
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 26 November 2018
Sec. Invertebrate Physiology
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.01678
This article is part of the Research Topic Insects at the Center of Interactions with Other Organisms View all 26 articles
 Rosanna Salvia1†
Rosanna Salvia1† Marisa Nardiello1†
Marisa Nardiello1† Carmen Scieuzo1†
Carmen Scieuzo1† Andrea Scala1†
Andrea Scala1† Sabino A. Bufo1
Sabino A. Bufo1 Asha Rao2
Asha Rao2 Heiko Vogel3
Heiko Vogel3 Patrizia Falabella2*
Patrizia Falabella2*Polydnaviruses (PDVs) are obligate symbionts of endoparasitoid wasps, which exclusively attack the larval stages of their lepidopteran hosts. The Polydnavirus is injected by the parasitoid female during oviposition to selectively infect host tissues by the expression of viral genes without undergoing replication. Toxoneuron nigriceps bracovirus (TnBV) is associated with Toxoneuron nigriceps (Hymenoptera: Braconidae) wasp, an endoparasitoid of the tobacco budworm larval stages, Heliothis virescens (Lepidoptera: Noctuidae). Previous studies showed that TnBV is responsible for alterations in host physiology. The arrest of ecdysteroidogenesis is the main alteration which occurs in last (fifth) instar larvae and, as a consequence, prevents pupation. TnBV induces the functional inactivation of H. virescens prothoracic glands (PGs), resulting in decreased protein synthesis and phosphorylation. Previous work showed the involvement of the PI3K/Akt/TOR pathway in H. virescens PG ecdysteroidogenesis. Here, we demonstrate that this cellular signaling is one of the targets of TnBV infection. Western blot analysis and enzyme immunoassay (EIA) showed that parasitism inhibits ecdysteroidogenesis and the phosphorylation of the two targets of TOR (4E-BP and S6K), despite the stimulation of PTTH contained in the brain extract. Using a transcriptomic approach, we identified viral genes selectively expressed in last instar H. virescens PGs, 48 h after parasitization, and evaluated expression levels of PI3K/Akt/TOR pathway genes in these tissues. The relative expression of selected genes belonging to the TOR pathway (tor, 4e-bp, and s6k) in PGs of parasitized larvae was further confirmed by qRT-PCR. The down-regulation of these genes in PGs of parasitized larvae supports the hypothesis of TnBV involvement in blocking ecdysteroidogenesis, through alterations of the PI3K/Akt/TOR pathway at the transcriptional level.
Toxoneuron nigriceps (Viereck) (Hymenoptera: Braconidae) is a solitary braconid endoparasitoid wasp of the tobacco budworm Heliothis virescens (Fabricius) (Lepidoptera: Noctuidae) larval stages. During oviposition, T. nigriceps female injects several maternal factors into the host (Lewis and Vinson, 1968; Pennacchio et al., 1993), which are able to modulate the host immune system and endocrine balance, in order to create a suitable environment for parasitoid progeny development (Beckage and Gelman, 2004; Pennacchio and Strand, 2006; Falabella et al., 2007; Moreau and Asgari, 2015). Maternal factors include protein rich venom (Laurino et al., 2016) and the ovarian calyx fluid, composed of ovarian proteins and an obligate symbiotic virus belonging to the Polydnaviridae family, the Toxoneuron nigriceps bracovirus (TnBV) (Lewis and Vinson, 1968). The genome of Polydnaviruses (PDVs) consists of multiple double-stranded segmented circular DNA, integrated as provirus into the wasp genome, specifically associated in a mutualistic symbiosis (Webb et al., 2000; Webb and Strand, 2005). PDVs integrated into the parasitoid genome replicate exclusively in wasp ovaries, where their circular genome is generated from linear DNA copies of insect chromosomes (Varricchio et al., 1999; Volkoff et al., 2010; Dupuy et al., 2011). PDVs express their genes, without any replication event, in several host tissues, causing alterations in their physiology and enabling parasitoid larvae to survive, grow and finally pupate in silken cocoons (Strand and Burke, 2014). Thus, successful development of parasitoid wasp ensures vertical transmission of the integrated viral genome to the next generation (Strand, 2010).
In H. virescens larvae parasitized by T. nigriceps, the main infection target tissues are haemocytes and prothoracic glands (PGs) (Stoltz and Vinson, 1979; Wyder et al., 2003), where TnBV expresses several genes belonging to different families encoding protein tyrosine phosphatases (PTPs), the largest gene family in bracovirus (Provost et al., 2004; Falabella et al., 2006, 2007; Webb et al., 2006), ankyrin motif protein (ANK) (Falabella et al., 2007; Salvia et al., 2017), UDP dehydrogenase, proteins containing Ben domains (Park and Kim, 2010; Falabella et al., unpublished data). Other viral genes expressed in the host are TnBV1, implicated in immune suppression (Varricchio et al., 1999; Malva et al., 2004) and activating caspase proteins (Lapointe et al., 2005) and TnBV2 encoding a retroviral aspartyl protease (Falabella et al., 2003). Previous studies reported that H. virescens PGs explanted from parasitized last (fifth) instar larvae do not respond to the stimulus of the prothoracicotropic hormone (PTTH), the neuropeptide that triggers ecdysone production (Pennacchio et al., 1997, 1998a). Besides the inhibition of ecdysteroidogenesis, a decrease of protein synthesis and a complete inhibition of PTTH-induced phosphorylation of some unidentified proteins in PGs of parasitized larvae were observed (Pennacchio et al., 1997, 1998a). Moreover, it was demonstrated that TnBV is responsible for the functional inactivation of PGs and thus for blocking host pupation (Pennacchio et al., 1998a) due to the reduction of ecdysone biosynthesis in parasitized last instar larvae. Recent work reported that the TOR pathway is involved in ecdysteroidogenesis in some lepidopteran species (Gu et al., 2012; Kemirembe et al., 2012), including H. virescens (Scieuzo et al., 2018).
In this work, we show that specific TnBV genes are expressed in PGs of H. virescens last instar larvae and we argue that they could be involved in blocking ecdysteroidogenesis, at least in part, through negative regulation of the PI3K/Akt/TOR pathway at the transcriptional level.
Toxoneuron nigriceps wasps were reared on larval stages of its host, Heliothis virescens, according to Vinson et al. (1973). Heliothis virescens larvae were maintained on a standard artificial diet developed by Vanderzant et al. (1962). Rearing temperature was maintained at 29 ± 1°C for the host, parasitized host larvae and cocoons. Toxoneuron nigriceps adults were kept at 25 ± 1°C and fed with water and honey. In both cases, a 16:8 light/dark photoperiod and a relative humidity of 70 ± 5% were adopted. Late 2 or early 3 days old last (fifth) instar larvae of H. virescens were individually parasitized by T. nigriceps and maintained as described by Pennacchio et al. (1994). H. virescens last instar larvae were staged according to Webb and Dahlman (1985) and synchronized as reported by Pennacchio et al. (1992).
The prothoracic glands (PGs) from parasitized and non-parasitized H. virescens 3 days old last instar larvae were dissected in 1X phosphate-buffered saline (PBS) as previously reported (Pennacchio et al., 1998a).
Glands were incubated at 25°C in 100 μl of Grace’s insect medium (Sigma-Aldrich, St. Louis, MO, United States) for 30 min (time of rest) in order to reduce the possibility of their activation by experimental manipulation, as reported for Manduca sexta PGs (Bollenbacher et al., 1983; Smith et al., 1986).
The brain extract containing PTTH (hereafter referred to as PTTH) was prepared by homogenizing brains dissected from an equal number of H. virescens 3 and early 4 days old last instar larvae and stored in ice-cold Grace’s insect medium (Sigma-Aldrich, St. Louis, MO, United States). The homogenate was placed in boiling water for 2 min, cooled to 4°C on ice and centrifuged at 15,000 g at 4°C for 5 min (Pennacchio et al., 1997). Before being used, PTTH extract was filtered with a 0.20 μm Sterile Syringe Filter (Corning Incorporated, Corning, NY, United States) and then diluted in Grace’s insect medium to 0.1 brain equivalent/μl (BE/μl) and either used immediately for the experiments described below or stored at -80°C.
The effect of parasitism on the phosphorylation of the TOR targets 4E-BP and S6K was studied in PGs explanted from parasitized and non-parasitized last instar larvae, under six different experimental conditions: basal PGs incubated in Grace’s insect medium (Sigma-Aldrich, St. Louis, MO, United States) from non-parasitized larvae; basal PGs incubated in Grace’s insect medium from parasitized larvae; stimulated PGs with 0.1 BE/μl PTTH from non-parasitized larvae; stimulated PGs with 0.1 BE/μl PTTH from parasitized larvae; PGs from non-parasitized larvae incubated with 1 μM rapamycin (Calbiochem, catalog number 553210, San Diego, CA, United States), a specific inhibitor of TOR; PGs from non-parasitized larvae incubated with 1 μM rapamycin and stimulated with 0.1 BE/μl PTTH.
After dissection and initial incubation (as described above), PGs were incubated for 3 h at 25°C, for all the experimental conditions, except for incubation with rapamycin, in which PGs were pre-incubated with rapamycin alone for 30 min, then transferred to fresh medium containing the same dose of the inhibitor with or without PTTH 0.1 BE/μl.
After the incubation, a pool of 20 PGs for each of these conditions was lysed directly in 2X Laemmli buffer (Laemmli, 1970), to block protease and phosphatase activity. The extracted proteins were separated by 12% polyacrylamide gel electrophoresis and then transferred on a Whatman nitrocellulose membrane (Protran, Dassel, Germany).
