 were removed after reading the full text. Finally, a total of 19 studies were included in this meta-analysis (Figure 1).
In general, the methodological quality of the included studies was good, without a high risk of bias. Four studies had different inclusion criteria for fQRS, and therefore the conclusions may be biased compared with other studies (Table 1). Three studies did not give a precise reason for the lack of follow-up, and consequently, the relationship between fQRS and the outcome may be different for completing and non-completing participants (Lorgis et al., 2012; Li et al., 2016; Dural et al., 2019). The funnel plot and Egger test did not suggest evidence of publication bias (Figure 2).
Four studies had different inclusion criteria (studies by Akgul, Yildirim, Kurtul, and Umapathy). Patients showing fQRS involving the infarct territory within 48 h of admission were included in the “fQRS group.” Patients who did not develop fQRS even after 48 h of admission were included in the “non-fQRS group.”
Of the remaining 15 studies, the first ECG upon admission showing fQRS was included in the “fQRS group” and the first ECG upon admission without fQRS was included in the “non-fQRS group.”
Table 2 shows an overview of studies investigating the role of fQRS in cardiovascular events. In terms of baseline data, the incidence of fQRS was about 40%. Most of the participants were male (76%) and received reperfusion interventional therapy (82%). Gender, history of previous MI, previous PCI, and smoking were statistically different between the fQRS and non-fQRS groups. However, hypertension and diabetes were not statistically different between the fQRS and non-fQRS groups while using statistical analysis (Table 3).
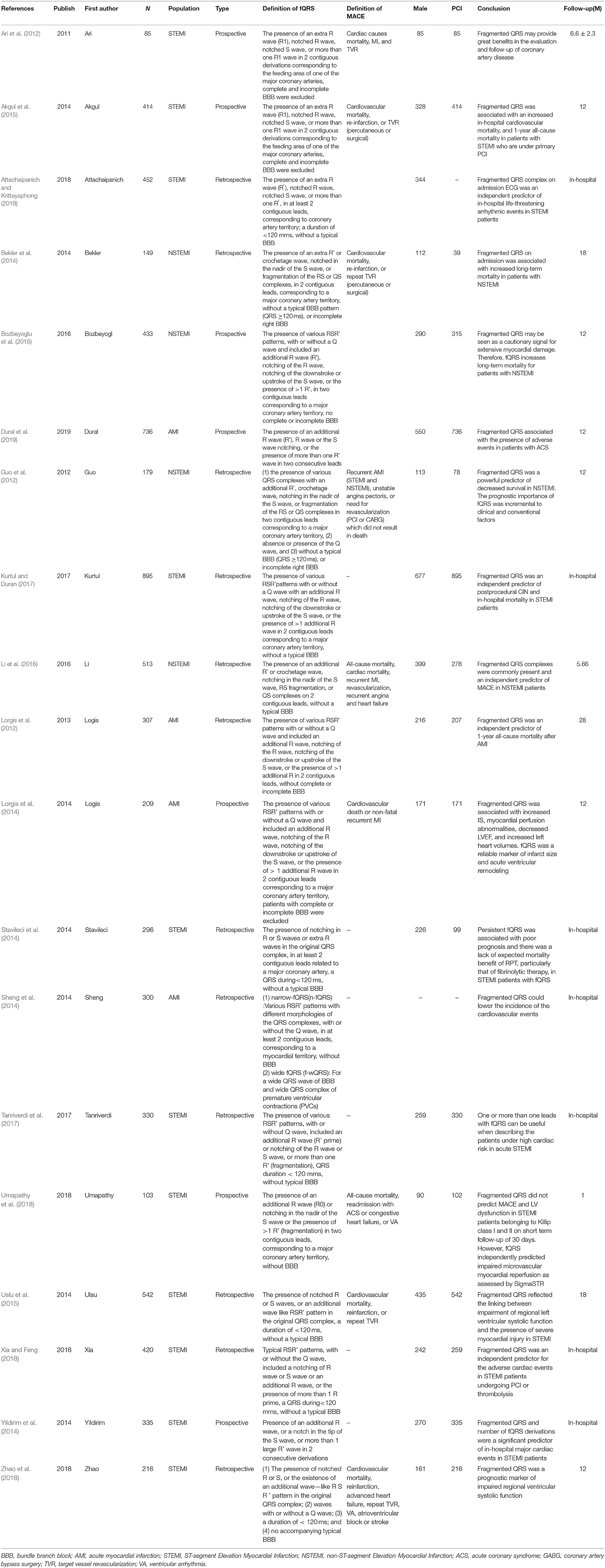
Table 2. Overview of studies investigating fragmented QRS complex in relation to Cardiovascular events in AMI.
All studies, except those conducted by Sheng et al., Li et al., and Logis et al. reported left ventricular ejection function (LVEF) in study groups. Of the 19 studies, 16 studies reported LVEF, and 13 reported LVEF as mean ± standard deviation. Thirteen studies demonstrated a significant negative correlation between LVEF in patients with fQRS and AMI (MD, −5.47; CI, [−7.03, −3.91]; p < 0.00001) with significant heterogeneity (I2 = 84%). Moreover, seven studies reported an obvious relationship between fQRS and triple vessel lesions (OR, 2.14; 95% CI, 1.31–3.51; p = 0.002) with significant heterogeneity (I2 = 79%).
