
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol., 03 September 2018
Sec. Cardiac Electrophysiology
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.01226
 Gary Tse1,2
Gary Tse1,2 Mengqi Gong3
Mengqi Gong3 Lei Meng3
Lei Meng3 Cheuk W. Wong4
Cheuk W. Wong4 George Bazoukis5
George Bazoukis5 Matthew T. V. Chan6
Matthew T. V. Chan6 Martin C. S. Wong7
Martin C. S. Wong7 Konstantinos P. Letsas5
Konstantinos P. Letsas5 Adrian Baranchuk8
Adrian Baranchuk8 Gan-Xin Yan9,10
Gan-Xin Yan9,10 Tong Liu3*
Tong Liu3* William K. K. Wu6*
William K. K. Wu6*Background: Acquired QT interval prolongation has been linked with malignant ventricular arrhythmias, such as torsade de pointes, in turn predisposing to sudden cardiac death. Increased dispersion of repolarization has been identified as a pro-arrhythmic factor and can be observed as longer Tpeak – Tend interval and higher Tpeak – Tend/QT ratio on the electrocardiogram. However, the values of these repolarization indices for predicting adverse outcomes in this context have not been systematically evaluated.
Method: PubMed, Embase and Cochrane Library databases were searched until 14th February 2018, identifying 232 studies.
Results: Five studies on acquired QT prolongation met the inclusion criteria and 308 subjects with drug-induced LQTS patients (mean age: 66 ± 18 years old; 46% male) were included in this meta-analysis. Tpeak – Tend intervals were longer [mean difference [MD]: 76 ms, standard error [SE]: 26 ms, P = 0.003; I2 = 98%] and Tpeak – Tend/QT ratios were higher (MD: 0.14, SE: 0.03, P = 0.000; I2 = 29%) in patients with torsade de pointes compared to those without these events.
Conclusion: Tpeak – Tend interval and Tpeak – Tend/QT ratio were higher in patients with acquired QT prolongation suffering from torsade de pointes compared to those who did not. These repolarization indices may provide additional predictive value for identifying high-risk individuals.
Acquired prolongation of QT interval increases the risk of ventricular tachycardia/fibrillation (VT/VF) and sudden cardiac death (SCD). However, patients with prolonged QT intervals are heterogeneous in that only a subset develop these adverse events and risk stratification remains difficult. Of the different electrophysiological mechanisms, exacerbated repolarization gradient across the myocardial wall has been identified as an important factor of arrhythmogenesis in the context of QT prolongation, as it can lead to unidirectional block and reentrant activity (Opthof et al., 2007; Coronel et al., 2009; Choy et al., 2016). On the electrocardiogram, the dispersion of repolarization can be estimated using the Tpeak – Tend Interval, defined as the time difference between the peak and the end of the T-wave. However, this parameter varies with heart rate (Gupta et al., 2008) and normalization with QT interval (Tpeak – Tend/QT ratio) gives a relatively constant range of values between 0.17 and 0.23 (Gupta et al., 2008). Despite their strong physiological basis, the predictive value of both parameters in drug-induced QT prolongation remains controversial.
A recent meta-analysis reported that higher Tpeak – Tend interval was predictive of adverse events that include ventricular tachyarrhythmias and sudden cardiac death in congenital long QT syndrome, but it did not investigate its value in cases of acquired QT interval prolongation (Tse et al., 2017a). Nevertheless, several reports have investigated the differences in Tpeak – Tend interval and/or Tpeak – Tend / QT ratio between event-positive and event-negative subjects with acquired QT prolongation with some conflicting results. In this study, we performed a systematic review and meta-analysis to elucidate the relationship between these parameters and adverse events in this patient population.
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISM) statement (Moher et al., 2015). PubMed, Embase and Cochrane Library were searched for studies that investigated the association between Tpeak – Tend or Tpeak – Tend/QT with arrhythmic or mortality endpoints in long QT syndrome. The following search terms were used for all three databases: [“Tpeak–Tend” OR “Tp-Te” OR “Tpeak-end” OR “Tp-e” OR “T(peak)-T(end)” OR “T wave peak-to-end” OR “T peak-T end” OR “Tpe” “TPEc” OR “T-peak to T-end” OR “Tpeak-to-Tend” AND “long QT”]. The search period was from the beginning of the database through to 14th February 2018 without language restrictions. The following inclusion criteria were used: (i) the study was conducted in humans, (ii) Tpeak – Tend intervals or Tpeak – Tend/QT ratios between event-positive (ventricular tachycardia/fibrillation, or sudden cardiac death) and event-negative groups were compared.
The quality assessment of these studies included in our meta-analysis was performed using the Newcastle–Ottawa Quality Assessment Scale (NOS; Marshall et al., 2007). The details of the NOS quality assessment are shown in Supplementary Tables 1, 2. No studies were excluded because of the quality score.
