- 1CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
Sea cucumbers exposed to stressful circumstances eviscerate most internal organs, and then regenerate them rapidly under favorable environments. Reversible protein phosphorylation and acetylation are major modifications regulating protein function. Herein, for the first time, we perform quantitative phospho- and acetyl proteomics analyses of intestine regeneration in a sea cucumber species Apostichopus japonicus. We identified 1,862 phosphorylation sites in 1,169 proteins, and 712 acetylation sites in 470 proteins. Of the 147 and 251 proteins differentially modified by phosphorylation and acetylation, respectively, most were related to cytoskeleton biogenesis, protein synthesis and modification, signal recognition and transduction, energy production and conversion, or substance transport and metabolism. Phosphorylation appears to play a more important role in signal recognition and transduction than acetylation, while acetylation is of greater importance in posttranslational modification, protein turnover, chaperones; energy production and conversion; amino acid and lipid transport and metabolism. These results expanded our understanding of the regulatory mechanisms of posttranslational modifications in intestine regeneration of sea cucumbers after evisceration.
Introduction
The ability to regenerate viscera is the most dramatic characteristic of sea cucumbers. Sea cucumbers exposed to stressful circumstances eviscerate most internal organs including the digestive tube, the haemal system, and the respiratory trees; and they can restore the lost organs in 20~100 days when placed in a favorable environment, and regain full functions (García-Arrarás and Greenberg, 2001). This phenomenon has aroused the interest of many researchers who are dedicated to studying mechanisms responsible for the regulation of this phenomenal regenerative capacity (Vickery et al., 2001; Carnevali, 2006; Candia-Carnevali et al., 2009; García-Arrarás and Dolmatov, 2010).
Over the past two decades, a number of researchers have investigated many mechanisms in sea cucumbers, including those related to morphological changes, cell differentiation, proliferation and migration, extracellular matrix remodeling, nerve regrowth, and gene regulatory mechanisms (García-Arrarás et al., 1998, 1999; García-Arrarás and Greenberg, 2001; Mashanov et al., 2011, 2013; Sun et al., 2013b; Miao et al., 2017). However, the mechanisms controlling regeneration have not been fully understood due to the complexity of this process and the interferences of multiple genes during transcription, post-transcriptional regulation, translation, and posttranslational modification at multiple levels (Zhao et al., 2016). Thanks to the rapid development of high-throughput sequencing technologies, considerable mRNA and microRNA expression profiles related to regeneration of sea cucumbers have been reported, which investigated the regenerative mechanism at the genome-wide scale (Ortiz-Pineda et al., 2009; Sun et al., 2011, 2013a, 2017a,b; Mashanov et al., 2014). These findings provide comprehensive insight into the underlying mechanisms of regeneration, and a roadmap for screening and functional analysis of key candidate genes. However, despite of this progress, few proteome studies related to sea cucumbers regeneration have been reported (Sun et al., 2017c).
In our previous study, we used isobaric tag for relative and absolute quantitation (iTRAQ) technology to probe proteomic changes during intestine regeneration in Apostichopus japonicus (Sun et al., 2017c). However, due to the complexity of regeneration, it's far from revealing the mechanism only at protein expression levels. The posttranslational modification (PTM) regulation of regeneration such as phosphorylation and acetylation need to be investigated. Protein phosphorylation is universally employed for temporary modulation of protein function, serving to alternatively induce or abolish enzyme activity, and facilitate or disrupt protein interactions (Huttlin et al., 2010). Protein acetylation is also an important regulatory modification that regulates diverse functions including DNA recognition, protein-protein interactions, and protein stability (Kouzarides, 2000). Both modifications play important and well-characterized roles in many biological processes related to regeneration including signal transduction, nervous system development (Cohen et al., 2011), hepatic regeneration (Revuelta-Cervantes et al., 2011), intestinal regeneration (Cai et al., 2010), development of male germ cells (Pang and Rennert, 2013), and regulation of embryonic stem cell transcription (Kim J. et al., 2010). Proteomic-based approaches provide powerful tools for investigation of complex biological processes (Franco et al., 2013). Moreover, quantitative phospho- and acetyl proteomics analyses have been successfully applied in many studies including the mechanism of aestivation in sea cucumbers (Chen et al., 2016), regulation of resistant rice (Li et al., 2015), regulation of development in the murine brain (Goswami et al., 2012), regulation of mouse cardiomyopathy (Kuzmanov et al., 2016), and pathological processes in type 2 diabetes (Du et al., 2015).
Herein, we employed tandem mas tag (TMT) labeling and immobilized metal affinity chromatography (IMAC) enrichment followed by tandem high resolution liquid chromatography-mass spectrometry (LC-MS/MS) quantitative phospho- and acetyl proteomics analyses of regenerative intestine at 3 days post evisceration (dpe) in A. japonicus (Figure 1). Intestine regeneration can be divided into five different stages: wound healing (0–3 dpe), blastema formation (3–7 dpe), lumen formation (7–14 dpe), intestine differentiation (14–21 dpe), and growth (21 dpe-) (García-Arrarás et al., 1998; Ortiz-Pineda et al., 2009). At 3 dpe, regeneration enters the blastema formation stage that includes the most crucial regulatory processes during intestine regeneration involving cell migration, dedifferentiation, and transdifferentiation, during which many genes are differentially expressed (García-Arrarás et al., 1998; Sun et al., 2013a). At this stage, intestine regeneration was found to be regulated by genes related to cytoskeletal changes, protein synthesis, signal recognition and transduction, energy production and conversion, and substance transport and metabolism (Sun et al., 2017c). This is the first analysis of PTM-related mechanisms regulating intestine regeneration in sea cucumbers, and the first to describe the importance of acetylation in sea cucumbers. The results provided new insight into the posttranslational regulatory mechanisms responsible for regeneration in sea cucumbers.
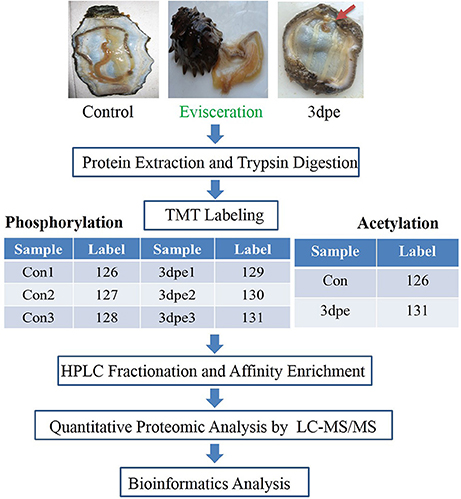
Figure 1. Outline of the experiment design for phosphorylation and acetylation modification study. Control, the normal intestine in the sea cucumber. 3 dpe, the regenerative intestine at 3 days post evisceration.
Materials and Methods
Animals and Samples
Adult A. japonicus (100 ± 10 g) were collected from the coast of Qingdao, Shandong Province, and acclimated in an aquarium containing seawater at ~15°C. Control sea cucumbers were fed once a day. For the regeneration experiment, sea cucumbers were induced to eviscerate all viscera, including intestine and respiratory trees by injecting ~2 mL 0.35 M KCl into the coelom (García-Arrarás and Greenberg, 2001; Suárez-Castillo, 2004; Rojas-Cartagena et al., 2007). For the phosphorylation study, regenerative intestine from 12 individuals (three biological replicates × four sea cucumbers per biological replicate) at 3 days post-evisceration (dpe) were included in the experiment group (three biological replicates: 3dpe1, 3dpe2, and 3dpe3). Normal intestine from 12 non-eviscerated sea cucumbers (three biological replicates × four sea cucumbers per biological replicate) were collected to serve as the control group (three biological replicates: Con1, Con2, and Con3). For the acetylation study, regenerative intestine from five individuals at 3 dpe were used for experiment group 3 dpe, and normal intestine from five non-eviscerated sea cucumbers served as controls. Normal and regenerative intestines were immediately frozen in liquid nitrogen and stored at −80°C.
Protein Extraction and Trypsin Digestion
Dissected intestines were first ground with liquid nitrogen, and the cell powder was transferred to a 5 mL centrifuge tube and sonicated three times on ice using a high intensity ultrasonic processor (Scientz) in lysis buffer (8 M urea, 2 mM EDTA, 10 mM DTT, and 2% phosphatase inhibitor cocktail V). The remaining debris was removed by centrifugation at 20,000 × g for 10 min at 4°C. Finally, protein was precipitated with cold 15% trichloroacetic acid (TCA) for 2 h at −20°C. After centrifugation at 4°C for 10 min, the supernatant was discarded, and the remaining precipitate was washed with cold acetone three times. Protein was redissolved in buffer (8 M urea, 100 mM NH4HCO3, pH 8.0) and the protein concentration was determined with a 2-D Quant kit (GE Healthcare, Piscataway NJ) according to the manufacturer's instructions.
