
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 10 January 2020
Sec. Computational Physiology and Medicine
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.01530
 Koyu Hori1†
Koyu Hori1† Yufeng Mao1†
Yufeng Mao1† Yumi Ono1
Yumi Ono1 Hiroki Ora1*
Hiroki Ora1* Yuki Hirobe1
Yuki Hirobe1 Hiroyuki Sawada2
Hiroyuki Sawada2 Akira Inaba2
Akira Inaba2 Satoshi Orimo2
Satoshi Orimo2 Yoshihiro Miyake1
Yoshihiro Miyake1Gait analysis is used widely in clinical practice to evaluate abnormal gait caused by disease. Conventionally, medical professionals use motion capture systems or make visual observations to evaluate a patient's gait. Recent biomedical engineering studies have proposed easy-to-use gait analysis methods employing wearable sensors with inertial measurement units (IMUs). IMUs placed on the shanks just above the ankles allow for long-term gait monitoring because the participant can walk with or without shoes during the analysis. To the knowledge of the authors, no IMU-based gait analysis method has been reported that estimates stride length, gait speed, stride duration, stance duration, and swing duration simultaneously. In the present study, we tested a proposed gait analysis method that uses IMUs attached on the shanks to estimate foot trajectory and temporal gait parameters. Our proposed method comprises two steps: stepwise dissociation of continuous gait data into multiple steps and three-dimensional trajectory estimation from data obtained from accelerometers and gyroscopes. We evaluated this proposed method by analyzing the gait of 19 able-bodied participants (mean age 23.9 years, 9 men and 10 women). Wearable sensors were attached on the participants' shanks, and we measured three-axis acceleration and three-axis angular velocity with the sensors to estimate foot trajectory during walking. We compared gait parameters estimated from the foot trajectory obtained with the proposed method and those measured with a motion capture system. Mean accuracy (± standard deviation) was 0.054 ± 0.031 m for stride length, 0.034 ± 0.039 m/s for gait speed, 0.002 ± 0.020 s for stride duration, 0.000 ± 0.017 s for stance duration, and 0.002 ± 0.024 s for swing duration. These results suggest that the proposed method is suitable for gait analysis, whereas there is a room for improvement of its accuracy and further development of this IMU-based gait analysis method will enable us to use such systems for clinical gait analysis.
Analysis of abnormal gait can provide important information about diseases and injuries. For example, patients with Parkinson's disease (PD) often exhibit shuffling, festinating, and freezing of gait. The most widely used clinical rating scale for PD, the Unified Parkinson's Disease Rating Scale, includes observation of gait (Goetz et al., 2008). Patients with cerebellar disorders sometimes have a wide-based (atactic) gait, and those with cerebral vascular disease sometimes exhibit a hemiplegic gait. Recent articles have reported changes in gait, such as reduced gait velocity and stride length, in diseases with gait disorders and in other conditions, such as Alzheimer's disease (Mielke et al., 2013) and depression (Lemke et al., 2000).
Clinical gait analysis is performed mostly by health-care providers using visual observation (Krebs et al., 1985). Although this method is the most readily accessible means of gait analysis available to health-care providers (Barker et al., 2006), it is a subjective and qualitative method that is inadequate for assessing changes in gait features during ongoing treatment interventions. It is also difficult for clinicians to share this information with health-care providers and patients. Motion capture systems are used in clinical research for gait analysis (Mcginley et al., 2009) and scientific research (Lieberman et al., 2010). Because they provide well-quantified and accurate results, these systems are currently considered to be the criterion standard for clinical gait analysis (Cameron and Wagner, 2011). However, because the special equipment needed for motion capture is expensive and requires a large space, few medical institutions can use these systems for clinical gait analysis (Barker et al., 2006).
Several studies have proposed gait analysis methods using inertial measurement units (IMUs) to solve the problems described above (Sabatini et al., 2005; Moore et al., 2007; Mariani et al., 2010; Rebula et al., 2013; Kitagawa and Ogihara, 2016). IMUs used in these methods are inexpensive and wearable (Fujiki et al., 2009). In particular, we focused on methods that estimate trajectories of a foot because such methods can be used to obtain several spatial gait parameters. Sabatini et al. (2005) proposed an IMU-based gait analysis method that estimates a two-dimensional trajectory in the sagittal plane of a foot during walking. Other studies have proposed gait analysis methods that estimate the three-dimensional foot trajectory during walking in a stepwise manner to obtain values of foot clearance (Mariani et al., 2010, 2012; Kitagawa and Ogihara, 2016). The trajectory estimation methods reported in several studies (Sabatini et al., 2005; Mariani et al., 2012; Kitagawa and Ogihara, 2016) use an IMU attached on the dorsum of the foot and are better for obtaining this gait feature. As described above, and to the knowledge of the authors, there is no report of a method that estimates three-dimensional foot trajectory from an IMU attached on the shank during waking in a stepwise manner to calculate simultaneously spatial and temporal clinical gait parameters, including stride length, gait speed, stride duration, stance duration, and swing duration.
In this study, we propose a novel gait analysis method for clinical purposes that uses IMUs attached on the shanks to estimate foot trajectory and to obtain estimated clinical gait parameters. We conducted an experimental evaluation of the proposed method. Here, we report an example of the application of the proposed method to PD patients.
