- 1Graduate School, Tokyo Ariake University of Medical Health Sciences, Tokyo, Japan
- 2Human Informatics Research Institute, National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan
- 3Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 4Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas, TX, United States
- 5Department of Biomedical Engineering, Toyo University, Kawagoe, Japan
- 6Center for General Education, Aichi Institute of Technology, Toyota, Japan
The augmented cerebral hemodynamic pulsatility could lead to the elevated risk of cerebrovascular disease. To determine the impact of an acute orthostatic challenge on a pulsatile component of cerebral hemodynamics, mild lower body negative pressure (LBNP, -30 mmHg) was applied to 29 men. Middle cerebral artery blood flow velocity (MCAv) was measured by transcranial Doppler technique. Stroke volume (SV) was estimated by the Modelflow method with adjustment by the Doppler ultrasound-measured SV at rest. SV, peak and pulsatile MCAv, and pulsatility index were significantly lower during the LBNP stimulation than those at the baseline (e.g., supine resting) (P < 0.05 for all), whereas mean arterial pressure and mean MCAv remained unchanged. The change in SV with the LBNP stimulation significantly correlated with corresponding changes in peak and pulsatile MCAv (r = 0.617, P < 0.001; r = 0.413, P = 0.026, respectively). These results suggest that pulsatile components of cerebrovascular hemodynamics are dampened during the transient period of orthostatic challenge (as simulated using LBNP) when compared to supine rest, and which is partly due to the modified SV.
Introduction
There is a gaining recognition that augmented cerebral hemodynamic pulsatility could lead to the elevated risk of cerebrovascular disease. The brain, a high flow organ, is particularly sensitive to excessive pressure and flow pulsatility (Mitchell, 2008). Normally, central elastic arteries (e.g., aorta and carotid artery) dampen blood flow and blood pressure fluctuations by expanding and recoiling vessel wall (called Windkessel function). On the other hand, arterial stiffening is associated with an impaired Windkessel function causes the brain’s chronic exposure to cyclic mechanical forces of cardiac pulsations, promoting abnormalities in the microvascular structure and function (O’Rourke and Safar, 2005; Mitchell et al., 2011).
Furthermore, cardiac ejection per se may influence cerebral hemodynamics. In the supine position, stroke volume (SV) is greater than that in the upright position because the reduced hydrostatic pressure facilitates the venous return, and which consequently increases cardiac preload (Rowell, 1986). However, the impact of posture on cerebral hemodynamics, particularly pulsatility, is fully unknown (Bode, 1991). Accordingly, the aim of this study was to determine the impact of an acute orthostatic challenge on a pulsatile component of cerebral hemodynamics. We hypothesized that pulsatile components of cerebrovascular hemodynamics are dampened during the transient period of an orthostatic challenge when compared to supine rest because of the decreased SV.
Materials and Methods
Twenty-nine healthy men (age = 21 ± 2 year, height = 174 ± 6 cm, body mass = 63.6 ± 8.4 kg, body mass index = 21.0 ± 2.1 kg/m2; means ± standard deviation) volunteered for the present study. Each subject provided written informed consent after all of the potential risks and procedures were explained. All experimental procedures and protocol conformed to the Declaration of Helsinki and were approved by the institutional research board of National Institute of Advanced Industrial Science and Technology (No. 2013-434). All subjects were not smokers, had no known cardiovascular or pulmonary disorders, had no history of head injury, and were not taking any prescribed medication known to influence systemic or cerebrovascular function.
The experiment consisted assessment of body composition, cardiac echocardiography test at rest, and measurement of cerebral and systemic hemodynamics at rest and -30 mmHg of lower body negative pressure (LBNP). All measurements were conducted in an environmentally controlled laboratory with a quiet and air-conditioned room (24–25°C). At first, the subjects underwent body composition assessment (e.g., weight and height), and supine rest at least 20 min. Then, echocardiographic examinations were performed to obtain the reference value of SV in the left lateral decubitus position using an ultrasound devise (CX50 × MATRIX, Philips Ultrasound., Bothell, WA, United States) equipped with a multi-frequency probe (2.5-MHz transducer), as we previously reported (Tomoto et al., 2015, 2018). Briefly, Doppler velocity time integral (VTI) was obtained from LV ejection velocity waveform recorded via an apical three-chamber view. SV was computed from multiplying the VTI by the cross-sectional area of the LV outflow tract (LVOT) via a parasternal long axis view, as previously reported (Lewis et al., 1984). LVOT diameter was measured at the base of the aortic valve right after the valve was fully open.
