- 1Department of Nutrition, Dietetics, and Hospitality Management, Auburn University, Auburn, AL, United States
- 2Laboratory of Diabetes and Exercise Metabolism, Department of Physiology, Midwestern University, Glendale, AZ, United States
Skeletal muscle utilizes both free fatty acids (FFAs) and glucose that circulate in the blood stream. When blood glucose levels acutely increase, insulin stimulates muscle glucose uptake, oxidation, and glycogen synthesis. Under these conditions, skeletal muscle preferentially oxidizes glucose while the oxidation of fatty acids (FAs) oxidation is reciprocally decreased. In metabolic disorders associated with insulin resistance, such as diabetes and obesity, both glucose uptake, and utilization muscle are significantly reduced causing FA oxidation to provide the majority of ATP for metabolic processes and contraction. Although the causes of this metabolic inflexibility or disrupted “glucose-fatty acid cycle” are largely unknown, a diet high in fat and sugar (HFS) may be a contributing factor. This metabolic inflexibility observed in models of obesity or with HFS feeding is detrimental because high rates of FA oxidation in skeletal muscle can lead to the buildup of toxic metabolites of fat metabolism and the accumulation of pro-inflammatory cytokines, which further exacerbate the insulin resistance. Further, HFS leads to skeletal muscle atrophy with a decrease in myofibrillar proteins and phenotypically characterized by loss of muscle mass and strength. Overactivation of ubiquitin proteasome pathway, oxidative stress, myonuclear apoptosis, and mitochondrial dysfunction are some of the mechanisms involved in muscle atrophy induced by obesity or in mice fed with HFS. In this review, we will discuss how HFS diet negatively impacts the various physiological and metabolic mechanisms in skeletal muscle.
Introduction
Obesity is defined as an excess in adipose tissue mass that may have adverse consequences on health (Ofei, 2005). Obesity is a serious global public health concern in terms of contribution to human diseases. Obesity increases the likelihood of developing other serious conditions, including cardiovascular diseases, osteoporosis, certain cancers, sleep apnea, cognitive and behavioral disorders (Warburton et al., 2006). Obesity increases these risks both independently and in association with other diseases (Kopelman, 2000) and life expectancy is typically reduced in patients with obesity (Hurt et al., 2010). The hallmark of obesity is insulin resistance, which eventually increases the incidence of type 2 diabetes mellitus (T2DM), a metabolic disorder characterized by abnormal glucose homeostasis, elevated rates of lipolysis and cardiovascular disease (Kannel and McGee, 1979; Lieb et al., 2009). In obesity, excess adipose tissue leads to hyperleptinemia and leptin resistance as well as increased production of pro-inflammatory cytokines (Maffei et al., 1995; Marsh et al., 1999; Schwartz et al., 2000; Fantuzzi, 2005) resulting in decreases in whole body insulin sensitivity and disruption in appetite control and regulation of food intake (Hellstrom, 2013). These metabolic alterations depict the relationship between obesity and T2DM. Despite being a leading preventable through lifestyle modification, the unprecedented prevalence of T2DM during the last 10 years has stimulated a great deal of interest into the understanding of the complex mechanisms associated with obesity as well as finding solutions to prevent the progression of this public health crisis.
Over the past decade, research has revealed a variety of causative agents for insulin resistance and the main focus has been to recognize correlational studies in order to appropriately elucidate the underlying molecular mechanisms. Despite the causation, most of the current research considers insulin resistance in skeletal muscle to be integral to overall glucose metabolism and disposal (Keller and Attie, 2010). In studies comparing normal glucose tolerant myocytes to insulin resistant myocytes, insulin resistant myocytes demonstrate impaired post-insulin receptor mechanisms, deficient glucose transporter content (GLUT4) and signaling proteins as well as impaired glucose metabolism (Koves et al., 2007). There is no debate that insulin resistance has detrimental effects on cell function, but the major question involves the causation, not the effect, of the insulin resistance, and the most probable cause can be avoided by making healthier diet choices such as reducing the intake of energy-rich foods containing high in fat and sugar (HFS).
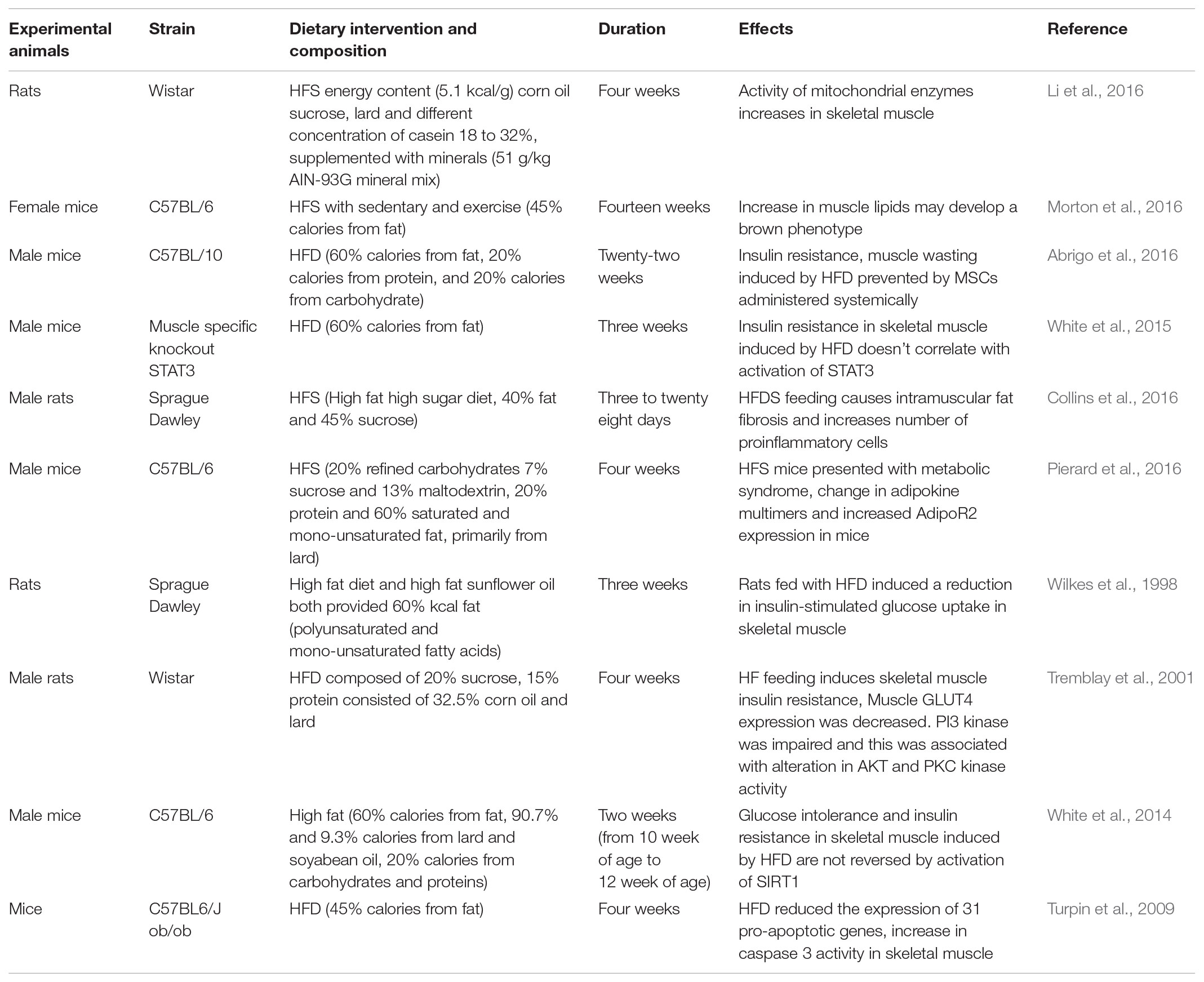
In skeletal muscle, free fatty acids (FFAs) increases protein kinase C (PKC) activation, which in turn, inhibit the metabolic insulin signaling pathways (Griffin et al., 1999; Itani et al., 2002). One mechanism behind this theory suggests changes in PKC, diacylglyerol (DAG), and IKB-Kinase (IκB-α) are induced by an increased concentration of FAs in the plasma membrane. This study provided many avenues explaining how lipids induce resistance, especially with correlations suggesting that PKC (activated by increased DAG concentrations) could cause insulin resistance by affecting the insulin receptor substrate (IRS) and other components of the insulin-signaling cascade. Similarly, another study examined how the effect of altered skeletal muscle lipase expression may actually cause the increase of accumulation of DAG and therefore disrupt insulin signaling. It is hypothesized that the imbalance between hormone sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) influences DAG accumulation and insulin resistance (Badin et al., 2011). Evidence suggests targeting of skeletal muscle lipases may be an effective approach to overcome resistance for insulin (Badin et al., 2011). Peripheral insulin resistance is reported in rodent models of spontaneous obesity, including leptin receptor-deficient Leprdb/db and leptin-deficient Lepob/ob mice, and the obese Zucker rat (Table 1). Depending on the age of these rodents, insulin resistance can result in hypertension and increasing the likelihood of a greater fibrocellular proliferation response to disruption in endothelial integrity of major vessels as well as to changes in the pattern in energy production, including a decrease in glucose oxidation (Kurtz et al., 1989; Tschöp and Heiman, 2001). Further, evidence based on fasting insulin levels in patents with impaired glucose tolerance indicates that insulin action is linked to both capillary blood supply to muscle and the muscle fiber characteristics (Lindgärde et al., 1982; Lillioja et al., 1987). Reduced capillary density in skeletal muscle is observed in obesity and this can limit the diffusion of insulin, the oxidative capacity of muscle fibers, and the tyrosine kinase activity of the insulin receptor, with the latter associated with muscle fiber type (James et al., 1986). Indeed, type I muscle fibers are associated with greater oxidative capacity, capillary density, and insulin sensitivity. Muscle biopsies obtained from overweight human patients show increase in type IIb muscle fibers and less type I muscle fibers as compared to humans having normal weight (Lillioja et al., 1987; Houmard et al., 2000). Type IIb fibers are less response to the effects of insulin on glucose uptake and is correlated with reduced capillary density and in the obese patient (Lillioja et al., 1987).