For the phosphorylation study, the protein solution was reduced with 10 mM dithiothreitol (DTT) for 1 h at 37°C and alkylated with 20 mM iodoacetamide (IAA) for 45 min at room temperature in darkness. For the acetylation study, the protein solution was reduced with 5 mM DTT for 30 min at 56°C and alkylated with 11 mM IAA for 15 min at room temperature in darkness. For trypsin digestion, the method was the same for both studies. Briefly, protein samples were diluted by adding 100 mM TEAB to less than 2 M urea, and trypsin was added at a 1:50 trypsin:protein mass ratio for the first digestion overnight, followed by a second 1:100 trypsin:protein digestion for 4 h.
TMT Labeling, HPLC Fractionation, and Affinity Enrichment
After trypsin digestion, peptides were desalted using a Strata X C18 SPE column (Phenomenex) and vacuum-dried. The peptides were reconstituted in 0.5 M TEAB and processed according to the manufacturer's protocol supplied with the 6-plex TMT kit (Figure 1). Briefly, one unit of TMT reagent (defined as the amount of reagent required to label 1 mg of protein) was thawed and reconstituted in acetonitrile (ACN). Peptide mixtures were then incubated for 2 h at room temperature, pooled, desalted, and dried by vacuum centrifugation.
For the phosphorylation study, the sample was fractionated by high pH reversed-phase HPLC with an Agilent 300Extend C18 column (5 μm particles, 4.6 mm inside diameter, 250 mm length). Peptides were first separated into 80 fractions with a gradient of 2–60% ACN in 10 mM ammonium bicarbonate (pH 10) over 80 min. The peptide mixtures were first incubated with an IMAC microsphere suspension and shaken gently. IMAC microspheres enriched with phosphopeptides were collected by centrifugation, and the supernatant was removed. For the phosphorylation study, to remove non-specifically adsorbed peptides, IMAC microspheres were sequentially washed with 50% ACN/6% TFA and 30% ACN/0.1% TFA. To elute the enriched phosphopeptides from the IMAC microspheres, elution buffer containing 10% NH4OH was added and gently shaken. The supernatant containing phosphopeptides was collected and lyophilised for LC-MS/MS analysis.
For the acetylation study, the sample was fractionated by high pH reversed-phase HPLC using a Thermo Betasil C18 column (5 μm particles, 4.6 mm ID, 250 mm length). The peptides were first separated into 60 fractions with a gradient of 8–32% ACN (pH 9.0) over 60 min. The peptides were then combined into eight fractions and dried by vacuum centrifuging. The peptides were dissolved in IP buffer solution (100 mM NaCl, 1 mM EDTA, 50 mM TRIS-HCl, and 0.5% NP-40, pH 8.0) and the supernatant was transferred to acetylated resin (PTM104 from PTM Bio Company). Following incubation with shaking at 4°C overnight, and washing sequentially with 50% ACN/6% TFA and 30% ACN/0.1% TFA, salt was removed from the acetylated peptides using C18 ZipTips prior to LC-MS/MS analysis.
Quantitative Proteomic Analysis by LC-MS/MS
Phosphorylation Analysis
Peptides were dissolved in solvent A consisting of 0.1% formic acid (FA) in 2% ACN and directly loaded onto a reversed-phase pre-column (Acclaim PepMap 100, Thermo Fisher Scientific). Peptide separation was performed using a reversed-phase analytical column (Acclaim PepMap RSLC, Thermo Fisher Scientific) with a linear gradient of 4–22% solvent B (0.1% FA in 98% ACN) for 50 min, 22–35% solvent B for 12 min, 35–80% solvent B for 4 min, and holding at 80% for the last 4 min, all at a constant flow rate of 400 nL/min, on an EASY-nLC 1000 UPLC system. The resulting peptides were analyzed by a Q Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific).
Peptides were subjected to an NSI source followed by tandem mass spectrometry (MS/MS) in a Q Exactive Plus (Thermo Fisher Scientific) coupled online to the UPLC. Intact peptides were detected in the Orbitrap at a resolution of 70,000. Peptides were selected for MS/MS using an NCE setting of 28, 30, and ion fragments were detected in the Orbitrap at a resolution of 17,500. A data-dependent procedure that alternated between one MS scan followed by 20 MS/MS scans was applied for the top 20 precursor ions above a threshold ion count of 5.0E3 in the MS survey scan with 15.0 s dynamic exclusion. The electrospray voltage applied was 2.0 kV. Automatic gain control (AGC) was used to prevent overfilling of the orbitrap, and 5E4 ions were accumulated for generation of MS/MS spectra. For MS scans, the m/z scan range was 350–1,800. The fixed first mass was set as 100 m/z.
Acetylation Analysis
Tryptic peptides were dissolved in 0.1% FA (solvent A), directly loaded onto a homemade reversed-phase analytical column (15 cm length, 75 μm inside diameter). The gradient comprised an increase from 6 to 23% solvent B (0.1% FA in 98% ACN) over 26 min, 23–35% over 8 min, an increase to 80% over 3 min, then holding at 80% for the last 3 min, all at a constant flow rate of 400 nL/min on an EASY-nLC 1000 UPLC system. The peptides were subjected to an NSI source followed by MS/MS analysis in an Orbitrap Fusion Tribrid (Thermo Fisher Scientific) coupled online to the UPLC. The intact peptides were detected in the Orbitrap at a resolution of 60,000. The peptides were selected for MS/MS using an NCE setting of 35, and ion fragments were detected in the Orbitrap at a resolution of 15,000. A data-dependent procedure that alternated between one MS scan followed by 20 MS/MS scans was applied for the top 20 precursor ions above a threshold intensity greater than 5E3 in the MS survey scan with 15.0 s dynamic exclusion. The electrospray voltage applied was 2.0 kV. AGC was used to prevent overfilling of the Orbitrap, and 5E4 ions were accumulated for generation of MS/MS spectra. For MS scans, the m/z scan range was 350–1,550. The fixed first mass was set as 100 m/z.
The resulting MS/MS data were processed using MaxQuant with the integrated Andromeda search engine (v.1.5.2.8). Tandem mass spectra were searched against A. japonicus transcriptome and genome databases (Sun et al., 2011; Du et al., 2012; Zhang et al., 2017) concatenated with the reverse decoy database. Trypsin/P was specified as the cleavage enzyme, allowing up to two missed cleavages, five modifications per peptide, and five charges. The mass error was set to 10 ppm for precursor ions and 0.02 Da for fragment ions. Carbamidomethylation on Cys was specified as a fixed modification, and oxidation on Met, phosphorylation on Ser, Thr, Tyr, and acetylation on the protein N-terminus were specified as variable modifications. The false discovery rate (FDR) threshold for proteins, peptides, and modification sites was 1%. Minimum peptide length was set at seven residues. For the quantification method, TMT-6plex was selected. All other parameters in MaxQuant were set to default values. The site localization probability was set as >0.5. We checked the mass error of all identified peptides, and the distribution was close to zero, and most were less than 0.02 Da, confirming that the mass accuracy of the MS data was acceptable. We next verified the length of most peptides was between eight and 20 residues, consistent with the properties of tryptic peptides, confirming that sample preparation conformed to the required standard.
A p < 0.05 from t-tests and a fold-change >1.50 or < 0.67 were set as the thresholds for differential phosphorylation. Because of the relatively lower protein recognition ration in acetylation study, a p < 0.05 by the t-test and a fold-change >1.20 or < 0.83 were set as the thresholds for differential acetylation to get a comprehensive understanding.
Bioinformatics Analysis
Gene ontology (GO; http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) databases were used to classify and group the identified proteins, and hypergeometric tests were used to define significantly enriched GO terms and pathways of differentially expressed proteins. A p < 0.05 was considered significant.
Domain annotation was performed using InterProScan (a sequence analysis application) based on the protein sequence alignment method. InterPro (http://www.ebi.ac.uk/interpro/) is a database that integrates diverse information about protein families, domains, and functional sites, and makes it freely available to the public via Web-based interfaces and services. Central to the database are diagnostic models, known as signatures, against which protein sequences can be searched to determine their potential functions. InterPro has utility in the large-scale analysis of whole genomes and meta-genomes, as well as in characterizing individual protein sequences.
Pan Acetylation Western Blotting
The same extracted proteins discussed above in section Protein Extraction and Trypsin Digestion were used in this experiment. Western blotting samples containing equal amounts of protein (20 μg/condition) were prepared by heating in 12% polyacrylamide buffer for 10 min at 95°C. Gel electrophoresis was then performed initially for 30 min at 80 V, then at 120 V until the bromophenol blue had run off the front of the gel. Following transfer to PVDF membranes (Millipore, Bedford, MA, USA) for 2 h at 80 V and 4°C, the membranes were blocked in phosphate-buffered saline (PBS) containing 5% non-fat milk and 0.1% tween-20 for 1 h at RT. Antibody incubation was performed by rinsing with TBST for 10 min three times, incubating at 4°C overnight with primary antibody (anti-acetyllysine antibody, PTM-101) diluted 1:1,000 in PBS with 1% non-fat powdered milk and 0.1% tween-20, rinsing with TBST for 10 min three times, incubating for 1 h at RT with secondary antibody (Thermo, Pierce, goat anti-mouse IgG, H+L, peroxidase-conjugated, 31430) diluted 1:5,000 in PBS with 1% non-fat powdered milk and 0.1% Tween-20), rinsing with TBST for 10 min three times, and staining with ECL western blot detection reagent (Beyotime, Beijing, China) followed by quantification using NIH Image 1.63 software (Syngene, Cambridge, UK).