A total of 4,633 patients from 11 studies were included in the analysis of in-hospital mortality (Figure 3). Fragmented QRS was significantly associated with a higher risk of in-hospital mortality (OR, 3.97; 95% CI, 2.45–6.44; p < 0.00001) with obvious heterogeneity (I2 = 58% p = 0.008). The summary positive LR was 1.94 (95% CI, 1.49–2.52) and the negative LR was 0.55(95% CI, 0.41–0.75). The positive and negative LRs revealed significant heterogeneity (I2 = 91.9%, p < 0.0001) (I2 = 67.2%, p = 0.0007) (Figure 4). The heterogeneity was verified by identifying three studies by Yildirim et al. (2014), Akgul et al. (2015), and Kurtul and Duran (2017), using meta-regression analysis. According to the fQRS criteria, we divided the patients into two subgroups: (1) the fQRS positive group (an arbitrary ECG group), if fQRS appeared in any ECG within 48 h; the OR was 23.32 (95% CI, 6.38–85.20); there was no heterogeneity in the subgroup (I2 = 0%, p = 0.8). (2) the fQRS positive group (specific time group), if fQRS was found in the ECG on admission or at the 48th h; the OR was 2.77 (95% CI. 2.06–3.72); there was no heterogeneity in the subgroup (I2 = 0%, p = 0.64). Meanwhile, the heterogeneity was verified by identifying two studies of Akgul et al. and Kurtul et al. using sensitivity analysis. After removing them in the analysis, the overall pooled estimate of OR (2.82, 95% CI, 2.11-3.78) was reduced but removed any significant heterogeneity (I2 = 0%, p = 0.56).
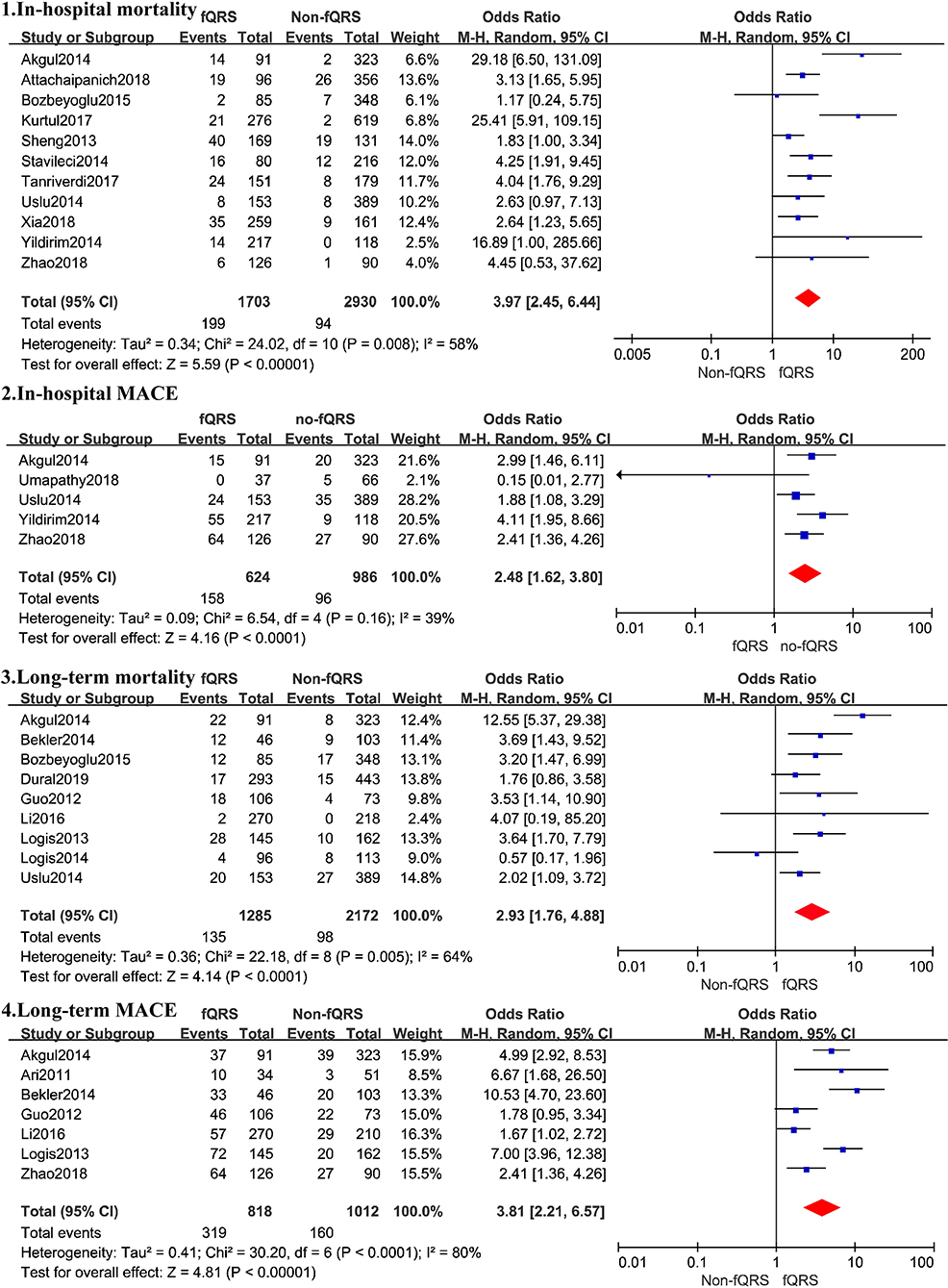
Figure 3. Summary of the odds risk of mortality and MACE in patients with fQRS. The size of the square for each study is proportional to the sample size. Weighting refers to the contribution of each study to the pooled result and was determined using the random effects model (the endpoints reported in the forest plot are the odds risk of in-hospital mortality, in-hospital MACE, long-term mortality, and long-term MACE, respectively).