Effects of Obesity on Skeletal Muscle
Peripheral insulin signaling, ambulation, and glucose homeostasis are dependent on skeletal muscle mass. Obesity also leads to severe loss of skeletal muscle mass and function (Hilton et al., 2008) as a result of impaired regenerative function (Akhmedov and Berdeaux, 2013). Obesity-related loss of skeletal muscle creates a cycle of increasing glucose metabolism abnormalities with associated liver dysfunction and further exacerbating muscle loss (Kalyani et al., 2014). One possible mechanism explaining impaired regeneration of skeletal muscle in models of obesity is the involvement of the prolyl hydroxylase (PHD) domain family of enzymes that regulate vascular endothelial growth factor (VEGF) expression and hypoxia-inducible factor-1 alpha (HIF-1α) (Matsuura et al., 2013; Michailidou et al., 2015). Decreased protein expression of HIF-1α and VEGF is associated with decreased muscle endurance in wild type mice and impaired angiogenesis in the leptin-deficient obese diabetic mouse (Mace et al., 2007; Mason and Johnson, 2007), whereas upregulation of HIF-1α and VEGF promotes muscle regeneration (Beckman et al., 2013). Under normoxic conditions, PHD enzymes degrade HIF-1α and inhibit the hypoxia-response pathway (Appelhoff et al., 2004), while with hypoxia, PHD2, the most predominant isoform in skeletal muscle, is inactivated leading to increased activity of the hypoxia-response pathway (Lijkwan et al., 2014). To examine the role of PHD, HIF-1α, and VEGF on muscle regeneration in obesity, 5-month-old male C57Bl/6J mice were fed a HFD for a period of 16 weeks. As expected, these mice exhibited reduced muscle regenerative function, expressed as fiber cross sectional area, in response to muscle cryoinjury compared to mice fed a regular diet. Cryoinjured skeletal muscle from obese mice displayed increased protein expression of PHD2 and reduced VEGF expression, suggesting that prior HFD followed by cold stress impairs skeletal muscle regeneration. This regenerative defect induced by HFD was improved following inhibition of PHD2 using dimethyloxalyglycine (Sinha et al., 2017b).
In recent observations, impaired regeneration and reduction of AMPK activity occur in satellite cells isolated from injured muscles of DIO mice (Fu et al., 2016). These pathways were significantly impaired by insulin resistance associated with obesity and can variably inhibit muscle regeneration. In skeletal muscle, insulin growth factor-1 (IGF-1) is responsible is responsible for mediating insulin signaling pathway (Fuentes et al., 2011). Glycolytic, type II muscle fibers, mediated by Brg1/Brm-associated factor (Baf60c) in skeletal muscle undergoes a protective shift due to obesity (Meng et al., 2013, 2014; Brown et al., 2015). Skeletal muscle regeneration and maintenance has close relationship with obesity-associated liver dysfunction. Sarcopenia or degenerative loss of skeletal muscle is well-correlated with non-alcoholic fatty liver disease mostly observed in overweight individuals with no insulin resistance (Hong et al., 2014; Milić et al., 2014). Further, as leptin resistance is often reported in obesity and T2DM (Maffei et al., 1995), enervate the regeneration of skeletal muscle in the ob/ob and db/db mouse models of obesity, also suggests that leptin deficiency or resistance could contribute to poor muscle regeneration and satellite cell function (Nguyen et al., 2011).
Chronic, low-grade skeletal muscle inflammation is another key hallmark in obesity (Xu, 2013). Studies show that HFS feeding can trigger skeletal muscle and the liver to release an increased amount of pro-inflammatory cytokines (Park et al., 2004), including IL-6, IL-1-beta, and TNF-α. IL-6 is one of the main deleterious pro-inflammatory cytokines and overexpression of IL-6 can cause severe muscle atrophy (Park et al., 2004). In addition, skeletal muscle from obese individuals contains decreased amounts of mitochondrial content, leading to impaired energy production and skeletal muscle wasting (Greco et al., 1995; Brandenburg et al., 1999). In obese patients, skeletal muscle wasting can also be caused by reduced expression of AMPK. This key metabolic regulator increases GLUT4 expression and glucose metabolism and also has an important role in the up regulation of myogenin, myogenic factor 5, MyoD, and paired box protein 7 (Pax7) and which are important to muscle growth (Ridgeway and Skerjanc, 2001; Hernández-Hernández et al., 2017).
In addition, the obese state also exacerbates the effects of sarcopenia, a degenerative loss in muscle mass and function (Tomlinson et al., 2016). Obesity and sarcopenia act synergistically causing attenuation in muscle mass and elevation in accumulation of fat. The loss of muscle mass associated with sarcopenia may occur with the natural aging process (Moataz and Hamrick, 2010) or in the presence of obesity or with HF feeding (secondary sarcopenia) where the severity of muscle wasting is increased, leading to severe atrophy and a decrease in muscle strength. One of the main mechanisms involved with this form of sarcopenia is myostatin (growth/differentiation factor 8), which inhibits skeletal muscle cell growth (Moataz and Hamrick, 2010).
An important feature contributing to longevity is muscle mass (Srikanthan and Karlamangla, 2014). In the obese patient with sarcopenia (Stenholm et al., 2008), both intramuscular adipose accumulation and muscle fibrosis (Tardif et al., 2014) are observed. This can be explained, in part, to the accumulation of inflammatory cells and intramuscular fat observed at the onset of insulin resistance (Muoio, 2012). The effect of HFD on skeletal muscle mass in both humans and preclinical models is largely based on formation of inflammatory cells, mitochondrial dysfunction, metabolism, and regulation of glucose (Pagliassotti et al., 1994; Chicco et al., 2003; de Wilde et al., 2008; Fink et al., 2014; Jordy et al., 2014; Paglialunga et al., 2015) and on indirect observations of intramuscular lipid deposition (Fink et al., 2014). Furthermore, these studies were often conducted in the presence of obesity. Intramuscular lipid accumulation occurring chronically over time attenuates the anabolic properties of skeletal muscle protein and reduces its activity, which eventually leads to a significant reduction of long term integrity and dynamics of skeletal muscle (Tardif et al., 2014). Induction of obesity with HFS alters muscle integrity (Collins et al., 2016) and the early changes of morphology, protein synthesis, and function in skeletal muscle in obesity caused by HFD remain to be determined (Fink et al., 2014; Warren et al., 2014). One study reported that a lower muscle mass is associated with fewer insulin-responsive target tissues, which would promote insulin resistance and ultimately lead to obesity (Badin et al., 2011). This not only suggests that sarcopenia can potentially lead to obesity, but also explains how sarcopenia may promote a sedentary lifestyle for individuals prone to obesity, which in turn, leads to decreased physical activity and therefore increases the risk for obesity (Abdul-Ghani and DeFronzo, 2010).