Results
Overview of Phospho- and Acetyl Proteomics Data
All acquired data are available via ProteomeXchange under identifier PXD008374. In this study, we quantified dynamic changes in phosphorylation during intestine regeneration in A. japonicus, identifying 2,584 phosphorylation sites in 1,531 proteins, among which 1,862 phosphorylation sites in 1,169 proteins were quantified (Table 1). In all, 127 phosphorylation sites were upregulated in 113 proteins, and 38 phosphorylation sites were downregulated in 34 proteins (Table 1, Data Sheet 1).
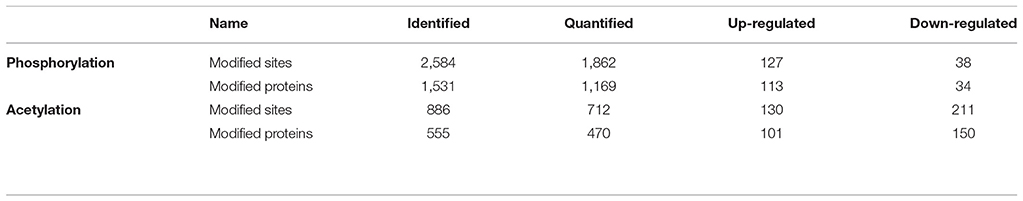
Table 1. Summary of identified, quantified, and differentially quantified sites and proteins of phosphorylation and acetylation.
We also quantified the dynamic changes in acetylation during intestine regeneration in A. japonicus, and identified 886 acetylation sites in 555 proteins, among which 712 acetylation sites in 470 proteins were quantified (Table 1). In all, 130 acetylation sites were upregulated in 101 proteins, and 211 acetylation sites were downregulated in 150 proteins (Table 2, Data Sheet 2).
GO, Domain, KEGG Pathway, and Subcellular Localization Analyses
To further understand the functions and features of the differentially phosphorylated and acetylated proteins, we annotated data based on GO (Figure 2), domains (Figure 3), pathways (Figure 4), and subcellular localization (Figure 5).
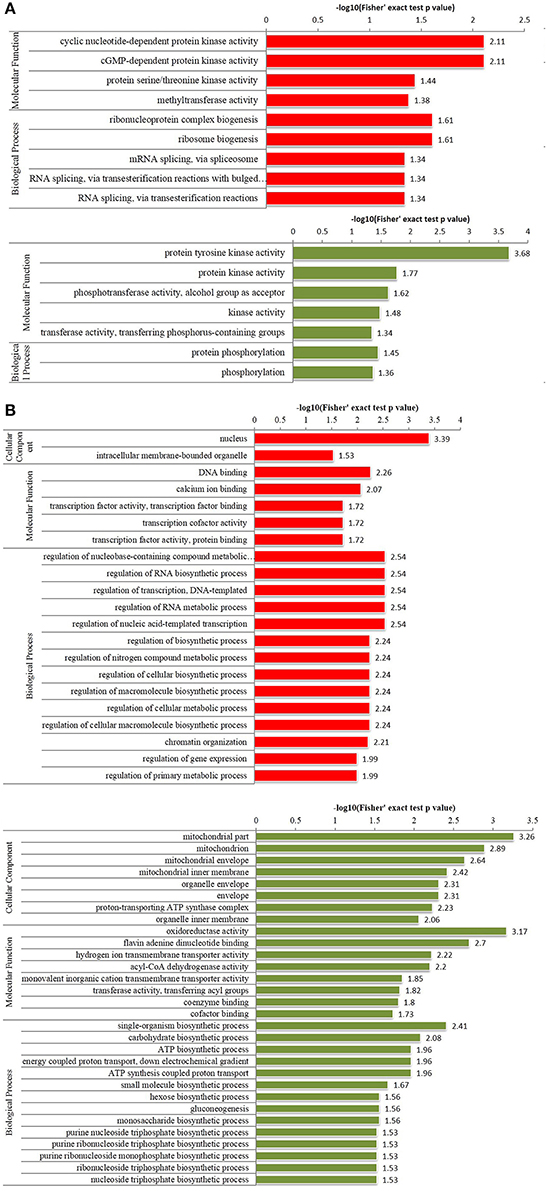
Figure 2. Enriched gene ontology (GO) analysis of differentially phosphorylated and acetylated proteins (p < 0.05). (A) phosphorylation. (B) Acetylation. Red, GO terms of proteins for which the modification was upregulated. Green, GO terms of proteins for which the modification was downregulated.
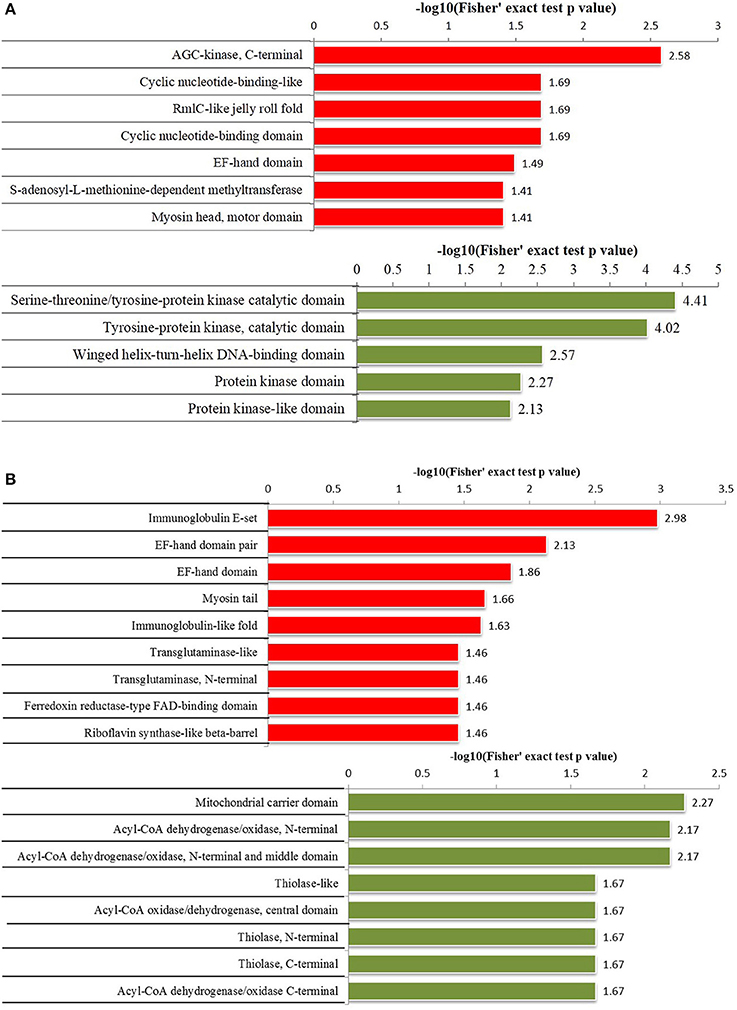
Figure 3. Protein domain enrichment analysis of differentially phosphorylated and acetylated proteins (p < 0.05). (A) phosphorylation. (B) Acetylation. Red, Protein domain terms for which the modification was upregulated. Green, Protein domain terms for which the modification was downregulated.
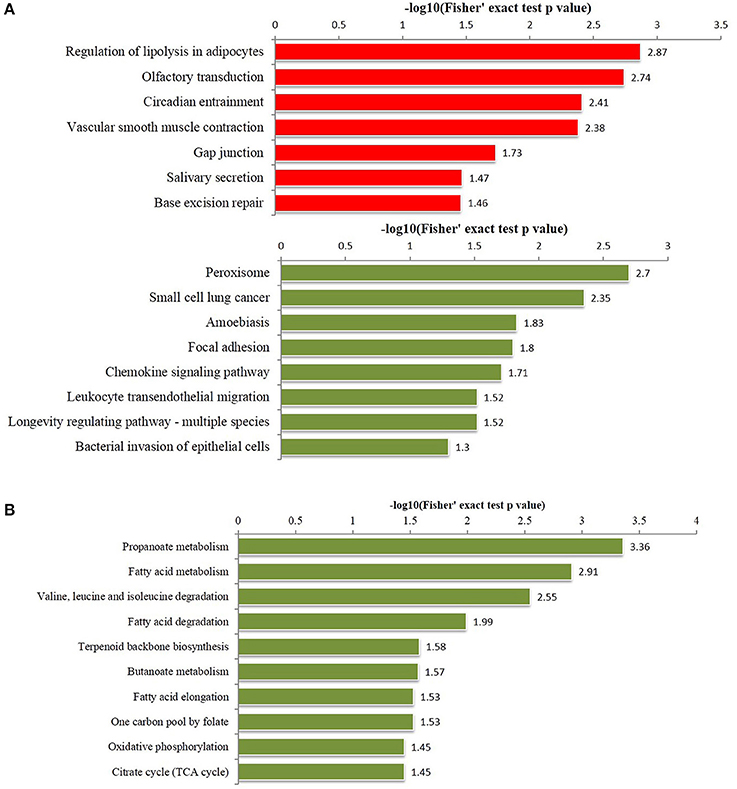
Figure 4. Enriched pathways analysis of differentially phosphorylated and acetylated proteins (all p < 0.05). (A) phosphorylation. (B) Acetylation. Red, Pathways terms of proteins for which the modification was upregulated. Green, Pathways terms of proteins for which the modification was downregulated.
