
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Physiol., 28 May 2019
Sec. Membrane Physiology and Membrane Biophysics
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.00641
This article is part of the Research TopicConnections to Membrane Trafficking Where you Least Expect Them: Diseases, Dynamics, Diet and DistanceView all 11 articles
 Caroline Beaudoin-Chabot1
Caroline Beaudoin-Chabot1 Lei Wang1†
Lei Wang1† Alexey V. Smarun2
Alexey V. Smarun2 Dragoslav Vidović3
Dragoslav Vidović3 Mikhail S. Shchepinov2
Mikhail S. Shchepinov2 Guillaume Thibault1*
Guillaume Thibault1*Chemically reinforced essential fatty acids (FAs) promise to fight numerous age-related diseases including Alzheimer’s, Friedreich’s ataxia and other neurological conditions. The reinforcement is achieved by substituting the atoms of hydrogen at the bis-allylic methylene of these essential FAs with the isotope deuterium. This substitution leads to a significantly slower oxidation due to the kinetic isotope effect, inhibiting membrane damage. The approach has the advantage of preventing the harmful accumulation of reactive oxygen species (ROS) by inhibiting the propagation of lipid peroxidation while antioxidants potentially neutralize beneficial oxidative species. Here, we developed a model system to mimic the human dietary requirement of omega-3 in Caenorhabditis elegans to study the role of deuterated polyunsaturated fatty acids (D-PUFAs). Deuterated trilinolenin [D-TG(54:9)] was sufficient to prevent the accumulation of lipid peroxides and to reduce the accumulation or ROS. Moreover, D-TG(54:9) significantly extended the lifespan of worms under normal and oxidative stress conditions. These findings demonstrate that D-PUFAs can be used as a food supplement to decelerate the aging process, resulting in extended lifespan.
Sensitive to oxidative damage, the brain consumes around 20% of oxygen despite making only 2% of body weight. The brain is rich in polyunsaturated fatty acids (PUFAs) and oxygen in the lipid bilayer is particularly high reaching millimolar levels (Subczynski and Hyde, 1983). This tissue requires large quantity of ATP to maintain intracellular ion homeostasis resulting in high oxygen uptake. Iron also accumulates in the brain becoming problematic in late-life by catalyzing free radical reactions (Zecca et al., 2004). As the brain produces more mitochondria-generated superoxide compared to skeletal muscle, neuron produces more reactive oxygen species (ROS) with low levels of endogenous antioxidants (Malinska et al., 2009). As a result, the brain spends a quarter of its energy to maintain and repair lipid membranes damaged from ROS (Brenna and Carlson, 2014). Several studies demonstrated that blocking the production of lipid peroxides can be beneficial to prevent the development of Alzheimer’s disease (AD), Parkinson’s disease, and Huntington’s disease (Huang et al., 1999; Lee et al., 2011; Reed, 2011; Gandhi et al., 2012; Shichiri, 2014; Deas et al., 2016).
Senescence is a major cause of age-related diseases, correlating with an accumulation of ROS (McHugh and Gil, 2018). Enhanced formation of ROS oxidizes lipids to generate peroxides and aldehydes. Unlike short-lived ROS, these lipid peroxidation (LPO) products can produce damage throughout the cell due to their non-radical nature. Thus, lipid peroxidation is also strongly linked to aging and the accumulation of LPO products has been observed in AD, Parkinson’s disease, stroke, rheumatic arthritis, and cancer (Davies and Guo, 2014; Shichiri, 2014). However, clinical studies on the efficiency of antioxidants for these age-associated diseases have been disappointing.
The rate liming step of PUFA autoxidation is an ROS-driven hydrogen abstraction off a bis-allylic (between double bounds) methylene group, which is followed quickly by a series of transformations, generating toxic end-products (Shchepinov, 2007). During the nineties, there was a prominent research interest in dietary antioxidants generating hope as potential agent to slow aging and to prevent the development of ROS-related diseases. Disappointing results emerged from the studies conducted in humans (Moller and Loft, 2002). Thus, it appeared that human cells generate a certain amount of beneficial free radicals, playing important roles in cellular functions (Halliwell, 2011; Sena and Chandel, 2012; Yan, 2014). A balance of antioxidants and ROS must be kept in vivo, and supplementation of dietary antioxidants might compromise this critical equilibrium (Sies et al., 2017). This may be due to several reasons, including (1) the near-saturating amount of antioxidants already present in living cells and the stochastic nature of the ROS-inflicted damage, (2) the importance of ROS in cell signaling and hormetic upregulation of protective mechanisms, (3) the pro-oxidant nature of some antioxidants such as vitamin E, and (4) the non-radical nature of PUFA peroxidation products, which can no longer be quenched with most antioxidants (Shchepinov, 2011).
Mitochondrial membranes are rich in cardiolipin, predominantly containing linoleic acid (LA) and α-linolenic acid (ALA) FAs (Paradies et al., 2002; Figure 1A). Thus, as PUFAs are essential nutrients in human, supplementing a diet with deuterated bis-allylic methylenes could represent the most promising approach to fight against ROS-initiated attacks leading to alleviate the influence of aging and age-associated diseases on human life. In Saccharomyces cerevisiae, deuterated PUFAs (D-PUFAs) have been shown to reduce oxidative stress (Hill et al., 2011) by protecting mitochondria against ROS (Andreyev et al., 2015). In addition, D-PUFA prevented lipid peroxidation in primary co-cultures of neurons and astrocytes (Angelova et al., 2015) and in Friedreich ataxia model system (Cotticelli et al., 2013). More recently, D-PUFAs significantly ameliorated performance in cognitive and memory tests using the AD mouse model Aldh2-/- (Elharram et al., 2017). This recent finding suggests that D-PUFAs might be sufficient to reduce the generation of AD-induced lipid peroxidation and to prevent the cognitive decline in AD. In human, a recent randomized clinical trial has been conducted, demonstrating the safety and potential protective effect against Friedreich’s ataxia (Zesiewicz et al., 2018). Friedreich’s ataxia is a neurodegenerative disease associated with an increase of oxidative stress (Ventura et al., 2009). More than a decade ago, supplementing the diet with D-PUFAs was predicted to delay aging (Shchepinov, 2007). However, there is still no study reporting the role of D-PUFAs in modulating lifespan.
