
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 25 June 2020
Sec. Developmental Physiology
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.00639
This article is part of the Research TopicThe Role of the Fetal Membranes in Pregnancy and BirthView all 17 articles
 Wenxu Qi1
Wenxu Qi1 Peinan Zhao1
Peinan Zhao1 Wei Wang2
Wei Wang2 Zhexian Sun1,3
Zhexian Sun1,3 Xiao Ma1,3
Xiao Ma1,3 Hui Wang1,4
Hui Wang1,4 Wenjie Wu1,3
Wenjie Wu1,3 Zichao Wen1
Zichao Wen1 Zulfia Kisrieva-Ware1
Zulfia Kisrieva-Ware1 Pamela K. Woodard2
Pamela K. Woodard2 Qing Wang2
Qing Wang2 Robert C. McKinstry2
Robert C. McKinstry2 Yong Wang1,2,3,4*
Yong Wang1,2,3,4*In approximately 8% of term births and 33% of pre-term births, the fetal membrane (FM) ruptures before delivery. In vitro studies of FMs after delivery have suggested the series of events leading to rupture, but no in vivo studies have confirmed this model. In this study, we used a three-dimensional constructive interference in steady state (3D-CISS) sequence to examine the FM at the cervical internal os zone during pregnancy; 18 pregnant women with one to three longitudinal MRI scans were included in this study. In 14 women, the FM appeared normal and completely intact. In four women, we noted several FM abnormalities including cervical funneling, chorioamniotic separation, and chorion rupture. Our data support the in vitro model that the FM ruptures according to a sequence starting with the stretch of chorion and amnion, then the separation of amnion from chorion, next the rupture of chorion, and finally the rupture of amnion ruptures. These findings hold great promise to help to develop an in vivo magnetic resonance imaging marker that improves examination of the FMs.
During pregnancy, the fetus is surrounded by amniotic fluid contained within a fetal membrane (FM). FM is composed of the amnion, which faces the fetus, and the chorion, which contacts the maternal decidua. In a healthy pregnancy, the FM is critical for maintaining a pregnancy until delivery (Parry and Strauss, 1998; Menon and Richardson, 2017). However, in about 8% of pregnancies, the FM ruptures before labor, which is called premature rupture of membranes (PROM). FM rupture before 37 weeks of gestation, termed preterm prelabor rupture of membranes (PPROM), is responsible for approximately one-third of preterm births and is the most common identifiable factor associated with preterm birth (Mathews and MacDorman, 2010; Waters and Mercer, 2011; Martin et al., 2012). Currently, there is no easy way to predict PPROM in early pregnancy, and thus the prevention is very limited.
To solve this problem, we first need to understand the mechanisms of FM rupture. Several investigators have attempted to do so by performing in vitro mechanical test on FM after delivery (Artal et al., 1976; Lavery and Miller, 1979; Helmig et al., 1993; Oyen et al., 2004). For example, data from Arikat et al. and Strohol et al. suggest that FM rupture follows this sequence: (1) Amnion and chorion stretch together under load; (2) amnion separates from chorion; (3) chorion ruptures; (4) amnion distends further, non-elastically; and (5) amnion ruptures (Arikat et al., 2006; Strohl et al., 2010). Ultrasound, an imaging modality widely used clinically to monitor pregnancy in vivo, can detect some signs associated with PROM and PPROM, such as FM thickness (Frigo et al., 1998; Severi et al., 2008; Başaran et al., 2014; Nunes et al., 2016) and chorioamniotic separation (Devlieger et al., 2003). The FM region that appears to be most prone to rupture is near the internal cervical os (McLaren et al., 1999). However, this para-cervical weak zone is often difficult to visualize by transvaginal ultrasound because of the low contrast between the FM and the maternal decidua (Severi et al., 2008). Strong in vivo evidence is still absent in the literature.
Here, we proposed to visualize the FM near the internal cervical os using magnetic resonance (MR) images acquired with a sequence named three-dimensional constructive interference in steady state (3D-CISS). This sequence provides both high spatial resolution and excellent contrast between the cerebrospinal fluid (high signal from water) and tissue structures (lower signal). And thus it is commonly used in clinical procedures to evaluate fine structures, such as cranial nerves surrounded by cerebrospinal fluid (Yoshino et al., 2003; Yousry et al., 2005). In MR images, the difference of signal intensity between amniotic fluid (high signal) and the FM (low/intermediate signal) is similar to the difference of signal intensity between cerebrospinal fluid and nerves, and the FM has similar thickness as nerves. Therefore, the 3D-CISS sequence is able to visualize the FM near the internal cervical os. In our study, we performed 3D-CISS MR imaging on 18 women at one to three time points between 20 and 36 weeks of gestation. And we report the result of four women who had evidence of abnormal FM structure. Our data suggest that the in vivo FM rupture sequence matches what proposed from in vitro studies (Arikat et al., 2006; Strohl et al., 2010).
This study was approved by the Washington University in St. Louis Institutional Review Board (protocols 201612140, 201707152). Participants were recruited by research nurses from the patient population attending the Obstetrics and Gynecology Clinic and the Women’s Health Center in the Barnes-Jewish Hospital Center for Outpatient Health. Participants were included if they were 18 years of age or older and had a healthy singleton pregnancy. Participants were excluded if they had a twin pregnancy or a contraindication to MRI. Before imaging, all patients were screened for MRI safety and provided written informed consent. Age, body mass index, and other clinical information were recorded for all participants. Pregnancy outcomes were collected from the medical records. Term birth was defined as birth between 37 0/7 weeks of gestation and 42 0/7 weeks of gestation (Goldenberg et al., 2008). Preterm birth was defined as birth between 20 0/7 weeks of gestation and 36 6/7 weeks of gestation (Goldenberg et al., 2008). PROM was defined as rupture of membranes before labor. PPROM was defined as rupture of membranes followed by labor before 37 weeks of gestation (Simhan and Canavan, 2005; Goldenberg et al., 2008).