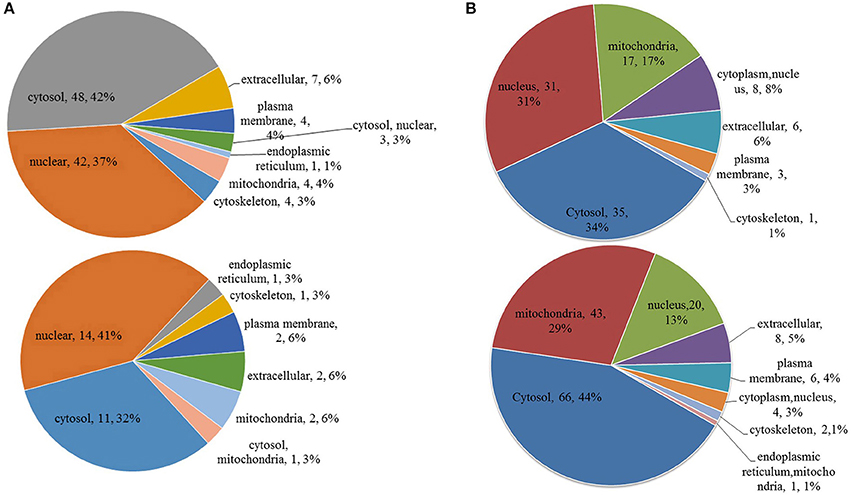
Figure 5. Subcellular location of differentially phosphorylated and acetylated proteins. (A) phosphorylation. (B) acetylation. Up, subcellular location of proteins for which the modification was upregulated. Down, subcellular location of proteins for which the modification was downregulated.
GO analysis showed significant enrichment of upregulated phosphorylated proteins in 4 terms of molecular function (MF) including cyclic nucleotide-dependent protein kinase activity and cGMP-dependent protein kinase activity etc., and five terms of biological process (BP), including ribonucleoprotein complex biogenesis, and ribosome biogenesis etc. (Figure 2A). Downregulated phosphorylated proteins were significantly enriched in 5 terms of MF including protein tyrosine kinase activity and protein kinase activity etc., and in two terms of BP including protein phosphorylation and phosphorylation (Figure 2A). Upregulated acetylated proteins were enriched in 2 terms of cellular component (CC) including nucleus and intracellular membrane-bounded organelle, five terms of MF including DNA binding and calcium ion binding etc., and 14 terms of BP including regulation of nucleobase-containing compound metabolic processes, and regulation of RNA biosynthetic processes etc. (Figure 2B). Downregulated acetylated proteins were enriched in 8 terms of CC including mitochondrion and organelle envelope etc., eight terms of MF including oxidoreductase activity and flavin adenine dinucleotide binding etc., and 14 terms of BP including single organism biosynthetic processes, and carbohydrate biosynthetic processes etc. (Figure 2B).
Protein domain enrichment analysis revealed that upregulated phosphorylated proteins were significantly enriched in 7 terms including AGC-kinase, C-terminal, cyclic nucleotide-binding-like, and RmlC-like jelly roll fold etc., while downregulated phosphorylated proteins were significantly enriched in 5 terms including the serine-threonine/tyrosine-protein kinase catalytic domain, tyrosine-protein kinase catalytic domain, and winged helix-turn-helix DNA-binding domain etc. (Figure 3A). Upregulated acetylated proteins were significantly enriched in 9 terms including immunoglobulin E-set, EF-hand domain pair, and myosin tail etc., while downregulated acetylated proteins were significantly enriched in 8 terms including mitochondrial carrier domain, and Acyl-CoA dehydrogenase/oxidase N-termini etc. (Figure 3B).
KEGG pathway-based enrichment analysis revealed 7 pathways for upregulated phosphorylated proteins including regulation of lipolysis in adipocytes, olfactory transduction, and circadian entrainment, while 8 pathways for downregulated phosphorylated proteins included peroxisome, small cell lung cancer, and amoebiasis (Figure 4A). Regarding acetylation, no pathways were significantly enriched for upregulated proteins, but 10 pathways were identified for downregulated acetylated proteins including propanoate metabolism, fatty acid metabolism, and valine, leucine and isoleucine degradation (Figure 4B).
Subcellular location annotation information of differentially phosphorylated and acetylated proteins is shown in Figure 5. Regarding phosphorylation, most up- and downregulated proteins were mainly localized to the nucleus and cytosol (Figure 5A). For acetylation, most upregulated proteins were localized to the nucleus and cytosol, whereas most downregulated proteins to the cytosol and mitochondria (Figure 5B).
Differentially Phosphorylated and Acetylated Proteins
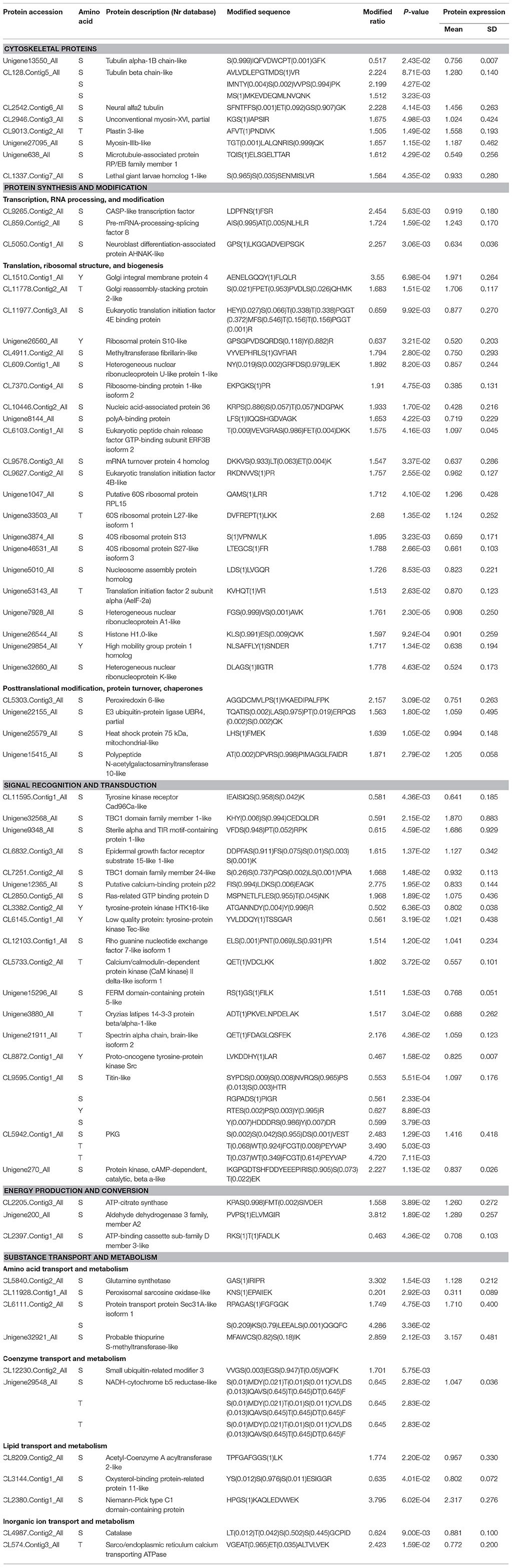
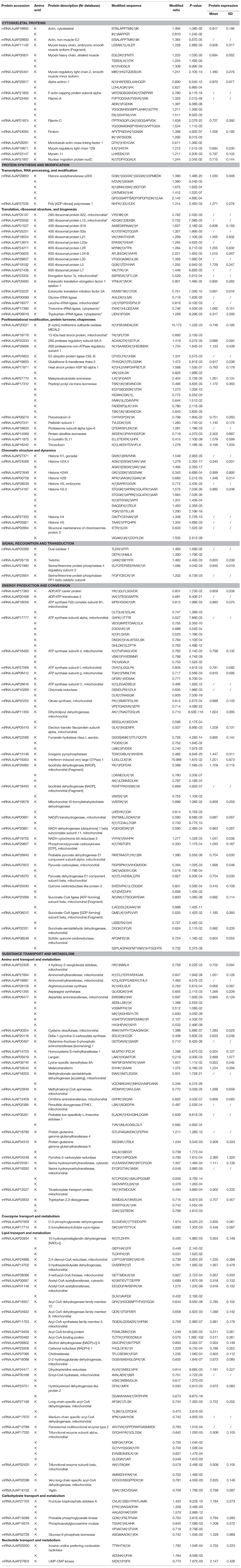
Proteins differentially phosphorylated and acetylated during intestine regeneration were mainly related to cytoskeleton biogenesis (P: 8; A: 14), protein synthesis and modification (P: 29; A: 44), signal recognition and transduction (P: 18; A: 4), energy production and conversion (P: 3; A: 30), and substance transport and metabolism (P: 11; A: 58) (Tables 2, 3, Figure 6). Phosphorylated sites were mainly serine (S), followed by tyrosine (Y) and threonine (T). Most acetylated sites were lysine (K). Further studies showed the proteins regulate “protein synthesis and modification” at different levels, including transcription factors, RNA processing and modification (P: 3, A: 2), translation, ribosomal structure and biogenesis (P: 22, A: 18), posttranslational modification, protein turnover, chaperones (P: 4, A: 15), and chromatin structure and dynamics (A: 9; Tables 2, 3, Figure 6). All differentially modified proteins “in substance transport and metabolism” were divided into six groups: amino acid transport and metabolism (P: five; A:25,), coenzyme transport and metabolism (P:2; A: 2), lipid transport and metabolism (P:3; A: 25), carbohydrate transport and metabolism (A:4), inorganic ion transport and metabolism (P:2), and nucleotide transport and metabolism (A: 2; Tables 2, 3, Figure 6). Moreover, most of the phosphorylated proteins played important roles in signal recognition and transduction. Whilemost acetylated proteins were related to posttranslational modification, protein turnover, chaperones (15), energy production and conversion (30), as well as amino acid and lipid transport and metabolism (25). Additionally, in order to investigate the significance of the observed phosphorylation and acetylation, the results were compared with our previous protein expression data (Sun et al., 2017c).