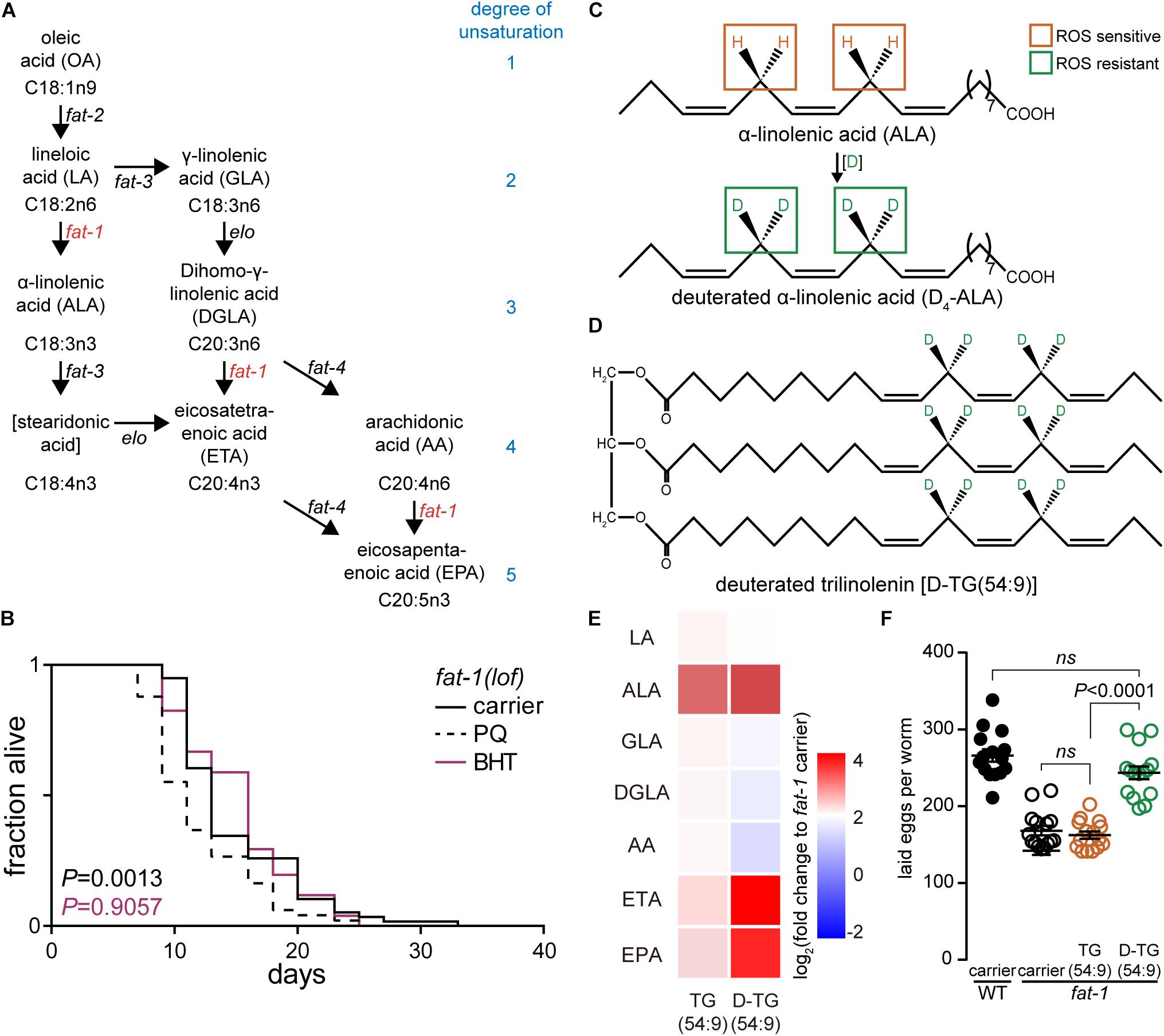
Figure 1. Deuterated trilinolenin rescues fat-1 infertility phenotype. (A) Polyunsaturated fatty acid (PUFA) biosynthesis in C. elegans. Key enzymes fat-1 and fat-2 are highlighted in red. Adapted from Shmookler Reis et al. (2011). (B) Lifespan assay of fat-1(lof) subjected to carrier, 0.2 mM butylated hydroxytoluene (BHT), or 1 mM PQ. Log-rank test to carrier. (C) α-linolenic acid (ALA) is susceptible to oxidation attacks while deuterated ALA (D4-ALA) is protected. (D) Schematic representation of deuterated trilinolenin [D-TG(54:9)]. (E) Heat map of PUFAs extracted in fat-1(lof) supplemented with carrier, 0.48 mM trilinolenin [TG(54:9)] or 0.48 mM deuterated trilinolenin [D-TG(54:9)]. (F) Number of laid eggs per worm of WT and fat-1(lof) animals treated as in (E).
In this study, we characterized the role of D-PUFAs against ROS in the multicellular model organism C. elegans. This organism was recently reported to be sensitive to deuterated lysine (5,5-D2-lysine) which strongly affected its development (Korneenko et al., 2017). To mimic the dietary requirement of omega-3 in human, we selected the omega-3 fatty acid desaturase fat-1(loss-of-function; lof) mutant worm which is also sensitive to the oxidative stress agent paraquat. Triglyceride with three omega-3 PUFAs, trilinolenin [TG(54:9)], was sufficient to induce lipid peroxidation while deuterated TG(54:9) [D-TG(54:9)] was protective. D-TG(54:9) reduced the oxidative stress response in addition of significantly extending the lifespan of fat-1(lof) under normal and oxidative stress conditions. These findings demonstrate that D-TG(54:9) is adequate to prevent the propagation of ROS-induced molecular damages resulting in a significant extension of lifespan.
Error bars indicate standard error of the mean (SEM), calculated from at least three biological replicates, unless otherwise indicated. P-values were calculated using one-way ANOVA with Tukey’s test or log-rank test for lifespan, unless otherwise indicated and reported as P-values. All statistical tests were performed using GraphPad Prism 7 software.
All strains were grown at 20°C using standard C. elegans methods as previously described (Koh et al., 2018). Nematode growth media (NGM) agar plates were seeded with Escherichia coli strain OP50 for normal growth. C. elegans strains wild type N2, fat-1(bx24), mev-1(tk22), gst-4p::GFP::NLS(cl2166), sod-3p::GFP(cf1553) and bacteria strains OP50 were gifted from the Caenorhaditis Genetics Center. Trilinolenin [TG(54:9)] was obtained from Nu-Chek Prep and deuterated at bis-allylic position as previously described (Smarun et al., 2017) to obtained a mixture of 76% deuterated TG(54:9) [D-TG(54:9)]. Lipids were stored into an atmosphere of argon to prevent lipid oxidation. Lipids were freshly dissolved in PBS buffer (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.4) containing 0.1% Triton X-100 or kept at -80°C for later use prior to supplementing NGM agar plate as previously described (Deline et al., 2013). Butylated hydroxytoluene (BHT) and paraquat (PQ) were obtained from Sigma and Acros Organics, respectively.
Lifespan assays were performed at 20°C as previously described (Apfeld and Kenyon, 1999). Synchronized animals were transferred to NGM plates containing 0.2 mM BHT, 1 mM paraquat (PQ), 0.48 mM TG(54:9), or 0.48 mM D-TG(54:9), when indicated. Pyrimidine analog 5-fluoro-2’-deoxyuridine (FUdR, Sigma; St. Louis) was added at 50 μM to pre-fertile young adult worms to prevent development of progeny. Adults were scored manually as dead or alive every 2–3 day. Nematodes which ceased pharyngeal pumping and had no respond to gentle stimulation were recorded as dead. Those worms that were male, crawled off the plate or non-natural death were censored.
Synchronized L1 animals were transferred to NGM plates containing 0.48 mM TG(54:9), 0.48 mM D-TG(54:9) or carrier. Approximately 10,000 L4 to young adult worms were harvested and washed thoroughly with M9 buffer and lyophilised overnight (Vertis, Warminster, PA, United States). FAs were esterified to fatty acid methyl esters (FAME) with 300 μl of 1.25 M HCl-methanol for 1 h at 80°C. FAMEs were extracted three times with 1 ml of hexane. Combined extracts were dried under nitrogen, resuspended in 100 μl hexane. FAMEs were separated by gas chromatography with flame ionization detector (GC-FID; GC-2014; Shimadzu, Kyoto, Japan) using an ULBON HR-SS-10 50 m × 0.25 mm column (Shinwa, Tokyo, Japan). Supelco 37 component FAME mix was used to identify corresponding FAs (Sigma-Aldrich, St. Louis, MO, United States). Data was normalized using internal standard pentadecanoic acid (C15:0) and worm dry weight.