Data from the different studies were entered in pre-specified spreadsheets in Microsoft Excel (Version 2016). All search entries were retrieved as complete manuscripts and assessed to determine whether they met the inclusion criteria. The extracted data elements consisted of: (i) publication details: last name of first author, publication year; (ii) study design; (iii) endpoint(s); (iv) the quality score; and (v) the characteristics of the population including sample size, gender, age and number of subjects. Two reviewers (GT and MG) reviewed each included study independently. Disagreements were resolved by consulting with a third reviewer (TL).
Mean differences with 95% confidence interval (CI) for Tpeak – Tend interval and Tpeak – Tend/QT ratio were extracted from each study and subsequently pooled. Heterogeneity between studies was determined using Cochran's Q-value, the weighted sum of squared differences between individual study effects and the pooled effect across studies, and I2 from the standard chi-square test, which describes the percentage of the variability in effect estimates resulting from heterogeneity. I2 > 50% was considered to reflect significant statistical heterogeneity. A fixed effects model was used if I2 < 50%, otherwise the random-effects model using the inverse variance heterogeneity method was used. To locate the origin of the heterogeneity, sensitivity analysis excluding one study at a time were performed. Funnel plots, Begg and Mazumdar rank correlation test and Egger's test were used to assess for possible publication bias.
A flow diagram of the search strategy is shown in Supplementary Figure 1. Our search retrieved 99 entries from PubMed, 116 entries from Embase and 17 from Cochrane Library. Of these, five studies were relevant for acquired LQTS and were included in our final meta-analysis (Yamaguchi et al., 2003; Topilski et al., 2007; Darbar et al., 2008; Couderc et al., 2010; Subbiah et al., 2010). A total of 308 patients were included (mean age: 66 ± 18 years old; 46% male). Table 1 shows the baseline characteristics of these studies and of the study populations.
The Tpeak – Tend intervals for event-negative (Figure 1A, top panel) and event-positive groups (Figure 1A, middle panel) were 96 ± 14 and 165 ± 15, respectively, with a significant mean difference of 76 ± 26 ms (P = 0.003, I2 = 98%) (Figure 1A, bottom panel). Moreover, the Tpeak – Tend/QT ratio for event-negative (Figure 1B, top panel) and event-positive (Figure 1B, middle panel) groups were 0.19 ± 0.01 and 0.29 ± 0.04, respectively, with a significant mean difference of 0.14 ± 0.03 (P < 0.0001; I2 = 29%) (Figure 1B, bottom panel). The results from bias analyses are shown in the Supplementary Material.
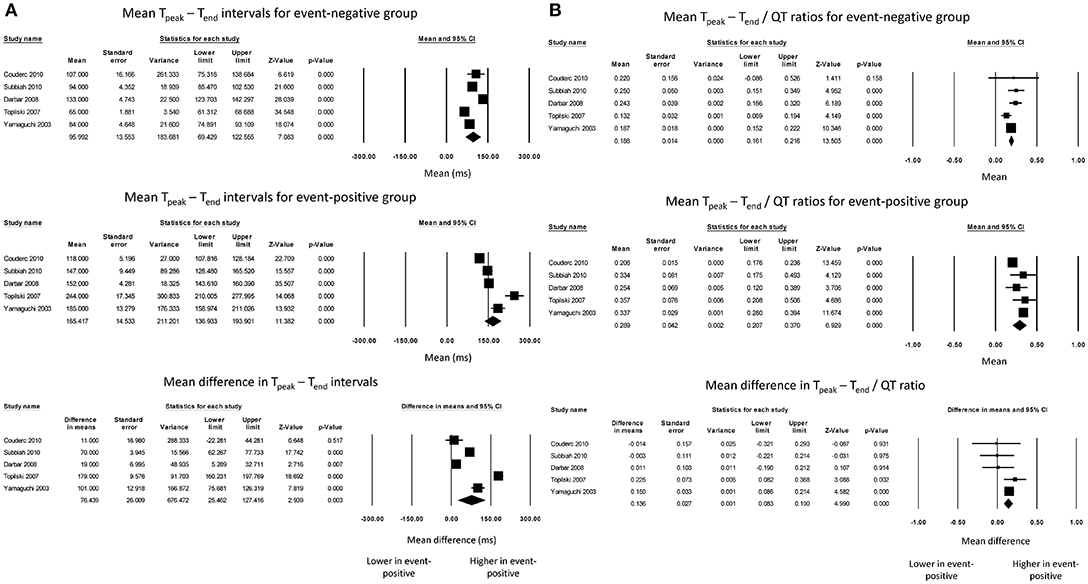
Figure 1. Forest plot demonstrating the (A) mean Tpeak – Tend intervals in the event-negative group (top panel), event-positive group (middle panel) and difference between both groups (bottom panel) in acquired QT prolongation, and (B) mean Tpeak – Tend/QT ratios in the event-negative group (top panel), event-positive group (middle panel) and difference between both groups (bottom panel) in acquired QT prolongation.