Specific antibodies were used to evaluate phosphorylation of the two TOR targets: anti-phospho-4E-BP (Cell Signaling catalog number 2855S, Danvers, MA, United States) and anti-phospho-S6K (Millipore catalog number 04-393, Temecula, CA, United States) (Gu et al., 2012; Scieuzo et al., 2018). Signals obtained with the anti-actin antibody (Abcam, catalog number 75186, Cambridge, United Kingdom) were used as loading controls as reported for other Lepidoptera (Smith et al., 2014). All antibodies were diluted 1:1000 in tris-buffered saline and 0.1% Tween 20 (TBS-T) with 5% bovine serum albumin (BSA), and the incubation was carried out for 16 h. Membranes were sequentially incubated with each of the three antibodies. Goat anti-rabbit conjugated to horseradish peroxidase (Invitrogen, Carlsbad, CA, United States), diluted 1:15,000 in TBS-T, was used as secondary antibody. Detection was carried out using enhanced chemiluminescence (ECL) (LiteAB Blot Kit – Euroclone, Pavia, Italy) and signals were measured by a ChemidocTM MP System (Bio-Rad, Milan, Italy).
Following the dissection of PGs, Grace’s insect medium was replaced with a fresh medium containing stimulators or inhibitors, as described in section Analysis of Protein Phosphorylation. Ecdysone released in the medium was determined by a competitive enzyme immunoassay (EIA), using anti-ecdysone as primary antibody and 20-hydroxyecdysone-peroxidase conjugated as tracer, as previously described (Kingan, 1989; Scieuzo et al., 2018). All experiments were performed on a single PG, in three technical replicates for each of the six biological replicates.
In order to identify genes differentially expressed in H. virescens PGs from parasitized and non-parasitized last instar larvae, a transcriptome analysis was conducted. Total RNA from 300 PGs explanted from 3 days old last instar parasitized (48 h post-parasitism) and synchronized non-parasitized larvae, was extracted using TRI-Reagent (Sigma-Aldrich, St. Louis, MO, United States), according to the manufacturer’s protocol. An additional DNase (Turbo DNase, Ambion Inc., Austin, TX, United States) treatment was carried out before the second purification step to remove any remaining DNA. The DNase enzyme was removed, and the RNA was further purified by using the RNeasy MinElute Clean up Kit (Qiagen, Venio, Netherlands), following the manufacturer’s protocol, and eluted in 20 μl of RNA Storage Solution (Ambion Inc., Austin, TX, United States). RNA integrity was verified on an Agilent 2100 Bioanalyzer using the RNA Nano chips (Agilent Technologies, Palo Alto, CA, United States) and RNA quantity was determined by Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, United States). Poly(A)+ RNA was isolated from 5 μg total RNA for PGs from parasitized and non-parasitized larvae using the Ambion MicroPoly(A) Purist Kit according to the manufacturer’s instructions (Life Technologies, Carlsbad, CA, United States).
Sequencing was carried out by the Max Planck Genome Center1 using standard TruSeq procedures on an Illumina HiSeq2500 sequencer, generating approximately 40 mio paired-end (2 × 100 bp) reads for each of the tissue samples. Quality control measures, including the filtering of high-quality reads based on the score given in FASTQ files, removal of reads containing primer/adaptor sequences and trimming of read lengths, were carried out using CLC Genomics Workbench v9.12. The de novo transcriptome assembly was carried out with the same software, selecting the presumed optimal consensus transcriptome as previously described (Vogel et al., 2014). All obtained sequences (contigs) were used as query for a BLASTX searches (Altschul et al., 1997) against the non-redundant database of the National Center for Biotechnology Information (NCBI), considering all hits with an e-value < 1E-3. The transcriptome was annotated using BLAST, Gene Ontology (GO) and InterPro terms (InterProScan, EBI), enzyme classification (EC) codes, and metabolic pathways (Kyoto Encyclopedia of Genes and Genomes, KEGG) as implemented in BLAST2GO v4. 13. Based on the BLAST hits, the contigs were assigned to either insect or virus (i.e., TnBV) origin. To optimize the annotation of data, we used GO slim, which uses a subset of the complete GO terms that provides a broader overview of the transcriptome ontology content.
Digital gene expression analysis was carried out by using CLC Genomics workbench v9.1 to generate BAM (mapping) files and QSeq Software (DNAStar Inc.) to remap the Illumina reads onto the reference transcriptome and then counting the sequences to estimate expression levels, using previously described parameters for read mapping and normalization (Vogel et al., 2014; Jacobs et al., 2016). In particular, the expression abundance of each conting was calculated based on the reads per kilobase per million mapped reads (RPKM) method (Mortazavi et al., 2008), using the formula:
RPKM (A) = (10,00,000 × C × 1,000)/(N × L), where RPKM (A) is the abundance of gene A, C is the number of reads that uniquely aligned to gene A, N is the total number of reads that uniquely aligned to all genes, and L is the number of bases in gene A. The RPKM method is able to eliminate the influence of different gene lengths and sequencing discrepancy in the calculation of expression abundance.
To evaluate the relative expression of tor, 4ebp, and s6k genes in parasitized and non-parasitized H. virescens PGs, quantitative RT-PCR (qRT-PCR) experiments were carried out on an ABI PRISM® 7500 Fast Real-Time PCR System Thermal Cycler (Applied Biosystems, Foster City, CA, United States), with cDNA samples prepared from 3 days old last instar parasitized and non-parasitized PGs, following the guidelines reported in Minimum Information Required for Publication of Quantitative Real-Time PCR experiments (MIQE) (Bustin et al., 2009) and minimum information necessary for quantitative real-time PCR experiments (Johnson et al., 2014). Based on their relative expression levels obtained from our RNAseq data showing that five candidate control genes were not affected by parasitization (Supplementary Figure S1), Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), elongation factor 1-alpha (ef1a) and ribosomal protein L13 (rp13) were chosen as reference genes for normalization of qRT-PCR data. Specific primers for each H. virescens gene (tor, s6k, 4ebp) and reference genes were designed using Primer 3.04 (Supplementary Table S1).
Genes of interest and reference gene sequences were obtained from the PG de novo transcriptome assembly (Scieuzo et al., 2018).
PCR amplifications were performed using GoTaq qPCR Master Mix (Promega, Madison, WI, United States). The reactions were carried out in a 20 μl volume containing 20 ng cDNA and 0.3 μmol/L final primer concentration. Cycling conditions for all genes were: 2 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 58°C. At the end of each run, a melting curve analysis was performed to confirm the specificity of PCR products. All amplification reactions were run in triplicate (technical replicates) and included negative controls (non-template reactions, replacing cDNA with ultrafiltered sterile water). All qRT-PCR analyses were performed for a set of three biological replicates. In order to evaluate gene expression levels, relative quantification was performed using equations described by Liu and Saint (2002), based on PCR amplification efficiencies of reference and target genes. Amplification efficiency of each target gene and endogenous genes was determined according to the equation E = 10-1/S - 1 (Lee et al., 2006), where S is the slope of the standard curve generated from three serial 10-fold dilutions of cDNA. Quantification analysis of amplification was performed using the comparative CT (ΔCT) method. The efficiencies of the amplicons were approximately equal (gapdh parasitized = 0.98; gapdh non-parasitized = 0.96; ef parasitized = 0.82; ef non-parasitized = 0.86; rpl13 parasitized = 0.91; rpl13 non-parasitized = 0.93; tor parasitized = 0.89; tor non-parasitized = 0.88; 4ebp parasitized = 0.97; 4ebp non-parasitized = 0.94; s6k parasitized = 0.88; s6k non-parasitized = 0.87).
qRT-PCR data were expressed as mean ± SEM (standard error of mean) of independent biological replicates and were compared by a one-way analysis of variance (ANOVA) and Bonferroni post hoc test using GraphPad Prism 6.00 software for Windows (GraphPad Software, La Jolla, CA, United States5). Enzyme immunoassay data were expressed as mean ± SEM (standard error of mean) of independent biological replicates and were compared by a two-way analysis of variance (ANOVA) with Treatment (3 levels: Parasitized, non-parasitized Control and non-parasitized Rapamycin-treated) as the first factor and PTTH-stimulated (2 levels: yes, no) as the second factor. Post hoc Means Comparison test was done with both Tukey and SNK tests. In order to correct heteroscedasticity and non-normality, checked with Levene and Shapiro–Wilk tests, data were square root transformed before the analysis. All the statistical analysis was done using Systat 13 (Systat Software, Inc., San Jose, CA, United States6).
In order to verify the effect of parasitism on the TOR pathway and a potential impact on Heliothis virescens ecdysteroidogenesis, western blot analyses were performed on protein extracts from PGs, previously incubated under different conditions (basal, PTTH, rapamycin, PTTH added with rapamycin) explanted from non-parasitized and parasitized last instar (fifth) larvae. The phosphorylation of the main targets of TOR kinase was detected using antibodies against phospho-4E-BP and phospho-S6K, respectively.
The in vitro exposure of PGs explanted from 3 days old last instar non-parasitized larvae to PTTH contained in brain extract enhanced the phosphorylation level of both 4E-BP and S6K proteins. No phosphorylation signals were detected in PGs explanted from non-parasitized larvae, treated with rapamycin (with or without PTTH stimulation) and from parasitized larvae (with or without PTTH stimulation) (Figure 1).