Our proposed gait analysis system is illustrated in Figure 1A. For gait analysis, we used two IMUs (TSND121, ATR-Promotions, Kyoto, Japan; Figure 1B) with a triaxial accelerometer (±8 G range), triaxial gyroscope (±1,000° per range), and Android OS tablet (ZenPad10, ASUSTeK Computer Inc., Taipei, Taiwan; Figure 1C). Raw accelerometer and gyroscope signals were sampled at 100 Hz (16 bits per sample). The size of the IMU is 37 mm × 46 mm × 12 mm and its weight is about 22 g. IMUs are attached on the shanks (just above the ankles) with bands (Figure 1B). The inertial coordinate system used to represent foot orientation and position is shown in Figure 1B. Acceleration and angular velocity data of both the shanks measured during walking are transmitted to the tablet through Bluetooth.
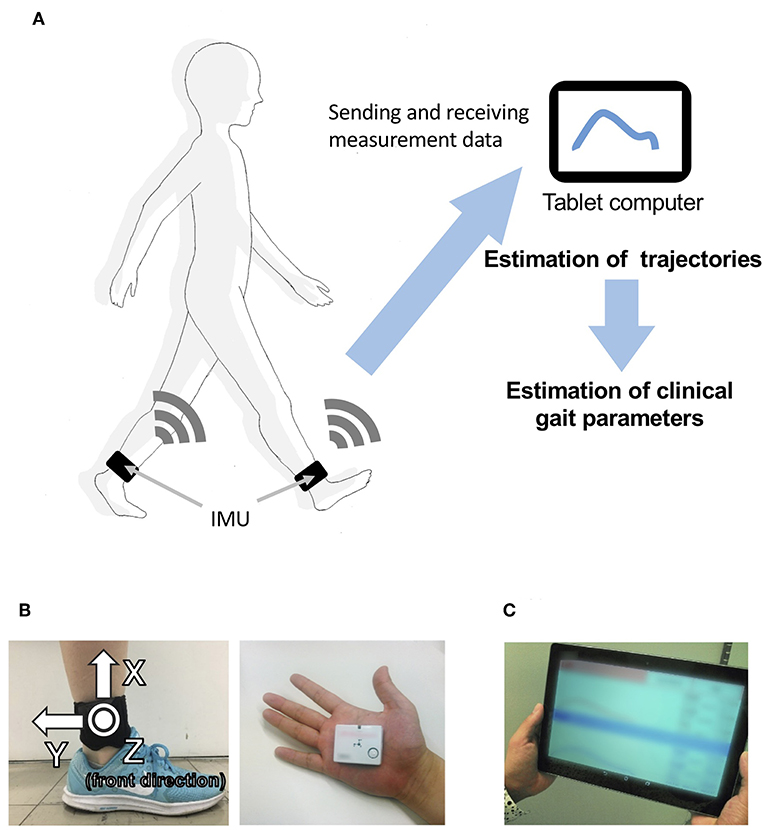
Figure 1. Overview of the system. (A) System configuration in the proposed method, including (B) a wearable sensor and its attachment on the shanks (just above the ankles), and (C) a tablet computer.
Our proposed method comprises two steps: dissociation of continuous gait data into multiple steps and three-dimensional trajectory estimation in a stepwise manner. Each process is described as follows.
We dissociated the signals into four steps: (1) smoothing and finding the local maximums; (2) finding the heel-strike (HS) and toe-off (TO) points; (3) quadratic regression; and (4) calculating the split point. One walking cycle is defined as a single step, and the starting point of each cycle is defined as the split point between HS and immediate TO points. We identify the split point in each cycle based on raw angular velocity data in the z-axis ωz of the gyroscope (right-handed; Figure 1B, left).
The raw data contained much noise, and a median filter (window length: 5) was first used to smooth the data. We found the local maximum lk that is larger than the threshold (200°/s; Figure 2A).
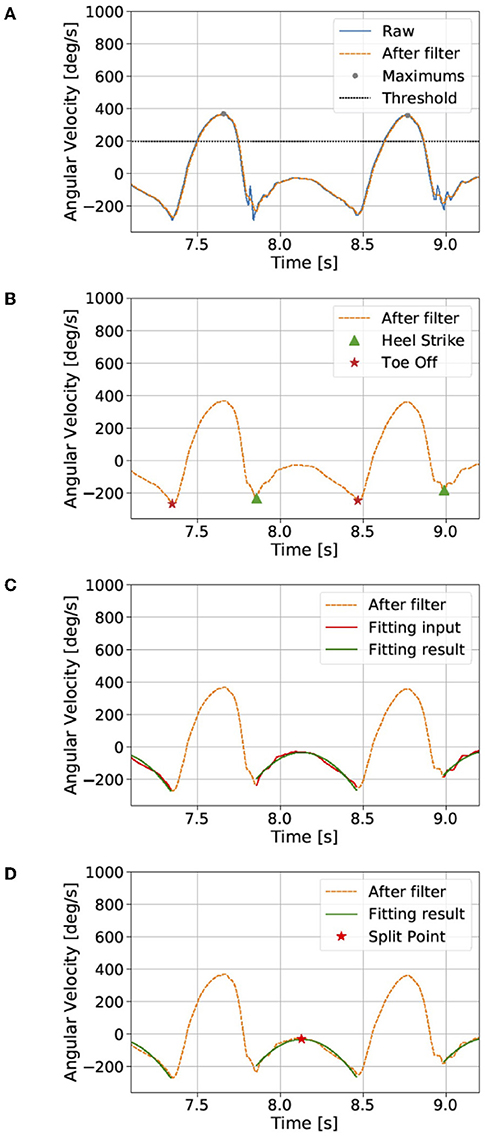
Figure 2. Dissociation of the continuous gait signal (see the section Proposed method). The raw data contains much noise, and a median filter was first used to smooth the data. (A) We found local maxima that are larger than a threshold and (B) then the heel-strike and toe-off points. (C) We assume a quadratic curve between the heel-strike and toe-off points. (D) Finally, the split point was defined as the maximum point of the quadratic fitting result. The gait cycle was defined by the data from the split point to the next split point.
