
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 04 January 2019
Sec. Integrative Physiology
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.01830
This article is part of the Research Topic Peritoneal Dialysis and Its Local and Systemic Complications: From the Bench to the Clinic. View all 16 articles
 Ewa Wojtaszek1*
Ewa Wojtaszek1* Agnieszka Grzejszczak1
Agnieszka Grzejszczak1 Katarzyna Grygiel2
Katarzyna Grygiel2 Jolanta Małyszko1
Jolanta Małyszko1 Joanna Matuszkiewicz-Rowińska1
Joanna Matuszkiewicz-Rowińska1Background: The peritoneal dialysis (PD) urgent-start pathway, without typical 2-week break-in period, was meant for late-referral patients able and prone to join PD-first program, with its main advantages such as: keeping the vascular system intact, preserving their residual renal function and retaining life-style flexibility. We compared the short- and long-term outcomes of consecutive 35 patients after urgent- and 94 patients after the planned start of PD as the first choice.
Methods: The study included all incident end-stage renal disease patients starting PD program between January 2005 and December 2015, classified into two groups: those with urgent (unplanned) and those with elective (planned) start. Urgent PD was initiated as an overnight automatic procedure (APD) with dwell volume gradually increased, and after 2–3 weeks, target PD method was established.
Results: The mean time between catheter implantation and PD start was 3.5 ± 2.3 in urgent and 16.2 ± 1.7 days in planned-start groups (p < 0.00001). 51% of the patients in the urgent-start group required PD during first 48 h after catheter insertion. Mean follow-up of 17.6 ± 11.09 months (median: 19.0) was in the urgent-start group and 28.6 ± 26.6 months (median: 19.5) in the planned-start group. The early mechanical complications were observed more often in the urgent-start group (29 vs. 4%, p = 0.00005). The only significant predictors of early mechanical complications were serum albumin (p = 0.02) and time between the catheter insertion and PD start. The first year patient survival and technique survival censored for death and kidney transplantation were not significantly different between groups. In Cox proportional analysis the independent risk factors for patient survival as well as for method and patient survival appeared Charlson Comorbidity Index CCI (HR 1.4; p = 0.01 and 1.24; p = 0.02) and time from catheter implantation to PD start with HR 5.11; p = 0.03 and 4.29; p = 0.04 for <2 days, while time >14 days lost its predictive value (p = 0.07).
Conclusion: Peritoneal dialysis may be a feasible and safe alternative to HD in patients who need to start dialysis urgently without established dialysis access, with an acceptable complications rates, as well as patient and technique survival.
Almost 3 million people worldwide are affected by end stage renal disease (ESRD), and the vast majority of those who cannot undergo renal transplantation are treated with hemodialysis (HD), while less than 10% are on peritoneal dialysis (PD) (Liyanage et al., 2015; Li and Kwong, 2017). Moreover, despite many advantages, comparable survival rates for both techniques and the efforts of PD societies, the numbers of PD patients are still declining, year by year (Mehrotra et al., 2016; Li et al., 2017). The problem is complex, with the main causes being: an inadequate education and training of the nephrologists, shortages of trained nurses, easy access to HD, lack of patients education, and, in some countries, financial issues. Another important aspect is that as much as 40–60% of the patients initiate dialysis urgently, with no dialysis access, due to a late referral (Perl et al., 2011; Blake et al., 2013).
Since the insertion of central venous catheter is readily available and much easier than PD catheter placement, HD becomes the typical initial modality in such situations and many remain on HD, taking the path of least resistance. This can be overcome by introducing a so-called PD urgent-start program, which has attracted increased interest in recent years (Povlsen and Ivarsen, 2006; Lobbedez et al., 2008; Sharma et al., 2008; Oliver et al., 2012; Arramreddy et al., 2014; Mahnensmith, 2014).
A program of this kind was introduced in the Department of Nephrology of the Medical University of Warsaw in 2004. The aim of our study was to compare the impact of planned and unplanned (urgent) initiation of PD therapy in ESRD patients on infectious and non-infectious complications rate, and patients as well as patient technique short- and long-term survival.
All incident patients with ESRD starting a PD program in the Department of Nephrology at the Medical University of Warsaw between January 2005 and December 2015 were enrolled into the study. The protocol was approved by the Bioethical Committee of Medical University of Warsaw. All patients gave written informed consent in accordance with the Declaration of Helsinki.
In accordance with the International Society for PD (ISPD) (Li et al., 2016) and the European Renal Best Practice (ERBP) guidelines (Dombros et al., 2005), they were classified into two groups: those with urgent (unplanned) and those with elective (planned) start, depending on whether PD initiation took place during less than 14 days after peritoneal access creation or later.