Every patient underwent MRI examination one, two, or three times between 20 and 36 weeks of gestation. A Siemens Magnetom Vida 3T whole body MRI scanner and a 30-channel phased-array torso coil (Erlangen, Germany) were used to acquire a series of sagittal view T2 weighted images (T2WI), with a half-Fourier acquisition single-shot turbo spin echo sequence and the following parameters: repetition time, 1800 ms; echo time, 94 ms; matrix, 320 × 650; flip angle, 140°; layer thickness, 4.0 mm; slice spacing, 0.8 mm; number of layers, 25. For the 3D-CISS sequence, parameters were as follows: repetition time, 7.71 ms; echo time, 3.70 ms; flip angle, 50°; acquisition number, 1; acquisition matrix, 640 × 640; field of view, 300 mm × 300 mm; bandwidth, 460 Hz per pixel; slice thickness, 1 mm; and in-plane resolution, 0.33 mm × 0.33 mm. The total acquisition time for both T2WI and 3D-CISS was 7 min.
Magnetic resonance images were independently analyzed by two radiologists (WQ and WW, with 10-year and 1-year of experience, respectively, in analyzing abdominal MR images) who were blinded to pregnancy outcomes. A consensus was reached in cases of discordance. The following imaging characteristics were evaluated: cervical funneling, chorioamniotic separation, and chorion or amnion rupture.
Between April 2019 and February 2020, 18 pregnant women were recruited for this study. Their mean age was 33.5 ± 12.1 years, and their mean body mass index at first prenatal visit was 23.8 ± 5.3 kg/m2. Demographic and clinical details of the 18 women included in this study are presented in Table 1. A total of 43 MRI scans were performed on these 18 patients.
Fourteen patients had normal-appearing FM in which the amnion, chorion, and decidua were intact and indistinguishable from one another at all imaging time points. For example, in the patient images shown in Figures 1A–C, the FM was completely intact at 20, 32, and 36 weeks’ gestation, though we noted some suspended FM material in the cervical canal at all three time points. None of the 14 patients with normal, intact FM had PPROM or PROM, and all 14 delivered at term.