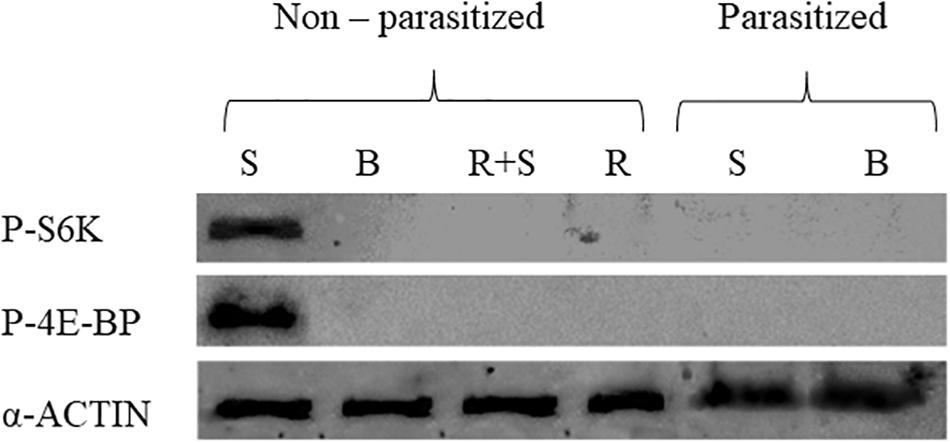
FIGURE 1. Phosphorylation of TOR target proteins, 4E-BP and S6K, in prothoracic glands, explanted from parasitized and non-parasitized larvae, under different experimental conditions. Basal prothoracic glands (B) or stimulated prothoracic glands with PTTH extract (S), rapamycin (R), explanted from parasitized or non-parasitized larvae. Prothoracic glands (PGs) from 3 days old last instar parasitized and non-parasitized larvae were incubated with 0.1 BE/μl PTTH (stimulated, S). Non-parasitized PGs were also pre-incubated with 1 μM rapamycin for 30 min, then transferred to fresh medium containing the same dose of the inhibitor with (stimulated, R+S) or without (R) 0.1 BE/μl PTTH. Parasitized and non-parasitized (basal, B) glands were incubated in Grace’s insect medium. Incubation was maintained for 3 h in each condition at 25°C. Glands lysed in Laemmli buffer 2X, were analyzed by western blot using antibodies against phospho-4E-BP (20 kDa), phospho-S6K (70 kDa). Each lane represents the equivalent of 20 prothoracic glands. The quantity of loaded proteins was verified by the endogenous control, α-Actin (42 kDa).
The in vitro biosynthetic activity of PGs explanted from H. virescens 3 days old last instar parasitized and non-parasitized larvae, in response to activators or inhibitors, is reported in Figure 2 and Supplementary Table S2.
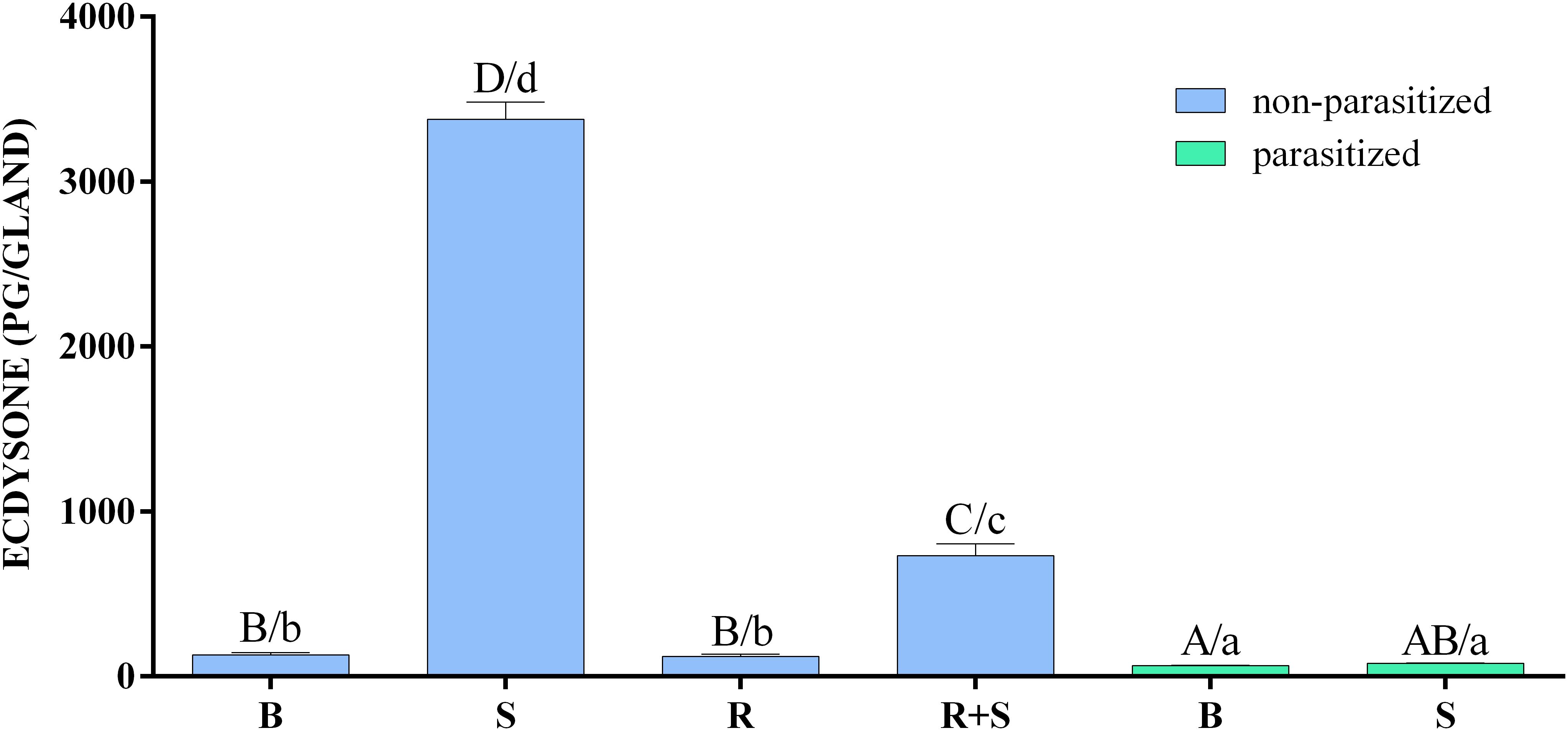
FIGURE 2. Inhibitory effect of parasitism and rapamycin on the synthesis of ecdysone by prothoracic glands of H. virescens. Prothoracic glands (PGs) explanted from parasitized and non-parasitized 3 days old last instar larvae were incubated in Grace’s insect medium with (stimulated, S) or without (basal, B) 0.1 BE/μl PTTH. Non-parasitized PGs were also pre-incubated with 1 μM rapamycin for 30 min, then transferred to fresh medium containing the same dose of the inhibitor with (rapamycin stimulated, R+S) or without (rapamycin basal, B) 0.1 BE/μl PTTH. Parasitized and non-parasitized (basal) glands were incubated in Grace’s insect medium. Incubation was maintained for 3 h in each condition at 25°C, after which the ecdysone produced was determined by enzyme immunoassay (EIA). Data are expressed as mean ± SEM of n = 6 experiments. Statistical analysis was performed by a two-way analysis of variance (ANOVA). Post hoc Means Comparison test was done with both Tukey and SNK tests. Different letters indicate significant differences (p < 0.05). Uppercase letters refer to the Tukey post hoc test and lowercase letters to the SNK test.
Ecdysone production was strongly enhanced by PTTH stimulation of non-parasitized PGs in comparison to all other experimental conditions. In the presence of rapamycin, the ecdysone production following PTTH-stimulation, although significantly lower compared to stimulation conditions, was significantly higher than basal or rapamycin-treated non-parasitized PGs, and basal or PTTH-stimulated parasitized PGs. In both PTTH-stimulated and non-stimulated parasitized PGs the titre of ecdysone was lower to that released by basal non-parasitized PGs (Supplementary Table S3).
For functional annotations, all sequences were subjected to Gene Ontology (GO) analyses in Blast2GO revealing that of the total number of contigs, 61% (40,290) shared significant similarity to proteins with assigned molecular functions in the GO database (Altschul et al., 1997). The analyses of the combined transcriptome allowed the identification of 18 contigs of viral origin selectively transcribed and putatively expressed in parasitized PGs. Among these, 6 contigs could be assigned to general bracovirus genes, while 12 could be assigned specifically to TnBV, as visualized in the heat map with the normalized mapped read (RPKM) counts (Figure 3).