In every late referral case the decision to initiate PD urgently was made individually in three steps. First, in patients with life-threatening metabolic disturbances (hyperkalemia, severe acidosis, severe volume overload, or marked uremia) the PD catheter implantation was preceded by 1–3 emergency HD procedures performed via temporary femoral vein access. Secondly, any medical or social contradictions to PD were carefully checked. In the last step, eligible patients were offered an informed choice of dialysis modality.
In those who opted for PD, a Tenckhoff catheter (straight or coiled) was inserted by open surgery under local or general anesthesia. PD was initiated as an overnight automatic procedure (APD) in supine position with 800–1000 mL dwell volume (for patients <, and ≥ 60 kg, respectively) and a dry day. The dwell volumes were gradually increased, and after 2–3 weeks patients remained on standard APD or were converted to continuous ambulatory PD (CAPD).
Follow up analyses of mechanical (leak, hernia, catheter migration, catheter obstruction, and bleeding) and infectious (peritonitis, exit site/tunnel infection) complications were performed after first 4 weeks, 90 days, and 12 months. Patients and technique survival rates were evaluated after first 90 days, 12 months, and at the end of the observation. The patients were observed for 3306 patient-moths (for entire group mean 25.6 ± 23.8, median 19 months); for planned-start group 28.6 ± 26.6, median 19.5 months, for urgent-start group 17.6 ± 0.9, median 19.0 months. Peritonitis and ESI/TI rates are expressed as episodes per year of treatment. Catheter migration as reflected by clinical suspicion was defined as lack/drop of ultrafiltration or problems with dialysate outflow. This suspicion was confirmed by X-ray of abdominal cavity; Catheter obstruction did not occur in our patients. Pericatheter leak (exit-site leak) was defined as fluid leak around the catheter exit-site. Abdominal leak on clinical suspicion seen as drop of ultrafiltration, asymmetric swelling of abdominal wall tissues and “orange peel” syndrome; was confirmed on radiological examination with contrast given to the catheter or during CT.
Statistical analysis was performed with STATISTICA software package (version12), StatSoft Poland. Continuous variables with normal distribution were presented as mean (standard deviation [SD]) and compared between two groups using Student’s t-test. Non-normal variables were expressed as median (interquartile range [IQR]) and compared with Mann–Whitney U-test. Categorical variables were presented as frequencies and percentages, and compared using Chi2 test. The survival rates were analyzed using the Kaplan–Meier technique and log-rank test. The multivariate Cox proportional hazards model was used in survival analysis to adjust outcomes for confounding variables such as: estimated glomerular filtration rate (eGFR), serum albumin and blood hemoglobin concentration, Charlson Comorbidity Index (CCI), time between catheter insertion and PD start, any complication appearance during first 4 weeks of the treatment and the urgent start per se. The statistical difference was considered to be significant for p < 0.05.
One hundred twenty nine incident patients (56% men), aged 50.8 ± 17.8 years entered the study. Among them, 35 (27%) started PD urgently and the remaining 94 (73%) in the planned manner. The main demographic and clinical data of the unplanned and planned beginners are presented in Table 1. There were no significant differences between study groups in terms of age, sex, and CCI. The patients who started PD urgently had worse kidney function, lower serum albumin, and blood hemoglobin concentrations at the beginning of the treatment.
The mean time between peritoneal access creation and PD start was 3.5 ± 2.3 and 16.2 ± 1.7 days, in urgent- and planned-start groups, respectively (p < 0.00001). Fifty-one percent of patients in the urgent-start group required PD during first 48 h after catheter insertion, and in 3 of them with life-threatening clinical symptoms – 2–3 short emergency HD procedures via femoral access were performed before PD catheter placement. In the remaining patients who started the treatment urgently, the time to PD commencement ranged between 3 and 8 days.
All mechanical complications are presented in Table 2. The early ones were observed more often in the urgent-start group (29% of patients vs. 4%, p = 0.00005), however, with no need for surgical intervention or temporary transition to HD. The most frequent early complication was dialysate leakage, which occurred in four patients – all of them started PD within 48 h after catheter implementation. In these patients, PD was postponed for 3–5 days and subsequently resumed with low-volume dwells.
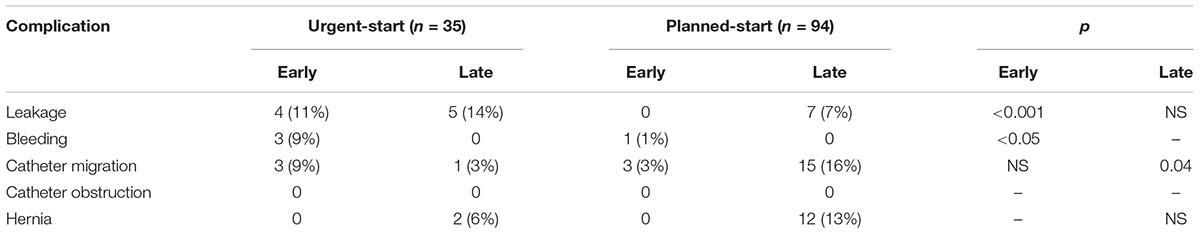
Table 2. Rate of early (first 4 weeks) and late (>4 weeks) mechanical complications in the studied groups.
