
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol., 24 April 2019
Sec. Clinical and Translational Physiology
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.00413
This article is part of the Research TopicType 2 Diabetes Management: A Focus on Metabolic DefectsView all 15 articles
 Katrine D. Galsgaard1,2
Katrine D. Galsgaard1,2 Jens Pedersen1,3
Jens Pedersen1,3 Filip K. Knop2,4,5
Filip K. Knop2,4,5 Jens J. Holst1,2*
Jens J. Holst1,2* Nicolai J. Wewer Albrechtsen1,6,7*
Nicolai J. Wewer Albrechtsen1,6,7*Glucagon is secreted from the pancreatic alpha cells upon hypoglycemia and stimulates hepatic glucose production. Type 2 diabetes is associated with dysregulated glucagon secretion, and increased glucagon concentrations contribute to the diabetic hyperglycemia. Antagonists of the glucagon receptor have been considered as glucose-lowering therapy in type 2 diabetes patients, but their clinical applicability has been questioned because of reports of therapy-induced increments in liver fat content and increased plasma concentrations of low-density lipoprotein. Conversely, in animal models, increased glucagon receptor signaling has been linked to improved lipid metabolism. Glucagon acts primarily on the liver and by regulating hepatic lipid metabolism glucagon may reduce hepatic lipid accumulation and decrease hepatic lipid secretion. Regarding whole-body lipid metabolism, it is controversial to what extent glucagon influences lipolysis in adipose tissue, particularly in humans. Glucagon receptor agonists combined with glucagon-like peptide 1 receptor agonists (dual agonists) improve dyslipidemia and reduce hepatic steatosis. Collectively, emerging data support an essential role of glucagon for lipid metabolism.
Glucagon is processed from its precursor, proglucagon, by prohormone convertase 2 and secreted from pancreatic alpha cells (Rouille et al., 1994). The role of glucagon in glucose metabolism has been intensively studied, and comprehensive reviews are found elsewhere (Jiang and Zhang, 2003; Ramnanan et al., 2011; Ahren, 2015; Holst et al., 2017a). In addition to regulating glucose metabolism, glucagon also seems important for minute-to-minute regulation of amino acid metabolism as part of the recently described liver-alpha cell axis (Solloway et al., 2015; Dean et al., 2017; Galsgaard et al., 2017; Holst et al., 2017b; Kim et al., 2017), in which amino acids stimulate glucagon secretion and glucagon in turn stimulates hepatic amino acid uptake and metabolism (ureagenesis) and, thus, circulating amino acid concentrations as well as increased hepatic NADH/NAD+ ratio. The actions of glucagon are mediated via the glucagon receptor, a seven transmembrane receptor coupled to Gαs- and Gq-proteins, which regulate adenylate cyclase (AC) and phospholipase C activities when activated (Wakelam et al., 1986; Jelinek et al., 1993; Aromataris et al., 2006). The glucagon receptor is primarily expressed in the liver, but it is also expressed in varying amounts in the central nervous system, kidneys, gastro-intestinal tract, heart (controversial), and pancreas (Svoboda et al., 1994).
Glucagon receptor expression has been reported in rat adipocytes (Svoboda et al., 1994; Hansen et al., 1995), where a lipolytic effect of glucagon may be of physiological relevance. As type 2 diabetic hyperglucagonaemia (Faerch et al., 2016) contributes to the hyperglycemic state of patients with type 2 diabetes (T2D) (Unger and Orci, 1975; Baron et al., 1987), inhibition of glucagon receptor signaling has been investigated as glucose-lowering therapy in T2D patients (Kazda et al., 2016; Kazierad et al., 2016, 2018; Vajda et al., 2017; Pettus et al., 2018). Interestingly, potential adverse effects of this therapeutic approach include increased low-density lipoprotein (LDL) plasma concentrations and increased hepatic fat accumulation (Guzman et al., 2017). Furthermore, hepatocyte studies have shown that glucagon stimulates beta-oxidation (Pegorier et al., 1989), inhibits lipogenesis and decrease triglyceride (TG) and very-low-density lipoprotein (VLDL) secretion (Guettet et al., 1988; Bobe et al., 2003) emphasizing a potentially important role of glucagon in lipid metabolism.
Lipolysis in adipocytes depends on activation of AC and thereby increased protein kinase A (PKA) activity. PKA phosphorylates (hence activates) perilipins (Greenberg et al., 1991) and hormone-sensitive lipase (HSL) (Stralfors et al., 1984; Garton et al., 1988; Anthonsen et al., 1998), and two additional lipases, resulting in hydrolysis of TGs and release of glycerol and free fatty acids (FFAs), e.g., palmitate (Egan et al., 1992; Lass et al., 2006; Granneman et al., 2009; Shen et al., 2009; Wang et al., 2009; Figure 1). Circulating levels of FFAs and glycerol therefore reflect the rate of lipolysis (Schweiger et al., 2014). For glucagon to directly influence adipocyte function, its cognate receptor must be expressed. Glucagon receptor mRNA has been detected in rat adipocytes (Svoboda et al., 1994; Hansen et al., 1995), but to determine the physiological relevance of glucagon receptor mRNA expression, it is necessary to investigate whether the mRNA is actually translated into a functional receptor. Specific antibodies directed against the glucagon receptor are necessary in addressing this question, but development of specific antibodies against glucagon receptors has been challenging and the antibodies available are unspecific and therefore not suitable for receptor localization (van der Woning et al., 2016). As an example, one study reported localization of the glucagon receptor in rat adipocytes using a monoclonal antibody (Iwanij and Vincent, 1990) whereas another using autoradiography, glucagon receptors were not found to be expressed (Watanabe et al., 1998), and no studies have demonstrated presence of glucagon receptors on human adipocytes (Carranza et al., 1993). Clearly, future studies should investigate glucagon receptor expression using antibody and antibody-independent methods.
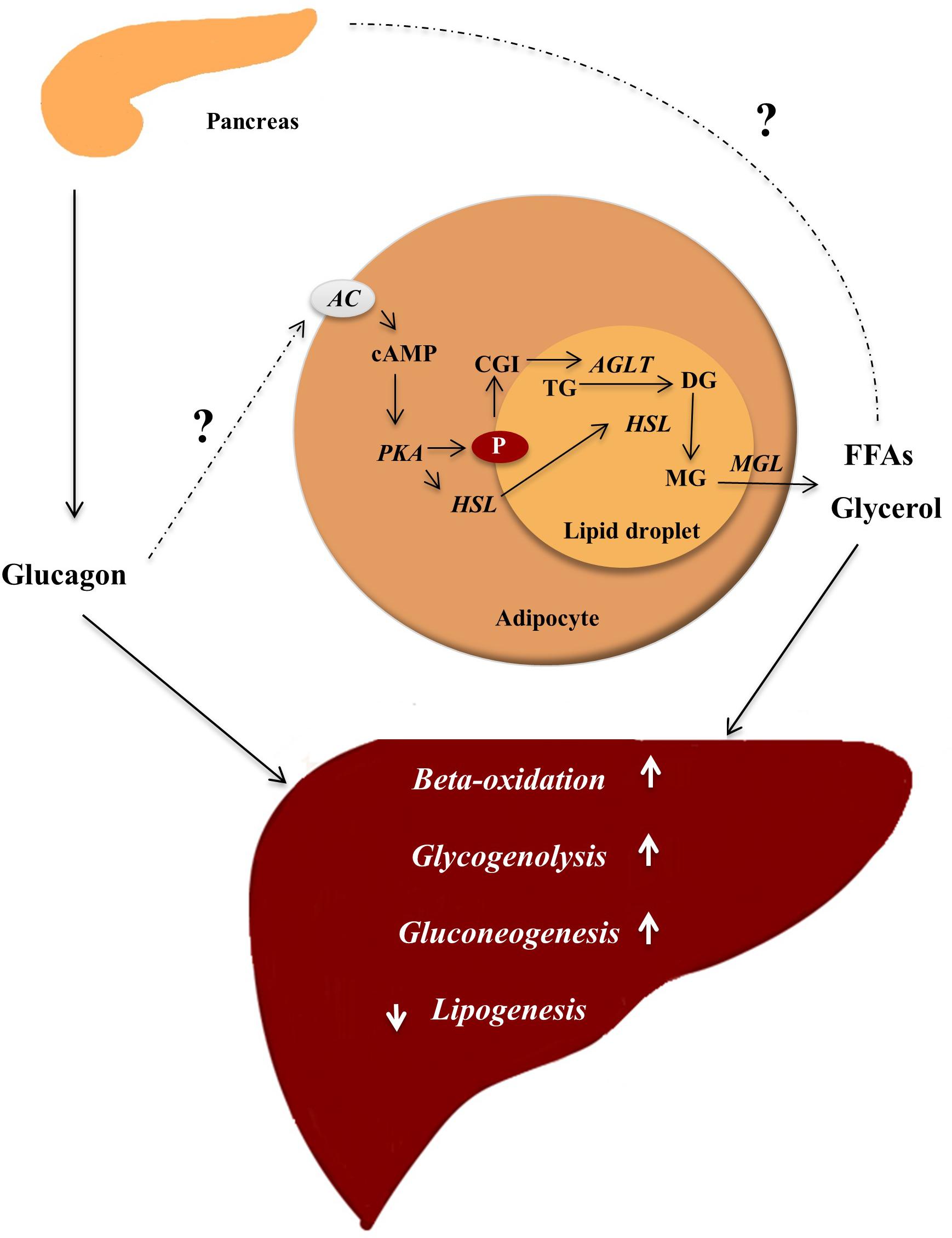
Figure 1. Glucagon ensures energy supply by mobilizing lipids. In the fasting state, glucagon is secreted and insulin concentrations are not sufficient to inhibit lipolysis in adipocytes, where lipids are stored in lipid droplets consisting of a core of triglycerols (TG) and sterols esters coated with perilipins (P) (proteins restricting access to the lipid core). In response to an appropriate stimuli, e.g., epinephrine and possibly glucagon, AC found in the plasma membrane of the adipocyte is activated, leading to increased intracellular concentrations of cAMP stimulating protein kinase A (PKA) activity. PKA phosphorylates (hence activates) hormone sensitive lipase (HSL) and P. The phosphorylation of P results in dissociation of the protein CGI-58. CGI-58 activates adipose triglycerol lipase (ATGL), which converts TGs to diaglycerols (DG). The phosphorylated P bind HSL and allows it to access the lipid droplet where it coverts DGs to monoglycerols (MG). The monoglycerols are hydrolyzed by monoacylglycerol lipase (MGL), yielding free fatty acids (FFAs) and glycerol, which are released to the blood. FFAs may stimulate glucagon secretion, and glucagon in turn stimulates hepatic gluconeogenesis (using FFAs and glycerol as substrates), glycogenolysis, and beta-oxidation thus providing substrates for the liver to secure sufficient energy supply to metabolically active tissue. Enzymes are written in italic and arrows indicate stimulation.