Synchronized L1 mev-1 mutants were transferred to NGM plates containing 0.1 mM BHT, 0.48 mM TG(54:9), 0.48 mM D-TG(54:9) or carrier for 54 h, after which they were transferred on supplemented plates with 8 mM paraquat when indicated for 24 h. Approximately 1,000 L4 to adult worms were harvested and washed thoroughly with M9 buffer, resuspended in 300 μl RIPA buffer (50 mM Tris–HCL pH7.5, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS), lysed with 1 mm silica beads by bead beating. Protein concentration was carried out using the Bicinchoninic Acid (BCA) Protein Assays kit following manufacturer’s protocol (Sigma; St. Louis). Assays for lipid peroxidation, using the thiobarbituric acid reactive substrate (TBARS) kit were performed following manufacturer’s protocol (Cayman Chemical).
Synchronized L1 mev-1 animals were transferred to NGM plates containing 0.1 mM BHT, 0.48 mM TG(54:9), 0.48 mM D-TG(54:9) or carrier and supplemented with 5 mM paraquat when indicated. L4 to young adult worms were transferred to 10 μM BODIPY 581/591 undecanoic acid (BODIPY581-591-C11) solution and stained for 30 min and washed three times with M9. Images were captured using confocal fluorescence microscope Zeiss LSM 710 microscope with a 20 × objective (Carl Zeiss MicroImaging). Oxidized and non-oxidized BODIPY581-591-C11 were excited at 488 and 568 and images were collected from emission at 530(30) and 590(30) nm, respectively. Fluorescence signal ratio of oxidized to non-oxidized BODIPY was normalized to carrier.
Synchronized L1 gst-4p::GFP or sod-3p::GFP worms were transferred to NGM plates containing 0.48 mM TG(54:9), 0.48 mM D-TG(54:9) or carrier and supplemented with 5 mM paraquat when indicated. To quantify GFP signal, worms were immobilized with 25 mM tetramisole and mounted on 2% agarose pad. Images were captured using Zeiss Axiovert 200 M fluorescence microscope with a 20 × objective. Images were stitched, and total fluorescence were quantified using Fiji ImageJ software.
Synchronized L1 WT and fat-1(lof) animals were transferred to NGM plates containing 0.48 mM TG(54:9), or 0.48 mM D-TG(54:9) and supplemented with 5 μM 5-fluoro-2’-deoxyuridine (FUdR, Sigma; St. Louis) to facilitate the counting of the eggs. The fertility of the worms was scored by counting eggs on day 5 and 6 after the transfer of the adults to a new plate.
In human, FAs lineloic acid (LA; omega-6) and α-linolenic acid (ALA; omega-3) are essentials (MacLean et al., 2004). In contrast, C. elegans synthesize both LA and ALA consequently we selected omega-3 fatty acid desaturase fat-1(loss-of-function; lof) mutant animal to mimic the human dietary requirement of ALA (Watts and Browse, 2002; Figure 1A). We tested different ROS agents including paraquat (PQ) and 95% oxygen (data not shown). Paraquat is reduced into radical from the mitochondrial leaking electrons to induce the production of superoxide radicals such as superoxide anion radical (O2∙-). In turn, hydroperoxyl radicals HO2∙ modify PUFAs through oxidative damage in a chain-propagation fashion (Bielski et al., 1983; De Grey, 2002). As low levels of oxidative stress has been reported to increase the lifespan of C. elegans (Wei and Kenyon, 2016) while high levels decrease lifespan (Schaar et al., 2015), we carried out a lifespan assay with fat-1(lof) worms. As expected, high concentration of 1 mM paraquat significantly reduced the lifespan of fat-1(lof) worms compared to carrier (Figure 1B and Table 1). This result indicates that fat-1(lof) worms are sensitive to the production of ROS by the toxic level of paraquat. We also subjected fat-1(lof) worms to the antioxidant BHT but the lifespan was unchanged compared to carrier. This result suggests that fat-1(lof) basal level of ROS is not harmful and neutralising ROS further with antioxidant does not influence fat-1(lof) longevity.
The protective effect of D-PUFAs against ROS has been demonstrated in yeast (Hill et al., 2011; Cotticelli et al., 2013; Andreyev et al., 2015), in primary co-cultures of neurons and astrocytes (Angelova et al., 2015) as well as in AD mouse model Aldh2-/- (Elharram et al., 2017) by supplementing the media with deuterated LA or ALA (Figure 1C; Hill et al., 2011; Cotticelli et al., 2013; Andreyev et al., 2015). We generated deuterated ALA from trilinolenin [TG(54:9)] to obtain deuterated trilinolenin [D-TG(54:9)] as triglycerides reflect human intake mainly consisting of esterified FAs (Figure 1D). The replacement of hydrogen by deuterium at the potential four bis-allylic CH2 group between the two double bonds was effective at 97.5% (data not shown) using our recently reported site-specific deuteration of polyunsaturated alkenes method (Smarun et al., 2017). To ensure that C. elegans ingests and metabolizes D-TG(54:9) as well as non-deuterated TG(54:9), we fed L1 larvae fat-1(lof) mutant with standard diet on NGM plates supplemented with 0.48 mM TG(54:9), D-TG(54:9), or the carrier (0.1% Triton X-100 in PBS). C. elegans is typically fed E. coli OP50 bacteria in which PUFAs are naturally absent but can be easily incorporated if supplemented to the media (Webster et al., 2013). Worms were harvested at stage larva 4 (L4)/young adult and lyophilized. Dried worms were subjected to derivatisation with hydrogen chloride in methanol to generate FAME. FAMEs were extracted with hexane and separated on GC-FID using a capillary column (Ulbon HR-SS-10). We observed a significant accumulation of eicosatetraenoic acid (ETA) and eicosapentaenoic acid (EPA) in fat-1(lof) fed D-TG(54:9) while the other changes were statistically non-significant compared to carrier (Figure 1E). This result indicates that deuterated α-linolenic acid is metabolized by C. elegans as a precursor to synthesize PUFAs with higher degrees of unsaturation.
Next, we asked if D-TG(54:9) is toxic to fat-1(lof) mutant by measuring its fertility which is diminished by ROS (Alcantar-Fernandez et al., 2018). As Δ9 fatty acid desaturases fat-6;fat-7 double mutant exhibits lower fertility (Brock et al., 2007), we monitored the number of laid eggs in wild-type (WT, N2) and fat-1(lof) mutant with standard diet on NGM plates supplemented with 0.48 mM TG(54:9), D-TG(54:9), or the carrier. On carrier, fat-1(lof) mutant laid a significant lower amount of eggs compared to WT (Figure 1F). Similarly, fat-1(lof) mutant, fed TG(54:9), laid a similar amount of eggs per worm compared to fat-1(lof) mutant on carrier. To our surprise, fat-1(lof) mutant, fed D-TG(54:9), laid a similar amount of eggs per worm compared to WT. This result suggests that D-TG(54:9) promotes fertility in fat-1(lof) mutant while TG(54:9) might promote lipid peroxidation.