Subgroup analysis based on the cause of acquired LQTS was performed. A meta-analysis of three studies examining drug-induced LQTS found that Tpeak – Tend intervals for event-negative (Figure 2A, top panel) and event-positive groups (Figure 2A, middle panel) took values of 108 ± 19 and 149 ± 16, respectively. However, the mean difference of 44 ± 28 ms did not reach statistical significance (P = 0.12; I2 = 94%; Figure 2A, bottom panel). Tpeak – Tend / QT ratios took values of 0.20 ± 0.02 and 0.27 ± 0.05 for event-negative (Figure 2B, top panel) and event-positive (Figure 2B, middle panel) groups, respectively, with a significant mean difference between both groups (0.13 ± 0.03, P < 0.0001; I2 = 22%; Figure 2B, bottom panel).
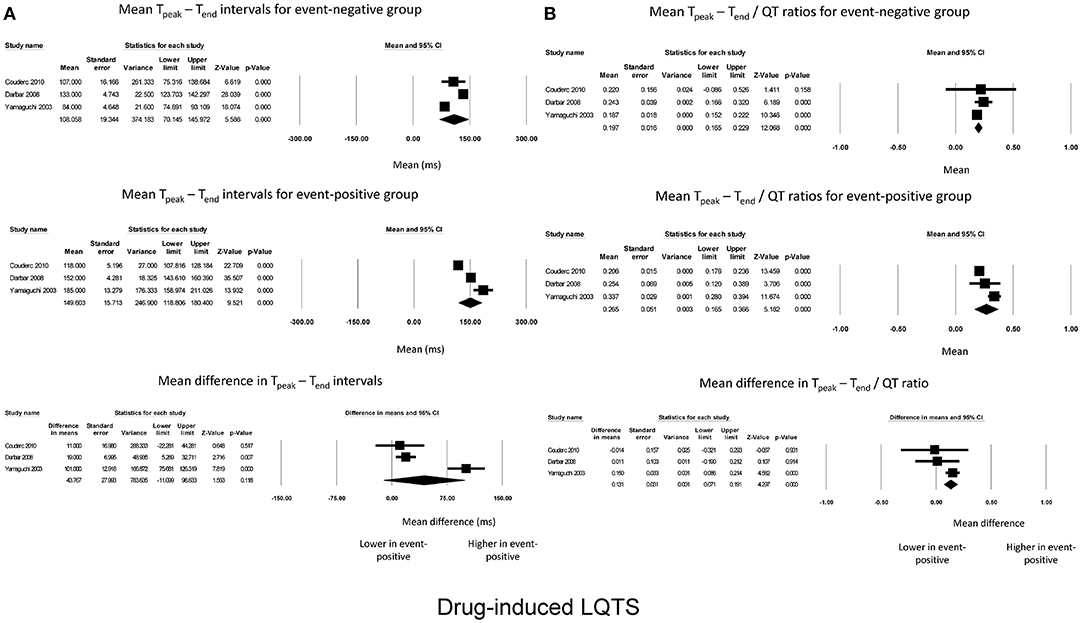
Figure 2. Forest plot demonstrating the (A) mean Tpeak – Tend intervals in the event-negative group (top panel), event-positive group (middle panel) and difference between both groups (bottom panel) in acquired QT prolongation, and (B) mean Tpeak – Tend/QT ratios in the event-negative group (top panel), event-positive group (middle panel) and difference between both groups (bottom panel) in drug-induced QT prolongation.
For AV block-induced LQTS, two studies were meta-analyzed. Tpeak – Tend intervals for event-negative (Figure 3A, top panel) and event-positive groups (Figure 3A, middle panel) were 79 ± 14 and 194 ± 49, respectively, leading to a significant mean difference of 124 ± 54 ms (P = 0.02; I2 = 99%; Figure 3A, bottom panel). By contrast, Tpeak – Tend / QT ratios took values of 0.18 ± 0.06 and 0.5 ± 0.06 for event-negative (Figure 3B, top panel) and event-positive (Figure 3B, middle panel) groups, respectively, but the mean difference between both groups was not significant (0.13 ± 0.11, P = 0.27; I2 = 66%; Figure 3B, bottom panel).
