- 1Department of Geriatric Cardiology, Chinese People’s Liberation Army General Hospital, Beijing, China
- 2Department of Cardiology and Hainan Branch, Chinese People’s Liberation Army General Hospital, Beijing, China
- 3Department of Pharmaceutical Care, Chinese People’s Liberation Army General Hospital, Beijing, China
Heart failure (HF) is a primary cause of morbidity and mortality worldwide. As the most widely studied and commonly applied natriuretic peptide (NP), B-type natriuretic peptide (BNP) has the effects of diuresis, natriuresis, vasodilation, anti-hypertrophy, and anti-fibrosis and it inhibits the renin-angiotensin-aldosterone and sympathetic nervous systems to maintain cardiorenal homeostasis and counteract the effects of HF. Both BNP and N-terminal pro B-type natriuretic peptide (NT-proBNP) are applied as diagnostic, managing, and prognostic tools for HF. However, due to the complexity of BNP system, the diversity of BNP forms and the heterogeneity of HF status, there are biochemical, analytical, and clinical issues on BNP not fully understood. Current immunoassays cross-react to varying degrees with pro B-type natriuretic peptide (proBNP), NT-proBNP and various BNP forms and cannot effectively differentiate between these forms. Moreover, current immunoassays have different results and may not accurately reflect cardiac function. It is essential to design assays that can recognize specific forms of BNP, NT-proBNP, and proBNP to obtain more clinical information. Not only the processing of proBNP (corin/furin) and BNP (neprilysin), but also the effects of glycosylation on proBNP processing and BNP assays, should be targeted in future studies to enhance their diagnostic, therapeutic, and prognostic values.
Introduction
Heart failure is a primary cause of morbidity and mortality worldwide. As the most widely studied and commonly applied NP, BNP has the effects of diuresis, natriuresis, vasodilation, anti-hypertrophy and anti-fibrosis and it inhibits the renin-angiotensin-aldosterone and sympathetic nervous systems to maintain cardiorenal homeostasis and counteract the effects of HF (Semenov and Katrukha, 2016). Both BNP and NT-proBNP are established as HF biomarkers and recommended by international guidelines (Thygesen et al., 2012; Fu et al., 2018). Although affected by age, gender, obesity, renal function, and other factors, plasma BNP levels are closely related to HF severity and applied as diagnostic, managing and prognostic tools for HF (Vasile and Jaffe, 2017). However, due to the complexity of BNP system, the diversity of BNP forms and the heterogeneity of HF status, there are biochemical, analytical and clinical issues on BNP not fully understood (Iwaz and Maisel, 2016).
Biochemical Issues
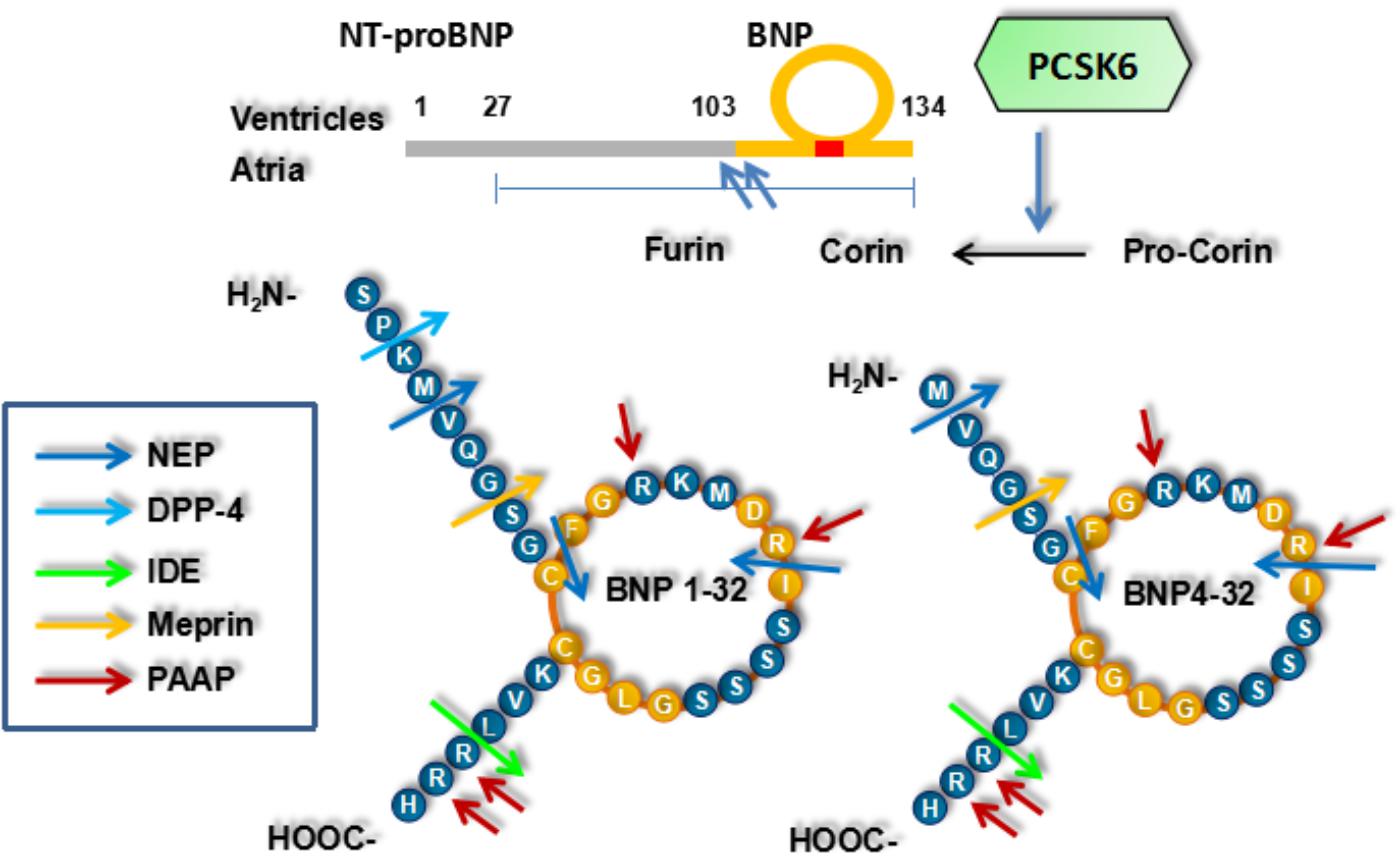
The 134-aa preproBNP is synthesized in the cardiomyocytes, and 108-aa proBNP is then produced by removing a 26-aa signal peptide (Figure 1). Enzyme-mediated processing of proBNP produces BNP (32-aa) with mature function and NT-proBNP (76-aa), which are released into the blood when the ventricular wall is stretched because of increased pressure or volume overload (Palazzuoli et al., 2010; Semenov et al., 2010). proBNP is degraded into NT-proBNP and BNP in a 1:1 ratio. NT-proBNP has no known bioactivity and is present at higher levels than BNP, perhaps due to slower clearance from blood (Bayes-Genis et al., 2004).