As PUFAs contribute to the generation of oxidative stress through the propagation of lipid peroxide (Porter et al., 1995; Yin et al., 2011; Figure 2A), we hypothesized that TG(54:9) will intensify paraquat-induced lipid peroxidation. Lipid peroxidation was monitored by measuring thiobarbituric acid reactive substances (TBARS) (Lagman et al., 2015) in lipid peroxidation-sensitive mev-1(lof) mutant worms (Labuschagne et al., 2013). The protein MEV-1 is the homolog of succinate dehydrogenase cytochrome b560 subunit of the mitochondrial respiratory chain complex II (Ishii et al., 1998). Synchronized L1 mev-1(lof) mutants were grown on NGM plates seeded with bacteria supplemented with carrier, BHT, TG(54:9), or D-TG(54:9). Subsequently, L4/young adult mev-1(lof) mutants were exposed to 24 h of paraquat. Paraquat alone was insufficient to significantly increase the accumulation of lipid peroxide compared to carrier alone (Figure 2B). On the other hand, mev-1(lof) mutants grown on TG(54:9) and subjected to paraquat exhibited a dramatic increase in lipid peroxides compared to paraquat alone and to TG(54:9) in the absence of paraquat. This result reinforces the role of PUFAs in catalyzing the propagation of ROS through lipid peroxidation (Yang et al., 2016). D-TG(54:9) was sufficient to prevent the propagation of paraquat-induced ROS through lipid peroxidation. This result indicates that D-PUFAs, in the form of triacylglycerol, are resistant to ROS and thus preventing further cellular damages.
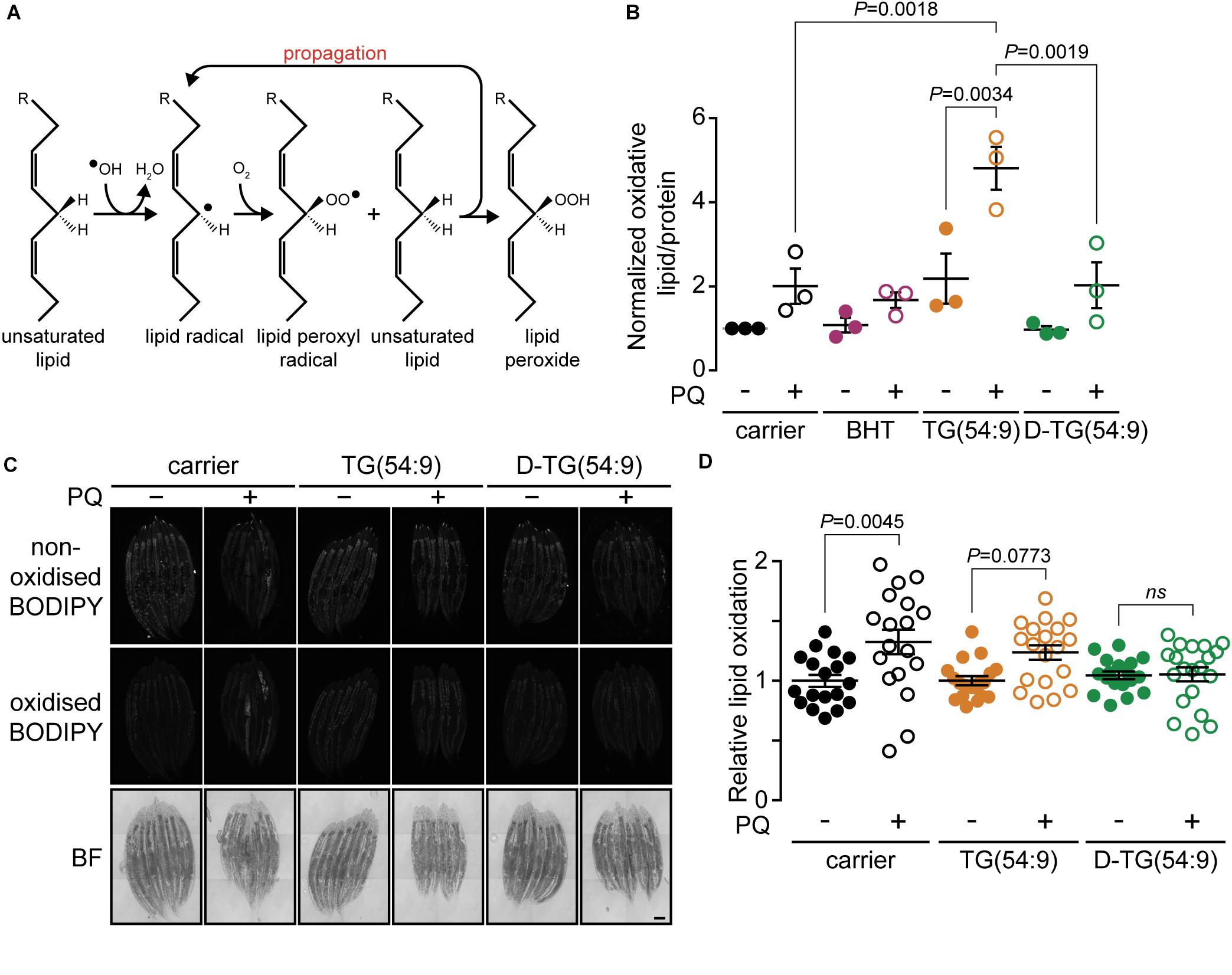
Figure 2. Trilinolenin is sufficient to induce lipid peroxidation while deuterated trilinolenin is protective. (A) Schematic representation of lipid peroxidation. (B) Normalized oxidized lipids of mev-1(lof) worms fed OP50 supplemented with carrier, 0.1 mM BHT, 0.48 mM trilinolenin [TG(54:9)] or 0.48 mM deuterated trilinolenin [D-TG(54:9)] and subjected to 8 mM paraquat (PQ) when indicated. (C) Representative confocal fluorescence images of mev-1(lof) worms fed OP50 supplemented with carrier, 0.48 mM trilinolenin [TG(54:9)] or 0.48 mM deuterated trilinolenin [D-TG(54:9)] and subjected to 5 mM paraquat (PQ) when indicated. Scale bar, 100 μm. (D) Quantification of oxidized to non-oxidized BODIPY581/591-C11 from (C).
To further assess the role of D-TG(54:9) in preventing the formation of lipid peroxides, we monitored in vivo fluorescence of the sensor BODIPY581/591 undecanoic acid (BODIPY581/591-C11). The sensor emission peak shifts from 590 to 510 nm when the polyunsaturated butadienyl domain is oxidized (Naguib, 1998; Aldini et al., 2001). mev-1(lof) mutants were grown and treated as for the TBARS assay. In contrast to the TBARS assay, paraquat alone was sufficient to significantly induce lipid peroxidation (Figures 2C,D). This suggests that BODIPY581/591-C11 is more sensitive than the TBARS assay to monitor lipid peroxidation in C. elegans as previously reported in different cell types (Dominguez-Rebolledo et al., 2010). In contrast to the TBARS assay, TG(54:9) combined with paraquat did not induce lipid peroxidation further compared to paraquat alone in mev-1(lof) mutants. D-TG(54:9) was sufficient to protect mev-1(lof) mutants against paraquat-induced lipid peroxidation. As the BODIPY-C11 lipid peroxidation reporter assay yielded small differences, BODIPY possibly failed to be incorporated evenly through the animal and perhaps not sufficiently where most of lipid peroxidation occurs. Together with the TBARS assay, these results demonstrate that D-PUFA is sufficient to prevent lipid peroxidation in C. elegans.
