
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol., 13 August 2019
Sec. Craniofacial Biology and Dental Research
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.01029
This article is part of the Research TopicSaliva and Oral Microbiota: From Physiology to Diagnostic and Therapeutic ImplicationsView all 15 articles
 Khrystyna Zhurakivska1*
Khrystyna Zhurakivska1* Giuseppe Troiano1
Giuseppe Troiano1 Vito Carlo Alberto Caponio1
Vito Carlo Alberto Caponio1 Mario Dioguardi1
Mario Dioguardi1 Luigi Laino2
Luigi Laino2 Angela Bruna Maffione1
Angela Bruna Maffione1 Lorenzo Lo Muzio1
Lorenzo Lo Muzio1Background: Nitric Oxide (NO) has a role in immunitary defense, regulation of mucosal blood flow and mucus production, regulation of smooth muscle contraction, cerebral blood flow, glucose regulation, and mitochondrial function. NO can be synthetized endogenously through the L-arginine-NO pathway or it can be absorbed by the human intestine through the dietary intake. Most of the ingested NO is in the form of nitrate (NO−3NO−3). NO−3NO−3 is a substrate of oral and intestinal microbiota and, at the end of the catabolic pathway, NO is released. Using antibacterial mouthwashes leads to an alteration of salivary NO−3NO−3 metabolism, however, with unclear consequences on the circulating NO levels. The aim of this study is to perform a systematic review in order to elucidate if the alterations of oral microbiota lead to modifications in plasma NO content.
Methods: Electronic databases were screened, using the following terms: [“oral bacteria” and (nitrate OR nitrite OR nitric)]. Clinical studies reporting NO−3NO−3 and NO−2NO−2 measurements in blood and their correlation to oral microbiota variations were included. We focused on the correlation between the changes in oral microbiota and plasma concentrations of nitrites (primary outcome). Subsequently, we investigated if modifications in oral microbiota could lead to changes in blood pressure and salivary NO−2NO−2 concentration (secondary outcome).
Results: Six studies, for a total of 82 participants were included in this review. In four studies, the use of mouthwash correlated to a reduction of plasma nitrite concentration (p < 0.05); Two studies did not find any difference in plasma nitrate or nitrite concentration. In five studies, a correlation between blood pressure (BP) changes and antibacterial mouthwashing emerged. Anyway, only three studies suggested a significant increase of systolic BP following mouthwashing compared with controls.
Conclusions: Although, the role of oral bacteria has been unequivocally demonstrated in the regulation of salivary NO−3NO−3 metabolism, their influence on plasma concentration of NO species remains ambiguous. Further studies with larger sample size are required in order to demonstrate if an alteration in oral microbiota composition may influence the blood content of NO−3NO−3/NO−2NO−2/NO and all the linked biological processes.
The specific contributions of oral microbiota in human physiopathology has not been explored yet, although different studies report how the composition of oral microbiota has a role in oral and systemic diseases (Santarelli et al., 2015; Sampaio-Maia et al., 2016; Aarabi et al., 2018; Cardoso et al., 2018).
In particular, some resident bacteria in the oral cavity are able to reduce the dietary intake of nitrates (NO−3NO−3), producing nitrites (NO−2NO−2). Among these, Neisseria, Veillonella, Haemophilus, Porphyromonas, Fusobacterium, Prevotella, Leptotrichia, Brevibacillus, and Granulicatella are mainly involved in this process (Doel et al., 2005; Hyde et al., 2014). The main dietary source of nitrate is represented by vegetables (Ysart et al., 1999). Once ingested, nitrate is absorbed in the gastrointestinal tract and enters in the bloodstream. Here, it mixes to the endogenous nitrate, which mainly derives from the L-arginine-NO pathway (Leaf et al., 1989). Most of the nitrate is then excreted in the urine, while up to 25% of plasma nitrate is taken up by the salivary glands and placed in the enterosalivary circulation (Spiegelhalder et al., 1976). Salivary nitrate is metabolized to nitrite by oral commensal bacteria and it can be further reduced to nitric oxide (NO). NO−2NO−2 can be stored in blood and tissues and used when there is a decreased endogenous production of NO (Bryan and Loscalzo, 2011). Consequently, NO may contribute to a myriad of biological processes, among which: immunitary defense, regulation of mucosal blood flow and mucus production, regulation of smooth muscle contraction, cerebral blood flow, glucose regulation, and mitochondrial function (Bryan and Loscalzo, 2011). The above described processes are known as “nitrate-nitrite-nitric oxide pathway” (Zweier et al., 1995). Considering this physiological pathway, the oral microbiota gained a fundamental role; changes in its composition were assumed to affect the final NO production (Hyde et al., 2014). In particular, since the 1980s, it has been proposed that antimicrobial mouthwashing could inhibit the nitrite formation in saliva (Tannenbaum et al., 1976). Subsequently, it was supposed that such alterations could also lead to variations in the systemic availability of NO (Govoni et al., 2008; Kapil et al., 2013; Woessner et al., 2016). Plasma analysis may contribute to better understand the NO blood availability because of changes in the oral microbiota.