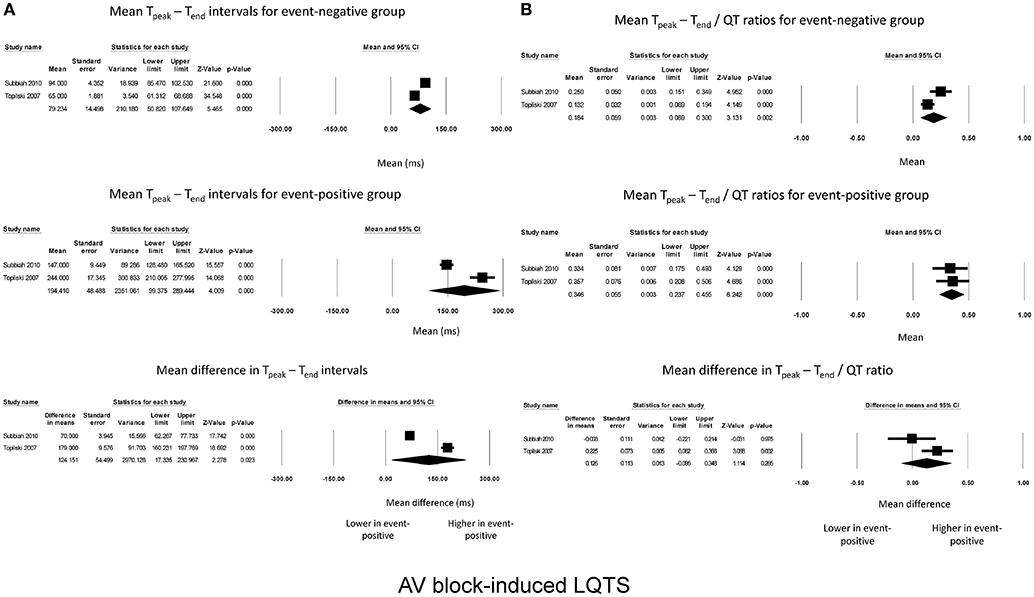
Figure 3. Forest plot demonstrating the (A) mean Tpeak – Tend intervals in the event-negative group (top panel), event-positive group (middle panel) and difference between both groups (bottom panel) in acquired QT prolongation, and (B) mean Tpeak – Tend/QT ratios in the event-negative group (top panel), event-positive group (middle panel) and difference between both groups (bottom panel) in AV block-induced QT prolongation.
Finally, QTc intervals for event-negative (Figure 4A, top panel) and event-positive groups (Figure 4A, middle panel) took values of 412 ± 104 and 594 ± 17, respectively, with a significant difference between both groups (180 ± 80 ms; P = 0.02; I2 = 99%; Figure 4A, bottom panel). Heart rates for event-negative (Figure 4B, top panel) and event-positive groups (Figure 4B, middle panel) took values of 56 ± 9 and 58 ± 9, respectively, with no significant mean difference between both groups (2 ± 1 ms; P = 0.26; I2 = 0%; Figure 4B, bottom panel).
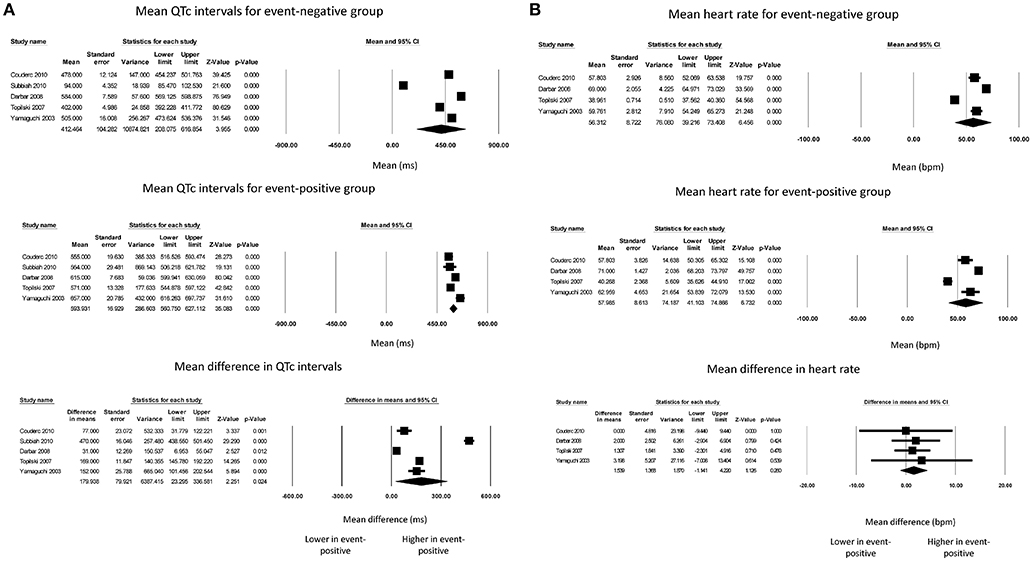
Figure 4. Forest plot demonstrating the (A) mean QTc intervals in the event-negative group (top panel), event-positive group (middle panel) and difference between both groups (bottom panel) in acquired QT prolongation, and (B) mean heart rate in the event-negative group (top panel), event-positive group (middle panel) and difference between both groups (bottom panel) in acquired QT prolongation.