After cardiac data acquirement, the LBNP testing was performed as previously reported (Sugawara et al., 2017; Tomoto et al., 2018). Each participant was placed in a supine position inside the LBNP testing chamber. Once inside, they straddled a wood seat with their feet clear of the base of the chamber and sealed at the level the iliac crest. Following 6 min rest at ambient barometric pressure, negative pressure was gradually induced using a commercially available vacuum and quantified with pressure transducer was invoked. The LBNP test was terminated when the participant completed 4 min at -30 mmHg of lower body pressure. During this experimental protocol, negative pressure was gradually increased and carefully monitored the signs of impending presyncope include dizziness, nausea, profuse sweating, or a rapid change in blood pressure defined as either a decrease in systolic blood pressure by 25 mmHg or a decrease in diastolic blood pressure by 15 mmHg within 1 min. When these signs were reported or observed, the experimental was stopped. From the start of baseline to the end of LBNP stimulation, electrocardiography (ECG) was recorded using a three-lead system for calculation of heart rate (HR) (ML 132 Bio Amp, AD Instruments Inc., Colorado Springs, CO, United States). Radial arterial pressure waveforms were recorded at the left wrist by an applanation tonometry-based blood pressure measurement device (Jentow, Nihon Colin Co., Komaki, Japan), and those were calibrated with oscillometry-derived brachial blood pressure. Middle cerebral artery blood flow velocity (MCAv) was continuously recorded over the right temporal window ipsilaterally using 2-MHz transcranial Doppler probe (EZ Dop; DWL, Sipplingen, Germany). The sampling depth was set from 42 to 55 mm, and the angle of the Doppler probe and the sampling depth were adjusted to optimize the signal quality for each subject according to standard procedures (Tomoto et al., 2015, 2018). Throughout data collection, subjects were instructed to breathe normally. End-tidal CO2 (EtCO2) was monitored by a metabolic cart equipped with a respiratory gas-analyzing system (AE280S; Minato Medical Science, Tokyo, Japan). Analog signals of ECG, radial arterial pressure, the spectral envelope of MCAv, and EtCO2 were continuously stored into a computer using a data acquisition system (PowerLab, AD Instrument Inc., Colorado Springs, CO, United States) throughout the experiment, and analyzed off-line by an analysis software (LabChart, AD Instrument Inc., Colorado Springs, CO, United States). Systolic blood pressures (SBP), diastolic blood pressures (DBP), and pulse pressure (PP) were obtained from the radial arterial pressure waveforms. Time-averaged mean arterial pressure (MAP) was also calculated from the radial arterial pressure waveforms. Systemic hemodynamic pulsatility was assessed as PP/MAP. SV was computed from radial arterial pressure waveforms via the Modelflow method (BeatScope 1.1a, Finapres Medical System BV, Amsterdam, Netherlands) (Wesseling et al., 1993; Sugawara et al., 2003). Modelflow technique could provide reliable information, at least regarding the relative changes in SV during postural alteration (van Lieshout et al., 2003) and submaximal exercise (Wesseling et al., 1993; Sugawara et al., 2003). In addition, the Modelflow technique combined with calibration can quantitatively replace thermodilution estimates. In the present study, in order to minimize estimation error, Modelflow-derived SV was calibrated by Echo-Doppler (LVOT)-derived SV. Cardiac output (CO) was calculated as SV × HR. Systemic vascular conductance (SVC) was obtained as CO/MAP. Time-averaged (mean), peak (systolic), end-diastolic (diastolic), and pulsatile (=peak – end-diastolic) MCAv were calculated from the spectral envelope of MCAv. Pulsatility index (PI) was calculated as pulsatile MCAv/mean MCAv. Cerebrovascular conductance index (CVCi) was calculated as mean MCAv/MAP. Aforementioned systemic and cerebral hemodynamic variables were obtained beat-to-beat and reported averaged values for the last 1 min of each condition (i.e., the baseline and the LBNP condition).