Corin and furin are the possible proBNP activating enzymes produced in the cardiomyocytes and both the soluble circulating and membrane-bound forms may be involved in proBNP processing (Clerico et al., 2011). Corin and furin are present at the cell surface with the ability to process proBNP and have soluble forms in blood raising the possibility of proBNP processing in blood (Jiang et al., 2011). Corin and furin may produce different BNP forms: BNP4-32 and BNP1-32, respectively (Semenov et al., 2011). However, other enzymes cannot be excluded in humans (Koo et al., 2006; Gladysheva et al., 2008). Soluble corin is significantly decreased in HF, reminding us that it may have an attenuated activation and be applied as a biomarker in HF (Miyazaki et al., 2016). Moreover, corin and furin activation may correlate with BNP bioactivity, whereas their deficiency may correlate with hypertension and HF (Chan et al., 2005). Corin and furin overexpression may be beneficial in experimental HF models. Unprocessed proBNP has higher levels in patients with HF, suggesting that their activities are rate-limiting factors in HF (Gladysheva et al., 2013). The various BNP forms have different cGMP activating properties and proBNP and NT-proBNP have reduced cGMP activities (Heublein et al., 2007; Dong et al., 2012). However, there have been almost no studies with the specific purpose of assessing proBNP processing in blood (Clerico et al., 2015a). proBNP processing is significantly disturbed in HF and may be a novel target for drugs (Del Ry et al., 2013; Clerico et al., 2015a).
A disintegrin and metalloprotease 10 mediates corin shedding and decreases corin bioactivity at the cell surface (Jiang et al., 2011). In humans, corin is expressed not only in the heart, but also in the kidney (proximal convoluted tubules and medullary collecting ducts) (Ichiki et al., 2011). In proximal tubular epithelial cells, corin is expressed in the apical membrane, whereas neprilysin is expressed in the brush border (Dong et al., 2016). Reduced renal corin expression and urine soluble corin in CKD may prevent local function of NPs (Fang et al., 2013). Reduced renal NP autocrine may contribute to NP resistance and further disturb cardiorenal homeostasis, if it is not compensated by increased cardiac NP endocrine (Dong et al., 2016). ADAM 10-mediated shedding may reduce corin levels in the kidney. Future studies are needed to analyze renal corin expression and ADAM 10-mediated shedding in patients with HF and/or CKD.
Proteolytic cleavage (Arg801-Ile802) may activate corin, with PCSK6 as an activating enzyme (Chen et al., 2015; Volpe and Rubattu, 2016). Among nine members of proprotein convertase subtilisin/kexin (PCSK) family, PCSK6 overexpression can enhance corin activation. Meanwhile, selective PCSK6 gene silencing by small interfering RNAs can abolish corin activation. PCSK6 gene expression can be detected in cells expressing corin, and corin variants without the cleavage site for PCSK6 are resistant to PCSK6 activation (Wang et al., 2008). However, previous studies have demonstrated that not only proBNP levels, but also cardiac function and hypertrophy, cannot be changed by affecting either corin or PCSK6, in spite of reduced proANP levels and effective control of hypertension (Chen et al., 2015). PCSK family includes a series of serine endoproteases with many substrates. Other substrates of PCSK6 are cytokines of TGF-β family, nodal growth differentiation factor pro-protein and aggrecanases (Turpeinen et al., 2013). Atrial natriuretic peptide (ANP) has an anti-hypertrophic effect on the heart through TGF-β signaling (Calvieri et al., 2012). PCSK6 may inhibit the cytokines of TGF-β family, and counteract the anti-hypertrophic effect of ANP. Another member of PCSK family, PCSK9, mediates degradation of low-density lipoprotein cholesterol and has been recommended as a target for a novel lipid-lowering drug (Navarese et al., 2015). As a corin activating enzyme, PCSK6 may be a novel target for drugs in HF by mediating proBNP degradation and increasing endogenous BNP (Volpe et al., 2016).
B-type natriuretic peptide is degraded by neprilysin, dipeptidyl peptidase-4 (DPP-4) and insulin-degrading enzyme (IDE) (Ralat et al., 2011). Peptidyl arginine aldehyde protease has been shown to degrade BNP at the sites with arginine, because its inhibitors reduce BNP degradation (Belenky et al., 2004). Meprin has been shown to degrade BNP in animals but not in humans. Whether other enzymes, including meprin, degrade BNP remains undetermined in humans (Dickey and Potter, 2010). BNP is degraded by neprilysin at several sites, but not at these sites simultaneously. proBNP differs from BNP with a 76-aa N-terminal extension and may not be a substrate of neprilysin, suggesting potential effects of NP length and N-terminal extension on neprilysin degradation (Pankow et al., 2009). Similarly, urodilatin is a N-terminal extended form of ANP and less rapidly degraded than ANP. D-type NP is not degraded and has the longest extension among NPs (Pankow et al., 2009). Neither glycosylated nor non-glycosylated forms of proBNP are sensitive to neprilysin degradation, suggesting no effect of glycosylation on proBNP resistance to neprilysin. Moreover, BNP may be a poorer substrate of neprilysin than ANP (Dickey and Potter, 2011). However, neprilysin has inconsistent effects on BNP degradation, perhaps because of different experimental conditions and race specificities.