Glucagon has been reported to activate HSL (Vaughan et al., 1964; Slavin et al., 1994) and lipolysis in rat adipocytes (Vaughan and Steinberg, 1963; Rodbell and Jones, 1966; Prigge and Grande, 1971; Manganiello and Vaughan, 1972; Lefebvre et al., 1973; Livingston et al., 1974) within minutes (Honnor et al., 1985) at concentrations as low as 6 × 10−10 M (Lefebvre and Luyckx, 1969) and 10−11 M (Heckemeyer et al., 1983). Glucagon has also been shown to stimulate lipolysis in birds, rabbits (Richter et al., 1989; Wu et al., 1990), and human adipocytes in vitro (Perea et al., 1995) at concentrations near 10−8 M (Richter et al., 1989). At physiological plasma concentrations (1–40 pM), a lipolytic effect of glucagon in human adipocytes has been difficult to demonstrate (Mosinger et al., 1965; Vizek et al., 1979; Gravholt et al., 2001). One of the first human studies reporting a lipolytic effect of glucagon, demonstrated that an injection of 7.5 μg glucagon into the branchial artery resulted in a rapid increase in FFA plasma concentrations in the corresponding vein (Pozza et al., 1971) but this was not replicated in a similar study with mean increases of glucagon plasma concentrations by 237 pM in overnight fasted subjects (Pozefsky et al., 1976). An increase in FFA plasma concentrations has been demonstrated upon glucagon infusion (mean glucagon increment 209 ± 15 pM) (Schneider et al., 1981) and intravenous injection of glucagon [reaching plasma concentrations of >1,000 pM (Schade and Eaton, 1975)]. Since supra-physiological glucagon concentrations were applied, these studies may lack specificity because of interaction of glucagon with other related G protein-coupled receptors (e.g., the glucagon-like peptide 1 (GLP-1) receptor) (Hjorth et al., 1994). Pharmacological concentrations of glucagon also stimulate secretion of catecholamines and growth hormone, both of which have powerful lipolytic effects (Mitchell et al., 1969; Stallknecht et al., 1995), possibly as part of a generalized sympathetic nervous system discharge (Paschoalini and Migliorini, 1990). Glucagon was not found to have any lipolytic effects in clinical studies using glucagon concentrations ranging from 19 to 64 pM (Wu et al., 1990; Jensen et al., 1991; Gravholt et al., 2001; Xiao et al., 2011). In some clinical studies investigating the lipolytic effect of supra-physiological glucagon concentrations, the lipolytic effect of glucagon could be abolished by insulin (Samols et al., 1965; Goldfine et al., 1972; Liljenquist et al., 1974; Schade and Eaton, 1975; Schneider et al., 1981), and in rat adipocytes insulin is a potent inhibitor of lipolysis (Rodbell and Jones, 1966; Lefebvre and Luyckx, 1969; Prigge and Grande, 1971; Liljenquist et al., 1974; Gerich et al., 1976). A lipolytic effect of glucagon, if any, on human adipocytes may therefore only be physiologically relevant when insulin secretion is low. Supporting this, a 2-h infusion of 1 ng/kg × min glucagon (presumably resulting in physiologically relevant elevations) and somatostatin in insulin-deficient diabetic subjects caused a two to three-fold increase in FFA and glycerol plasma concentrations, compared to infusion of somatostatin alone. However, when insulin, somatostatin, and glucagon were infused together, glucagon had no lipolytic effect (Gerich et al., 1976). Furthermore, infusion with saline only gave the same increase in FFA as compared to glucagon infusion. In another study glucagon was infused at 1.2 ng/kg × min (high but also relevant) together with somatostatin for 2 h, but there was no lipolytic effect of glucagon at insulin concentrations of 38 pM (Jensen et al., 1991). In contrast, a 2-h glucagon infusion at 1.3 ng/kg × min, during a mean insulin plasma concentration of 65 pM, increased the rate of appearance of labeled FFA and glycerol by 40 and 36%, respectively (Carlson et al., 1993). As glucagon receptors are expressed on beta cells (Adriaenssens et al., 2016; Svendsen et al., 2018) and may stimulate insulin secretion through both GLP-1 and glucagon receptors (Svendsen et al., 2018) it may be speculated that intraislet regulation of insulin through glucagon may contribute to its effect on lipid metabolism.
It is important to note that FFA and glycerol in plasma are not only determined by release from adipocytes, but also by rate of uptake and re-esterification in other tissues. A lack of effect of glucagon on the free plasma pool of FFA and glycerol, does therefore not rule out that glucagon has a direct effect on lipid metabolism in adipocytes and hepatocytes (Figure 1).
In hepatocytes, glucagon action increases the transcription factor cAMP responsive element binding (CREB) protein, which induces the transcription of carnitine acyl transferase 1 (CPT-1) (Longuet et al., 2008). CPT-1 enables catabolism of long-chain fatty acids by converting fatty acids to acyl-carnitines, which are transported into the mitochondria and subjected to beta-oxidation (Kim et al., 2000; Stephens et al., 2007). During beta-oxidation the fatty acids are degraded into acetate, which ultimately enters the citric acid cycle (DiMarco and Hoppel, 1975). Furthermore, through PKA-dependent phosphorylation, glucagon receptor signaling inactivates acetyl-CoA carboxylase, the enzyme catalyzing the formation of malonyl-CoA. Malonyl-CoA is the first intermediate in fatty acid synthesis and inhibits CPT-1 (i.e., inhibits beta-oxidation). By inhibiting the formation of malonyl-CoA, glucagon diverts FFAs to beta-oxidation rather than re-esterification into TGs (Figure 2). Periportal and perivenous hepatocytes receive different concentrations of substrates and oxygen and as a consequence periportal hepatocytes primarily mediate oxidative processes, including beta-oxidation, whereas perivenous hepatocytes preferentially mediate glucose uptake and lipogenesis (Jungermann, 1988; Guzman and Castro, 1989).
