- 1Department of Stomatology, Guangzhou Hospital of Integrated Traditional and West Medicine, Guangzhou, China
- 2Center for Evidence-based and Translational Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
- 3Department of Gynecology, Guangdong Women and Children Hospital, Guangzhou, China
Objectives: TP53 is an important tumor suppressor gene to maintain genomic integrity, and its mutations increase the susceptibility to oral carcinoma. Previous published studies have reported the relation of TP53 codon 72 polymorphism with the risk of oral carcinoma, but the results remain controversial and inconclusive.
Methods: We therefore utilized meta-analysis based on a comprehensive search in PubMed, EMBASE, and Google of Scholar databases up to August 19, 2017.
Results: Total 3,525 cases and 3,712 controls from 21 case-control studies were selected. We found no significant association between TP53 codon 72 polymorphism and oral carcinoma susceptibility in all genetic contrast models, including subgroup analysis based on control source and ethnicity. Furthermore, TP53 codon 72 polymorphism was not significant associated with oral carcinoma susceptibility in tobacco or alcohol use, and HPV infection status. Our results were confirmed by sensitivity analysis and no publication bias was found.
Conclusions: Taken together, our data indicate that TP53 codon 72 polymorphism is not associated with the susceptibility to oral carcinoma.
Introduction
Based on the GLOBOCAN2012 investigations, oral carcinoma is regarded as one of the most common causes of cancer related morbidity and mortality, contributing to 3.8% of all cancer cases and 3.6% of cancer related deaths (Warnakulasuriya, 2009; Ferlay et al., 2015; Shield et al., 2017). The long-term survival rate of oral carcinoma is < 50% despite improved treatment schedules such as surgery, radiation and chemotherapy (Coleman et al., 2008; De Angelis et al., 2014). Oral carcinoma is highly associated with tobacco smoking, alcohol consumption and the exposure to a variety of exogenous or endogenous carcinogens (Petti, 2009). However, the etiology of oral carcinoma remains poorly understood. Furthermore, not all individuals exposing to these risk factors are subject to oral carcinoma, and additional genetic factors may also contribute to oral carcinoma susceptibility (Chen et al., 2010; Anantharaman et al., 2011; Niu et al., 2012).
The human TP53 is well-known tumor suppressor gene and plays an important role in DNA damage response by inducing cell cycle arrest or apoptosis (Slee et al., 2004; Harris and Levine, 2005). TP53 mutation is frequently found in human tumors, including oral carcinoma (Olivier et al., 2002). A common single nucleotide polymorphism at TP53 codon 72 is crucial for its tumor suppressor function (Suzuki and Matsubara, 2011). Several meta-analysis demonstrated that TP53 codon 72 polymorphism was associated with the susceptibility to a variety of cancers, such as colorectal cancer (Du et al., 2017), esophageal cancer (Steccanella et al., 2017), nasopharyngeal cancer (Zhuo et al., 2009a), and non-Hodgkin lymphomas (Xu et al., 2017).
Själander et al. demonstrated that the distribution of TP53 genotypes differed among different ethnicities, which is a notable confounding factor in carcinoma risk (Själander et al., 1995). Tobacco and alcohol use are known risk factors for oral carcinoma (Hashibe et al., 2007). In addition, TP53 codon 72 mutation spectrum has been shown to be altered with Human papillomavirus (HPV) infection, an emerging oral carcinoma risk factor (Chor et al., 2016). So far many case-control studies investigated the association of functional polymorphism of TP53 codon 72 with susceptibility to oral carcinoma, but the results remain conflicting and inconclusive (Tandle et al., 2001; Nagpal et al., 2002; Hsieh et al., 2005; Wang et al., 2012; Saleem et al., 2013). We therefore conducted this meta-analyses to evaluate the relationship of TP53 codon 72 polymorphism with tobacco and/or alcohol use and HPV infection in the susceptibility to oral carcinoma.
Materials and Methods
Search Strategy
PubMed, EMBASE, and Google of Scholar databases up to August 19, 2017 were searched with a combination of the keywords as follows: [(oral OR tongue OR mouth OR buccal OR oropharynx) AND (tumor OR carcinoma OR cancer) AND (TP53 OR P53 OR Arg72Pro) AND (variant* OR mutation OR polymorphism*)].
Inclusion and Exclusion Criteria
Inclusion criteria were: (i) evaluated the association between tobacco and/or alcohol uses, TP53 codon 72 polymorphism, HPV infection, and susceptibility to oral carcinoma; (ii) case-control researches published in English or Chinese; (iii) definite histopathologic diagnosis or clearly reported the type; (iv) sufficient data to evaluate the ORs and 95%CI, and P-value; (v) genotype distribution was in Hardy-Weinberg equilibrium (HWE). Major exclusion criteria were: (i) Reviews, conference abstracts, case reports; (ii) only-case study; (iii) genotype distribution was inconsistent with HWE; (iv) when duplicated studies published, only the study with the large sample size was included.
Data Extraction
Data extraction was performed independently by two authors using a standardized form. Data such as: first author, country, ethnicity, year of publication, source of the controls, genotype distribution of cases and controls. Discrepancies were settled by discussion, with disagreements resolved by consensus.
Statistical Analysis
The association was determined by calculating odds ratios (ORs) with corresponding 95% credible interval (95%CI). Q-test and I2 statistics were used to quantify statistical heterogeneity. The random-effect model was conducted if the heterogeneity was significant (P < 0.05) (DerSimonian and Laird, 1986); otherwise, the fixed effect model was utilized (Mantel and Haenszel, 1959). The sensitivity analysis was carried out through sequential exclusion of any one individual study. Begg's funnel plot and the Egger's test was performed to assess the potential publication bias of the researches (Begg and Mazumdar, 1994; Egger et al., 1997). The present meta-analysis was carried out by STATA 12.0 (Stata, College Station, TX, USA). P < 0.05 was considered statistically significant.
