
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 16 July 2019
Sec. Exercise Physiology
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.00903
This article is part of the Research TopicExploration of the Physiological Effects of Exercise in Cardiovascular DiseasesView all 10 articles
Background: We hypothesized that a 2-week twice daily aquatic endurance plus calisthenics exercise training program: (i) increases aerobic exercise capacity (peak oxygen uptake/O2peak), (ii) improves endothelium-dependent flow-mediated vasodilation (FMD), and (iii) reduces circulating markers of low-grade inflammation and hemostasis, as compared to land-based endurance plus calisthenics exercise training or no exercise in patients undergoing short-term residential cardiac rehabilitation after a recent coronary artery disease (CAD) event.
Methods: Patients with a recent myocardial infarction or revascularization procedure were randomized into two interventional groups and a control group. The interventional groups underwent supervised aerobic endurance plus calisthenics exercise training either in thermo-neutral water or on land at moderate intensity (60–80% of the peak heart rate achieved during symptom-limited graded exercise testing) for 30 min twice daily for 2 weeks (i.e., 24 sessions). The control group was deferred from supervised exercise training for the 2-week duration of the intervention, but was advised low-to-moderate intensity physical activity at home while waiting. At baseline and after the intervention period, all participants underwent estimation of aerobic exercise capacity, brachial artery flow-mediated dilatation (FMD, measured ultrasonographically at rest and during reactive hyperemia after 4.5 min of forearm cuff inflation), markers of cardiac dysfunction (NT-proBNP), inflammation (hsCRP, IL-6, IL-8, IL-10), cell adhesion (ICAM, P-selectin), and hemostasis (fibrinogen, D-dimer).
Results: A total of 89 patients (mean age 59.9 ± 8.2 years, 77.5% males, O2peak at baseline 14.8 ± 3.5 ml kg-1 min-1) completed the study. Both exercise modalities were safe (no significant adverse events recorded) and associated with a significant improvement in O2peak as compared to controls: age and baseline O2peak-adjusted end-of-study O2peak increased to 16.7 (95% CI 16.0–17.4) ml kg-1 min-1 with land-based training (p < 0.001 for change from baseline) and to 18.6 (95% CI 17.9–19.3) ml kg-1 min-1 with water-based training (p < 0.001 for change from baseline), but not in controls (14.9 ml kg-1 min-1; 95% CI 14.2–15.6; p = 0.775 for change from baseline). FMD also increased in both intervention groups (from 5.5 to 8.8%, p < 0.001 with land-based, and from 7.2 to 9.2%, p < 0.001 with water-based training, respectively), as compared to controls (p for change 0.629). No significant changes were detected in biomarkers of inflammation, cell adhesion or hemostasis, whereas levels of NT-proBNP (marker of cardiac dysfunction) decreased in the water-based training group (p = 0.07 vs. controls).
Conclusion: Endurance plus calisthenics exercise training in thermo-neutral water is safe, and improves aerobic exercise capacity and vascular function in patients undergoing short-term residential cardiac rehabilitation after a recent CAD event.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier NCT02831829.
Exercise-based cardiac rehabilitation remains a cornerstone of management and secondary prevention in patients with coronary artery disease (CAD) (Piepoli et al., 2010; Anderson et al., 2016). Acute coronary events – such as a recent myocardial infarction and/or coronary artery bypass grafting (CABG) procedure – may impair the ability of individuals to engage in exercise because of cardiac dysfunction, risks associated with the acute effects of exercise, post-procedure recovery, or immediate post-event psychological concerns (Anderson et al., 2016). In this respect, cardiac rehabilitation – either in outpatient settings or as an intensive short-term residential program – provides sufficient monitoring and reassurance to patients in the immediate aftermath of a recent CAD event, thus empowering them to confidently adopt long-term regular exercise and a healthy lifestyle (Mampuya, 2012; Menezes et al., 2014).
Aerobic exercise training on land (such as cycling, walking, jogging, or rowing) – either alone, or supplemented by non-weight bearing exercises (calisthenics) or low-weight resistance training – has been the most studied and therefore the most widely implemented (Bjarnason-Wehrens et al., 2010) exercise modality in cardiac rehabilitation programs. On the one hand, this exercise modality is safe in CAD patients, purportedly because it provides a regulated cardiac output increase to meet the perfusion demand of large exercising muscle groups, thus minimizing safety concerns over raised pre- and after-load in high-risk cardiac patients with low aerobic exercise capacity – such as those after a recent myocardial infarction or revascularization procedure (Balady et al., 2007). On the other hand, this exercise modality has been shown to mitigate risk factors and metabolic abnormalities (Casillas et al., 2007; Ismail et al., 2013), which contribute to CAD and its progression, as well as to improve endothelial dysfunction, which plays a central role in all stages of atherosclerosis. In patients with CAD, endothelium-dependent vascular dysfunction may be associated with impaired systemic (skeletal muscle) and coronary (myocardial) perfusion, resulting in exercise intolerance and myocardial ischemia at exertion, respectively (Bruning and Sturek, 2015). Moreover, endothelial dysfunction in CAD is associated with low-grade inflammation and increased monocyte adhesion, which promote atherosclerotic plaque build-up and rupture, as well as with increased hemostatic activity, which promotes coronary thrombosis (Kokkinos and Myers, 2010; Winzer et al., 2018). Conversely, land-based exercise training has been shown to revert or reduce these abnormalities. In a seminal study by Hambrecht et al. (2000), 4 weeks of in–hospital bicycle ergometer training improved endothelium–dependent arterial vasomotion in patients with CAD, likely through restoring the balance between synthesis and depletion of vasoactive and vasoprotective nitric oxide (NO) (Hambrecht et al., 2003). Mechanistically, this is largely due to a response to exercise-induced shear stress (Kokkinos and Myers, 2010; Bruning and Sturek, 2015). In addition, regular aerobic exercise training on land is associated with a reduction in low-grade inflammation [as assessed by high-sensitive C-reactive protein (hsCRP) levels], cell adhesion (as assessed by cell adhesion molecules, such as ICAM and P-selectin) and hemostatic activity (as assessed by markers of coagulation and fibrinolysis, such as fibrinogen and D-dimer) (Womack et al., 2003; Pedersen, 2017), suggesting an interplay between the effects of aerobic exercise on the endothelium, low-grade inflammation, cell adhesion, and hemostasis in patients with CAD (Kokkinos and Myers, 2010; Winzer et al., 2018).