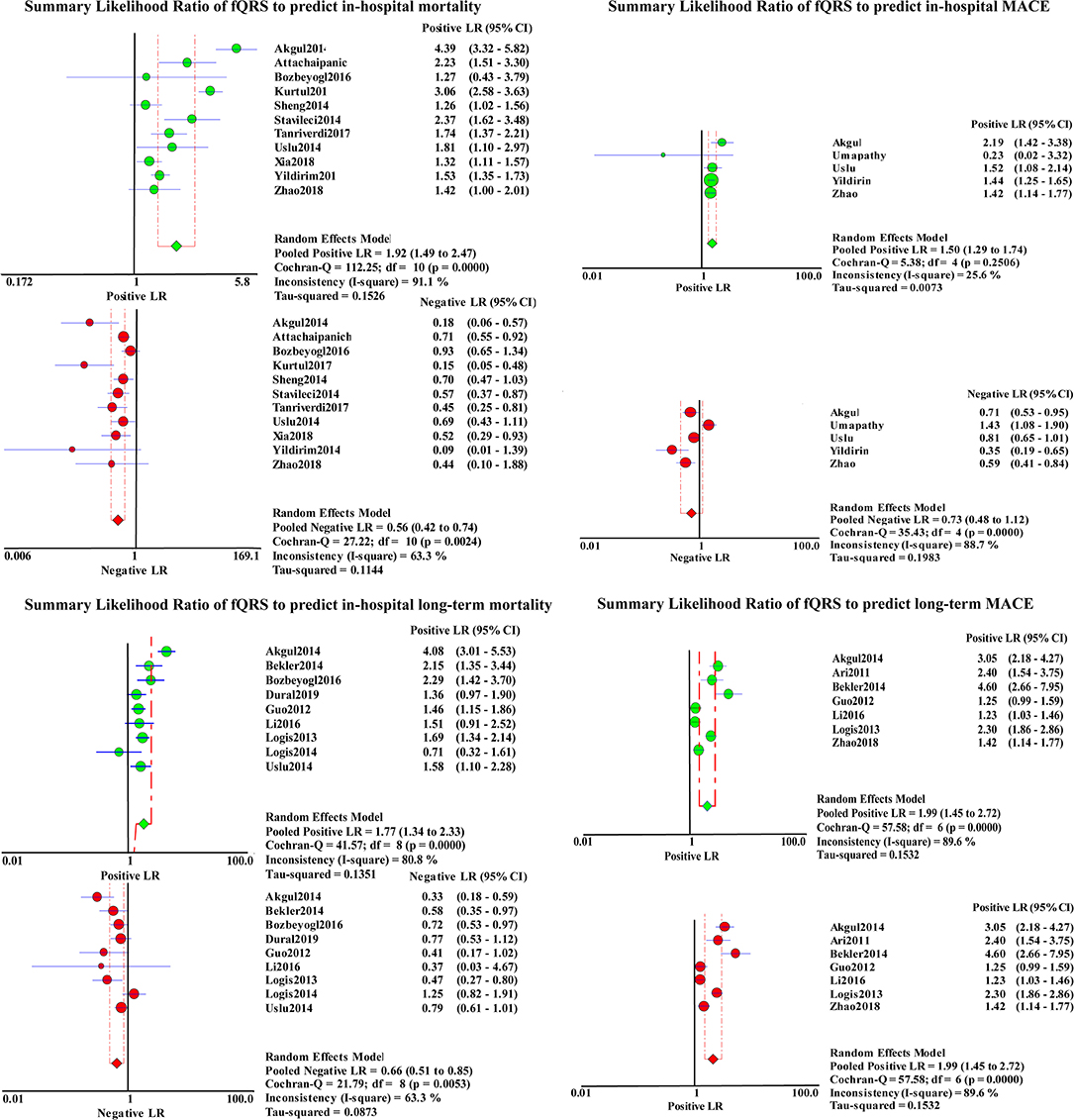
Figure 4. Summary LRs of fQRS to predict in-hospital mortality, in-hospital MACE, long-term mortality, and long-term MACE.
Six studies reported all-cause in-hospital mortality (OR, 3.77; 95% CI, 2.06–6.91; p < 0.00001), five reported in-hospital cardiovascular mortality (OR, 4.31; 95% CI, 2.45–6.44; p = 0.002), and nine merely calculated the mortality in STEMI patients (OR, 4.92; 95% CI, 2.93–8.28) (Table 4). However, there was significant heterogeneity between these studies before the sensitivity analysis identified three studies by Akgul et al., Kurtul et al., and Yildirim et al. as outliers (Yildirim et al., 2014; Akgul et al., 2015; Kurtul and Duran, 2017). After removing the studies by Kurtul and Duran (2017) and Yildirim et al. (2014) the overall pooled OR (2.32, 95% CI, 1.68–3.21) in the meta-analysis for all-cause in-hospital mortality was reduced, with less heterogeneity between studies (I2 = 13%, p = 0.25). After removing the study by Akgul et al. (2015) alone, the overall pooled OR (2.91, 95% CI 1.73–4.87) in the meta-analysis for in-hospital cardiovascular mortality also somewhat decreased, without any heterogeneity between studies (I2 = 0%, p = 0.60). The pooled OR (3.38 95% CI, 2.40–4.77) in the meta-analysis for the in-hospital mortality in STEMI patients decreased, without any heterogeneity between studies (I2 = 0%, p = 0.86) after removing Akgul et al. and Yildirim et al.'s studies (Yildirim et al., 2014; Akgul et al., 2015). Summary estimates in other subgroups are given in Table 4.