The late mechanical complications occurred in 33.5% of all studied patients, 20% in urgent-start and 31% in the planned-start group (p = 0.15). The rate of late non-infectious complications was similar in both groups, with the exception of PD catheter migration, more frequently seen in the planned-start group.
In the regression logistic analysis, serum albumin as continuous variable (0.18; CI: 0.04–0.77; p = 0.02) and time between the catheter insertion and PD start expressed as categorical variables: ≤48 h (1,79; CI: 0,45–7,18; p = 0,02), and >14 days (0.08; CI: 0.01–0.5; p = 0.003) occurred the only significant predictors of early mechanical complications. In regard to long-term follow-up, neither the time between the catheter insertion and PD start, the urgent start per se nor any early complication influenced the occurrence of late mechanical events.
There were no infectious complications in any studied patient during the first 4 weeks of PD treatment. During the whole observation at least one episode of peritonitis was observed—in 12 patients (34%) in the urgent-start group and in 31 (33%) patients in the planned-start group. There were no differences between the groups regarding peritonitis and/or exit-site/tunnel infection rates during the first year of PD therapy as well as during the whole observation period (Tables 3, 4). In the regression logistic analysis none of analyzed parameters had an influence on peritonitis or exit-site/tunnel infection occurrence.
The patients were observed for 3306 patient-months, with mean follow-up of 17.6 ± 11.09 months (median: 19.0, range: 1.0–44 months) in the urgent-start group and 28.6 ± 26.6 months (median: 19.5, range: 1.0–103 months) in the planned-start group. The short- and long-term outcomes in studied patients are presented in Table 5. The analysis revealed that PD urgent-start was associated with reduced survival but only in the first 90 days of the therapy (86 vs. 99%; p < 0.0001), and at the end of observation the rates were similar. During the study period five patients died of peritonitis (1 from US group and 4 from PLS group), which creates quite a high mortality rate due to PD peritonitis in our population (5/56 episodes). A review of these cases revealed: all patients had high comorbidity: CCI 7–9; all episodes occurred > 12 months from PD start; in two cases polymicrobial peritonitis was secondary to bowel perforation; in one case due to Gram negative (E. coli); in one case due polymicrobial (E. coli, Enterococcus fecal, Enterobacter cloacae, Candida spp.) infection without proven bowel perforation; in one case it occurred in the course of sepsis secondary to the complications after surgical treatment of lower limb ischemia. Thus, peritonitis was one of the contributing cause of death in these seriously ill patients. The causes of death are shown in Table 6.
At the end of observation only 27 (21%) of all studied patients (17% from urgent- and 22% from planned-start group) stayed on PD. The most frequent reason for PD cessation was kidney transplantation – 34% of all patients (29 and 34%, respectively), death – 23% of all patients (37 and 17%, respectively), and transition to HD – 23% of all patients (17 and 25%, respectively). The causes of transition to HD are presented in Table 7.
Cox proportional analysis was performed for patient survival, method survival and both in 90 days, 1 year and all follow-up period. The results are presented in Table 8.
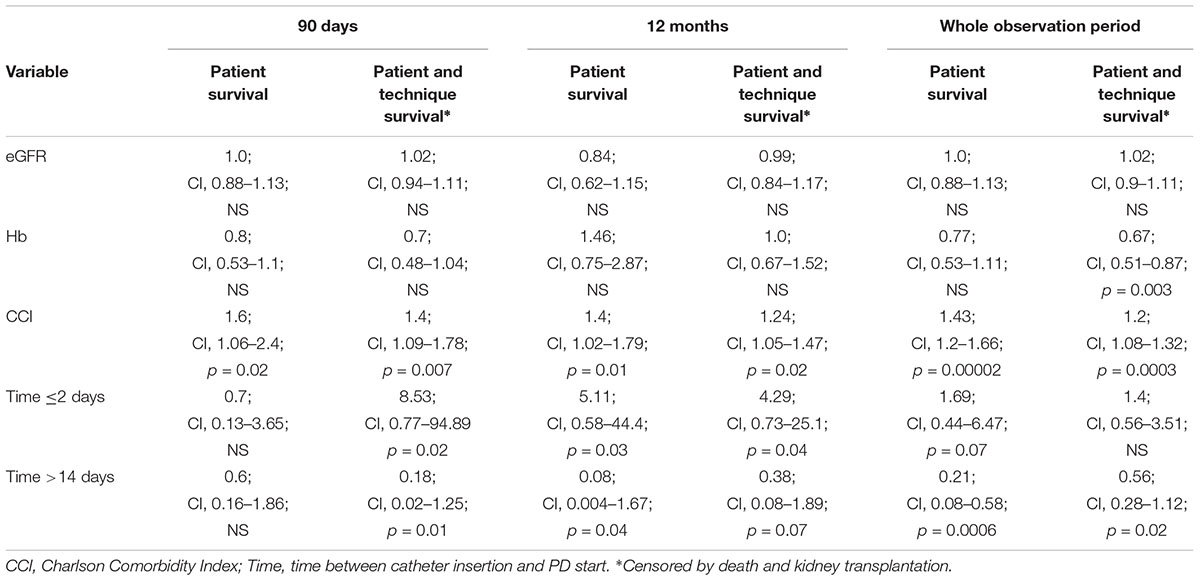
Table 8. Independent risk factors for patient and patient-method outcomes (Cox proportional analysis).