Both proBNP and NT-proBNP are glycosylated in blood and the potential glycosylation sites are Thr36, Ser37, Ser44, Thr48, Ser53, Thr58, and Thr71 within the N-terminal region (aa residue 1–76), but not within the BNP (aa residue 77–108) (Seferian et al., 2008). All these glycosylation sites are complete except Thr36 and Thr58 (Schellenberger et al., 2006). NT-proBNP is glycosylated in the central region (aa residue 28–56), but not in the C-terminal region (aa residue 61–76) (Seferian et al., 2008). proBNP is glycosylated not only in the central region, but also in the region near the cleavage site (aa residue 63–76) (Semenov et al., 2009). Percentages of glycosylated proBNP and NT-proBNP are dependent on the individual. Patients with chronic HF, but not those with acute HF, have the highest percentage of glycosylated proBNP (Vodovar et al., 2014). Meanwhile, furin bioactivity, but not its levels, is greater in patients with acute HF than in those with chronic HF (Vodovar et al., 2014). proBNP processing may have different mechanisms: patients with acute HF have increased BNP production due to more acute fluid overload and patients with chronic HF have limited proBNP degradation due to less acute fluid overload. Glycosylation, especially at the Thr71 near the cleavage site, may inhibit corin- and furin-mediated degradation of proBNP in HF (Schellenberger et al., 2006). This effect remains undetermined but may correlate with whether proBNP is processed in blood (Peng et al., 2011; Halfinger et al., 2017).
Analytical Issues
Current NT-proBNP immunoassays have the same antibodies and calibrators from RocheTM with small systematic differences (Clerico et al., 2012). However, current BNP immunoassays (Table 1) have different antibodies and calibrators with large systematic differences (Clerico et al., 2005). The most common BNP immunoassays are sandwich immunoassays with two monoclonal or polyclonal antibodies binding to two separate epitopes: one binds to the ring structure to recognize the active form and the other binds to the N-terminal or C-terminal region (Franzini et al., 2013). The one binding to the C-terminal region [ShionogiTM IRMA and Siemens ADVIA for the Centaur platform] with same monoclonal antibodies (the epitope 27–32 and 14–21) may not recognize cleaved forms of BNP like BNP1-27, while the one binding to the N-terminal region [AlereTM and Beckman CoulterTM Triage BNP assays with the same monoclonal (the epitope 5–13) and polyclonal (the possible epitope 1–10) antibodies] may not recognize cleaved forms of BNP like BNP3-32 (Belenky et al., 2004). The single-epitope sandwich (SES)-BNPTM immunofluorescent assay needs only one epitope by two different monoclonal antibodies, including the first monoclonal antibody (24C5) binding to the epitope 11–17, which is the most stable within the ring structure, and the second monoclonal antibody (Ab-BNP2) binding to the immune complex (the epitope 11–17 and 24C5) (Tamm et al., 2008). As a highly sensitive assay, it stabilizes the immune complex and increases epitope affinity but recognizes not only BNP forms, but also glycosylated and non-glycosylated forms of proBNP.
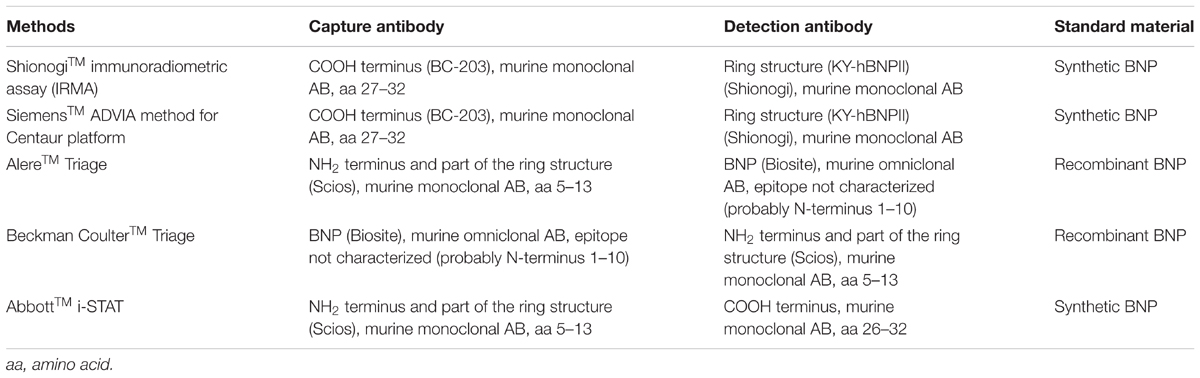
TABLE 1. Antibodies and standard materials used in commercial B-type natriuretic peptide (BNP) immunoassays.
Current BNP immunoassays substantially reflect total levels of proBNP and BNP forms (Liang et al., 2007). Although proBNP processing occurs before or during secretion, unprocessed proBNP is present in blood at even higher levels than BNP and represents a significant part of BNP immunoreactivity in healthy individuals and HF patients (Costello-Boerrigter et al., 2013). Corin and furin cannot process all the proBNP when proBNP production is obviously increased in patients with HF, or proBNP glycosylation occurs before secretion, especially at the Thr71 (Clerico et al., 2015a). Because proBNP shares a 32-aa structure with BNP, proBNP can mediate physiological functions like BNP. However, as the predominant form of BNP immunoreactivity in HF, unprocessed proBNP has an obviously decreased physiological function compared with BNP (Liang et al., 2007). Meanwhile, due to the cleavage of amino-terminal dipeptide from BNP1-32 by DPP-4 and neprilysin, BNP3-32 and 5–32 are present in blood. BNP3-32 rather than BNP1-32 may be the predominant form of BNP (Suzuki et al., 2017). Compared with BNP1-32, other BNP forms, such as BNP3-32, BNP4-32, BNP5-32, BNP5-31, BNP1-27, BNP 1-26, and BNP 1-25, have an obviously decreased physiological function and increased degradation rate by neprilysin (Brandt et al., 2006). However, due to race-specific proteases, BNP forms may have no effect on the resistance of human BNP to neprilysin (Brandt et al., 2006). Current BNP immunoassays overestimate BNP1-32 levels, because they also recognize less active BNP forms (Lewis et al., 2017).
Antibody detection of NT-proBNP and proBNP may also be affected by glycosylation (Peng et al., 2011). Glycosylation suppresses binding of antibodies and makes them lose immunoreactivity (Luckenbill et al., 2008). Current NT-proBNP immunoassays cross-react with non-glycosylated proBNP, and do not detect glycosylated NT-proBNP and proBNP. NT-proBNP immunoassays may be improved by antibodies detecting glycosylated or non-glycosylated NT-proBNP and antibodies detecting NT-proBNP not affected by glycosylation (Rosjo et al., 2015). In current proBNP immunoassays, glycosylated proBNP cross-reacts more than non-glycosylated proBNP with BNP and NT-proBNP (Emdin et al., 2011). There are inter-individual differences in NT-proBNP and proBNP glycosylation in patients with and without HF (Saenger et al., 2017). The N- and C-terminal regions of NT-proBNP and BNP are degraded in blood, which occurs between Pro2-Leu3, Leu3-Gly4, Pro6-Gly7, and Pro75-Arg76 of NT-proBNP (Foo et al., 2013). Thus, detecting N- and C-terminal cleaved forms of BNP and NT-proBNP is another challenge and it is difficult to develop an antibody not affected by glycosylation or terminal cleavage.