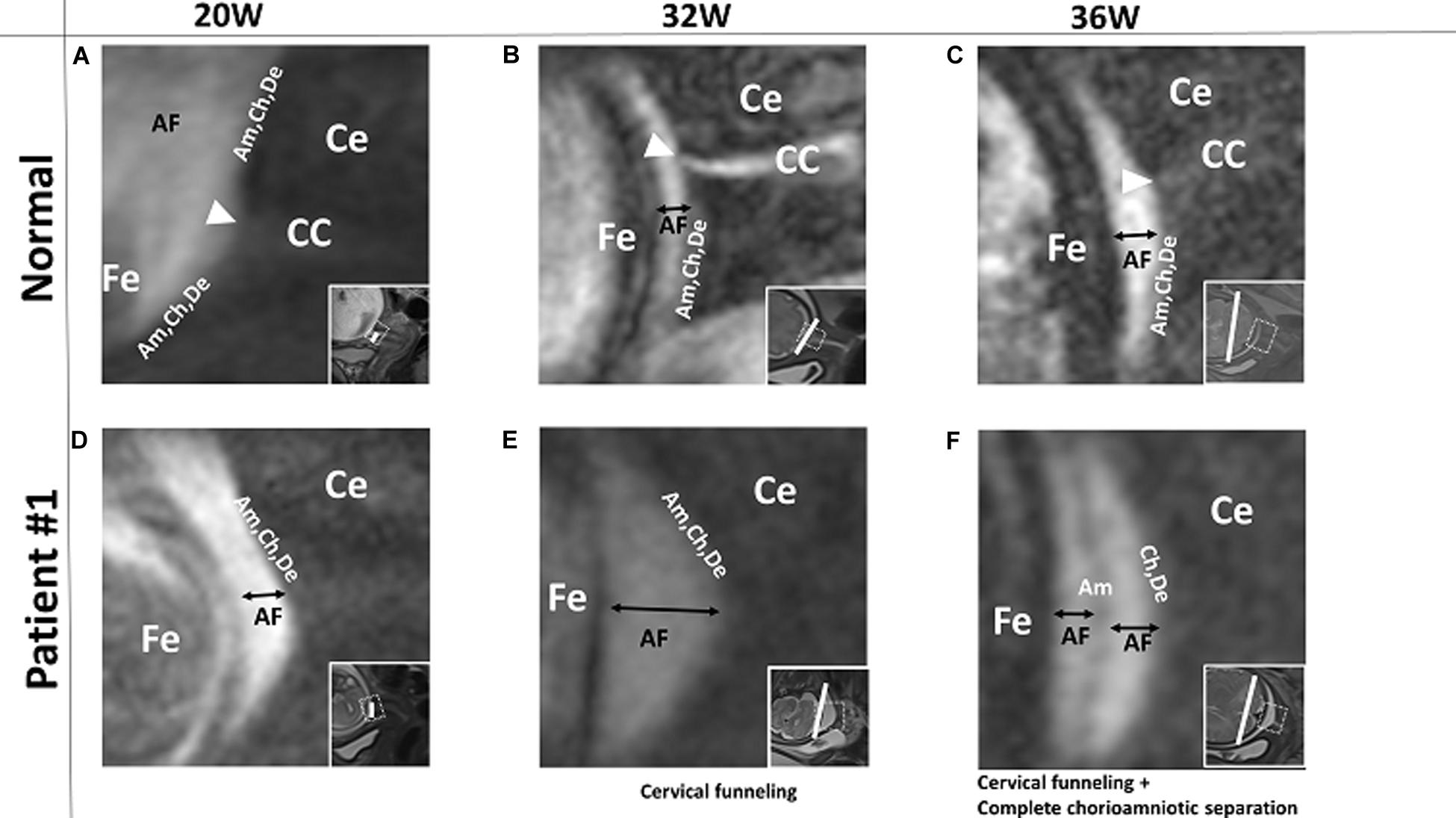
Figure 1. A pregnant woman with normal FM and Patient #1. (A–C) 3D-CISS images from a pregnant woman with normal, intact FM at the indicated time points. The white triangle indicates FM suspended in the cervical canal region. Images from patient #1, showing normal FM at 20 weeks (D), cervical funneling at 32 weeks (E), and cervical funneling and complete chorioamniotic separation at 36 weeks (F). Insets show T2WI images of the same regions. The white lines indicate the diameter of the cervix anatomical internal os. AF, amniotic fluid; Am, amnion; CC, cervical canal; Ce, cervix; Ch, chorion; De, decidua; Fe, fetus.
Four patients had both cervical funneling, in which the FM protruded into the cervix, and chorioamniotic separation, in which amniotic fluid was visible between the amnion and chorion, detectable in at least one of their MRI scans.
In patient #1, the FM appeared normal at 20 weeks (Figure 1D). However, at 32 weeks, this patient had cervical funneling with amniotic fluid and FM protruding into the cervix (Figure 1E). At 36 weeks, amniotic fluid was visible between amnion and chorion, indicating chorioamniotic separation (Figure 1F). This patient did not have PPROM or PROM and delivered at term.
In patient #2, the FM showed cervical funneling and partial chorioamniotic separation at 32 weeks and complete chorioamniotic separation at 36 weeks (Figures 2A,B). This patient did not have PPROM or PROM and delivered at term.
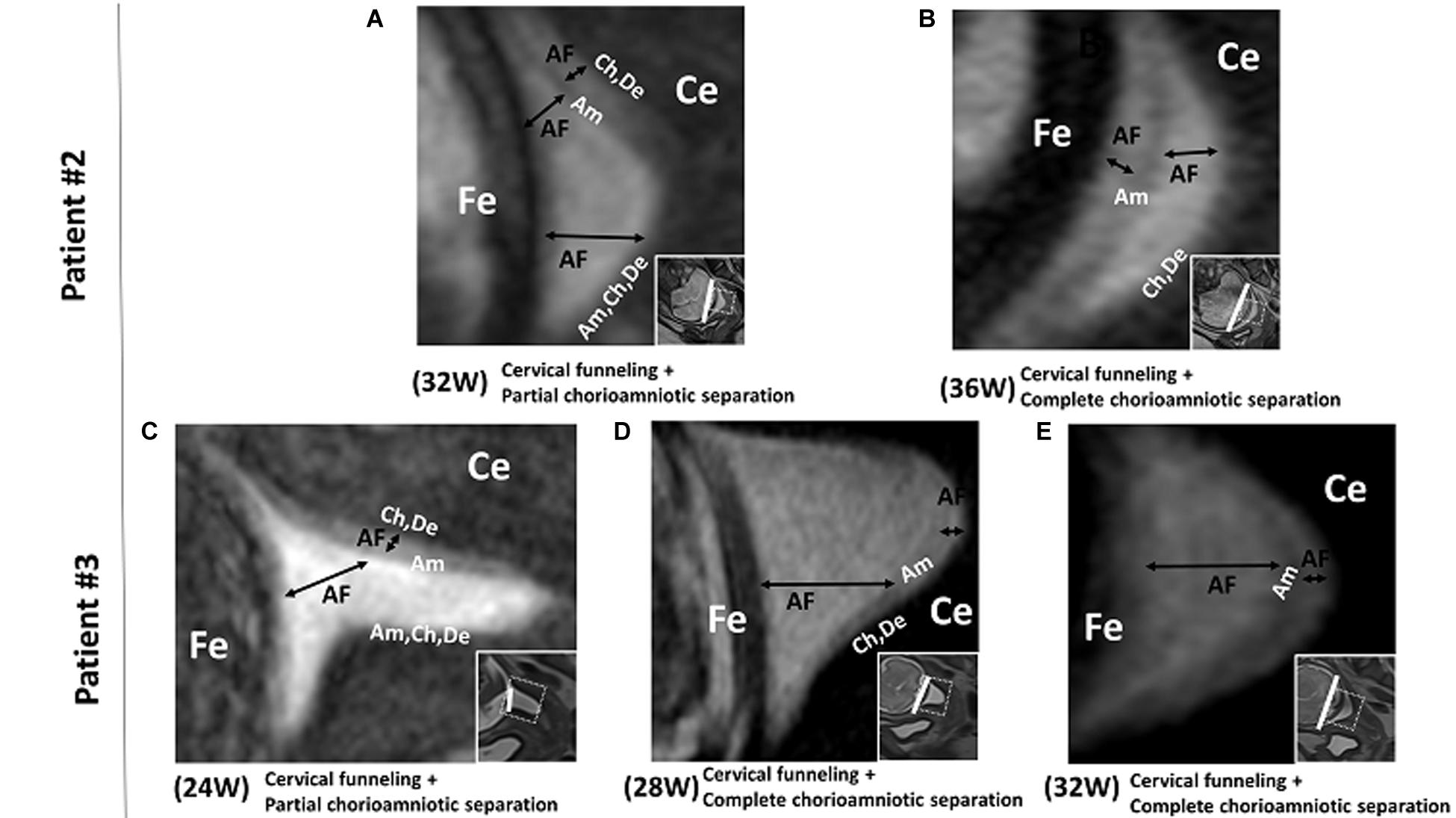
Figure 2. Patients # 2 and # 3. 3D-CISS images from patient #2, showing cervical funneling and partial chorioamniotic separation at 32 weeks (A) and cervical funneling and complete chorioamniotic separation at 36 weeks (B). Images from patient #3, showing cervical funneling and partial chorioamniotic separation at 24 weeks (C) and cervical funneling and complete chorioamniotic separation at 28 weeks (D) and 32 weeks (E). Insets show T2WI images of the same regions. The white lines indicate the diameter of the cervix anatomical internal os. AF, amniotic fluid; Am, amnion; Ce, cervix; Ch, chorion; De, decidua; Fe, fetus.