During the first 90 days of dialysis therapy the only important negative predictor for patient survival appeared to be CCI (HR 1.6 [CI: 1.06–2.4]; p = 0.02); however, later on, both CCI and time from catheter implantation to PD start were found to be significant. The same applies for both method and patient survival: CCI (HR 1.24 [CI: 1.05–1.47]; p = 0.02), time from catheter implantation (continuous variable HR 0.66 [CI: 0.48–0.91]; p = 0.01) and ≤ 2 days (HR 4.29 [CI: 0.73–25.1]; p = 0.04) were found to be predictive during the whole analyzed period, although after 1 year – time >14 days lost its predictive value (p = 0.07). There were no predictors for method survival alone in any analyzed period.
In our nephrology clinic, the PD urgent-start pathway, without typical 2-week break-in period, was opened 14 years ago; its outline is presented in Figure 1. It was meant for late-referral patients, able and prone to join PD-first program, with its main advantages such as: keeping the vascular system intact, preserving their residual renal function and retaining life-style flexibility. There was also our hope that early unplanned PD may be a good method to increase use this option of RRT. Finally, for patients who ultimately decided to change the treatment for HD, PD treatment continued until arteriovenous fistula maturation enabled to avoid temporary vascular access catheter placement. In this paper we compare the short- and long-term outcomes of consecutive 35 patients after urgent- and 94 patients after the planned start, who have commenced PD as a first RRT in our unit during this period.
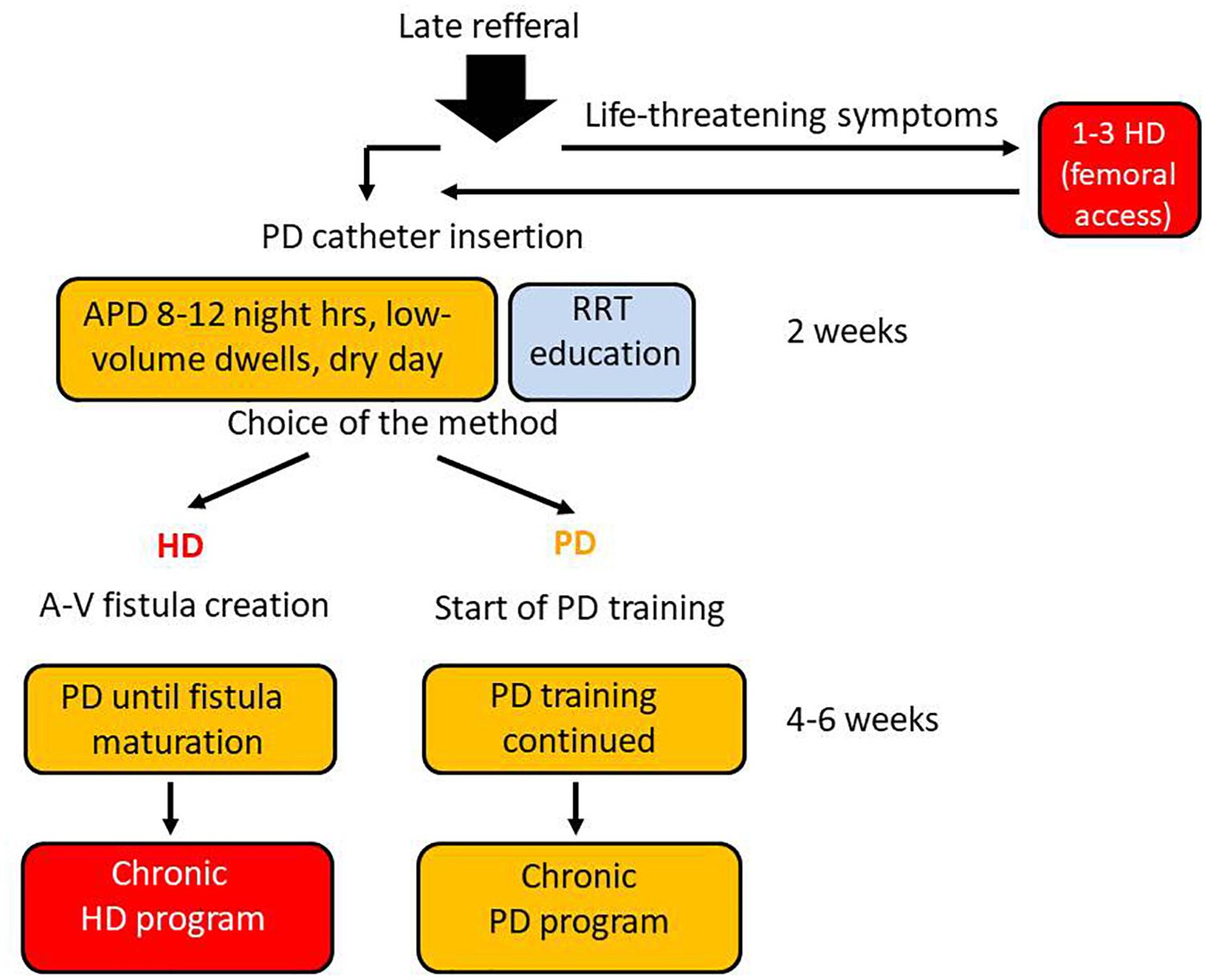
Figure 1. PD urgent–start program realized in our nephrology unit. Every late referral patient is quickly but carefully evaluated. Those who have no contradictions to PD and give an informed consent for PD as a bridge to final therapy choice are qualified to the program. The patients with life-threatening clinical symptoms like: pulmonary edema, severe hyperkalemia or acidosis, are given 1–3 short HD treatments via acute femoral catheter, before peritoneal access creation. The peritoneal catheter is introduced by a surgeon from our team, and the low-volume 8–12 night hours APD is started, with concomitant RRT education program for the patient and his family. If – after receiving sufficient information – the patients decide to remain on PD, the PD training is started, while in case when he/she considers HD as a more suitable RRT option – a arteriovenous fistula is created and PD continued until matured.