In contrast to exercise on land, the implementation of aquatic exercises in cardiac rehabilitation programs remains debated (Lazar et al., 2013). Concerns have been traditionally raised over possible adverse cardiovascular effects of exercising in water – namely, an increased preload due to water immersion (i.e., hydrostatically driven raise in venous return and central venous pressure, possibly yielding ventricular dysfunction) (Meyer, 2006; Pendergast et al., 2015; Shah et al., 2017) and/or an increased afterload due to temperatures below thermo-neutrality (i.e., vasoconstriction in cold water associated with a risk of dysrhythmias) (Schmid et al., 2009). On the other hand, the very same hemodynamic effects of thermo-neutral water immersion in patients with CAD have been associated with favorable improvements in cardiac performance and peripheral vascular reactivity after as little as 3 weeks of aquatic cardiac rehabilitation, suggesting pronounced exercise-induced shear stress as a possible mechanism (Mourot et al., 2010). Yet, previous research on the impact of aquatic exercise in patients with CAD is limited when compared with land-based modalities (Schmid et al., 2007, 2009; Volaklis et al., 2007; Laurent et al., 2009; Teffaha et al., 2011; Choi et al., 2015), and marred by small number of participants, selective patient inclusion [limited to stable CAD (Volaklis et al., 2007; Teffaha et al., 2011) or to patients achieving > 7 METs at exercise testing, (Tokmakidis et al., 2008) thus not reflecting the patient populations referred for cardiac rehabilitation in the immediate aftermath of a CAD event], and inferior study design [e.g., pre-post studies without comparator groups (Korzeniowska-Kubacka et al., 2016) or non-randomized patient separation] (Tokmakidis et al., 2008), with four notable exceptions. Volaklis et al., 2007 randomized 24 patients with stable CAD to 16 weeks of either water cycling plus water games, land cycling plus resistance training, or no exercise at all, and showed that water- and land-based protocols comparably improved exercise test time, muscle strength and blood lipid profiles. Conversely, Lee et al. (2017) randomized 60 older (>65 years old) patients with CAD and osteoarthritis to 24 weeks of either aqua walking or treadmill walking, and showed that both protocols comparably improved aerobic exercise capacity, but the improvements in body composition and lipid levels were significantly more pronounced with aqua walking. Teffaha et al. (2011) randomized 24 patients with CAD and 24 patients with heart failure to 3 weeks of cardiac rehabilitation, comprising land cycling plus calisthenics either on land or in water; both protocols were associated with significant increase in aerobic exercise capacity in patients with CAD. Similarly, Laurent et al. (2009) randomized 24 patients with CAD and 24 patients with heart failure to 3 weeks of cardiac rehabilitation, comprising land cycling plus gymnastics either on land or in water; both protocols improved aerobic exercise capacity in patients with CAD, but only water training was associated with increased levels of NO metabolites after the intervention period – indirectly suggesting an improvement in endothelial function with aquatic exercise. Given the specific hemodynamic responses to exercising in xiphoid-level water, with increased peripheral blood flow and enhanced shear stress yielding endothelial nitric oxide synthase up-regulation (Di Francescomarino et al., 2009; Ayme et al., 2014), improvements in endothelium-dependent vascular function may be hypothesized, and have indeed been confirmed with aquatic exercise in prehypertensive adults (Nualnim et al., 2012) and in patients with osteoarthritis (Alkatan et al., 2016), but not in patients with CAD. Thus, while several studies confirmed the relative cardiovascular safety of thermo-neutral water immersion in patients with CAD, only a limited number of trials assessed the efficacy of aquatic training modalities on aerobic exercise capacity, and none appraised the effect of such training on vascular function or explored its interplay with low-grade inflammation and hemostasis.
Therefore, we sought to compare the effect of a land- and a water-based exercise training program on aerobic exercise capacity and vascular function. In addition, we assumed that the improvements in endothelium-dependent vascular function would be accompanied by a reduction in markers of low-grade inflammation, endothelial adhesion and coagulation, given the association between inflammation and endothelial dysfunction, and the central role of endothelial integrity in promoting cell adhesion and coagulation. Hence, we hypothesized that a 2-week, twice-daily aquatic endurance plus calisthenics exercise training program would (i) increase aerobic exercise capacity (peak oxygen uptake/O2peak), (ii) improve endothelium-dependent flow-mediated vasodilation (FMD), and (iii) reduce markers of low-grade inflammation, cell adhesion, and hemostasis as compared to land-based endurance plus calisthenics exercise training and to no exercise in patients undergoing short-term residential cardiac rehabilitation after a recent CAD event.
The study was designed as a prospective, randomized, open-label clinical trial with three parallel groups (two intervention groups and one control group) (Figure 1).
The study was carried out at the Centre for Cardiac Rehabilitation in Šmarješke Toplice, Slovenia. The center provides residential cardiac rehabilitation for patients after a myocardial infarction or open-heart surgery, with a live-in 14-day program encompassing twice-daily supervised exercise training sessions, education, dietary and smoking cessation advice, medical supervision, and psychological counseling for individuals without access to outpatient rehabilitation services.
Patients after a recent CAD event [2–4 weeks after myocardial infarction, percutaneous coronary intervention (PCI) and/or coronary artery by-pass surgery (CABG)] with a left ventricular ejection fraction (LVEF) above 40% were invited to participate. Recruitment took place between May and October 2016. Patients with uncontrolled/decompensated valve diseases necessitating specific (surgical or percutaneous) management, patients after valve replacement, with uncontrolled dysrhythmias or presence of a permanent pacemaker, with contraindications to exercise, unable to perform exercise testing or to swim, with mental impairment, severe anemia, severe obstructive/restrictive lung disease, recent thromboembolic events, hepatic dysfunction, and/or age over 80 years were excluded.
Patients were randomized into (a) a water-based exercise training group, (b) a land-based exercise training group, or (c) a control group, using adaptive urn-randomization with sealed envelopes and allocation concealment from the recruiting investigator.