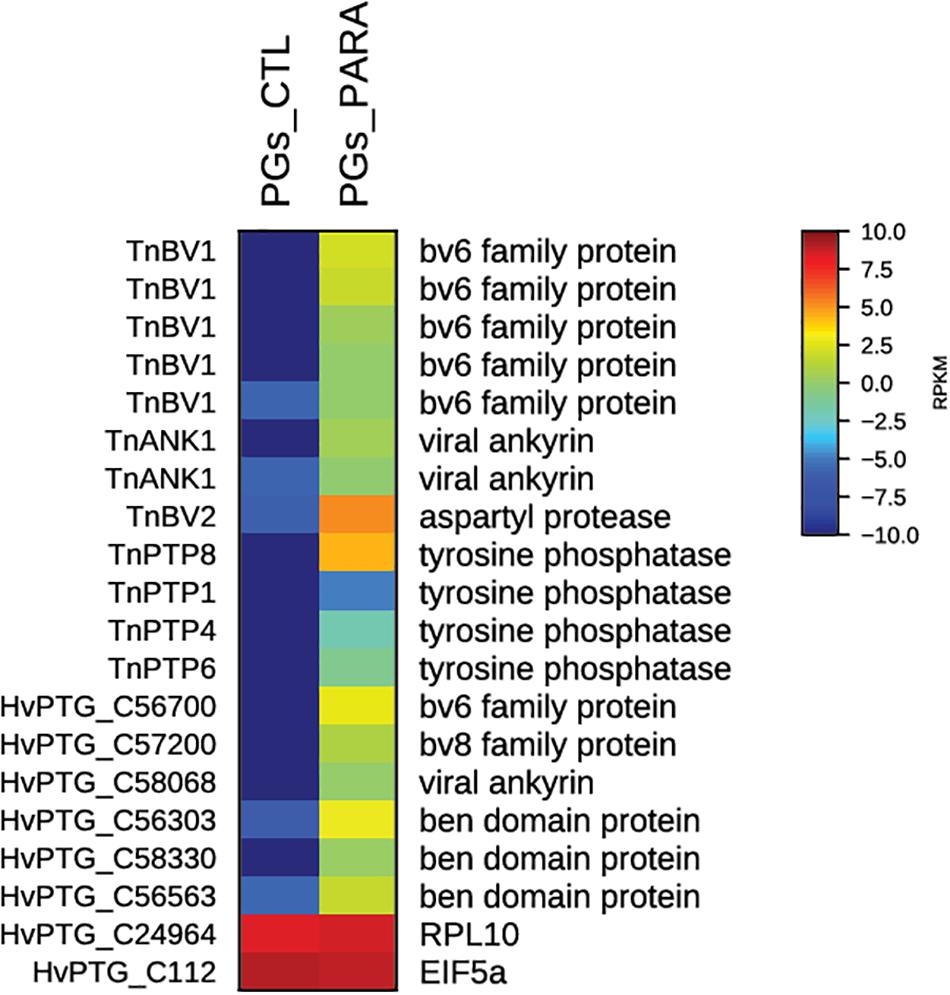
FIGURE 3. Heat map showing relative expression levels of TnBV genes in PGs from parasitized (PGs_PARA) and non-parasitized (PGs_CTL) H. virescens larvae. Viral genes are exclusively expressed in PGs from parasitized larvae. The housekeeping genes RPl10 and EIF5a are used for normalization and are shown to confirm the uniform expression of these control genes across samples. The map is based on log2-transformed RPKM values shown in the gradient heat map (blue represents weakly-expressed genes, and red represents strongly-expressed genes).
Using the transcriptome and RNAseq mapping data, it was also possible to evaluate the transcript levels of all genes encoding for proteins involved in the cell signaling pathway PI3K/Akt/TOR. As shown in the respective heat map (Figure 4), in PGs extracted from parasitized larvae all of the identified genes are downregulated compared to control (non-parasitized) PGs.

FIGURE 4. Heat map showing relative expression levels of all TOR pathway genes in PGs from parasitized (PGs_PARA) and non-parasitized (PGs_CTL) larvae. Genes belonging to the TOR pathway are downregulated in parasitized larvae compared to the control (non-parasitized). The housekeeping genes RPl10 and EIF5a are used for normalization and are shown to confirm the uniform expression of these control genes across samples. The map is based on log2- transformed RPKM values shown in the gradient heat map (blue represents weakly-expressed genes, and red represents strongly-expressed genes).
Moreover, the transcript levels of genes codifying for proteins involved both in MAPK pathway and in the biosynthesis of ecdysone (Halloween genes) were evaluated. In PGs extracted from parasitized larvae, 57 of the 58 identified transcripts encoding for proteins belonging to the MAPK pathway are downregulated compared to control (non-parasitized larvae PGs) (Figure 5). The downregulation of 5 among the 6 identified contigs corresponding to Halloween genes is also observed in PGs extracted from parasitized larvae compared to non-parasitized larvae PGs (control) (Figure 6).
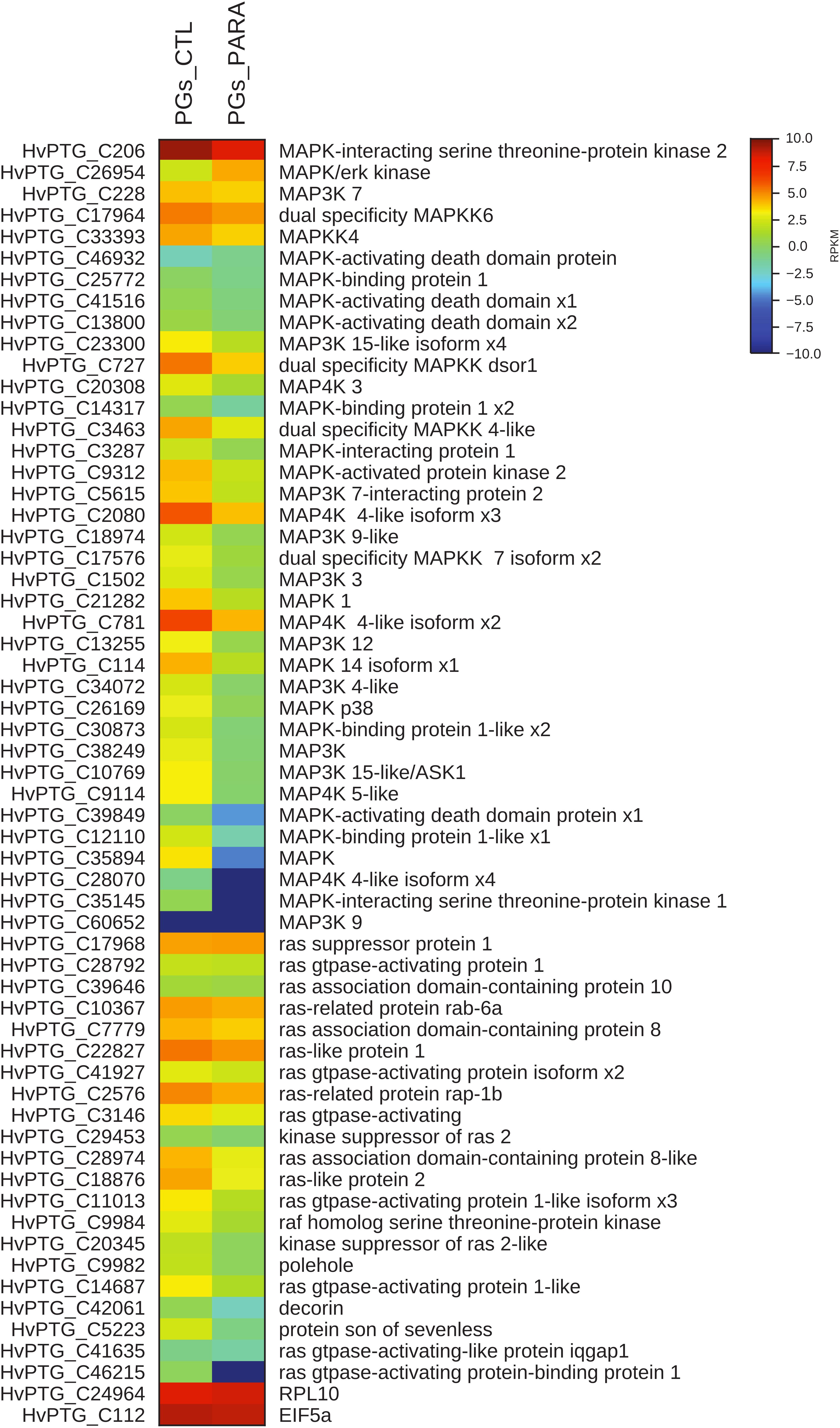
FIGURE 5. Heat map showing relative expression levels of MAPK pathway genes in PGs from parasitized (PGs_PARA) and non-parasitized (PGs_CTL) larvae. Genes belonging to the MAPK pathway are downregulated in parasitized larvae compared to the control (non-parasitized). The housekeeping genes RPl10 and EIF5a are used for normalization and are shown to confirm the uniform expression of these control genes across samples. The map is based on log2- transformed RPKM values shown in the gradient heat map (blue represents weakly-expressed genes, and red represents strongly-expressed genes).

FIGURE 6. Heat map showing relative expression levels of Halloween genes in PGs from parasitized (PGs_PARA) and non-parasitized (PGs_CTL) larvae. Halloween genes are downregulated in parasitized larvae compared to the control (non-parasitized). The housekeeping genes RPl10 and EIF5a are used for normalization and are shown to confirm the uniform expression of these control genes across samples. The map is based on log2- transformed RPKM values shown in the gradient heat map (blue represents weakly-expressed genes, and red represents strongly-expressed genes).
In order to confirm the transcriptomic analysis, showing a downregulation of TOR pathway genes, we analyzed the relative expression of tor, 4ebp, and s6k genes by quantitative Real time PCR (qRT-PCR) in parasitized and non-parasitized PGs. Our results showed that all tested genes displayed lower expression levels in parasitized PGs. The expression levels were statistically significantly different compared to those in non-parasitized samples (Figure 7).