We then found the k-th local minimums near HS mhsk and TO mtok points between the lk and lk+1. mhsk is the local minimum closest to the right of lk, and mtok is the local minimum closest to the left of lk+1 (Figure 2B).
We assume ωz between mhsk and mtok points to represent a quadratic curve (Figure 2C):
where is the best-fitting result for angular velocity in the z-axis.
A quadratic regression is then used to fit the raw data and to calculate the parameters ak, bk, ck:
where Mk is the number of points between mhsk and mtok.
Finally, the segmentation point spk was defined as the maximum point of the quadratic fitting result (Figure 2D):
The k-th cycle was defined by the data between spk and spk+1. In each cycle, we estimate the trajectory.
The foot trajectory in each cycle can be calculated by integrating the acceleration between each segmentation point. Two coordinate systems are applied: a laboratory coordinate system (e) and a sensor coordinate system (s). Because the raw data from the sensor are represented by the time-variant sensor coordinates, we need to transpose them into time-invariant laboratory coordinates. Acceleration in the laboratory coordinates can be converted from measured sensor acceleration using a rotational matrix:
where θ, ϕ, and ψ are the Euler angles around the x-, y-, and z-axes.
We divide the estimation process of the foot trajectory into five steps: (1) calculate the initial Euler angles; (2) calculate the time derivative of the Euler angles; (3) calculate the Euler angles using the integral; (4) transpose the accelerations using a rotational matrix; and (5) calculate the trajectory using the double integral.
At the beginning of each cycle, we can assume that the foot is in full contact with the floor and is momentarily stationary. The accelerometer is assumed to detect only gravitational acceleration g at the outset:
where as,0 is the initial acceleration vector in the sensor coordinate, and ϕ0 and ψ0 are the initial Euler angles around the y- and z-axes.
Therefore, the initial Euler angles vector θ0 can be calculated as:
The relation between the ith angular velocity ωi from the gyroscope and the time derivation of Euler angles can be calculated as:
where ϕi and ψi are the ith Euler angles around the y- and z-axes.
Euler angles are derived by the integration of Euler angle derivation:
where Δt is the sampling rate.
The rotational matrix calculated by Equation (3) is used to estimate the acceleration in laboratory coordinates Rs→eas, which contains the gravitational acceleration. Linear acceleration ae in laboratory coordinates can then be calculated by simply subtracting gravity:
The i-th velocity ve,i in the laboratory coordinates is estimated by integration of linear acceleration ae,i:
and the i-th foot trajectory pe,i is estimated by integration of ve,i:
Integration may drift because of IMU sensor error, and the calculations of velocity and trajectory are corrected by the constraint condition. In each cycle, both the initial value and the end value of the velocity in three directions and the trajectory in the vertical direction can be assumed as 0. The algorithm below shows how to estimate velocity.
First, the forward integral and back integral are calculated separately from linear acceleration ae,i:
where the superscripts f and b mean forward and backward, respectively. The correction result can then be derived by the weighted average of the forward and backward integral:
where wi is the weight and wi ∈ [0, 1].
Because is more accurate near the starting point and is more accurate near the end point, the function for calculating wi should increase monotonically. Here, we choose the sigmoid function to calculate wi:
where N is the number of points in the current cycle and m is a hyperparameter calculated from experiment, which we choose as m = 0.1. The trajectory in the vertical direction can also be calculated using the algorithm above.
The gait events included the HS and TO points, which were extracted first. The HS and TO events detection algorithm was based on the peak detection of the raw angular velocity in sagittal plane ωz. At the end of the swing period, several of negative peaks can be observed in ωz and the first one is associated with the HS instant (Salarian et al., 2004). Before the swing period, a negative peak is associated with the TO instant (Salarian et al., 2004).
For the definition of each gait event search region, the pattern of shank tilt angle, which was inspired from an instep-based previous study (Tunca et al., 2017) was introduced. At the end of the swing period, the shank will rotate around the knee axis caused by knee extension and reach the maximum forward. Then, the rotation of the shank around the ankle axis will start and the foot contact with the ground will produce the HS instant. In this process, θz will appear to increase first and then decrease. Thus a positive peak will appear in θz before the HS instant where θz was computed via the integration of ωzwith the sampling interval Δt. For the convenience of the description, we refer to this instant where peak occurrence as shank-max-forward (SMF). Similarly, after the TO instant, the ankle will lift slightly and rotate until reaching a certain height. Then it will start to rotate and present a negative peak in θz. We refer to this instant as the shank-max-backward (SMB) for convenience. As a result, SMF and SMB of θz can be used to define a proper search interval of the HS and TO. We found the SMF and SMB via a peak search algorithm signal.find_peaks in SciPy (version 1.2.0) which can find proper peaks via the prominence (define intrinsic height of a peak) and the distance (define the distance between peaks) properties. Then, SMFs are the positive peaks and SMBs are the negative peaks whose prominence is larger than 0.2 [rad] and the distance is at least 0.4 [s]. Finally, HS is defined as the first peak appears after each SMF instant, and the TO is the minimum of angular velocity in the interval (SMB − 0.3 [s], SMB).