Effects of HFS on Expression of Skeletal Muscle Proteins
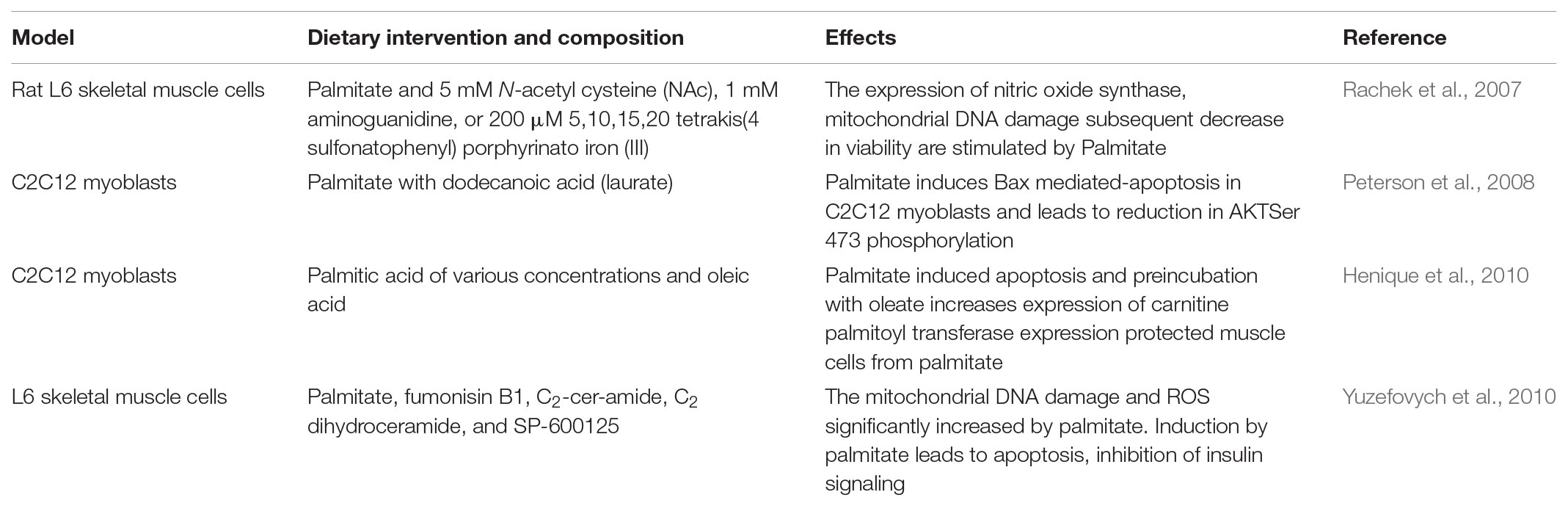
One of the most detrimental consequences of a HFS diet is muscle atrophy (Cabello-Verrugio et al., 2015). HFS diet induces over-activation of the pathway causing the increased expression of muscle ring finger protein 1 (MuRF-1), muscle atrophy F-box/atrogin-1, and muscle specific ubiquitin E3-ligase F-box proteins (Abrigo et al., 2016). Induction of skeletal muscle atrophy by HFS diet also occurs as a result of ubiquitin proteasome pathway (UPP) overactivation, increased oxidative stress and activation of myonuclear apoptosis (Sishi et al., 2011; Cabello-Verrugio et al., 2015). Elevated levels of toxic lipid intermediates from HFS promotes insulin and leptin resistance and increases the expression of pro-inflammatory cytokines, contributing to decreased regenerative capacity of skeletal muscle (Akhmedov and Berdeaux, 2013). In differentiated L6 muscle cells incubated with FAs or lipid metabolites, the expression of the myogenic transcription factor myogenin is markedly reduced (Mebarek et al., 2007). In addition, several studies show increased apoptosis in differentiated L6 and C2C12 muscle cells incubated either with the FA palmitate or silencing of stearoyl-CoA desaturase (SCD1) (Table 2) (Rachek et al., 2007; Peterson et al., 2008; Turpin et al., 2009; Henique et al., 2010; Yuzefovych et al., 2010). Acute triglyceride infusion in gastrocnemius muscle of mice results increase in FFAs, diacyl glycerol levels and apoptosis (Turpin et al., 2009). In gastrocnemius muscle of mice fed with HFS diet for 16 weeks, increase in caspase 3 activity is likely due to increase in oxidative stress, dysfunction of mitochondria and formation of reactive oxygen species (ROS) (Bonnard et al., 2008). Short-term (3 weeks). The impairment in regeneration of skeletal muscle due to injury by cold reduces the number of satellite cells in young mice fed with HFS (Woo et al., 2011). In a study by Nguyen et al. (2011), there was no significant impairment in the size of regenerative fibers of extensor digitorium longus muscle in mice fed with HFS for 12 weeks and injured with cardiotoxin. In transgenic mice over-expressing human lipoprotein lipase (LPL), FFA content is increased in gastrocnemius muscle during lipid overload. Transgenic mice develop severe myopathy from apoptosis and increase in degradation of protein (Levak-Frank et al., 1995; Tamilarasan et al., 2012). In skeletal muscle, diacylgycerol, and ceramides leads impairment of insulin-like growth factor (IGF)-1/Akt signaling directly or via JNK and IKKβ pathways ultimately leading to insulin resistance (Yu et al., 2002; Li et al., 2004; Samuel et al., 2010; Turban and Hajduch, 2011). Ceramides attenuates uptake of amino acids in L6 myotubes by reducing expression of sodium coupled membrane associated amino acid transporter SNAT2, protein synthesis and phosphorylation ribosomal protein S6 kinase beta-1 (p70S6) kinase (Hyde et al., 2005). Studies also have shown that the increase in apoptosis and reduction in AKT/mTOR phosphorylation correlates with upregulation of TNF-α receptors in skeletal muscle of rats fed with HFS diet (Sishi et al., 2011). Rats with HFS diet showed an increase in both gene and protein expression of musclin (Chen et al., 2017). Rhesus monkey fed with HFS induces a shift from slow to fast myosin heavy chain (MHC) in the plantaris, soleus, and digitorum longus muscles (Hyatt et al., 2016). In rodents and human muscle, HFS causes reduction in expression and percentage of fibers expressing type 1 MHC which suggests reduction in glucose tolerance, insulin sensitivity and overall oxidative capacity (Lillioja et al., 1987; Abou Mrad et al., 1992; Hickey et al., 1995; Kriketos et al., 1996) (Table 3). The oxidative characteristic of soleus muscle is generally fatigue resistant (Alford et al., 1987; Hodgson et al., 2001; Asmussen et al., 2003) and administration of HFS decreases sensory and motor conduction velocity in soleus muscle (Obrosova et al., 2007; Davidson et al., 2010; Guilford et al., 2011). Pathologically elevated levels of prolyl hydroxyl domain-2 in mice fed HFS drives impairment in muscle regeneration and limits VEGF expression (Sinha et al., 2017a).
High in fat and sugar obese mice exhibit a down regulation of pro-apoptotic genes when compared to lean mice (Ghosh et al., 2004). This suggests that the molecular adaptation to excess lipid overload by HFS suppresses pro-apoptotic genes which is critical in preventing lipo-apoptosis from occurring in skeletal muscle (Ghosh et al., 2004). Evidence suggests that in addition to apoptosis, HFS or palmitate treatment leads to the production of sphingosines and ceramides which is associated with glucose intolerance and insulin resistance (Chavez et al., 2005; Hu et al., 2009). In addition, skeletal muscle IKK and JNK activities are elevated in obese, diabetic, or HFS feeding mice (Hirosumi et al., 2002; Solinas et al., 2006). Effect of long-term HFS diet on skeletal muscle and calcium regulation were analyzed and a profound effect was observed on the expression levels of fast-TnT versus slow-TnT proteins in skeletal muscle. TnT proteins play a role in regulating the thin myofilament’s conformational changes during excitation–contraction coupling (Eshima et al., 2017). Long term HFS feeding for more than 4 weeks resulted in a decrease in the fast-TnT proteins (Eshima et al., 2017). There is also evidence to support that a decline in fast-TnT proteins causes a decrease in skeletal muscle contractile force. Mice fed with HFS also leads to overexpression of serine/threonine protein kinase 25 (STK25) which is associated with ectopic fat storage and decreased insulin responsiveness in skeletal muscle. These mice led to hyperinsulinemia and impaired total body insulin homeostasis (Chursa et al., 2017). This provides the first evidence supporting the claim that STK25 is involved in ectopic lipid formation and insulin responsiveness in skeletal muscle. The lipid formation found in this study was mostly intramyocellular lipids (IMCLs). Accumulation of IMCLs is associated with a decline in mitochondrial function, insulin resistance, and a decrease in exercise endurance by impairing the sacromeric ultrastructure in skeletal muscle (Chursa et al., 2017).