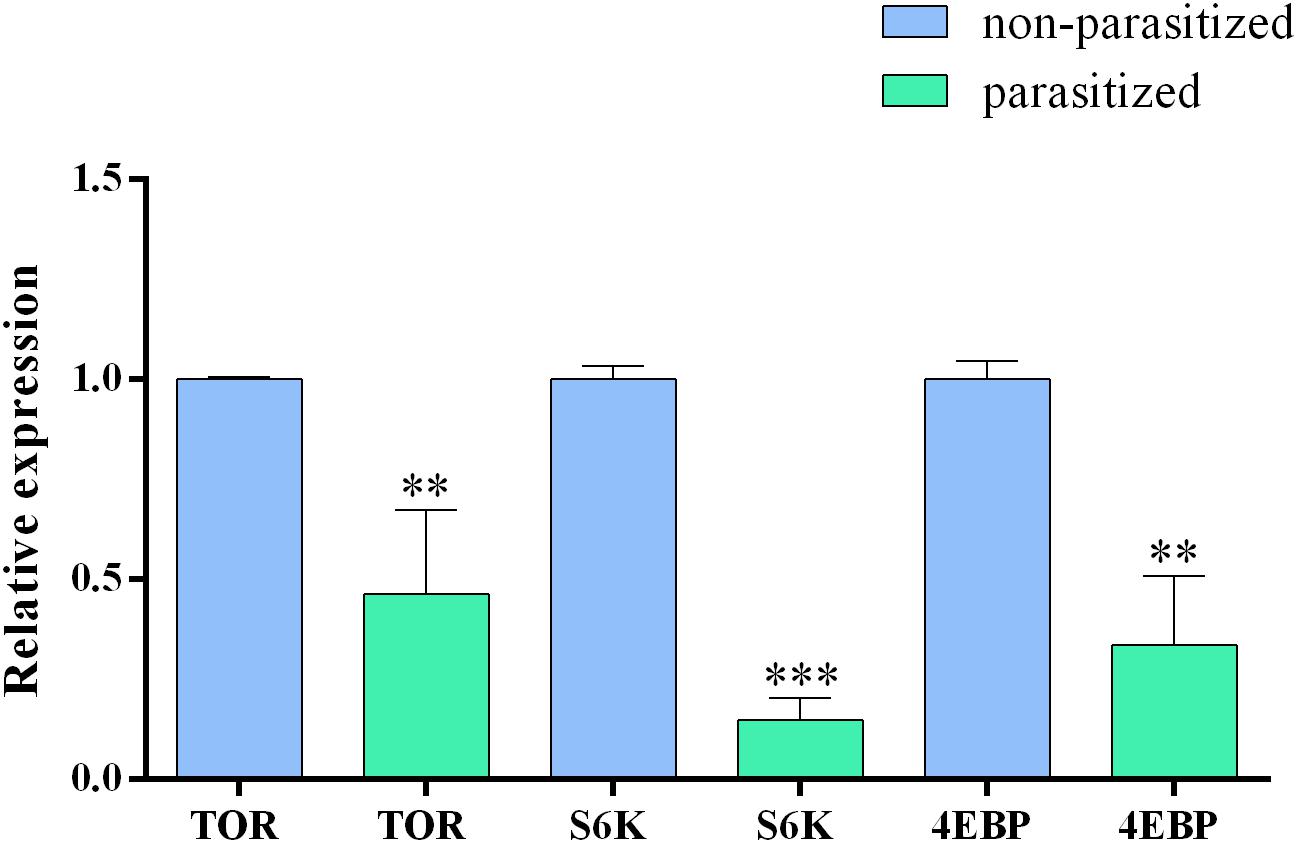
FIGURE 7. Relative expression level of tor, s6k, and 4ebp genes in basal parasitized and basal non-parasitized PGs. Gene expression levels were quantified by qRT-PCR. Data represent the mean of three independent replicates ± SEM. Statistically significant differences between samples are indicated with asterisk (∗∗p ≤ 0.01 and ∗∗∗p ≤ 0.001, one way ANOVA and Bonferroni post hoc <0.05). Reference genes: gapdh, rp13, and ef. Calibrator sample: non-parasitized PGs.
The serine/threonine protein kinase Target of Rapamycin (TOR) is one of the key proteins involved in the control of several cell processes. TOR belongs to the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway and its activation is modulated by the combination of signaling pathways following intracellular stimuli, such as nutrients and growth factors. The TOR protein and other proteins belonging to its cellular cascade are highly conserved among eukaryotes from yeast to mammals, including insects (Zhang et al., 2000; Rexin et al., 2015; Gonzalez and Hall, 2017). The alteration of the PI3K/Akt pathway, which leads to the overexpression or the inactivation of TOR kinase, seems to be critical in a number of diseases, such as cancer and diabetes (Ali et al., 2017), or neurological diseases such as Parkinson (Xu et al., 2014; Tiwari and Pal, 2017) and Alzheimer (Tang et al., 2015; Tramutola et al., 2015). TOR therefore represents one of the major therapeutic targets in these pathological alterations. The macrolide rapamycin and its analogs are highly specific inhibitors of TOR and they are considered therapeutic molecules with antitumor (Calimeri and Ferreri, 2017; Faes et al., 2017; Liu et al., 2017) and immunosuppressive activity (McMahon et al., 2011; Hamdani et al., 2017; Yee and Tan, 2017), and with promising activity against neurological disease (Wang et al., 2014; Maiese, 2016). These molecules have a unique mechanism of action: they first bind FK506-binding protein (FKBP12), a 12 kDa immunophilin, and then this complex inhibits the serine/threonine kinase TOR (Russell et al., 2011).
The use of rapamycin or its analogs has also side effects: dose-dependent cytopenia, hyperlipidemia, thrombosis and pulmonary, cardiovascular, skin or bone damages (Bhat et al., 2013). The identification of new genes and molecules able to modulate/inhibit the TOR pathway with a similar or different mechanism as rapamycin can be considered an important goal to enrich the strategy and tools employable in cancer therapy or in pathology related to TOR pathway deregulation.
Beside the important roles regarding the regulation of cell growth and proliferation in response to environmental and nutritional conditions, in insects the TOR pathway is involved in ecdysone biosynthesis by PGs or analogous organs, stimulated by the PTTH. In previous work we demonstrated the involvement of the PI3K/Akt/TOR pathway in ecdysteroidogenesis stimulated by PTTH, contained in the brain extract, in Heliothis virescens PGs (Scieuzo et al., 2018). Here we show that this cellular signaling pathway is one of the targets of infection by TnBV, the Polydnavirus associated with the endoparasitoid wasp Toxoneuron nigriceps.
T. nigriceps oviposits into all larval instars of H. virescens, which can reach the stage of mature larva (last instar) but become developmentally arrested failing pupation. The PGs functional inactivation is responsible for blocking pupation of the parasitized host last instar larvae (Tanaka and Vinson, 1991; Pennacchio et al., 1997, 1998a,b, 2001). The reduced biosynthetic activity of host PGs was due to their infection by transcriptionally active TnBV, suggesting that viral gene expression in PGs might play a role in the disruption of the PTTH signal transduction pathway (Pennacchio et al., 1998a).
Our recent studies showing a PI3K/Akt/TOR signaling involvement in PTTH-stimulated ecdysteroidogenesis by H. virescens PGs, confirmed that PTTH rapidly enhanced the phosphorylation of translational repressor 4E-binding protein (4E-BP) and p70 ribosomal protein S6 kinase (S6K), two well-known downstream targets of TOR. Moreover, we also demonstrated that rapamycin blocked phosphorylation of 4E-BP and S6K in PTTH-stimulated PGs and strongly inhibited PTTH-stimulated ecdysteroidogenesis (Scieuzo et al., 2018).
In the present study the possible role of TnBV on the PI3K/Akt/TOR pathway in PTTH-stimulated ecdysone biosynthesis in H. virescens PGs was investigated. Interestingly, our results confirm that a parasitism event completely inhibits PTTH-mediated stimulation of ecdysone biosynthesis in PGs of parasitized larvae, as suggested in previous work (Pennacchio et al., 1997, 1998a; Falabella et al., 2006), and demonstrate that one of the TnBV effects is linked to PI3K/Akt/TOR pathway alteration. The impact of TnBV on ecdysteroidogenesis is more dramatic than the effect of rapamycin: parasitism totally inhibited ecdysone production of PTTH-stimulated PGs, whereas the effect of rapamycin was partial. This difference can be explained on the basis of our previous study (Scieuzo et al., 2018) and other studies on lepidopteran species (Lin and Gu, 2007; Gu et al., 2011) which demonstrated that both MAPK and PI3K/Akt/TOR pathways are independently involved in PTTH-stimulated ecdysteroidogenesis, but rapamycin only affects the TOR pathway. Evidently the expression of TnBV genes in H. virescens PGs affects PTTH-stimulated ecdysteroidogenesis pathways at different levels and also with different mechanisms. Moreover, the PTTH-stimulated phosphorylation of 4E-BP and S6K, detected only in non-parasitized PGs, indicates that the PI3K/Akt/TOR pathway is directly stimulated by the neuropeptide hormone, as previously demonstrated (Scieuzo et al., 2018); no phosphorylation signal was detected in parasitized PGs, both basal and stimulated, apparently similar to the effect of PG incubation with rapamycin (Gu et al., 2011, 2012; Scieuzo et al., 2018). In vitro ecdysone biosynthesis evaluation showed that non-parasitized PGs, treated with rapamycin (R) and stimulated with PTTH extract (R+S), produced a significantly lower amount of ecdysone in comparison to non-parasitized PGs stimulated with PTTH extract (S), but a significantly higher amount of ecdysone in comparison to both untreated parasitized PGs (B) and those stimulated with PTTH extract (S), confirming that a parasitism event completely blocks ecdysteroidogenesis. This confirms, above all, that PI3K/Akt/TOR is not the only pathway involved in H. virescens ecdysteroidogenesis, suggesting that parasitization affects all the signaling pathways involved in ecdysteroidogenesis (Scieuzo et al., 2018).
Taken together, our results indicate that the infection of host PGs by TnBV alters ecdysone production, at least in part, by modulating the TOR pathway through the expression of one or more viral genes. In support of this hypothesis, we identified all viral genes expressed in PGs, comparing the expression levels of transcripts in parasitized and non-parasitized PGs. Among these were previously identified and, in some cases, functionally characterized TnBV genes (Varricchio et al., 1999; Falabella et al., 2003; Provost et al., 2004) such as TnBV1, TnBV2, TnBVank1, ptp1, ptp4, ptp6, and ptp8. TnBVank1 displays significant sequence similarity with members of the IkB family (Silverman and Maniatis, 2001; Thoetkiattikul et al., 2005; Falabella et al., 2007; Bitra et al., 2012; Salvia et al., 2017). These proteins are generally involved in the control of NF-kB signaling pathways both in insects and vertebrates (Silverman and Maniatis, 2001).