Averaged values for the last 1 min of each condition were calculated and compared. Data were reported as means and standard deviation. Each hemodynamic variable was compared between the baseline and the LBNP condition by a paired t-test. Correlation between variables of interests was analyzed by Pearson’s correlation analysis. Statistical significance was set a priori at P < 0.05.
Results
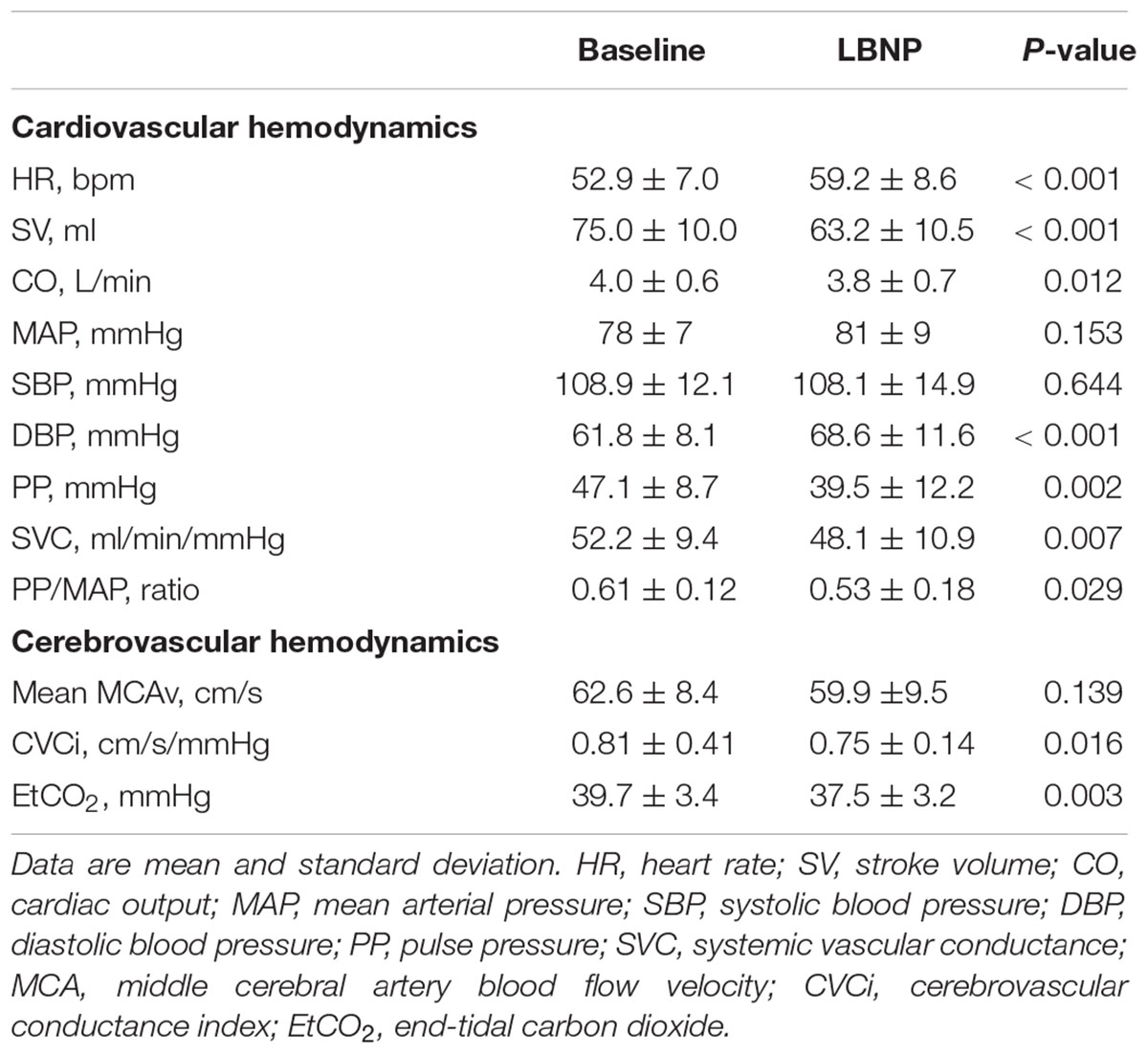
No subject showed the signs of impending presyncope during the LNBP testing. Table 1 presents the responses of systemic and cerebral hemodynamics to the LBNP stimulation. SBP, MAP, and mean MCAv remained unchanged during the LBNP stimulation. DBP and HR elevated significantly, and PP, PP/MAP, SV, CO, SVC, CVCi, and EtCO2 were significantly reduced during the LBNP stimulation.