Effects of HFS Diet on Mitochondrial Function in Skeletal Muscle
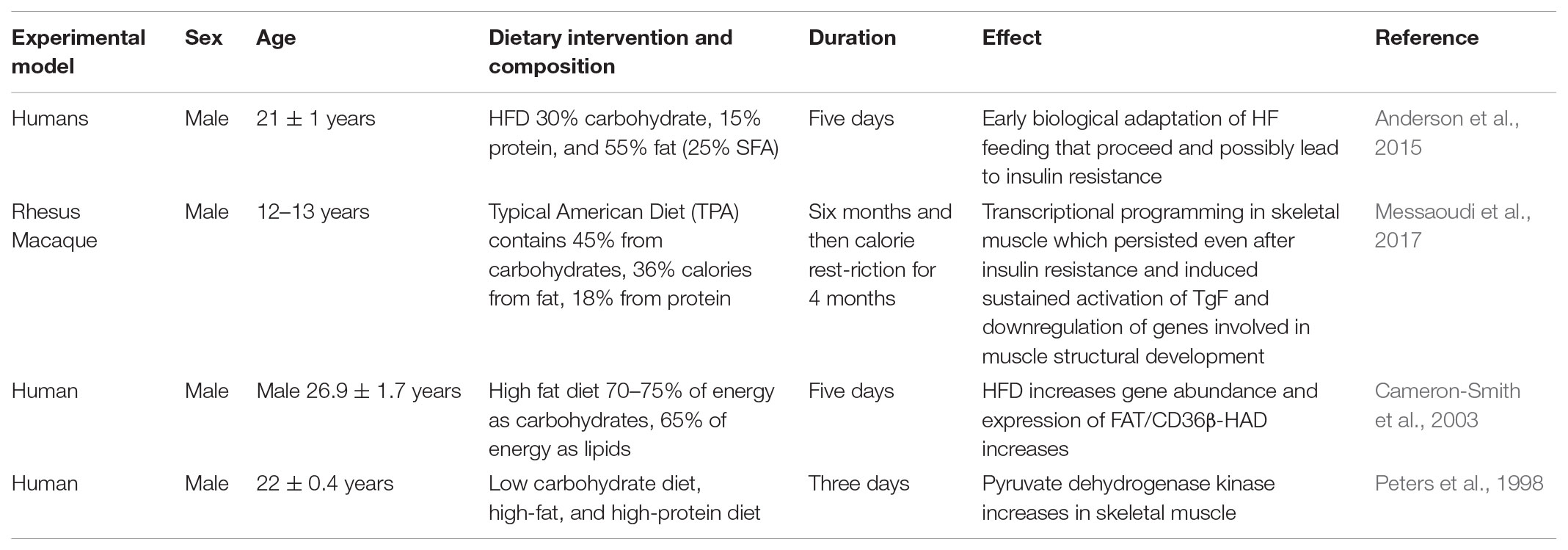
In addition to the changes in structural and contractile proteins in skeletal muscle, obesity, and insulin resistance are associated with reduced mitochondrial oxidative phosphorylation activity and decreased synthesis of genes involved in oxidative phosphorylation (Kelley et al., 2002). Several factors are involved in this decline in oxidative activity, including inherited genetics, physical inactivity, environmental and physiological factors. In elderly patients with insulin resistance, there is a reduction in mitochondrial protein synthesis (Guillet et al., 2004). Inducing insulin resistance by HFS feeding decreases state 3 ADP-dependent respiration in the presence of glutamate (complex 1-linked substrate) and consequently reducing overall ATP production (Chanseaume et al., 2006). Six weeks of HFS feeding also increases intramyocellular triglyceride content in soleus of Wistar rats and reduces superoxide anion radical production as a possible mitochondrial adaptation to excess energy intake against ROS (Chanseaume et al., 2006). In response to the same diet given to Wistar rats, de novo mitochondrial protein synthesis rates were increased in soleus, presumably to maintain mitochondrial protein function (Chanseaume et al., 2007) and compensate for the increased protein breakdown previously reported in the poorly controlled T2DM patient (Bell et al., 2006). In a similar study, to assess whether impaired ATP synthesis in mitochondria is associated with corresponding changes in resting muscle energetics, in vivo analysis of high-energy phosphate levels and flux was determined using 31P-nuclear magnetic resonance in diet-induced obese rats (Chanseaume et al., 2008). With HF and high sucrose feeding, muscle ATP content was increased whereas phosphocreatine (PCr) was decreased, resulting in a decrease in the PCr to ATP ratio and creatine content. However, rates of ATP exchange between PCr and γ-ATP were increased by creatine kinase, suggesting that increased catalytic activity of ATP synthesis may be a compensatory mechanism for impaired mitochondrial ATP production in the obese insulin-resistant rat (Chanseaume et al., 2008). Long term HFS feeding (16 weeks) decreased mitochondrial cyclooxygenase (COX1)/COX3 gene expression and citrate synthase activity in the oxidoglycolytic gastrocnemius muscle (Bonnard et al., 2008). These changes were induces dramatic decrease in the number of mitochondrial subsarcolemmal layer and intermyofibillar as well as in the expression of key genes involved in mitochondrial biogenesis, including PPARγ coactivator PGC1α and Mfn2. Unlike earlier studies (Chanseaume et al., 2007), in which a decrease in ROS activity was observed, 16 weeks of HFS increased the synthesis of the mitochondrial ROS markers uncoupling proteins 2 and 3 in response to elevated oxidative stress (Bonnard et al., 2008). In their study, Bonnard et al. (2008) observed that HFS feeding in mice induced a severe diabetic phenotype, characterized by hyperleptinemia and elevated levels of FFAs in plasma. Mitochondrial oxidative phosphorylation of complex 1-linked substrates (glutamate/malate) was decreased in state 3 respiration as well as in the presence of octanoyl- and palmitoyl-carnitine, demonstrating that β-oxidation is also reduced in the diet-induced diabetic mouse. Structurally, mitochondrial subpopulations exhibit swelling and disarrayed cristae to long-term HFS feeding (Bonnard et al., 2008). Since mitochondrial ROS production is implicated in insulin resistance, recent work has investigated whether targeting mitochondrial ROS production can alleviate insulin resistance by decreasing oxidative stress in muscle (Paglialunga et al., 2012). ROS sequestering can be accomplished by using the Skulachev ion (SkQ, plastoquinonyl decyltriphenylphosphonium), an oral mitochondrial-specific antioxidant that targets the inner membrane of mitochondria and scavenges ROS from complex I (Skulachev et al., 2009). As expected, mouse fed an obesogenic HFS Western diet developed the typical alterations in state 3 mitochondrial respiration as well as a reduction in maximum carbonyl cyanide p-trifluoromethoxyphenylhydrazone-induced respiration with pyruvate, indicative of maximum electron transport chain activity. ROS sequestering with SkQ had no effect with any given substrate (Paglialunga et al., 2012). However, with SkQ, oxidative stress was reversed but without an improvement muscle insulin sensitivity and insulin signaling as evaluated by pAkt and downstream phosphorylated GSK3. It appears that under these feeding conditions, insulin resistance and oxidative stress are indirectly related.
Myodegeneration
In inclusion body myositis (IBM), the degeneration of muscle fiber and inflammation of mononuclear-cell are the known pathological features, but their involvement in the etiology of this disorder is not clear (Askanas and Engel, 2006, 2007, 2008; Engel and Askanas, 2006; Askanas et al., 2009). The degenerative features involve deposition of misfolded, congophilic, ubiquitinated, and vacuolization of multiple protein aggregates in intramuscular fibers (Askanas and Engel, 2007, 2008; Askanas et al., 2009). Amyloid-beta (Aβ) and phospho-tau, which are the pathological hallmarks of Alzheimer’s disease (AD) accumulate during the progression of IBM (Askanas et al., 1993). Based on previous findings, increased formation of Aβ, particularly Aβ1-42, causes impairment in motor function in skeletal muscle of mouse models (Fukuchi et al., 1998; Jin et al., 1998; Sugarman et al., 2002; Kitazawa et al., 2006; Moussa et al., 2006). In addition to Aβ and phospho-tau, alpha-synuclein (α-Syn) ubiquitin, and apolipoprotein E acculate in muscle, highlighting common pathological parallels between AD and IBM (Albrecht and Bilbao, 1993; Askanas et al., 1994; Mirabella et al., 1996; Askanas and Engel, 2006). Recently, we found that a HFS diet in C57BL/6 mice leads to deposition of myostatin, inflammatory, and amyloid markers in skeletal muscle. In addition to Aβ deposition, there increased autophagy, ubiquitinated proteins, and deposition of misfolded protein in skeletal muscle of mice fed with HFS diet were observed (Rasool et al., unpublished).
