- 1VasoActive Research Group, School of Health and Sport Sciences, University of the Sunshine Coast, Sunshine Coast, QLD, Australia
- 2Centre for Research on Exercise, Physical Activity and Health, School of Human Movement and Nutrition Sciences, The University of Queensland, Brisbane, QLD, Australia
- 3Queensland Research Centre for Peripheral Vascular Disease, James Cook University, Townsville, QLD, Australia
- 4Department of Vascular and Endovascular Surgery, Townsville Hospital, Townsville, QLD, Australia
Markers of chronic inflammation increase with aging, and are associated with cardiovascular disease prevalence and mortality. Increases in fitness with exercise training have been associated with lower circulating concentrations of cytokines known to have pro-inflammatory actions (such as interleukin-6 [IL-6]) and higher circulating concentrations of anti-inflammatory cytokines (interleukin-10 [IL-10]). However, the effect of cardiorespiratory fitness on acute cytokine responses to a single bout of exercise in healthy older individuals is unknown. We compared the response of plasma cytokines IL-6, tumor necrosis factor-alpha (TNF-α) and IL-10 to a bout of moderate-intensity continuous and higher-intensity interval exercise between older individuals with higher and lower levels of cardiorespiratory fitness. Sixteen lower-fit (VO2peak: 22.6±2.8 mL.kg−1.min−1) and fourteen higher-fit participants (VO2peak: 37.4±5.9 mL.kg−1.min−1) completed three 24 min experimental protocols in a randomized order: (1) moderate-intensity continuous exercise (40% of peak power output [PPO]); (2) higher-intensity interval exercise (12 × 1 min intervals at 70% PPO separated by 1 min periods at 10% PPO); or (3) non-exercise control. Plasma cytokines were measured at rest, immediately after, and during 90 min of recovery following exercise or control. Plasma IL-6 concentrations at baseline were greater in the higher-fit compared to the lower-fit group (P = 0.02), with no difference in plasma IL-10 or TNF-α concentrations at baseline between groups. Plasma IL-6 and IL-10 concentrations in both groups increased immediately after all protocols (IL-6: P = 0.02, IL-10: P < 0.01). However, there was no difference in the IL-6 and IL-10 response between the exercise and non-exercise (control) protocols. After all protocols, no changes in plasma TNF-α concentrations were observed in either the higher- or lower-fit groups. In this study, basal concentrations of circulating IL-6 were elevated in older individuals with higher levels of cardiorespiratory fitness. However, changes in plasma cytokine concentrations after exercise were not different to changes after non-exercise control in both the lower- and higher-fit groups.
Introduction
Chronic, low-grade inflammation is a common feature of various age-related chronic diseases (Himmerich et al., 2006). Such diseases are a major cause of global morbidity and mortality (Das et al., 2017), and this burden is expected to increase with an aging population (Yazdanyar and Newman, 2009). Regular exercise facilitates the creation of an anti-inflammatory environment (Petersen and Pedersen, 2005), leading to reduced basal inflammatory-, and increased anti-inflammatory, cytokine concentrations in both younger and older adults (Monzillo et al., 2003; Goldhammer et al., 2005; Santos et al., 2012). These long-term changes in circulating cytokines are believed to be mediated by the repeated acute inflammatory, and subsequent anti-inflammatory cytokine responses during recovery from single bouts of exercise (Reihmane and Dela, 2014).
In response to a short bout of exercise, acute increases in IL-6 have been proposed to suppress pro-inflammatory TNF-α (Starkie et al., 2003), and up-regulate anti-inflammatory cytokines such as IL-10 (Steensberg et al., 2003; Lira et al., 2009), creating an anti-inflammatory milieu for several hours after the cessation of exercise (Mendham et al., 2015). While this response is well established in young people, the response in older adults is more varied, with reports that the skeletal muscle (Hamada et al., 2005) and circulating (Reihmane et al., 2013, 2016) cytokine responses to a single bout of exercise may be supressed compared with those observed in young participants.