Using Drosophila melanogaster as a model to functionally characterize TnBV genes, it was shown that TnBVank1 expression in host germ cells altered the microtubule network in oocytes (Duchi et al., 2010; Valzania et al., 2014). Subsequently, Valzania et al. (2014) confirmed that the expression of TnBVank1 in PG cells strongly reduced ecdysone biosynthesis and, as a consequence, inhibited the transition of D. melanogaster larval to pupal stage, mimicking the developmental arrest observed in H. virescens larvae parasitized by T. nigriceps.
These results support the hypothesis that TnBVank1, expressed in H. virescens PGs, could actively participate in inducing ecdysone titer reduction. TnBVank1 influences different physiological pathways involved in both the disruption of cytoskeletal structure of PG cells and in affecting the sterol delivery from endosomal compartments trough the interaction with Alix, as reported for D. melanogaster (Valzania et al., 2014). This multifunctional activity of TnBVank1 is not surprising. The expression of this gene in H. virescens immune cells was demonstrated to induce apoptosis through the interaction with Alix, besides its irreversible inhibition of NF-kB translocation in cell nuclei, thus blocking the expression of key genes and inducing apoptotic phenomena (Falabella et al., 2007; Salvia et al., 2017). The reduced gland size observed in D. melanogaster larvae (Valzania et al., 2014) and the low basal production of ecdysteroids in PGs of H. virescens parasitized larvae were also reported (Pennacchio et al., 1997, 1998b). Here we demonstrate that in naturally parasitized larvae these symptoms were associated with a disruption of PTTH signaling by active TnBV infection of PGs (Pennacchio et al., 1998a). It is highly plausible that at least part of these effects could be attributable to the expression of TnBVank1 in the parasitized host.
Although the effects of other viral genes should be analyzed in vivo or in vitro, we can speculate on possible roles of different TnBV genes in the suppression of ecdysteroidogenesis.
Among the 13 putative PTPs identified in the TnBV genome, 8 PTP genes have a full protein tyrosine phosphatase domain (Provost et al., 2004; Falabella et al., 2006).
Our results demonstrate that PTP1, 4, 6, and 8 are specifically expressed in parasitized PGs. These PTPs together with PTP7 (Provost et al., 2004; Falabella et al., 2006), could dephosphorylate PG proteins phosphorylated by tyrosine kinases following PTTH stimulation. The expression of TnBV PTP7 (24 h after parasitism) (Falabella et al., 2006) and of TnBV PTP1, PTP4, PTP6, and PTP8 (reported here) specifically in PGs, confirms that PTP expression is host tissue-specific.
Members of the TnBV PTP gene family, expressed at different times during parasitism, could be good candidates for a functional involvement in host PG inactivation through dephosphorylation of regulatory proteins. These proteins are possibly involved in the extremely intricate PTTH-stimulated ecdysone secretion pathway. However, at least in case of H. virescens and other Lepidoptera, this pathway is not yet fully identified. Since TnBV PTPs belong to the classical PTPs (Provost et al., 2004), they dephosphorylate only tyrosine residues, and it is reasonable to hypothesize that TnBV PTPs do not directly affect the TOR pathway (Brunn et al., 1997; Aoki et al., 2001). The target/s of viral PTPs still remain/s unknown.
The effect of parasitism on PGs could act on different levels to ensure the total inhibition of PG biosynthetic activity. Indeed, we demonstrate an alternative way to control the TOR pathway at the transcriptional level through expression of one or more TnBV genes in PGs of parasitized larvae. The down-regulation of TOR genes, especially tor, s6k, and 4ebp, was confirmed by qRT-PCR experiments. Although it remains unclear which of the TnBV genes are involved, TnBVank1 could play a role, also because of its ability to block NF-kB-mediated gene expression regulation (Falabella et al., 2007; Dan et al., 2008; Oeckinghaus et al., 2011).
The expression of TnBV genes in PGs seems to alter TOR metabolic pathway, influencing essential steps for the synthesis of ecdysone. However in H. virescens at least two independent pathways contribute to ecdysteroidogenesis: MAPK and PI3K/Akt/TOR cellular signaling (Scieuzo et al., 2018). RNA-seq data showed a down-regulation of genes involved in both PI3K/Akt/TOR, MAPK pathways, and in the biosynthesis of ecdysone (Halloween genes). These findings suggest that the massively reduced amounts of ecdysone following T. nigriceps parasitism could be ascribed to the expression of TnBV genes in PGs. Further studies are needed to obtain more information regarding the possible mechanism of action of TnBV proteins on these pathways. Further characterization of other viral proteins would allow a better understanding of the mechanisms involved in the inhibition of ecdysone synthesis, and could provide a range of candidates potentially capable of inhibiting more steps of the PI3K/Akt/TOR pathway. The possible use of viral proteins in synergy with rapamycin or its analogs can be a turning point in medical treatment with the PI3K/Akt/TOR pathway as possible therapeutic target.
The short read data have been deposited in the EBI short read archive (SRA) with the following sample accession numbers: ERS2859514-ERS2859515 (PGs of non-parasitized H. virescens larvae) and ERS2859516 (PGs of T. nigriceps parasitized H. virescens larvae). The complete study can also be accessed directly using the following URL: http://www.ebi.ac.uk/ena/data/view/PRJEB29401.
Insects used in this work were treated as well as possible given the constraints of the experimental design.
PF designed the experiments, wrote and critically revised the paper. HV, RS, MN, CS, AS, AR, and SB contributed to the data interpretation and critically revised the paper. RS and CS performed the western blot experiments. AS, MN, and RS performed the samples collection and RT-qPCR. MN and CS performed the enzyme immunoassay. HV performed the de novo transcriptome assembly and analysis. All authors read and approved the manuscript.
This work was supported by the Max Planck Society and the University of Basilicata (RIL funds).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Prof. Paolo Fanti, University of Basilicata, for the assistance in statistical analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.01678/full#supplementary-material
FIGURE S1 | Heat map showing relative expression levels of five candidate reference genes in PGs from parasitized (PGs_PARA) and non-parasitized (PGs_CTL) larvae. Eukaryotic translation initiation factor 5A-1 (eif5a), ribosomal protein L10 (rpl10), Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), elongation factor 1-alpha (ef1a) and ribosomal protein L13 (rp13) were pre-selected as candidate reference genes for normalization of qRT-PCR data since they were not affected by parasitization. Gapdh, ef1a and rp13 were subsequently chosen as reference genes.
TABLE S1 | Primers used for qRT-PCR. F: forward, R: reverse.
TABLE S2 | Ecdysone released by prothoracic glands in different experimental conditions. Data are expressed as mean of ecdysone concentrations (pg/gland) ± SEM of n = 6 experiments. Different letters indicate significant differences (p < 0.05). Uppercase letters refer to the Tukey post hoc test and lowercase letters to the SNK test.
TABLE S3 | Raw data of enzyme immunoassay (EIA) (a) and Two-Way ANOVA statistical output (b).
4E-BP, translational repressor 4E-binding protein; Akt, protein kinase B; ANOVA, analysis of variance; BE, brain equivalent; BLAST, basic local alignment search tool; BSA, bovine serum albumin; ECL, enhanced chemo luminescence; EIA, enzyme immunoassay; GO, Gene Ontology; NCBI, National Center for Biotechnology Information; PBS, phosphate-buffered saline; PDV, polydnavirus; PG, prothoracic gland; PI3K, phosphoinositide 3-kinase; PTP, protein tyrosine phosphatase; PTTH, prothoracicotropic hormone; S6K, p70 ribosomal protein S6 kinase; SEM, standard error of mean; TBS-T, tris-buffered saline-Tween 20; TnBV, Toxoneuron nigriceps bracovirus; TOR, target of rapamycin.