A highly sensitive immunoassay for proBNP is not affected by proBNP glycosylation, because it has a capture monoclonal antibody binding to the epitope 26–32 of BNP and a detection monoclonal antibody binding to the epitope 13–20 of proBNP, neither of which are glycosylation sites (Seferian et al., 2007). Another immunoassay for proBNP has no significant cross-reaction with both NT-proBNP and BNP, because it has a polyclonal antibody binding to BNP and a monoclonal antibody binding to the cleavage site of proBNP, an epitope only belonging to proBNP (Macheret et al., 2011). Meanwhile, a radioimmunoassay for proBNP binds to the N-terminal of proBNP (the epitope 1–10) and recognizes both proBNP and NT-proBNP (Goetze et al., 2002). Automated immunoassays specific for both proBNP and BNP1-32 may be useful to determine both production and bioactivity of BNP forms. Moreover, proBNP and BNP immunoassays have been combined to better predict poor prognosis in patients with HF (Dries et al., 2010). Two immunoassays can be simultaneously applied to the same sample. However, BNP1-32 immunoassays with chromatography and mass spectrometry are unsuitable for routine application and there is no commercially available immunoassay that can recognize only active BNP1-32 (Miller et al., 2011).
Neprilysin inhibition may have varied effects on plasma BNP levels as a result of different immunoassays. The N-terminal Met4-Phe5 is the initial cleavage site and no BNP immunoassay has any antibody binding to it. Another cleavage site is located within the ring structure (Arg17-Ile18), which is cleaved before other sites, such as Lys14-Met15, Gly23-Leu24, and Val28-Leu29, and BNP immunoassays (ShionogiTM and SiemensTM) with antibodies binding to the epitopes 14–21 may be sensitive to neprilysin degradation (Clerico et al., 2015b). With only one epitope and without space between epitopes, SES-BNPTM assay is not sensitive to neprilysin degradation.
Blood samples for BNP assays should be drawn only in plastic tubes because BNP is unstable in glass tubes due to kallikrein activation (Apple et al., 2005). EDTA plasma is the only recommended specimen for BNP assays and serum is the recommended specimen for NT-proBNP assays. There are significant differences between serum and plasma levels of NPs with various detection platforms. Anticoagulant type is also significant. BNP is stable during storage at room temperature for 24 h or at 30°C for 12 h. Protease inhibitor (aprotinin) can be added to increase BNP storage time. NT-proBNP is stable during storage in serum, heparinized plasma or EDTA plasma at room temperature or at 4°C for 72 h or at -80°C for up to 1 year (Dong et al., 2012). It is essential to validate the effect of freeze-thaw cycles on the stability of BNP and NT-proBNP assays (Apple et al., 2005).
Clinical Issues
Point of care testing is performed near the patients outside of the central laboratory with a rapid turnaround (Iwaz and Maisel, 2016). POCT for BNP and NT-proBNP can effectively facilitate not only home-monitoring and community-service outside of hospitals, but also emergency testing and BNP-guided therapy in hospitals (Christenson et al., 2014). However, due to poor performance (particularly sensitivity and precision) compared with laboratory assays, POCT for BNP and NT-proBNP has not been finally approved by authoritative organizations and is not widely available for clinical application (Jungbauer et al., 2012). POCT for BNP with untreated fingertip capillary whole blood (AlereTM Heart Check) closely correlates with POCTs for BNP with venipuncture EDTA plasma (AlereTM Triage) or EDTA whole blood (AbbottTM i-STAT) (Maisel et al., 2013; Prontera et al., 2015). AbbottTM i-STAT produces a result within 10 min, whereas AlereTM Triage and AlereTM Heart Check produces their results within 15 min. It remains undetermined whether POCTs have good correlation with and similar precision to laboratory assays (Shah et al., 2010). AbbottTM i-STAT and AlereTM Triage reduce HF diagnosis compared with laboratory assays (Kosowsky et al., 2006). Both POCT and laboratory assays have systematic differences caused by cross-reaction with glycosylated or non-glycosylated forms (Clerico et al., 2015b).
In the emergency room, rapid assay for BNP and NT-proBNP can discriminate the origin of acute dyspnea (acute HF versus bronchial asthma) (Fu et al., 2018). A plasma NT-proBNP level of 300 pg/ml is appropriate for ruling out acute HF. Age-dependent cutoff levels of plasma NT-proBNP are appropriate for ruling in acute HF: 450 pg/ml in patients <50 years of age, 900 pg/ml in patients ≥50 years of age, and 1800 pg/ml in patients >75 years of age (McMurray et al., 2012). Plasma BNP levels of 100 and 400 pg/ml are appropriate for ruling out and ruling in acute HF, respectively (Dickstein et al., 2008).
The recombinant form of BNP (nesiritide) has been applied as a conventional drug in HF. It is currently considered to improve clinical symptoms and cardiac function and has no effect on patient prognosis (Fu et al., 2012). An angiotensin receptor inhibitor and neprilysin inhibitor (ARNI, LCZ696) has been approved as a novel drug in HF by US Food and Drug Administration (McMurray et al., 2013). Because LCZ696 affects plasma BNP levels through inhibiting BNP degradation by neprilysin, it makes plasma BNP levels not accurately reflect cardiac function and produces a challenge for using BNP as a biomarker, making HF diagnostically ambiguous and therapeutically misleading (Packer et al., 2015). It could be argued that NT-proBNP is insensitive to neprilysin degradation and therefore can be applied as a biomarker in patients with LCZ696. However, this assumption is based on a simplified model of a complex biological phenomenon, and more studies are essential to analyze this biological phenomenon. Due to the complexity of BNP system (proBNP and BNP forms), neprilysin inhibition does not have a straightforward effect on plasma BNP levels (Pemberton et al., 2012). It remains undetermined how LCZ696 affects plasma BNP and NT-proBNP levels. Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial has shown that plasma BNP levels increase while plasma NT-proBNP levels decrease in patients with LCZ696. Angiotensin receptor inhibitor and neprilysin inhibitor (ARNI, LCZ696) may inhibit both BNP degradation and proBNP processing. Increased BNP levels may inhibit proBNP production and thus decreased NT-proBNP levels may not be caused by improved cardiac function. Moreover, beneficial effects of LCZ696 in HF may be accomplished by increasing plasma ANP and C-type natriuretic peptide (CNP) levels but not plasma BNP levels. However, considering the presence of BNP in blood and its sensitivity to neprilysin, a relatively modest increase in plasma BNP levels may also account for improved prognosis with LCZ696 in patients with HF (McMurray et al., 2014). Several studies have realized improved mortality and admission with BNP-guided therapy (Valle et al., 2011). However, previous studies have yielded inconsistent results on NT-proBNP-guided therapy (Sanders-van Wijk et al., 2014). Current randomized clinical trials on BNP-guided therapy may further evaluate BNP-guided therapy in patients with HF (Januzzi and Troughton, 2013).