Before and after the intervention period (on Day 0 and Day 14), all participants underwent cardiopulmonary exercise testing, ultrasonographic assessment of FMD of the brachial artery, and blood sample collection. The primary outcomes were change from baseline of O2peak and FMD. Exploratory outcomes included change from baseline of biomarkers of low-grade inflammation (hsCRP, IL6, IL8, IL10) and endothelial activation (ICAM, P-selectin), hemostasis (D-dimer, fibrinogen), and neurohormonal activity (NT-proBNP).
Written informed consent was obtained for each participant. The study complied with the World Medical Association Declaration of Helsinki on ethics in medical research and was approved by a local medical research ethics committee (0120-655/2016-2). This study is registered at ClinicalTrials.gov, number NCT02831829.
The intervention was designed as either a water- or a land-based endurance plus calisthenics exercise program during 2-week residential cardiac rehabilitation. The exercise programs consisted of 30-min training sessions twice daily, 6 days a week (24 sessions in total).
Other aspects of rehabilitation (including lifestyle education and provision of a Mediterranean-style diet, medical supervision, psychological support) were identical for both intervention groups. The principles of treatment were not changed over the intervention period, but medication adjustment was allowed at the discretion of the treating cardiologist to ensure optimal control of risk factors.
Water-based exercise training comprised two daily training sessions in a heated swimming pool (32.8°C), with water depth at the xiphoid process level (1.5 m). The exercise program consisted of two 30-min sessions daily, namely aerobic endurance and calisthenics. Aerobic endurance exercise comprised 5 min of warm-up, 20 min of conditioning (water walking, side-stepping, cycling with arms) at 60–80% peak heart rate achieved during symptom limited graded exercise testing, and 5 min of cool-down. Calisthenics comprised 5 min of warm-up, 20 min of conditioning (engaging muscle groups of the upper and lower limbs, such as triceps extensions, triceps dips, modified leg press, leg abduction/adduction, wall push-ups at 60–80% peak heart rate), and 5 min of cool-down.
Land-based exercise training comprised two 30-min sessions daily, namely bicycle ergometer training (5 min warm-up, 20 min at 60–80% peak heart rate, and 5 min of cool-down) and calisthenics (5 min of warm-up, 20 min of exercises engaging muscle groups of the upper and lower limbs at 60–80% peak heart rate with a progressive increase in speed and the number of repetitions), and cool-down.
Patients in the control group were given lifestyle advice, and made aware of the beneficial effects of exercise and advised to engage in regular physical activities (i.e., usual daily activities, such as walking), but were asked to refrain from enrolling in a supervised exercise program for the duration of the intervention period (i.e., 2 weeks).
Aerobic exercise capacity was determined by measuring O2peak by cardiopulmonary bicycle exercise testing (CPET) using the cycle-ergometer Schiller CS-200 (Schiller A.G. Baar, Switzerland) with the Ganshorn Power Cube gas analysis unit (Ganshorn Deutschland GmbH). Calibration of primary sensors for flow, O2 and CO2 gas measurement were performed before each exercise test. All the participants underwent a symptom-limited exercise test. They were advised to adhere to normal medical regimes, avoid exercise and heavy meals on the day of testing. Resting data including ECG were monitored 3 min before starting the test. Participants were tested using a maximal incremental protocol: after 3 min of unloaded cycling (“0 W”), the work rate was continuously increased on the computer-controlled cycle ergometer in a ramp-like fashion to achieve the predicted maximal workload after 10 min. Predicted maximal workload on the bicycle ergometer was estimated based on age, gender, and body surface area. The test was considered completed if the respiratory exchange ratio achieved was ≥1.1. During exercise, participants wore a mouthpiece connected with the gas analysis unit, thus measuring oxygen and carbon dioxide flow (O2 and CO2, respectively). ECG and heart rate were continuously monitored, and records were made every 2 min. Blood pressure was measured at rest and every 2 min during the test and cool-down period. Monitoring of the participants and the mentioned parameters continued for 6 min after test termination. There were no clinically relevant adverse effects during the exercise testing. To assess the reproducibility of exercise testing, 10 subjects were selected randomly and tested twice before the intervention started. The intra-class correlation coefficient (ICC for single measure) for O2peak was 0.861, p = 0.004.
Endothelial function was assessed by flow-mediated dilatation (FMD) of the right brachial artery with ultrasound scanning (Philips ultrasound system iE 33 with a high resolution linear-array vascular probe with a frequency of 10 MHz), under standardized conditions and in accordance with current recommendations (Flammer et al., 2012). The brachial artery was imaged 2–10 cm above the elbow fossa. To determine the endothelium-dependent vasodilatation, the forearm was tightened with the sphygmomanometer cuff until a pressure of 50 mmHg higher than the systolic pressure value was achieved. The grip was released after 4.5 min. Flow was measured within 15–20 s, and artery diameter 60–90 s after releasing the grip. After 15 min of rest, endothelium-independent vasodilatation was measured, induced by 0.4 mg of nitroglycerin (Nitrolingual spray®) upon sublingual spray application. The diameter of the brachial artery and the average velocity of blood flow were measured 3–4 min after dosing. FMD was expressed as percentage change from rest [(brachial artery diameter at peak hyperemia – diameter at rest) × 100/diameter at rest]. To assess the reproducibility of FMD, 10 subjects were selected randomly. The intra-class correlation coefficient for FMD was 0.855, p = 0.004.
All patients had venous blood samples taken in the fasting state, in the morning, after 30 min of rest in the supine position, from the cubital vein into 4.5 mL vacuum tubes containing 0.11 mol/L sodium citrate (Becton Dickinson, Vacutainer System Europe, Germany). Plasma was prepared within 30 min with 20-min centrifugation at 2,000 × g. In fresh plasma, the concentrations of fibrinogen (Dade® Thrombin Reagent) and D-dimer (Innovance D-dimer, both Siemens Healthcare Diagnostics, Marburg, Germany) were determined on an automated coagulation analyzer CS2100i (Sysmex, Kobe, Japan). The remaining plasma was aliquoted, snap frozen in liquid nitrogen and stored at –75°C until analysis. In thawed plasma, NT-proBNP was determined on a Stratus® CS Acute CareTM analyzer based upon solid phase Radial Partition Immunoassay (RPIA) technology (Siemens Healthcare Diagnostics, Marburg, Germany). Plasma CRP, ICAM-1, IL-6, IL-8, IL-10, and P-selectin were measured with the xMAP® Technology utilizing magnetic beads coupled with specific antibodies (all R&D Systems, Minneapolis, United States) on a MagPix instrument (Luminex Corporation, Austin, United States).