Ali, M., Bukhari, S. A., and Lee, H. W. (2017). Upstream signalling of mTORC1 and its hyperactivation in type 2 diabetes (T2D). BMB Rep. 50, 601–609. doi: 10.5483/BMBRep.2017.50.12.206
Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. doi: 10.1093/nar/25.17.3389
Aoki, M., Blazek, E., and Vogt, P. K. (2001). A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl. Acad. Sci. U.S.A. 98, 136–141. doi: 10.1073/pnas.98.1.136
Beckage, N. E., and Gelman, D. B. (2004). Wasp parasitoid disruption of host development: implications for new biologically based strategies for insect control. Annu. Rev. Entomol. 49, 299–330. doi: 10.1146/annurev.ento.49.061802.123324
Bhat, M., Sonenberg, N., and Gores, G. J. (2013). The mTOR pathway in hepatic malignancies. Hepatology 58, 810–818. doi: 10.1002/hep.26323
Bitra, K., Suderman, R. J., and Strand, M. R. (2012). Polydnavirus Ank proteins bind NF-κB homodimers and inhibit processing of relish. PLoS Pathog. 8:e1002722. doi: 10.1371/journal.ppat.1002722
Bollenbacher, W. E., O’Brien, M. A., Katahira, E. J., and Gilbert, L. I. (1983). A kinetic analysis of the action of insect prothoracicotropic hormone. Mol. Cell. Endocrinol. 32, 27–46. doi: 10.1016/0303-7207(83)90096-5
Brunn, G. J., Fadden, P., Haystead, T. A., and Lawrence, J. C. (1997). The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J. Biol. Chem. 272, 32547–32550. doi: 10.1074/jbc.272.51.32547
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. doi: 10.1373/clinchem.2008.112797
Calimeri, T., and Ferreri, A. J. M. (2017). m-TOR inhibitors and their potential role in haematological malignancies. Br. J. Haematol. 177, 684–702. doi: 10.1111/bjh.14529
Dan, H. C., Cooper, M. J., Cogswell, P. C., Duncan, J. A., Ting, J. P. Y., and Baldwin, A. S. (2008). Akt-Dependent Regulation of NF-κB Is Controlled by mTOR and Raptor in Association with IKK. Genes Dev. 22, 1490–1500. doi: 10.1101/gad.1662308
Duchi, S., Cavaliere, V., Fagnocchi, L., Grimaldi, M. R., Falabella, P., Graziani, F., et al. (2010). The impact on microtubule network of a bracovirus IkappaB-like protein. Cell. Mol. Life Sci. 67, 1699–1712. doi: 10.1007/s00018-010-0273-2
Dupuy, C., Periquet, G., Serbielle, C., Bézier, A., Louis, F., and Drezen, J. M. (2011). Transfer of a chromosomal Maverick to endogenous bracovirus in a parasitoid wasp. Genetica 139, 489–496. doi: 10.1007/s10709-011-9569-x
Faes, S., Santoro, T., Demartines, N., and Dormond, O. (2017). Evolving significance and future relevance of anti-angiogenic activity of mTOR inhibitors in cancer therapy. Cancers 9:E152. doi: 10.3390/cancers9110152
Falabella, P., Caccialupi, P., Varricchio, P., Malva, C., and Pennacchio, F. (2006). Protein tyrosine phosphatases of Toxoneuron nigriceps bracovirus as potential disrupters of host prothoracic gland function. Arch. Insect Biochem. Physiol. 61, 157–169. doi: 10.1002/arch.20120
Falabella, P., Varricchio, P., Gigliotti, S., Tranfaglia, A., Pennacchio, F., and Malva, C. (2003). Toxoneuron nigriceps polydnavirus encodes a putative aspartyl protease highly expressed in parasitized host larvae. Insect Mol. Biol. 12, 9–17. doi: 10.1046/j.1365-2583.2003.00382.x
Falabella, P., Varricchio, P., Provost, B., Espagne, E., Ferrarese, R., Grimaldi, A., et al. (2007). Characterization of the IκB-like gene family in polydnaviruses associated with wasps belonging to different Braconid subfamilies. J. Gen. Virol. 88, 92–104. doi: 10.1099/vir.0.82306-0
Gonzalez, A., and Hall, M. N. (2017). Nutrient sensing and TOR signalling in yeast and mammals. EMBO J. 36, 397–408. doi: 10.15252/embj.201696010
Gu, S. H., Yeh, W. L., Young, S. C., Lin, P. L., and Li, S. (2012). TOR signaling is involved in PTTH stimulated ecdysteroidogenesis by prothoracic glands in the silkworm. Bombyx mori. Insect Biochem. Mol. Biol. 42, 296–303. doi: 10.1016/j.ibmb.2011.12.010
Gu, S. H., Young, S. C., Lin, J. L., and Lin, P. L. (2011). Involvement of PI3K/Akt signaling in PTTH-stimulated ecdysteroidogenesis by prothoracic glands of the silkworm. Bombyx mori. Insect Biochem. Mol. Biol. 41, 197–202. doi: 10.1016/j.ibmb.2010.12.004
Hamdani, S., Thiolat, A., Naserian, S., Grondin, C., Moutereau, S., Hulin, A., et al. (2017). Delayed and short course of rapamycin prevents organ rejection after allogeneic liver transplantation in rats. World J. Gastroenterol. 14, 6962–6972. doi: 10.3748/wjg.v23.i38.6962
Jacobs, C., Steiger, S., Heckel, D. G., Wielsch, N., Vilcinskas, A., and Vogel, H. (2016). Sex, offspring and carcass determine antimicrobial peptide expression in the burying beetle. Sci. Rep. 6:25409. doi: 10.1038/srep25409
Johnson, G., Nour, A. A., Nolan, T., Huggett, J., and Bustin, S. (2014). Minimum information necessary for quantitative real-time PCR experiments. Methods Mol. Biol. 1160, 5–17. doi: 10.1007/978-1-4939-0733-5_2
Kemirembe, K., Liebmann, K., Bootes, A., Smith, W. A., and Suzuki, Y. (2012). Amino acids and TOR signaling promote prothoracic gland growth and the initiation of larval molts in the tobacco hornworm Manduca sexta. PLoS One 7:e44429. doi: 10.1371/journal.pone.0044429
Kingan, T. G. (1989). A competitive enzyme-linked immunosorbent assay: applications in the assay of peptides, steroids, and cyclic nucleotides. Anal. Biochem. 183, 283–289. doi: 10.1016/0003-2697(89)90481-8
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. doi: 10.1038/227680a0
Lapointe, R., Wilson, R., Vilaplana, L., O’Reilly, D. R., Falabella, P., Douris, V., et al. (2005). Expression of a Toxoneuron nigriceps polydnavirus-encoded protein causes apoptosis-like programmed cell death in lepidopteran insect cells. J. Gen. Virol. 86(Pt 4), 963–971. doi: 10.1099/vir.0.80834-0
Laurino, S., Grossi, G., Pucci, P., Flagiello, A., Bufo, S. A., and Bianco, G. (2016). Identification of major Toxoneuron nigriceps venom proteins using an integrated transcriptomic/proteomic approach. Insect Biochem. Mol. Biol. 76, 49–61. doi: 10.1016/j.ibmb.2016.07.001
Lee, C., Kim, J., Shin, S. G., and Hwang, S. (2006). Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 123, 273–280. doi: 10.1016/j.jbiotec.2005.11.014
Lewis, W. J., and Vinson, S. B. (1968). Egg and larval development of Cardiochiles nigriceps. Ann. Entomol. Soc. Am. 61, 561–656. doi: 10.1093/aesa/61.3.561
Lin, J. L., and Gu, S. H. (2007). In vitro and in vivo stimulation of extracellular signal-regulated kinase (ERK) by the prothoracicotropic hormone in prothoracic gland cells and its developmental regulation in the silkworm. Bombyx mori. J. Insect Physiol. 53, 622–631. doi: 10.1016/j.jinsphys.2007.03.004
Liu, W., and Saint, D. A. (2002). A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal. Biochem. 302, 52–59. doi: 10.1006/abio.2001.5530
Liu, Y., Pandeswara, S., Dao, V., Padrón, A., Drerup, J. M., Lao, S., et al. (2017). Biphasic rapamycin effects in lymphoma and carcinoma treatment. Cancer Res. 77, 520–531. doi: 10.1158/0008-5472.CAN-16-1140
Maiese, K. (2016). Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br. J. Clin. Pharmacol. 82, 1245–1266. doi: 10.1111/bcp.12804
Malva, C., Varricchio, P., Falabella, P., La Scaleia, R., Graziani, F., and Pennacchio, F. (2004). Physiological and molecular interaction in the host- parasitoid system Heliothis virescens-Toxoneuron Nigriceps: current status and future perspectives. Insect Biochem. Mol. Biol. 34, 177–183. doi: 10.1016/j.ibmb.2003.09.008
McMahon, G., Weir, M. R., Li, X. C., and Mandelbrot, D. A. (2011). The evolving role of mTOR inhibition in transplantation tolerance. J. Am. Soc. Nephrol. 22, 408–415. doi: 10.1681/ASN.2010040351
Moreau, S., and Asgari, S. (2015). Venom proteins from parasitoid wasps and their biological functions. Toxins 7, 2385–2412. doi: 10.3390/toxins7072385
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L., and Wold, B. (2008). Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628. doi: 10.1038/nmeth.1226
Oeckinghaus, A., Hayden, M. S., and Ghosh, S. (2011). Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 12, 695–708. doi: 10.1038/ni.2065
Park, B., and Kim, Y. (2010). Transient transcription of a putative RNase containing BEN domain encoded in Cotesia plutellae bracovirus induces an immunosuppression of the diamondback month. Plutella xylostella. J. Invertebr. Pathol. 105, 156–163. doi: 10.1016/j.jip.2010.06.003
Pennacchio, F., Falabella, P., and Vinson, S. B. (1998a). Regulation of Heliothis virescens prothoracic glands by Cardiochiles nigriceps polydnavirus. Arch. Insect Biochem. Physiol. 38, 1–10.
Pennacchio, F., Falabella, P., Sordetti, R., Varricchio, P., Malva, C., and Vinson, S. B. (1998b). Prothoracic gland inactivation in Heliothis virescens (F.) (Lepidoptera:Noctuidae) larvae parasitized by Cardiochiles nigriceps (V.) (Hymenoptera:Braconidae). J. Insect Physiol. 44, 845–857. doi: 10.1016/S0022-1910(98)00016-X
Pennacchio, F., Malva, C., and Vinson, S. B. (2001). “Regulation of host endocrine system by the endophagous braconid Cardiochiles nigriceps and its polydnavirus,” in Endocrine Interactions of Insect Parasites and Pathogens, eds J. P. Edward and R. J. Weaver (Oxford: BIOS), 123–132.