Conclusion
High in fat and sugar diet promotes atrophy of skeletal muscle and induces protein degradation and peripheral inflammation. Prolonged HFS diet accelerates skeletal muscle atrophy, function, and impairs peripheral glucose transport. This implies that there is no relevant compensation in demand and supply of energy supply as evident by increase in weight and accelerated loss of muscle mass when atrophy is activated. Interestingly, attenuation in protein synthesis in response to obesity has been associated with insulin resistance caused by HFS. In addition, HFS also decreases the rate of ATP synthesis and the ability of muscle to respond to growth signals, which impairs recovery from injuries, accelerates the effects of aging, and adversely affects glucose homeostasis. HFS has a potential of inducing skeletal muscle atrophy and leads to phenotype of myositis or IBM.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the Auburn University start-up funds to TG, Alabama Agricultural Experimental Station, Hatch/Multistate Funding Program to JRB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdul-Ghani, M. A., and DeFronzo, R. A. (2010). Pathogenesis of Insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 476, 279–298. doi: 10.1155/2010/476279
Abou Mrad, J., Yakubu, F., Lin, D., Peters, J. C., Atkinson, J. B., and Hill, J. O. (1992). Skeletal muscle composition in dietary obesity-susceptible and dietary obesity-resistant rats. Am. J. Physiol. 262, 684–688. doi: 10.1152/ajpregu.1992.262.4.R684
Abrigo, J., Rivera, J. C., Aravena, J., Cabrera, D., Simon, F., Ezquer, F., et al. (2016). High fat diet induced skeletal muscle wasting is decreased by mesenchymal stem cells administration: implications on oxidative stress, ubiquitin proteasome pathway activation, and myonuclear apoptosis. Oxid. Med. Cell. Longev. 2016:9047821. doi: 10.1155/2016/9047821
Akhmedov, D., and Berdeaux, R. (2013). The effects of obesity on skeletal muscle regeneration. Front. Physiol. 17:371. doi: 10.3389/fphys.2013.00371
Albrecht, S., and Bilbao, J. M. (1993). Ubiquitin expression in inclusion body myositis. An immunohistochemical study. Arch. Pathol. Lab. Med. 117,789–793.
Alford, E. K., Roy, R. R., Hodgson, J. A., and Edgerton, V. R. (1987). Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hind limb suspension. Exp. Neurol. 96, 635–649. doi: 10.1016/0014-4886(87)90225-1
Anderson, A. S., Haynie, K. R., McMillan, R. P., Osterberg, K. L., Boutagy, N. E., Frisard, M. I., et al. (2015). Early skeletal muscle adaptations to short-term high-fat diet in humans before changes in insulin sensitivity. Obesity 23, 720–724. doi: 10.1002/oby.21031
Appelhoff, R. J., Tian, Y. M., Raval, R. R., Turley, H., Harris, A. L., Pugh, C. W., et al. (2004). Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279, 38458–38465. doi: 10.1074/jbc.M406026200
Askanas, V., Alvarez, R. B., and Engel, W. K. (1993). beta-Amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann. Neurol. 34, 551–560. doi: 10.1002/ana.410340408
Askanas, V., and Engel, W. K. (2006). Inclusion body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition. Neurology 66(2 Suppl. 1), S39–S48. doi: 10.1212/01.wnl.0000192128.13875.1e
Askanas, V., and Engel, W. K. (2007). Inclusion-body myositis, a multifactorial muscle disease associated with aging: current concepts of pathogenesis. Curr. Opin. Rheumatol. 19, 550–559. doi: 10.1097/BOR.0b013e3282efdc7c
Askanas, V., and Engel, W. K. (2008). Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer’s and Parkinson’s disease brains. Acta Neuropathol. 116, 583–595. doi: 10.1007/s00401-008-0449-0
Askanas, V., Engel, W. K., and Nogalska, A. (2009). Inclusion body myositis: a degenerative muscle disease associated with intra-muscle fiber multi-protein aggregates, proteasome inhibition, endoplasmic reticulum stress and decreased lysosomal degradation. Brain Pathol. 19, 493–506. doi: 10.1111/j.1750-3639.2009.00290.x
Askanas, V., Mirabella, M., Engel, W. K., Alvarez, R. B., and Weisgraber, K. H. (1994). Apolipoprotein E immunoreactive deposits in inclusion-body muscle diseases. Lancet 343, 364–365. doi: 10.1016/S0140-6736(94)91208-4
Asmussen, G., Schmalbruch, I., Soukup, T., and Pette, D. (2003). Contractile properties, fiber types, and myosin isoforms in fast and slow muscles of hyperactive Japanese waltzing mice. Exp. Neurol. 184, 758–766. doi: 10.1016/S0014-4886(03)00294-2
Badin, P. M., Louche, K., Mairal, A., Liebisch, G., Schmitz, G., and Rustan, A. C. (2011). Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans diabetes. 60, 1734–1742. doi: 10.2337/db10-1364
Beckman, S. A., Chen, W. C., Tang, Y., Proto, J. D., Mlakar, L., Wang, B., et al. (2013). Beneficial effect of mechanical stimulation on the regenerative potential of muscle-derived stem cells is lost by inhibiting vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 33, 2004–2012. doi: 10.1161/ATVBAHA.112.301166
Bell, J. A., Volpi, E., Fujita, S., Cadenas, J. G., Sheffield-Moore, M., and Rasmussen, B. B. (2006). Skeletal muscle protein anabolic response to increased energy and insulin is preserved in poorly controlled type 2 diabetes. J. Nutr. 136, 1249–1255. doi: 10.1093/jn/136.5.1249
Bonnard, C., Durand, A., Peyrol, S., Chanseaume, E., Chauvin, M. A., Morio, B., et al. (2008). Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Invest. 118, 789–800. doi: 10.1172/JCI32601
Brandenburg, S. L., Reusch, J. E., Bauer, T. A., Jeffers, B. W., Hiatt, W. R., and Regensteiner, J. G. (1999). Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care 1640–1646. doi: 10.2337/diacare.22.10.1640
Brown, L. A., Lee, D. E., Patton, J. F., Perry, R. A. Jr., Brown, J. L., Baum, J. I., et al. (2015). Dietinduced obesity alters anabolic signalling in mice at the onset of skeletal muscle regeneration. Acta Physiol. 251, 46–57. doi: 10.1111/apha.12537
Cabello-Verrugio, C., Morales, M. G., Rivera, J. C., Cabrera, D., and Simon, F. (2015). Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med. Res. Rev. 35, 437–463. doi: 10.1002/med.21343
Cameron-Smith, D., Burke, L. M., Angus, D. J., Tunstall, R. J., Cox, G. R., Bonen, A., et al. (2003). A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am. J. Clin. Nutr. 77, 313–318. doi: 10.1093/ajcn/77.2.313
Chanseaume, E., Bielicki, G., Tardy, A. L., Renou, J. P., Freyssenet, D., Boirie, Y., et al. (2008). Impaired resting muscle energetics studied by (31)P-NMR in diet-induced obese rats. Obesity 16, 572–577. doi: 10.1038/oby.2007.91
Chanseaume, E., Giraudet, C., Gryson, C., Walrand, S., Rousset, P., Boirie, Y., et al. (2007). Enhanced muscle mixed and mitochondrial protein synthesis rates after a high-fat or high-sucrose diet. Obesity 15, 853–859. doi: 10.1038/oby.2007.582
Chanseaume, E., Malpuech-Brugère, C., Patrac, V., Bielicki, G., Rousset, P., Couturier, K., et al. (2006). Diets high in sugar, fat, and energy induce muscle type-specific adaptations in mitochondrial functions in rats. J. Nutr. 136, 2194–2200. doi: 10.1093/jn/136.8.2194
Chavez, J. A., Holland, W. L., Bär, J., Sandhoff, K., and Summers, S. A. (2005). Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 280, 20148–20153. doi: 10.1074/jbc.M412769200