Estimated stride length SL is calculated by the trajectory in the y and z direction:
where . Estimated stride duration is calculated as the time from one HS to the next ipsilateral HS. Estimated gait speed is calculated as the value obtained by dividing stride length by stride duration. Estimated stance duration is calculated as the time from HS to ipsilateral TO. Estimated swing duration time is calculated as the time from TO to the next ipsilateral HS.
We conducted an experimental evaluation of our proposed method to validate the accuracy of the trajectory estimation of the shanks (just above the ankles) to verify whether it allows the analysis of gait for clinical purposes. Twenty healthy people participated in the experiment, and we used a motion capture system as the criterion standard for the evaluating gait in a clinical setting. We evaluated the accuracy of our proposed method for calculating the estimated trajectory and clinical gait parameters. We used an IMU attached on the shanks for the experimental evaluation.
An optical motion capture system (Nobby Tech. Ltd., Tokyo, Japan) was used as the reference system. We used 12 cameras, and the motion capture volume was about 2 m × 7 m × 1 m (width, length, and height; Figure 3A). The position error of the markers of the motion capture system during the calibration was <1 mm. The dimensions of the floor of the room were 18 m × 7 m (length and width; Figure 3A). Three optical markers were attached on each foot as shown in Figure 3B. Two of the three markers were attached on the heel and the toe (metatarsal head II) to assess gait parameters. The third marker was attached on the IMU to evaluate the trajectory estimation. To synchronize the IMU and the motion capture system, the participants hit their heel to the floor before the gait measurement. The peaks of the spike waveforms, that were caused by the heel hits of the participants, in both the IMU signals and the motion capture system data were used to define time 0.
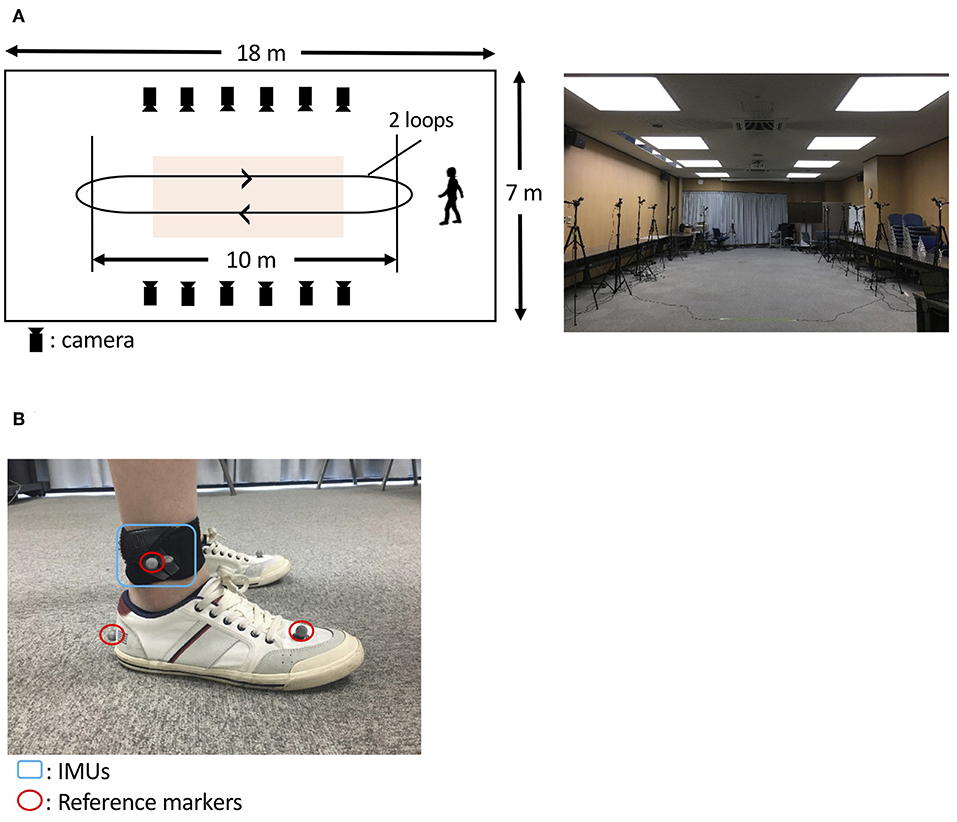
Figure 3. Protocol to evaluate the proposed method. (A) A room at the Tokyo Institute of Technology was used (A, right). The orange-shaded area shows the measurement area for gait analysis. The participants with IMUs and optical markers (B) walked around the room twice, as indicated by the arrows (A, left).
We recruited 20 able-bodied volunteer participants, with no history of gait abnormalities, from the Tokyo Institute of Technology for the experimental evaluation. The Ethics Committee of Tokyo Institute of Technology approved the protocols for the evaluation, and all participants provided their written informed consent. Because of technical problems with the motion capture system, the data for one of the 20 volunteer participants were excluded from the analyses. Ultimately, we used data from 19 participants (mean age 23.9 years, 9 men and 10 women, mean height 1.66 ± 0.07 m and mean body mass index 20.2 ± 2.7).
As an example of the application of the proposed method to PD patients, we used the method to analyze gait in one healthy elderly participant and four patients with PD. The healthy elderly participant was recruited from a public interest incorporated association in Machida City, Tokyo that provides human resource services for elderly people. Patients with PD were recruited from Kanto Central Hospital, Tokyo. PD had been diagnosed by a physician. The exclusion criteria for this study were past history of other neurological or orthopedic disorders that can affect gait or posture (excluding PD). The healthy elderly participant and the four PD participants provided written informed consent in accordance with requirements of the Ethics Committee of Tokyo Institute of Technology. The Kanto Central Hospital Ethics Committee and the Ethics Committee of Tokyo Institute of Technology approved the protocol for the application of the proposed method.