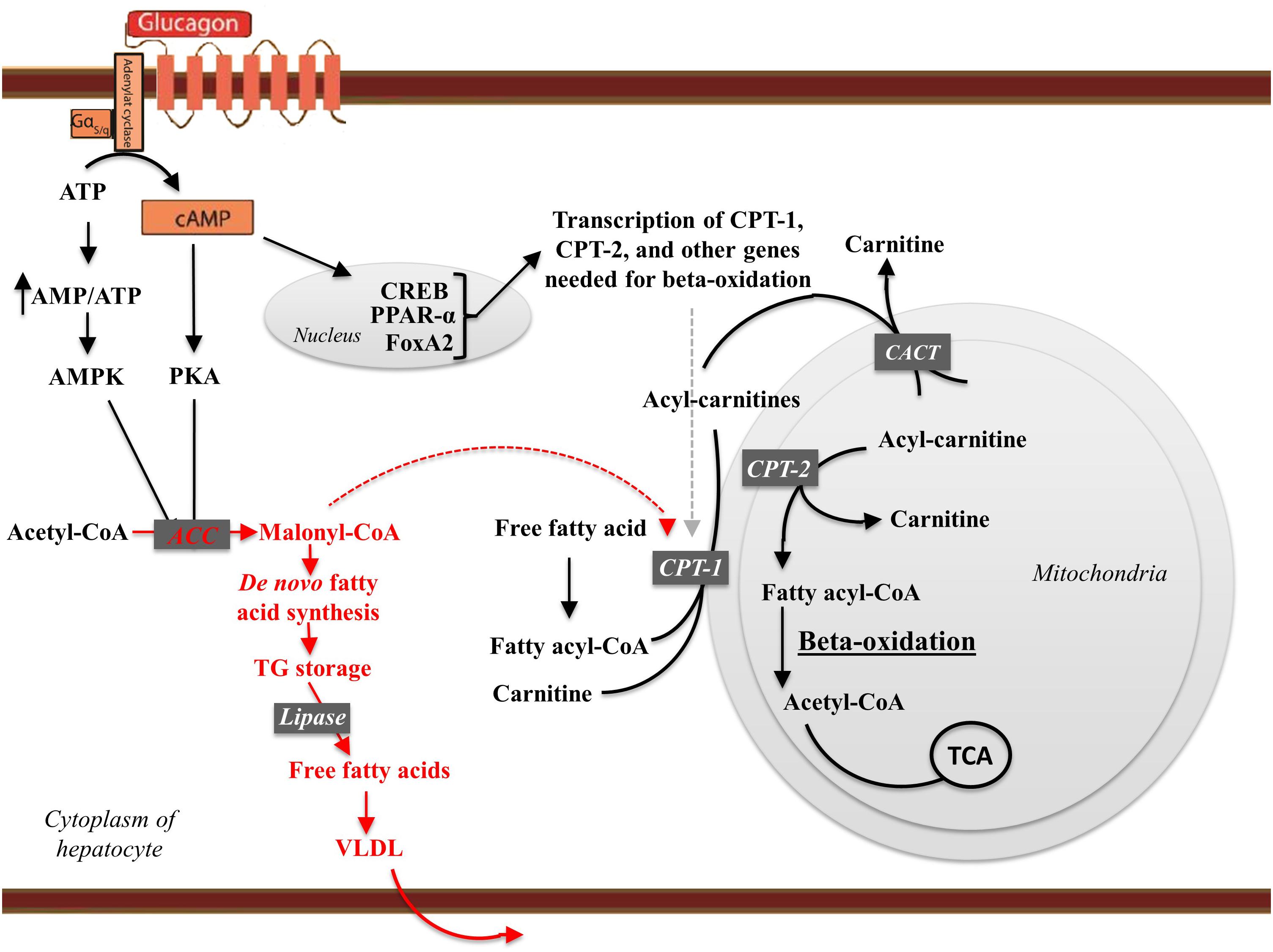
Figure 2. The effects of glucagon receptor signaling on hepatic lipid metabolism. Glucagon activates its cognate receptor, a seven transmembrane receptor coupled to a Gs protein, resulting in AC activity and cAMP production. The increase in intracellular cAMP activates protein kinase A (PKA), which phosphorylates (hence inactivates) acetyl-CoA carboxylase (ACC). Glucagon thus inhibit malonyl-CoA formation and the subsequent de novo fatty acid synthesis. When formed, the fatty acids are, after re-esterification, stored as trigycerides in and released from the hepatocytes in the form of very-low density lipoprotein (VLDL). Thus, glucagon leads the free fatty acids toward beta-oxidation and decreases de novo fatty acid synthesis and VLDL release. cAMP accumulation in hepatocytes activates the cAMP responsible binding element (CREB) protein, which induces the transcription of carnitine acyl transferase-1 (CPT-1), and other genes needed for beta-oxidation. CPT-1 catalyzes the attachment of carnitine to fatty acyl-CoA, forming acyl-carnitine. The acyl-carnitines transverse the mitochondrial membrane mediated via the carnitine-acylcarnitine translocase (CACT). Once in the mitochondrial matrix, carnitine acyl transferase-2 (CPT-2) is responsible for transferring the acyl-group from the acyl-carnitine back to CoA. Carnitine leaves the mitochondria matrix through the carnitine-acylcarnitine translocase. During beta-oxidation, the fatty acid chains are degraded into acetate. Acetate reacts with CoA to yield acetyl-CoA, which reacts with oxaloacetate to form citrate that inhibits glycolysis through inhibition of pyruvate dehydrogenase and phosphofructokinase-1. Finally, citrate enters the citric acid cycle (TCA). Thus, glucagon increases fatty acid catabolism, inhibits glycolysis, and fuels the TCA cycle. By increasing AC activity glucagon increase the AMP/ATP ratio sufficient to activate AMP-activated kinase (AMPK), which phosphorylates ACC, leading to transcriptional activation of peroxisome proliferator-activated receptor-α (PPARα). PPARα stimulates the transcription of genes involved in beta-oxidation including CPT-1, CPT-2, and acetyl-CoA oxidase. Glucagon stimulates FoxA2 activity, which induces transcription of genes such as CPT-1, very-, and medium- long-chain acyl-CoA dehydrogenase. Enzymes and pathways inhibited by glucagon are shown in red, while enzymes and pathways stimulated by glucagon are shown in black.
In hepatocytes, glucagon may bring about an energy-depleted state (increasing the AMP/ATP ratio) sufficient to activate AMP-activated kinase (Berglund et al., 2009), which phosphorylates acetyl-CoA carboxylase (Peng et al., 2012) and p38 mitogen-activated protein kinase, leading to transcriptional activation of peroxisome proliferator-activated receptor-α (PPARα) (Longuet et al., 2008). PPARα stimulates the transcription of genes involved in beta-oxidation including CPT-1, CPT-2, and acetyl-CoA oxidase (Patsouris et al., 2006), and the transcription of fibroblast growth factor 21, which is produced in the liver in response to glucagon (Xu et al., 2009; Cyphert et al., 2014). Glucagon also stimulates forkhead transcription factor A2 activity (FoxA2), which induces transcription of genes involved in beta-oxidation, such as CPT-1, very-, and medium- long-chain acyl-CoA dehydrogenase (Wolfrum and Stoffel, 2006; von Meyenn et al., 2013). Subsequent to activating its receptors on hepatocytes, insulin suppresses most of these pathways, and the metabolic state in the hepatocytes may therefore be determined by the insulin-glucagon ratio, rather than by the hormone concentrations per se (Parrilla et al., 1974). Insulin inhibits lipolysis in adipocytes and by reducing the amount of substrate (FFA and glycerol) reaching the liver may reduce (Perry et al., 2015) hepatic gluconeogenesis.
To investigate the physiological effects of glucagon in lipid metabolism, several studies have relied on glucagon receptor knockout (Gcgr−/−) mice or animals treated with GRA. In the livers of Gcgr−/− mice there is an increase in glycolysis and a decrease in gluconeogenesis and citric acid cycle activity, which results in decreased acetyl-CoA oxidation and acetyl-CoA accumulation. The accumulation of acetyl-CoA in the cytosol of hepatocytes results in increased lipogenesis. Supporting this, genes involved in lipogenesis, e.g., ATP citrate lyase and fatty acid synthase, were found to be upregulated in livers of Gcgr−/− mice at both the mRNA and protein level (Longuet et al., 2008; Yang et al., 2011), while CPT-1 and -2 levels, and other enzymes necessary for beta-oxidation, were downregulated (Yang et al., 2011). Hepatic beta-oxidation is essential for the production of both glucose and ketones since it provides the substrates acetyl-CoA and acetate and mitochondrial energy supply (ATP/NADH) needed for gluconeogenesis (Staehr et al., 2003). The hepatic gene expression profile changes markedly in response to fasting, and major differences have been reported in expression levels of genes involved in lipid metabolism between the fed and fasted state (Longuet et al., 2008; Zhang et al., 2011). Following a prolonged fast (16 h), wild-type mice had an increased hepatic expression of genes involved in beta-oxidation, such as CPT-1, CPT-2, and acyl-CoA dehydrogenase, but this was not observed in Gcgr−/− mice, which displayed an impaired beta-oxidation in both the fasted and fed state (Longuet et al., 2008) and Gcgr−/− mice failed to change the hepatic energy state in response to fasting (Berglund et al., 2009). Furthermore, Gcgr−/− mice showed increased hepatic TG secretion and increased plasma concentrations of TG and FFA after a 16 h fasting period, but not after 5 h of fasting (Longuet et al., 2008). Others (Gelling et al., 2003) also found similar TG and FFA plasma concentrations in Gcgr−/− and wild-type mice after a short-term fast; they did, however, find increased plasma concentrations of LDL in Gcgr−/− mice. Glucagon thus seems to regulate hepatic metabolism in response to fasting by stimulating glucose-producing processes, including beta-oxidation. When challenged with a high fat diet (HFD) for 8 weeks, Gcgr−/− mice did not increase the amount of inguinal and epididymal fat, whereas the amount of both doubled in wild-type mice (Longuet et al., 2008). In line with this, others (Gelling et al., 2003) showed a decrease in white adipose tissue mass and an increase in lean body mass in Gcgr−/− compared to wild-type mice, without changes in bodyweight, food consumption, or energy expenditure and one group (Conarello et al., 2007) found that Gcgr−/− mice had lower amounts of white adipose tissue when fed both a HFD and a low fat diet compared to wild-type mice, and thus seemed to be resistant to diet-induced obesity. This could reflect an inability of Gcgr−/− mice to mobilize the hepatic lipid storage; instead adipocyte lipolysis (by catecholamines) maintain the energy supply to other metabolically active tissues.