The results confirm that PD can be a safe method of introducing RRT in unplanned acute dialysis settings. In general they are comparable with those of other authors, presented in Table 9 (Song et al., 2000; Banli et al., 2005; Povlsen and Ivarsen, 2006; Jo et al., 2007; Lobbedez et al., 2008; Yang et al., 2011; Casaretto et al., 2012; Ghaffari, 2012; Koch et al., 2012; Masseur et al., 2014; Alkatheeri et al., 2016; Bitencourt Dias et al. 2016, 2017; Jin et al., 2016; Pai et al., 2016; Wong et al., 2016; Xu et al., 2017; Wang et al., 2017; Nayak et al., 2018). However, there are many methodological differences among these studies concerning patients population, the technique of peritoneal catheter placement, or length of break-in period. In most of them the observation period was rather short (mostly 3–6 months), and only some of them have the control groups. In the presented study the median of the observation time was 19 months.
The biggest concern about acute PD start is the risk of early mechanical complications: dialysate leaks as well as the catheter dysfunction. It may be that an early rise in peritoneal pressure negatively affects the wound healing and facilitates leakage. This can be further intensified by hypoalbuminemia, relatively often present in ESRD patients who need urgent dialysis start. In the presented study a short break-in period together with low serum albumin occurred to be the independent predictors for early dialysate leakage. The risk of this complication in patients who started PD urgently was 11% (vs. 0% in planned start group, p < 0.001), and its overall incidence was higher than described by some other authors (Povlsen and Ivarsen, 2006; Lobbedez et al., 2008; Sharma et al., 2008; Yang et al., 2011; Liu et al., 2014). There was also a higher incidence of bleeding into peritoneal cavity in urgent PD start group. However, after several days of peritoneal rest PD was resumed, and – unlike in some other studies – none of the patients needed a surgical intervention, and PD technique success at 3 months as well as during the whole observation was similar in both groups. The rates of late non-infectious complications were in both groups similar, with an exception of PD catheter migration, more frequently seen in the planned-start group. It did not affect the technique and patient survival either.
Contrary to the common perception that early use of peritoneal catheter may increase a risk of infectious complications such as exit-site/tunnel infections and peritonitis, we did not observe any of these in the early 4-week period of the treatment. The incidence rates during the whole observation period were in both studied groups similar. A shorter time to first peritonitis episode in urgent-start group shorter (p = 0.04) seems to be rather a consequence of a shorter observation period in this group. In the regression logistic analysis none of analyzed parameters had influence on peritonitis or exit-site/tunnel infection occurrence.