Results
Characteristics of the Selected Studies
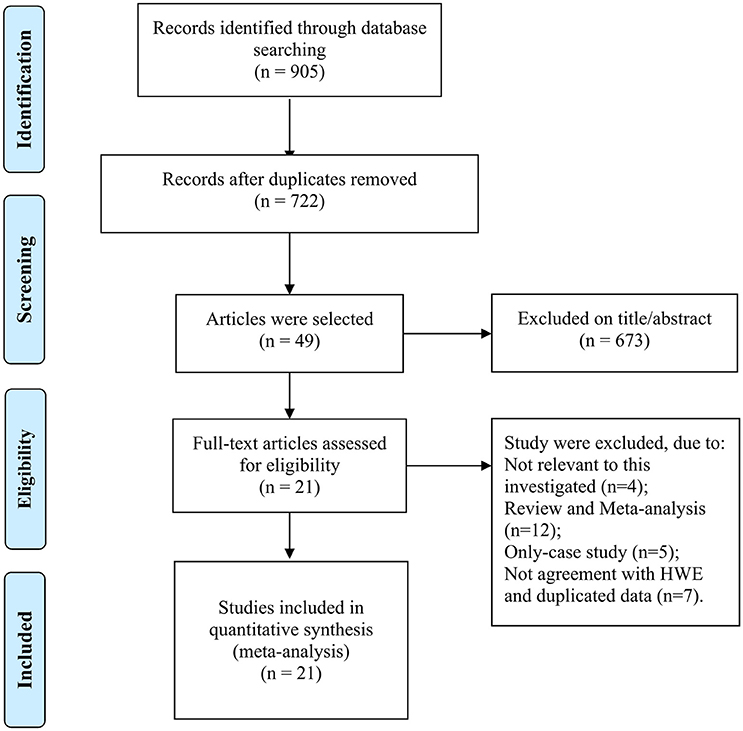
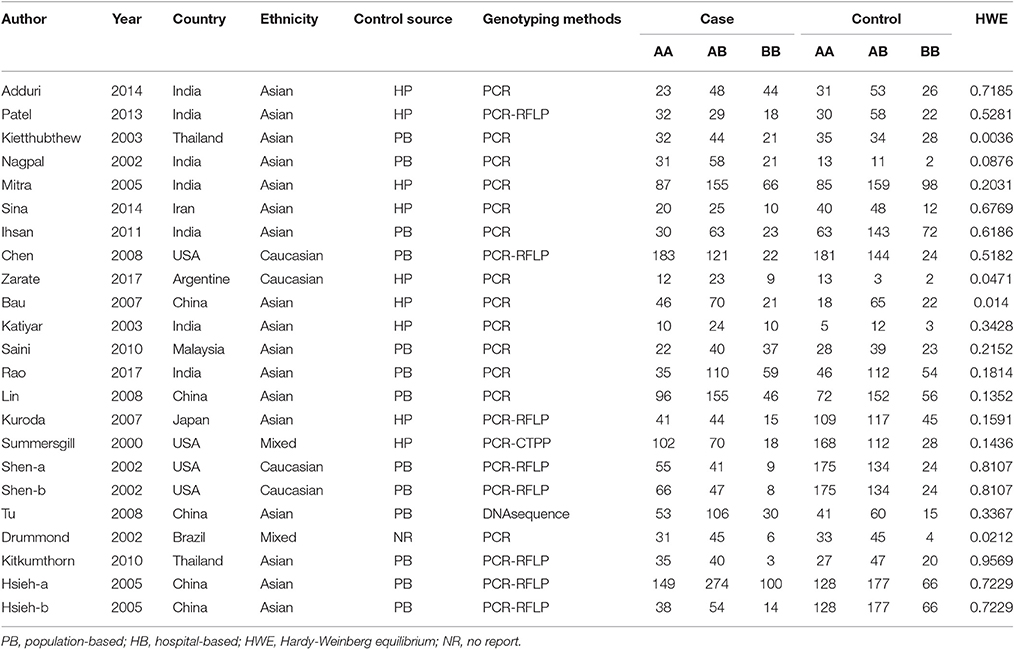
The selection of eligible studies to be included in this meta-analysis was shown in Figure 1, 905 potentially relevant researches were initially obtained from the PubMed, EBMASE, and Google of Scholar databases. After the exclusion of irrelevant studies, a total of 28 studies were included. Among the remaining articles, four articles (Tandle et al., 2001; Jing et al., 2012; Saleem et al., 2013; Nagam et al., 2017) were not in agreement with HWE (P < 0.001) and three duplicated data publications were further excluded (Ji et al., 2008; Misra et al., 2009; Wang et al., 2012). We included 21 case-control study involving 3,525 oral carcinoma patients and 3,712 controls (Summersgill et al., 2000; Drummond et al., 2002; Nagpal et al., 2002; Shen et al., 2002; Katiyar et al., 2003; Kietthubthew et al., 2003; Hsieh et al., 2005; Mitra et al., 2005; Bau et al., 2007; Kuroda et al., 2007; Chen et al., 2008; Lin et al., 2008; Tu et al., 2008; Kitkumthorn et al., 2010; Ihsan et al., 2011; Saini et al., 2011; Patel et al., 2013; Adduri et al., 2014; Sina et al., 2014; Rao et al., 2017; Zarate et al., 2017). The characteristics of the selected studies are shown in Table 1.
Meta-Analysis Results
Based on 21 case-control studies no significant association was found between TP53 Arg72Pro polymorphism and susceptibility to oral carcinoma in any genetic model (ArgPro vs. ArgArg: OR = 1.0, 95%CI = 0.90–1.11; ProPro vs. ArgArg: OR = 0.97, 95%CI = 0.84–1.12; Pro vs. Arg: OR = 1.0, 95%CI = 0.90–1.12; ArgPro+ProPro vs. ArgArg: OR = 1.01, 95%CI = 0.86–1.18; ProPro vs. ArgPro+ArgArg: OR = 0.96, 95% = 0.85–1.09). Based on subgroup analysis, stratified by control source or ethnicity, we obtained similar results (Figure 2, Table 2).