In patient #3, the FM showed cervical funneling and partial chorioamniotic separation at 24 weeks and complete chorioamniotic separation at 32 and 36 weeks (Figures 2C–E). This patient did not have PPROM or PROM and delivered at term.
In patient #4, the FM showed deeper cervical funneling, chorioamniotic separation, and chorionic rupture at 36 weeks (Figure 3). This patient developed PPROM 6 h after the MRI scan and delivered preterm (36 2/7 weeks).
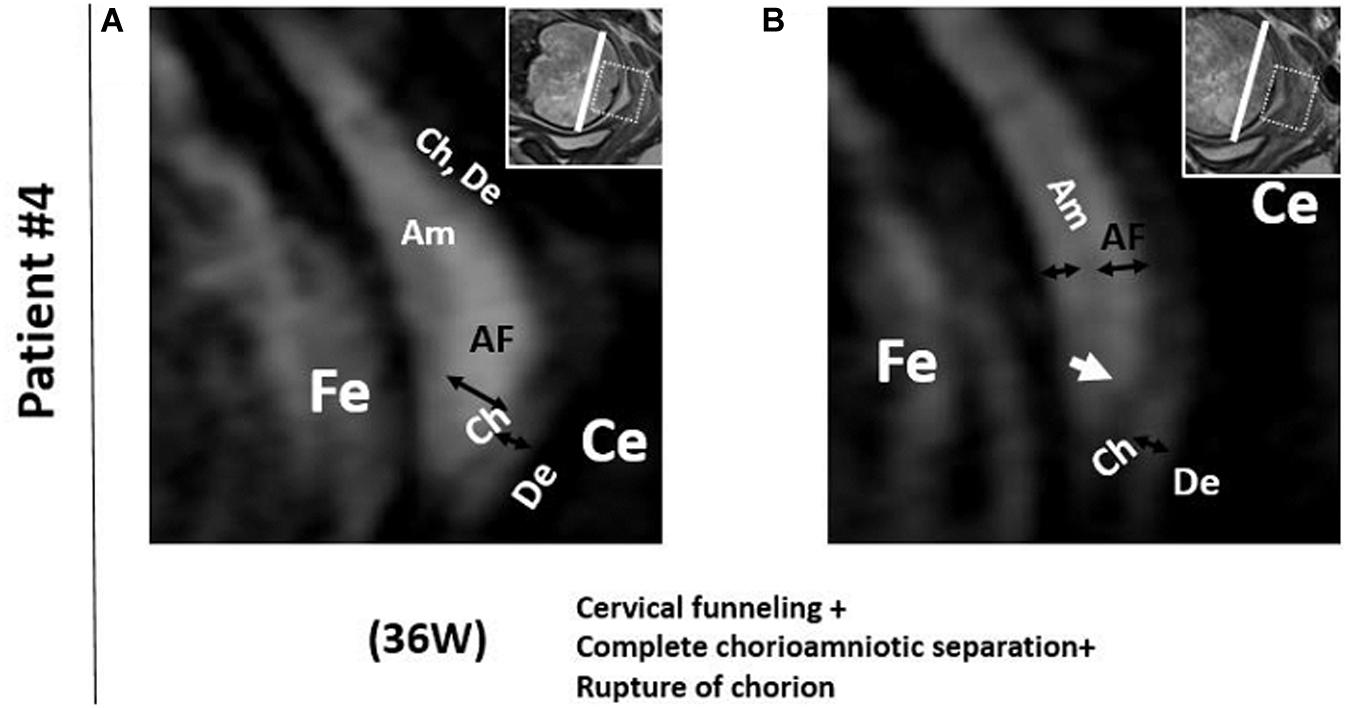
Figure 3. Patient 4. 3D-CISS images from patient #4 showing cervical funneling, chorioamniotic separation, and chorionic rupture at 36 weeks. The white arrow indicates the point of chorionic rupture. Insets show T2WI images of the same regions. The white lines indicate the diameter of the cervix anatomical internal os. AF, amniotic fluid; Am, amnion; Ce, cervix; Ch, chorion; De, decidua; Fe, fetus.