Data are presented as mean (standard deviation) for normally distributed continuous variables and as median (interquartile range) otherwise. Differences in the baseline characteristics of patients between groups were tested by ANOVA or Kruskal–Wallis test, as appropriate. Differences in the primary objective, end-of-study VO2 max, between study groups were tested using ANCOVA, controlling for baseline VO2 max and age of patients. ANCOVA with age and baseline measurement included as a covariate was used for all other normally distributed variables. Post hoc differences were tested by Sidak test. Variables, measured in percentages (FMD and NMD) were logarithmized prior the analysis. For the logarithmized FMD, the assumption of homogeneity of regression slopes was violated, therefore, between and within group ANOVA with age as a covariate was used to test the between-group differences. In all other non-normally distributed variables, the change from baseline for each patient was calculated and Kruskal–Wallis test was used to test the differences in change from baseline between the study groups. When statistically significant differences between groups were found, Mann–Whitney U test was used to test pairwise differences. The differences with p < 0.05 were treated as statistically significant. All analyses were done using the software SPSS, v. 21.
Sample size calculation suggests an 80% power at 0.05 significance level for the detection of a between-group difference (and assumed between-subject standard deviation) of 1 MET (3.5 ml kg-1 min-1) with the inclusion of 90 patients (30 per group).
The differences with p < 0.05 were treated as statistically significant. All analyses were done using the software SPSS, v. 21.
Eighty-nine patients completed the study: 30 patients in the land-based training group, 29 in the water-based training group, and 30 in the control group; one participant dropped out during the course of the study due to upper respiratory tract infection (Figure 1). Mean age of the participants who completed the study was 59.9 ± 8.2, and 77.5% were male. All the participants had CAD, and 77 (86.5%) had suffered a myocardial infarction. Patients randomized to the land-based group were significantly older and achieved a lower O2 peak at baseline (Table 1).
Both exercise modalities were associated with a statistically significant increase in O2peak as compared with controls (Figure 2). After controlling for baseline O2peak and patients’ age (ANCOVA), mean estimate end-of-study O2peak increased by 15.3% (16.7 ml kg-1 min-1; 95% CI 16.0–17.4 ml kg-1 min-1; p < 0.001 for change from baseline) with land-based training, and by 27.4% (18.6 ml kg-1 min-1; 95% CI 17.9–19.3 ml kg-1 min-1; p < 0.001 for change from baseline) with water-based training, but not in controls (a 0.6% increase, i.e., 14.9 ml kg-1 min-1, 95% CI 14.2–15.6; 14.9 ml kg-1 min-1; p = 0.775 for change from baseline). The effect size (d) was moderate in the land-based group (d = 0.61), and large in the water-based group (d = 1.02). Time-to-exhaustion and peak workload also increased significantly in both intervention groups compared to controls (Table 2).
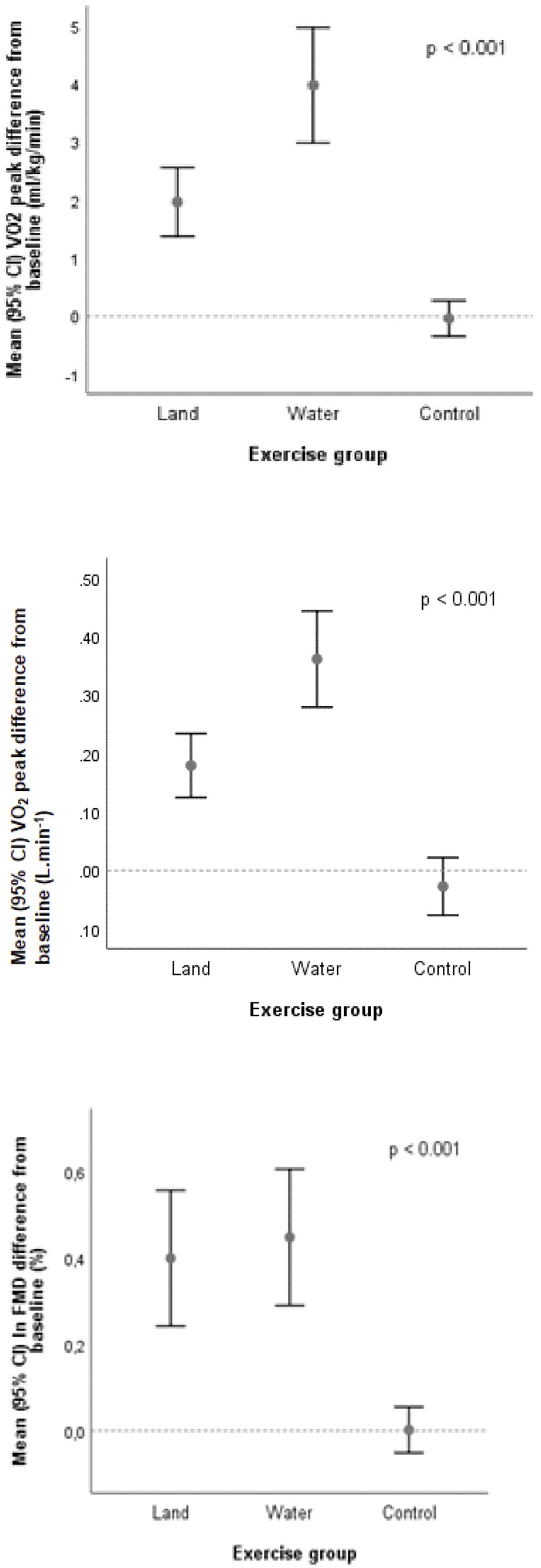
Figure 2. Changes in aerobic exercise capacity (O2peak) and flow-mediated dilatation (FMD) expressed on the logarithmic scale.