Nine studies representing 3,457 patients with fQRS were included in this meta-analysis for long-term mortality (Figure 3). Of 3,457 patients, 233 died during the mean/median follow up of 5.66–28 months. Mortality was more common in patients with fQRS than in controls (135/1285 vs. 98/2172) (OR, 2.93; 95% CI, 1.76–4.88; p < 0.0001), with significant heterogeneity between studies (I2 =66%; p < 0.0001). The pooled positive and negative LRs were 1.77 and 1.66, respectively, with significant heterogeneity in positive LR (I2 = 80.8%, p < 0.0001) and negative LR (I2 =63.3%, p = 0.0053) between studies (Figure 4).
Four studies reported all-cause long-term mortality (OR, 4.22; 95% CI, 1.68–11.60; p < 0.003) and eight reported long-term cardiovascular mortality (OR, 3.22; 95% CI, 1.79–5.80; p < 0.0001), respectively, both with significant heterogeneity between studies (I2 = 75%, p = 0.003, and I2 = 66%, p < 0.0001, respectively). The sensitivity analysis showed that the study by Akgul et al. (2015) was the outlier, which shared the same cause of heterogeneity in in-hospital mortality. The presence of fQRS was still found to be correlated with long-term mortality risk (OR, 2.40; 95% CI, 1.67–3.47; p < 0.00001), all-cause mortality risk (OR, 2.81; 95% CI, 1.40–5.65; p = 0.004), and cardiovascular mortality risk (OR, 2.58; 95% CI, 1.69–3.94; p < 0.0001) after ruling out this study from the analysis, with reduced heterogeneity (I2 = 25, 37%, and 22%; p = 0.23, 0.21, and 0.22, respectively) (Table 4).
The result was not statistically significant (OR, 4.91; 95% CI, 0.82–29.44; p = 0.0006; I2 = 91%) when only patients with STEMI were included (n = 956). The fQRS was still found to be correlated with a higher risk of long-term mortality (OR, 3.44; 95% CI, 2.04–5.80; p < 0.00001), without heterogeneity (I2 = 0%, p = 1.00) when only patients with NSTEMI were considered (n = 1,248). In addition, in the presence of fQRS, whether or not these several studies with obvious heterogeneity are removed. The OR of in-hospital mortality is always higher than that of long-term mortality. In terms of the prediction of in-hospital mortality, the predictive value for in-hospital cardiovascular mortality may be higher than that for all-cause mortality. In terms of long-term mortality, however, the predictive value for all-cause mortality may be higher than that for cardiovascular mortality. Summary estimates in other subgroups are given in Table 4.
A total of 1,610 patients with STEMI in 5 studies were included in the analysis for in-hospital MACE (Figure 3). The incidence of MACE was higher in the fQRS group (158/624 vs. 96/986)(OR, 2.48; 95% CI, 1.62–3.80; p < 0.0001) than in controls, without significant heterogeneity (I2 = 39%,). The summary positive LR was 1.50 (95% CI, 1.29–1.74) (Figure 4) without significant heterogeneity (I2 = 25.6%, p = 0.32). The negative LR was 0.73 (95% CI, 0.48–1.12) with significant heterogeneity (I2 = 86.8%, p < 0.0001) (Figure 4).
Outcomes regarding long-term MACE were available in 7 studies (Figure 3). During a mean/median follow up of 5.66–28 months, 479 out of 1,830 patients died. MACE was more common in patients with fQRS (319/818 vs. 160/1012) (OR, 3.81; 95% CI, 2.21–6.57). Significant heterogeneity existed across studies (I2 = 82%, p < 0.0001). The summary positive LR was 1.99 (95% CI, 1.45–2.72) (Figure 4), and the negative LR was 0.54 (95% CI, 0.44–0.68) with significant heterogeneity in the positive LR (I2 = 89.6%, p < 0.0001) and the negative LR(I2 = 59.2%, p = 0.023). Sensitivity analysis and meta-regression analysis failed to identify a single study to account for heterogeneity (Figure 4).
Five studies including 839 patients and 1,188 patients in the fQRS and the non-fQRS group, respectively, reported heart failure (Table 4). There was an association between fQRS and heart failure (OR, 1.65; 95% CI, 1.02–2.66; p = 0.04) with significant heterogeneity (I2 = 64%, p < 0.0001). A total of 6 studies reported VT/VF events (Table 4). Among 2,218 patients, 223 had VT/VF. There was a significant association between fQRS and VT/VF events (OR, 2.76; 95% CI, 1.72–4.43; p < 0.0001) with significant heterogeneity (I2= 57%, p = 0.04). Sensitivity analysis and Meta-regression failed to identify the reason for the heterogeneity of the two studies.
This meta-analysis conducted a subgroup analysis on the main endpoint events based on whether the QRS duration was included ≥120 mms and whether the patients included in the study had a previous history of MI or PCI. The pooled OR of in-hospital mortality, MACE, and long-term MACE were higher than those in the studies with a previous history of MI. In the subgroup analysis of QRS duration, it was found that the total value of hospital deaths was lower in the group with a QRS duration of included ≥120 mms compared to the group with a QRS duration of <120 mms. The clinical value of other subgroups may be low because there was no statistical significance or small study sample size (Table 4).
The main findings of this meta-analysis are: (1) the fQRS on admission ECG was strongly associated with mortality and MACE in AMI patients during the in-hospital stay and follow-up; (2) fQRS was positively correlated with ventricular arrhythmia (VA) and heart failure in in-hospital patients; (3) fQRS also indicated a decreased LVEF and a high incidence of coronary artery triple vessel lesions in AMI patients.