The aim of this study is to perform a systematic review in order to elucidate if alterations of oral microbiota lead to modifications in plasma content of nitrogen species.
The protocol for this systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Liberati et al., 2009).
In addition, it was prospectively registered on the online database PROSPERO (International prospective register of systematic reviews) with the registration number CRD42019124473.
This systematic review was performed in order to answer the following question: “Do changes in oral microbiota influence variations in blood content of nitrogen species?”
Electronic databases PubMed, SCOPUS, and Web of Science were screened independently by two authors, KZ and GT, in order to select studies suitable for inclusion in this review. The following strategy of search was used: [“oral bacteria” AND (nitrate OR nitrite OR nitric)]. In addition, bibliographies of systematic reviews and included studies were manually revised in order to find other articles to be included in this study.
Only studies published in English language and fulfilling the following criteria were considered eligible for inclusion in this review:
- Original clinical studies reporting NO−3NO−3 and NO−2NO−2 blood measurements correlated with oral microbiota variations.
No restrictions were applied about the year of publication. Studies eligibility was independently assessed in a joint session by two authors (KZ and GT). Authors screened for articles by reading only title and abstract of the studies, according to the eligibility criteria. If the data reported in abstract were not sufficient to make a clear decision, the full text publication was examined. The selected papers were full-text evaluated in a second round and, if fulfilling the inclusion criteria, were included in the qualitative analysis. Any disagreement was solved in a discussion between reviewers in a joint session.
The following information was extracted from the included papers:
- Authors' names and year of publication, study design, number of participants, type of intervention, plasma NO−3NO−3 and NO−2NO−2 changes, salivary NO−3NO−3 and NO−2NO−2 changes, Blood Pressure Changes, Relative abundance of NO−3NO−3 reducing species.
We focused on the correlation between the changes in oral microbiota and plasma concentrations of nitrites (primary outcome). Subsequently, we also investigated if modifications in oral microbiota could lead to changes in blood pressure and salivary NO−2NO−2 concentration (secondary outcome).
A total of 200 records were retrieved after the application of search strategy. After the first screening and duplicates removing, 84 records were chosen for title and abstract evaluation. Fourteen studies, resulting after this step, were selected for full text examination. Eight studies were excluded because did not meet inclusion criteria (Lundberg et al., 2004; Doel et al., 2005; Hyde et al., 2014; Clodfelter et al., 2015; Qu et al., 2016; Koch et al., 2017; Kapil et al., 2018; Preshaw, 2018). The reasons for exclusion are detailed in Table 1. The flow-chart in Figure 1 summarizes the selection process of inclusion. At the end, six papers were included in the systematic review (Table 2; Govoni et al., 2008; Kapil et al., 2013; Bondonno et al., 2015; McDonagh et al., 2015; Sundqvist et al., 2016; Woessner et al., 2016). In these studies, the test groups (mouthwashing) were compared to control (no mouthwashing) and implications on the production of nitrogen species were evaluated. Other three studies with a different design were identified and deemed valid to be included in the present review (Burleigh et al., 2018; Vanhatalo et al., 2018; Liddle et al., 2019). They only investigated how changes in oral microbiota could lead to alterations in NO−3NO−3/NO−2NO−2 concentrations in human fluids. They were considered relevant for the development of the topic and are showed separately in the results (Table 3).