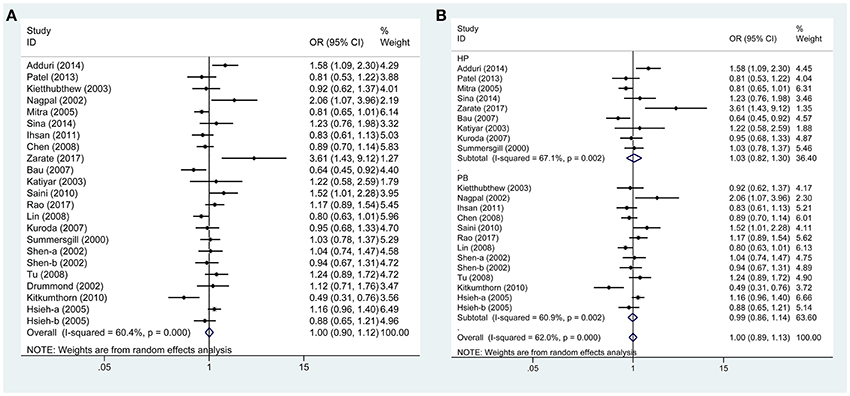
Figure 2. Forest plots demonstrated the association between TP53 codon 72 polymorphism and oral carcinoma susceptibility in the allele model. (A) Overall analysis. (B) Subgroup analysis by source of control.
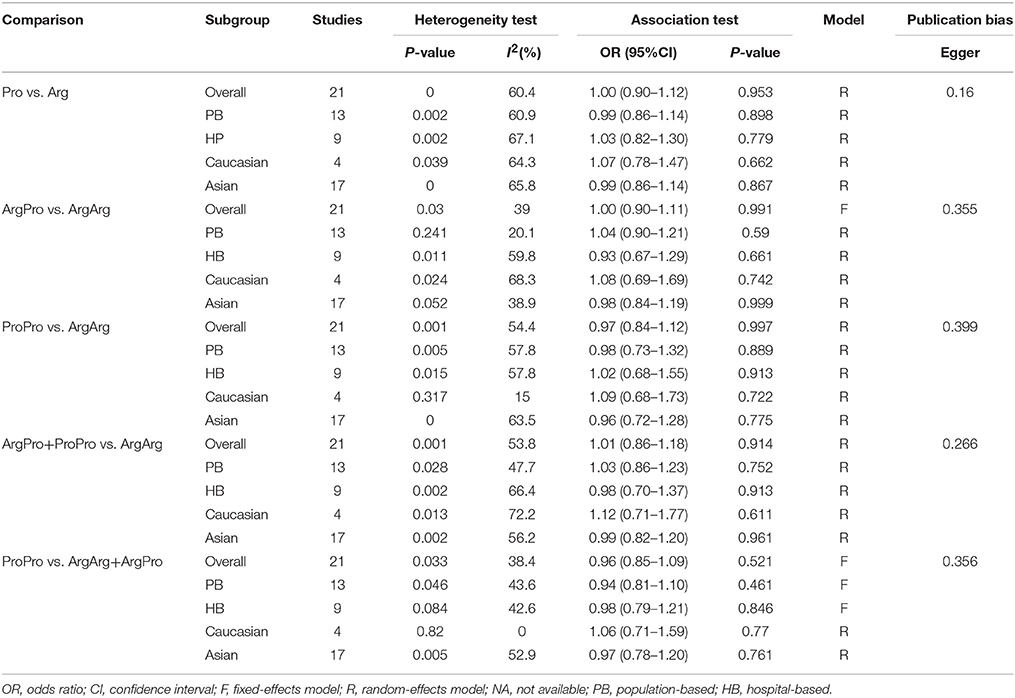
Table 2. Meta-analysis of the association between TP53 codon 72 polymorphism and oral carcinoma susceptibility.
In addition, subgroup analysis stratified by tobacco use (no vs. yes) was performed, and the association was still not significant in either tobacco users (OR = 0.88, 95%CI = 0.67–1.16) or non-users (OR = 0.84, 95%CI = 0.84–2.26). Similar results were found for subgroup analysis stratified by alcohol use or HPV infection status (Figures 3, 4, Table 3).
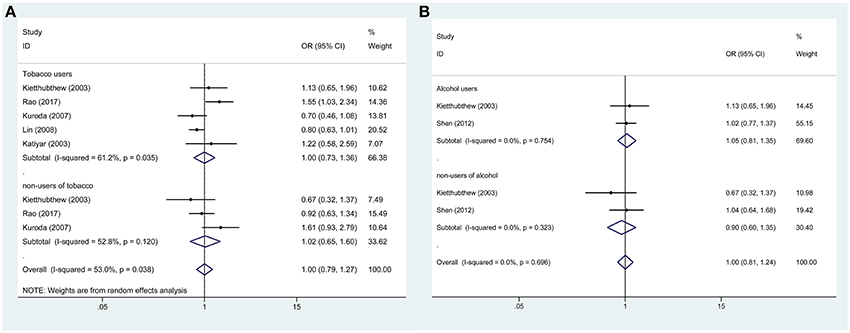
Figure 3. Forest plots demonstrated the association between TP53 codon 72 polymorphism and oral carcinoma susceptibility in the allele model. (A) Subgroup analysis by tobacco users. (B) Subgroup analysis by alcohol users.