In this meta-analysis, we found that the presence of fQRS on admission ECG was associated with a nearly 4-fold risk of in-hospital mortality and long-term MACE, and an almost 3-fold risk of MACE and VT/VF in the hospital, and mortality during the follow-up period. Thus, this meta-analysis suggests that fQRS has a definite predictive value for in-hospital and long-term cardiovascular events. In the subgroup analysis, we found that for patients with no previous history of MI and PCI, the emergence of fQRS suggests that the risk of in-hospital death, in-hospital MACE events, and long-term MACE events may be higher, which has not been reported in previous studies. We speculate that the reason for these opposing findings may be that fQRS mainly develops after MI, so the possibility of hemodynamic and electrophysiological instability is higher. Additionally, meta-analyses of previous studies showed that for ischemic cardiomyopathy, the longer the QRS duration, the greater the risk of death. On the contrary, in our meta-analysis, we calculated a higher OR value for studies with a QRS duration of <120 mms (Rosengarten et al., 2015). Unfortunately, the specific reason behind these inconsistencies is not clear; although, it may be related to the inclusion criteria for fQRS.
The comorbidities of MI negatively affect prognosis, especially in patients with diabetes mellitus, which can increase the incidence of adverse events including MACE and death. State of diabetes could cause downregulation of stem endothelial cells implied in regenerative post-STEMI events, and loss of epigenetic regulators implied in intracoronary vessels thrombosis, and prediabetes increases the inflammatory burden in pericoronary adipose tissue (Marfella et al., 2013, 2018a,b; Sardu et al., 2019; D'onofrio et al., 2020). There are often concerns for patients with these complications. However, our meta-analysis did not show a statistical difference in the incidence of diabetes between the fQRS and non-fQRS groups, possibly due to the different mechanisms of cardiovascular events associated with fQRS and diabetes. Moreover, our study found a negative correlation between fQRS and LVEF in patients with MI, which could be the result of multiple vascular diseases and larger infarct area and might also be one of the reasons for poor prognosis (Gungor et al., 2016).
Previous studies identified fQRS as a significant predictor of in-hospital mortality in patients with AMI (Das et al., 2007, 2009; Gungor et al., 2016; Attachaipanich and Krittayaphong, 2019). Our meta-analysis suggests that fQRS increases in-hospital and long-term mortality in AMI patients. The underlying mechanism may be related to myocardial scar formation, but there is still a lack of relevant research evidence (Rosengarten et al., 2015). A negative correlation between fQRS and LVEF was demonstrated in our study and previous studies. Moreover, data from this study shows that fQRS increases the risk of triple vessel lesions, indicating patients with advanced coronary artery disease (Thuijs et al., 2019). Previous studies have reported that fQRS is related to the size of the infarct area after MI (Pietrasik et al., 2007; Das et al., 2009; Lorgis et al., 2012), which may be responsible for the risk of mortality and MACE in patients with AMI. The risk of cardiovascular mortality was higher than that of all-cause mortality at the time of hospitalization in our meta-analysis. Bozbeyoglu et al. have suggested that cardiovascular mortality associated with fQRS may be due to VA, sudden cardiac death (SCD), and deterioration of left ventricular systolic function secondary to myocardial scarring (Bozbeyoglu et al., 2016). Fragmented QRS is also a predictor of progression to heart failure after MI (Korhonen et al., 2010; Akgul et al., 2015). This meta-analysis correlates heart failure with fQRS, which may lead to an increase in cardiovascular mortality.
It is noteworthy that fQRS had a higher predictive value for hospital mortality than follow-up mortality. Another study showed that the mortality in the fQRS group mainly occurred in the first month after discharge during the follow-up period (Akgul et al., 2015). Therefore, monitoring cardiac rhythm for longer than 24–48 h may be required in patients with fQRS (Attachaipanich and Krittayaphong, 2019), and MI patients with fQRS need more attention during hospitalization and for 1 month after discharge. In particular, fQRS significantly increased in-hospital mortality in the group with arbitrary ECG, according to the subgroup analysis. These three studies concluded that fQRS significantly increased the risk of in-hospital mortality, a finding consistent with our observation that one should not ignore the warning signs of transient fQRS found within 48 h of admission. Furthermore, virtually all patients developed fQRS within 48 h after primary PCI, especially within 24 to 48 h (Akbarzadeh et al., 2013; Sheng et al., 2014; Zhang et al., 2017). Therefore, an assessment based on the presence of fQRS on admission ECG may underestimate the predictive value of fQRS for mortality.
This is the first meta-analysis on the relationship between fQRS and in-hospital MACE in patients with AMI and is consistent with the results of previous studies that established fQRS as a significant predictor for in-hospital MACE in AMI (Yildirim et al., 2014; Bozbeyoglu et al., 2016). Although it is not as high as the value for mortality, this is a significant conclusion that cannot be ignored. Of the five studies included, four studies found a significant correlation between fQRS and MACE events. Stavileci et al. (2014) reported that persistent fQRS on admission ECG was associated with poor prognosis in patients with STEMI. Lorgis et al. (2012) have reported that persistent fQRS and BNP were associated with decreased survival in univariate analysis. However, these were not significant independent predictors of MACE in multivariate Cox regression analysis. This study found that fQRS was not a predictor of heart failure or VA in univariate analysis. Fragmented QRS did not predict in-hospital MACE in STEMI patients in a study by Umapathy et al. (2018), that included STEMI patients presenting Killip I and II with a lower incidence of triple-vessel disease (1.7%) and fQRS resolving in 26% of the patients, indicating better myocardial revascularization (Umapathy et al., 2018). In contrast, other study cohorts included all patients with fQRS who had a higher incidence of triple-vessel disease, larger infarct size, and worse left ventricular function. However, it cannot be ignored that our study only analyzed in-hospital MACE in patients with STEMI, because we did not find any reports of in-hospital MACE in patients with NSTEMI. Therefore, it is unclear whether our findings represent AMI. In terms of long-term MACE prediction, our meta-analysis found that fQRS is closely related to long-term MACE, but that heterogeneity was high, and no obvious abnormality was found in a single study through sensitivity analysis.