Administration of GRAs has been associated with increased hepatic fat content (assessed as hepatic fat fraction measured by magnetic resonance imaging) and increased plasma concentrations of LDL (Guzman et al., 2017). Furthermore, subjects with endogenous glucagon deficiency (pancreatectomized subjects) (Dresler et al., 1991) and rats (Sloop et al., 2004) and diabetic (db/db) mice (Liang et al., 2004) treated with glucagon antisense oligonucleotide have increased hepatic fat. These data suggest that inhibition of glucagon receptor signaling results in hepatic lipid accumulation. In addition, Gcgr−/− mice may be prone to steatosis when challenged with a high fat diet (HFD) for 8 weeks (Longuet et al., 2008). However, a study involving a similar HFD diet for 12 weeks and mice with the same sex, gene modification, and background (C57BL/6J), showed that Gcgr−/− mice were protected from steatosis (Conarello et al., 2007). Of notice, C57BL/6J mice do not consistently develop steatosis upon HFD feeding (Charlton et al., 2011), and this might have influenced the results. In rats, impaired glucagon action also associates with development of hepatic steatosis (Charbonneau et al., 2005a). Interestingly, HFD feeding has been reported to decrease glucagon receptor expression at the plasma membrane of rat hepatocytes (Charbonneau et al., 2005b, 2007). These data suggest that hepatic lipid accumulation may cause impaired glucagon receptor signaling, and that this (as demonstrated using GRAs) may contribute to and accelerate hepatic lipid accumulation.
Acute administration of 30 μg/kg glucagon decreased FFA and TG plasma concentrations and reduced hepatic TG content and secretion in mice (Longuet et al., 2008). Chronic hyperglucagonemia (injection of 10 μg glucagon every 8 h for 21 days) had hypolipidemic effects in rats, evident by a 70 and 38% decrease in plasma concentrations of TGs and phospholipids, respectively (Guettet et al., 1988). Consistent with this, glucagon inhibited synthesis and secretion of TGs in cultured hepatocytes (Longuet et al., 2008), in perfused rat livers (Penhos et al., 1966; Heimberg et al., 1969), and decreased the synthesis of hepatic VLDL in rats (Eaton, 1973). In humans, hyperglucagonemia (56 ± 20 pM), during a pancreatic clamp, reduced hepatic lipoprotein particle turnover (Xiao et al., 2011), and glucagon administration increased hepatic beta-oxidation in humans (Prip-Buus et al., 1990). In diet-induced obese (DIO) mice, a once-weekly treatment with 70 nmol/kg glucagon/GLP-1 receptor co-agonist resulted in loss of fat mass, which in the same study was also found, although less pronounced in GLP-1 receptor knockout mice, and improved hepatic lipid metabolism and steatosis within 4 weeks (Day et al., 2009). Another glucagon/GLP-1 co-agonist (1.9 μmol/kg daily for 14 days) decreased acetyl-CoA and malonyl-CoA concentrations and increased CPT-1 mRNA in the livers of DIO mice, whereas a selective GLP-1 receptor agonist had no effect (Pocai et al., 2009). Both of these dual agonists reduced hepatic steatosis, increased HSL activity in adipocytes, and improved dyslipidemia in DIO mice (Day et al., 2009; Pocai et al., 2009). Supporting these data, other glucagon/GLP-1 receptor co-agonists have been reported to lower plasma concentrations of TG and cholesterol (Clemmensen et al., 2014), decrease hepatic fat content (Henderson et al., 2016), and reduce adipose mass in rodent models of T2D and obesity (Evers et al., 2017; Zhou et al., 2017). Importantly, acute administration of 25 nmol/kg glucagon/GLP-1 co-agonist decreased plasma concentration of TGs, cholesterol, and LDL in DIO mice within 1 h, whereas liraglutide (a pure GLP-1 receptor agonist) administration had no effect (More et al., 2017). In addition, hepatic synthesis of VLDL and palmitate, and fatty acid esterification decreased, while beta-oxidation and LDL receptors expression increased upon co-agonist, but not liraglutide, administration (More et al., 2017). The inhibitory effect on hepatic lipogenesis and stimulatory effect on beta-oxidation therefore seems to be mediated by glucagon receptor signaling. Several clinical studies are currently investigating the potential treatment of obesity and T2D using glucagon/GLP-1 co-agonists (Capozzi et al., 2018).
FFAs are under certain circumstances insulin secretagogs (Boden and Carnell, 2003) but their ability to stimulate glucagon secretion remains debated (Gerich et al., 1974; Bollheimer et al., 2004; Gromada et al., 2007). Some clinical studies found a suppression of glucagon secretion at increased FFA concentrations (Madison et al., 1968; Edwards and Taylor, 1970; Luyckx and Lefebvre, 1970; Gerich et al., 1974) whereas isolated alpha cells were shown to secrete glucagon in response to FFA stimulation (Gross and Mialhe, 1986; Collins et al., 2008). In isolated rat pancreatic islets, palmitate stimulated glucagon secretion (Gremlich et al., 1997; Dumonteil et al., 2000). Others found palmitate to stimulate glucagon secretion in a glucose-dependent manner using isolated pancreatic islets; increasing at glucose concentrations of 2.8, 5.6, and 10 mM (Olofsson et al., 2004) but not at 16.7 mM (Bollheimer et al., 2004). Medium and long-chain fatty acids (>C5) have been reported to stimulate glucagon secretion by activation of FFA receptor G protein-coupled receptor 40 (GPR40) (Wang et al., 2011; Kristinsson et al., 2017) and GPR119 (Hansen et al., 2012; Li et al., 2018), both present in the pancreatic islets (Briscoe et al., 2003). FFAs may also function as metabolic substrate and stimulate alpha cell secretion through beta-oxidation (Kristinsson et al., 2017; Briant et al., 2018). FFAs decrease secretion of somatostatin (Gromada et al., 2001), and may lower the tonic inhibition of somatostatin on alpha cells (Gromada et al., 2007; Müller et al., 2017). A clinical study investigating the effects of ingestion of lipids on hormone secretion, found no change in glucagon secretion after intravenous or oral administration of a lipid emulsion (3 ml/kg) (Lindgren et al., 2011), neither did glucagon plasma concentrations change upon a 300 min lipid infusion raising FFA plasma concentrations from 0.4 to 0.8 mM (Staehr et al., 2003). No difference in glucagon secretion was observed between subjects consuming a HFD or a low-fat diet for 2 weeks (Raben et al., 2001). In contrast to this, ingestion of long–chain fatty acids (olive oil and C8 fatty acids) lead to increased plasma concentrations of glucagon 40 min after, whereas no increase was observed after ingestion of short-chain fatty acids (C4), however, glucose-dependent insulinotropic polypeptide (GIP) concentrations also increased upon ingestion of long-chain fatty acids and this may have caused an increase in glucagon secretion (Mandoe et al., 2015). Another study observed that a meal rich in mono-unsaturated fatty acids resulted in a larger glucagon response when compared to a control meal (Sloth et al., 2009). Others also observed an increase in glucagon concentrations upon fat-enriched meals (Radulescu et al., 2010; Niederwanger et al., 2014). The glucagon response observed upon a 90 min intraduodenal infusion of linoleic, oleic, and palmitic acids were significant lower than observed upon protein infusion (Ryan et al., 2013). Studies of ability of FFAs to stimulate glucagon secretion are complex, since FFAs are found in many forms and their stimulatory effect may vary (Radulescu et al., 2010) [as is the case for incretin secretion (Feltrin et al., 2004; Thomsen et al., 1999)]. Furthermore, the increased glucagon concentrations reported in some studies may result from other proglucagon products (e.g., glicentin or oxyntomodulin), since measurements of plasma glucagon concentrations have been marred with problems regarding sensitivity and specificity (Wewer Albrechtsen et al., 2016), and further studies investigating the regulation of glucagon secretion by FFAs are needed.