In our study, the longitudinal 3D-CISS MRI data provide the first in vivo evidence to support the first three steps of the model proposed by Arikat et al. regarding the sequence of events leading to FM rupture and PROM or PPROM. In the first step of their model, the FM stretches and protrudes into the cervix when the cervical internal os dilates to cause cervical funneling. This is evident in patient #1 at 32 weeks. In step 2, the amnion partially or completely separates from the chorion, as is evident in patient #1 at 36 weeks, patient #2 at 32 and 36 weeks, patient #3 at 24, 28, and 32 weeks, and patient #4 at 36 weeks. In step 3, further cervical internal os dilation leads to additional FM stretch and chorion rupture as seen in patient #4 at 36 weeks. In step 4, the amnion distends further. Finally, in step 5, the amnion ruptures, leading to PPROM or PROM. We present a schematic of the first three steps of this model in Figure 4.
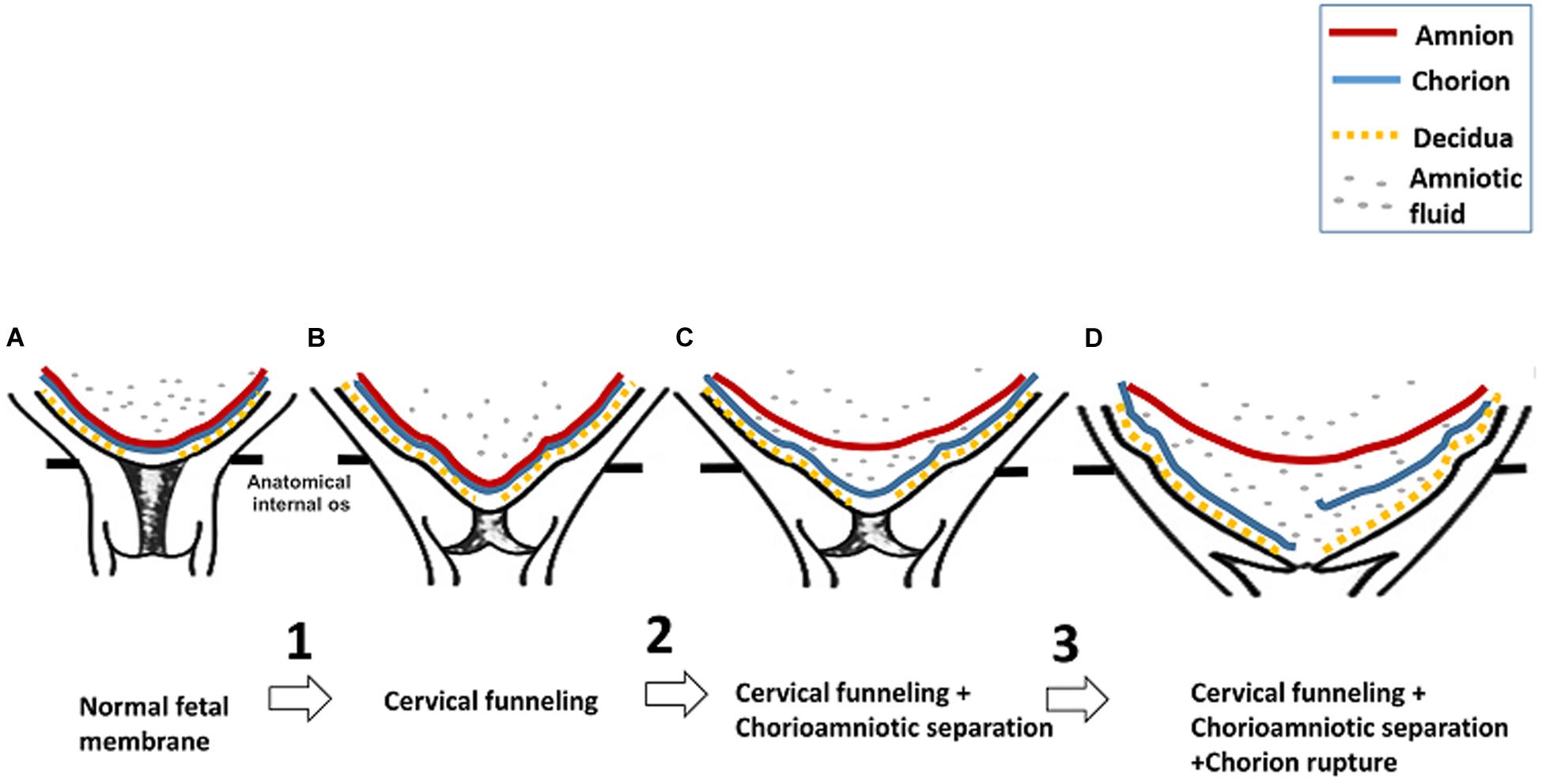
Figure 4. Schematic of the first three steps of premature FM rupture detected by 3D-CISS. (A) After 16 weeks’ gestation, the amnion (red) and chorion (blue) usually fuse, and the chorion is fused to the decidua (yellow) at the maternal–fetal interface. No amniotic fluid (gray dots) can be seen between the amnion and chorion or between the chorion and decidua. (B) In step 1, the FM stretches as it protrudes into the cervix when the internal cervical os dilates, causing cervical funneling. (C) In step 2, the amnion separates from the chorion, and amniotic fluid is detectable between the amnion and chorion. (D) In step 3, the FM undergoes additional stretch upon further internal cervical os dilation. This can result in chorion rupture.