Unlike the anti-inflammatory benefits of transient increases in IL-6 from skeletal muscle with acute exercise, elevations in IL-6 released from other sources (e.g., hepatocytes or adipose tissue), are associated with increases in pro-inflammatory TNF-α and C-reactive protein (CRP). Importantly, higher levels of cardiorespiratory fitness have been associated with lower circulating concentrations of both IL-6 and CRP at rest (Kohut et al., 2006), which raises the possibility that low cardiorespiratory fitness may also contribute to the cytokine response to acute exercise in older adults. In younger adults, who typically have higher levels of cardiorespiratory fitness, the cytokine response to exercise does not appear to be influenced by cardiorespiratory fitness (Scott et al., 2013; Landers-Ramos et al., 2014). However, few studies in middle-aged or older adults have attempted to directly address this question. In recreational cyclists (age: 43 ± 10 y) higher self-reported fitness was associated with a lower CRP response to prolonged competitive exercise (Kleiven et al., 2017). In contrast, a recent study comparing masters athletes with lower-fit untrained adults reported no difference in the anti-inflammatory IL-10 and TGF-β responses to a single bout of exercise (Minuzzi et al., 2017). Whether cardiorespiratory fitness influences the acute inflammatory response to exercise in older adults remains to be established.
Interval exercise enables periods of higher-intensity exercise interspersed with periods of recovery, beyond the intensity that could normally be sustained with continuous exercise. This format of exercise is increasingly being used and recommend as part of the prevention and management of various chronic conditions (Gibala et al., 2012). In older adults, high-intensity interval training has been reported to elicit greater improvements in cardiorespiratory fitness compared with continuous exercise (Hwang et al., 2016); and in young adults interval exercise training is reported to exert similar improvements in cardiometabolic health, despite a lower overall exercise volume (Gillen et al., 2016). Acute increases in IL-6 were reported to be augmented in young adults after high-intensity interval exercise compared to moderate-intensity continuous exercise of the same duration and workload (Leggate et al., 2010). In contrast, a recent study of older adults suggests that the IL-6 response to interval exercise was lower than that during work- and time-matched continuous exercise (Windsor et al., 2017), although this study only included individuals with relatively low levels of cardiorespiratory fitness.
We hypothesize that low levels of cardiorespiratory fitness may contribute to the suppressed inflammatory responses to exercise previously reported in older adults. Understanding the interaction between cardiorespiratory fitness, exercise intensity and the cytokine responses to short-term exercise in older adults may improve exercise training prescription to optimize the anti-inflammatory effects of exercise as part of the prevention and management of age- and inflammatory-related conditions. Therefore, the aim of this study was to compare the plasma IL-6, TNF-α, and IL-10 responses to moderate-intensity continuous and higher-intensity interval exercise between healthy older adults with lower and higher levels of cardiorespiratory fitness.
Methods
Subjects
Sixteen lower-fit participants and 14 higher-fit participants were recruited from a University alumni cohort and through local advertisement. Participants were included if they were aged 60–86 years, non-smokers (>12 months no smoking history) and able to undertake cycling exercise. Participants were excluded if they had a body mass index (BMI) >39, uncontrolled hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg), unstable angina or diagnosed reversible cardiac ischemia, diagnosed uncontrolled cardiac arrhythmia with recurrent episodes or symptoms on exertion, heart failure, symptomatic aortic stenosis, or chronic obstructive pulmonary disease. Participants were also excluded if they had a known inflammatory condition including rheumatoid arthritis, ankylosing spondylitis or chronic active hepatitis, or were prescribed anti-inflammatory medication (e.g., NSAIDS) for regular use. Participants were informed of the methods and study design verbally and in writing before providing written informed consent. The study was approved by the University of the Sunshine Coast Human Research Ethics Committee, and conducted in accordance with the Declaration of Helsinki.
Experimental Design and Study Overview
Participants attended a baseline assessment followed by three experimental visits. The baseline assessment involved anthropometric measurements and a maximal incremental cycling test for the determination of maximal work output and cardiorespiratory fitness (VO2peak). VO2peak data were used to stratify participants into either the higher-fit (VO2peak ≥ 32 mL.kg−1.min−1 for males, ≥28 mL.kg−1.min−1 for females) or lower-fit group (VO2peak ≤ 28.0 mL.kg−1.min−1 for males, ≤ 25 mL.kg−1.min−1 for females) based upon current normative values for older individuals (Heyward and Gibson, 2014). All participants then attended three additional experimental visits conducted in a randomized, cross-over design. During these visits, participants completed a non-exercise control, a moderate-intensity continuous or higher-intensity interval cycling exercise protocol. All exercise was performed in an upright position on an electro-magnetically braked cycle ergometer (Lode Corival, Lode B.V., Groningen, Netherlands) and each visit was separated by 3–10 days, during which time participants were asked to maintain their habitual diet and physical activity patterns. Participants were asked to refrain from drinking alcohol or consuming caffeine for 12 h, and from using pro re nata anti-inflammatory medication for 72 h prior to each visit. Participants were required to be fasted for 3 h prior to each visit after the consumption of a standardized snack (4 oat breakfast biscuits, 20 g CHO, 8 g fat). Testing was performed at the same time of day under consistent laboratory conditions (room temperature: 23 ± 1°C).