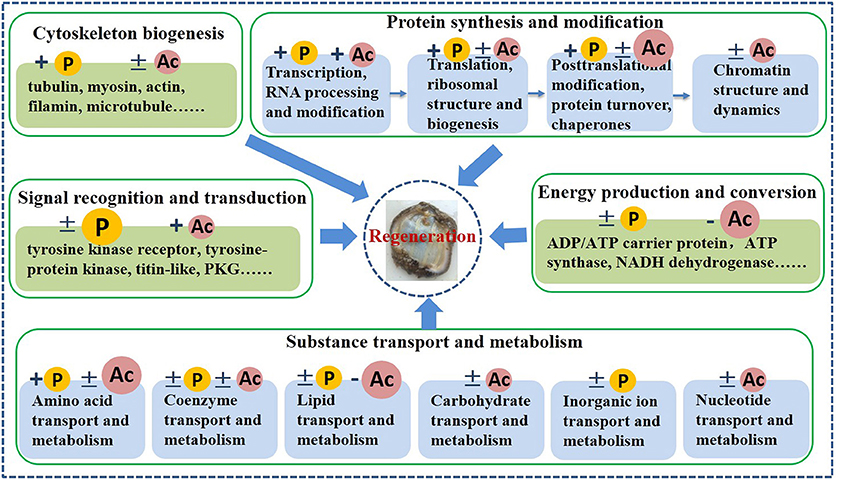
Figure 6. Schematic model of phosphorylation and acetylation during intestine regeneration in sea cucumbers. +, modification levels of almost all proteins were upregulated; −, modification levels of almost all proteins were downregulated; ±, modification levels of proteins were up- or downregulated. P, phosphorylation; Ac, acetylation; Big P or Ac, a large number of proteins were regulated by this reversible modification.
Pan-Acetylation Western Blotting Analyses
The overall acetylation regulation pattern of all proteins was determined by western blotting using a pan anti-acetyllysine antibody. As shown in Figure 7, many proteins were acetylated in both normal and regenerative intestine, but the overall acetylation levels were upregulated during intestine regeneration. Notably, consistent with the acetylated proteomic results, proteins with a molecular weight of ~10 kDa, which were most likely members of the histone family, were upregulated during intestine regeneration. Those findings confirmed that acetylation levels were significantly altered during intestine regeneration, suggesting acetylation probably played an important role in intestine regeneration in sea cucumbers.
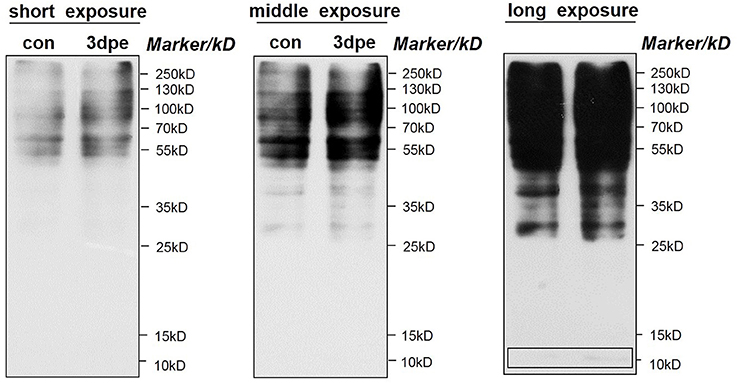
Figure 7. Western blotting of pan-acetylation during intestine regeneration in sea cucumbers. Cell lysate samples contain 20 μg of total protein per lane. Proteins with a molecular weight of ~10 kb are likely histone family member.
Discussion
PTM can influence protein folding, activity, stability, antigenicity, intracellular localization, and interaction with other proteins or with nucleic acids (Soppa, 2010). To date, more than 200 different PTMs have been described, among which reversible protein phosphorylation and acetylation are prominent and ubiquitous regulatory mechanisms (Van Noort et al., 2012). Herein, we analyzed PTM mechanisms during intestine regeneration in sea cucumbers for the first time, and revealed the importance of protein acetylation-based regulation.
Cytoskeleton Biogenesis
Cytoskeletal proteins play very important roles in cell dedifferentiation and migration during intestine regeneration in sea cucumbers (Murray and García-Arrarás, 2004; Ortiz-Pineda et al., 2009). In this study, phosphorylation and acetylation levels of most cytoskeletal proteins were upregulated, implying that intestine regeneration was regulated through protein phosphorylation (Tables 2, 3, Figure 6). S site hyperphosphorylation (>2-fold upregulation) of the tubulin beta chain-like and neural alfa2 tubulin was detected in regenerative intestine, even though protein expression remained relatively constant during intestine regeneration (Sun et al., 2017c). Acetylation modification of tubulin is known to alter the selectivity of kinesin-1 translocation, and lead to the formation of multiple axons, which is critical for neuronal development and function (Hammond et al., 2010). Interestingly, we found that phosphorylation levels were also upregulated during regeneration, which implied that PTM played an important role in regulating the functions of tubulin. These findings supported the “tubulin-code” hypothesis that predicts different tubulin genes or posttranslational modifications confer variation in the carboxy-terminal tail, resulting in unique interactions with microtubule-associated proteins for specific cellular functions (Sirajuddin et al., 2014).
Filamin-A and filamin-C showed significantly upregulated acetylation at multiple sites during intestine regeneration (Table 3). These actin-binding proteins are crucial in cell adhesion and spreading, which are critical for development, tissue remodeling, and wound healing (Kim H. et al., 2010; Fujita et al., 2012). It has been reported that filamin A was required in injured axons for HDAC5 activity and axon regeneration (Cho et al., 2015). Herein, we inferedthat filamin was modified by acetylation to regulate regeneration in sea cucumbers.
Protein Synthesis and Modification
Transcription Factors, RNA Processing, and Modification
Phosphorylation of CASP-like transcription factor was upregulated 2.454-fold, even though protein expression was not altered (Table 2). This CCAAT displacement protein transcription factor is believed to function in the tethering of transport vesicles and organization of the Golgi stack (Gillingham et al., 2002). Hence, we propose that phosphorylation of CASP-like transcription factor may play an important role in organizing the Golgi stack to regulate protein synthesis. Hyperphosphorylation of pre-mRNA-processing-splicing factor 8 (Prpf8) and neuroblast differentiation-associated protein AHNAK-like was detected in regenerative intestine, and upregulation was 1.724- and 2.257-fold compared with normal intestine (Table 2). Prpf8 is a highly conserved component of both major and U12-dependent minor spliceosomes, and this protein affects transcript splicing, cell survival, and myeloid differentiation in zebrafish (Keightley et al., 2013). AHNAK was found to be the most prominent component of extracellular vesicles, and this protein increases the motility of neighboring fibroblasts (Silva et al., 2016). The present study might suggest phosphorylation activates various factors associated with RNA processing and modification to promote intestine regeneration in sea cucumbers.
It is worth noting that the acetylation level of histone acetyltransferase (HAT) p300 was significantly upregulated at five K sites during intestine regeneration, while protein expression remained constant (Table 3). HATs catalyze histone acetylation which facilitates transcription (Carrozza et al., 2003). HAT p300 acetylates pax5 and strongly enhances pax5-mediated transcriptional activity (He et al., 2011). It has been reported that p300 targets both the epigenome and transcription to unlock a post-injury silent gene expression programme that supports axonal regeneration (Gaub et al., 2011). Hence, acetylation mediated transcription, which played a very important role in intestine regeneration in A. japonicus. The underlying mechanisms remain to be uncovered in future research.