The major findings of this systematic review and meta-analysis of 308 acquired LQTS subjects are that Tpeak – Tend intervals were longer by 76 ± 26 ms and Tpeak – Tend/QT ratios were higher by 0.14 ± 0.03 in those who exhibited adverse events compared with those who did not. QTc were also longer by 180 ± 80 ms but no difference in heart rate was observed.
Tpeak – Tend represent the global dispersion of repolarization (Xia et al., 2005) and its prolongation has been associated with arrhythmogenesis in conditions such as Brugada syndrome, heart failure, myocardial infarction (Tse et al., 2017a,b). Recently, our group conducted a meta-analysis of the predictive values of Tpeak – Tend and Tpeak – Tend / QT ratios in congenital LQTS (Tse et al., 2018). This study extends these findings by providing evidence that Tpeak – Tend intervals and Tpeak – Tend/QT ratios are valuable for risk stratification in acquired QT prolongation. Importantly, it shows that such have different risks of adverse outcomes, and that Tpeak – Tend intervals, Tpeak – Tend/QT ratios and QTc intervals can be used to identify those who are most at risk. Besides the studies included in this meta-analysis, a large retrospective study compared the outcomes between 293 acquired LQTS subjects and 542 subjects without LQTS (Yu et al., 2017). They found significantly longer QTC intervals associated with higher incidences of life-threatening ventricular arrhythmias as well as one-year mortality in the acquired LQTS group. However, there was no further risk stratification within the acquired LQTS group into survivors and non-survivors using the different ECG indices reported here. Further work is needed to examine whether Tpeak – Tend, Tpeak – Tend/QT ratio and QTc interval are significant predictors of arrhythmic and mortality events, and to compare their relative sensitivity and specificity.
In our meta-analysis, patients with acquired LQTS due to both drugs and complete AV block were included. Clearly, the mechanisms underlying QT prolongation in these respective groups are different. Thus, drugs that inhibit the outward potassium ion channels can lead to delayed ventricular repolarization, leading to action potential prolongation and lengthening of the QT interval. Whilst drug or metabolic causes of QT lengthening are more common, the original descriptions of torsade de pointes associated with QT prolongation by Dessertenne involved complete AV block (Dessertenne, 1966). The underlying causes may be dysfunction of more than one potassium ion channel (Topilski et al., 2007). Our subgroup analysis found that Tpeak – Tend/QT ratios, but not Tpeak – Tend intervals, were higher in event-positive subjects with drug-induced LQTS, whereas the converse was true for patients with AV block. However, this may be due to the small sample size and number of studies included.
In addition to QT, QTc, Tpeak – Tend intervals and Tpeak – Tend/QT ratio, additional ECG parameters have been proposed for risk stratification (Tse and Yan, 2016). Static markers such as JTPeak prolongation have been shown to predict adverse events in drug-induced LQTS beyond QTc (Johannesen et al., 2016). A new biomarker termed index of Cardiac Electrophysiological Balance (iCEB), an ECG surrogate of excitation wavelength (Tse, 2016), has been shown to predict ventricular arrhythmias associated with dofetilide-induced QT prolongation (Lu et al., 2013) and beyond (Robyns et al., 2016). Dynamic markers include T-wave alternans (TWA) (Zareba et al., 1994), microvolt TWAs (Takasugi et al., 2016), beat-to-beat variability in repolarization (Couderc et al., 2007, 2009; Hinterseer et al., 2008, 2009, 2010; Pueyo et al., 2011) and restitution indices (Nicolson et al., 2012, 2014), all of which have demonstrated predictability for arrhythmic outcomes. These dynamic markers may be provide additional value beyond QTc intervals, as illustrated by the findings that short-term variability in QT intervals was higher in drug-induced LQTS compared to controls, whereas QTc interval was not significantly different between these two groups (Hinterseer et al., 2008). It is unsurprising that variability in repolarization, rather than just the baseline repolarization properties, can further provide risk stratification because arrhythmias do not occur in every individual with prolonged QTc or Tpeak – Tend intervals. Instead, these dynamic factors can either initiate the arrhythmia or provide an additional abnormal substrate that further elevates the risk of re-entry (Thomsen et al., 2004; Tse et al., 2016b).