Fragmented QRS was an independent predictor of VA in AMI. Attachaipanich et al. showed that the time from admission to the last life-threatening arrhythmia event was longer in the fQRS than in the non-fQRS group (Attachaipanich and Krittayaphong, 2019). This meta-analysis found that fQRS was significantly associated with VT events. The impulse conduction velocity delay, reentrant circuit, and increased risk of automaticity due to fibroblast infiltration form an arrhythmogenic substrate associated with an increased risk of cardiac arrhythmia that originates from the peri-infarct area (Attachaipanich and Krittayaphong, 2019). This could also increase the mortality and the risk of MACE.
Overall, we found that fQRS is significantly associated with mortality and MACE in patients with AMI and other adverse events such as heart failure and VA. However, current research is still unclear whether the presence of fQRS leads to a reduction in LVEF and further development of electrophysiological instability, thereby further increasing mortality and events. It has also not yet been established whether the instability of hemodynamics and electrophysiological activities, caused by severe myocardial vascular lesions and larger infarct area, can lead to fQRS and increased risk of MACE and mortality of MI. However, this does not affect the predictive value of fQRS for patient mortality and MACE. The causality is not yet clear, so a series of trials will be needed to prove the cause of MACE and death by excluding other factors such as demographic data, clinical, and laboratory tests on patients at the same level.
(1) All studies included were observational, and most of them were retrospective. None of the studies evaluated the use of fQRS in a randomized fashion. (2) Long-term cardiovascular follow-up from 5 to 26 months extended over a long time. (3) Fragmented QRS was defined differently among included studies, contributing to the significant heterogeneity found in our meta-analysis. (4) The data extracted were not adjusted for other variables, such as age, past medical history, and ventricular function. Further studies may evaluate whether fQRS can be used as an independent predictor of in-hospital and long-term cardiovascular events in the AMI population. (5) In the exclusion criteria, patients with previous MI and PCI could not be excluded and these patients significantly influenced prognosis and the formation of fQRS.
The presence of fragmented QRS is associated with increased risk of in-hospital mortality, long-term mortality, and MACE in patients with AMI, while transient fQRS cannot be ignored. Moreover, it is related to an increased risk of in-hospital heart failure and VT/VF events. In the presence of fQRS, the OR for in-hospital mortality is higher than that for long-term mortality. The OR for long-term MACE is higher than that for in-hospital MACE. In terms of the prediction of in-hospital mortality, the predictive value for in-hospital cardiovascular mortality may be higher than that for all-cause mortality. In terms of long-term mortality, however, the predictive value for all-cause mortality may be higher than that for cardiovascular mortality. The in-hospital mortality in the fQRS group was significantly higher than that in the non-fQRS group when only patients with STEMI were included. The long-term mortality in the fQRS group was significantly higher than that in the non-fQRS group when only patients with NSTEMI were included. These results indicate that fQRS has a predictive value for both in-hospital and long-term adverse events. Even though the evidence for RCT trails is limited, it is still a predictor of clinical significance.
All datasets generated for this study are included in the article.
GL and QL participated in the study design, searched databases, extracted, and assessed data. GL drafted the manuscript. YP and ZZ revised the manuscript. GL, QL, and JD extracted data. ZZ designed the study. All authors approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.01027/full#supplementary-material
Akbarzadeh, F., Pourafkari, L., Ghaffari, S., Hashemi, M., and Sadeghi-Bazargani, H. (2013). Predictive value of the fragmented QRS complex in 6-month mortality and morbidity following acute coronary syndrome. Int. J. Gen. Med. 6, 399–404. doi: 10.2147/IJGM.S40050
Akgul, O., Uyarel, H., Pusuroglu, H., Surgit, O., Turen, S., Erturk, M., et al. (2015). Predictive value of a fragmented QRS complex in patients undergoing primary angioplasty for ST elevation myocardial infarction. Ann. Noninvasive Electrocardiol. 20, 263–272. doi: 10.1111/anec.12179
Ari, H., Cetinkaya, S., Ari, S., Koca, V., and Bozat, T. (2012). The prognostic significance of a fragmented QRS complex after primary percutaneous coronary intervention. Heart Vessels 27, 20–28. doi: 10.1007/s00380-011-0121-9
Attachaipanich, T., and Krittayaphong, R. (2019). Fragmented QRS as a predictor of in-hospital life-threatening arrhythmic complications in ST-elevation myocardial infarction patients. Ann. Noninvasive Electrocardiol. 24:e12593. doi: 10.1111/anec.12593