Translation, Ribosomal Structure, and Biogenesis
Phosphorylation levels of 20 proteins associated with translation, ribosomal structure, and biogenesis were upregulated, and acetylation levels of 18 proteins were altered, suggesting phosphorylation and acetylation played important roles in these aspects of protein synthesis during intestine regeneration (Tables 2, 3). Eukaryotic translation initiation factor 4B-like (eIF4B) and translation initiation factor 2 subunit alpha were hyperphosphorylated 1.757- and 1.513-fold at S and T sites, respectively (Table 2). The phosphorylation state of eukaryotic translation initiation factors positively correlated with both translation and growth rates in the cell (van Gorp et al., 2009). eIF4B, a well-known hyperphosphorylated protein, played a critical role during the initiation of protein synthesis, and its activity could be regulated by multiple phosphorylation events, with phosphorylation of serine essential for optimal translational activity (Duncan and Hershey, 1984; van Gorp et al., 2009). Thus, phosphorylation of translation-related proteins might promote protein synthesis, which is essential for intestine regeneration.
Acetylation levels of 11 ribosomal proteins were altered during intestine regeneration, which implied that acetylation was important for the functions of ribosomal proteins. Three ribosomal proteins, L7, S5, and S18, were acetylated in Salmonella typhimurium, which facilitated proton exchange and catalysis (Vetting et al., 2008), and 30 out of 68 ribosomal proteins were found to be N-terminal-acetylated in yeast (Arnold et al., 1999). Thus, acetylation regulated the function of ribosomal proteins, which facilitated intestine regeneration in sea cucumbers.
Posttranslational Modification, Protein Turnover, Chaperones
In this category, four proteins including E3 ubiquitin-protein ligase UBR4 etc. were found to be hyperphosphorylated during intestine regeneration, while protein expression levels remained constant (Table 2). Furthermore, 15 proteins, including [F-actin]-methionine sulfoxide oxidase MICAL2 etc., were differentially acetylated during intestine regeneration in A. japonicus (Table 3). Hence, acetylation appeared to regulate their functions more dramatically than phosphorylation during intestine regeneration. Acetylation levels of maleylacetoacetate isomerase, peptidyl-prolyl cis-trans isomerase, and protein disulphide-isomerase were significantly changed. We therefore speculated that the activity of the isomerase family members may be altered via acetylation and deacetylation, which might play a role in regeneration in A. japonicus. Similar results have been reported previously, including the role of Cyclophilin A in immunity and viral infection (Lammers et al., 2010), prolyl isomerase Pin1 in orchestrating p53 acetylation (Mantovani et al., 2007), and prolyl isomerase Pin1 in fibroblast growth factor 2-induced osteoblast differentiation (Yoon et al., 2014).
Chromatin Structure and Dynamics
Chromatin structure and dynamics have a major impact on all nuclear processes (Eberharter and Becker, 2002). Nine proteins related to this category were differentially acetylated or deacetylated (Table 3). Acetylation may be more vital to the regulation of chromatin structure and dynamics than phosphorylation. Among all regulated proteins, eight belonged to the histone family. Histone acetylation is the mostdocumented form of protein acetylation, and is known to direct histone assembly and help regulate the unfolding and activity of genes (Grunstein, 1997). Gene expression is affected by the positioning of nucleosomes relative to regulatory sequence elements, which are regulated by site-specific acetylation of nucleosomal histones (Eberharter and Becker, 2002). Histone acetylation can influence all three aspects of chromatin organization, which is central to the switch between permissive and repressive chromatin structure (Eberharter and Becker, 2002). In this study, we also concluded that acetylation modification might play a very important role in regulating gene expression to facilitate intestine regeneration in sea cucumbers.
Signal Recognition and Transduction
Rapid and accurate transmission of signals from cell surface receptors to the nucleus is clearly dependent on protein phosphorylation (Karin and Hunter, 1995), which is also verified by our present results t. Phosphorylation levels of 18 proteins were significantly altered (Table 2, Figure 6). From these results, we concludedthat protein phosphorylation might play a very important role in signal recognition and transduction during intestine regeneration. The cGMP-dependent protein kinase (PKG) was dramatically hyperphosphorylated by over 2.483- to 4.720-fold, during intestine regeneration. It has been demonstrated that phosphorylation plays a central role in regulating the activation and signaling lifetime of protein kinases (Newton, 2003). We also observed the phosphorylation levels of four sites in titin-like protein were downregulated, while the protein expression level was unaltered (Table 2). The role of titin phosphorylation in the muscle signaling mechanisms is well-documented (Tskhovrebova and Trinick, 2003), and evidence has suggested that both the Z-line and M-line ends of the molecule are components of signaling pathways that control tension- and protein-turnover-related processes (Tskhovrebova and Trinick, 2003).
Energy Production and Conversion
In contrast to the “signal recognition and transduction” category, almost all modified proteins related to the “energy production and conversion” term were regulated by deacetylation (Table 3, Figure 6). The results showed that 27 proteins were hypoacetylated, but only three proteins were hyperacetylated. Two points are noteworthy in this study; (1) acetylation levels of most proteins were downregulated, and (2) a large number of the modified proteins were related to ATP/ADP production and conversion. During the early stages of intestine regeneration, A. japonicus stops feeding due to loss of intestines after evisceration, hence an energy management strategy is vital to the smooth progress of regenerative processes. It has been established that reversible acetylation-deacetylation of PGC-1α, an important regulator of energy homeostasis, is related to energy sensors that regulate mitochondrial energy homeostasis to maintain appropriate energy levels (Jeninga et al., 2010). In our study, almost all proteins were hypoacetylated, which may be a wise way to save energy. ATP synthase family members including mitochondrial ATP synthase subunit alpha (d, f, g, and o), were significantly hypoacetylated, by 0.495- to 0.796-fold. ATP synthase, an evolutionarily conserved protein complex, uses chemiosmotic energy stored as a gradient across the mitochondrial inner membrane to convert ADP and orthophosphate to ATP (Vassilopoulos et al., 2014). A previous study verified that SIRT3 deacetylates ATP synthase F1 in response to nutrient- and exercise-induced stress (Vassilopoulos et al., 2014). For A. japonicus, intestine regeneration is a type of nutrient- or stimulation-related stress. Hence, deacetylation of proteins related to energy production and conversion may manipulate energy distribution in response to changes in nutrient and energy stress to ensure continuation of intestine regeneration processes.
Substance Transport and Metabolism
Substance metabolism must be affected by energy distribution and hypometabolism during intestine regeneration. From the results, we predicted that reversible acetylation of substance transport and metabolism-related proteins in regenerative intestine might be more vital than reversible phosphorylation, and regulation of reversible acetylation appeared mainly to involve amino acid and lipid transport and metabolism. Many amino acid transferases, including aminomethyl transferase, aspartate aminotransferase, and ornithine aminotransferase, were hyper- or hypoacetylated, implying that reversible acetylation altered the three-dimensional structure and enzymatic functions to regulate amino acid transport to support intestine regeneration. During the early stages of intestine regeneration, key nutrient substances must be transported in a timely manner to regenerative blastema. In our previous study, we found that over half of proteins related to amino acid transport and metabolism were upregulated (Sun et al., 2017c). Taken together, these results suggested that amino acid related to transport and metabolism were rapidly manipulated during blastema formation in the early stages of intestine regeneration in A. japonicus. Additionally, acetylation levels of almost all proteins related to lipid transport and metabolism were downregulated during intestine regeneration, which is comparable to our previous study showing that expression of all proteins related to this term were significantly downregulated at 3 dpe (Sun et al., 2017c). Hence, we speculated that lipid metabolism could be postponed to provide extra energy for the intermediate or later stages of regeneration. This finding might indicate that sea cucumbers could adopt appropriate energy and metabolism strategies to guarantee the smooth progress of regeneration.
Conclusions
This study provided a global view of the role of protein phosphorylation and acetylation in intestine regeneration in sea cucumbers. To the best of our knowledge, it is the first such phospho- and acetylproteomics study in sea cucumbers. We found that the identified differentially modified proteins regulated regeneration from five different aspects, and phosphorylation and acetylation played different roles. These results not only improved our understanding of the regulatory mechanisms of intestine regeneration, but also enhanced our understanding of phosphorylation- and acetylation-based regulation in sea cucumbers even echinoderm.
Ethics Statement
This study was carried out in accordance with the recommendations of Welfare ethics of experimental animals and safety inspection system of animal experiments, laboratory animal management and ethics Committee of IOCAS. The protocol was approved by the laboratory animal management and ethics Committee of IOCAS.