Figure 1 depicts responses of middle cerebral artery pulsatile hemodynamic measures to the LBNP stimulation. Peak and pulsatile MCAv (P < 0.0001 for both) and PI (P = 0.001) were significantly attenuated. The relative change in SV with the LBNP stimulation significantly correlated with corresponding changes in peak and pulsatile MCAv (r = 0.617, P = 0.0004; r = 0.413, P = 0.026, respectively) (Supplementary Figure). The relative change in PP with the LBNP stimulation did not significantly correlate with corresponding changes in peak MCAv (r = 0.260, P = 0.173), pulsatile MCAv (r = 0.329, P = 0.081), and PI (r = 0.117, P = 0.546). Likewise, the relative change in PP/MAP with the LBNP stimulation did not significantly correlate with corresponding changes in peak MCAv (r = 0.223, P = 0.245), pulsatile MCAv (r = 0.303, P = 0.110), and PI (r = 0.271, P = 0.156).
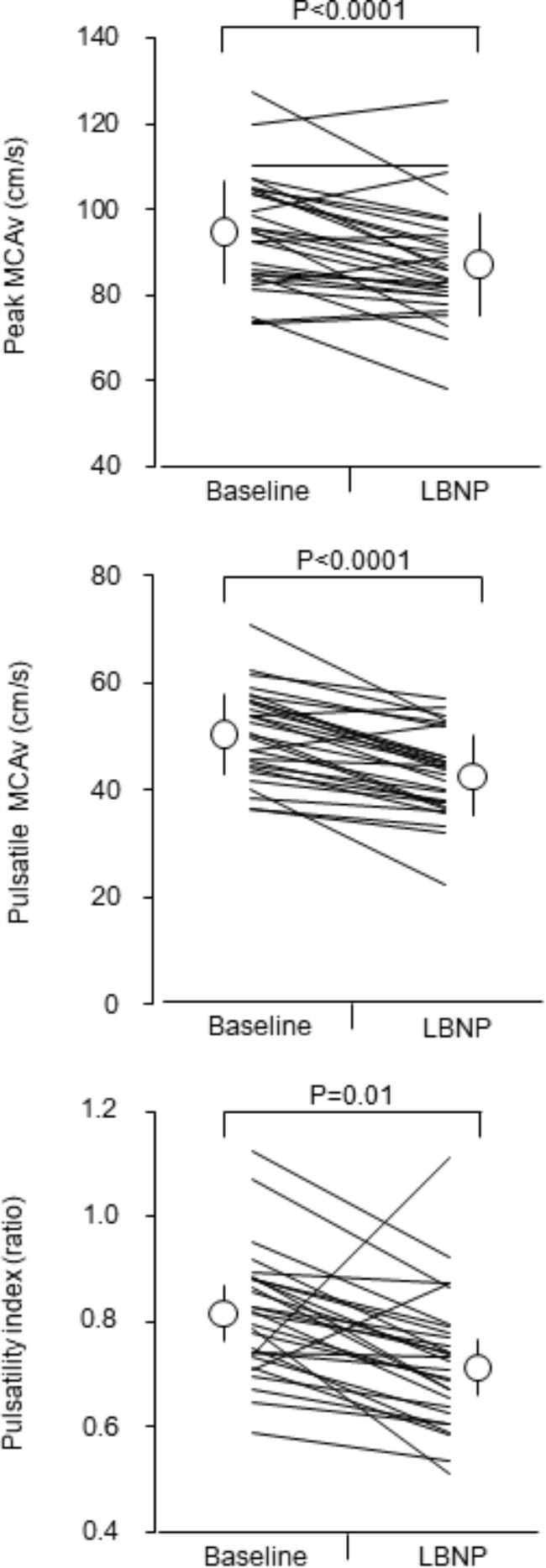
Figure 1. Responses of middle cerebral artery pulsatile hemodynamic measures to mild lower body negative pressure (LBNP) stimulation. Open circles and error bars are mean values and standard deviation. Thin lines indicate individual changes. MCAv = middle cerebral artery blood flow velocity.