Conclusion
B-type natriuretic peptide metabolism and its forms are complex and make assays particularly challenging, but critical to providing future insight into BNP application and HF heterogeneity. Current immunoassays cross-react to varying degrees with proBNP, NT-proBNP, and various BNP forms and cannot effectively differentiate between these forms. Moreover, current immunoassays have different results and may not accurately reflect cardiac function. It is essential to design assays that can recognize specific forms of BNP, NT-proBNP, and proBNP to obtain more clinical information. Considering the complexity of BNP system and the heterogeneity of HF status, not only the processing of proBNP (corin/furin) and BNP (neprilysin), but also the effects of glycosylation on proBNP processing and BNP assays, should be targeted in future studies to enhance their diagnostic, therapeutic and prognostic values.
Author Contributions
SF, PP, QZ, PY, and LL contributed to the study design, performed data collection and analyses, and drafted this paper.
Funding
This study was supported by grants from the National Key Basic Research Project (2012CB517503 and 2013CB530804), the Health Special Scientific Research Subject of Chinese People’s Liberation Army (12BJZ34 and 14BJZ12), the Sanya Medical and Health Science and Technology Innovation Project (2016YW21), and the Clinical Scientific Research Supporting Fund of Chinese People’s Liberation Army General Hospital (2017FC-CXYY-3009).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
aa, amino acid; ADAM, a disintegrin and metalloprotease; ARNI, angiotensin receptor inhibitor and neprilysin inhibitor; BNP, B-type natriuretic peptide; CKD, chronic kidney disease; EDTA, ethylenediaminetetraacetic acid; HF, heart failure; IRMA, immunoradiometric assay; NP, natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide; PCSK6, proprotein convertase subtilisin/kexin-6; POCT, point of care testing; proANP, pro-atrial natriuretic peptide; proBNP, pro B-type natriuretic peptide; TGF-β, transforming growth factor-β.
References
Apple, F. S., Panteghini, M., Ravkilde, J., Mair, J., Wu, A. H., Tate, J., et al. (2005). Quality specifications for B-type natriuretic peptide assays. Clin. Chem. 51, 486–493. doi: 10.1373/clinchem.2004.044594
Bayes-Genis, A., Santalo-Bel, M., Zapico-Muniz, E., Lopez, L., Cotes, C., Bellido, J., et al. (2004). N-terminal probrain natriuretic peptide (NT-proBNP) in the emergency diagnosis and in-hospital monitoring of patients with dyspnoea and ventricular dysfunction. Eur. J. Heart Fail. 6, 301–308. doi: 10.1016/j.ejheart.2003.12.013
Belenky, A., Smith, A., Zhang, B., Lin, S., Despres, N., Wu, A. H., et al. (2004). The effect of classspecific protease inhibitors on the stabilization of B-type natriuretic peptide in human plasma. Clin. Chim. Acta 340, 163–172. doi: 10.1016/j.cccn.2003.10.026
Brandt, I., Lambeir, A. M., Ketelslegers, J. M., Vanderheyden, M., Scharpe, S., and De Meester, I. (2006). Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin. Chem. 52, 82–87. doi: 10.1373/clinchem.2005.057638
Calvieri, C., Rubattu, S., and Volpe, M. (2012). Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides. J. Mol. Med. 90, 5–13. doi: 10.1007/s00109-011-0801-z
Chan, J. C., Knudson, O., Wu, F., Morser, J., Dole, W. P., and Wu, Q. (2005). Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc. Natl. Acad. Sci. U.S.A. 102, 785–790. doi: 10.1073/pnas.0407234102
Chen, S., Cao, P., Dong, N., Peng, J., Zhang, C., Wang, H., et al. (2015). PCSK6-mediated corin activation is essential for normal blood pressure. Nat. Med. 21, 1048–1053. doi: 10.1038/nm.3920
Christenson, E. S., Collinson, P. O., Defilippi, C. R., and Christenson, R. H. (2014). Heart failure biomarkers at point-of-care: current utilization and future potential. Expert Rev. Mol. Diagn. 14, 185–197. doi: 10.1586/14737159.2014.882772
Clerico, A., Franzini, M., Masotti, S., Prontera, C., and Passino, C. (2015a). State of the art of immunoassay methods for B-type natriuretic peptides: an update. Crit. Rev. Clin. Lab. Sci. 52, 56–69. doi: 10.3109/10408363.2014.987720
Clerico, A., Giannoni, A., Vittorini, S., and Passino, C. (2011). Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones. Am. J. Physiol. Heart Circ. Physiol. 301, H12–H20. doi: 10.1152/ajpheart.00226.2011
Clerico, A., Passino, C., Franzini, M., and Emdin, M. (2015b). Cardiac biomarker testing in the clinical laboratory: where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin. Chim. Acta 443, 17–24. doi: 10.1016/j.cca.2014.06.003
Clerico, A., Prontera, C., Emdin, M., Passino, C., Storti, S., Poletti, R., et al. (2005). Analytical performance and diagnostic accuracy of immunometric assays for the measurement of plasma BNP and NT-proBNP concentrations. Clin. Chem. 51, 445–447. doi: 10.1373/clinchem.2004.038281
Clerico, A., Zaninotto, M., Prontera, C., Giovannini, S., Ndreu, R., Franzini, M., et al. (2012). State of the art of BNP and NT-proBNP immunoassays: the CardioOrmoCheck study. Clin. Chim. Acta 414, 112–119. doi: 10.1016/j.cca.2012.07.017
Costello-Boerrigter, L. C., Lapp, H., Boerrigter, G., Lerman, A., Bufe, A., Macheret, F., et al. (2013). Secretion of prohormone of B-type natriuretic peptide, proBNP1-108, is increased in heart failure. JACC Heart Fail. 1,207–212. doi: 10.1016/j.jchf.2013.03.001