Pennacchio, F., Sordetti, R., Falabella, P., and Vinson, S. B. (1997). Biochemical and ultrastructural alterations in prothoracic glands of Heliothis virescens (F.) (Lepidoptera: Noctuidae) last instar larvae parasitized by Cardiochiles nigriceps (V.) (Hymenoptera: Braconidae). Insect Biochem. Mol. Biol. 27, 439–450. doi: 10.1016/S0965-1748(97)00016-7
Pennacchio, F., and Strand, M. R. (2006). Evolution of developmental strategies in parasitic hymenoptera. Ann. Rev. Entomol. 51, 233–258. doi: 10.1146/annurev.ento.51.110104.151029
Pennacchio, F., Vinson, S. B., and Tremblay, E. (1992). Host regulation effects of Heliothis virescens (F.) larvae induced by teratocytes of Cardiochiles nigriceps (V.) (Lepidoptera, Noctuidae – Hymenoptera, Braconidae). Arch. Insect Biochem. Physiol. 19, 177–192. doi: 10.1002/arch.940190304
Pennacchio, F., Vinson, S. B., and Tremblay, E. (1993). Growth and development of Cardiochiles nigriceps (V.) (Hymenoptera, Braconidae) larvae and their synchronization with some changes of the hemolymph composition of their host, Heliothis virescens (F.) (Lepidoptera, Noctuidae). Arch. Insect Biochem. Physiol. 24, 65–77. doi: 10.1002/arch.940240202
Pennacchio, F., Vinson, S. B., Tremblay, E., and Ostuni, A. (1994). Alteration of ecdysone metabolism in Heliothis virescens (F.) (Lepidoptera, Noctuidae) larvae induced by Cardiochiles nigriceps (V.) (Hymenoptera, Braconidae). Insect Biochem. Mol. Biol. 24, 383–394.
Provost, B., Varricchio, P., Arana, E., Espagne, E., Falabella, P., Huguet, E., et al. (2004). Bracovirus contain a large multigene family coding for protein tyrosine phosphatases. J. Virol. 78, 13090–13103. doi: 10.1128/JVI.78.23.13090-13103.2004
Rexin, D., Meyer, C., Robaglia, C., and Veit, B. (2015). TOR signalling in plants. Biochem. J. 15, 1–14. doi: 10.1042/BJ20150505
Russell, R. C., Fang, C., and Guan, K. L. (2011). An emerging role for TOR signalling in mammalian tissue and stem cell physiology. Development 138, 3343–3356. doi: 10.1242/dev.058230
Salvia, R., Grossi, G., Amoresano, A., Scieuzo, C., Nardiello, M., and Giangrande, C. (2017). The multifunctional polydnavirus TnBVANK1 protein: impact on host apoptotic pathway. Sci. Rep. 7:11775. doi: 10.1038/s41598-017-11939-x
Scieuzo, C., Nardiello, M., Salvia, R., Pezzi, M., Chicca, M., and Leis, M. (2018). Ecdysteroidogenesis and development in Heliothis virescens (Lepidoptera: Noctuidae): focus on PTTH-stimulated pathways. J. Insect Physiol. 15, 57–67. doi: 10.1016/j.jinsphys.2018.02.008
Silverman, N., and Maniatis, T. (2001). NF-kB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15, 2321–2342. doi: 10.1101/gad.909001
Smith, W. A., Combest, W. L., and Gilbert, L. I. (1986). Involvement of cyclic AMP-dependent protein kinase in prothoracicotropic hormone-stimulated ecdysone synthesis. Mol. Cell. Endocrinol. 47, 25–33. doi: 10.1016/0303-7207(86)90012-2
Smith, W. A., Lamattina, A., and Collins, M. (2014). Insulin signaling pathways in lepidopteran ecdysone secretion. Front. Physiol. 5:19. doi: 10.3389/fphys.2014.00019
Stoltz, D. B., and Vinson, S. B. (1979). Viruses and parasitism in insects. Adv. Virus Res. 24, 125–171. doi: 10.1016/S0065-3527(08)60393-0
Strand, M. R. (2010). “Polydnaviruses,” in Insect Virol, eds S. Asgari and K. N. Johnson (Norwich: Caister Academic Press), 216–241.
Strand, M. R., and Burke, G. R. (2014). Polydnaviruses: nature’s genetic engineers. Ann. Rev. Virol. 1, 333–354. doi: 10.1146/annurev-virology-031413-085451
Tanaka, T., and Vinson, S. B. (1991). Depression of prothoracic gland activity of Heliothis virescens by venom and calyx fluids from the parasitoid. Cardiochiles nigriceps. J. Insect Physiol. 37, 139–144. doi: 10.1016/0022-1910(91)90099-L
Tang, Z., Ioja, E., Bereczki, E., Hultenby, K., Li, C., Guan, Z., et al. (2015). mTOR mediates tau localization and secretion: implication for Alzheimer’s disease. Biochim. Biophys. Acta 1853, 1646–1657. doi: 10.1016/j.bbamcr.2015.03.003
Thoetkiattikul, H., Beck, M. H., and Strand, M. R. (2005). Inhibitor κB-like proteins from a polydnavirus inhibit NF-κB activation and suppress the insect immune response. Proc. Nat. Acad. Sci. U.S.A. 102, 11426–11431. doi: 10.1073/pnas.0505240102
Tiwari, P. C., and Pal, R. (2017). The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues Clin. Neurosci. 19, 71–80.
Tramutola, A., Triplett, J. C., Di Domenico, F., Niedowicz, D. M., Murphy, M. P., Coccia, R., et al. (2015). Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J. Neurochem. 133, 739–749. doi: 10.1111/jnc.13037
Valzania, L., Romani, P., Tian, L., Li, S., Cavaliere, V., Pennacchio, F., et al. (2014). A polydnavirus ANK protein acts as virulence factor by disrupting the function of prothoracic gland steroidogenic cells. PLoS One 9:e95104. doi: 10.1371/journal.pone.0095104
Vanderzant, E. S., Richardson, C. D., and Fort, S. W. Jr. (1962). Rearing of the bollworm on artificial diet. J. Econ. Entomol. 55:140. doi: 10.1673/031.007.3501
Varricchio, P., Falabella, P., Sordetti, R., Graziani, F., Malva, C., and Pennacchio, F. (1999). Cardiochiles nigriceps polydnavirus: molecular characterization and gene expression in parasitized Heliothis virescens larvae. Insect Biochem. Mol. Biol. 29, 1087–1096. doi: 10.1016/S0965-1748(99)00087-9
Vinson, S. B., Guillot, F. S., and Hays, D. B. (1973). Rearing of Cardiochiles nigriceps in the laboratory, with Heliothis virescens as hosts. Ann. Entomol. Soc. Am. 66:1172. doi: 10.1093/aesa/66.5.1170
Vogel, H., Badapanda, C., Knorr, E., and Vilcinskas, A. (2014). RNA-sequencing analysis reveals abundant developmental stage-specific and immunity-related genes in the pollen beetle Meligethes aeneus. Insect Mol. Biol. 23, 98–112. doi: 10.1111/imb.12067
Volkoff, A. N., Jouan, V., Urbach, S., Samain, S., Bergoin, M., and Wincker, P. (2010). Analysis of virion structural components reveals vestiges of the ancestral ichnovirus genome. PLoS Pathog. 6:e1000923. doi: 10.1371/journal.ppat.1000923
Wang, C., Yu, J. T., Miao, D., Wu, Z. C., Tan, M. S., and Tan, L. (2014). Targeting the mTOR signaling network for Alzheimer’s disease therapy. Mol. Neurobiol. 49, 120–135. doi: 10.1007/s12035-013-8505-8
Webb, B. A., Beckage, N. E., Hayakawa, Y., Krell, P. J., and Lanzrein, B. (2000). “Family polydnaviridae,” in Virus Taxonomy: Seventh Report of the International Committee on Taxonomy of Viruses, eds M. H. V. Van Regenmortel (Cambridge, MA: Academic Press), 253–260.
Webb, B. A., and Dahlman, D. L. (1985). Developmental pathology of Heliothis virescens larvae parasitized by Microplitis croceipes: parasite-mediated host developmental arrest. Arch. Insect Biochem. Physiol. 2, 1–139. doi: 10.1002/arch.940020203
Webb, B. A., and Strand, M. R. (2005). “The biology and genomics of polydnaviruses,” in Comprehensive Molecular Insect Science, Vol. 6, eds L. I. Gilbert, K. Iatrou, and S. S. Gill (San Diego: Elsevier), 260–323.
Webb, B. A., Strand, M. R., Dickey, S. E., Beck, M. H., Hilgarth, R. S., Barney, W. E., et al. (2006). Polydnavirus genomes reflect their dual roles as mutualists and pathogens. Virology 347, 160–174. doi: 10.1016/j.virol.2005.11.010
Wyder, S., Blank, F., and Lanzrein, B. (2003). Fate of polydnavirus DNA of the egg–larval parasitoid Chelonus inanitus in the host Spodoptera littoralis. J. Insect Physiol. 49, 491–500. doi: 10.1016/S0022-1910(03)00056-8
Xu, Y., Liu, C., Chen, S., Ye, Y., Guo, M., Ren, Q., et al. (2014). Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell. Signal. 26, 1680–1689. doi: 10.1016/j.cellsig.2014.04.009
Yee, M. L., and Tan, H. H. (2017). Use of everolimus in liver transplantation. World J. Hepatol. 18, 990–1000. doi: 10.4254/wjh.v9.i23.990
Keywords: Polydnavirus, TnBV, PI3K/Akt/TOR, ecdysteroidogenesis, prothoracic glands
Citation: Salvia R, Nardiello M, Scieuzo C, Scala A, Bufo SA, Rao A, Vogel H and Falabella P (2018) Novel Factors of Viral Origin Inhibit TOR Pathway Gene Expression. Front. Physiol. 9:1678. doi: 10.3389/fphys.2018.01678
Received: 02 June 2018; Accepted: 08 November 2018;
Published: 26 November 2018.
Edited by:
Guy Smagghe, Ghent University, BelgiumReviewed by:
Takashi Koyama, University of Copenhagen, DenmarkCopyright © 2018 Salvia, Nardiello, Scieuzo, Scala, Bufo, Rao, Vogel and Falabella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrizia Falabella, cGF0cml6aWEuZmFsYWJlbGxhQHVuaWJhcy5pdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.