Bekler, A., Gazi, E., Erbag, G., Peker, T., Barutcu, A., Altun, B., et al. (2014). Relationship between presence of fragmented qrs on admission and long term mortality in patients with non st-elevated myocardial infarction. Am. J. Cardiol. 113, S58–S59. doi: 10.1016/j.amjcard.2014.01.159
Bozbeyoglu, E., Yildirimtürk, Ö., Yazici, S., Ceylan, U. S., Erdem, A., Kaya, A., et al. (2016). Fragmented QRS on admission electrocardiography predicts long-term mortality in patients with non–ST-segment elevation myocardial infarction. Ann. Noninvasive Electrocardiol. 21, 352–357. doi: 10.1111/anec.12314
Cenko, E., Van Der Schaar, M., Yoon, J., Manfrini, O., Vasiljevic, Z., Vavlukis, M., et al. (2019). Sex-related differences in heart failure after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol 74, 2379–2389. doi: 10.1016/j.jacc.2019.08.1047
Das, M. K., Khan, B., Jacob, S., Kumar, A., and Mahenthiran, J. (2006). Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 113, 2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892
Das, M. K., Michael, M. A., Suradi, H., Peng, J., Sinha, A., Shen, C., et al. (2009). Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am. J. Cardiol. 104, 1631–1637. doi: 10.1016/j.amjcard.2009.07.046
Das, M. K., Saha, C., El Masry, H., Peng, J., Dandamudi, G., Mahenthiran, J., et al. (2007). Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm 4, 1385–1392. doi: 10.1016/j.hrthm.2007.06.024
D'onofrio, N., Sardu, C., Paolisso, P., Minicucci, F., Gragnano, F., Ferraraccio, F., et al. (2020). MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J. Cell. Physiol. 235, 1438–1452. doi: 10.1002/jcp.29064
Dural, M., Sunman, H., Algül, E., Hocamguliyev, H., Sahan, H. F., Çimen, T., et al. (2019). Relationship between serum bilirubin levels and presence of fragmented QRS in patients with acute coronary syndrome. Biomark. Med. 14, 65–73. doi: 10.2217/bmm-2018-0493
Eitel, I., De Waha, S., Wohrle, J., Fuernau, G., Lurz, P., Pauschinger, M., et al. (2014). Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 64, 1217–1226. doi: 10.1016/j.jacc.2014.06.1194
Gungor, B., Ozcan, K. S., Karatas, M. B., Sahin, I., Ozturk, R., and Bolca, O. (2016). Prognostic value of QRS fragmentation in patients with acute myocardial infarction: a meta-analysis. Ann. Noninvasive Electrocardiol. 21, 604–612. doi: 10.1111/anec.12357
Guo, R., Zhang, J., Li, Y., Xu, Y., Tang, K., and Li, W. (2012). Prognostic significance of fragmented QRS in patients with non-ST elevation myocardial infarction: results of a 1-year, single-center follow-up. Herz 37, 789–795. doi: 10.1007/s00059-012-3603-3
Hayden, J. A., Van Der Windt, D. A., Cartwright, J. L., Cote, P., and Bombardier, C. (2013). Assessing bias in studies of prognostic factors. Ann. Intern. Med. 158, 280–286. doi: 10.7326/0003-4819-158-4-201302190-00009
Korhonen, P., Husa, T., Konttila, T., Tierala, I., Mäkijärvi, M., Väänänen, H., et al. (2010). Fragmented QRS in prediction of cardiac deaths and heart failure hospitalizations after myocardial infarction. Ann. Noninvasive Electrocardiol. 15, 130–137. doi: 10.1111/j.1542-474X.2010.00353.x
Kurtul, A., and Duran, M. (2017). Fragmented QRS complex predicts contrast-induced nephropathy and in-hospital mortality after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Clin. Cardiol. 40, 235–242. doi: 10.1002/clc.22651
Li, M., Wang, X., Mi, S. H., Chi, Z., Chen, Q., Zhao, X., et al. (2016). Short-term prognosis of fragmented QRS complex in patients with non-ST elevated acute myocardial infarction. Chin. Med. J. 129, 518–522. doi: 10.4103/0366-6999.176989
Lorgis, L., Cochet, A., Chevallier, O., Angue, M., Gudjoncik, A., Lalande, A., et al. (2014). Relationship between fragmented QRS and no-reflow, infarct size, and peri-infarct zone assessed using cardiac magnetic resonance in patients with myocardial infarction. Can. J. Cardiol. 30, 204–210. doi: 10.1016/j.cjca.2013.11.026
Lorgis, L., Jourda, F., Richard, C., Zeller, M., Gudjoncik, A., Buffet, P., et al. (2012). Prognostic value of persistent vs. transient fragmented QRS on a 12-lead ECG in patients with acute myocardial infarction. Arch. Cardiovasc. Dis. Suppl. 4:7. doi: 10.1016/S1878-6480(12)70416-0
Marfella, R., Rizzo, M. R., Siniscalchi, M., Paolisso, P., Barbieri, M., Sardu, C., et al. (2013). Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: effects on myocardial salvage. Int. J. Cardiol. 168, 3954–3962. doi: 10.1016/j.ijcard.2013.06.053
Marfella, R., Sardu, C., Balestrieri, M. L., Siniscalchi, M., Minicucci, F., Signoriello, G., et al. (2018a). Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol. Metab. Syndr. 10:1. doi: 10.1186/s13098-017-0304-3
Marfella, R., Sardu, C., Calabrò, P., Siniscalchi, M., Minicucci, F., Signoriello, G., et al. (2018b). Non-ST-elevation myocardial infarction outcomes in patients with type 2 diabetes with non-obstructive coronary artery stenosis: effects of incretin treatment. Diabetes Obes. Metab. 20, 723–729. doi: 10.1111/dom.13122
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2015). Heart disease and stroke statistics−2015 update: a report from the American Heart Association. Circulation 131, e29–e322. doi: 10.1161/CIR.0000000000000152