Del Ry, S., Cabiati, M., and Clerico, A. (2013). Recent advances on natriuretic peptide system: new promising therapeutic targets for the treatment of heart failure. Pharmacol. Res. 76, 190–198. doi: 10.1016/j.phrs.2013.08.006
Dickey, D. M., and Potter, L. R. (2010). Human B-type natriuretic peptide is not degraded by meprin A. Biochem. Pharmacol. 80, 1007–1011. doi: 10.1016/j.bcp.2010.06.015
Dickey, D. M., and Potter, L. R. (2011). ProBNP1-108 is resistant to degradation and activates guanylyl cyclase-A with reduced potency. Clin. Chem. 57,1272–1278. doi: 10.1373/clinchem.2011.169151
Dickstein, K., Cohen-Solal, A., Filippatos, G., McMurray, J. J., Ponikowski, P., Poole-Wilson, P. A., et al. (2008). ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J. 29, 2388–2442. doi: 10.1093/eurheartj/ehn309
Dong, L., Wang, H., Dong, N., Zhang, C., Xue, B., and Wu, Q. (2016). Localization of corin and atrial natriuretic peptide expression in human renal segments. Clin. Sci. 130, 1655–1664. doi: 10.1042/CS20160398
Dong, N., Chen, S., Wang, W., Zhou, Y., and Wu, Q. (2012). Corin in clinical laboratory diagnostics. Clin. Chim. Acta 413, 378–383. doi: 10.1016/j.cca.2011.10.032
Dries, D. L., Ky, B., Wu, A. H., Rame, J. E., Putt, M. E., and Cappola, T. P. (2010). Simultaneous assessment of unprocessed ProBNP1-108 in addition to processed BNP32 improves identification of high-risk ambulatory patients with heart failure. Circ. Heart Fail. 3, 220–227. doi: 10.1161/CIRCHEARTFAILURE.109.903153
Emdin, M., Passino, C., and Clerico, A. (2011). Natriuretic peptide assays revisited: do we need pro-B-type natriuretic peptide? J. Am. Coll. Cardiol. 57, 1396–1398. doi: 10.1016/j.jacc.2010.09.075
Fang, C., Shen, L., Dong, L., Liu, M., Shi, S., Dong, N., et al. (2013). Reduced urinary corin levels in patients with chronic kidney disease. Clin. Sci. 124, 709–717. doi: 10.1042/CS20120517
Foo, J. Y., Wan, Y., Schulz, B. L., Kostner, K., Atherton, J., Cooper-White, J., et al. (2013). Circulating fragments of N-terminal pro-B-type natriuretic peptides in plasma of heart failure patients. Clin. Chem. 59, 1523–1531. doi: 10.1373/clinchem.2012.200204
Franzini, M., Masotti, S., Prontera, C., Ripoli, A., Passino, C., Giovannini, S., et al. (2013). Systematic differences between BNP immunoassays: comparison of methods using standard protocols and quality control materials. Clin. Chim. Acta 424, 287–291. doi: 10.1016/j.cca.2013.07.001
Fu, S., Ping, P., Wang, F., and Luo, L. (2018). Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 12:2. doi: 10.1186/s13036-017-0093-0
Fu, S., Yi, S., Zhu, B., Wang, L., Wang, H., Bai, Y., et al. (2012). Efficacy and safety of a modified dosage regimen of nesiritide in patients older than 75 years with acute heart failure. Aging Clin. Exp. Res. 24, 524–529. doi: 10.3275/8295
Gladysheva, I. P., Robinson, B. R., Houng, A. K., Kovats, T., and King, S. M. (2008). Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J. Mol. Cell. Cardiol. 44, 131–142. doi: 10.1016/j.yjmcc.2007.10.002
Gladysheva, I. P., Wang, D., McNamee, R. A., Houng, A. K., Mohamad, A. A., Fan, T. M., et al. (2013). Corin overexpression improves cardiac function, heart failure, and survival in mice with dilated cardiomyopathy. Hypertension 61, 327–332. doi: 10.1161/HYPERTENSIONAHA.112.193631
Goetze, J. P., Kastrup, J., Pedersen, F., and Rehfeld, J. F. (2002). Quantification of pro-B-type natriuretic peptide and its products in human plasma by use of an analysis independent of precursor processing. Clin. Chem. 48, 1035–1042.
Halfinger, B., Hammerer-Lercher, A., Amplatz, B., Sarg, B., Kremser, L., and Lindner, H. H. (2017). Unraveling the molecular complexity of O-glycosylated endogenous (N-Terminal) pro-B-type natriuretic peptide forms in blood plasma of patients with severe heart failure. Clin. Chem. 63, 359–368. doi: 10.1373/clinchem.2016.265397
Heublein, D. M., Huntley, B. K., Boerrigter, G., Cataliotti, A., Sandberg, S. M., Redfield, M. M., et al. (2007). Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension 49, 1114–1119. doi: 10.1161/HYPERTENSIONAHA.106.081083
Ichiki, T., Huntley, B. K., Heublein, D. M., Sandberg, S. M., McKie, P. M., Martin, F. L., et al. (2011). Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin. Chem. 57, 40–47. doi: 10.1373/clinchem.2010.153908
Iwaz, J. A., and Maisel, A. S. (2016). Recent advances in point-of-care testing for natriuretic peptides: potential impact on heart failure diagnosis and management. Expert Rev. Mol. Diagn. 16, 641–650. doi: 10.1586/14737159.2016.1158105
Januzzi, J. L., and Troughton, R. (2013). Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide measurements are useful in heart failure management. Circulation 127, 500–507. doi: 10.1161/CIRCULATIONAHA.112.120485
Jiang, J., Wu, S., Wang, W., Chen, S., Peng, J., Zhang, X., et al. (2011). Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J. Biol. Chem. 286, 10066–10072. doi: 10.1074/jbc.M110.185082
Jungbauer, C. G., Kaess, B., Buchner, S., Birner, C., Lubnow, M., Resch, M., et al. (2012). Equal performance of novel N-terminal proBNP (Cardiac proBNP) and established BNP (Triage BNP) point-of-care tests. Biomark. Med. 6, 789–796. doi: 10.2217/bmm.12.67
Koo, B. H., Longpre, J. M., Somerville, R. P., Alexander, J. P., Leduc, R., and Apte, S. S. (2006). Cell-surface processing of pro-ADAMTS9 by furin. J. Biol. Chem. 281, 12485–12494. doi: 10.1074/jbc.M511083200