To construct the spike waveforms for synchronization between the IMU and the motion capture system (for the synchronization method, see “Overview of the evaluation”), the participants hit their heel to the floor before the gait measurement. In two trials, the participants walked on a flat floor at their own self-selected natural pace and a slow pace. In each trial, the participants walk straight in the motion capture volume and turned outside of the motion capture volume. Thus, each trial comprised four straight walks (two round trips; Figure 3A, left). We used the gait data obtained as the participants walked in the motion capture volume and removed the gait data as the participants turned outside of the motion capture volume.
To consider the validity of the estimation of foot trajectory from IMUs attached on the shanks, we calculated correlations between stride length estimated with the proposed method, measured with a motion capture marker attached on the IMU, and measured with a motion capture marker attached on the heel.
To evaluate the proposed method, the shank (just above the ankle) trajectory and clinical gait parameters calculated by our proposed method were compared with those collected by the motion capture system. The comparisons of trajectory information were conducted in the sagittal plane. Five clinical gait parameters (stride length, gait speed, stride duration, stance duration, and swing duration) were compared.
The trajectories of our proposed method and the reference data are shown in Figure 4. The R value between displacement in the direction of forward movement calculated with the proposed method and measured with a marker attached on the IMU was 0.978 (Figure 5A, left). The R value between the maximum vertical displacement calculated with the proposed method and measured with a marker attached on the IMU was 0.925 (Figure 5A, right).
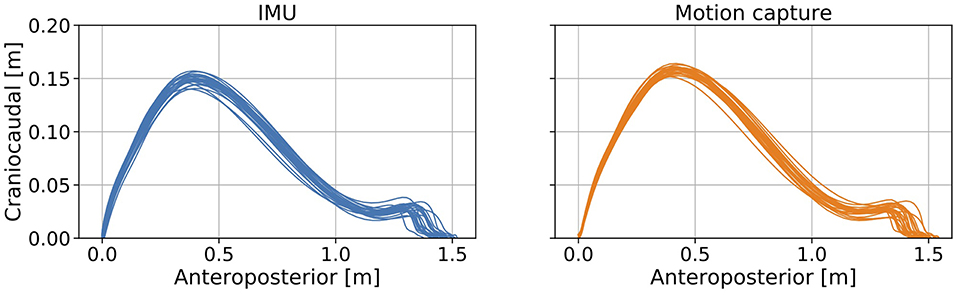
Figure 4. Errors between the estimated foot trajectory identified with our proposed method and reference data from the motion capture system projected in the sagittal plane.
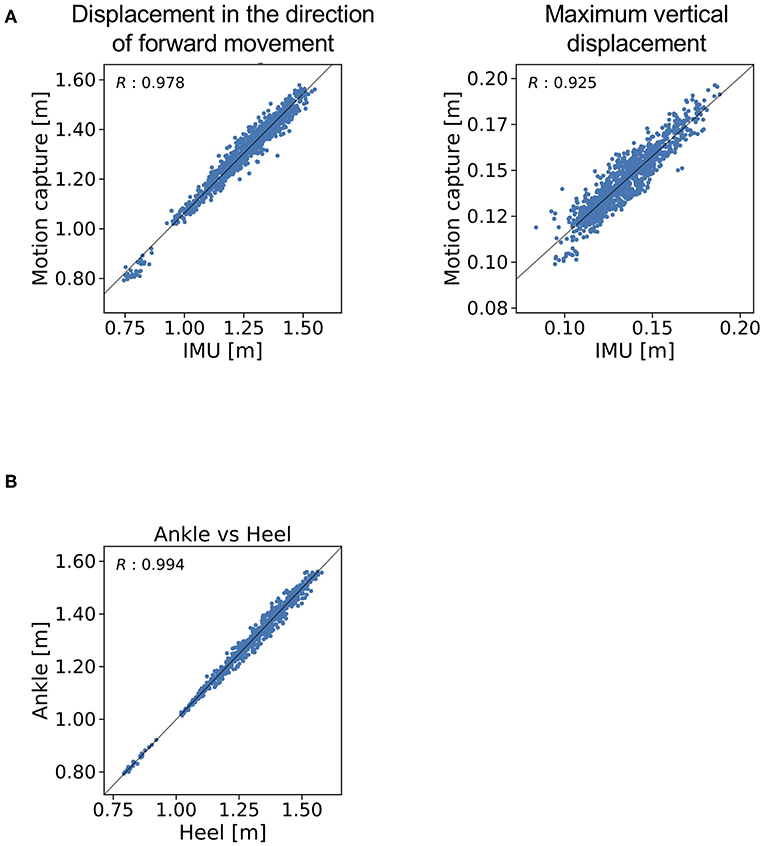
Figure 5. R values for (A) the displacement in the direction of forward movement and maximum vertical displacement estimated with the proposed method and (B) measured with a marker attached on the IMU and measured with a marker attached on the heel.
The R value between displacement in the direction of forward movement calculated with the marker attached on the IMU and that measured with the marker attached on the heel was 0.994 (Figure 5B).