Author Contributions
LS and HY conceived the paper. LS, CL, XL, and HY designed the experiments and analyzed the data. LS wrote the paper. LX, DH, JS and LZ revised the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 41776162, 41776161), NSFC-Shandong Joint Fund for Marine Science Research Centers (grant no. U1606404), the Agricultural Seed Project of Shandong Province (2016LZGC032), Open Research Fund Program of Guangxi Key Laboratory of Marine Biotechnology (GLMBT-201605), Qingdao applied basic research program (17-1-1-49-JCH), Creative Team Project of the Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology (LMEES-CTSP-2018-1), Taishan Scholars Program (Distinguished Taishan Scholars).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.00836/full#supplementary-material
Data Sheet 1. All phosphorylated proteins during intestine regeneration in sea cucumbers.
Data Sheet 2. All acetylated proteins during intestine regeneration in sea cucumbers.
References
Arnold, R. J., Polevoda, B., Reilly, J. P., and Sherman, F. (1999). The action of N-terminal acetyltransferases on yeast ribosomal proteins. J. Biol. Chem. 274, 37035–37040. doi: 10.1074/jbc.274.52.37035
Cai, J., Zhang, N., Zheng, Y., de Wilde, R. F., Maitra, A., and Pan, D. (2010). The hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Gene Dev. 24, 2383–2388. doi: 10.1101/gad.1978810
Candia-Carnevali, M. D., Thorndyke, M. C., and Matranga, V. (2009). Chapter 7: regenerating echinoderms: a promise to understand stem cells potential, in Stem Cells in Marine Organisms, eds B. Rinkevich and V. Matranga (Dordrecht: Springer), 165–186.
Carnevali, M. D. C. (2006). Regeneration in echinoderms: repair, regrowth, cloning. Inf. Syst. J. 3, 64–76.
Carrozza, M. J., Utley, R. T., Workman, J. L., and Cote, J. (2003). The diverse functions of histone acetyltransferase complexes. Trends Genet. 19, 321–329. doi: 10.1016/S0168-9525(03)00115-X
Chen, M., Zhu, A., and Storey, K. B. (2016). Comparative phosphoproteomic analysis of intestinal phosphorylated proteins in active versus aestivating sea cucumbers. J. Proteomics 135, 141–150. doi: 10.1016/j.jprot.2015.09.016
Cho, Y., Park, D., and Cavalli, V. (2015). Filamin A is required in injured axons for HDAC5 activity and axon regeneration. J. Biol. Chem. 290, 22759–22770. doi: 10.1074/jbc.M115.638445
Cohen, S., Gabel, H. W., Hemberg, M., Hutchinson, A. N., Sadacca, L. A., Ebert, D. H., et al. (2011). Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron 72, 72–85. doi: 10.1016/j.neuron.2011.08.022
Du, H., Bao, Z., Hou, R., Wang, S., Su, H., Yan, J., et al. (2012). Transcriptome sequencing and characterization for the sea cucumber Apostichopus japonicus(Selenka, 1867). PLoS ONE 7:e33311. doi: 10.1371/journal.pone.0033311
Du, Y., Cai, T., Li, T., Xue, P., Zhou, B., He, X., et al. (2015). Lysine malonylation is elevated in type 2 diabetic mouse models and enriched in metabolic associated proteins. Mol. Cell. Proteomics 14:227. doi: 10.1074/mcp.M114.041947
Duncan, R., and Hershey, J. W. (1984). Heat shock-induced translational alterations in HeLa cells. Initiation factor modifications and the inhibition of translation. J. Biol. Chem. 259:11882.
Eberharter, A., and Becker, P. B. (2002). Histone acetylation: a switch between repressive and permissive chromatin: second in review series on chromatin dynamics. EMBO Rep. 3, 224–229. doi: 10.1093/embo-reports/kvf053
Franco, C., Soares, R., Pires, E., Koci, K., Almeida, A. M., Santos, R., et al. (2013). Understanding regeneration through proteomics. Proteomics 13, 686–709. doi: 10.1002/pmic.201200397
Fujita, M., Mitsuhashi, H., Isogai, S., Nakata, T., Kawakami, A., Nonaka, I., et al. (2012). Filamin c plays an essential role in the maintenance of the structural integrity of cardiac and skeletal muscles, revealed by the medaka mutant zacro. Dev. Biol. 361, 79–89. doi: 10.1016/j.ydbio.2011.10.008
García-Arrarás, J., Díaz-Miranda, L., Torres, I. I., File, S., Jimenez, L. B., Rivera-Bermudez, K., et al. (1999). Regeneration of the enteric nervous system in the sea cucumber Holothuria glaberrima. J. Com. Neurol. 406, 461–475.
García-Arrarás, J. E., and Dolmatov, I. Y. (2010). Echinoderms; potential model systems for studies on muscle regeneration. Curr. Pharm. Design 16, 942. doi: 10.2174/138161210790883426
García-Arrarás, J. E., and Greenberg, M. J. (2001). Visceral regeneration in holothurians. Microsc. Res. Tech. 55, 438–451. doi: 10.1002/jemt.1189
García-Arrarás, J., Estrada-Rodgers, L., Santiago, R., Torres, I. I., Díaz-Miranda, L., and Torres-Avillán, I. (1998). Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). J. Exp. Zool. 281, 288–304.
Gaub, P., Joshi, Y., Wuttke, A., Naumann, U., Schnichels, S., Heiduschka, P., et al. (2011). The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain 134, 2134–2148. doi: 10.1093/brain/awr142
Gillingham, A. K., Pfeifer, A. C., and Munro, S. (2002). CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a golgi membrane protein related to giantin. Mol. Biol. Cell 13:3761. doi: 10.1091/mbc.e02-06-0349
van Gorp, A. G., van der Vos, K. E., Brenkman, A. B., Bremer, A., van den Broek, N., Zwartkruis, F., et al. (2009). AGC kinases regulate phosphorylation and activation of eukaryotic translation initiation factor 4B. Oncogene 28, 95–106. doi: 10.1038/onc.2008.367
Goswami, T., Li, X., Smith, A. M., Luderowski, E. M., Vincent, J., Rush, J. E., et al. (2012). Comparative phosphoproteomic analysis of neonatal and adult murine brain. Proteomics 12, 2185–2189. doi: 10.1002/pmic.201200003
Grunstein, M. (1997). Histone acetylation in chromatin structure and transcription. Nature 389, 349–352. doi: 10.1038/38664
Hammond, J. W., Huang, C. F., Kaech, S., Jacobson, C., Banker, G., and Verhey, K. J. (2010). Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell 21, 572–583. doi: 10.1091/mbc.e09-01-0044
He, T., Hong, S. Y., Huang, L., Xue, W., Yu, Z., Kwon, H., et al. (2011). Histone acetyltransferase p300 acetylates Pax5 and strongly enhances Pax5-mediated transcriptional activity. J. Biol. Chem. 286, 14137–14145. doi: 10.1074/jbc.M110.176289
Huttlin, E. L., Jedrychowski, M. P., Elias, J. E., Goswami, T., Rad, R., Beausoleil, S. A., et al. (2010). A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189. doi: 10.1016/j.cell.2010.12.001.