The following limitations should be recognized. Firstly, the heterogeneity was substantial in our meta-analysis of the mean difference for Tpeak – Tend intervals, with I2 taking a value of 98%. This compared to a low level of heterogeneity for Tpeak – Tend/QT ratios with I2 of 29%. The reason may be due to different methods for determining Tend, which significantly affects the values of Tpeak – Tend intervals obtained. Two studies used the tangent method by taking the intersection of a tangent to the steepest downslope of the dominant repolarization wave with the isoelectric line, whereas the remaining study used the point at which the T-wave intersected with the isoelectric line. By contrast, Tend cancels out in Tpeak – Tend/QT ratios as it appears in both the numerator and denominator, thereby reducing the influence of Tend on the calculated ratio. Secondly, considerable inter-observer variability is likely present because Tpeak – Tend were measured by investigators from different research groups. Thirdly, recall bias may have been present in the retrospective studies, which could have also contributed to some of the heterogeneity observed. Fourthly, the baseline characteristics of the included studies were different, in that one study examined outcomes in the pediatric population whereas two studies were on adult populations. Fifthly, electrolyte abnormalities, most frequently hypokalemia, can also lead to delayed repolarization and consequently QT lengthening (Lee et al., 2003; Hanton et al., 2007; Tse et al., 2016a,c). Thus, potassium levels can be a confounder in further altering the QT interval. Further studies should therefore specify the plasma K+ concentrations at the time of ECG measurements where possible. Finally, although traditionally prolonged QT intervals have been categorized into congenital and acquired causes, recent epidemiological studies reveal a complex relationship between genetics and the environment. Specifically, missense variants in ion channel genes such as the SCN5A that encode for the cardiac voltage-gated sodium channel, can potentiate the effects of hypokalaemia-induced QT prolongation (Akylbekova et al., 2014).
In the context of acquired QT prolongation, Tpeak – Tend intervals were longer and Tpeak – Tend / QT ratios were higher in patients showing adverse events than in those who did not suffer from these events. These repolarization indices may therefore be useful for risk stratification in this patient population.
GT: conception of study, data extraction and analysis, data interpretation, manuscript draft, critical revision of manuscript; MG: data extraction and analysis, data interpretation, manuscript draft, critical revision of manuscript; All authors: data interpretation, critical revision of manuscript; WW and TL: study supervision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
GT thanks the Croucher Foundation of Hong Kong for the support of their clinical assistant professorships.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.01226/full#supplementary-material
Akylbekova, E. L., Payne, J. P., Newton-Cheh, C., May, W. L., Fox, E. R., Wilson, J. G., et al. (2014). Gene-environment interaction between SCN5A-1103Y and hypokalemia influences QT interval prolongation in African Americans: the Jackson Heart Study. Am. Heart J. 167, 116–122 e111. doi: 10.1016/j.ahj.2013.10.009
Choy, L., Yeo, J. M., Tse, V., Chan, S. P., and Tse, G. (2016). Cardiac disease and arrhythmogenesis: Mechanistic insights from mouse models. Int. J. Cardiol. Heart Vasc. 12, 1–10. doi: 10.1016/j.ijcha.2016.05.005
Coronel, R., Wilms-Schopman, F. J., Opthof, T., and Janse, M. J. (2009). Dispersion of repolarization and arrhythmogenesis. Heart Rhythm 6, 537–543. doi: 10.1016/j.hrthm.2009.01.013
Couderc, J., Xia, J., Xu, X., Kaab, S., Hinteeser, M., and Zareba, W. (2010). Static and dynamic electrocardiographic patterns preceding torsades de pointes in the acquired and congenital long QT syndrome. Comput. Cardiol. 37, 357–360.
Couderc, J. P., Kaab, S., Hinterseer, M., Mcnitt, S., Xia, X., Fossa, A., et al. (2009). Baseline values and sotalol-induced changes of ventricular repolarization duration, heterogeneity, and instability in patients with a history of drug-induced torsades de pointes. J. Clin. Pharmacol. 49, 6–16. doi: 10.1177/0091270008325927
Couderc, J. P., Zareba, W., Mcnitt, S., Maison-Blanche, P., and Moss, A. J. (2007). Repolarization variability in the risk stratification of MADIT II patients. Europace 9, 717–723. doi: 10.1093/europace/eum131
Darbar, D., Kimbrough, J., Jawaid, A., Mccray, R., Ritchie, M. D., and Roden, D. M. (2008). Persistent atrial fibrillation is associated with reduced risk of torsades de pointes in patients with drug-induced long QT syndrome. J. Am. Coll. Cardiol. 51, 836–842. doi: 10.1016/j.jacc.2007.09.066
Dessertenne, F. (1966). Ventricular tachycardia with 2 variable opposing foci. Arch. Mal. Coeur Vaiss. 59, 263–272.