We found significantly higher mortality rate after first 90 days of the therapy in patients initiating PD urgently (14 vs. 1.1%, p < 0.0001). Although both groups were comparable in respect to age, many other factors may explain the reduced short-term survival in the unplanned starters (Lobbedez et al., 2008; Ivarsen and Povlsen, 2014). It is well-known that first 90 days of any dialysis therapy is a period of disproportionately high mortality and that in patients who start the treatment urgently and are often in a challenging clinical condition the outcomes are worst, with the percentages reaching even 30% in unplanned HD (where a part of which may be attributable to CVC (Khan et al., 1995; Collins et al., 2009; Mendelssohn et al., 2009; Baer et al., 2010; Panochia et al., 2016). Our urgent start patients were “sicker”: had more advanced uremia, lower albumin and hemoglobin levels and worse general clinical status, with necessity of dialysis within first 48 h in 51% of them, including 3 (8.6%) patients with life-threatening uremic symptoms in whom 2–3 short emergency HD via femoral access were performed before PD catheter placement.
The higher mortality rate in the urgent-start group persisted after 1 year (17 vs. 2%) and at the end of observation (37 vs. 17%), although the difference did not reach the statistical significance, possibly because small numbers of studied patients. The only significant predictor of patient death during first 90 days of dialysis therapy appeared to be CCI (HR 1.6 [CI: 1.06–2.4]; p = 0.02), however, later on both: CCI and time from catheter implantation to PD start were found to be significant.
The studied group consists of unselected incident patients started PD in our unit and seems to be fairly typical patients population treated with PD (age, comorbidity, ∼30% urgent start). The mortality rate in this group in the entire observation period (mean: 25.6 ± 23.8, median: 19 months) was 23%, and 10.6% in the first year (4.6%–90 days, 6%–90 days–12 months). It seems to be comparable with reported mortality rates in dialysis populations (USRDS, ERA-EDTA Registry), which despite some improvement in the last decade, remains very high (∼20%) with a universal phenomenon of increased mortality early after dialysis initiation (Robinson et al., 2014). The mortality rate was distinctly, however not statistically significant higher (effect of the sample size?) in urgent-starters in all time intervals, and this is good established observation that urgent dialysis start is an important factor of poor prognosis (short and long-term) irrespective of dialysis modality and generally results from various aspects related to uremic complications and comorbidities, as well as dialysis issues (i.e., CVC in HD patients). In our study, in Cox proportional analysis the time ≤ 2 days from catheter implantation to dialysis start (which may be a marker of necessity of dialysis initiation – more advanced uremia, worse general clinical status), and CCI proved to be an independent risk factors for patient survival in the whole observation.
With death and renal transplantation being the censored events, the PD technique survival was excellent in both, urgent and planned start groups, being respectively: 100 and 96% at 3 months, 97 and 87% at 12 months, and 83 and 76% at the end of the observation. These percentages are higher than reported by the other experienced urgent-start PD centers. In a French study by Lobbedez et al. (2008), which included 34 unplanned dialysis patients the actuarial PD technique survival was 90% at 6 months and 88% at 1 year. In the study done by Povlsen and Ivarsen, who compared outcomes of 52 urgent start with 52 planned-start patients, the 3-month PD technique censored survival rates were even lower, with corresponding values: 87 and 90% (Povlsen and Ivarsen, 2006).
Peritoneal dialysis may be a feasible and safe alternative to HD in patients who need to start dialysis urgently without established dialysis access, with an acceptable complications rates, as well as patient and technique survival. We found this method an important part of full RRT program in every reference dialysis unit, allowing for real free individual choice of the modality. However to be successful PD urgent start program needs adequate infrastructure, expertise, good organization with continuous availability of experienced surgeons or other doctors who place peritoneal catheters, dedication of the complete team, and a good RRT educational program, adjusted to the unplanned setting, to give the full possibility of unbiased final choice of the RRT method, that fits best to their lifestyle.
The study was exempted from the requirement of the Ethics Committee, as all the patients were in end-stage kidney disease and require renal replacement therapy in other planned or urgent manner. All of them singed the informed consent to start renal replacement therapy as required by the National Health found (routine informed consent for the procedure of increased risk).
EW, AG, and JM-R conceived the idea for the study and contributed to the design of the research. EW and JM-R performed the statistical analysis of the collected data and performed the analysis. EW, JM, and JM-R were involved in the preparation of the manuscript. EW, AG, and KG were involved in data collection. All the authors analyzed the data, edited, and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alkatheeri, A. M., Blake, P. G., Gray, D., and Jain, A. K. (2016). Success of urgent-start peritoneal dialysis in a large Canadian renal program. Perit. Dial. Int. 36, 171–176. doi: 10.3747/pdi.2014.00148
Arramreddy, R., Zheng, S., Saxena, A. B., Liebman, S. E., and Wong, L. (2014). Urgent-start peritoneal dialysis: a chance fo a new beginning. Am. J. Kidney Dis. 63, 390–395.
Baer, G., Lameire, N., and Van Biesen, W. (2010). Late referral of patients with end-stage renal disease: an in-depth review and suggestions for further actions. NDT Plus 3, 17–27. doi: 10.1093/ndtplus/sfp050
Banli, O., Altun, H., and Oztemel, A. (2005). Early start of CAPD with the Seldinger technique. Perit. Dial. Int. 25, 556–559.