Consistent with the in vitro studies, our in vivo study indicates that the stretch of FM is the first step in FM rupture. During pregnancy, outward pressure on FM from the amniotic fluid is balanced by inward pressure from the uterine wall. However, when the cervical internal os opens (cervical funneling), inward pressure on the FM overlying the cervix will decrease, and the FM will protrude into the cervical canal, causing the stretch of FM. Our longitudinal data suggest that the FM stretch in the para-cervical weak zone can lead to chorioamniotic separation. Data from in vitro studies suggest that the mechanical force applied to FM reduces the adhesiveness between amnion and chorion, leading to chorioamniotic separation (Strohl et al., 2010). This result is also supported by in vitro second harmonic generation microscopy studies of FM, revealing that the repeated mechanical loading affects the integrity of the amnion–chorion interface and can increase the risk of FM rupture (Mauri et al., 2013).
Before 14 weeks’ gestation, the chorion and amnion have not yet fused together, and the chorioamniotic separation is always normal. After 16 weeks, however, any chorioamniotic separation is identified as uncommon and anomalous (Kim et al., 2007; Bibbo et al., 2016). Such separation is dangerous, as the ultrasound-detected chorionic separation after 16 weeks is associated with adverse perinatal outcomes such as fetal extremity deformities, fetal death (Graf et al., 1997; Levine et al., 1998), and preterm delivery (Levine et al., 1998; Sydorak et al., 2002; Devlieger et al., 2003; Wilson et al., 2003). The 3D-CISS images can detect chorioamniotic separation, since the amniotic fluid lies between the chorion and amnion.
We observed that the chorioamniotic separation which occurs before FM rupture is consistent with three sets of previous data. First, in clinical observations, FM components are frequently separated at delivery after spontaneous rupture of the membranes before delivery (Strohl et al., 2010). Second, a video-recorded sequence of in vitro FM rupture revealed that the chorion and amnion separated before rupture (Arikat et al., 2006). Third, in in vitro mechanical tests, two peaks were noted in the force vs. displacement curve, suggesting that FM rupture occurs via separate rupture of the amnion and chorion (Artal et al., 1976; Lavery and Miller, 1977; Oxlund et al., 1990; Schober et al., 1994; El Khwad et al., 2005).
In patient 4, the chorion ruptured before the amnion, which is supported by in vitro studies (Artal et al., 1976; Lavery and Miller, 1979; Helmig et al., 1993; Oyen et al., 2004; Arikat et al., 2006). But some studies suggest that the amnion ruptures first (Artal et al., 1976; Lavery and Miller, 1979; Helmig et al., 1993; Oyen et al., 2004; Arikat et al., 2006). Our data are consistent with in vitro mechanical testing revealing that the amnion was consistently stronger, stiffer, and more ductile than the chorion (Arikat et al., 2006). The amnion may be stronger because it is composed of a dense layer of collagen fibrils, where the FM strength mainly comes from (Strauss, 2013).
The major strength of this work is the first ever use of 3D-CISS MRI to obtain in vivo images of the FM at much higher contrast and better resolution than other types of MRI or ultrasound. Clinical ultrasound is a series of 2D images acquired at several limited angles, which cannot provide a 3D description of the FM overlying the cervix. In comparison, 3D-CISS MRI is not operator dependent and can provide a high resolution, high contrast 3D spatial coverage of FM with multi-planar viewing angle capability. Therefore, 3D-CISS MRI provides a novel way to study the FM overlying the cervix. Additionally, by longitudinally imaging patients, we could define the sequences of events leading to FM rupture.
In this study, we used a 3.0 T MRI to image the FM of pregnant women. MRI has been used to evaluate obstetrical, placental, and fetal abnormalities in pregnant patients for more than 30 years, and its application during pregnancy is generally considered safe for the fetus (Patenaude et al., 2014; Radiology TACo, 2015; Ray et al., 2016). Compared with the current commonly used fetal MRI sequence, the 3D-CISS sequence was applied without exceeding either of the specific absorption rate and acoustic noise. Additionally, 3D-CISS is a high-speed sequence (4 min) and therefore reduced the patients’ exposure to the magnetic.
Our study has three main limitations. First, we had a small sample size and our data are qualitative in nature. Second, we did not measure other FM characteristics such as thickness and signal intensity. Lastly, our medical records did not separate PPROM from PTL in the history of preterm delivery.
In summary, our data support the in vitro model that the FM ruptures according to a sequence starting with stretch of the chorion and amnion together, then separation of the amnion from the chorion, next the rupture of the chorion, and finally the rupture of the amnion ruptures. An important next step is to conduct a larger longitudinal study to confirm these findings. If we can define an MRI marker that predicts FM rupture, we may be able to intervene to prevent PPROM.
All datasets presented in this study are included in the article/supplementary files.
The studies involving human participants were reviewed and approved by the Washington University in St. Louis Institutional Review Board (protocols 201612140 and 201707152). The patients/participants provided their written informed consent to participate in this study.
WQ and PZ designed the experiment. WQ and WiW evaluated magnetic resonance images. ZS, XM, HW, WnW, ZW, ZK-W, PW, and QW collected the data and aided in preparation of the manuscript. RM co-supervised the research. YW obtained funding for the project, supervised the work, and participated in preparation of the manuscript.