Maximal Incremental Cycling Test
Participants cycled for 3 min with no resistance at a self-selected pedal-rate between 60 and 90 RPM, which was then maintained throughout the test. Resistance then increased to 20 W for 1 min, and by a further 10 W each min until volitional cessation. All participants reached the criteria for maximum effort, defined by achieving the following end-points: (1) heart rate within 10 b·min−1 of age-predicted peak heart rate, (2) respiratory exchange ratio of >1.15, and (3) rating of perceived exertion (RPE) ≥9 on a 10 point scale (Borg, 1982). Heart rate was recorded continuously using a 12-lead ECG (Mortara Inc., Milwaukee, WI, USA) and RPE was recorded during the final 10 s of each stage. Gas exchange data were collected continuously and stored at 15 s intervals using a Parvo Medics TrueOne 2400 metabolic cart and software (Parvo Medics, East Sandy UT, USA). VO2peak was determined as the highest VO2 value in a given 15 s period over the final minute of exercise. Peak power output was then used to establish the exercise intensity for the subsequent experimental test visits.
Experimental Testing Visits
Each of the three experimental visits (visits 2–4) commenced with the participant lying in the supine position for 15 min. During this time, a 12-lead ECG was fitted, an antecubital forearm vein was cannulated and a resting blood sample was drawn. Participants then completed one of the following protocols: (1) control, rest in an upright seated position; (2) moderate-intensity continuous cycling exercise at 40% peak power output (W); (3) higher-intensity interval cycling exercise, consisting of 12 × 1 min intervals at 70% peak power output separated by 1 min periods of active recovery at 10% peak power output. Participants cycled at a self-selected pedal rate of 60–90 RPM, which was kept consistent between visits. Each protocol was matched for time (24 min), and the exercise protocols were matched for total work. Heart rate and RPE were recorded during the final 10 s of each bout of the interval exercise protocol, and at the same corresponding time (i.e., every 2 min) during the other protocols. After completing the protocol, participants were immediately returned to the supine position and were monitored for 90 min. In order to establish the cytokine responses to each experimental session, blood samples were drawn before and at 3 time points following exercise or control (immediately, 20 and 90 min after).
Physical Activity Recall
Upon arrival at the laboratory for each experimental visit, participants completed the International Physical Activity Questionnaire modified for the elderly (IPAQ-E). Briefly, participants were asked to recall their physical activity levels during the previous 7 days, including the amount of time doing moderate or vigorous physical activity. Examples of the types of physical activity deemed moderate or vigorous were supplied within the questionnaire (Hurtig-Wennlöf et al., 2010).
Blood Sampling and Biochemical Analyses
After discarding the first 4 ml of collected blood, samples were collected in K2EDTA Vacutainer™ tubes using standard aseptic techniques. Normal saline (0.9%) was used to flush the cannula after each blood draw to maintain patency. Plasma was separated by centrifugation (1,500 × g for 15 min at 22°C) and stored in 1.5 mL aliquots at −80°C until further analysis. For IL-6 and IL-10, an additional time point was included (20 min post-exercise) to ensure that any peak in cytokine concentrations, which is reported to occur shortly after exercise cessation, was captured in the dataset (Petersen and Pedersen, 2006; Gleeson et al., 2011). Plasma IL-6, IL-10, and TNF-α concentrations were measured using commercially available sandwich ELISA kits (eBioscience, San Diego CA, USA). The catalog number for each ELISA kit are as follows; IL-6: 88-7066, IL-10: 88-7106, TNF-α: 88-7346. Samples were analyzed in duplicate for all time points, and all techniques and materials were used according to the manufacturer's instructions. All samples for any one participant were analyzed using the same assay to eliminate inter-assay variance. The within assay coefficient of variation for the duplicate analyses performed were (mean ± SD): IL-6 4.7 ± 5.0%; IL-10 4.6 ± 5.3%; TNF 4.3 ± 4.3%. The between visit intra-class correlations for each cytokine at baseline were as follows: IL-6: 0.86 (95% confidence interval 0.75–0.93), IL-10: 0.97 (0.94–0.98), TNF-α: 0.99 (0.97–0.99). The assay sensitivity for each cytokine was calculated by multiplying the control value on each assay by two (IL-6: 2.0 pg/ml, IL-10: 1.4 pg/ml, TNF-α: 6.3 pg/ml).