The means and standard deviations (SD) of the gait parameters compared between the proposed method and the motion capture system are summarized in Table 1. Figure 6 shows the agreement between the proposed method and the motion capture system in Bland-Altman plots. The mean ±1 SD accuracy of stride length was 0.054 ± 0.031 m (Figure 6A). The R value between displacement in the direction of forward movement calculated with the proposed method and measured with a marker attached on the heel was 0.978 (Figure 6A). The mean ±1 SD accuracy were as follows: 0.034 ± 0.039 m/s for gait speed (Figure 6B); 0.002 ± 0.020 s for stride duration (Figure 6C); 0.000 ± 0.017 s for stance duration (Figure 6D); and 0.002 ± 0.024 s for swing duration (Figure 6E).
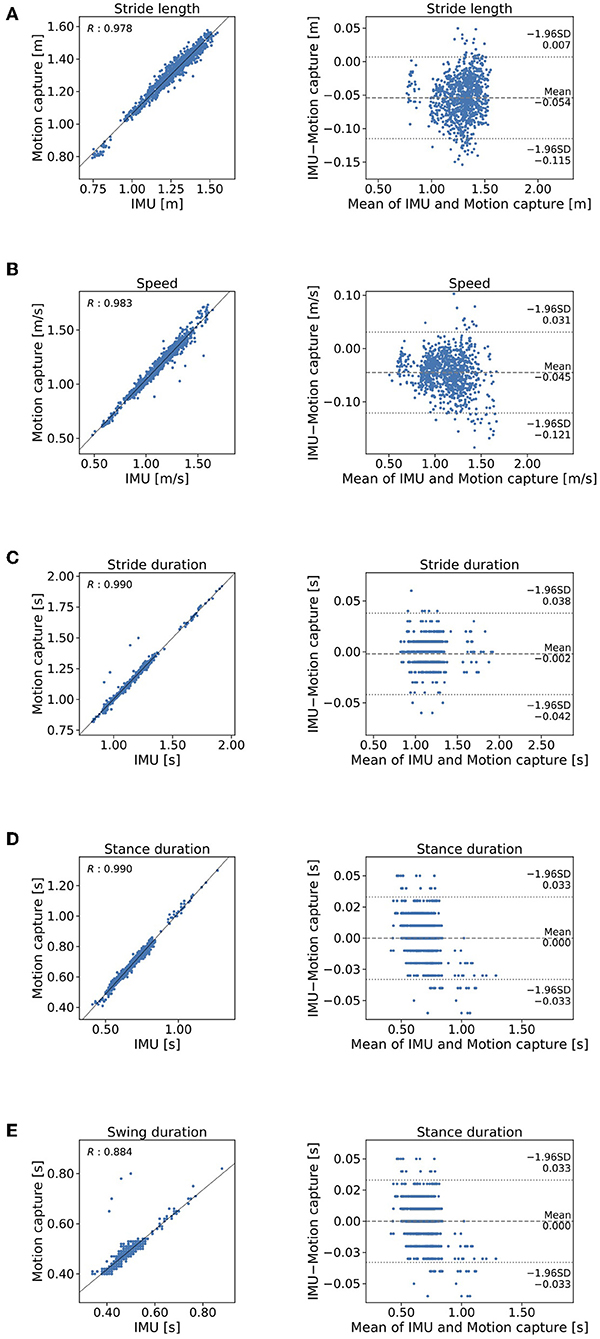
Figure 6. Comparison of the proposed method and the criterion standard in stepwise manner. Mocap, motion capture. Scatterplos and Bland-Altman plots of (A) stride length, (B) gait speed, (C) stride duration, (D) stance duration, and (E) swing duration.
The shank trajectory over 15 steps for each participant is shown in Figure 7. The mean clinical gait parameters of the PD patients are summarized in Table 2.
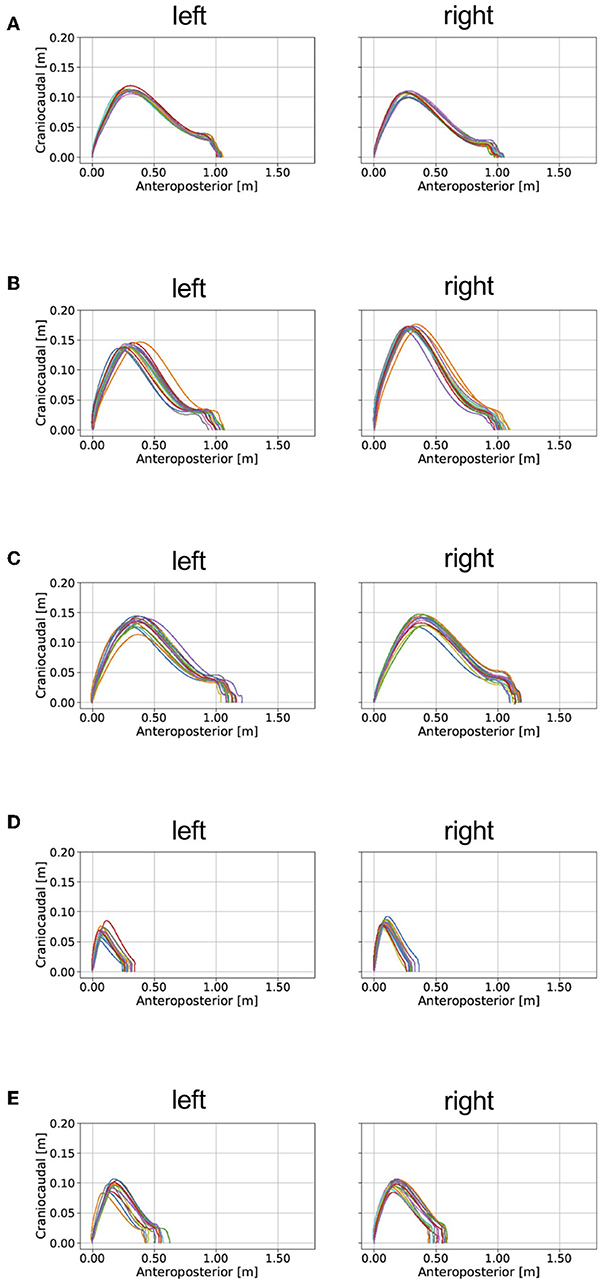
Figure 7. Examples of the application of our proposed method for analyzing gait in patients with PD and a healthy elderly subject. mH&Y, modified Hoehn and Yahr scale. The estimated gait trajectories of (A) a healthy elderly subject, (B) a patient with PD (mH&Y 2), (C) a patient with PD (mH&Y 2), (D) a patient with PD (mH&Y 4), and (E) a patient with PD (mH&Y 4) are plotted. The left panels shows the trajectory of the left foot, and the right panels shows the trajectory of the right foot.