Discussion
The main findings of this study are as follows: first, indices of cerebrovascular pulsatile hemodynamics (e.g., peak and pulsatile MCAv and PI) are significantly higher during the supine than the LBNP condition, whereas mean MCAv, reflecting cerebral perfusion, was maintained. Second, LBNP-induced change in SV correlated with the corresponding changes in peak and pulsatile MCAv. These results suggest that pulsatile components of cerebrovascular hemodynamics are dampened during the transient period of orthostatic challenge (as simulated using LBNP) when compared to supine rest partly due to modified SV. On the other hand, the change in systemic pressure pulsatility (assessed by PP/MAP) was not associated with responses of pulsatile cerebrovascular hemodynamics to the LBNP stimulation.
In this study, we examined whether pulsatile components of cerebrovascular hemodynamics are dampened during the transient period of the orthostatic challenge because of the reduced cardiac ejection (e.g., SV). Addressing this aim, hydrostatic pressure could be a confounding factor which influences cerebral hemodynamics. Therefore, we used LBNP as orthostatic stimulation. In addition, to minimize the change in transmural pressure, we applied mild LBNP stimulation (e.g., -30 mmHg). As expected, SV was significantly larger by 19 % in the supine position presumably due to the enhanced venous return and subsequent increase in cardiac preload (Rowell, 1986), whereas MAP remained unchanged. These systemic hemodynamic responses were similar to a previous study which used the head-up tilt as the orthostatic stimulation (Shaw et al., 2014). However, since the LBNP stimulation may not have the equivalent effects as orthostatic stimulation by the head-up tilt, a further study using the head-up tilt is ideal to clarify this research question.
Importantly, although mild orthostatic stimulation did not influence mean MCAv, the indices of the pulsatile component of cerebrovascular hemodynamics (e.g., peak and pulsatile velocity) were dampened by 8–15 % by the mild LBNP stimulation. The augmented cerebral flow and pressure fluctuations cause several cerebral small vessel diseases (O’Rourke and Safar, 2005; Mitchell et al., 2011; Tarumi et al., 2014). Therefore, our finding may support the notion that the prolonged bedridden develops and accelerates dementia is discussed repeatedly in lay publications. A future study to examine the impact of prolonged bedridden on cerebral hemodynamics and structure is warranted.
Large conduit arteries located cardiothoracic region (e.g., aorta and carotid artery) are highly compliant and dampen intermittent blood flow ejection from the left ventricle as “Windkessel” (a German term meaning elastic reservoir). This role is considered as an important function to protect vulnerable microvasculature, such as the brain, against cyclic mechanical forces of cardiac pulsations (O’Rourke and Safar, 2005). Since we studied young apparent healthy men in this study, it could be thought that the mechanical forces of cardiac pulsations were effectively dampened. Conversely, in individuals who have high arterial stiffness, it is plausible that cyclic mechanical forces of cardiac pulsations are not attenuated appropriately. As the next step, the impact of posture on cerebral hemodynamics, particularly pulsatile component, has to be examined in elderly peoples or hypertensives with the poor Windkessel function of large conduit arteries.
Conclusion
In conclusion, cerebral pulsatile hemodynamic measures are higher during the baseline (e.g., supine resting) than those during short-term mild orthostatic simulation by LBNP, and that the changes in pulsatile hemodynamic measures relate to LBNP-induced change in SV. These results suggest that pulsatile components of cerebrovascular hemodynamics are dampened during the transient period of orthostatic challenge (as simulated using LBNP) when compared to supine rest partly due to modified SV.
Author Contributions
JS and SO conceived and designed the research. TT, TI, and JS performed the experiments. YN, TT, TI, and JS analyzed the data. YN, TT, TI, SO, KT, and JS interpreted the results of experiments and approved the final version of the manuscripts. YN prepared the figures and drafted the manuscripts.