Kosowsky, J. M., Weiner, C., Aronson, A. A., and Morrissey, J. H. (2006). Impact of point-of-care Btype natriuretic peptide (BNP) measurement on medical decision-making for older emergency department patients with dyspnea. J. Emerg. Med. 31, 147–150. doi: 10.1016/j.jemermed.2005.10.001
Lewis, L. K., Raudsepp, S. D., Yandle, T. G., Prickett, T. C., and Richards, A. M. (2017). Development of a BNP1-32 immunoassay that does not cross-react with proBNP. Clin. Chem. 63, 1110–1117. doi: 10.1373/clinchem.2016.269712
Liang, F., O’Rear, J., Schellenberger, U., Tai, L., Lasecki, M., Schreiner, G. F., et al. (2007). Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J. Am. Coll. Cardiol. 49, 1071–1078. doi: 10.1016/j.jacc.2006.10.063
Luckenbill, K. N., Christenson, R. H., Jaffe, A. S., Mair, J., Ordonez-Llanos, J., Pagani, F., et al. (2008). Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for standardization of markers of cardiac damage. Clin. Chem. 54, 619–621. doi: 10.1373/clinchem.2007.097998
Macheret, F., Boerrigter, G., McKie, P., Costello-Boerrigter, L., Lahr, B., Heublein, D., et al. (2011). Pro-B-type natriuretic peptide 1-108 circulates in the general community: plasma determinants and detection of left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 57, 1386–1395. doi: 10.1016/j.jacc.2011.01.005
Maisel, A., Barnard, D., Jaski, B., Frivold, G., Marais, J., Azer, M., et al. (2013). Primary results of the HABIT Trial (Heart failure assessment with BNP in the home). J. Am. Coll. Cardiol. 61, 1726–1735. doi: 10.1016/j.jacc.2013.01.052
McMurray, J. J., Adamopoulos, S., Anker, S. D., Auricchio, A., Böhm, M., Dickstein, K., et al. (2012). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 33, 1787–1847. doi: 10.1093/eurheartj/ehs104
McMurray, J. J., Packer, M., Desai, A. S., Gong, J., Lefkowitz, M. P., Rizkala, A. R., et al. (2013). Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM-HF). Eur. J. Heart Fail. 15, 1062–1073. doi: 10.1093/eurjhf/hft052
McMurray, J. J., Packer, M., Desai, A. S., Gong, J., Lefkowitz, M. P., Rizkala, A. R., et al. (2014). Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004. doi: 10.1056/NEJMoa1409077
Miller, W. L., Phelps, M. A., Wood, C. M., Schellenberger, U., Van Le, A., Perichon, R., et al. (2011). Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-Type natriuretic peptide in patients with chronic heart failure. Circ. Heart Fail. 4, 335–360. doi: 10.1161/CIRCHEARTFAILURE.110.960260
Miyazaki, J., Nishizawa, H., Kambayashi, A., Ito, M., Noda, Y., Terasawa, S., et al. (2016). Increased levels of soluble corin in pre-eclampsia and fetal growth restriction. Placenta 48, 20–25. doi: 10.1016/j.placenta.2016.10.002
Navarese, E. P., Kolodziejczak, M., Schulze, V., Gurbel, P. A., Tantry, U., Lin, Y., et al. (2015). Effects of Proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann. Intern. Med. 163, 40–51. doi: 10.7326/M14-2957
Packer, M., McMurray, J. J., Desai, A. S., Gong, J., Lefkowitz, M. P., Rizkala, A. R., et al. (2015). Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 131, 54–61. doi: 10.1161/CIRCULATIONAHA.114.013748
Palazzuoli, A., Gallotta, M., Quatrini, I., and Nuti, R. (2010). Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. Vasc. Health Risk Manag. 6, 411–418. doi: 10.2147/VHRM.S5789
Pankow, K., Schwiebs, A., Becker, M., Siems, W. E., Krause, G., and Walther, T. (2009). Structural substrate conditions required for neutral endopeptidase-mediated natriuretic peptide degradation. J. Mol. Biol. 393, 496–503. doi: 10.1016/j.jmb.2009.08.025
Pemberton, C. J., Siriwardena, M., Kleffmann, T., Ruygrok, P., Palmer, S. C., Yandle, T. G., et al. (2012). First identification of circulating prepro-A-type natriuretic peptide (preproANP) signal peptide fragments in humans: initial assessment as cardiovascular biomarkers. Clin. Chem. 58, 757–767. doi: 10.1373/clinchem.2011.176990
Peng, J., Jiang, J., Wang, W., Qi, X., Sun, X. L., and Wu, Q. (2011). Glycosylation and processing of pro-B-type natriuretic peptide in cardiomyocytes. Biochem. Biophys. Res. Commun. 411, 593–598. doi: 10.1016/j.bbrc.2011.06.192
Prontera, C., Masotti, S., Franzini, M., Emdin, M., Passino, C., Zucchelli, G. C., et al. (2015). Comparison between BNP values measured in capillary blood samples with a POCT method and those measured in plasma venous samples with an automated platform. Clin. Chem. Lab. Med. 53, e125–e127. doi: 10.1515/cclm-2014-0873
Ralat, L. A., Guo, Q., Ren, M., Funke, T., Dickey, D. M., Potter, L. R., et al. (2011). Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. J. Biol. Chem. 286, 4670–4679. doi: 10.1074/jbc.M110.173252
Rosjo, H., Dahl, M. B., Jorgensen, M., Roysland, R., Brynildsen, J., Cataliotti, A., et al. (2015). Influence of glycosylation on diagnostic and prognostic accuracy of N-terminal pro-B-type natriuretic peptide in acute dyspnea: data from the Akershus cardiac examination 2 study. Clin. Chem. 61, 1087–1097. doi: 10.1373/clinchem.2015.239673
Saenger, A. K., Rodriguez-Fraga, O., Ler, R., Ordonez-Llanos, J., Jaffe, A. S., Goetze, J. P., et al. (2017). Specificity of B-Type natriuretic peptide assays: cross-reactivity with different BNP, NT-proBNP, and proBNP Peptides. Clin. Chem. 63, 351–358. doi: 10.1373/clinchem.2016.263749