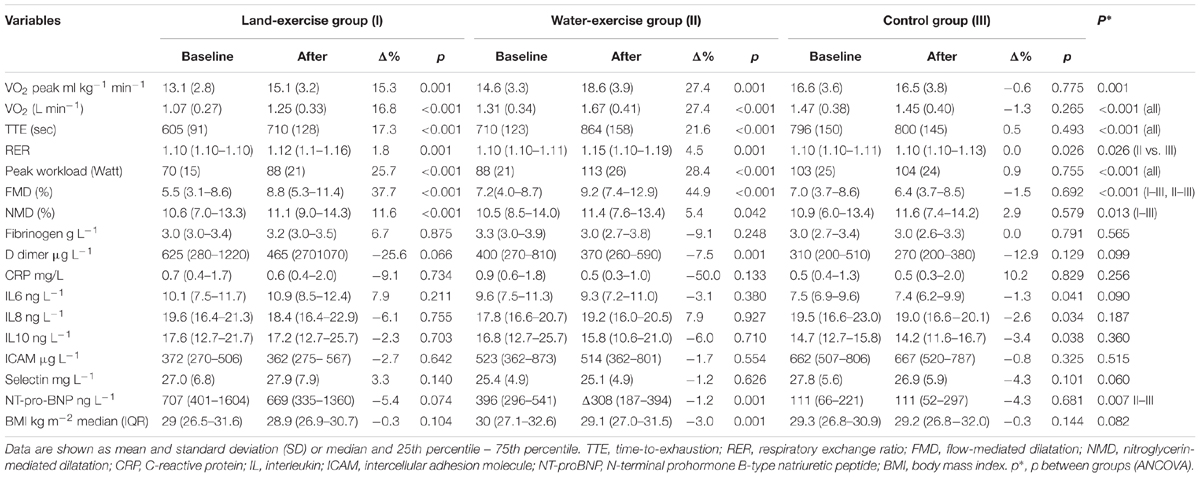
Table 2. Measurements for the three study groups at baseline and after training, and between group differences.
End-of-study FMD increased from 5.5 to 8.8% (p < 0.001) in the land-based training group, and from 7.2 to 9.2% (p < 0.001) in the water-based training group; no significant change was observed in the control group (p = 0.629). NMD increased in both intervention groups (with larger increments in the land-based exercise group); the increase in the intervention groups was statistically significant in comparison to controls (p < 0.001). See Table 2 and Figure 2.
No significant changes were detected in biomarkers of low-grade inflammation, whereas levels of NT-proBNP and D-dimer decreased in the water-based training group (p = 0.01 for both; Table 2).
Supervised short-term exercise training – either water-based or land-based – is safe, and improves aerobic exercise capacity and vascular function in patients with CAD. Despite concerns about the safety and effectiveness of aquatic exercise in patients after a recent CAD event, our study in patients undergoing residential cardiac rehabilitation demonstrated that a 2-week, twice-daily water-based training program was not associated with adverse cardiovascular events, and improved aerobic exercise capacity (as determined by O2peak) and endothelial function (as determined by FMD). To our knowledge, this is the largest study of water- vs. land-based training in patients after a recent CAD event, and the first to address vascular function in this context. Our results contribute to the growing body of evidence on the safety and effectiveness of aquatic exercise in cardiovascular patients, and suggest that aquatic exercise modalities may be a suitable option for cardiac rehabilitation of selected patients after a recent CAD event, such as those with concomitant musculoskeletal conditions, frailty or at risk of falls, or for those who might prefer aquatic exercise.
In our study, both land- and water-based training were associated with a significant increase in exercise capacity (O2peak, time-to-exhaustion, and peak workload) as compared to controls. The magnitude of baseline-adjusted O2peak increase – in the range of 2–4 ml kg-1 min-1, roughly corresponding to 1 MET – was comparable in the two intervention groups, and represents a clinically significant achievement (Conraads et al., 2015). O2peak improvements in our study were larger than those reported in previous randomized trials of short-term (3 weeks) aquatic rehabilitation (in the range of 2.0–2.4 ml kg-1 min-1) (Laurent et al., 2009; Teffaha et al., 2011). Both Laurent et al. (2009) and Teffaha et al. (2011), however, enrolled more stable and younger CAD patients (mean age 52 and 54 years, respectively), which was reflected in higher baseline O2peak (20 and 22 ml kg-1 min-1, respectively) and therefore possibly disposed the study population to diminishing returns in aerobic exercise capacity improvements. In fact, the end-of-study O2peak in our population (16.7 and 18.4 ml kg-1 min-1, respectively) was still lower than that reported in studies with CAD patients (Casillas et al., 2007; Winzer et al., 2018), but nonetheless higher when compared to studies of water-based training in patients with chronic heart failure. This likely reflects the specifics of our patient population (i.e., after a recent myocardial infarction and/or revascularization procedure in the risk spectrum between stable CAD at one end, and clinically manifest cardiac dysfunction on the other); of note, our patient population represents the population of CAD patients traditionally referred for cardiac rehabilitation.
End-of-study O2peak in our trial was also significantly higher in the aquatic exercise group (p < 0.001 after co-variance analysis adjusting for age and baseline capacity). Larger improvements with water- vs. land-based exercise training in CAD were observed previously (Teffaha et al., 2011; Lee et al., 2017). However, the magnitude of difference (2 ml kg-1 min-1) was larger in our study when compared to previous reports (1 ml kg-1 min-1) (Mourot et al., 2010; Teffaha et al., 2011), but the confidence intervals for our estimations were large and the study was underpowered to provide a definite conclusion as to whether differences in end-of-study O2peak between aquatic and land-based training are indeed relevant. Methodologically, the difference favoring water-based over land-based exercise in our study may still be attributable to randomization failure (with patients in the land-based group being older and having lower baseline O2peak). Alternatively, larger improvements may derive from either the specific physiology of water immersion or the higher intensity of aquatic exercise. In terms of specific physiology, water immersion is linked with hemodynamic and peripheral responses associated with improved myocardial efficiency and endurance (DiCarlo et al., 1991; Tei et al., 1995; Mourot et al., 2010). Previous studies (Teffaha et al., 2011; Lee et al., 2017) compared water- vs. land-based calisthenics on top of land-based endurance training (cycling), whereas our aquatic training protocol (both endurance and calisthenics) was entirely carried out in xiphoid-level water. In terms of the intensity, exercise prescription was based on peak heart rate achieved during symptom-limited graded exercise testing on a land bicycle ergometer. On the one hand, the hypothesized intensity may not be directly interchanged between aerobic exercise in water and cycling on ambient air. On the other hand, peak heart rate-derived intensity may not provide a precise measure of metabolic stress in comparison to, for instance, ventilatory thresholds, especially in patients with cardiovascular disease (Carvalho and Mezzani, 2010). The challenges of threshold appraisal in clinical practice, however, partially explain why the majority of research – including ours – continues to favor the use of peak heart rate to prescribe exercise (Mann et al., 2013). Moreover, our intervention protocol did employ the aquatic heart rate adjustment (i.e., to a maximum rate 13% or 10 bpm lower than in land-based exercise) (Gabrielsen et al., 2000). In this respect, previous research yielded inconclusive results: some studies have corroborated the higher intensity (and thus the rationale for heart rate adjustment) of aquatic exercise (Lee et al., 2017), while others – including research in CAD populations exercising to up to 75% O2peak – have not (Tokmakidis et al., 2008; Laurent et al., 2009; Teffaha et al., 2011). These inconclusive results may be reconciled by addressing the specific protocols of aquatic exercise, suggesting that the level of water may play an important role. In our study, deeper water (xiphoid process level) – which increases buoyancy and decreases resistance (Cider et al., 2003) – may have provided comparable intensity of aquatic exercise, but a significant hydrostatically induced hemodynamic response to immersion.