To further assess the role of D-TG(54:9) in preventing the propagation of lipid peroxide, we monitored the oxidative stress response in vivo. We monitored the expression of the reporter protein GFP under the promotor sod-3 (sod-3p::GFP) (Xu and Kim, 2012). SOD-3 is a mitochondrial superoxide dismutase which has been reported to increase transcriptionally as a result of oxidative stress (An and Blackwell, 2003). These worms were grown and treated as per the TBARS assay. No significant increase in the expression of sod-3p::GFP was observed across the different conditions (Figures 3A,B). As this assay was inconclusive, we used the transgenic worm expressing GFP tagged with a nuclear localisation signal under the promotor gst-4 (gst-4p::GFP::NLS) (Paek et al., 2012). GST-4 is a glutathione S-transferase protein that responds to oxidative stress by an increase at the transcription and translational levels (Link and Johnson, 2002). Glutathione S-transferases protect cells against lipid peroxidation (Yang et al., 2001). gst-4p::GFP::NLS worms were grown with triacylglycerol and paraquat as per the TBARS assay. The expression of gst-4p::GFP::NLS was significantly increased in the presence of paraquat and further increase with the addition of TG(54:9) (Figures 3C,D). On the other hand, no significant change in GFP expression was observed in animals fed D-TG(54:9) in the absence or the presence of paraquat. Consistent with the TBARS assay, these findings further demonstrate that TG(54:9) is harmful by exponentially propagating paraquat-induced ROS while D-TG(54:9) exhibit a strong protective effect.
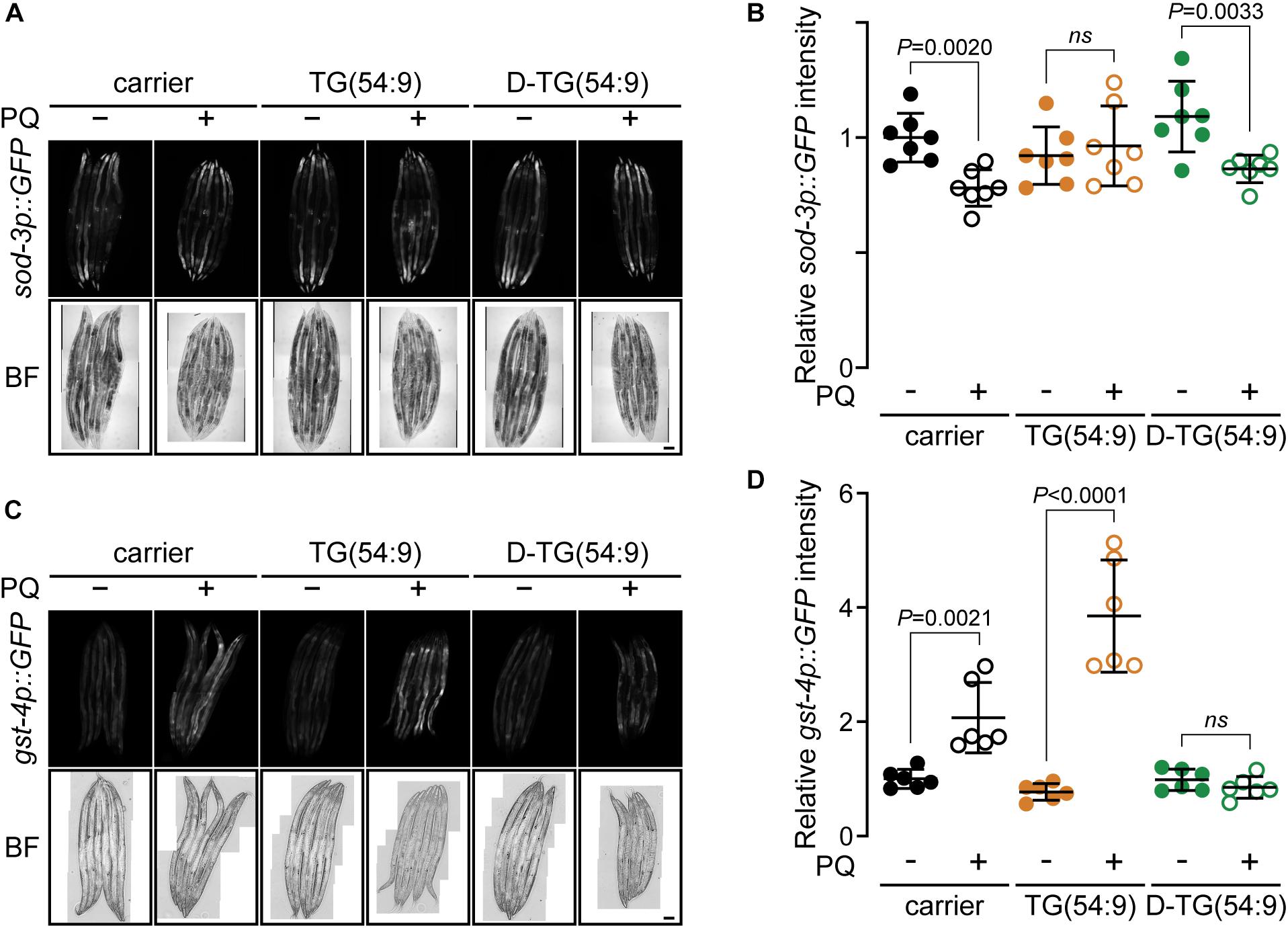
Figure 3. Deuterated trilinolenin reduces oxidative stress response. (A) Representative confocal fluorescence images of sod-3p::GFP transgenic worms fed OP50 supplemented with carrier, trilinolenin [TG(54:9)] or deuterated trilinolenin [D-TG(54:9)] and subjected to 5 mM paraquat (PQ) when indicated. Scale bar, 100 μm. (B) Quantification of A. (C) Representative confocal fluorescence images of gst-4p::GFP::NLS transgenic worms treated as in A fed OP50 supplemented with carrier, trilinolenin [TG(54:9)] or deuterated trilinolenin [D-TG(54:9)] and subjected to 5 mM paraquat (PQ) when indicated. Scale bar, 100 μm. (D) Quantification of C.
As D-TG(54:9) reduces oxidative stress, we carried out a lifespan assay of fat-1(lof) worms fed normal diet supplemented with either TG(54:9) or D-TG(54:9). D-TG(54:9) significantly extended the lifespan of worms compared to the supplementation with TG(54:9) (Figure 4A and Table 1). This suggests that D-TG(54:9) might be sufficient to prevent endogenous ROS-induced cellular damages associated with aging. To further assess the role of D-TG(54:9) on the lifespan of fat-1(lof) worms, we exposed the animals to paraquat from L1 stage. As expected, the lifespan of fat-1(lof) worms exposed to paraquat was not significantly extended by TG(54:9) (Figure 4B and Table 1). As fat-1(lof) worms are unable to synthesize several PUFAs (Figure 1A), TG(54:9) might be necessary as a precursor of PUFAs ETA and EPA to extend the lifespan although the animals are under oxidative stress condition. It should be noted that worms were exposed to a fifth of the paraquat concentration used to monitor lipid peroxidation and oxidative stress. The lifespan of fat-1(lof) worms exposed to paraquat was further extended with D-TG(54:9) compared to paraquat alone and to TG(54:9) in combination with paraquat. This result indicates that D-TG(54:9) is sufficient to promote longevity. Potentially, supplementing a human diet with D-PUFAs might be sufficient to decelerate aging and to prevent the progression of age-associated diseases.