Gupta, P., Patel, C., Patel, H., Narayanaswamy, S., Malhotra, B., Green, J. T., et al. (2008). T(p-e)/QT ratio as an index of arrhythmogenesis. J. Electrocardiol. 41, 567–574. doi: 10.1016/j.jelectrocard.2008.07.016
Hanton, G., Yvon, A., Provost, J. P., Racaud, A., and Doubovetzky, M. (2007). Quantitative relationship between plasma potassium levels and QT interval in beagle dogs. Lab. Anim. 41, 204–217. doi: 10.1258/002367707780378050
Hinterseer, M., Beckmann, B. M., Thomsen, M. B., Pfeufer, A., Dalla Pozza, R., Loeff, M., et al. (2009). Relation of increased short-term variability of QT interval to congenital long-QT syndrome. Am. J. Cardiol. 103, 1244–1248. doi: 10.1016/j.amjcard.2009.01.011
Hinterseer, M., Beckmann, B. M., Thomsen, M. B., Pfeufer, A., Ulbrich, M., Sinner, M. F., et al. (2010). Usefulness of short-term variability of QT intervals as a predictor for electrical remodeling and proarrhythmia in patients with nonischemic heart failure. Am. J. Cardiol. 106, 216–220. doi: 10.1016/j.amjcard.2010.02.033
Hinterseer, M., Thomsen, M. B., Beckmann, B. M., Pfeufer, A., Schimpf, R., Wichmann, H. E., et al. (2008). Beat-to-beat variability of QT intervals is increased in patients with drug-induced long-QT syndrome: a case control pilot study. Eur. Heart J. 29, 185–190. doi: 10.1093/eurheartj/ehm586
Johannesen, L., Vicente, J., Mason, J. W., Erato, C., Sanabria, C., Waite-Labott, K., et al. (2016). Late sodium current block for drug-induced long QT syndrome: Results from a prospective clinical trial. Clin. Pharmacol. Therapeut. 99, 214–223. doi: 10.1002/cpt.205
Lee, S., Harris, N. D., Robinson, R. T., Yeoh, L., Macdonald, I. A., and Heller, S. R. (2003). Effects of adrenaline and potassium on QTc interval and QT dispersion in man. Eur. J. Clin. Invest. 33, 93–98. doi: 10.1046/j.1365-2362.2003.01123.x
Lu, H. R., Yan, G. X., and Gallacher, D. J. (2013). A new biomarker – index of Cardiac Electrophysiological Balance (iCEB) – plays an important role in drug-induced cardiac arrhythmias: beyond QT-prolongation and Torsades de Pointes (TdPs). J. Pharmacol. Toxicol. Methods. 68, 250–259. doi: 10.1016/j.vascn.2013.01.003
Marshall, S. C., Molnar, F., Man-Son-Hing, M., Blair, R., Brosseau, L., Finestone, H. M., et al. (2007). Predictors of driving ability following stroke: a systematic review. Top. Stroke Rehabil. 14, 98–114. doi: 10.1310/tsr1401-98
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4:1. doi: 10.1186/2046-4053-4-1
Nicolson, W. B., Mccann, G. P., Brown, P. D., Sandilands, A. J., Stafford, P. J., Schlindwein, F. S., et al. (2012). A novel surface electrocardiogram-based marker of ventricular arrhythmia risk in patients with ischemic cardiomyopathy. J. Am. Heart Assoc. 1:e001552. doi: 10.1161/JAHA.112.001552
Nicolson, W. B., Mccann, G. P., Smith, M. I., Sandilands, A. J., Stafford, P. J., Schlindwein, F. S., et al. (2014). Prospective evaluation of two novel ECG-based restitution biomarkers for prediction of sudden cardiac death risk in ischaemic cardiomyopathy. Heart 100, 1878–1885. doi: 10.1136/heartjnl-2014-305672
Opthof, T., Coronel, R., Wilms-Schopman, F. J., Plotnikov, A. N., Shlapakova, I. N., Danilo, P. Jr., et al. (2007). Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm 4, 341–348. doi: 10.1016/j.hrthm.2006.11.022
Pueyo, E., Corrias, A., Virág, L., Jost, N., Szél, T., Varró, A., et al. (2011). A multiscale investigation of repolarization variability and its role in cardiac arrhythmogenesis. Biophys. J. 101, 2892–2902. doi: 10.1016/j.bpj.2011.09.060
Robyns, T., Lu, H. R., Gallacher, D. J., Garweg, C., Ector, J., Willems, R., et al. (2016). Evaluation of index of cardio-electrophysiological balance (iCEB) as a new biomarker for the identification of patients at increased arrhythmic risk. Ann. Noninvas. Electrocardiol. 21, 294–304. doi: 10.1111/anec.12309
Subbiah, R. N., Gollob, M. H., Gula, L. J., Davies, R. W., Leong-Sit, P., Skanes, A. C., et al. (2010). Torsades de pointes during complete atrioventricular block: Genetic factors and electrocardiogram correlates. Can. J. Cardiol. 26, 208–212. doi: 10.1016/S0828-282X(10)70369-X