Pietrasik, G., Goldenberg, I., Zdzienicka, J., Moss, A. J., and Zareba, W. (2007). Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q-wave myocardial infarction. Am. J. Cardiol. 100, 583–586. doi: 10.1016/j.amjcard.2007.03.063
Rosengarten, J. A., Scott, P. A., and Morgan, J. M. (2015). Fragmented QRS for the prediction of sudden cardiac death: a meta-analysis. Europace 17, 969–977. doi: 10.1093/europace/euu279
Sardu, C., D'onofrio, N., Torella, M., Portoghese, M., Loreni, F., Mureddu, S., et al. (2019). Pericoronary fat inflammation and Major Adverse Cardiac Events (MACE) in prediabetic patients with acute myocardial infarction: effects of metformin. Cardiovasc. Diabetol. 18:126. doi: 10.1186/s12933-019-0931-0
Sheng, Q. H., Hsu, C. C., Li, J. P., Hong, T., and Huo, Y. (2014). Correlation between fragmented QRS and the short-term prognosis of patients with acute myocardial infarction. J. Zhejiang Univ. Sci. B 15, 67–74. doi: 10.1631/jzus.B1300091
Stavileci, B., Cimci, M., Ikitimur, B., Barman, H. A., Ozcan, S., Ataoglu, E., et al. (2014). Significance and usefulness of narrow fragmented QRS complex on 12-lead electrocardiogram in acute ST-segment elevation myocardial infarction for prediction of early mortality and morbidity. Ann. Noninvasive Electrocardiol. 19, 338–344. doi: 10.1111/anec.12133
Stone, G. W., Selker, H. P., Thiele, H., Patel, M. R., Udelson, J. E., Ohman, E. M., et al. (2016). Relationship between infarct size and outcomes following primary pci: patient-level analysis from 10 randomized trials. J. Am. Coll. Cardiol. 67, 1674–1683. doi: 10.1016/j.jacc.2016.01.069
Tanriverdi, Z., Dursun, H., Colluoglu, T., and Kaya, D. (2017). Single derivation fragmented QRS can predict poor prognosis in successfully revascularized acute STEMI patients. Arq. Bras. Cardiol. 109, 213–221. doi: 10.5935/abc.20170099
Thuijs, D., Kappetein, A. P., Serruys, P. W., Mohr, F. W., Morice, M. C., Mack, M. J., et al. (2019). Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 394, 1325–1334. doi: 10.1016/S0140-6736(19)31997-X
Umapathy, S., Yadav, R., Goswami, K. C., Karthikeyan, G., Parakh, N., and Bahl, V. K. (2018). Prognostic significance of fragmented QRS in patients with ST-elevation myocardial infarction undergoing revascularization. Indian Heart J. 70(Suppl. 3), S126–S132. doi: 10.1016/j.ihj.2018.07.014
Uslu, N., Gul, M., Cakmak, H. A., Atam, A., Pusuroglu, H., Satilmisoglu, H., et al. (2015). The assessment of relationship between fragmented QRS complex and left ventricular wall motion score index in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Ann. Noninvasive Electrocardiol. 20, 148–157. doi: 10.1111/anec.12180
Vignoli, A., Tenori, L., Giusti, B., Takis, P. G., Valente, S., Carrabba, N., et al. (2019). NMR-based metabolomics identifies patients at high risk of death within two years after acute myocardial infarction in the AMI-Florence II cohort. BMC Med. 17:3. doi: 10.1186/s12916-018-1240-2
Wise, R. A., Chapman, K. R., Scirica, B. M., Bhatt, D. L., Daoud, S. Z., Zetterstrand, S., et al. (2019). Effect of aclidinium bromide on major cardiovascular events and exacerbations in high-risk patients with chronic obstructive pulmonary disease: the ASCENT-COPD randomized clinical trial. JAMA 321, 1693–1701. doi: 10.1001/jama.2019.4973
Wright, R. S., Anderson, J. L., Adams, C. D., Bridges, C. R., Casey, D. E. Jr, Ettinger, S. M., et al. (2011). 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/Non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 57, e215–e367. doi: 10.1016/j.jacc.2011.02.011
Xia, W., and Feng, X. Y. (2018). Fragmented QRS (fQRS) complex predicts adverse cardiac events of ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention and thrombolysis. Med. Sci. Monit. 24, 4634–4640. doi: 10.12659/MSM.908712
Yildirim, E., Karaçimen, D., Özcan, K. S., Osmonov, D., Türkkan, C., Altay, S., et al. (2014). The relationship between fragmentation on electrocardiography and in-hospital prognosis of patients with acute myocardial infarction. Med. Sci. Monit. 20, 913–919. doi: 10.12659/MSM.890201
Zhang, R., Chen, S., Zhao, Q., Sun, M., Yu, B., and Hou, J. (2017). Fragmented QRS complex is a prognostic marker of microvascular reperfusion and changes in LV function occur in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Exp. Ther. Med. 13, 3231–3238. doi: 10.3892/etm.2017.4380
Keywords: cardiovascular events, acute myocardial infarction, fragmented QRS, electrocardiogram, mortality
Citation: Luo G, Li Q, Duan J, Peng Y and Zhang Z (2020) The Predictive Value of Fragmented QRS for Cardiovascular Events in Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. Front. Physiol. 11:1027. doi: 10.3389/fphys.2020.01027
Received: 13 May 2020; Accepted: 27 July 2020;
Published: 06 October 2020.
Edited by:
Ruben Coronel, University of Amsterdam, NetherlandsReviewed by:
Tong Liu, Tianjin Medical University, ChinaCopyright © 2020 Luo, Li, Duan, Peng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Zhang, emhhbmdjY3VAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.