Glucagon may, aside from its physiological actions on glucose and amino acid metabolism, also be important for lipid metabolism via effects on hepatic beta-oxidation and lipogenesis, and potentially increased lipolysis in adipocytes. A direct role of glucagon on adipocytes may be of importance in rodents, as glucagon stimulates lipolysis (Vaughan and Steinberg, 1963; Rodbell and Jones, 1966; Prigge and Grande, 1971; Manganiello and Vaughan, 1972; Lefebvre et al., 1973; Livingston et al., 1974), whereas in humans an adipocyte-dependent lipolysis of glucagon is more complex. In both rodents and humans, glucagon is a powerful regulator of hepatic lipid metabolism (Day et al., 2009; Xiao et al., 2011) as highlighted in studies using GRAs (Guzman et al., 2017). The clinical use of GRAs is further challenged by glucagon’s role in amino acid metabolism, and blocking the glucagon receptor results in hyperaminoacidemia and eventually alpha cell hyperplasia (Holst et al., 2017b). Treatment of diabetes using the current GRAs may therefore not be feasible, however, one may speculate that targeted antagonism of glucagon signaling may circumvent these unwarranted side-effects. Currently glucagon receptor agonists, combined with GLP-1 and GIP receptor agonists, are investigated as possible therapeutic agents (Gu et al., 2011; Sadry and Drucker, 2013; Sanchez-Garrido et al., 2017; Capozzi et al., 2018). In preclinical studies, these agents improve steatosis and dyslipidemia, possibly as a consequence of regulation of hepatic lipid metabolism by glucagon agonism (Day et al., 2009).
Taken together, glucagon seems to play an important physiological role in the acute regulation of lipid metabolism but clearly further studies particularly in humans are warranted.
All authors wrote and approved the final edition of the manuscript.
All funding sources have been submitted. NNF Tandem Programme (31526), NNF Project support in Endocrinology and Metabolism – Nordic Region (34250), and Excellence Emerging Investigator Grant – Endocrinology and Metabolism (NNF19OC0055001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adriaenssens, A. E., Svendsen, B., Lam, B. Y., Yeo, G. S., Holst, J. J., Reimann, F., et al. (2016). Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 59, 2156–2165. doi: 10.1007/s00125-016-4033-1
Ahren, B. (2015). Glucagon-early breakthroughs and recent discoveries. Peptides 67, 74–81. doi: 10.1016/j.peptides.2015.03.011
Anthonsen, M. W., Ronnstrand, L., Wernstedt, C., Degerman, E., and Holm, C. (1998). Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 273, 215–221. doi: 10.1074/jbc.273.1.215
Aromataris, E. C., Roberts, M. L., Barritt, G. J., and Rychkov, G. Y. (2006). Glucagon activates Ca2+ and Cl- channels in rat hepatocytes. J. Physiol. 573(Pt 3), 611–625. doi: 10.1113/jphysiol.2006.109819
Baron, A. D., Schaeffer, L., Shragg, P., and Kolterman, O. G. (1987). Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, 274–283. doi: 10.2337/diab.36.3.274
Berglund, E. D., Lee-Young, R. S., Lustig, D. G., Lynes, S. E., Donahue, E. P., Camacho, R. C., et al. (2009). Hepatic energy state is regulated by glucagon receptor signaling in mice. J. Clin. Invest. 119, 2412–2422. doi: 10.1172/JCI38650
Bobe, G., Ametaj, B. N., Young, J. W., and Beitz, D. C. (2003). Effects of exogenous glucagon on lipids in lipoproteins and liver of lactating dairy cows. J. Dairy Sci. 86, 2895–2903. doi: 10.3168/jds.S0022-0302(03)73886-7
Boden, G., and Carnell, L. H. (2003). Nutritional effects of fat on carbohydrate metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 17, 399–410.
Bollheimer, L. C., Landauer, H. C., Troll, S., Schweimer, J., Wrede, C. E., Scholmerich, J., et al. (2004). Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations. Metabolism 53, 1443–1448. doi: 10.1016/j.metabol.2004.06.011
Briant, L. J. B., Dodd, M. S., Chibalina, M. V., Rorsman, N. J. G., Johnson, P. R. V., Carmeliet, P., et al. (2018). CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep. 23, 3300–3311. doi: 10.1016/j.celrep.2018.05.035
Briscoe, C. P., Tadayyon, M., Andrews, J. L., Benson, W. G., Chambers, J. K., Eilert, M. M., et al. (2003). The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278, 11303–11311. doi: 10.1074/jbc.M211495200
Capozzi, M. E., DiMarchi, R. D., Tschop, M. H., Finan, B., and Campbell, J. E. (2018). Targeting the incretin/glucagon system with triagonists to treat diabetes. Endocr. Rev. 39, 719–738. doi: 10.1210/er.2018-00117
Carlson, M. G., Snead, W. L., and Campbell, P. J. (1993). Regulation of free fatty acid metabolism by glucagon. J. Clin. Endocrinol. Metab. 77, 11–15.
Carranza, M. C., Simon, M. A., Torres, A., Romero, B., and Calle, C. (1993). Identification of glucagon receptors in human adipocytes from a liposarcoma. J. Endocrinol. Invest. 16, 439–442. doi: 10.1007/BF03348878
Charbonneau, A., Couturier, K., Gauthier, M. S., and Lavoie, J. M. (2005a). Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training. Int. J. Sports Med. 26, 432–441.
Charbonneau, A., Melancon, A., Lavoie, C., and Lavoie, J. M. (2005b). Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise. Am. J. Physiol. Endocrinol. Metab. 289, E8–E14.
Charbonneau, A., Unson, C. G., and Lavoie, J. M. (2007). High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. J. Physiol. 579(Pt 1), 255–267. doi: 10.1113/jphysiol.2006.121954
Charlton, M., Krishnan, A., Viker, K., Sanderson, S., Cazanave, S., McConico, A., et al. (2011). Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G825–G834. doi: 10.1152/ajpgi.00145.2011
Clemmensen, C., Chabenne, J., Finan, B., Sullivan, L., Fischer, K., Kuchler, D., et al. (2014). GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes 63, 1422–1427. doi: 10.2337/db13-1609
Collins, S. C., Salehi, A., Eliasson, L., Olofsson, C. S., and Rorsman, P. (2008). Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia 51, 1689–1693. doi: 10.1007/s00125-008-1082-0
Conarello, S. L., Jiang, G., Mu, J., Li, Z., Woods, J., Zycband, E., et al. (2007). Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50, 142–150. doi: 10.1007/s00125-006-0481-3
Cyphert, H. A., Alonge, K. M., Ippagunta, S. M., and Hillgartner, F. B. (2014). Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9:e94996. doi: 10.1371/journal.pone.0094996
Day, J. W., Ottaway, N., Patterson, J. T., Gelfanov, V., Smiley, D., Gidda, J., et al. (2009). A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 5, 749–757. doi: 10.1038/nchembio.209
Dean, E. D., Li, M., Prasad, N., Wisniewski, S. N., Von Deylen, A., Spaeth, J., et al. (2017). Interrupted glucagon signaling reveals hepatic alpha-cell axis and role for l-glutamine in alpha-cell proliferation. Cell Metab. 25, 1362–1373.e5. doi: 10.1016/j.cmet.2017.05.011
DiMarco, J. P., and Hoppel, C. (1975). Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. J. Clin. Invest. 55, 1237–1244. doi: 10.1172/JCI108042
Dresler, C. M., Fortner, J. G., McDermott, K., and Bajorunas, D. R. (1991). Metabolic consequences of (regional) total pancreatectomy. Ann. Surg. 214, 131–140. doi: 10.1097/00000658-199108000-00007
Dumonteil, E., Magnan, C., Ritz-Laser, B., Ktorza, A., Meda, P., and Philippe, J. (2000). Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology 141, 174–180. doi: 10.1210/endo.141.1.7230
Eaton, R. P. (1973). Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. J. Lipid Res. 14, 312–318.