Bitencourt, Dias D, Mendes, M. L., Burgugi, Banin V, Barretti, P., and Ponce, D. (2017). Urgent-start peritoneal dialysis: the first year of brazilian experience. Blood Purif. 44, 283–287. doi: 10.1159/000478970
Blake, P. G., Quinn, R. R., and Oliver, M. J. (2013). Peritoneal dialysis and the process of modality selection. Perit. Dial. Int. 33, 233–241. doi: 10.3747/pdi.2012.00119
Casaretto, A., Rosario, R., Kotzker, W. R., Pagan-Rosario, Y., Groenhoff, C., and Guest, S. (2012). Urgent-start peritoneal dialysis: report from a U.S. private nephrology practice. Adv. Perit. Dial. 28, 102–105.
Collins, A. J., Foley, R. N., Gilbertson, D. T., and Chen, S. C. (2009). The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin. J. Am. Soc. Nephrol. 4, S5–S11. doi: 10.2215/CJN.05980809
Dias, D. B., Banin, V., Mendes, M. L., Barretti, P., and Ponce, D. (2016). Peritoneal dialysis can be an option for unplanned chronic dialysis: initial results from a developing country. Int. Urol. Nephrol. 48, 901–906. doi: 10.1007/s11255-016-1243-x
Dombros, N., Dratwa, M., Feriani, M., Gokal, R., Heimburger, O., Krediet, R., et al. (2005). European best practice guidelines for peritoneal dialysis. 3 peritoneal access. Nephrol. Dial. Transplant. 20(Suppl. 9), ix8–ix12.
Ghaffari, A. (2012). Urgent-start peritoneal dialysis: a quality improvement report. Am. J. Kidney Dis. 59, 400–408. doi: 10.1053/j.ajkd.2011.08.034
Ivarsen, P., and Povlsen, J. V. (2014). Can peritoneal dialysis be applied for unplanned initiation of chronic dialysis? Nephrol. Dial. Transplant. 29, 2201–2206. doi: 10.1093/ndt/gft487
Jin, H., Fang, W., Zhu, M., Yu, Z., Fang, Y., Yan, H., et al. (2016). Urgent-start peritoneal dialysis and hemodialysis in esrd patients: complications and outcomes. PLoS One 11:e0166181. doi: 10.1371/journal.pone.0166181
Jo, Y. I., Shin, S. K., Lee, J. H., Song, J. O., and Park, J. H. (2007). Immediate initiation of CAPD following percutaneous catheter placement without break-in procedure. Perit. Dial. Int. 27, 179–183.
Khan, I. H., Catto, G. R., Edward, N., and MacLeod, A. M. (1995). Death during the first 90 days of dialysis: a case control study. Am. J. Kidney Dis. 25, 276–280.
Koch, M., Kohnle, M., Trapp, R., Haastert, B., Rump, L. C., and Aker, S. (2012). Comparable outcome of acute unplanned peritoneal dialysis and haemodialysis. Nephrol. Dial. Transplant. 27, 375–380. doi: 10.1093/ndt/gfr262
Li, P. K., Chow, K. M., Van de Luijtgaarden, M. W., Johnson, D. W., Jager, K. J., Mehrotra, R., et al. (2017). Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 13, 90–103. doi: 10.1038/nrneph.2016.181
Li, P. K., and Kwong, V. W. K. (2017). Current challanges and opportunities in PD. Semin. Nephrol. 37, 2–9. doi: 10.1016/j.semnephrol.2016.10.002
Li, P. K. T., Szeto, C. C., Piraino, B., de Artega, J., Fan, S., Figueiredo, A. E., et al. (2016). ISPD peritonitis recommendations:update on prevention and treatment. Perit. Dial. Int. 36, 481–508.
Liu, F. X., Ghaffari, A., Dhatt, H., Kumar, V., Balsera, C., Wallace, E., et al. (2014). Economic evaluation of urgent-start peritoneal dialysis versus urgent-start hemodialysis in the United States. Medicine 93, 1–7. doi: 10.1097/MD.0000000000000293
Liyanage, T., Ninomiya, T., Jha, V., Neal, B., Patrice, H. M., Okpechi, I., et al. (2015). Worldwide access to tretment for end-stage kidney disease: a systematic review. Lancet 385, 1975–1982. doi: 10.1016/S0140-6736(14)61601-9
Lobbedez, T., Lecouf, A., Ficheux, M., Henri, P., Hurault de Ligny, B., and Ryckelynck, J. P. (2008). Is rapid initiation of peritoneal dialysis feasible in unplanned dialysis patients? A single-centre experience. Nephrol. Dial. Transplant. 23, 3290–3294. doi: 10.1093/ndt/gfn213
Mahnensmith, R. L. (2014). Urgent-start peritoneal dialysis; what are the problems and their solutions? Semin. Dial. 27, 291–294.