Chen, W. J., Liu, Y., Sui, Y. B., Yang, H. T., Chang, J. R., Tang, C. S., et al. (2017). Positive association between musclin and insulin resistance in obesity: evidence of a human study and an animal experiment. Nutr. Metab. 1014, 46–57. doi: 10.1186/s12986-017-0199-x
Chicco, A., D’Alessandro, M. E., Karabatas, L., Pastorale, C., Basabe, J. C., and Lombardo, Y. B. (2003). Muscle lipid metabolism and insulin secretion are altered in insulin-resistant rats fed a high sucrose diet. J. Nutr. 133, 127–133. doi: 10.1093/jn/133.1.127
Chursa, U., Nuñez-Durán, E., Cansby, E., Amrutkar, M., Sütt, S., Ståhlman, M., et al. (2017). Overexpression of protein kinase STK25 in mice exacerbates ectopic lipid accumulation, mitochondrial dysfunction and insulin resistance in skeletal muscle. Diabetologia 60, 553–567. doi: 10.1007/s00125-016-4171-5
Collins, K. H., Hart, D. A., Reimer, R. A., Seerattan, R. A., Waters-Banker, C., Sibole, S. C., et al. (2016). High-fat high-sucrose diet leads to dynamic structural and inflammatory alterations in the rat vastus lateralis muscle. J. Orthop. Res. 34, 2069–2078. doi: 10.1002/jor.23230
Davidson, E. P., Coppey, L. J., Calcutt, N. A., Oltman, C. L., and Yorek, M. A. (2010). Diet-induced obesity in Sprague-Dawley rats causes microvascular and neural dysfunction. Diabetes Metab. Res. Rev. 26, 306–318. doi: 10.1002/dmrr.1088
de Wilde, J., Mohren, R., van den Berg, S., Boekschoten, M., Dijk, K. W., de Groot, P., et al. (2008). Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiol. Genomics 32, 360–369. doi: 10.1152/physiolgenomics.00219.2007
Engel, W. K., and Askanas, V. (2006). Inclusion-body myositis: clinical, diagnostic, and pathologic aspects. Neurology 66, 20–29. doi: 10.1212/01.wnl.0000192260.33106.bb
Eshima, H., Tamura, Y., Kakehi, S., Kurebayashi, N., Murayama, T., Nakamura, K., et al. (2017). Long-term, but not short-term high fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol. Rep. 5:13250. doi: 10.14814/phy2.13250-13261
Fantuzzi, G. (2005). Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115, 911–919. doi: 10.1016/j.jaci.2005.02.023
Fink, L. N., Costford, S. R., Lee, Y. S., Jensen, T. E., Bilan, P. J., Oberbach, A., et al. (2014). Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity 3, 747–757. doi: 10.1002/oby.20615
Fu, X., Zhu, M., Zhang, S., Foretz, M., Viollet, B., and Du, M. (2016). Obesity impairs skeletal muscle regeneration through inhibition of AMPK. Diabetes Metab. Res. Rev. 65, 188–200. doi: 10.2337/db15-0647
Fuentes, E. N., Björnsson, B. T., Valdés, J. A., Einarsdottir, I. E., Lorca, B., Alvarez, M., et al. (2011). IGF-I/PI3K/Akt and IGF-I/MAPK/ERK pathways in vivo in skeletal muscle are regulated by nutrition and contribute to somatic growth in the fine flounder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1532–R1542. doi: 10.1152/ajpregu.00535.2010
Fukuchi, K., Pham, D., Hart, M., Li, L., and Lindsey, J. R. (1998). Amyloid-beta deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am. J. Pathol. 153, 1687–1693. doi: 10.1016/S0002-9440(10)65682-9
Ghosh, S., An, D., Pulinilkunnil, T., Qi, D., Lau, H. C., Abrahani, A., et al. (2004). Role of dietary fatty acids and acute hyperglycemia in modulating cardiac cell death. Nutrition 20, 916–923. doi: 10.1016/j.nut.2004.06.013
Greco, A. V., Tataranni, P. A., Mingrone, G., de Gaetano, A., Manto, A., Cotroneo, P., et al. (1995). Daily energy metabolism in patients with type 1 diabetes mellitus. J. Am. Coll. Nutr. 14, 286–291. doi: 10.1080/07315724.1995.10718509
Griffin, M. E., Marcucci, M. J., Cline, G. W., Bell, K., Barucci, N., Lee, D., et al. (1999). Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes Metab. Res. Rev. 48, 1270–1274. doi: 10.2337/diabetes.48.6.1270
Guilford, B. L., Ryals, J. M., and Wright, D. E. (2011). Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Exp. Diabetes Res. 848, 307–320. doi: 10.1155/2011/848307
Guillet, C., Prod’homme, M., Balage, M., Gachon, P., Giraudet, C., Morin, L., et al. (2004). Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 18, 1586–1587. doi: 10.1096/fj.03-1341fje
Hellstrom, P. M. (2013). Satiety signals and obesity. Curr. Opin. Gastroenterol. 29, 222–227. doi: 10.1097/MOG.0b013e32835d9ff8
Henique, C., Mansouri, A., Fumey, G., Lenoir, V., Girard, J., Bouillaud, F., et al. (2010). Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J. Biol. Chem. 285, 36818–36827. doi: 10.1074/jbc.M110.170431
Hernández-Hernández, J. M., García González, E. G., Brun, C. E., and Rudnicki, M. A. (2017). The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 72, 10–18. doi: 10.1016/j.semcdb.2017.11.010
Hickey, M. S., Carey, J. O., Azevedo, J. L., Houmard, J. A., Pories, W. J., Israel, R. G., et al. (1995). Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am. J. Physiol. 268, 453–457. doi: 10.1152/ajpendo.1995.268.3.E453
Hilton, T. N., Tuttle, L. J., Bohnert, K. L., Mueller, M. J., and Sinacore, D. R. (2008). Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys. Ther. 88, 1336–1344. doi: 10.2522/ptj.20080079
Hirosumi, J., Tuncman, G., Chang, L., Görgün, C. Z., Uysal, K. T., Maeda, K., et al. (2002). A central role for JNK in obesity and insulin resistance. Nature 420, 333–336. doi: 10.1038/nature01137
Hodgson, J. A., Wichayanuparp, S., Recktenwald, M. R., Roy, R. R., McCall, G., Day, M. K., et al. (2001). Circadian force and EMG activity in hindlimb muscles of rhesus monkeys. J. Neurophysiol. 86, 1430–1144. doi: 10.1152/jn.2001.86.3.1430
Hong, H. C., Hwang, S. Y., Choi, H. Y., Yoo, H. J., Seo, J. A., Kim, S. G., et al. (2014). Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 59, 1772–1778. doi: 10.1002/hep.26716
Houmard, J. A., Weidner, M. L., Koves, T. R., Hickner, R. C., and Cortright, R. L. (2000). Association between muscle fiber composition and blood pressure levels during exercise in men. Am. J. Hypertens. 13, 586–592. doi: 10.1016/S0895-7061(99)00259-9
Hu, W., Bielawski, J., Samad, F., Merrill, A. H. Jr., and Cowart, L. A. (2009). Palmitate increases sphingosine-1-phosphate in C2C12 myotubes via upregulation of sphingosine kinase message and activity. J. Lipid Res. 50, 1852–1862. doi: 10.1194/jlr.M800635-JLR200
Hurt, R. T., Kulisek, C., Buchanan, L. A., and McClave, S. A. (2010). The obesity epidemic: challenges, health initiatives and implications for gastroenterologists. Gastroenterol. Hepatol. 6, 780–792.
Hyatt, J. P., Nguyen, L., Hall, A. E., Huber, A. M., Kocan, J. C., Mattison, J. A., et al. (2016). Muscle specific myosin heavy chain shifts in response to a long term high fat/high sugar dietand resveratrol treatment in nonhuman primates. Front. Physiol. 7:77. doi: 10.3389/fphys.2016.00077
Hyde, R., Hajduch, E., Powell, D. J., Taylor, P. M., and Hundal, H. S. (2005). Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 19, 461–463. doi: 10.1096/fj.04-2284fje
Itani, S. I., Ruderman, N. B., Schmieder, F., and Boden, G. (2002). Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase, C., and IkappaB-alpha. Diabetes Metab. Res. Rev. 51, 2005–2011.
James, D. E., Zorzano, A., Böni-Schnetzler, M., Nemenoff, R. A., Powers, A., Pilch, P. F., et al. (1986). Intrinsic differences of insulin receptor kinase activity in red and white muscle. J. Biol. Chem. 261, 14939–14944.