Sanders-van Wijk, S., Maeder, M. T., Nietlispach, F., Rickli, H., Estlinbaum, W., Erne, P., et al. (2014). Long-term results of intensified, N-terminal-pro-B-type natriuretic peptide-guided versus symptom-guided treatment in elderly patients with heart failure: five-year follow-up from TIME-CHF. Circ. Heart Fail. 7, 131–139. doi: 10.1161/CIRCHEARTFAILURE.113.000527
Schellenberger, U., O’Rear, J., Guzzetta, A., Jue, R. A., Protter, A. A., and Pollitt, N. S. (2006). The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch. Biochem. Biophys. 451, 160–166. doi: 10.1016/j.abb.2006.03.028
Seferian, K. R., Tamm, N. N., Semenov, A. G., Mukharyamova, K. S., Tolstaya, A. A., Koshkina, E. V., et al. (2007). The brain natriuretic peptide (BNP) precursor is the major immunoreactive form of BNP in patients with heart failure. Clin. Chem. 53, 866–873. doi: 10.1373/clinchem.2006.076141
Seferian, K. R., Tamm, N. N., Semenov, A. G., Tolstaya, A. A., Koshkina, E. V., Krasnoselsky, M. I., et al. (2008). Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin. Chem. 54, 866–873. doi: 10.1373/clinchem.2007.100040
Semenov, A. G., and Katrukha, A. G. (2016). Different susceptibility of B-Type natriuretic peptide (BNP) and BNP precursor (proBNP) to cleavage by neprilysin: the N-Terminal part does matter. Clin. Chem. 62, 617–622. doi: 10.1373/clinchem.2016.254524
Semenov, A. G., Postnikov, A. B., Tamm, N. N., Seferian, K. R., Karpova, N. S., Bloshchitsyna, M. N., et al. (2009). Processing of probrain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin. Chem. 55, 489–498. doi: 10.1373/clinchem.2008.113373
Semenov, A. G., Seferian, K. R., Tamm, N. N., Artem’eva, M. M., Postnikov, A. B., Bereznikova, A. V., et al. (2011). Human pro-B-type natriuretic peptide is processed in the circulation in a rat model. Clin. Chem. 57, 883–890. doi: 10.1373/clinchem.2010.161125
Semenov, A. G., Tamm, N. N., Seferian, K. R., Postnikov, A. B., Karpova, N. S., Serebryanaya, D. V., et al. (2010). Processing of pro-Btype natriuretic peptide: furin and corin as candidate convertases. Clin. Chem. 56, 1166–1176. doi: 10.1373/clinchem.2010.143883
Shah, K., Terracciano, G. J., Jiang, K., Maisel, A. S., and Fitzgerald, R. L. (2010). Comparability of results between point-of-care and automated instruments to measure B-type natriuretic peptide. West. J. Emerg. Med. 11, 44–48.
Suzuki, T., Israr, M. Z., Heaney, L. M., Takaoka, M., Squire, I. B., and Ng, L. L. (2017). Prognostic role of molecular forms of B-Type natriuretic peptide in acute heart failure. Clin. Chem. 63, 880–886. doi: 10.1373/clinchem.2016.265140
Tamm, N. N., Seferian, K. R., Semenov, A. G., Mukharyamova, K. S., Koshkina, E. V., Krasnoselsky, M. I., et al. (2008). Novel immunoassay for quantification of brain natriuretic peptide and its precursor in human blood. Clin. Chem. 54, 1511–1518. doi: 10.1373/clinchem.2007.100545
Thygesen, K., Mair, J., Mueller, C., Huber, K., Weber, M., Plebani, M., et al. (2012). Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur. Heart J. 33, 2001–2006. doi: 10.1093/eurheartj/ehq509
Turpeinen, H., Ortutay, Z., and Pesu, M. (2013). Genetics of the first seven proprotein convertase enzymes in health and disease. Curr. Genomics 14, 453–467. doi: 10.2174/1389202911314050010
Valle, R., Aspromonte, N., Milani, L., Peacock, F. W., Maisel, A. S., Santini, M., et al. (2011). Optimizing fluid management in patients with acute decompensated heart failure (ADHF): the emerging role of combined measurement of body hydration status and brain natriuretic peptide (BNP) levels. Heart Fail. Rev. 16, 519–529. doi: 10.1007/s10741-011-9244-4
Vasile, V. C., and Jaffe, A. S. (2017). Natriuretic peptides and analytical barriers. Clin. Chem. 63, 50–58. doi: 10.1373/clinchem.2016.254714
Vodovar, N., Seronde, M. F., Laribi, S., Gayat, E., Lassus, J., Boukef, R., et al. (2014). Post-translational modifications enhance NT-proBNP and BNP production in acute decompensated heart failure. Eur. Heart J. 35, 3434–3441. doi: 10.1093/eurheartj/ehu314
Volpe, M., Battistoni, A., and Mastromarino, V. (2016). Natriuretic peptides and volume handling in heart failure: the paradigm of a new treatment. Eur. J. Heart Fail. 18, 442–444. doi: 10.1002/ejhf.468
Volpe, M., and Rubattu, S. (2016). Novel insights into the mechanisms regulating pro-atrial natriuretic peptide cleavage in the heart and blood pressure regulation: proprotein convertase subtilisin/kexin 6 is the corin activating enzyme. Circ. Res. 118, 196–198. doi: 10.1161/CIRCRESAHA.115.307875
Keywords: a disintegrin and metalloprotease, B-type natriuretic peptide, chronic kidney disease, corin, furin, heart failure, LCZ696, proprotein convertase subtilisin/kexin-6
Citation: Fu S, Ping P, Zhu Q, Ye P and Luo L (2018) Brain Natriuretic Peptide and Its Biochemical, Analytical, and Clinical Issues in Heart Failure: A Narrative Review. Front. Physiol. 9:692. doi: 10.3389/fphys.2018.00692
Received: 04 March 2018; Accepted: 17 May 2018;
Published: 05 June 2018.
Edited by:
Gabriele Giacomo Schiattarella, Università degli Studi di Napoli Federico II, ItalyReviewed by:
Matilde Otero-Losada, Instituto de Investigaciones Cardiológicas (ININCA), ArgentinaDennis W. T. Nilsen, Stavanger University Hospital, Norway
Gennaro Pagano, King’s College London, United Kingdom
Copyright © 2018 Fu, Ping, Zhu, Ye and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Ye, c2NpMzAxQDEyNi5jb20= Leiming Luo, bGxlaW1Ac2luYS5jb20=
†These authors are joint first authors.
 Shihui Fu
Shihui Fu Ping Ping3†
Ping Ping3†