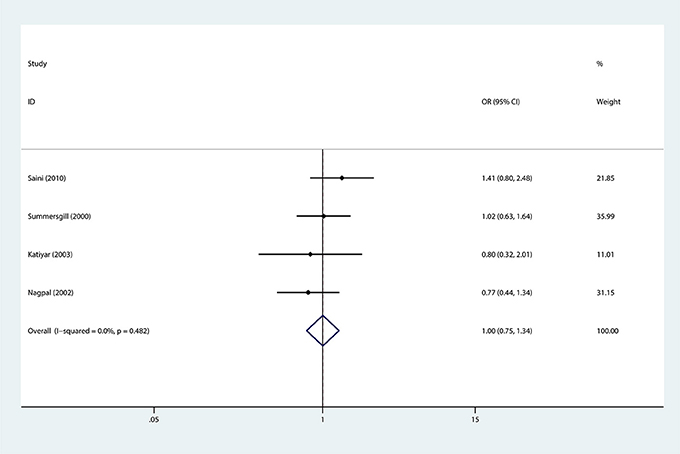
Figure 4. Forest plots demonstrated the association between TP53 codon 72 polymorphism and oral carcinoma susceptibility stratified by HPV infection status in the allele model.
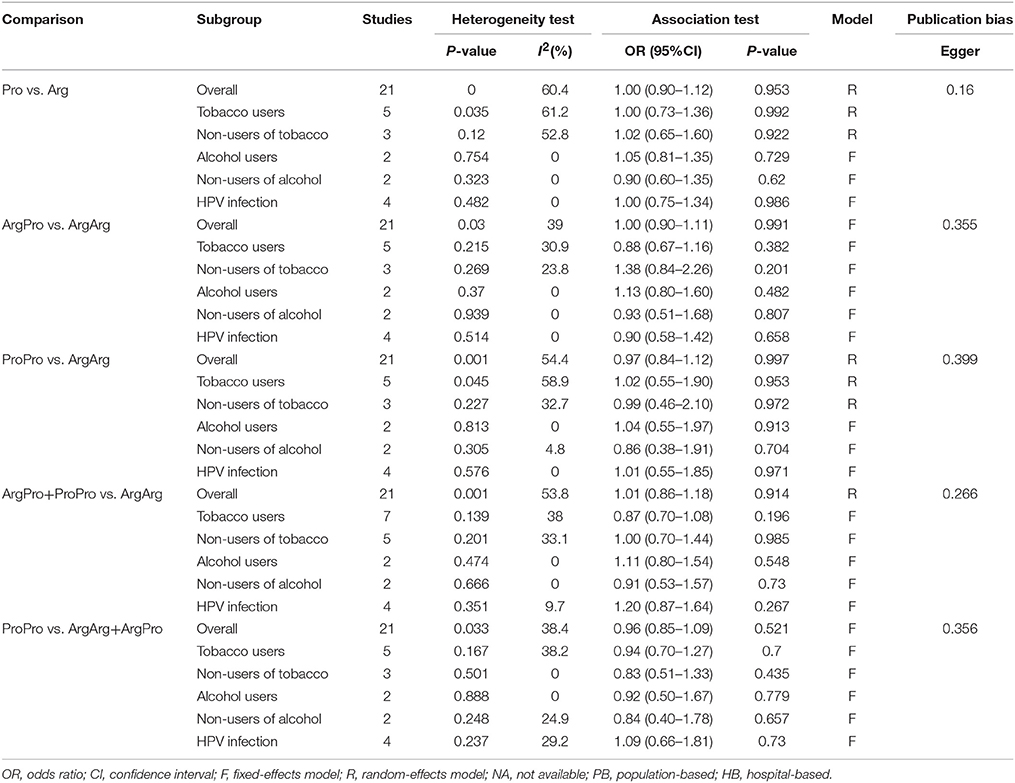
Table 3. Meta-analysis of the association between TP53 codon 72, tobacco or alcohol uses, HPV-infection status and Oral carcinoma susceptibility.
Sensitivity and Heterogeneity Analysis
Between-study heterogeneity was examined and significant heterogeneity (P < 0.05) was detected in some genetic comparisons, so random-effects model was adopted (DerSimonian and Laird, 1986); otherwise, the fixed-effect model was utilized (Mantel and Haenszel, 1959). The sensitivity analysis was carried out through sequential exclusion of any one individual study, and the results showed that our conclusion was robust and credible (Figure 5).
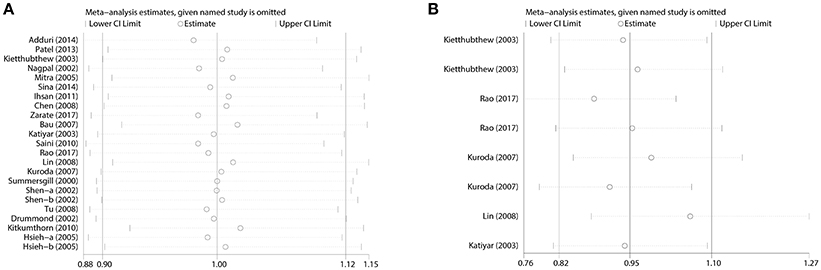
Figure 5. Sensitivity analysis for the influences of TP53 codon 72 polymorphism and oral carcinoma susceptibility under the allele model. (A) Overall analysis. (B) Subgroup analysis by tobacco users.
Publication Bias
Begg's and Egger's test was utilized to examine the potential publication bias of the studies (Begg and Mazumdar, 1994; Egger et al., 1997). As shown in Figure 6, there was no significant publication bias (Tables 2, 3).
Figure 6. Funnel plot of publication biases on the association between TP53 codon 72 polymorphism and oral carcinoma susceptibility.