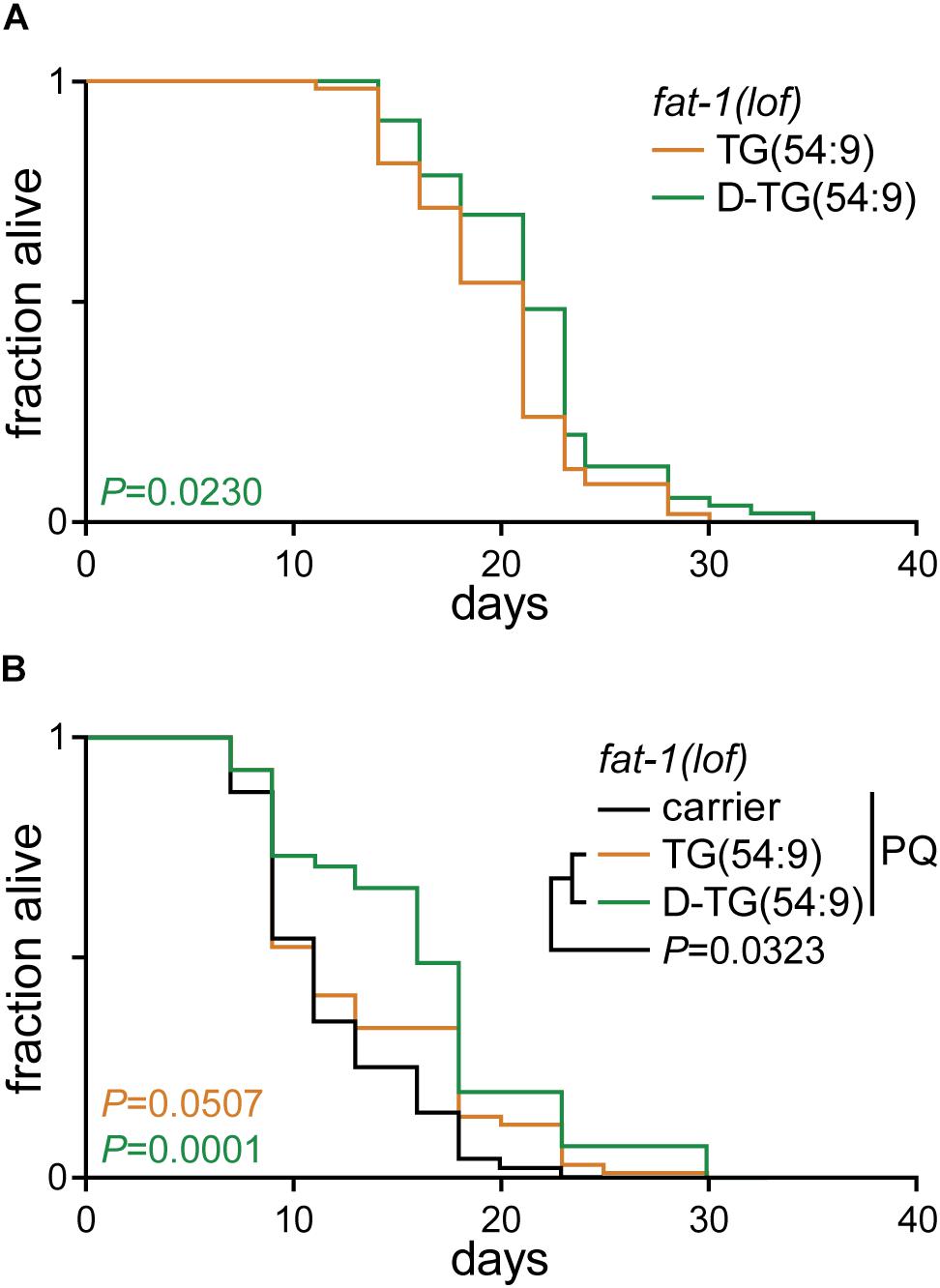
Figure 4. Deuterated trilinolenin reduces lipid peroxidation. (A) Lifespan assay of fat-1(lof) worms fed OP50 supplemented with 0.48 mM trilinolenin [TG(54:9)] or 0.48 mM deuterated trilinolenin [D-TG(54:9)]. Log-rank test to control. (B) Lifespan assay of worms fed OP50 supplemented with carrier, trilinolenin [TG(54:9)] or deuterated trilinolenin [D-TG(54:9)] and subjected to 1 mM paraquat (PQ). Log-rank test to control.
Chemically reinforced essential FAs have the potential to target age-related diseases such as Alzheimer’s, Friedreich’s ataxia and other neurological conditions. Substituting the atoms of hydrogen at the bis-allylic methylene of essential polyunsaturated FAs with isotope deuterium prevents substantial chemical damage. Recently, we developed a novel synthesis approach to reinforced natural FAs (Smarun et al., 2017), which could potentially be scaled up to industrial quantities. These deuterated FAs have the advantage to prevent harmful accumulation of ROS by disfavouring the formation of lipid peroxides while antioxidants are poorly transported within the cell. The results presented in this study show that D-TG(54:9) is sufficient to prevent the accumulation of lipid peroxides in animals and to reduce the accumulation of ROS. We used four different approaches which, together, clearly demonstrate the protective effect of deuterated trilinolenin against paraquat-induced oxidative stress while non-deuterated trilinolenin promotes the propagation of lipid peroxide. Supplementing the diet with D-PUFAs was anticipated to delay aging (Shchepinov, 2007). As predicted, D-TG(54:9) significantly extends the lifespan of worms under both normal and oxidative stress conditions when compared to non-deuterated trilinolenin [TG(54:9)]. It should be noted that D-PUFA must be in low abundance to be beneficial (Kinghorn et al., 2015). Taken together, we have developed a toolkit to monitor the protective role of deuterated FAs against age-related model diseases as well as longevity, allowing future high-throughput discoveries.
During the late nineties, a large boom of research on dietary antioxidants generated hopes as potential agents to slow aging and to prevent the development of ROS-related diseases. However, mixed results emerged from studies conducted in humans (Moller and Loft, 2002). It became clear that human cells generate a certain number of free radicals that play an important role in cellular functions (Halliwell, 2011; Sena and Chandel, 2012; Yan, 2014). A balance of antioxidants and reactive species must be kept in vivo, and supplementation of dietary antioxidants might compromise this delicate equilibrium. More importantly, as PUFAs belong to the group of essential nutrients that must be supplied with diet, it was proposed that PUFAs with deuterated bis-allylic methylenes could represent a novel approach to fight against ROS-initiated attacks, leading to lessening the influence of aging and age-associated diseases on human life.
The protective effect of D-PUFAs against ROS has been demonstrated in yeast and in primary co-cultures of neurons and astrocytes (Hill et al., 2011; Cotticelli et al., 2013; Andreyev et al., 2015; Angelova et al., 2015). As unicellular organisms uniformly absorb FAs, it is imperative to assess the protective role of D-PUFA that is absorbed in the intestine and disseminated to different tissues of a multicellular organism. Recently, D-PUFAs was shown to improve cognition and to reduce lipid peroxidation in the brain of several neurodegenerative disease models (Elharram et al., 2017; Hatami et al., 2018; Raefsky et al., 2018). Similarly in C. elegans, we have demonstrated that H-PUFA promotes, while D-PUFA reduces, both lipid peroxidation and oxidative stress during paraquat-induced oxidative stress. For the first time, we have demonstrated the beneficial effect of D-PUFAs in extending longevity. In future, C. elegans could be used to validate the beneficial role of D-PUFAs in modulating the lifespan of different disease models.
All datasets generated for this study are included in the manuscript and/or the supplementary files.
GT conceived the study, wrote the original draft of the manuscript, and acquired the funding. CB-C and GT performed the methodology. CB-C and LW contributed to formal analysis. CB-C investigated the study. CB-C, AS, MS, and DV performed the resources. MS and GT wrote, reviewed, and edited the manuscript.