Jeninga, E. H., Schoonjans, K., and Auwerx, J. (2010). Reversible acetylation of PGC-1: connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene 29, 4617–4624. doi: 10.1038/onc.2010.206
Karin, M., and Hunter, T. (1995). Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr. Biol. 5, 747–757. doi: 10.1016/S0960-9822(95)00151-5
Keightley, M., Crowhurst, M. O., Layton, J. E., Beilharz, T. H., Markmiller, S., Varma, S., et al. (2013). In vivo mutation of pre-mRNA processing factor 8 (Prpf8) affects transcript splicing, cell survival and myeloid differentiation. FEBS Lett. 587, 2150–2157. doi: 10.1016/j.febslet.2013.05.030
Kim, H., Nakamura, F., Lee, W., Shifrin, Y., Arora, P. D., and McCulloch, C. A. (2010). Filamin A is required for vimentin-mediated cell adhesion and spreading. Am. J. Physiol. Cell Physiol. 298, C221–C236. doi: 10.1152/ajpcell.00323.2009
Kim, J., Woo, A. J., Chu, J., Snow, J. W., Fujiwara, Y., Kim, C. G., et al. (2010). A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell 143, 313–324. doi: 10.1016/j.cell.2010.09.010
Kouzarides, T. (2000). Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 19, 1176–1179. doi: 10.1093/emboj/19.6.1176
Kuzmanov, U., Guo, H., Buchsbaum, D., Cosme, J., Abbasi, C., Isserlin, R., et al. (2016). Global phosphoproteomic profiling reveals perturbed signaling in a mouse model of dilated cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 113, 12592–12597. doi: 10.1073/pnas.1606444113
Lammers, M., Neumann, H., Chin, J. W., and James, L. C. (2010). Acetylation regulates cyclophilin A catalysis, immunosuppression and HIV isomerization. Nat. Chem. Biol. 6, 331–337. doi: 10.1038/nchembio.342
Li, Y., Ye, Z., Nie, Y., Zhang, J., Wang, G.-L., and Wang, Z. (2015). Comparative phosphoproteome analysis of Magnaporthe oryzae-responsive proteins in susceptible and resistant rice cultivars. J. Proteomics 115, 66–80. doi: 10.1016/j.jprot.2014.12.007
Mantovani, F., Tocco, F., Girardini, J. E., Smith, P., Gasco, M., Lu, X., et al. (2007). The prolyl isomerase Pin1 orchestrates p53 acetylation and dissociation from the apoptosis inhibitor iASPP. Nat. Struct. Mol. Biol. 14, 912–920. doi: 10.1038/nsmb1306
Mashanov, V. S., Zueva, O. R., and García-Arrarás, J. E. (2011). Expression of Wnt9, TCTP, and Bmp1/Tll in sea cucumber visceral regeneration. Gene Expr. Pattern 12, 24–35. doi: 10.1016/j.gep.2011.10.003
Mashanov, V. S., Zueva, O. R., and García-Arrarás, J. E. (2013). Radial glial cells play a key role in echinoderm neural regeneration. BMC Biol. 11:49. doi: 10.1186/1741-7007-11-49
Mashanov, V. S., Zueva, O. R., and García-Arrarás, J. E. (2014). Transcriptomic changes during regeneration of the central nervous system in an echinoderm. BMC Genomics 15:357. doi: 10.1186/1471-2164-15-357
Miao, T., Wan, Z., Sun, L., Li, X., Xing, L., Bai, Y., et al. (2017). Extracellular matrix remodeling and matrix metalloproteinases (ajMMP-2 like and ajMMP-16 like) characterization during intestine regeneration of sea cucumber Apostichopus japonicus. Comp. Biochem. Phys. B 212, 12–23. doi: 10.1016/j.cbpb.2017.06.011
Murray, G., and García-Arrarás, J. (2004). Myogenesis during holothurian intestinal regeneration. Cell Tissue Res. 318, 515–524. doi: 10.1007/s00441-004-0978-3
Newton, A. C. (2003). Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 370, 361–371. doi: 10.1042/bj20021626
Ortiz-Pineda, P. A., Ramírez-Gómez, F., Pérez-Ortiz, J., González-Díaz, S., Santiago-De, Jesús, F., Hernández-Pasos, J., et al. (2009). Gene expression profiling of intestinal regeneration in the sea cucumber. BMC Genomics 10:262. doi: 10.1186/1471-2164-10-262
Pang, A., and Rennert, O. (2013). Protein acetylation and spermatogenesis. Reprod. Syst. Sex. Disord. S1:005. doi: 10.4172/2161-038X.S1-005
Revuelta-Cervantes, J., Mayoral, R., Miranda, S., González-Rodríguez, Á., Fernández, M., Martín-Sanz, P., et al. (2011). Protein tyrosine phosphatase 1B (PTP1B) deficiency accelerates hepatic regeneration in mice. Am. J. Pathol. 178, 1591–1604. doi: 10.1016/j.ajpath.2010.12.020
Rojas-Cartagena, C., Ortiz-Pineda, P. A., Ramirez-Gomez, F., Suarez-Castillo, E. C., Matos-Cruz, V., Rodriguez, C., et al. (2007). Distinct profiles of expressed sequence tags during intestinal regeneration in the sea cucumber Holothuria glaberrima. Physiol. Genomics 31, 203–215. doi: 10.1152/physiolgenomics.00228.2006
Silva, T. A., Smuczek, B., Valadao, I. C., Dzik, L. M., Iglesia, R. P., Cruz, M. C., et al. (2016). AHNAK enables mammary carcinoma cells to produce extracellular vesicles that increase neighboring fibroblast cell motility. Oncotarget 7, 49998–50016. doi: 10.18632/oncotarget.10307
Sirajuddin, M., Rice, L. M., and Vale, R. D. (2014). Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 16, 335–344. doi: 10.1038/ncb2920
Soppa, J. (2010). Protein acetylation in archaea, bacteria, and eukaryotes. Archaea 2010, 5399–5406. doi: 10.1155/2010/820681
Suárez-Castillo, E. C, Medina-Ortíz, W. E., Roig-López, J. L., and García-Arrarás, J. E. (2004). Ependymin, a gene involved in regeneration and neuroplasticity in vertebrates, is overexpressed during regeneration in the echinoderm Holothuria glaberrima. Gene 334, 133–143. doi: 10.1016/j.gene.2004.03.023
Sun, L., Chen, M., Yang, H., Wang, T., Liu, B., Shu, C., et al. (2011). Large scale gene expression profiling during intestine and body wall regeneration in the sea cucumber Apostichopus japonicus. Comp. Biochem. Phys. D 6, 195–205. doi: 10.1016/j.cbd.2011.03.002
Sun, L., Sun, J., Li, X., Zhang, L., Yang, H., and Wang, Q. (2017a). Understanding regulation of microRNAs on intestine regeneration in the sea cucumber Apostichopus japonicus using high-throughput sequencing. Comp. Biochem. Phys. D 22, 1–9. doi: 10.1016/j.cbd.2017.01.001
Sun, L., Sun, J., Xu, Q., Li, X., Zhang, L., and Yang, H. (2017b). Metabolic responses to intestine regeneration in sea cucumbers Apostichopus japonicus. Comp. Biochem. Phys. D 22, 32–38. doi: 10.1016/j.cbd.2017.02.003.
Sun, L., Xu, D., Xu, Q., Sun, J., Xing, L., Zhang, L., et al. (2017c). iTRAQ reveals proteomic changes during intestine regeneration in the sea cucumber Apostichopus japonicus. Comp. Biochem. Phys. D 22, 39–49. doi: 10.1016/j.cbd.2017.02.004
Sun, L., Yang, H., Chen, M., Ma, D., and Lin, C. (2013a). RNA-seq reveals dynamic changes of gene expression in key stages of intestine regeneration in the sea cucumber Apostichopus japonicus. PLoS ONE 8:e69441. doi: 10.1371/journal.pone.0069441
Sun, L. N., Yang, H. S., Chen, M. Y., and Xu, D. X. (2013b). Cloning and expression analysis of Wnt6 and Hox6 during intestinal regeneration in the sea cucumber Apostichopus japonicus. Genet. Mol. Res. 12, 5321–5334. doi: 10.4238/2013.November.7.7
Tskhovrebova, L., and Trinick, J. (2003). Titin: properties and family relationships. Nat. Rev. Mol. Cell Biol. 4, 679–689. doi: 10.1038/nrm1198
Van Noort, V., Seebacher, J., Bader, S., Mohammed, S., Vonkova, I., Betts, M. J., et al. (2012). Cross-talk between phosphorylation and lysine acetylation in a genome-reduced bacterium. Mol. Syst. Biol. 8, 571–571. doi: 10.1038/msb.2012.4
Vassilopoulos, A., Pennington, J. D., Andresson, T., Rees, D. M., Bosley, A. D., Fearnley, I. M., et al. (2014). SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid. Redox Sign. 21, 551–564. doi: 10.1089/ars.2013.5420
Vetting, M. W., Bareich, D. C., Yu, M., and Blanchard, J. S. (2008). Crystal structure of RimI from Salmonella typhimurium LT2, the GNAT responsible for Nα-acetylation of ribosomal protein S18. Protein Sci. 17, 1781–1790. doi: 10.1110/ps.035899.108
Vickery, M. C. L., Vickery, M. S., Amsler, C. D., and McClintock, J. B. (2001). Regeneration in echinoderm larvae. Microsc. Res. Techn. 55, 464–473. doi: 10.1002/jemt.1191
Yoon, W., Cho, Y., Kim, W., Bae, H., Islam, R., Woo, K., et al. (2014). Prolyl isomerase Pin1-mediated conformational change and subnuclear focal accumulation of Runx2 are crucial for fibroblast growth factor 2 (FGF2)-induced osteoblast differentiation. J. Biol. Chem. 289, 8828–8838. doi: 10.1074/jbc.M113.516237
Zhang, X., Sun, L., Yuan, J., Sun, Y., Gao, Y., Zhang, L., et al. (2017). The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biol. 15:e2003790. doi: 10.1371/journal.pbio.2003790.
Keywords: sea cucumber, intestine regeneration, phosphoproteomics, acetyl proteomics, posttranslational modification
Citation: Sun L, Lin C, Li X, Xing L, Huo D, Sun J, Zhang L and Yang H (2018) Comparative Phospho- and Acetyl Proteomics Analysis of Posttranslational Modifications Regulating Intestine Regeneration in Sea Cucumbers. Front. Physiol. 9:836. doi: 10.3389/fphys.2018.00836
Received: 10 April 2018; Accepted: 14 June 2018;
Published: 03 July 2018.
Edited by:
Youji Wang, Shanghai Ocean University, ChinaReviewed by:
Tony De Tomaso, University of California, Santa Barbara, United StatesZhigang Shen, Huazhong Agricultural University, China;
Pablo Andres Ortiz-Pineda, National Training Service, Colombia
Copyright © 2018 Sun, Lin, Li, Xing, Huo, Sun, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongsheng Yang, aHNoeWFuZ0BxZGlvLmFjLmNu
 Lina Sun
Lina Sun Chenggang Lin1,2
Chenggang Lin1,2