The designs of the experiments were heterogeneous among studies, so it was not possible to perform a quantitative analysis. A total of 82 participants were enrolled in the included studies. The effect of various mouthwashes on NO−3NO−3 metabolism was investigated, providing discordant results among the studies. Four studies (Govoni et al., 2008; Kapil et al., 2013; McDonagh et al., 2015; Woessner et al., 2016) detected a significant negative influence of mouthwashing on plasma nitrite concentration (p < 0.05), meanwhile other two studies (Bondonno et al., 2015; Sundqvist et al., 2016) found no significant alteration of plasma nitrate and nitrite, comparing mouthwash administration against placebo.
The types of mouthwashes used in the studies were different; nevertheless all of them contained chlorhexidine. In two studies more than one mouthwash was tested (McDonagh et al., 2015; Woessner et al., 2016), resulting in some differences in the outcomes (Table 2). Results regarding the secondary outcome:
- Effects of mouthwashing on the salivary NO−2NO−2 changes: there were significant differences between the test and the control groups in all the studies (Govoni et al., 2008; Kapil et al., 2013; Bondonno et al., 2015; McDonagh et al., 2015; Sundqvist et al., 2016; Woessner et al., 2016).
- Effects of mouthwashing on Blood Pressure were evaluated in five studies: in three studies, Systolic Blood Pressure resulted significantly higher in the test groups compared to the controls (Kapil et al., 2013; Bondonno et al., 2015; Woessner et al., 2016). One study did not report any difference between test and control group (Sundqvist et al., 2016). In one study, the differences emerged only in some conditions (i.e., during treadmill walking), but not in others (McDonagh et al., 2015).
Three of the included studies, identified the types of oral nitrate reducing species and correlated their abundance with plasma NO−2NO−2 changes (Burleigh et al., 2018; Vanhatalo et al., 2018; Liddle et al., 2019). The most abundant species emerged to be: Prevotella melaninogenica, Veillonella dispar, Hemophilus parainfluenzae, Neisseria subflava, Veillonella parvula, Fusobacterium nucleatum subsp. nucleatum, Campylobacter concisus, Leptorichia buccalis, Prevotella intermedia. In two of these studies no correlation emerged between the abundance of NO−3NO−3 reducing species and plasma NO−2NO−2 changes (Burleigh et al., 2018; Liddle et al., 2019). Furthermore, in one study emerged how NO−3NO−3 supplementation influenced the salivary microbiome, leading to an increase in the relative abundance of Proteobacteria (+225%) and decrease of Bacteroidetes (−46%; P < 0.05; Vanhatalo et al., 2018). High relative quantities of Rothia and Neisseria and low presence of Prevotella and Veillonella were correlated with greater increases in plasma [NO−2NO−2] in response to NO−3NO−3 supplementation (Vanhatalo et al., 2018).
Numerous bacteria express nitrate reductase genes and are capable to reduce nitrate to nitrite in vitro (Torres et al., 2016). In humans a physiologically relevant nitrate reduction occurs by means of some facultative anaerobic bacteria localized in crypts of the tongue (Duncan et al., 1995; Doel et al., 2005). Among the highest nitrate-reducers species, identified in the oral cavity, there are: Firmicutes (Staphylococcus, Streptococcus, and Veillonella) and Actinobacteria (Actinomyces), followed by numerous other taxa, including Pasteurella, Rothia, Neisseria, Haemophilus, Granulicatella (Smith et al., 1999; Palmerini et al., 2003; Hyde et al., 2014). Using dietary nitrate supplementation as selective pressure for bacteria capable of nitrate reduction, several studies found proliferation of Veillonella (Koopman et al., 2016), Neisseria (Velmurugan et al., 2016), and Rothia species (Velmurugan et al., 2016), all previously identified as high nitrate reducers. These data, as well as those of the studies examined in this review, confirm a close link between the oral microbiota and the salivary circulation of nitrates. However, the scientific evidence of a systemic repercussion is still poor today.
In order to evaluate the efficacy and/or alteration of conversion of oral nitrate to estimate the availability of NO pool, most of the studies, including our analysis, used plasma nitrite concentration (or changes in concentration), since the direct measurement of NO is difficult. This approach is justified by the relationships between plasma nitrite concentrations and physiological effects, as demonstrated by James et al. (2015). For this reason, only studies reporting blood measurements of nitrogen species were included in this review. Given this, the aim of the review was to verify even the alteration of oral microbiota could have a direct impact on plasma changes in nitrite concentration and, as proposed by some studies (Bondonno et al., 2015; Tribble et al., 2019), repercussions on Blood pressure or other biological processes (Joshipura et al., 2017).