This work was supported by funds from the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2018-T2-1-002) and Nanyang Assistant Professorship program from Nanyang Technological University, Singapore (GT).
MS owns stocks in Retrotope.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank members of Thibault lab for critical reading of the manuscript. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Alcantar-Fernandez, J., Navarro, R. E., Salazar-Martinez, A. M., Perez-Andrade, M. E., and Miranda-Rios, J. (2018). Caenorhabditis elegans respond to high-glucose diets through a network of stress-responsive transcription factors. PLoS One 13:e0199888. doi: 10.1371/journal.pone.0199888
Aldini, G., Yeum, K. J., Russell, R. M., and Krinsky, N. I. (2001). A method to measure the oxidizability of both the aqueous and lipid compartments of plasma. Free Radic. Biol. Med. 31, 1043–1050. doi: 10.1016/s0891-5849(01)00684-0
An, J. H., and Blackwell, T. K. (2003). SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 17, 1882–1893. doi: 10.1101/gad.1107803
Andreyev, A. Y., Tsui, H. S., Milne, G. L., Shmanai, V. V., Bekish, A. V., Fomich, M. A., et al. (2015). Isotope-reinforced polyunsaturated fatty acids protect mitochondria from oxidative stress. Free Radic. Biol. Med. 82, 63–72. doi: 10.1016/j.freeradbiomed.2014.12.023
Angelova, P. R., Horrocks, M. H., Klenerman, D., Gandhi, S., Abramov, A. Y., and Shchepinov, M. S. (2015). Lipid peroxidation is essential for alpha-synuclein-induced cell death. J. Neurochem. 133, 582–589. doi: 10.1111/jnc.13024
Apfeld, J., and Kenyon, C. (1999). Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809. doi: 10.1038/45544
Bielski, B. H., Arudi, R. L., and Sutherland, M. W. (1983). A study of the reactivity of HO2/O2- with unsaturated fatty acids. J. Biol. Chem. 258, 4759–4761.
Brenna, J. T., and Carlson, S. E. (2014). Docosahexaenoic acid and human brain development: evidence that a dietary supply is needed for optimal development. J. Hum. Evol. 77, 99–106. doi: 10.1016/j.jhevol.2014.02.017
Brock, T. J., Browse, J., and Watts, J. L. (2007). Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics 176, 865–875. doi: 10.1534/genetics.107.071860
Cotticelli, M. G., Crabbe, A. M., Wilson, R. B., and Shchepinov, M. S. (2013). Insights into the role of oxidative stress in the pathology of friedreich ataxia using peroxidation resistant polyunsaturated fatty acids. Redox. Biol. 1, 398–404. doi: 10.1016/j.redox.2013.06.004
Davies, S. S., and Guo, L. (2014). Lipid peroxidation generates biologically active phospholipids including oxidatively N-modified phospholipids. Chem. Phys. Lipids 181, 1–33. doi: 10.1016/j.chemphyslip.2014.03.002
De Grey, A. D. (2002). HO2∗: the forgotten radical. DNA Cell Biol. 21, 251–257. doi: 10.1089/104454902753759672
Deas, E., Cremades, N., Angelova, P. R., Ludtmann, M. H., Yao, Z., Chen, S., et al. (2016). Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in parkinson’s disease. Antioxid. Redox. Signal. 24, 376–391. doi: 10.1089/ars.2015.6343
Deline, M. L., Vrablik, T. L., and Watts, J. L. (2013). Dietary supplementation of polyunsaturated fatty acids in Caenorhabditis elegans. J. Vis. Exp. 2013:50879. doi: 10.3791/50879
Dominguez-Rebolledo, A. E., Martinez-Pastor, F., Fernandez-Santos, M. R., del Olmo, E., Bisbal, A., Ros-Santaella, J. L., et al. (2010). Comparison of the TBARS assay and BODIPY C11 probes for assessing lipid peroxidation in red deer spermatozoa. Reprod. Domest. Anim. 45, e360–e368. doi: 10.1111/j.1439-0531.2009.01578.x
Elharram, A., Czegledy, N. M., Golod, M., Milne, G. L., Pollock, E., Bennett, B. M., et al. (2017). Deuterium-reinforced polyunsaturated fatty acids improve cognition in a mouse model of sporadic Alzheimer’s disease. FEBS J. 284, 4083–4095. doi: 10.1111/febs.14291
Gandhi, S., Vaarmann, A., Yao, Z., Duchen, M. R., Wood, N. W., and Abramov, A. Y. (2012). Dopamine induced neurodegeneration in a PINK1 model of Parkinson’s disease. PLoS One 7:e37564. doi: 10.1371/journal.pone.0037564
Halliwell, B. (2011). Free radicals and antioxidants - quo vadis? Trends Pharmacol. Sci. 32, 125–130. doi: 10.1016/j.tips.2010.12.002
Hatami, A., Zhu, C., Relano-Gines, A., Elias, C., Galstyan, A., Jun, M., et al. (2018). Deuterium-reinforced linoleic acid lowers lipid peroxidation and mitigates cognitive impairment in the Q140 knock in mouse model of Huntington’s disease. FEBS J. doi: 10.1111/febs.14590 [Epub ahead of print].
Hill, S., Hirano, K., Shmanai, V. V., Marbois, B. N., Vidovic, D., Bekish, A. V., et al. (2011). Isotope-reinforced polyunsaturated fatty acids protect yeast cells from oxidative stress. Free Radic. Biol. Med. 50, 130–138. doi: 10.1016/j.freeradbiomed.2010.10.690
Huang, X., Atwood, C. S., Hartshorn, M. A., Multhaup, G., Goldstein, L. E., Scarpa, R. C., et al. (1999). The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 38, 7609–7616. doi: 10.1021/bi990438f
Ishii, N., Fujii, M., Hartman, P. S., Tsuda, M., Yasuda, K., Senoo-Matsuda, N., et al. (1998). A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature 394, 694–697. doi: 10.1038/29331
Kinghorn, K. J., Castillo-Quan, J. I., Bartolome, F., Angelova, P. R., Li, L., Pope, S., et al. (2015). Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction. Brain 138(Pt 7), 1801–1816. doi: 10.1093/brain/awv132
Koh, J. H., Wang, L., Beaudoin-Chabot, C., and Thibault, G. (2018). Lipid bilayer stress-activated IRE-1 modulates autophagy during endoplasmic reticulum stress. J. Cell Sci. 131:jcs217992. doi: 10.1242/jcs.217992
Korneenko, T. V., Pestov, N. B., Hurski, A. L., Fedarkevich, A. M., Shmanai, V. V., Brenna, J. T., et al. (2017). A strong developmental isotope effect in Caenorhabditis elegans induced by 5,5-deuterated lysine. Amino Acids 49, 887–894. doi: 10.1007/s00726-017-2386-5
Labuschagne, C. F., Stigter, E. C., Hendriks, M. M., Berger, R., Rokach, J., Korswagen, H. C., et al. (2013). Quantification of in vivo oxidative damage in Caenorhabditis elegans during aging by endogenous F3-isoprostane measurement. Aging Cell 12, 214–223. doi: 10.1111/acel.12043
Lagman, M., Ly, J., Saing, T., Kaur Singh, M., Vera Tudela, E., Morris, D., et al. (2015). Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS One 10:e0118436. doi: 10.1371/journal.pone.0118436
Lee, J., Kosaras, B., Del Signore, S. J., Cormier, K., McKee, A., Ratan, R. R., et al. (2011). Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington’s disease mice. Acta Neuropathol. 121, 487–498. doi: 10.1007/s00401-010-0788-5
Link, C. D., and Johnson, C. J. (2002). Reporter transgenes for study of oxidant stress in Caenorhabditis elegans. Methods Enzymol. 353, 497–505. doi: 10.1016/s0076-6879(02)53072-x
MacLean, C. H., Mojica, W. A., Morton, S. C., Pencharz, J., Hasenfeld Garland, R., Tu, W., et al. (2004). Effects of Omega-3 Fatty Acids on Lipids and Glycemic Control in Type II Diabetes and the Metabolic Syndrome and on Inflammatory Bowel Disease, Rheumatoid Arthritis, Renal Disease, Systemic Lupus Erythematosus, and Osteoporosis. Evidence Report/Technology Assessment no. 89. Rockville: Agency for Healthcare Research and Quality (AHRQ).