This study was primarily supported by the grants from the NIH/National Institute of Child Health and Human Development (R01HD094381; to PIs YW and AGC), and was also supported, in part, by grants from the March of Dimes (March of Dimes Prematurity Research Center at Washington University), the NIH/National Institute of Aging (R01AG053548; to T. Benzinger and YW), and the BrightFocus Foundation (A2017330S; to YW). The funder had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the article for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Molly Stout for constructive suggestions and Deborah Frank for editing the manuscript. We also thank Jessica Chubiz and Megan Steiner for coordinating the clinical team to enroll patients.
Arikat, S., Novince, R. W., Mercer, B. M., Kumar, D., Fox, J. M., Mansour, J. M., et al. (2006). Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am. J. Obstet. Gynecol. 194, 211–217. doi: 10.1016/j.ajog.2005.06.083
Artal, R., Sokol, R. J., Neuman, M., Burstein, A. H., and Stojkov, J. (1976). The mechanical properties of prematurely and non–prematurely ruptured membranes. Methods and preliminary results. Am. J. Obstet. Gynecol. 125, 655–659. doi: 10.1016/0002-9378(76)90788-2
Başaran, D., Özyüncü, Ö, Kara, Ö, Şahin, N., Turğal, M., and Önderoğlu, L. S. (2014). Ultrasonographic measurement of amniochorionic membrane in asymptomatic pregnant women is not a useful tool for preterm birth prediction. J. Obstet. Gynaecol. Res. 40, 62–66. doi: 10.1111/jog.12121
Bibbo, C., Little, S. E., Bsat, J., Botka, K. A., Benson, C. B., and Robinson, J. N. (2016). Chorioamniotic separation found on obstetric ultrasound and perinatal outcome. AJP Rep. 6, e337–e343. doi: 10.1055/s-0036-1593407
Devlieger, R., Scherjon, S. A., Oepkes, D., Meerman, R., Timmerman, D., and Vandenbussche, F. P. (2003). Ultrasound visualization of fetal membrane detachment at the uterine cervix: the ‘moon sign’. Ultrasound Obstet. Gynecol. 22, 431–432. doi: 10.1002/uog.234
El Khwad, M., Stetzer, B., Moore, R. M., Kumar, D., Mercer, B., Arikat, S., et al. (2005). Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol. Reprod. 72, 720–726. doi: 10.1095/biolreprod.104.033647
Frigo, P., Lang, C., Sator, M., Ulrich, R., and Husslein, P. (1998). Membrane thickness and PROM–high-frequency ultrasound measurements. Prenat. Diagn. 18, 333–337. doi: 10.1002/(sici)1097-0223(199804)18:4<333::aid-pd264>3.0.co;2-h
Goldenberg, R. L., Culhane, J. F., Iams, J. D., and Romero, R. (2008). Epidemiology and causes of preterm birth. Lancet 371, 75–84. doi: 10.1016/S0140-6736(08)60074-4
Graf, J. L., Bealer, J. F., Gibbs, D. L., Adzick, N. S., and Harrison, M. R. (1997). Chorioamniotic membrane separation: a potentially lethal finding. Fetal Diagn. Ther. 12, 81–84. doi: 10.1159/000264436
Helmig, R., Oxlund, H., Petersen, L. K., and Uldbjerg, N. (1993). Different biomechanical properties of human fetal membranes obtained before and after delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 48, 183–189. doi: 10.1016/0028-2243(93)90086-r
Kim, Y. N., Jeong, D. H., Jeong, S. J., Sung, M. S., Kang, M. S., and Kim, K. T. (2007). Complete chorioamniotic membrane separation with fetal restrictive dermopathy in two consecutive pregnancies. Prenat. Diagn. 27, 352–355. doi: 10.1002/pd.1673
Lavery, J. P., and Miller, C. E. (1977). The viscoelastic nature of chorioamniotic membranes. Obstet. Gynecol. 50, 467–472.
Lavery, J. P., and Miller, C. E. (1979). Deformation and creep in the human chorioamniotic sac. Am. J. Obstet. Gynecol. 134, 366–375. doi: 10.1016/s0002-9378(16)33077-0
Levine, D., Callen, P. W., Pender, S. G., McArdle, C. R., Messina, L., Shekhar, A., et al. (1998). Chorioamniotic separation after second-trimester genetic amniocentesis: importance and frequency. Radiology 209, 175–181. doi: 10.1148/radiology.209.1.9769829
Martin, J. A., Hamilton, B. E., Ventura, S. J., Osterman, M. J., Wilson, E. C., and Mathews, T. J. (2012). Births: final data for 2010. Natl. Vital. Stat. Rep. 61, 1–72.
Mathews, T. J., and MacDorman, M. F. (2010). Infant mortality statistics from the 2006 period linked birth/infant death data set. Natl. Vital. Stat. Rep. 58, 1–31.