Both water- and land-based exercise training improved vascular function (between 2 and 3 absolute percentage change improvements). Similar improvements have been reported with land-based aerobic training (Di Francescomarino et al., 2009), but not yet with aquatic exercise in patients with CAD. FMD – a marker of endothelial function and thus cardiovascular health (Flammer et al., 2012) – increased significantly after 2 weeks of exercise training in both intervention groups. While most previous studies employed exercise programs of longer duration, our intervention was relatively short-term and suggests that an increase in endothelial function can be detected as soon as 2 weeks after exercise training initiation; these results are in line with previous reports in both human trials (Tinken et al., 2008) and animal models (Graham and Rush, 2004). Improved endothelial function – as determined indirectly by increased levels of plasma nitric oxide metabolites – has been reported in water-based exercise training in patients with CAD (Conraads et al., 2015), while FMD increases have been documented in prehypertensive adults (Nualnim et al., 2012) and patients with osteoarthritis undergoing aquatic exercise training (Alkatan et al., 2016). Contrary to our findings, however, Alkatan et al. (2016) – in the only previous study comparing water-based training (swimming) with land-based training (cycling) – reported on larger improvements in endothelial function with the former (4 vs. 1% absolute percentage change improvement). Exercise type (swimming) and duration (8 weeks) may partly explain such differences; alternatively, Alkatan et al. (2016) enrolled cardiovascular disease-free individuals with osteoarthritis, whereas in our CAD population the vascular damage may have been too pronounced for a discernible difference between water- and land-based training to be detected.
Contrary to the increased aerobic exercise capacity and vascular function, neither inflammation nor endothelial activation markers improved. Atherosclerosis in general, and CAD in particular, are characterized by low-grade inflammation, which may be reduced with regular long-term exercise training (Nimmo et al., 2013). We hypothesized that improvements in endothelium-dependent vascular function would be accompanied by a reduction in the markers of low-grade inflammation, endothelial adhesion and coagulation, given the association between inflammation and endothelial dysfunction, and the central role of endothelial integrity in promoting cell adhesions and coagulation. However, 2 weeks of exercise training may have been too short to achieve such changes; aquatic exercise trials of longer duration in osteoarthritis (Alkatan et al., 2016) have reported improved vascular function and inflammation markers with water-based exercise. Adding to these observations, our study suggests that improvements in FMD – unparalleled by a reduction in the markers of inflammation (interleukins and hsCRP) and inflammation-induced endothelial activation (P-selectin and ICAM) – more likely derive from immediate exercise-induced hemodynamic changes rather than from a reversal in inflammation-caused vascular dysfunction. Alternatively, it is also possible that exercise-induced long-term changes in body mass, composition and metabolism might in the long run reverse low-grade chronic inflammation in cardiovascular disease; however, neither our 2-week exercise program nor longer 12-week trials (Mohammadi et al., 2018) achieved significant changes in the body mass index, and did not appraise potential body composition changes, which may be brought about by aquatic exercise.
We have identified several limitations. Firstly, this was a single-center study involving a limited number of patients with a recent CAD event. The results can therefore not be extrapolated to other cardiovascular patients. Secondly, we assessed relevant but surrogate endpoints, and the clinical relevance of our findings should be confirmed in larger clinical trials. Also, our study focused on exercise capacity, vascular function and low-grade inflammation; whilst providing some insight into aquatic exercise in patients with CAD, our findings convey only limited inferences about the potential (patho)physiologic responses to water- vs. land-based exercise training in this patient population. Specific impacts of aquatic exercise in patients with CAD – such as on body composition and metabolism – should therefore be addressed in further studies. Thirdly, baseline between-group differences suggest randomization failure and have required statistical adjustment, which calls for our study to be regarded as pilot and hypothesis-generating. Fourthly, while both intervention groups underwent residential cardiac rehabilitation (controlling for some confounders, such as diet), the control group did not, which may have yielded overestimation of the effect of both interventions as compared to controls. Lastly, we chose a specific type (xiphoid-level endurance plus calisthenics training) and duration of exercise (2-week intervention), which can only address immediate physiological responses, but not sustainable effects of regular training.
Aquatic exercise is a safe and effective training modality for patients undergoing short-term residential cardiac rehabilitation after a recent CAD event. As compared to land-based exercise, endurance plus calisthenics exercise training in thermo-neutral water provides comparable improvements in exercise capacity and vascular function in patients with CAD. Our pilot study therefore represents a starting point for further research into optimal exercise modalities in CAD patients, with water-based training likely emerging as a suitable exercise option.
Patients who corresponded to the inclusion criteria were invited to participate in the study. A written informed consent was obtained for each participant. The study complied with the World Medical Association Declaration of Helsinki on ethics in medical research and was approved by the local medical research ethics committee (0120-655/2016-2).
DV contributed to drafting the work, acqusition, analysis, and interpretation of the data for the work, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MN and MB substantially contributed to the conception of the work. BB substantially contributed to the design of the work. BJ contributed to the drafting the work, revising it critically for important intellectual content, and provided approval for the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are particularly grateful to all the participants in the study, for their assistance in the realization of the study. We owe our gratitude to the nurses and physiotherapists at the Centre for Cardiac Rehabilitation, Terme Krka, Šmarješke Toplice. We would like to thank Vanja Erčulj, M.Sc., for her assistance with the statistical analysis.