From our analysis emerged some discordant results. Most of the evaluated studies report a correlation between oral microbiota and nitrites variations in plasma (Govoni et al., 2008; Kapil et al., 2013; McDonagh et al., 2015; Woessner et al., 2016). Two studies found no effect of mouthwashing on changes in plasma nitrites (Bondonno et al., 2015; Sundqvist et al., 2016). Govoni et al. (2008), despite having found a marked attenuation after mouthwash, noted that a significant rise in plasma nitrite occurred. Interestingly, this occurred despite the fact that the nitrite formation in the mouth has been completely abolished by the intervention, excluding its salivary origin. They proposed the existence of an alternative origin of nitrite, giving two possible explanations for this phenomenon: in the first hypothesis, they supposed a possible contribute of the gastrointestinal microflora to the nitrate reduction; in the second one, they hypothesized that mammalian cells in the gut wall o in another district are capable of nitrate reduction. However, despite this interesting hypothesis, results show that the sudden rise in plasma nitrite after nitrate ingestion is mainly deriving from bacterial nitrate reduction in the oral cavity and the use of antibacterial mouthwashes may have an impact on this chain. Even if, results of the interventions analyzed in the present review are statistically significant, the clinical repercussions need further investigation. Among these, the effects of the mouthwashes on Blood Pressure were those more analyzed, given the strong effect that the intake of rich in nitrates food has on blood pressure (Ashworth and Bescos, 2017). Following mouthwashing, a certain increase in blood pressure occurred in three studies (Kapil et al., 2013; Bondonno et al., 2015; Woessner et al., 2016), although, it should be noted that in one of these studies (Bondonno et al., 2015), the increase in BP was not associated with a statistically significant reduction in plasma nitrites. This controversial result could be linked to a bias in the kind of the patients included. The experiments were conducted in hypertensive men and women already in treatment with antihypertensive medication.
Another important risk of bias in interpreting the results, is given by the heterogeneity of the experiments conducted in various studies, in particular by the different timing of the fluid analysis, which could lead to mismatch in the comparison of the results, since the nitrate metabolism is subject to sudden changes in association with time.
Although, most of the included studies demonstrate a statistically significant correlation between an alteration of the oral microbiota due to the use of antibacterial mouthwash and plasma changes in nitrite concentration, this link has not been established yet. Further RCT studies with larger samples and well defined designs are necessary in order to demonstrate if an alteration in oral microbiota composition may influence the blood content of NO−3NO−3/NO−2NO−2/NO and, consequently, all the biological processes that depends on them.
All datasets for this study are included in the manuscript and the supplementary files.
KZ and GT performed online research and collected data. VC analyzed data and results. LLa and MD contribute to write the manuscript. LLo and AM conceived and supervised the research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aarabi, G., Schnabel, R. B., Heydecke, G., and Seedorf, U. (2018). Potential impact of oral inflammations on cardiac functions and atrial fibrillation. Biomolecules 8:E66. doi: 10.3390/biom8030066
Ashworth, A., and Bescos, R. (2017). Dietary nitrate and blood pressure: evolution of a new nutrient? Nutr. Res. Rev. 30, 208–219. doi: 10.1017/S0954422417000063
Bondonno, C. P., Liu, A. H., Croft, K. D., Considine, M. J., Puddey, I. B., Woodman, R. J., et al. (2015). Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am. J. Hypertens. 28, 572–575. doi: 10.1093/ajh/hpu192