Masseur, A., Guest, S., and Kumar, V. (2014). Early technique success after initiation of treatment with urgent-start peritoneal dialysis. Adv. Perit. Dial. 30, 36–39.
Mehrotra, R., Devuyst, O., Davies, S. J., and Johnson, D. W. (2016). The current state of peritoneal dialysis. J. Am. Soc. Nephrol. 27, 3238–3252.
Mendelssohn, D. C., Malmberg, C., and Hamandi, B. (2009). An integrated review of ‘unplanned’ dialysis initiation: reframing the terminology to ‘suboptimal’ initiation. BMC Nephrol. 10:22. doi: 10.1186/1471-2369-10-22
Nayak, K. S., Subhramanyam, S. V., Pavankumar, N., Antony, S., and Sarfaraz, Khan MA (2018). Emergent start peritoneal dialysis for end-stage renal disease: outcomes and advantages. Blood Purif. 45, 313–319. doi: 10.1159/000486543
Oliver, M. J., Verrelli, M., Zacharias, J. M., Blake, P. G., Garg, A. X., Johnson, J. F., et al. (2012). Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol. Dial. Transplant. 27, 810–816. doi: 10.1093/ndt/gfr289
Pai, M. F., Yang, J. Y., Chen, H. Y., Hsu, S. P., Chiu, Y. L., Wu, H. Y., et al. (2016). Comparing long-term outcomes between early and delayed initiation of peritoneal dialysis following catheter implantation. Ren. Fail. 38, 875–881. doi: 10.3109/0886022X.2016.1165069
Panochia, N., Tazza, L., Stazzio, E. D., Liberatori, M., Vulpio, C., Giungi, S., et al. (2016). Mortality in hospitalized chronic kidney disease patients starting unplanned urgent haemodialysis. Nephrology 21, 62–67. doi: 10.1111/nep.12561
Perl, J., Wald, R., McFarlane, P., Bargman, J. M., Vonesh, E., Na, Y., et al. (2011). Hemodialysis vascular access modifies the association between dialysis modality and survival. J. Am. Soc. Nephrol. 22, 1113–1121. doi: 10.1681/ASN.2010111155
Povlsen, J. V., and Ivarsen, P. (2006). How to start the late referred ESRD patient urgently on chronic APD. Nephrol. Dial. Transplant. 21, ii56–ii59.
Robinson, B. M., Zhang, J., Morgenstern, H., Bradbury, B. D., Ng, L. J., McCullough, K. P., et al. (2014). Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 85, 158–165. doi: 10.1038/ki.2013.252
Sharma, A. P., Mandhani, A., Daniel, S. P., and Filler, G. (2008). Shorter break-in period is a viable option with tighter PD catheter securing during the insertion. Nephrology 13, 672–676. doi: 10.1111/j.1440-1797.2008.01000.x
Song, J. H., Kim, G. A., Lee, S. W., and Kim, M. J. (2000). Clinical outcomes of immediate full-volume exchange one year after peritoneal catheter implantation for CAPD. Perit. Dial. Int. 20, 194–199.
Wang, C., Fu, X., Yang, Y., Deng, J., Zhang, H. Q., Deng, H. M., et al. (2017). A comparison between intermittent peritoneal dialysis and automatic peritoneal dialysis on urgent peritoneal dialysis. Am. J. Nephrol. 45, 540–548. doi: 10.1159/000477178
Wong, L. P., Li, N.-C., Kansal, S., Lacson, E. Jr., Maddux, F., Kessler, J., et al. (2016). Urgent peritoneal dialysis starts for ESRD: initial multicenter experiences in the United States. Am. J. Kidney Dis. 68, 499–502.
Xu, D., Liu, T., and Dong, J. (2017). Urgent-start peritoneal dialysis complications: prevalence and risk factors. Am. J. Kidney Dis. 70, 102–110.
Keywords: peritoneal dialysis, short-term outcomes, mechanical complication, technique survival, patient survival, long-term outcomes, infectious complications
Citation: Wojtaszek E, Grzejszczak A, Grygiel K, Małyszko J and Matuszkiewicz-Rowińska J (2019) Urgent-Start Peritoneal Dialysis as a Bridge to Definitive Chronic Renal Replacement Therapy: Short- and Long-Term Outcomes. Front. Physiol. 9:1830. doi: 10.3389/fphys.2018.01830
Received: 24 September 2018; Accepted: 06 December 2018;
Published: 04 January 2019.
Edited by:
Janusz Witowski, Poznan University of Medical Sciences, PolandReviewed by:
Robert Ekart, University Clinical Centre Maribor, SloveniaCopyright © 2019 Wojtaszek, Grzejszczak, Grygiel, Małyszko and Matuszkiewicz-Rowińska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ewa Wojtaszek, ZXdvanRhc3pla0B3dW0uZWR1LnBs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.