Discussion
TP53 inactivation is a frequent event in cancer and involves point mutations and allelic loss (Baker et al., 1990; Tommasino et al., 2003). Moreover, TP53 polymorphisms could affect cancer susceptibility (Whibley et al., 2009). Pro72 allele has been implicated in coronary artery disease (Khan et al., 2016), systemic lupus erythematosus (Lee et al., 2012) and ulcerative colitis (Vaji et al., 2011). In contrast, Arg72 allele is implicated in pilocytic astrocytoma (Mascelli et al., 2016). Codon 72 TP53 polymorphisms have shown different associations with the risk of carcinomas in different populations, including oral carcinoma susceptibility (Tandle et al., 2001; Nagpal et al., 2002; Hsieh et al., 2005; Wang et al., 2012, 2014; Dahabreh et al., 2013; Saleem et al., 2013).
Although many case-control studies investigated the association of tobacco and/or alcohol uses, TP53 codon 72 polymorphism, and HPV infection with oral carcinoma susceptibility, the results were inconclusive. A past case-control studies failed to detect any significant association of TP53 codon 72 polymorphism with oral carcinoma susceptibility (Summersgill et al., 2000). In 2007, Bau et al. reported that the ArgArg genotype seemed to increase the susceptibility to oral carcinoma 2.7-fold in Chinese (Bau et al., 2007). Previous several meta-analyses reported the lack of association between TP53 codon 72 polymorphism and the risk of oral carcinoma (Zhuo et al., 2009b; Zeng et al., 2014; Hou et al., 2015), but these studies did not stratify the conditions such as tobacco and/or alcohol uses, HPV-infection status to perform subgroup analysis. Therefore, we performed the present meta-analysis to provide better estimate on the association of TP53 codon 72 polymorphism with oral carcinoma susceptibility.
Tobacco smoking is a well-known risk factor of cancer and could affect gene polymorphism in oral carcinoma (Ye et al., 2008). To evaluate the association between TP53 polymorphism and oral carcinoma susceptibility in tobacco users, we analyzed all available data extracted from the included studies, and found no significant association, indicating that TP53 codon 72 polymorphism is not a potential risk factor of oral carcinoma in tobacco users.
Regular alcohol consumption is associated with an increased risk for oral cancer. Such association is dose-dependent. Indeed, among individuals consuming 4–5 drinks daily, the risk for cancer of the oral cavity is 2–3-fold higher than among non-drinkers (Baan et al., 2007; Seitz and Stickel, 2007; Wiseman, 2008). To further investigate a possible association between oral carcinoma susceptibility and TP53 codon 72 polymorphism in alcohol users, we extracted relevant data from two studies and the results also failed to suggest a market correlation, demonstrated that TP53 codon 72 polymorphism may not be a risk of oral carcinoma in alcohol use status.
HPV infection has been suggested as one of the contributing factors for oral carcinoma. It was suggested that the interaction of TP53 codon 72 polymorphism with HPV was associated with oral carcinoma susceptibility (Kitkumthorn et al., 2010). However, this opinion was challenged by other studies (Summersgill et al., 2000; Lin et al., 2008). In this study we performed subgroup analysis on the interaction of p53 gene polymorphism with HPV infection on oral cancer susceptibility and the results indicated that TP53 codon 72 polymorphism is not a risk factor of oral carcinoma no matter HPV infection status.
The present study had several limitations. Firstly, only studies written in English or Chinese were included in the meta-analysis. This means that eligible studies published in other languages may have been overlooked, which may have introduced selection bias. Secondly, the sample size of some studies was limited and the results should be interpreted carefully. Thirdly, this study had statistical heterogeneity, although this is extremely common in meta-analyses of genetic association studies. We thus conducted subgroup analyses to identify all factors that contribute to the heterogeneity. Finally, other factors such as the age, gender, life-style that may affect the interaction of TP53 codon 72 polymorphism with oral carcinoma could not be analyzed due to the lack of original data.
In summary, this meta-analysis suggests that there is no statistical association between TP53 codon 72 polymorphism and oral cancer susceptibility, independent of tobacco and/or alcohol use and HPV-infection status. However, this conclusion should be confirmed by multi-center and large-scale studies based on multiple ethnic groups.
Author Contributions
Y-ML, JS, X-TZ, and R-HL conceived and designed the experiments. Y-ML performed the experiments. X-HY, CH, and X-WJ analyzed the data. X-WJ, Y-DY, C-JW, E-MZ, and Z-XY contributed reagents, materials, analysis tools. Y-ML wrote the paper. Y-ML, and X-TZ methods analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adduri, R. S., Katamoni, R., Pandilla, R., Madana, S. N., Paripati, A. K., Kotapalli, V., et al. (2014). TP53 Pro72 allele is enriched in oral tongue cancer and frequently mutated in esophageal cancer in India. PLoS ONE 9:e114002. doi: 10.1371/journal.pone.0114002
Anantharaman, D., Samant, T. A., Sen, S., and Mahimkar, M. B. (2011). Polymorphisms in tobacco metabolism and DNA repair genes modulate oral precancer and cancer risk. Oral Oncol. 47, 866–872. doi: 10.1016/j.oraloncology.2011.06.015
Baan, R., Straif, K., Grosse, Y., Secretan, B., El Ghissassi, F., Bouvard, V., et al. (2007). Carcinogenicity of alcoholic beverages. Lancet Oncol. 8, 292–293. doi: 10.1016/S1470-2045(07)70099-2
Baker, S. J., Preisinger, A. C., Jessup, J. M., Paraskeva, C., Markowitz, S., Willson, J. K., et al. (1990). p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 50, 7717–7722.
Bau, D. T., Tsai, M. H., Lo, Y. L., Hsu, C. M., Tsai, Y., Lee, C. C., et al. (2007). Association of p53 and p21(CDKN1A/WAF1/CIP1) polymorphisms with oral cancer in Taiwan patients. Anticancer Res. 27, 1559–1564.
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101.