Mauri, A., Perrini, M., Mateos, J. M., Maake, C., Ochsenbein-Koelble, N., Zimmermann, R., et al. (2013). Second harmonic generation microscopy of fetal membranes under deformation: normal and altered morphology. Placenta 34, 1020–1026. doi: 10.1016/j.placenta.2013.09.002
McLaren, J., Malak, T. M., and Bell, S. C. (1999). Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum. Reprod. 14, 237–241. doi: 10.1093/humrep/14.1.237
Menon, R., and Richardson, L. S. (2017). Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin. Perinatol. 41, 409–419. doi: 10.1053/j.semperi.2017.07.012
Nunes, V., Cross, J., Speich, J. E., Morgan, D. R., Strauss, J. F., and Ramus, R. M. (2016). Fetal membrane imaging and the prediction of preterm birth: a systematic review, current issues, and future directions. BMC Pregnancy Childbirth 16:387. doi: 10.1186/s12884-016-1176-5
Oxlund, H., Helmig, R., Halaburt, J. T., and Uldbjerg, N. (1990). Biomechanical analysis of human chorioamniotic membranes. Eur. J. Obstet. Gynecol. Reprod. Biol. 34, 247–255. doi: 10.1016/0028-2243(90)90078-f
Oyen, M. L., Cook, R. F., and Calvin, S. E. (2004). Mechanical failure of human fetal membrane tissues. J. Mater. Sci. Mater. Med. 15, 651–658. doi: 10.1023/b:jmsm.0000030205.62668.90
Parry, S., and Strauss, J. F. (1998). Premature rupture of the fetal membranes. N. Engl. J. Med. 338, 663–670. doi: 10.1056/NEJM199803053381006
Patenaude, Y., Pugash, D., Lim, K., Morin, L., Bly, S., Butt, K., et al. (2014). The use of magnetic resonance imaging in the obstetric patient. J. Obstet. Gynaecol. Can. 36, 349–363. doi: 10.1016/s1701-2163(15)30612-5
Radiology TACo, (2015). ACR–SPR Practice Parameter for the Safe and Optimal Performance of Fetal Magnetic Resonance Imaging (MRI). Virginia: ACR.
Ray, J. G., Vermeulen, M. J., Bharatha, A., Montanera, W. J., and Park, A. L. (2016). Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 316, 952–961. doi: 10.1001/jama.2016.12126
Schober, E. A., Kusy, R. P., and Savitz, D. A. (1994). Resistance of fetal membranes to concentrated force applications and reconciliation of puncture and burst testing. Ann. Biomed. Eng. 22, 540–548. doi: 10.1007/bf02367090
Severi, F. M., Bocchi, C., Voltolini, C., Borges, L. E., Florio, P., and Petraglia, F. (2008). Thickness of fetal membranes: a possible ultrasound marker for preterm delivery. Ultrasound Obstet. Gynecol. 32, 205–209. doi: 10.1002/uog.5406
Simhan, H. N., and Canavan, T. P. (2005). Preterm premature rupture of membranes: diagnosis, evaluation and management strategies. BJOG 112, (Suppl. 1), 32–37. doi: 10.1111/j.1471-0528.2005.00582.x
Strauss, J. F. (2013). Extracellular matrix dynamics and fetal membrane rupture. Reprod. Sci. 20, 140–153. doi: 10.1177/1933719111424454
Strohl, A., Kumar, D., Novince, R., Shaniuk, P., Smith, J., Bryant, K., et al. (2010). Decreased adherence and spontaneous separation of fetal membrane layers–amnion and choriodecidua–a possible part of the normal weakening process. Placenta 31, 18–24. doi: 10.1016/j.placenta.2009.10.012
Sydorak, R. M., Hirose, S., Sandberg, P. L., Filly, R. A., Harrison, M. R., Farmer, D. L., et al. (2002). Chorioamniotic membrane separation following fetal surgery. J. Perinatol. 22, 407–410. doi: 10.1038/sj.jp.7210753
Waters, T. P., and Mercer, B. (2011). Preterm PROM: prediction, prevention, principles. Clin. Obstet. Gynecol. 54, 307–312. doi: 10.1097/GRF.0b013e318217d4d3
Wilson, R. D., Johnson, M. P., Crombleholme, T. M., Flake, A. W., Hedrick, H. L., King, M., et al. (2003). Chorioamniotic membrane separation following open fetal surgery: pregnancy outcome. Fetal Diagn. Ther. 18, 314–320. doi: 10.1159/000071972
Yoshino, N., Akimoto, H., Yamada, I., Nagaoka, T., Tetsumura, A., Kurabayashi, T., et al. (2003). Trigeminal neuralgia: evaluation of neuralgic manifestation and site of neurovascular compression with 3D CISS MR imaging and MR angiography. Radiology 228, 539–545. doi: 10.1148/radiol.2282020439
Keywords: amnion, chorion, fetal membrane, preterm birth, premature rupture of membranes, preterm premature rupture of membranes, magnetic resonance imaging
Citation: Qi W, Zhao P, Wang W, Sun Z, Ma X, Wang H, Wu W, Wen Z, Kisrieva-Ware Z, Woodard PK, Wang Q, McKinstry RC and Wang Y (2020) In vivo Assessment of Supra-Cervical Fetal Membrane by MRI 3D CISS: A Preliminary Study. Front. Physiol. 11:639. doi: 10.3389/fphys.2020.00639
Received: 02 April 2020; Accepted: 20 May 2020;
Published: 25 June 2020.
Edited by:
Ramkumar Menon, The University of Texas Medical Branch at Galveston, United StatesReviewed by:
Marian Kacerovsky, University Hospital Hradec Kralove, CzechiaCopyright © 2020 Qi, Zhao, Wang, Sun, Ma, Wang, Wu, Wen, Kisrieva-Ware, Woodard, Wang, McKinstry and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Wang, d2FuZ3lvbmdAd3VzdGwuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.