We have proposed a new method for gait analysis that uses IMUs attached on the shanks to estimate foot trajectory and then to obtain estimated clinical gait parameters. The gait parameters obtained with the proposed method consists of stride length, gait speed, stride duration, stance duration, and swing duration. The experimental results show that the proposed method can be used to calculate clinical gait parameters by estimating foot trajectory.
The proposed gait analysis method comprises two IMUs with a triaxial accelerometer, triaxial gyroscope, and tablet computer. This method can be applied in a variety of locations outside of the gait laboratory and is less expensive than conventional gait analysis methods such as motion capture systems. The clinical advantage is that the patient burden is low because of the light weight (about 24 g) and easy attachment of the IMUs. We therefore anticipate that the proposed method would be suitable for clinical gait analysis. As for the location of the IMUs, the R value between displacement in the direction of forward movement as measured with the marker attached on the IMU and as measured with the marker attached on the heel (0.994) indicates that the location of the IMUs is valid at least for estimating the stride length. The R value between displacement in the direction of forward movement as estimated by the proposed method and as measured with the marker of the motion capture system attached on the IMU indicates that displacement in the direction of forward movement estimated by the proposed method explained 96% of the variation in displacement in the direction of forward movement as measured with the motion capture system.
The mean error of stride length estimated with the proposed method was 0.054 ± 0.031 m (Table 1). This result suggests that the proposed method can estimate clinical gait parameters such as stride length. A previous method in which the location of a IMU is on the dorsum of a foot found that the accuracy mean accuracy ± precision was 0.015 ± 0.068 m (Mariani et al., 2010). The IMU location on the shank may cause bias of this order of accuracy. We expected that further development of the method will overcome this limitation of performance. Several studies (Stolze et al., 2001; Curtze et al., 2015) have found that stride length is shorter in patients with PD than it is in healthy controls as observed in the example of the application of the proposed method (Table 2). For example, Morris et al. (2005) reported that stride length in PD patients in the off state was 0.96 ± 0.19 m, which was shorter than the stride length of 1.46 ± 0.08 m as measured in healthy age-matched controls. The R value between displacement in the direction of forward movement estimated by the proposed method and measured with the marker of the motion capture system attached on the heel indicates that stride length estimated by the proposed method explained 96% of the variation measured with the motion capture system. This result suggests that IMUs are potentially useful in clinical gait analysis. We expect that further development of the proposed method to evaluate the gait in people with PD.
We expect that further development of this method or other methods will enable us to evaluate quantitatively the effects of drugs and interventions such as rehabilitation in patients with gait disorders. In the future, we plan to assess patients with gait abnormalities, such as those caused by PD. We will validate the proposed method to determine whether it can identify abnormal gait patterns, including shuffle, short-steppage, and hemiplegic gaits. The sampling frequency that we used in the present study was 100 Hz. We plan to investigate the effect of the sampling frequency on the estimation of the gait parameter in the next study.
Our results suggest that the proposed method is suitable for gait analysis whereas there is a room for improvement of its accuracy. Unlike methods that use motion capture systems, this method can be used in a variety of locations, such as in the corridor of a medical center. Further development of our proposed method is expected to enable clinicians to share objective information about gait features with health-care providers and patients.
The datasets for this article are not made publicly available because there is an agreement for data exchange between Tokyo Institute of Technology and Kanto Central Hospital.
The Ethics Committee of Tokyo Institute of Technology approved the protocols for this study, and all participants provided written informed consent.
KH, YO, YH, YMa, HO, SO, and YMi designed the research. YO, KH, YH, HS, and AI performed the experiments. YMa, YO, HO, KH, and YMi analyzed the data. HO, YO, and YMi wrote the paper.
This study was partly supported by the Core Research for Evolutional Science and Technology (CREST) Program Nano inertia detection device and system of the Japan Science and Technology Agency (JST), a Ministry of Education, Culture, Sports, Science and Technology/Japan Society for the Promotion of Science (MEXT/JSPS) grant (16K12950), and a COI-JST grant to the Research Center for the Earth Inclusive Sensing Empathizing with Silent Voices.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Mr. Masatoshi Seki for assistance.