Edwards, J. C., and Taylor, K. W. (1970). Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Biochim. Biophys. Acta 215, 310–315. doi: 10.1016/0304-4165(70)90029-2
Egan, J. J., Greenberg, A. S., Chang, M. K., Wek, S. A., Moos, M. C. Jr., and Londos, C. (1992). Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc. Natl. Acad. Sci. U.S.A. 89, 8537–8541. doi: 10.1073/pnas.89.18.8537
Evers, A., Haack, T., Lorenz, M., Bossart, M., Elvert, R., Henkel, B., et al. (2017). Design of novel exendin-based dual glucagon-like peptide 1 (GLP-1)/glucagon receptor agonists. J. Med. Chem. 60, 4293–4303. doi: 10.1021/acs.jmedchem.7b00174
Faerch, K., Vistisen, D., Pacini, G., Torekov, S. S., Johansen, N. B., Witte, D. R., et al. (2016). Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 65, 3473–3481. doi: 10.2337/db16-0240
Feltrin, K. L., Little, T. J., Meyer, J. H., Horowitz, M., Smout, A. J., Wishart, J., et al. (2004). Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R524–R533. doi: 10.1152/ajpregu.00039.2004
Galsgaard, K. D., Winther-Sørensen, M., Ørskov, C., Kissow, H., Poulsen, S. S., and Vilstrup, H. (2017). Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver - alpha-cell axis. Am. J. Physiol. Endocrinol. Metab. 314, E93–E103. doi: 10.1152/ajpendo.00198.2017
Garton, A. J., Campbell, D. G., Cohen, P., and Yeaman, S. J. (1988). Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett. 229, 68–72. doi: 10.1016/0014-5793(88)80799-3
Gelling, R. W., Du, X. Q., Dichmann, D. S., Romer, J., Huang, H., Cui, L., et al. (2003). Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 100, 1438–1443. doi: 10.1073/pnas.0237106100
Gerich, J. E., Langlois, M., Schneider, V., Karam, J. H., and Noacco, C. (1974). Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. J. Clin. Invest. 53, 1284–1289. doi: 10.1172/JCI107675
Gerich, J. E., Lorenzi, M., Bier, D. M., Tsalikian, E., Schneider, V., Karam, J. H., et al. (1976). Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. J. Clin. Invest. 57, 875–884. doi: 10.1172/JCI108364
Goldfine, I. D., Cerasi, E., and Luft, R. (1972). Glucagon stimulation of insulin release in man: inhibition during hypoglycemia. J. Clin. Endocrinol. Metab. 35, 312–315. doi: 10.1210/jcem-35-2-312
Granneman, J. G., Moore, H. P., Krishnamoorthy, R., and Rathod, M. (2009). Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J. Biol. Chem. 284, 34538–34544. doi: 10.1074/jbc.M109.068478
Gravholt, C. H., Moller, N., Jensen, M. D., Christiansen, J. S., and Schmitz, O. (2001). Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. J. Clin. Endocrinol. Metab. 86, 2085–2089. doi: 10.1210/jc.86.5.2085
Greenberg, A. S., Egan, J. J., Wek, S. A., Garty, N. B., Blanchette-Mackie, E. J., and Londos, C. (1991). Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 266, 11341–11346.
Gremlich, S., Bonny, C., Waeber, G., and Thorens, B. (1997). Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J. Biol. Chem. 272, 30261–30269. doi: 10.1074/jbc.272.48.30261
Gromada, J., Franklin, I., and Wollheim, C. B. (2007). Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 28, 84–116. doi: 10.1210/er.2006-0007
Gromada, J., Hoy, M., Buschard, K., Salehi, A., and Rorsman, P. (2001). Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G(i2)-dependent activation of calcineurin and depriming of secretory granules. J. Physiol. 535(Pt 2), 519–532.
Gross, R., and Mialhe, P. (1986). Free fatty acids and pancreatic function in the duck. Acta Endocrinol. 112, 100–104. doi: 10.1530/acta.0.1120100
Gu, W., Lloyd, D. J., Chinookswong, N., Komorowski, R., Sivits, G. Jr., Graham, M., et al. (2011). Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice. J. Pharmacol. Exp. Ther. 338, 70–81. doi: 10.1124/jpet.111.179986
Guettet, C., Mathe, D., Riottot, M., and Lutton, C. (1988). Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Biochim. Biophys. Acta 963, 215–223. doi: 10.1016/0005-2760(88)90283-4
Guzman, C. B., Zhang, X. M., Liu, R., Regev, A., Shankar, S., Garhyan, P., et al. (2017). Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes. Metab. 19, 1521–1528. doi: 10.1111/dom.12958
Guzman, M., and Castro, J. (1989). Zonation of fatty acid metabolism in rat liver. Biochem. J. 264, 107–113. doi: 10.1042/bj2640107
Hansen, H. S., Rosenkilde, M. M., Holst, J. J., and Schwartz, T. W. (2012). GPR119 as a fat sensor. Trends Pharmacol. Sci. 33, 374–381. doi: 10.1016/j.tips.2012.03.014
Hansen, L. H., Abrahamsen, N., and Nishimura, E. (1995). Glucagon receptor mRNA distribution in rat tissues. Peptides 16, 1163–1166. doi: 10.1016/0196-9781(95)00078-X
Heckemeyer, C. M., Barker, J., Duckworth, W. C., and Solomon, S. S. (1983). Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Endocrinology 113, 270–276. doi: 10.1210/endo-113-1-270
Heimberg, M., Weinstein, I., and Kohout, M. (1969). The effects of glucagon, dibutyryl cyclic adenosine 3’,5’-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J. Biol. Chem. 244, 5131–5139.
Henderson, S. J., Konkar, A., Hornigold, D. C., Trevaskis, J. L., Jackson, R., Fritsch Fredin, M., et al. (2016). Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab. 18, 1176–1190. doi: 10.1111/dom.12735
Hjorth, S. A., Adelhorst, K., Pedersen, B. B., Kirk, O., and Schwartz, T. W. (1994). Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. J. Biol. Chem. 269, 30121–30124.
Holst, J. J., Holland, W., Gromada, J., Lee, Y., Unger, R. H., Yan, H., et al. (2017a). Insulin and glucagon: partners for life. Endocrinology 158, 696–701. doi: 10.1210/en.2016-1748
Holst, J. J., Wewer Albrechtsen, N. J., Pedersen, J., and Knop, F. K. (2017b). Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, 235–240. doi: 10.2337/db16-0994
Honnor, R. C., Dhillon, G. S., and Londos, C. (1985). cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steady-state relationship with lipolytic and antilipolytic modulators. J. Biol. Chem. 260, 15130–15138.
Iwanij, V., and Vincent, A. C. (1990). Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. J. Biol. Chem. 265, 21302–21308.
Jelinek, L. J., Lok, S., Rosenberg, G. B., Smith, R. A., Grant, F. J., Biggs, S., et al. (1993). Expression cloning and signaling properties of the rat glucagon receptor. Science 259, 1614–1616. doi: 10.1126/science.8384375
Jensen, M. D., Heiling, V. J., and Miles, J. M. (1991). Effects of glucagon on free fatty acid metabolism in humans. J. Clin. Endocrinol. Metab. 72, 308–315. doi: 10.1210/jcem-72-2-308
Jiang, G., and Zhang, B. B. (2003). Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 284, E671–E678. doi: 10.1152/ajpendo.00492.2002
Jungermann, K. (1988). Metabolic zonation of liver parenchyma. Semin. Liver Dis. 8, 329–341. doi: 10.1055/s-2008-1040554
Kazda, C. M., Ding, Y., Kelly, R. P., Garhyan, P., Shi, C., Lim, C. N., et al. (2016). Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care 39, 1241–1249. doi: 10.2337/dc15-1643
Kazierad, D. J., Bergman, A., Tan, B., Erion, D. M., Somayaji, V., Lee, D. S., et al. (2016). Effects of multiple ascending doses of the glucagon receptor antagonist PF-06291874 in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 18, 795–802. doi: 10.1111/dom.12672
Kazierad, D. J., Chidsey, K., Somayaji, V. R., Bergman, A. J., and Calle, R. A. (2018). Efficacy and safety of the glucagon receptor antagonist PF-06291874: a 12-week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Diabetes Obes. Metab. 20, 2608–2616. doi: 10.1111/dom.13440
Kim, J., Okamoto, H., Huang, Z., Anguiano, G., Chen, S., Liu, Q., et al. (2017). Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha-cell hyperplasia in mice. Cell Metab. 25, 1348–1361.e8. doi: 10.1016/j.cmet.2017.05.006
Kim, J. Y., Hickner, R. C., Cortright, R. L., Dohm, G. L., and Houmard, J. A. (2000). Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 279, E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039
Kristinsson, H., Sargsyan, E., Manell, H., Smith, D. M., Gopel, S. O., and Bergsten, P. (2017). Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Sci. Rep. 7:4657. doi: 10.1038/s41598-017-04730-5
Lass, A., Zimmermann, R., Haemmerle, G., Riederer, M., Schoiswohl, G., Schweiger, M., et al. (2006). Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 3, 309–319. doi: 10.1016/j.cmet.2006.03.005
Lefebvre, P., Luyckx, A., and Bacq, Z. M. (1973). Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Horm. Metab. Res. 5, 245–250. doi: 10.1055/s-0028-1093959
Lefebvre, P. J., and Luyckx, A. S. (1969). Effect of insulin on glucagon enhanced lipolysis in vitro. Diabetologia 5, 195–197. doi: 10.1007/BF01213680
Li, N. X., Brown, S., Kowalski, T., Wu, M., Yang, L., Dai, G., et al. (2018). GPR119 agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67, 1401–1413. doi: 10.2337/db18-0031
Liang, Y., Osborne, M. C., Monia, B. P., Bhanot, S., Gaarde, W. A., Reed, C., et al. (2004). Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 53, 410–417. doi: 10.2337/diabetes.53.2.410
Liljenquist, J. E., Bomboy, J. D., Lewis, S. B., Sinclair-Smith, B. C., Felts, P. W., Lacy, W. W., et al. (1974). Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. J. Clin. Invest. 53, 190–197. doi: 10.1172/JCI107537
Lindgren, O., Carr, R. D., Deacon, C. F., Holst, J. J., Pacini, G., Mari, A., et al. (2011). Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J. Clin. Endocrinol. Metab. 96, 2519–2524. doi: 10.1210/jc.2011-0266
Livingston, J. N., Cuatrecasas, P., and Lockwood, D. H. (1974). Studies of glucagon resistance in large rat adipocytes: 125I-labeled glucagon binding and lipolytic capacity. J. Lipid Res. 15, 26–32.