Jin, L. W., Hearn, M. G., Ogburn, C. E., Dang, N., Nochlin, D., Ladiges, W. C., et al. (1998). Transgenic mice overexpressing the C-99 fragment of betaPP with an alpha-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am. J. Pathol. 153, 1679–1686. doi: 10.1016/S0002-9440(10)65681-7
Jordy, A. B., Serup, A. K., Karstoft, K., Pilegaard, H., Kiens, B., and Jeppesen, J. (2014). Insulin sensitivity is independent of lipid binding protein trafficking at the plasma membrane in human skeletal muscle: effect of a 3-day, high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, 1136–1145. doi: 10.1152/ajpregu.00124.2014
Kalyani, R. R., Corriere, M., and Ferrucci, L. (2014). Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 819–829. doi: 10.1016/S2213-8587(14)70034-8
Kannel, W. B., and McGee, D. L. (1979). Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 2, 120–126. doi: 10.2337/diacare.2.2.120
Keller, M. P., and Attie, A. D. (2010). Physiological insights gained from gene expression analysis in obesity and diabetes. Annu. Rev. Nutr. 30, 341–364. doi: 10.1146/annurev.nutr.012809.104747
Kelley, D. E., He, J., Menshikova, E. V., and Ritov, V. B. (2002). Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes Metab. Res. Rev. 51, 2944–2950. doi: 10.2337/diabetes.51.10.2944
Kitazawa, M., Green, K. N., Caccamo, A., and LaFerla, F. M. (2006). Genetically augmenting Abeta42 levels in skeletal muscle exacerbates inclusion body myositis-like pathology and motor deficits in transgenic mice. Am. J. Pathol. 168, 1986–1997. doi: 10.2353/ajpath.2006.051232
Koves, T. R., Ussher, J. R., Noland, R. C., Slentz, D., Mosedale, M., Ilkayeva, O., et al. (2007). Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7, 45–56. doi: 10.1016/j.cmet.2007.10.013
Kriketos, A. D., Pan, D. A., Lillioja, S., Cooney, G. J., Baur, L. A., Milner, M. R., et al. (1996). Interrelationships between muscle morphology, insulin action, and adiposity. Am. J. Physiol. 270, 1332–1339. doi: 10.1152/ajpregu.1996.270.6.R1332
Kurtz, T. W., Morris, R. C., and Pershadsingh, H. A. (1989). The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension 13, 896–901. doi: 10.1161/01.HYP.13.6.896
Levak-Frank, S., Radner, H., Walsh, A., Stollberger, R., Knipping, G., Hoefler, G., et al. (1995). Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J. Clin. Invest. 96, 976–986. doi: 10.1172/JCI118145
Li, X., Higashida, K., Kawamura, T., and Higuchi, M. (2016). Alternate-day high-fat diet induces an increase in mitochondrial enzyme activities and protein content in rat skeletal muscle. Nutrients 4, 203–212. doi: 10.3390/nu8040203
Li, Y., Soos, T. J., Li, X., Wu, J., Degennaro, M., Sun, X., et al. (2004). Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser (1101). J. Biol. Chem. 279, 45304–45307. doi: 10.1074/jbc.C400186200
Lieb, W., Sullivan, L. M., Harris, T. B., Roubenoff, R., Benjamin, E. J., Levy, D., et al. (2009). Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care 32, 612–616. doi: 10.2337/dc08-1596
Lijkwan, M. A., Hellingman, A. A., Bos, E. J., van der Bogt, K. E., Huang, M., Kooreman, N. G., et al. (2014). Short hairpin RNA gene silencing of prolyl hydroxylase-2 with a minicircle vector improves neovascularization of hindlimb ischemia. Hum. Gene Ther. 25, 41–49. doi: 10.1089/hum.2013.110
Lillioja, S., Young, A. A., Culter, C. L., Ivy, J. L., Abbott, W. G., Zawadzki, J. K., et al. (1987). Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J. Clin. Invest. 80, 415–424. doi: 10.1172/JCI113088
Lindgärde, F., Eriksson, K. F., Lithell, H., and Saltin, B. (1982). Coupling between dietary changes, reduced body weight, muscle fibre size and improved glucose tolerance in middle-aged men with impaired glucose tolerance. Acta Med. Scand. 212, 99–106. doi: 10.1111/j.0954-6820.1982.tb03179.x
Mace, K. A., Yu, D. H., Paydar, K. Z., Boudreau, N., and Young, D. M. (2007). Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 15, 636–645. doi: 10.1111/j.1524-475X.2007.00278.x
Maffei, M., Halaas, J., Ravussin, E., Pratley, R. E., Lee, G. H., Zhang, Y., et al. (1995). Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1, 1155–1161. doi: 10.1038/nm1195-1155
Marsh, D. J., Hollopeter, G., Huszar, D., Laufer, R., Yagaloff, K. A., Fisher, S. L., et al. (1999). Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat. Genet. 21, 119–122.
Mason, S., and Johnson, R. S. (2007). The role of HIF-1 in hypoxic response in the skeletal muscle. Adv. Exp. Med. Biol. 618, 229–244. doi: 10.1007/978-0-387-75434-5_18
Matsuura, H., Ichiki, T., Inoue, E., Nomura, M., Miyazaki, R., Hashimoto, T., et al. (2013). Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation 127, 2078–2087. doi: 10.1161/CIRCULATIONAHA.113.001742
Mebarek, S., Komati, H., Naro, F., Zeiller, C., Alvisi, M., Lagarde, M., et al. (2007). Inhibition of de novo ceramide synthesis upregulates phospholipase D and enhances myogenic differentiation. J. Cell Sci. 120, 407–416. doi: 10.1242/jcs.03331
Meng, Z. X., Li, S., Wang, L., Ko, H. J., Lee, Y., Jung, D. Y., et al. (2013). Baf60c drives glycolytic metabolism in the muscle and improves systemic glucose homeostasis through Deptor-mediated Akt activation. Nat. Med. 19, 640–645. doi: 10.1038/nm.3144
Meng, Z. X., Wang, L., Xiao, Y., and Lin, J. D. (2014). The Baf60c/Deptor pathway links skeletal muscle inflammation to glucose homeostasis in obesity. Diabetes Metab. Res. Rev. 63, 1533–1545. doi: 10.2337/db13-1061
Messaoudi, I., Handu, M., Rais, M., Sureshchandra, S., Park, B. S., Fei, S. S., et al. (2017). Long-lasting effect of obesity on skeletal muscle transcriptome. BMC Genomics 18:411. doi: 10.1186/s12864-017-3799-y
Michailidou, Z., Morton, N. M., Moreno Navarrete, J. M., West, C. C., Stewart, K. J., Fernández-Real, J. M., et al. (2015). Adipocyte pseudohypoxia suppresses lipolysis and facilitates benign adipose tissue expansion. Diabetes Metab. Res. Rev. 64, 733–745. doi: 10.2337/db14-0233
Milić, S., Lulić, D., and Štimac, D. (2014). Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J. Gastroenterol. 20, 9330–9337. doi: 10.3748/wjg.v20.i28.9330
Mirabella, M., Alvarez, R. B., Bilak, M., Engel, W. K., and Askanas, V. (1996). Difference in expression of phosphorylated tau epitopes between sporadic inclusion body myositis and hereditary inclusion-body myopathies. J. Neuropathol. Exp. Neurol. 55, 774–786. doi: 10.1097/00005072-199607000-00003
Moataz, N. E., and Hamrick, M. W. (2010). Myostatin (GDF-8) as a key factor linking muscle mass and skeletal form. J. Musculoskelet. Neuronal Interact. 10, 56–63.