Takasugi, N., Goto, H., Takasugi, M., Verrier, R. L., Kuwahara, T., Kubota, T., et al. (2016). Prevalence of microvolt T-wave alternans in patients with long QT syndrome and its association with torsade de pointes. Circ. Arrhythm. Electrophysiol. 9:e003206. doi: 10.1161/CIRCEP.115.003206
Thomsen, M. B., Verduyn, S. C., Stengl, M., Beekman, J. D., De Pater, G., Van Opstal, J., et al. (2004). Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation 110, 2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8
Topilski, I., Rogowski, O., Rosso, R., Justo, D., Copperman, Y., Glikson, M., et al. (2007). The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J. Am. Coll. Cardiol. 49, 320–328. doi: 10.1016/j.jacc.2006.08.058
Tse, G. (2016). Both transmural dispersion of repolarization and of refractoriness are poor predictors of arrhythmogenicity: a role for iCEB (QT/QRS)? J. Geriatr. Cardiol. 13, 813–814. doi: 10.11909/j.issn.1671-5411.2016.09.007
Tse, G., Gong, M., Meng, L., Wong, C. W., Georgopoulos, S., Bazoukis, G., et al. (2018). Meta-analysis of Tpeak-Tend and Tpeak-Tend/QT ratio for risk stratification in congenital long QT syndrome. J. Electrocardiol. 51, 396–401. doi: 10.1016/j.jelectrocard.2018.03.001
Tse, G., Gong, M., Wong, W. T., Georgopoulos, S., Letsas, K. P., Vassiliou, V. S., et al. (2017a). The Tpeak - Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: A systematic review and meta-analysis. Heart Rhythm 14, 1131–1137. doi: 10.1016/j.hrthm.2017.05.031
Tse, G., Tse, V., and Yeo, J. M. (2016a). Ventricular anti-arrhythmic effects of heptanol in hypokalaemic, Langendorff-perfused mouse hearts. Biomed. Rep. 4, 313–324. doi: 10.3892/br.2016.577
Tse, G., Wong, C. W., Gong, M., Meng, L., Letsas, K. P., Li, G., et al. (2017b). Meta-analysis of T-wave indices for risk stratification in myocardial infarction. J. Geriat. Cardiol. 14, 776–779. doi: 10.11909/j.issn.1671-5411.2017.12.009
Tse, G., Wong, S. T., Tse, V., Lee, Y. T., Lin, H. Y., and Yeo, J. M. (2016b). Cardiac dynamics: alternans and arrhythmogenesis. J. Arrhythm. 32, 411–417. doi: 10.1016/j.joa.2016.02.009
Tse, G., Wong, S. T., Tse, V., and Yeo, J. M. (2016c). Restitution analysis of alternans using dynamic pacing and its comparison with S1S2 restitution in heptanol-treated, hypokalaemic Langendorff-perfused mouse hearts. Biomed. Rep. 4, 673–680. doi: 10.3892/br.2016.659
Tse, G., and Yan, B. P. (2016). Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace 9, 712–721. doi: 10.1093/europace/euw280.
Xia, Y., Liang, Y., Kongstad, O., Holm, M., Olsson, B., and Yuan, S. (2005). Tpeak-Tend interval as an index of global dispersion of ventricular repolarization: evaluations using monophasic action potential mapping of the epi- and endocardium in swine. J. Interv. Card. Electrophysiol. 14, 79–87. doi: 10.1007/s10840-005-4592-4
Yamaguchi, M., Shimizu, M., Ino, H., Terai, H., Uchiyama, K., Oe, K., et al. (2003). T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin. Sci. 105, 671–676. doi: 10.1042/CS20030010
Yu, H., Zhang, L., Liu, J., Liu, Y., Kowey, P. R., Zhang, Y., et al. (2017). Acquired long QT syndrome in hospitalized patients. Heart Rhythm 14, 974–978. doi: 10.1016/j.hrthm.2017.03.014
Keywords: Tpeak—Tend, Tpeak—Tend/QT, dispersion of repolarization, risk stratification, ventricular arrhythmia, sudden cardiac death
Citation: Tse G, Gong M, Meng L, Wong CW, Bazoukis G, Chan MTV, Wong MCS, Letsas KP, Baranchuk A, Yan G-X, Liu T and Wu WKK (2018) Predictive Value of Tpeak – Tend Indices for Adverse Outcomes in Acquired QT Prolongation: A Meta-Analysis. Front. Physiol. 9:1226. doi: 10.3389/fphys.2018.01226
Received: 14 May 2018; Accepted: 14 August 2018;
Published: 03 September 2018.
Edited by:
Zhilin Qu, University of California, Los Angeles, United StatesReviewed by:
Milan Stengl, Charles University, CzechiaCopyright © 2018 Tse, Gong, Meng, Wong, Bazoukis, Chan, Wong, Letsas, Baranchuk, Yan, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:Tong Liu, bGl1dG9uZ2RvY0AxMjYuY29t
William K. K. Wu, d3VrYWtlaUBjdWhrLmVkdS5oaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.