Longuet, C., Sinclair, E. M., Maida, A., Baggio, L. L., Maziarz, M., Charron, M. J., et al. (2008). The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 8, 359–371. doi: 10.1016/j.cmet.2008.09.008
Luyckx, A. S., and Lefebvre, P. J. (1970). Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Proc. Soc. Exp. Biol. Med. 133, 524–528. doi: 10.3181/00379727-133-34511
Madison, L. L., Seyffert, W. A. Jr., Unger, R. H., and Barker, B. (1968). Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17, 301–304. doi: 10.1016/0026-0495(68)90097-8
Mandoe, M. J., Hansen, K. B., Hartmann, B., Rehfeld, J. F., Holst, J. J., and Hansen, H. S. (2015). The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Am. J. Clin. Nutr. 102, 548–555. doi: 10.3945/ajcn.115.106799
Manganiello, V., and Vaughan, M. (1972). Selective loss of adipose cell responsiveness to glucagon with growth in the rat. J. Lipid Res. 13, 12–16.
Mitchell, M. L., Byrne, M. J., and Silver, J. (1969). Growth-hormone release by glucagon. Lancet 1, 289–290. doi: 10.1016/S0140-6736(69)91041-1
More, V. R., Lao, J., McLaren, D. G., Cumiskey, A. M., Murphy, B. A., Chen, Y., et al. (2017). Glucagon like receptor 1/ glucagon dual agonist acutely enhanced hepatic lipid clearance and suppressed de novo lipogenesis in mice. PLoS One 12:e0186586. doi: 10.1371/journal.pone.0186586
Mosinger, B., Kuhn, E., and Kujalová, V. (1965). Action of adipokinetic hormones on human adipose tissue in vitro. J. Lab. Clin. Med. 66, 380–389.
Müller, T. D., Finan, B., Clemmensen, C., DiMarchi, R. D., and Tschöp, M. H. (2017). The new biology and pharmacology of glucagon. Physiol. Rev. 97, 721–766. doi: 10.1152/physrev.00025.2016
Niederwanger, A., Ciardi, C., Tatarczyk, T., Khan, M. I., Hermann, M., Mittermair, C., et al. (2014). Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells. Am. J. Clin. Nutr. 100, 1222–1231. doi: 10.3945/ajcn.114.092023
Olofsson, C. S., Salehi, A., Gopel, S. O., Holm, C., and Rorsman, P. (2004). Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Diabetes 53, 2836–2843. doi: 10.2337/diabetes.53.11.2836
Parrilla, R., Goodman, M. N., and Toews, C. J. (1974). Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, 725–731. doi: 10.2337/diab.23.9.725
Paschoalini, M. A., and Migliorini, R. H. (1990). Participation of the CNS in the control of FFA mobilization during fasting in rabbits. Physiol. Behav. 47, 461–465. doi: 10.1016/0031-9384(90)90109-H
Patsouris, D., Reddy, J. K., Muller, M., and Kersten, S. (2006). Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 147, 1508–1516. doi: 10.1210/en.2005-1132
Pegorier, J. P., Garcia-Garcia, M. V., Prip-Buus, C., Duee, P. H., Kohl, C., and Girard, J. (1989). Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Biochem. J. 264, 93–100. doi: 10.1042/bj2640093
Peng, I. C., Chen, Z., Sun, W., Li, Y. S., Marin, T. L., Hsu, P. H., et al. (2012). Glucagon regulates ACC activity in adipocytes through the CAMKKbeta/AMPK pathway. Am. J. Physiol. Endocrinol. Metab. 302, E1560–E1568. doi: 10.1152/ajpendo.00504.2011
Penhos, J. C., Wu, C. H., Daunas, J., Reitman, M., and Levine, R. (1966). Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes 15, 740–748. doi: 10.2337/diab.15.10.740
Perea, A., Clemente, F., Martinell, J., Villanueva-Penacarrillo, M. L., and Valverde, I. (1995). Physiological effect of glucagon in human isolated adipocytes. Horm. Metab. Res. 27, 372–375. doi: 10.1055/s-2007-979981
Perry, R. J., Camporez, J. G., Kursawe, R., Titchenell, P. M., Zhang, D., Perry, C. J., et al. (2015). Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758. doi: 10.1016/j.cell.2015.01.012
Pettus, J., Reeds, D., Cavaiola, T. S., Boeder, S., Levin, M., Tobin, G., et al. (2018). Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: a randomized controlled trial. Diabetes Obes. Metab. 20, 1302–1305. doi: 10.1111/dom.13202
Pocai, A., Carrington, P. E., Adams, J. R., Wright, M., Eiermann, G., Zhu, L., et al. (2009). Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 58, 2258–2266. doi: 10.2337/db09-0278
Pozefsky, T., Tancredi, R. G., Moxley, R. T., Dupre, J., and Tobin, J. D. (1976). Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, 128–135. doi: 10.2337/diab.25.2.128
Pozza, G., Pappalettera, A., Melogli, O., Viberti, G., and Ghidoni, A. (1971). Lipolytic effect of intra-arterial injection of glucagon in man. Horm. Metab. Res. 3, 291–292. doi: 10.1055/s-0028-1096783
Prigge, W. F., and Grande, F. (1971). Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds. Comp. Biochem. Physiol. B 39, 69–82. doi: 10.1016/0305-0491(71)90254-9
Prip-Buus, C., Pegorier, J. P., Duee, P. H., Kohl, C., and Girard, J. (1990). Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Biochem. J. 269, 409–415. doi: 10.1042/bj2690409
Raben, A., Holst, J. J., Madsen, J., and Astrup, A. (2001). Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Am. J. Clin. Nutr. 73, 177–189. doi: 10.1093/ajcn/73.2.177
Radulescu, A., Gannon, M. C., and Nuttall, F. Q. (2010). The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. J. Clin. Endocrinol. Metab. 95, 3385–3391. doi: 10.1210/jc.2009-2559
Ramnanan, C. J., Edgerton, D. S., Kraft, G., and Cherrington, A. D. (2011). Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes. Metab. 13(Suppl. 1), 118–125. doi: 10.1111/j.1463-1326.2011.01454.x
Richter, W. O., Robl, H., and Schwandt, P. (1989). Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, 333–335. doi: 10.1016/0196-9781(89)90039-9
Rodbell, M., and Jones, A. B. (1966). Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J. Biol. Chem. 241, 140–142.
Rouille, Y., Westermark, G., Martin, S. K., and Steiner, D. F. (1994). Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells. Proc. Natl. Acad. Sci. U.S.A. 91, 3242–3246. doi: 10.1073/pnas.91.8.3242
Ryan, A. T., Luscombe-Marsh, N. D., Saies, A. A., Little, T. J., Standfield, S., Horowitz, M., et al. (2013). Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 98, 300–311. doi: 10.3945/ajcn.113.061333
Sadry, S. A., and Drucker, D. J. (2013). Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat. Rev. Endocrinol. 9, 425–433. doi: 10.1038/nrendo.2013.47
Samols, E., Marri, G., and Marks, V. (1965). Promotion of insulin secretion by glucogen. Lancet 2, 415–416. doi: 10.1016/S0140-6736(65)90761-0
Sanchez-Garrido, M. A., Brandt, S. J., Clemmensen, C., Muller, T. D., DiMarchi, R. D., and Tschop, M. H. (2017). GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia 60, 1851–1861. doi: 10.1007/s00125-017-4354-8
Schade, D. S., and Eaton, R. P. (1975). Modulation of fatty acid metabolism by glucagon in man. I. Effects in normal subjects. Diabetes 24, 502–509. doi: 10.2337/diabetes.24.5.502
Schneider, S. H., Fineberg, S. E., and Blackburn, G. L. (1981). The acute metabolic effects of glucagon and its interactions with insulin in forearm tissue. Diabetologia 20, 616–621. doi: 10.1007/BF00257430
Schweiger, M., Eichmann, T. O., Taschler, U., Zimmermann, R., Zechner, R., and Lass, A. (2014). Measurement of lipolysis. Methods Enzymol. 538, 171–193. doi: 10.1016/B978-0-12-800280-3.00010-4
Shen, W. J., Patel, S., Miyoshi, H., Greenberg, A. S., and Kraemer, F. B. (2009). Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J. Lipid Res. 50, 2306–2313. doi: 10.1194/jlr.M900176-JLR200
Slavin, B. G., Ong, J. M., and Kern, P. A. (1994). Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J. Lipid Res. 35, 1535–1541.