Morton, T. L., Galior, K., McGrath, C., Wu, X., Uzer, G., Uzer, G. B., et al. (2016). Exercise increases and browns muscle lipid in high-fat diet-fed mice. Front. Endocrinol. 30:80. doi: 10.3389/fendo.2016.00080
Moussa, C. E., Fu, Q., Kumar, P., Shtifman, A., Lopez, J. R., Allen, P. D., et al. (2006). Transgenic expression of beta-APP in fast-twitch skeletal muscle leads to calcium dyshomeostasis and IBM-like pathology. FASEB J. 20, 2165–2167. doi: 10.1096/fj.06-5763fje
Muoio, D. M. (2012). Revisiting the connection between intramyocellular lipids and insulin resistance: a long and winding road. Diabetologia 55, 2551–2554. doi: 10.1007/s00125-012-2597-y
Nguyen, M. H., Cheng, M., and Koh, T. J. (2011). Impaired muscle regeneration in ob/ob and db/db mice. Sci. World J. 28, 1525–1535. doi: 10.1100/tsw.2011.137
Obrosova, I. G., Ilnytska, O., Lyzogubov, V. V., Pavlov, I. A., Mashtalir, N., Nadler, J. L., et al. (2007). High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes Metab. Res. Rev. 56, 2598–2608. doi: 10.2337/db06-1176
Paglialunga, S., Ludzki, A., Root-McCaig, J., and Holloway, G. P. (2015). In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia 58, 1071–1080. doi: 10.1007/s00125-015-3531-x
Paglialunga, S., van Bree, B., Bosma, M., Valdecantos, M. P., Amengual-Cladera, E., Jörgensen, J. A., et al. (2012). Targeting of mitochondrial reactive oxygen species production does not avert lipid-induced insulin resistance in muscle tissue from mice. Diabetologia 55, 2759–2768. doi: 10.1007/s00125-012-2626-x
Pagliassotti, M. J., Knobel, S. M., Shahrokhi, K. A., Manzo, A. M., and Hill, J. O. (1994). Time course of adaptation to a high-fat diet in obesityresistant and obesity-prone rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 267, 659–664. doi: 10.1152/ajpregu.1994.267.3.R659
Park, S. Y., Choi, G. H., Choi, H. I., Ryu, J., Jung, C. Y., and Lee, W. (2004). Depletion of mitochondrial DNA causes impaired glucose utilization and insulin resistance in L6 GLUT4myc myocytes. J. Biol. Chem. 280, 9855–9864. doi: 10.1074/jbc.M409399200
Peters, S. J., St Amand, T. A., Howlett, R. A., Heigenhauser, G. J., and Spriet, L. L. (1998). Human skeletal muscle pyruvate dehydrogenase kinase activity increases after a low-carbohydrate diet. Am. J. Physiol. 275, 980–986. doi: 10.1152/ajpendo.1998.275.6.E980
Peterson, J. M., Wang, Y., Bryner, R. W., Williamson, D. L., and Alway, S. E. (2008). Bax signaling regulates palmitate-mediated apoptosis in C(2) C(12) myotubes. Am. J. Physiol. Endocrinol. Metab. 295, E1307-E1314. doi: 10.1152/ajpendo.00738.2007
Pierard, M., Conotte, S., Tassin, A., Boutry, S., Uzureau, P., Boudjeltia, K. Z., et al. (2016). Interactions of exercise training and highfat diet on adiponectin forms and muscle receptors in mice. Nutr. Metab. 3, 75–87. doi: 10.1186/s12986-016-0138-2
Rachek, L. I., Musiyenko, S. I., Ledoux, S. P., and Wilson, G. L. (2007). Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology 148, 293–299. doi: 10.1210/en.2006-0998
Ridgeway, A. G., and Skerjanc, I. S. (2001). Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. J. Biol. Chem. 276, 19033–19039. doi: 10.1074/jbc.M011491200
Samuel, V. T., Petersen, K. F., and Shulman, G. I. (2010). Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375, 2267–2277. doi: 10.1016/S0140-6736(10)60408-4
Schwartz, M. W., Woods, S. C., Porte, D. Jr., Seeley, R. J., and Baskin, D. G. (2000). Central nervous system control of food intake. Nature 404, 661–671. doi: 10.1038/35007534
Sinha, I., Sakthivel, D., Olenchock, B. A., Kruse, C. R., Williams, J., Varon, D. E., et al. (2017a). Prolyl hydroxylase domain-2 inhibition improves skeletal muscle regeneration in a male murine model of obesity. Front. Endocrinol. 8:153. doi: 10.3389/fendo.2017.00153
Sinha, I., Sakthivel, D., and Varon, D. E. (2017b). Systemic regulators of skeletal muscle regeneration in obesity. Front. Endocrinol. 16:29. doi: 10.3389/fendo.2017.00029
Sishi, B., Loos, B., Ellis, B., Smith, W., du Toit, E. F., and Engelbrecht, A. M. (2011). Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp. Physiol. 96, 179–193. doi: 10.1113/expphysiol.2010.054189
Skulachev, V. P., Anisimov, V. N., Antonenko, Y. N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., et al. (2009). An attempt to prevent senescence: a mitochondrial approach. Biochim. Biophys. Acta 1787, 437–461. doi: 10.1016/j.bbabio.2008.12.008
Solinas, G., Naugler, W., Galimi, F., Lee, M. S., and Karin, M. (2006). Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc. Natl. Acad. Sci. U.S.A. 103, 16454–16459. doi: 10.1073/pnas.0607626103
Srikanthan, P., and Karlamangla, A. S. (2014). Muscle mass index as a predictor of longevity in older adults. Am. J. Med. 127, 547–553. doi: 10.1016/j.amjmed.2014.02.007
Stenholm, S., Harris, T. B., Rantanen, T., Visser, M., Kritchevsky, S. B., and Ferrucci, L. (2008). Sarcopenic obesity: definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 11, 6693–6700. doi: 10.1097/MCO.0b013e328312c37d
Sugarman, M. C., Yamasaki, T. R., Oddo, S., Echegoyen, J. C., Murphy, M. P., Golde, T. E., et al. (2002). Inclusion body myositis-like phenotype induced by transgenic overexpression of beta APP in skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 99, 6334–6339. doi: 10.1073/pnas.082545599
Tamilarasan, K. P., Temmel, H., Das, S. K., Al Zoughbi, W., Schauer, S., Vesely, P. W., et al. (2012). Skeletal muscle damage and impaired regeneration due to LPL mediated lipotoxicity. Cell Death Dis. 19, 354–361. doi: 10.1038/cddis.2012.91
Tardif, N., Salles, J., Guillet, C., Tordjman, J., Reggio, S., Landrier, J. F., et al. (2014). Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2α activation. Aging Cell 13, 1001–1011. doi: 10.1111/acel.12263
Tomlinson, D. J., Erskine, R. M., Morse, C. I., Winwood, K., and Onambélé-Pearson, G. (2016). The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 17, 467–483. doi: 10.1007/s10522-015-9626-4
Tremblay, F., white Lavigne, C., Jacques, H., and Marette, A. (2001). Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (zeta/lambda) activities. Diabetes 50, 1901–1910. doi: 10.2337/diabetes.50.8.1901
Tschöp, M., and Heiman, M. L. (2001). Rodent obesity models: an overview. Exp. Clin. Endocrinol. Diabetes 109, 307–319. doi: 10.1055/s-2001-17297
Turban, S., and Hajduch, E. (2011). Protein kinase C isoforms: mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Lett. 585, 269–274. doi: 10.1016/j.febslet.2010.12.022
Turpin, S. M., Ryall, J. G., Southgate, R., Darby, I., Hevener, A. L., Febbraio, M. A., et al. (2009). Examination of ‘lipotoxicity’ in skeletal muscle of high-fat fed and ob/ob mice. J. Physiol. 587, 1593–1605. doi: 10.1113/jphysiol.2008.166033
Warburton, D. E., Nicol, C. W., and Bredin, S. S. (2006). Health benefits of physical activity: the evidence. CMAJ 174, 801–809. doi: 10.1503/cmaj.051351
Warren, B. E., Lou, P. H., Lucchinetti, E., Zhang, L., Clanachan, A. S., Affolter, A., et al. (2014). Early mitochondrial dysfunction in glycolytic muscle, but not oxidative muscle, of the fructose-fed insulin resistant rat. Am. J. Physiol. Endocrinol. Metab. 306, 658–667. doi: 10.1152/ajpendo.00511.2013
White, A. T., LaBarge, S. A., McCurdy, C. E., and Schenk, S. (2015). Knockout of STAT3 in skeletal muscle does not prevent high-fat diet-induced insulin resistance. Mol. Metab. 4, 569–575. doi: 10.1016/j.molmet.2015.05.001
White, A. T., Philp, A., Fridolfsson, H. N., Schilling, J. M., Murphy, A. N., Hamilton, D. L., et al. (2014). High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am. J. Physiol. Endocrinol. Metab. 307, 764–772. doi: 10.1152/ajpendo.00001.2014
Wilkes, J. J., Bonen, A., and Bell, R. C. (1998). A modified high-fat diet induces insulin resistance in rat skeletal muscle but not adipocytes. Am. J. Physiol. 275, 679–686. doi: 10.1152/ajpendo.1998.275.4.E679
Woo, M., Isganaitis, E., Cerletti, M., Fitzpatrick, C., Wagers, A. J., Jimenez Chillaron, J., et al. (2011). Early life nutrition modulates muscle stem cell number: implications for muscle mass and repair. Stem Cells Dev. 20,1763–1769. doi: 10.1089/scd.2010.0349
Xu, H. (2013). Obesity and metabolic inflammation. Drug Discov. Today Dis. Mech. 21–25. doi: 10.1016/j.ddmec.2013.03.006
Yu, C., Chen, Y., Cline, G. W., Zhang, D., Zong, H., Wang, Y., et al. (2002). Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277, 50230–50236. doi: 10.1074/jbc.M200958200
Keywords: high fat diet, high sugar, skeletal muscle, myodegeneration, myostatin
Citation: Rasool S, Geetha T, Broderick TL and Babu JR (2018) High Fat With High Sucrose Diet Leads to Obesity and Induces Myodegeneration. Front. Physiol. 9:1054. doi: 10.3389/fphys.2018.01054
Received: 13 December 2017; Accepted: 16 July 2018;
Published: 05 September 2018.
Edited by:
Elisabeth Lambert, Swinburne University of Technology, AustraliaReviewed by:
Alina Maloyan, Oregon Health & Science University, United StatesThomas J. Hawke, McMaster University, Canada
Adolfo Andrade-Cetto, Universidad Nacional Autónoma de México, Mexico
Copyright © 2018 Rasool, Geetha, Broderick and Babu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thangiah Geetha, dGhhbmdnZUBhdWJ1cm4uZWR1 Jeganathan R. Babu, amVnYW5yYkBhdWJ1cm4uZWR1
 Suhail Rasool
Suhail Rasool Thangiah Geetha
Thangiah Geetha Tom L. Broderick
Tom L. Broderick Jeganathan R. Babu
Jeganathan R. Babu