Alkatan, M., Machin, D. R., and Baker, J. R. (2016). Effects of swimming and cycling exercise intervention on vascular function in patients with osteoarthritis. Am. J. Cardiol. 117, 114–145. doi: 10.1016/j.amjcard.2015.10.017
Anderson, L., Thompson, D. R., Oldridge, N., Zwisler, A. D., Rees, K., Martin, N., et al. (2016). Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane systematic review and meta-analysis. JACC 67, 1–12.
Ayme, K., Gavarry, O., Rossi, P., Desruelle, A. V., Regnard, J., and Boussuges, A. (2014). Effect of head-out water immersion on vascular function in healthy subjects. Appl. Physiol. Nutr. Metab. 39, 425–431. doi: 10.1139/apnm-2013-0153
Balady, G. J., Williams, M. A., Ades, P. A., Bittner, V., Comoss, P., Foody, J. M., et al. (2007). Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the american heart association exercise, cardiac rehabilitation, and prevention committee, the council on clinical cardiology the councils o. Circulation 115, 2675–2682. doi: 10.1161/circulationaha.106.180945
Bjarnason-Wehrens, B., McGee, H., Zwisler, A. D., Piepoli, M., Benzer, W., Schmid, J. P., et al. (2010). on behalf of the cardiac rehabilitation section European Association of Cardiovascular Prevention and Rehabilitation. Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur. J. Card. Prev. Rehab. 17, 410–418. doi: 10.1093/eurheartj/ehr178
Bruning, R. S., and Sturek, M. (2015). Benefits of exercise training in coronary artery disease patients. Prog. Cardiovasc. Dis. 57, 443–453. doi: 10.1016/j.pcad.2014.10.006
Carvalho, V. O., and Mezzani, A. (2010). Aerobic exercise training intensity in patients with chronic heart failure: principles of assessment and prescription. Eur. J. Cardiovasc. Prev. Rehab. 18, 5–14. doi: 10.1097/hjr.0b013e32833a9c63
Casillas, J. M., Gremeaux, V., Damak, S., Feki, A., and Pérennou, D. (2007). Exercise training for patients with cardiovascular disease. Ann. Readapt. Med. Phys. 50, 403–418.
Choi, J. H., Kim, B. R., Joo, S. J., Han, E. Y., Kim, S. Y., Kim, S. M., et al. (2015). Comparison of cardiorespiratory responses during aquatic and land treadmill exercise in patients with coronary artery disease. J. Cardio. Rehabil. Prev. 35, 140–146. doi: 10.1097/HCR.0000000000000094
Cider, A., Schaufelberger, H., and Sunnerhagen, K. S. (2003). Anderson B. Hydrotherapy – a new approach to improve function in the older patient with chronic heart failure. Eur. J. Heart Fail 5, 527–535. doi: 10.1016/s1388-9842(03)00048-5
Conraads, V. M., Pattyn, N., De Maeyer, C., Beckers, P. J., Coeckelberghs, E., Cornelissen, V. A., et al. (2015). Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX_CAD study. Int. J. Cardiol. 20, 203–210. doi: 10.1016/j.ijcard.2014.10.155
Di Francescomarino, S., Sciartilli, A., Di Valerio, V., Di Baldassarre, A., and Gallina, S. (2009). The effect of physical exercise on endothelial function. Sports Med. 39, 797–812. doi: 10.2165/11317750-000000000-00000
DiCarlo, L. J., Sparling, P. B., Millard-Stafford, M. L., and Rupp, J. C. (1991). Peak heart rates during maximal running and swimming: implications for exercise presribtion. Int. J. Sports Med. 12, 309–312. doi: 10.1055/s-2007-1024687
Flammer, A. J., Anderson, T., CElermajer, D. S., Creager, M. A., Deanfield, J., Ganz, P., et al. (2012). The assessment of endothelial function from research into clinical practice. Circulation 126, 753–767.
Gabrielsen, A., Sørensen, V. B., Pump, B., Galatius, S., Videbaek, R., Bie, P., et al. (2000). Cardiovascular and neuroendocrine responses to water immersion in compensated heart failure. Am. J. Physiol. Heart Circ. Physiol. 275, 1931–1940.
Graham, D. A., and Rush, J. W. (2004). Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J. Appl. Physiol. 96, 2088–2096. doi: 10.1152/japplphysiol.01252.2003
Hambrecht, R., Adams, V., Erbs, S., Linke, A., Krankel, N., Shu, Y., et al. (2003). Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107, 3152–3231.
Hambrecht, R., Wolf, A., Gielen, S., Linke, A., Hofer, J., Erbs, S., et al. (2000). Effect of exercise on coronary endothelial function in patients with coronary artery disease. N. Engl. J. Med. 342, 454–460.
Ismail, H., McFarlane, J. R., Nojoumian, H., Dieberg, G., and Smart, N. A. (2013). Cinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure. JACC 1, 514–522. doi: 10.1016/j.jchf.2013.08.006
Kokkinos, P., and Myers, J. (2010). Exercise and physical activity: clinical outcomes and applications. Circulation 122, 1637–1648. doi: 10.1161/circulationaha.110.948349
Korzeniowska-Kubacka, I., Biliñska, M., Dobraszkiewicz-Wasilewska, B., Baranowski, R., Piotrowicz, E., and Piotrowicz, R. (2016). The influence of water-based training on arrhythmia I patients with stable coronary artery disease and preserved left ventricular function. Cardiol. J. 23, 93–99. doi: 10.5603/CJ.a2015.0065
Laurent, M., Daline, T., Malika, B., Fawzi, O., Philippe, V., Benoit, D., et al. (2009). Training-induced increase in nitric oxide metabolites in chronic heart failure and coronary artery disease: an extra benefit of water – based exercises? Eur. J. Cardiovasc. Prev. Rehabil. 16, 215–221. doi: 10.1097/HJR.0b013e3283292fcf
Lazar, J. M., Khanna, N., Chesler, R., and Salciccioli, L. (2013). Swimming and the heart. Int. J. Cardiol. 168, 19–25.