Bryan, N. S., and Loscalzo, J. (2011). Nitrite and Nitrate in Human Health and Disease. Cham: Springer. doi: 10.1007/978-1-60761-616-0
Burleigh, M. C., Liddle, L., Monaghan, C., Muggeridge, D. J., Sculthorpe, N., Butcher, J. P., et al. (2018). Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic. Biol. Med. 120, 80–88. doi: 10.1016/j.freeradbiomed.2018.03.023
Cardoso, E. M., Reis, C., and Manzanares-Céspedes, M. C. (2018). Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 130, 98–104. doi: 10.1080/00325481.2018.1396876
Clodfelter, W. H., Basu, S., Bolden, C., Dos Santos, P. C., King, S. B., and Kim-Shapiro, D. B. (2015). The relationship between plasma and salivary NOx. Nitric Oxide 47, 85–90. doi: 10.1016/j.niox.2015.04.003
Doel, J. J., Benjamin, N., Hector, M. P., Rogers, M., and Allaker, R. P. (2005). Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 113, 14–19. doi: 10.1111/j.1600-0722.2004.00184.x
Duncan, C., Dougall, H., Johnston, P., Green, S., Brogan, R., Leifert, C., et al. (1995). Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1, 546–551. doi: 10.1038/nm0695-546
Govoni, M., Jansson, E. A., Weitzberg, E., and Lundberg, J. O. (2008). The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19, 333–337. doi: 10.1016/j.niox.2008.08.003
Hyde, E. R., Andrade, F., Vaksman, Z., Parthasarathy, K., Jiang, H., Parthasarathy, D. K., et al. (2014). Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS ONE 9:e88645. doi: 10.1371/journal.pone.0088645
James, P. E., Willis, G. R., Allen, J. D., Winyard, P. G., and Jones, A. M. (2015). Nitrate pharmacokinetics: taking note of the difference. Nitric Oxide 48, 44–50. doi: 10.1016/j.niox.2015.04.006
Joshipura, K. J., Muñoz-Torres, F. J., Morou-Bermudez, E., and Patel, R. P. (2017). Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric Oxide 71, 14–20. doi: 10.1016/j.niox.2017.09.004
Kapil, V., Haydar, S. M., Pearl, V., Lundberg, J. O., Weitzberg, E., and Ahluwalia, A. (2013). Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 55, 93–100. doi: 10.1016/j.freeradbiomed.2012.11.013
Kapil, V., Rathod, K. S., Khambata, R. S., Bahra, M., Velmurugan, S., Purba, A., et al. (2018). Sex differences in the nitrate-nitrite-NO(*) pathway: role of oral nitrate-reducing bacteria. Free Radic. Biol. Med. 126, 113–121. doi: 10.1016/j.freeradbiomed.2018.07.010
Koch, C. D., Gladwin, M. T., Freeman, B. A., Lundberg, J. O., Weitzberg, E., and Morris, A. (2017). Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic. Biol. Med. 105, 48–67. doi: 10.1016/j.freeradbiomed.2016.12.015
Koopman, J. E., Buijs, M. J., Brandt, B. W., Keijser, B. J., Crielaard, W., and Zaura, E. (2016). Nitrate and the origin of saliva influence composition and short chain fatty acid production of oral microcosms. Microb. Ecol. 72, 479–492. doi: 10.1007/s00248-016-0775-z
Leaf, C. D., Wishnok, J. S., and Tannenbaum, S. R. (1989). L-arginine is a precursor for nitrate biosynthesis in humans. Biochem. Biophys. Res. Commun. 163, 1032–1037. doi: 10.1016/0006-291X(89)92325-5
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 62, e1–e34. doi: 10.1016/j.jclinepi.2009.06.006
Liddle, L., Burleigh, M. C., Monaghan, C., Muggeridge, D. J., Sculthorpe, N., Pedlar, C. R., et al. (2019). Variability in nitrate-reducing oral bacteria and nitric oxide metabolites in biological fluids following dietary nitrate administration: an assessment of the critical difference. Nitric Oxide 83, 1–10. doi: 10.1016/j.niox.2018.12.003
Lundberg, J. O., Weitzberg, E., Cole, J. A., and Benjamin, N. (2004). Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2, 593–602. doi: 10.1038/nrmicro929
McDonagh, S. T., Wylie, L. J., Winyard, P. G., Vanhatalo, A., and Jones, A. M. (2015). The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int. J. Sports Med. 36, 1177–1185. doi: 10.1055/s-0035-1554700