Sloop, K. W., Cao, J. X., Siesky, A. M., Zhang, H. Y., Bodenmiller, D. M., Cox, A. L., et al. (2004). Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J. Clin. Invest. 113, 1571–1581. doi: 10.1172/JCI20911
Sloth, B., Due, A., Larsen, T. M., Holst, J. J., Heding, A., and Astrup, A. (2009). The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period. Br. J. Nutr. 101, 1846–1858. doi: 10.1017/S0007114508137710
Solloway, M. J., Madjidi, A., Gu, C., Eastham-Anderson, J., Clarke, H. J., Kljavin, N., et al. (2015). Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of alpha-cell mass. Cell Rep. 12, 495–510. doi: 10.1016/j.celrep.2015.06.034
Staehr, P., Hother-Nielsen, O., Landau, B. R., Chandramouli, V., Holst, J. J., and Beck-Nielsen, H. (2003). Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes 52, 260–267. doi: 10.2337/diabetes.52.2.260
Stallknecht, B., Simonsen, L., Bulow, J., Vinten, J., and Galbo, H. (1995). Effect of training on epinephrine-stimulated lipolysis determined by microdialysis in human adipose tissue. Am. J. Physiol. 269(6 Pt 1), E1059–E1066. doi: 10.1152/ajpendo.1995.269.6.E1059
Stephens, F. B., Constantin-Teodosiu, D., and Greenhaff, P. L. (2007). New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 581(Pt 2), 431–444. doi: 10.1113/jphysiol.2006.125799
Stralfors, P., Bjorgell, P., and Belfrage, P. (1984). Hormonal regulation of hormone-sensitive lipase in intact adipocytes: identification of phosphorylated sites and effects on the phosphorylation by lipolytic hormones and insulin. Proc. Natl. Acad. Sci. U.S.A. 81, 3317–3321. doi: 10.1073/pnas.81.11.3317
Svendsen, B., Larsen, O., Gabe, M. B. N., Christiansen, C. B., Rosenkilde, M. M., Drucker, D. J., et al. (2018). Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 25, 1127–1134.e2. doi: 10.1016/j.celrep.2018.10.018
Svoboda, M., Tastenoy, M., Vertongen, P., and Robberecht, P. (1994). Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol. Cell. Endocrinol. 105, 131–137. doi: 10.1016/0303-7207(94)90162-7
Thomsen, C., Rasmussen, O., Lousen, T., Holst, J. J., Fenselau, S., Schrezenmeir, J., et al. (1999). Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am. J. Clin. Nutr. 69, 1135–1143. doi: 10.1093/ajcn/69.6.1135
Unger, R. H., and Orci, L. (1975). The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1, 14–16. doi: 10.1016/S0140-6736(75)92375-2
Vajda, E. G., Logan, D., Lasseter, K., Armas, D., Plotkin, D. J., Pipkin, J. D., et al. (2017). Pharmacokinetics and pharmacodynamics of single and multiple doses of the glucagon receptor antagonist LGD-6972 in healthy subjects and subjects with type 2 diabetes mellitus. Diabetes Obes. Metab. 19, 24–32. doi: 10.1111/dom.12752
van der Woning, B., De Boeck, G., Blanchetot, C., Bobkov, V., Klarenbeek, A., Saunders, M., et al. (2016). DNA immunization combined with scFv phage display identifies antagonistic GCGR specific antibodies and reveals new epitopes on the small extracellular loops. MAbs 8, 1126–1135. doi: 10.1080/19420862.2016.1189050
Vaughan, M., Berger, J. E., and Steinberg, D. (1964). Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. J. Biol. Chem. 239, 401–409.
Vaughan, M., and Steinberg, D. (1963). Effect of hormones on lipolysis and esterification of free fatty acids during incubation of adipose tissue in vitro. J. Lipid Res. 4, 193–199.
Vizek, K., Razova, M., and Melichar, V. (1979). Lipolytic effect of TSH, glucagon and hydrocortisone on the adipose tissue of newborns and adults in vitro. Physiol. Bohemoslov. 28, 325–331.
von Meyenn, F., Porstmann, T., Gasser, E., Selevsek, N., Schmidt, A., Aebersold, R., et al. (2013). Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab. 17, 436–447. doi: 10.1016/j.cmet.2013.01.014
Wakelam, M. J., Murphy, G. J., Hruby, V. J., and Houslay, M. D. (1986). Activation of two signal-transduction systems in hepatocytes by glucagon. Nature 323, 68–71. doi: 10.1038/323068a0
Wang, H., Hu, L., Dalen, K., Dorward, H., Marcinkiewicz, A., Russell, D., et al. (2009). Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J. Biol. Chem. 284, 32116–32125. doi: 10.1074/jbc.M109.006726
Wang, L., Zhao, Y., Gui, B., Fu, R., Ma, F., Yu, J., et al. (2011). Acute stimulation of glucagon secretion by linoleic acid results from GPR40 activation and [Ca2+]i increase in pancreatic islet {alpha}-cells. J. Endocrinol. 210, 173–179. doi: 10.1530/JOE-11-0132
Watanabe, M., Hayasaki, H., Tamayama, T., and Shimada, M. (1998). Histologic distribution of insulin and glucagon receptors. Braz. J. Med. Biol. Res. 31, 243–256. doi: 10.1590/S0100-879X1998000200008
Wewer Albrechtsen, N. J., Kuhre, R. E., Windelov, J. A., Orgaard, A., Deacon, C. F., Kissow, H., et al. (2016). Dynamics of glucagon secretion in mice and rats revealed using a validated sandwich ELISA for small sample volumes. Am. J. Physiol. Endocrinol. Metab. 311, E302–E309. doi: 10.1152/ajpendo.00119.2016
Wolfrum, C., and Stoffel, M. (2006). Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 3, 99–110. doi: 10.1016/j.cmet.2006.01.001
Wu, M. S., Jeng, C. Y., Hollenbeck, C. B., Chen, Y. D., Jaspan, J., and Reaven, G. M. (1990). Does glucagon increase plasma free fatty acid concentration in humans with normal glucose tolerance? J. Clin. Endocrinol. Metab. 70, 410–416. doi: 10.1210/jcem-70-2-410
Xiao, C., Pavlic, M., Szeto, L., Patterson, B. W., and Lewis, G. F. (2011). Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 60, 383–390. doi: 10.2337/db10-0763
Xu, J., Stanislaus, S., Chinookoswong, N., Lau, Y. Y., Hager, T., Patel, J., et al. (2009). Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models–association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 297, E1105–E1114. doi: 10.1152/ajpendo.00348.2009
Yang, J., MacDougall, M. L., McDowell, M. T., Xi, L., Wei, R., Zavadoski, W. J., et al. (2011). Polyomic profiling reveals significant hepatic metabolic alterations in glucagon-receptor (GCGR) knockout mice: implications on anti-glucagon therapies for diabetes. BMC Genom. 12:281. doi: 10.1186/1471-2164-12-281
Zhang, F., Xu, X., Zhou, B., He, Z., and Zhai, Q. (2011). Gene expression profile change and associated physiological and pathological effects in mouse liver induced by fasting and refeeding. PLoS One 6:e27553. doi: 10.1371/journal.pone.0027553
Keywords: glucagon, lipid, liver, adipose tissue, alpha cell
Citation: Galsgaard KD, Pedersen J, Knop FK, Holst JJ and Wewer Albrechtsen NJ (2019) Glucagon Receptor Signaling and Lipid Metabolism. Front. Physiol. 10:413. doi: 10.3389/fphys.2019.00413
Received: 21 October 2018; Accepted: 26 March 2019;
Published: 24 April 2019.
Edited by:
Tongzhi Wu, The University of Adelaide, AustraliaReviewed by:
Greg Smith, The University of New South Wales, AustraliaCopyright © 2019 Galsgaard, Pedersen, Knop, Holst and Wewer Albrechtsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jens J. Holst, ampob2xzdEBzdW5kLmt1LmRr Nicolai J. Wewer Albrechtsen, aGdrNzk1QGt1LmRr; bmljb2xhaS5hbGJyZWNodHNlbkBzdW5kLmt1LmRr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.