Barker, S., Craik, R., Freedman, W., Herrmann, N., and Hillstrom, H. (2006). Accuracy, reliability, and validity of a spatiotemporal gait analysis system. Med. Eng. Phys. 28, 460–467. doi: 10.1016/j.medengphy.2005.07.017
Cameron, M. H., and Wagner, J. M. (2011). Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr. Neurol. Neurosci. Rep. 11, 507–515. doi: 10.1007/s11910-011-0214-y
Curtze, C., Nutt, J. G., Carlson-Kuhta, P., Mancini, M., and Horak, F. B. (2015). Levodopa is a double-edged sword for balance and gait in people with Parkinson's disease. Mov. Disord. 30, 1361–1370. doi: 10.1002/mds.26269
Fujiki, Y., Tsiamyrtzis, P., and Pavlidis, I. (2009). “Making sense of accelerometer measurements in pervasive physical activity applications,” in Conference: Proceedings of the 27th International Conference on Human Factors in Computing Systems, CHI 2009, Extended Abstracts Volume (Boston, MA: ACM), 3425–3430. doi: 10.1145/1520340.1520497
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., et al. (2008). Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170. doi: 10.1002/mds.22340
Kitagawa, N., and Ogihara, N. (2016). Estimation of foot trajectory during human walking by a wearable inertial measurement unit mounted to the foot. Gait Posture 45, 110–114. doi: 10.1016/j.gaitpost.2016.01.014
Krebs, D. E., Edelstein, J. E., and Fishman, S. (1985). Reliability of observational kinematic gait analysis. Phys. Ther. 65, 1027–1033. doi: 10.1093/ptj/65.7.1027
Lemke, M. R., Wendorff, T., Mieth, B., Buhl, K., and Linnemann, M. (2000). Spatiotemporal gait patterns during over ground locomotion in major depression compared with healthy controls. J. Psychiatr. Res. 34, 277–283. doi: 10.1016/S0022-3956(00)00017-0
Lieberman, D. E., Venkadesan, M., Werbel, W. A., Daoud, A. I., D'andrea, S., Davis, I. S., et al. (2010). Foot strike patterns and collision forces in habitually barefoot versus shod runners. Nature 463, 531–535. doi: 10.1038/nature08723
Mariani, B., Hoskovec, C., Rochat, S., Büla, C., Penders, J., and Aminian, K. (2010). 3D gait assessment in young and elderly subjects using foot-worn inertial sensors. J. Biomech. 43, 2999–3006. doi: 10.1016/j.jbiomech.2010.07.003
Mariani, B., Rochat, S., Büla, C. J., and Aminian, K. (2012). Heel and toe clearance estimation for gait analysis using wireless inertial sensors. IEEE Trans. Biomed. Eng. 59, 3162–3168. doi: 10.1109/TBME.2012.2216263
Mcginley, J. L., Baker, R., Wolfe, R., and Morris, M. E. (2009). The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait Posture 29, 360–369. doi: 10.1016/j.gaitpost.2008.09.003
Mielke, M. M., Roberts, R. O., Savica, R., Cha, R., Drubach, D. I., Christianson, T., et al. (2013). Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 68, 929–937. doi: 10.1093/gerona/gls256
Moore, S. T., MacDougall, H. G., Gracies, J. M., Cohen, H. S., and Ondo, W. G. (2007). Long-term monitoring of gait in Parkinson's disease. Gait Posture 26, 200–207. doi: 10.1016/j.gaitpost.2006.09.011
Morris, M., Iansek, R., Mcginley, J., Matyas, T., and Huxham, F. (2005). Three-dimensional gait biomechanics in Parkinson's disease: evidence for a centrally mediated amplitude regulation disorder. Mov. Disord. 20, 40–50. doi: 10.1002/mds.20278
Rebula, J. R., Ojeda, L. V., Adamczyk, P. G., and Kuo, A. D. (2013). Measurement of foot placement and its variability with inertial sensors. Gait Posture 38, 974–980. doi: 10.1016/j.gaitpost.2013.05.012
Sabatini, A. M., Martelloni, C., Scapellato, S., and Cavallo, F. (2005). Assessment of walking features from foot inertial sensing. IEEE Trans. Biomed. Eng. 52, 486–494. doi: 10.1109/TBME.2004.840727
Salarian, A., Russmann, H., Vingerhoets, F. J., Dehollain, C., Blanc, Y., Burkhard, P. R., et al. (2004). Gait assessment in Parkinson's disease: toward an ambulatory system for long-term monitoring. IEEE Trans. Biomed. Eng. 51, 1434–1443. doi: 10.1109/TBME.2004.827933
Stolze, H., Kuhtz-Buschbeck, J. P., Drücke, H., Jöhnk, K., Illert, M., and Deuschl, G. (2001). Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 70, 289–297. doi: 10.1136/jnnp.70.3.289
Keywords: gait analysis, IMU, abnormal gait, inertial-measurement unit, wearable sensors
Citation: Hori K, Mao Y, Ono Y, Ora H, Hirobe Y, Sawada H, Inaba A, Orimo S and Miyake Y (2020) Inertial Measurement Unit-Based Estimation of Foot Trajectory for Clinical Gait Analysis. Front. Physiol. 10:1530. doi: 10.3389/fphys.2019.01530
Received: 16 April 2019; Accepted: 05 December 2019;
Published: 10 January 2020.
Edited by:
Ahsan H. Khandoker, Khalifa University, United Arab EmiratesReviewed by:
Hanatsu Nagano, Victoria University, AustraliaCopyright © 2020 Hori, Mao, Ono, Ora, Hirobe, Sawada, Inaba, Orimo and Miyake. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroki Ora, b3JhQGMudGl0ZWNoLmFjLmpw
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.