Chen, X., Sturgis, E. M., El-Naggar, A. K., Wei, Q., and Li, G. (2008). Combined effects of the p53 codon 72 and p73 G4C14-to-A4T14 polymorphisms on the risk of HPV16-associated oral cancer in never-smokers. Carcinogenesis 29, 2120–2125. doi: 10.1093/carcin/bgn191
Chen, X., Sturgis, E. M., Lei, D., Dahlstrom, K., Wei, Q., and Li, G. (2010). Human papillomavirus seropositivity synergizes with MDM2 variants to increase the risk of oral squamous cell carcinoma. Cancer Res. 70, 7199–7208. doi: 10.1158/0008-5472.CAN-09-4733
Chor, J. S., Vlantis, A. C., Chow, T. L., Fung, S. C., Ng, F. Y., Lau, C. H., et al. (2016). The role of human papillomavirus in head and neck squamous cell carcinoma: a case control study on a southern Chinese population. J. Med. Virol. 88, 877–887. doi: 10.1002/jmv.24405
Coleman, M. P., Quaresma, M., Berrino, F., Lutz, J. M., De Angelis, R., Capocaccia, R., et al. (2008). Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 9, 730–756. doi: 10.1016/S1470-2045(08)70179-7
Dahabreh, I. J., Schmid, C. H., Lau, J., Varvarigou, V., Murray, S., and Trikalinos, T. A. (2013). Genotype misclassification in genetic association studies of the rs1042522 TP53 (Arg72Pro) polymorphism: a systematic review of studies of breast, lung, colorectal, ovarian, and endometrial cancer. Am. J. Epidemiol. 177, 1317–1325. doi: 10.1093/aje/kws394
De Angelis, R., Sant, M., Coleman, M. P., Francisci, S., Baili, P., Pierannunzio, D., et al. (2014). Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE−5-a population-based study. Lancet Oncol. 15, 23–34. doi: 10.1016/S1470-2045(13)70546-1
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188.
Drummond, S. N., De Marco, L., Pordeus Ide, A., Barbosa, A. A., and Gomez, R. S. (2002). TP53 codon 72 polymorphism in oral squamous cell carcinoma. Anticancer Res. 22, 3379–3381.
Du, L., Kim, J. J., Shen, J., Chen, B., and Dai, N. (2017). KRAS and TP53 mutations in inflammatory bowel disease-associated colorectal cancer: a meta-analysis. Oncotarget 8, 22175–22186. doi: 10.18632/oncotarget.14549
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634.
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. doi: 10.1002/ijc.29210
Harris, S. L., and Levine, A. J. (2005). The p53 pathway: positive and negative feedback loops. Oncogene 24, 2899–2908. doi: 10.1038/sj.onc.1208615
Hashibe, M., Brennan, P., Benhamou, S., Castellsague, X., Chen, C., Curado, M. P., et al. (2007). Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 99, 777–789. doi: 10.1093/jnci/djk179
Hou, J., Gu, Y., Hou, W., Wu, S., Lou, Y., Yang, W., et al. (2015). P53 codon 72 polymorphism, human papillomavirus infection, and their interaction to oral carcinoma susceptibility. BMC Genet. 16:72. doi: 10.1186/s12863-015-0235-7
Hsieh, L. L., Huang, T. H., Chen, I. H., Liao, C. T., Wang, H. M., Lai, C. H., et al. (2005). p53 polymorphisms associated with mutations in and loss of heterozygosity of the p53 gene in male oral squamous cell carcinomas in Taiwan. Br. J. Cancer 92, 30–35. doi: 10.1038/sj.bjc.6602271
Ihsan, R., Devi, T. R., Yadav, D. S., Mishra, A. K., Sharma, J., Zomawia, E., et al. (2011). Investigation on the role of p53 codon 72 polymorphism and interactions with tobacco, betel quid, and alcohol in susceptibility to cancers in a high-risk population from North East India. DNA Cell Biol. 30, 163–171. doi: 10.1089/dna.2010.1119
Ji, X., Neumann, A. S., Sturgis, E. M., Adler, K., Dahlstrom, K. R., Schiller, J. T., et al. (2008). p53 codon 72 polymorphism associated with risk of human papillomavirus-associated squamous cell carcinoma of the oropharynx in never-smokers. Carcinogenesis 29, 875–879. doi: 10.1093/carcin/bgn039
Jing, G., Lv, K., and Jiao, X. (2012). The p53 codon 72 polymorphism and the risk of oral cancer in a Chinese Han population. Genet. Test. Mol. Biomarkers 16, 1149–1152. doi: 10.1089/gtmb.2012.0138
Katiyar, S., Thelma, B. K., Murthy, N. S., Hedau, S., Jain, N., Gopalkrishna, V., et al. (2003). Polymorphism of the p53 codon 72 arg/pro and the risk of HPV type 16/18-associated cervical and oral cancer in India. Mol. Cell. Biochem. 252, 117–124. doi: 10.1023/A:1025546610920
Khan, S., Phulukdaree, A., Ramkaran, P., Moodley, D., and Chuturgoon, A. A. (2016). The Arg72 variant of the p53 functional polymorphism (rs1042522) is associated with coronary artery disease in young South Africans of Indian ancestry. Gene 593, 261–264. doi: 10.1016/j.gene.2016.07.040
Kietthubthew, S., Sriplung, H., Au, W. W., and Ishida, T. (2003). The p53 codon 72 polymorphism and risk of oral cancer in Southern Thailand. Asian Pac. J. Cancer Prev. 4, 209–214.
Kitkumthorn, N., Yanatatsaneejit, P., Rabalert, J., Dhammawipark, C., and Mutirangura, A. (2010). Association of P53 codon 72 polymorphism and ameloblastoma. Oral Dis. 16:631–635. doi: 10.1111/j.1601-0825.2010.01664.x
Kuroda, Y., Nakao, H., Ikemura, K., and Katoh, T. (2007). Association between the TP53 codon72 polymorphism and oral cancer risk and prognosis. Oral Oncol. 43, 1043–1048. doi: 10.1016/j.oraloncology.2006.12.001
Lee, Y. H., Bae, S. C., Choi, S. J., Ji, J. D., and Song, G. G. (2012). Associations between the p53 codon 72 polymorphisms and susceptibility to systemic lupus erythematosus and rheumatoid arthritis: a meta-analysis. Lupus 21, 430–437. doi: 10.1177/0961203315583543
Lin, Y. C., Huang, H. I., Wang, L. H., Tsai, C. C., Lung, O., Dai, C. Y., et al. (2008). Polymorphisms of COX-2−765G>C and p53 codon 72 and risks of oral squamous cell carcinoma in a Taiwan population. Oral Oncol. 44, 798–804. doi: 10.1016/j.oraloncology.2007.10.006
Mantel, N., and Haenszel, W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748.