Lee, J. Y., Joo, K. C., and Brubaker, P. H. (2017). Aqua walking as an alternative exercise modality during cardiac rehabilitationfor coronary artery disease in older patients with lower extremity osteoarthritis. BMC Cardiovasc. Disord. 17:252. doi: 10.1186/s12872-017-0681-4
Mampuya, W. M. (2012). Cardiac rehabilitation past, present and future: an overview. Cardiovasc. Diagn. Ther. 2, 38–49. doi: 10.3978/j.issn.2223-3652.2012.01.02
Mann, T., Lamberts, R. P., and Lambert, I. (2013). Methods of prescribing relative exercise intensity: physiological and practical considerations. Sports Med. 43, 613–625. doi: 10.1007/s40279-013-0045-x
Menezes, A. R., Lavie, C. J., Milani, R. V., Forman, D. E., King, M., and Wiliams, M. A. (2014). Cardiac rehabilitation in United States. Prog. Cardiovasc. Dis. 56, 522–529.
Meyer, K. (2006). Left ventricular dysfunction and chronic heart failure: should aqua therapy and swimming be allowed? Br. J. Sports Med. 40, 817–818. doi: 10.1136/bjsm.2006.029165
Mohammadi, H. R., Khoshnam, M. S., and Khoshnam, E. (2018). Effects of different modes of exercise training on body composition and risk factors for cardiovascular disease in middle-aged men. Int. J. Prev. Med. 9:9. doi: 10.4103/ijpvm.IJPVM_209_16
Mourot, L., Teffaha, D., Bouhaddi, M., Ounissi, F., Vernochet, P., Dugue, B., et al. (2010). Exercise rehablitation restores physiological cardiovascular responses to short-term head-out water immersion in patients with heart failure. J. Cardiopulm. Rehab. Prevent. 30, 22–27. doi: 10.1097/hcr.0b013e3181c8595c
Nimmo, M. A., Leggate, M., Vianna, J. L., and King, J. A. (2013). The effect of physical activity on mediators of inflammation. Diabet. Obes. Metab. 15(Suppl. 3), 51–60. doi: 10.1111/dom.12156
Nualnim, N., Parkhurst, K., Dhindsa, M., Tarumi, T., Vavrek, J., and Tanaka, H. (2012). Effects of swimming training on blood pressure and vascular function in adults > 50 years of age. Am. J. Cardiol. 109, 1005–1010. doi: 10.1016/j.amjcard.2011.11.029
Pedersen, B. K. (2017). Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur. J. Clin. Invest. 47, 600–611. doi: 10.1111/eci.12781
Pendergast, D. R., Moon, R. E., Krasney, J. J., Held, H. E., and Zamparo, P. (2015). Human physiology in an aquatic enviroment. Compr. Physiol. 5, 1705–1750. doi: 10.1002/cphy.c140018
Piepoli, M. F., Corra, U., Benzer, W., Bjarnason-Wehrens, B., Dendale, P., Gaita, D., et al. (2010). Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 17, 1–17. doi: 10.1097/HJR.0b013e3283313592
Schmid, J. P., Morger, C., Noveanu, M., Binder, R. K., Anderegg, M., Saner, H., et al. (2009). Haemodynamic and arrhythmic effects of moderately cold (22C) water immersion and swimming in patients with stable coronary artery disease and heart failure. Eur. J. Heart Fail. 11, 903–909. doi: 10.1093/eurjhf/hfp114
Schmid, J. P., Noveanu, M., Morger, C., Gaillet, R., Capoferri, M., Anderegg, M., et al. (2007). Influence of water immersion, water gymnastics and swimming on cardiac output in patients with heart failure. Heart 93, 722–727. doi: 10.1136/hrt.2006.094870
Shah, P., Pellicori, P., Macnamara, A., Urbinati, A., and Clark, A. L. (2017). Is swimming safe in heart failure? Syst. Rev. Cardiol. Rev. 25, 321–325. doi: 10.1097/CRD.0000000000000154
Teffaha, D., Maurot, L., Vernochet, P., Ounissi, F., Regnard, J., Monpère, C., et al. (2011). Relevance of water gymnastics in rehabilitation programs in patients with chronic heart failure or coronary artery disease with normal left ventricular function. J. Cardiac. Fail. 17, 676–683. doi: 10.1016/j.cardfail.2011.04.008
Tei, C., Horikiri, Y., Park, J. C., Jeong, J. W., Chang, K. S., Toyama, Y., et al. (1995). Acute hemodynamic improvement by thermal vasodilatation in congestive heart failure. Circuation 91, 2582–2590. doi: 10.1161/01.cir.91.10.2582
Tinken, T. M., Thijssen, D. H. J., Black, M. A., Cable, N. T., and Green, D. J. (2008). Time course of change in vasodulatator function and capacity in response to exercise training in humans. J. Physiol. 586, 5003–5012. doi: 10.1113/jphysiol.2008.158014
Tokmakidis, S., Spassis, A., and Volaklis, K. (2008). Training, detraining and retraining effects after a water-based exercise program in patients with coronary artery disease. Cardiology 111, 257–264. doi: 10.1159/000127737
Volaklis, K. A., Spassis, A. T., and Tokmadis, S. P. (2007). Land versus water exercise in patients with coronary artey disease: effects on body composition, blood lipids and physical fitness. Am. Heart J. 154, 560.e1–560.e6. doi: 10.1016/j.ahj.2007.06.029
Winzer, E. B., Woitek, F., and Linke, A. (2018). Physical activity in the prevention and treatment of coronary artery disease. J. Am. Heart Asoc. 7:e007725.
Keywords: coronary artery disease, cardiac rehabilitation, aquatic exercise, myocardial infarction, exercise training
Citation: Vasić D, Novaković M, Božič Mijovski M, Barbič Žagar B and Jug B (2019) Short-Term Water- and Land-Based Exercise Training Comparably Improve Exercise Capacity and Vascular Function in Patients After a Recent Coronary Event: A Pilot Randomized Controlled Trial. Front. Physiol. 10:903. doi: 10.3389/fphys.2019.00903
Received: 24 December 2018; Accepted: 28 June 2019;
Published: 16 July 2019.
Edited by:
Markos Klonizakis, Sheffield Hallam University, United KingdomReviewed by:
Ahmad Alkhatib, University of Taipei, TaiwanCopyright © 2019 Vasić, Novaković, Božič Mijovski, Barbič Žagar and Jug. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Borut Jug, Ym9ydXQuanVnQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.