Palmerini, C. A., Palombari, R., Perito, S., and Arienti, G. (2003). NO synthesis in human saliva. Free Radic. Res. 37, 29–31. doi: 10.1080/1071576021000028398
Preshaw, P. M. (2018). Mouthwash use and risk of diabetes. Br. Dent. J. 225, 923–926. doi: 10.1038/sj.bdj.2018.1020
Qu, X. M., Wu, Z. F., Pang, B. X., Jin, L. Y., Qin, L. Z., and Wang, S. L. (2016). From nitrate to nitric oxide: the role of salivary glands and oral bacteria. J. Dent. Res. 95, 1452–1456. doi: 10.1177/0022034516673019
Sampaio-Maia, B., Caldas, I. M., Pereira, M. L., Pérez-Mongiovi, D., and Araujo, R. (2016). The oral microbiome in health and its implication in oral and systemic diseases. Adv. Appl. Microbiol. 97, 171–210. doi: 10.1016/bs.aambs.2016.08.002
Santarelli, A., Mascitti, M., Rubini, C., Bambini, F., Zizzi, A., Offidani, A., et al. (2015). Active inflammatory biomarkers in oral lichen planus. Int. J. Immunopathol. Pharmacol. 28, 562–568. doi: 10.1177/0394632015592101
Smith, A., Benjamin, N., Weetman, D., Mackenzie, D., and Macfarlane, T. (1999). The microbial generation of nitric oxide in the human oral cavity. Microb. Ecol. Health Dis. 11, 23–27. doi: 10.3402/mehd.v11i1.7880
Spiegelhalder, B., Eisenbrand, G., and Preussmann, R. (1976). Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet. Toxicol. 14, 545–548. doi: 10.1016/S0015-6264(76)80005-3
Sundqvist, M. L., Lundberg, J. O., and Weitzberg, E. (2016). Effects of antiseptic mouthwash on resting metabolic rate: a randomized, double-blind, crossover study. Nitric Oxide 61, 38–44. doi: 10.1016/j.niox.2016.10.003
Tannenbaum, S. R., Weisman, M., and Fett, D. (1976). The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet. Toxicol. 14, 549–552. doi: 10.1016/S0015-6264(76)80006-5
Torres, M. J., Simon, J., Rowley, G., Bedmar, E. J., Richardson, D. J., Gates, A. J., et al. (2016). Nitrous oxide metabolism in nitrate-reducing bacteria: physiology and regulatory mechanisms. Adv. Microb. Physiol. 68, 353–432. doi: 10.1016/bs.ampbs.2016.02.007
Tribble, G. D., Angelov, N., Weltman, R., Wang, B. Y., Eswaran, S. V., Gay, I. C., et al. (2019). Frequency of tongue cleaning impacts the human tongue microbiome composition and enterosalivary circulation of nitrate. Front. Cell. Infect. Microbiol. 9:39. doi: 10.3389/fcimb.2019.00039
Vanhatalo, A., Blackwell, J. R., L'heureux, J. E., Williams, D. W., Smith, A., Van Der Giezen, M., et al. (2018). Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 124, 21–30. doi: 10.1016/j.freeradbiomed.2018.05.078
Velmurugan, S., Gan, J. M., Rathod, K. S., Khambata, R. S., Ghosh, S. M., Hartley, A., et al. (2016). Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 103, 25–38. doi: 10.3945/ajcn.115.116244
Woessner, M., Smoliga, J. M., Tarzia, B., Stabler, T., Van Bruggen, M., and Allen, J. D. (2016). A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide 54, 1–7. doi: 10.1016/j.niox.2016.01.002
Ysart, G., Miller, P., Barrett, G., Farrington, D., Lawrance, P., and Harrison, N. (1999). Dietary exposures to nitrate in the UK. Food Addit. Contam. 16, 521–532. doi: 10.1080/026520399283669
Keywords: oral microbiota, oral bacteria, nitrite (nitrate) [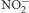 (
(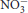 )], mouthwash, nitric oxide—NO
)], mouthwash, nitric oxide—NO
Citation: Zhurakivska K, Troiano G, Caponio VCA, Dioguardi M, Laino L, Maffione AB and Lo Muzio L (2019) Do Changes in Oral Microbiota Correlate With Plasma Nitrite Response? A Systematic Review. Front. Physiol. 10:1029. doi: 10.3389/fphys.2019.01029
Received: 27 March 2019; Accepted: 25 July 2019;
Published: 13 August 2019.
Edited by:
Giovanna Orsini, Marche Polytechnic University, ItalyReviewed by:
Heleni Vastardis, National and Kapodistrian University of Athens, GreeceCopyright © 2019 Zhurakivska, Troiano, Caponio, Dioguardi, Laino, Maffione and Lo Muzio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khrystyna Zhurakivska, a2hyeXN0eW5hLnpodXJha2l2c2thQHVuaWZnLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.