Mascelli, S., Nozza, P., Jones, D. T., Colin, C., Pistorio, A., Milanaccio, C., et al. (2016). TP53 codon 72 polymorphism may predict early tumour progression in paediatric pilocytic astrocytoma. Oncotarget 7, 47918–47926. doi: 10.18632/oncotarget.10295
Misra, C., Majumder, M., Bajaj, S., Ghosh, S., Roy, B., and Roychoudhury, S. (2009). Polymorphisms at p53, p73, and MDM2 loci modulate the risk of tobacco associated leukoplakia and oral cancer. Mol. Carcinog. 48, 790–800. doi: 10.1002/mc.20523
Mitra, S., Sikdar, N., Misra, C., Gupta, S., Paul, R. R., Roy, B., et al. (2005). Risk assessment of p53 genotypes and haplotypes in tobacco-associated leukoplakia and oral cancer patients from eastern Idia. Int. J. Cancer 117, 786–793. doi: 10.1002/ijc.21263
Nagam, S. L., Katta, S., and Prasad, V. V. (2017). Gender specific association of TP53 polymorphisms (EX4 215G>C Arg72Pro, IVS3+40-41ins16, and IVS6+62G>A), with risk of oral cancer subtypes and overall survival of the patients. Mol. Carcinog. 56, 895–912. doi: 10.1002/mc.22543
Nagpal, J. K., Patnaik, S., and Das, B. R. (2002). Prevalence of high-risk human papilloma virus types and its association with P53 codon 72 polymorphism in tobacco addicted oral squamous cell carcinoma (OSCC) patients of Eastern India. Int. J. Cancer 97, 649–653. doi: 10.1002/ijc.10112
Niu, Y., Shen, M., Li, H., Ni, X., Zhou, J., Zeng, X. T., et al. (2012). No association between MTHFR A1298C gene polymorphism and head and neck cancer risk: a meta-analysis based on 9,952 subjects. Asian Pac. J. Cancer Prev. 13, 3943–3947. doi: 10.7314/APJCP.2012.13.8.3943
Olivier, M., Eeles, R., Hollstein, M., Khan, M. A., Harris, C. C., and Hainaut, P. (2002). The IARC TP53 database: new online mutation analysis and recommendations to users. Hum. Mutat. 19, 607–614. doi: 10.1002/humu.10081
Patel, K. R., Vajaria, B. N., Begum, R., Shah, F. D., Patel, J. B., Shukla, S. N., et al. (2013). Association between p53 gene variants and oral cancer susceptibility in population from Gujarat, West India. Asian Pac. J. Cancer Prev. 14, 1093–1100. doi: 10.7314/APJCP.2013.14.2.1093
Petti, S. (2009). Lifestyle risk factors for oral cancer. Oral Oncol. 45, 340–350. doi: 10.1016/j.oraloncology.2008.05.018
Rao, A. K. D. M., Manikandan, M., Arunkumar, G., Revathidevi, S., Vinothkumar, V., et al. (2017). Prevalence of p53 codon 72, p73 G4C14-A4T14 and MDM2 T309G polymorphisms and its association with the risk of oral cancer in South Indians. Gene Reports 7, 106–112. doi: 10.1016/j.genrep.2017.03.003
Saini, R., Tang, T. H., Zain, R. B., Cheong, S. C., Musa, K. I., Saini, D., et al. (2011). Significant association of high-risk human papillomavirus (HPV) but not of p53 polymorphisms with oral squamous cell carcinomas in Malaysia. J. Cancer Res. Clin. Oncol. 137, 311–320. doi: 10.1007/s00432-010-0886-8
Saleem, S., Azhar, A., Hameed, A., Khan, M. A., Abbasi, Z. A., Qureshi, N. R., et al. (2013). P53 (Pro72Arg) polymorphism associated with the risk of oral squamous cell carcinoma in gutka, niswar and manpuri addicted patients of Pakistan. Oral Oncol. 49, 818–823. doi: 10.1016/j.oraloncology.2013.04.004
Seitz, H. K., and Stickel, F. (2007). Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 7, 599–612. doi: 10.1038/nrc2191
Shen, H., Zheng, Y., Sturgis, E. M., Spitz, M. R., and Wei, Q. (2002). P53 codon 72 polymorphism and risk of squamous cell carcinoma of the head and neck: a case-control study. Cancer Lett. 183, 123–130. doi: 10.1016/S0304-3835(02)00117-9
Shield, K. D., Ferlay, J., Jemal, A., Sankaranarayanan, R., Chaturvedi, A. K., Bray, F., et al. (2017). The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 67, 51–64. doi: 10.3322/caac.21384
Sina, M., Pedram, M., Ghojazadeh, M., Kochaki, A., and Aghbali, A. (2014). P53 gene codon 72 polymorphism in patients with oral squamous cell carcinoma in the population of northern Iran. Med. Oral Patol. Oral Cir. Bucal 19, e550–e555. doi: 10.4317/medoral.19794
Själander, A., Birgander, R., Kivelä, A., and Beckman, G. (1995). p53 polymorphisms and haplotypes in different ethnic groups. Hum. Hered. 45, 144–149. doi: 10.1159/000154275
Slee, E. A., O'Connor, D. J., and Lu, X. (2004). To die or not to die: how does p53 decide? Oncogene 23, 2809–2818. doi: 10.1038/sj.onc.1207516