Malinska, D., Kudin, A. P., Debska-Vielhaber, G., Vielhaber, S., and Kunz, W. S. (2009). Chapter 23 Quantification of superoxide production by mouse brain and skeletal muscle mitochondria. Methods Enzymol. 456, 419–437. doi: 10.1016/S0076-6879(08)04423-6
McHugh, D., and Gil, J. (2018). Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 217, 65–77. doi: 10.1083/jcb.201708092
Moller, P., and Loft, S. (2002). Oxidative DNA damage in human white blood cells in dietary antioxidant intervention studies. Am. J. Clin. Nutr. 76, 303–310. doi: 10.1093/ajcn/76.2.303
Naguib, Y. M. (1998). A fluorometric method for measurement of peroxyl radical scavenging activities of lipophilic antioxidants. Anal. Biochem. 265, 290–298. doi: 10.1006/abio.1998.2931
Paek, J., Lo, J. Y., Narasimhan, S. D., Nguyen, T. N., Glover-Cutter, K., Robida-Stubbs, S., et al. (2012). Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metab. 16, 526–537. doi: 10.1016/j.cmet.2012.09.007
Paradies, G., Petrosillo, G., Pistolese, M., and Ruggiero, F. M. (2002). Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 286, 135–141. doi: 10.1016/s0378-1119(01)00814-9
Porter, N. A., Caldwell, S. E., and Mills, K. A. (1995). Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30, 277–290. doi: 10.1007/bf02536034
Raefsky, S. M., Furman, R., Milne, G., Pollock, E., Axelsen, P., Mattson, M. P., et al. (2018). Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid beta-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging 66, 165–176. doi: 10.1016/j.neurobiolaging.2018.02.024
Reed, T. T. (2011). Lipid peroxidation and neurodegenerative disease. Free Radic. Biol. Med. 51, 1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027
Schaar, C. E., Dues, D. J., Spielbauer, K. K., Machiela, E., Cooper, J. F., Senchuk, M., et al. (2015). Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet. 11:e1004972. doi: 10.1371/journal.pgen.1004972
Sena, L. A., and Chandel, N. S. (2012). Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167. doi: 10.1016/j.molcel.2012.09.025
Shchepinov, M. S. (2007). Reactive oxygen species, isotope effect, essential nutrients, and enhanced longevity. Rejuvenation Res. 10, 47–59. doi: 10.1089/rej.2006.0506
Shchepinov, M. S. (2011). Alleviating Oxidative Stress Disorders With Pufa Derivatives. U.S. Patent No 10052299. Los Altos: Retrotope Inc.
Shichiri, M. (2014). The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 54, 151–160. doi: 10.3164/jcbn.14-10
Shmookler Reis, R. J., Xu, L., Lee, H., Chae, M., Thaden, J. J., Bharill, P., et al. (2011). Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging 3, 125–147. doi: 10.18632/aging.100275
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative Stress. Annu. Rev. Biochem. 86, 715–748. doi: 10.1146/annurev-biochem-061516-045037
Smarun, A. V., Petkovic, M., Shchepinov, M. S., and Vidovic, D. (2017). Site-specific deuteration of polyunsaturated alkenes. J. Org. Chem. 82, 13115–13120. doi: 10.1021/acs.joc.7b02169
Subczynski, W. K., and Hyde, J. S. (1983). Concentration of oxygen in lipid bilayers using a spin-label method. Biophys. J. 41, 283–286. doi: 10.1016/S0006-3495(83)84439-7
Ventura, N., Rea, S. L., Schiavi, A., Torgovnick, A., Testi, R., and Johnson, T. E. (2009). p53/CEP-1 increases or decreases lifespan, depending on level of mitochondrial bioenergetic stress. Aging Cell 8, 380–393. doi: 10.1111/j.1474-9726.2009.00482.x
Watts, J. L., and Browse, J. (2002). Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 99, 5854–5859. doi: 10.1073/pnas.092064799
Webster, C. M., Deline, M. L., and Watts, J. L. (2013). Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans. Dev. Biol. 373, 14–25. doi: 10.1016/j.ydbio.2012.10.002
Wei, Y., and Kenyon, C. (2016). Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 113, E2832–E2841. doi: 10.1073/pnas.1524727113
Xu, X., and Kim, S. K. (2012). The GATA transcription factor egl-27 delays aging by promoting stress resistance in Caenorhabditis elegans. PLoS Genet. 8:e1003108. doi: 10.1371/journal.pgen.1003108
Yan, L. J. (2014). Positive oxidative stress in aging and aging-related disease tolerance. Redox. Biol. 2C, 165–169. doi: 10.1016/j.redox.2014.01.002
Yang, W. S., Kim, K. J., Gaschler, M. M., Patel, M., Shchepinov, M. S., and Stockwell, B. R. (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U.S.A. 113, E4966–E4975. doi: 10.1073/pnas.1603244113
Yang, Y., Cheng, J. Z., Singhal, S. S., Saini, M., Pandya, U., Awasthi, S., et al. (2001). Role of glutathione S-transferases in protection against lipid peroxidation. Overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J. Biol. Chem. 276, 19220–19230. doi: 10.1074/jbc.M100551200
Yin, H., Xu, L., and Porter, N. A. (2011). Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111, 5944–5972. doi: 10.1021/cr200084z
Zecca, L., Youdim, M. B., Riederer, P., Connor, J. R., and Crichton, R. R. (2004). Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 5, 863–873. doi: 10.1038/nrn1537
Keywords: polyunsaturated fatty acid (PUFA), deuterated fatty acid, oxidative stress, lipid peroxidation, lifespan, C. elegans, essential fatty acids, linolenic acid
Citation: Beaudoin-Chabot C, Wang L, Smarun AV, Vidović D, Shchepinov MS and Thibault G (2019) Deuterated Polyunsaturated Fatty Acids Reduce Oxidative Stress and Extend the Lifespan of C. elegans. Front. Physiol. 10:641. doi: 10.3389/fphys.2019.00641
Received: 10 March 2019; Accepted: 06 May 2019;
Published: 28 May 2019.
Edited by:
Mauricio Antonio Retamal, Universidad del Desarrollo, ChileReviewed by:
Andrey Y. Abramov, University College London, United KingdomCopyright © 2019 Beaudoin-Chabot, Wang, Smarun, Vidović, Shchepinov and Thibault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guillaume Thibault, dGhpYmF1bHRAbnR1LmVkdS5zZw==
†Present address: Lei Wang, Department of Physiology and Biophysics, Miller School of Medicine, University of Miami, Miami, FL, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.