Funding
This research was supported by the special coordination funds of the Japanese Ministry of Education, Culture, Sports, Science, and Technology (25702045 and 26670116, JS).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the study volunteers for their participation. The authors thank Ms. Mitsuho Watanabe for her technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.00230/full#supplementary-material
References
Bode, H. (1991). Cerebral blood flow velocities during orthostasis and physical exercise. Eur. J. Pediatr. 150, 738–743. doi: 10.1007/BF01958769
Lewis, J. F., Kuo, L. C., Nelson, J. G., Limacher, M. C., and Quinones, M. A. (1984). Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation 70, 425–431. doi: 10.1161/01.CIR.70.3.425
Mitchell, G. F. (2008). Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J. Appl. Physiol. 105, 1652–1660. doi: 10.1152/japplphysiol.90549.2008
Mitchell, G. F., van Buchem, M. A., Sigurdsson, S., Gotal, J. D., Jonsdottir, M. K., Kjartansson, O., et al. (2011). Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility–Reykjavik study. Brain 134(Pt 11), 3398–3407. doi: 10.1093/brain/awr253
O’Rourke, M. F., and Safar, M. E. (2005). Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46, 200–204. doi: 10.1161/01.HYP.0000168052.00426.65
Rowell, L. B. (1986). Human Circulation: Regulation During Physical Stress. New York, NY: Oxford University Press.
Shaw, B. H., Loughin, T. M., Mackey, D. C., Robinovitch, S. N., and Claydon, V. E. (2014). The effect of orthostatic stress type on cardiovascular control. Blood Press. Monit. 19, 327–338. doi: 10.1097/MBP.0000000000000067
Sugawara, J., Tanabe, T., Miyachi, M., Yamamoto, K., Takahashi, K., Iemitsu, M., et al. (2003). Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol. Scand. 179, 361–366. doi: 10.1046/j.0001-6772.2003.01211.x
Sugawara, J., Tomoto, T., Imai, T., Maeda, S., and Ogoh, S. (2017). Impact of mild orthostatic stress on aortic-cerebral hemodynamic transmission: insight from the frequency domain. Am. J. Physiol. Heart Circ. Physiol. 312, H1076–H1084. doi: 10.1152/ajpheart.00802.2016
Tarumi, T., Ayaz Khan, M., Liu, J., Tseng, B. Y., Parker, R., Riley, J., et al. (2014). Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J. Cereb. Blood Flow Metab. 34, 971–978. doi: 10.1038/jcbfm.2014.44
Tomoto, T., Imai, T., Ogoh, S., Maeda, S., and Sugawara, J. (2018). Relationship between aortic compliance and impact of cerebral blood flow fluctuation to dynamic orthostatic challenge in endurance athletes. Front. Physiol. 9:25. doi: 10.3389/fphys.2018.00025
Tomoto, T., Sugawara, J., Nogami, Y., Aonuma, K., and Maeda, S. (2015). The influence of central arterial compliance on cerebrovascular hemodynamics: insights from endurance training intervention. J. Appl. Physiol. 119, 445–451. doi: 10.1152/japplphysiol.00129.2015
van Lieshout, J. J., Toska, K., van Lieshout, E. J., Eriksen, M., Walloe, L., and Wesseling, K. H. (2003). Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur. J. Appl. Physiol. 90, 131–137. doi: 10.1007/s00421-003-0901-8
Keywords: orthostatic stress, lower body negative pressure, transcranial Doppler method, pulsatility index, stroke volume
Citation: Ninomiya Y, Tomoto T, Ogoh S, Imai T, Takahashi K and Sugawara J (2019) Effects of Mild Orthostatic Stimulation on Cerebral Pulsatile Hemodynamics. Front. Physiol. 10:230. doi: 10.3389/fphys.2019.00230
Received: 28 September 2018; Accepted: 21 February 2019;
Published: 12 March 2019.
Edited by:
Pamela Davern, Baker Heart and Diabetes Institute, AustraliaReviewed by:
Jie Liu, Rutgers, The State University of New Jersey, United StatesMark Butlin, Macquarie University, Australia
Copyright © 2019 Ninomiya, Tomoto, Ogoh, Imai, Takahashi and Sugawara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Sugawara, anVuLnN1Z2F3YXJhQGFpc3QuZ28uanA=
 Yuka Ninomiya
Yuka Ninomiya Tsubasa Tomoto
Tsubasa Tomoto Shigehiko Ogoh
Shigehiko Ogoh Tomoko Imai
Tomoko Imai Koki Takahashi
Koki Takahashi Jun Sugawara
Jun Sugawara