Steccanella, F., Costanzo, A., and Petrelli, F. (2017). Editorial on role of p53 in esophageal cancer from a meta-analysis of 16 studies by Fisher et al. J. Thorac. Dis. 9, 1450–1452. doi: 10.21037/jtd.2017.05.72
Summersgill, K. F., Smith, E. M., Kirchner, H. L., Haugen, T. H., and Turek, L. P. (2000). p53 polymorphism, human papillomavirus infection in the oral cavity, and oral cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 90, 334–339. doi: 10.1067/moe.2000.107359
Suzuki, K., and Matsubara, H. (2011). Recent advances in p53 research and cancer treatment. J. Biomed. Biotechnol. 2011:978312. doi: 10.1155/2011/978312
Tandle, A. T., Sanghvi, V., and Saranath, D. (2001). Determination of p53 genotypes in oral cancer patients from India. Br. J. Cancer 84, 739–742. doi: 10.1054/bjoc.2000.1674
Tommasino, M., Accardi, R., Caldeira, S., Dong, W., Malanchi, I., Smet, A., et al. (2003). The role of TP53 in Cervical carcinogenesis. Hum. Mutat. 21, 307–312. doi: 10.1002/humu.10178
Tu, H. F., Chen, H. W., Kao, S. Y., Lin, S. C., Liu, C. J., and Chang, K. W. (2008). MDM2 SNP 309 and p53 codon 72 polymorphisms are associated with the outcome of oral carcinoma patients receiving postoperative irradiation. Radiother. Oncol. 87, 243–252. doi: 10.1016/j.radonc.2008.03.018
Vaji, S., Salehi, Z., and Aminian, K. (2011). Association of p53 codon 72 genetic polymorphism with the risk of ulcerative colitis in northern Iran. Int. J. Colorectal Dis. 26, 235–238. doi: 10.1007/s00384-010-1021-7
Wang, F., Wang, P., Wang, B., Fu, Z. J., Yuan, Y., Yan, S. L., et al. (2014). Association between TP53 Arg72Pro polymorphism and thyroid carcinoma risk. Tumour Biol. 35, 2723–2728. doi: 10.1007/s13277-013-1359-x
Wang, Z., Sturgis, E. M., Zhang, Y., Huang, Z., Zhou, Q., Wei, Q., et al. (2012). Combined p53-related genetic variants together with HPV infection increase oral cancer risk. Int. J. Cancer 131, E251–E258. doi: 10.1002/ijc.27335
Warnakulasuriya, S. (2009). Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 45, 309–316. doi: 10.1016/j.oraloncology.2008.06.002
Whibley, C., Pharoah, P. D., and Hollstein, M. (2009). p53 polymorphisms: cancer implications. Nat. Rev. Cancer 9, 95–107. doi: 10.1038/nrc2584
Wiseman, M. (2008). The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc. Nutr. Soc. 67, 253–256. doi: 10.1017/S002966510800712X
Xu, P., Liu, X., Ouyang, J., and Chen, B. (2017). TP53 mutation predicts the poor prognosis of non-Hodgkin lymphomas: evidence from a meta-analysis. PLoS ONE 12:e0174809. doi: 10.1371/journal.pone.0174809
Ye, Y., Lippman, S. M., Lee, J. J., Chen, M., Frazier, M. L., Spitz, M. R., et al. (2008). Genetic variations in cell-cycle pathway and the risk of oral premalignant lesions. Cancer 113, 2488–2495. doi: 10.1002/cncr.23854
Zarate, A. M., Don, J., Secchi, D., Carrica, A., Galindez Costa, F., Panico, R., et al. (2017). Study of the TP53 codon 72 polymorphism in oral cancer and oral potentially malignant disorders in argentine patients. Tumor Biol. 39, 1–7. doi: 10.1177/1010428317699113
Zeng, X. T., Luo, W., Geng, P. L., Guo, Y., Niu, Y. M., and Leng, W. D. (2014). Association between the TP53 codon 72 polymorphism and risk of oral squamous cell carcinoma in Asians: a meta-analysis. BMC Cancer 14:469. doi: 10.1186/1471-2407-14-469
Zhuo, X. L., Cai, L., Xiang, Z. L., Zhuo, W. L., Wang, Y., and Zhang, X. Y. (2009a). TP53 codon 72 polymorphism contributes to nasopharyngeal cancer susceptibility: a meta-analysis. Arch. Med. Res. 40, 299–305. doi: 10.1016/j.arcmed.2009.03.006
Keywords: TP53 codon 72, susceptibility, oral cancer, polymorphism, meta-analysis
Citation: Lin Y-M, Shao J, Yin X-H, Huang C, Jia X-W, Yuan Y-D, Wu C-J, Zhen E-M, Yao Z-X, Zeng X-T and Liu R-H (2018) Meta-Analysis Results on the Association Between TP53 Codon 72 Polymorphism With the Susceptibility to Oral Cancer. Front. Physiol. 9:1014. doi: 10.3389/fphys.2018.01014
Received: 19 March 2018; Accepted: 09 July 2018;
Published: 02 August 2018.
Edited by:
Thimios Mitsiadis, Universität Zürich, SwitzerlandReviewed by:
Vincenzo Desiderio, Università degli Studi della Campania “Luigi Vanvitelli” Naples, ItalyMichele Caraglia, Università degli Studi della Campania “Luigi Vanvitelli” Caserta, Italy
Copyright © 2018 Lin, Shao, Yin, Huang, Jia, Yuan, Wu, Zhen, Yao, Zeng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-Hua Liu, cmhsaXU2MkAxNjMuY29t
†Co-first authors.
 Ying-Mei Lin
Ying-Mei Lin Jun Shao1†
Jun Shao1† Xian-Tao Zeng
Xian-Tao Zeng