Data Analysis
Sample size estimates were calculated (G*Power software, v 3.1) based on the mean change in IL-6 concentrations in response to short-duration exercise (1.5 ± 0.2 pg/ml) in both older and younger populations (Kinugawa et al., 2003; Castellano et al., 2008; Landers-Ramos et al., 2014). With 80% power and an alpha level of 0.05, an estimated sample size of five subjects per group is needed to detect this same difference between groups and conditions. Comparisons of baseline cytokines between groups were carried out using a two-way linear mixed model (LMM) analysis. Heart rate and RPE during exercise, and cytokine concentrations after each experimental protocol were compared between fitness groups (higher, lower), between protocols (moderate-intensity continuous, higher-intensity interval, control), and across time (before each protocol, immediately post-, 20 min post- and 90 min post-protocol) using a three-way LMM (group*protocol*time). Data that were not normally distributed were transformed as appropriate before statistical analyses. Statistically significant interactions were further investigated with multiple comparisons using Fisher's least significant difference approach (Rothman, 1990; Perneger, 1998). The strength of the relationships between self-reported physical activity levels, cardiorespiratory fitness levels, cytokine concentrations at baseline and changes in cytokine concentrations (delta) in response to exercise were assessed using Pearson correlation coefficient. Analyses were conducted using the Statistical Package for Social Sciences (Version 22; IBM SPSS Inc., Chicago, IL). Statistical significance was delimited at P < 0.05 and exact P-values are cited. Data are presented in the text as mean (95% confidence interval) unless otherwise stated.
Results
Baseline Participant Characteristics
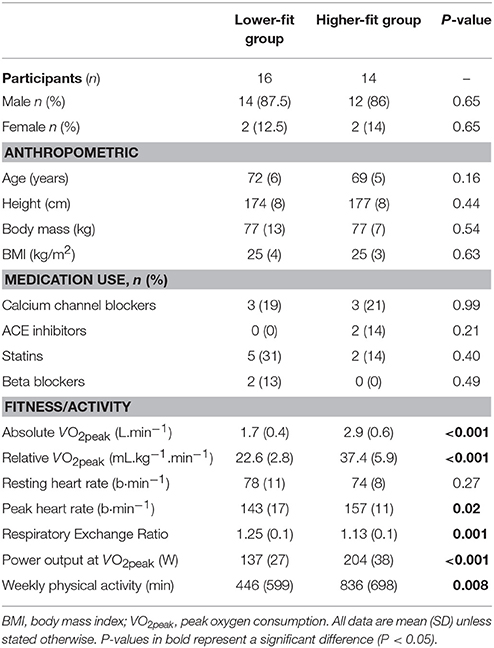
The baseline participant characteristics are presented in Table 1. Absolute and relative VO2peak were greater in the higher-fit group (P < 0.001). There were no significant differences between groups in terms of age, height, body mass and BMI. The higher-fit group reported completing 390 min (95% CI, 105–675, P = 0.008) more activity minutes per week than the lower-fit group.
Experimental Exercise Responses
Mean power output (W) during exercise was greater in the higher-fit group [moderate-intensity continuous: mean = 81 W, (95% CI, 71–91); higher-intensity interval: mean = 143 W (95% CI, 133–152)] compared to the lower-fit group [moderate-intensity continuous: mean = 55 W (95% CI, 45–64); higher-intensity interval: mean = 96 W (95% CI, 86–105), P < 0.001]. Mean heart rate during higher-intensity interval exercise was greater in the higher-fit compared to the lower-fit group [mean heart rate 111 b·min−1 (95% CI, 105–117) vs. 103 b·min−1 (95% CI, 98–109), P = 0.05], but mean heart rate was similar between groups during moderate-intensity continuous exercise [98 b·min−1 (95% CI, 93–104) vs. 96 b·min−1 (95% CI, 91–101), P = 0.51)] and control [58 b·min−1 (95% CI, 52–63) vs. 63 b·min−1 (95% CI, 58–68), P = 0.18]. Mean RPE was 4 (95% CI, 3–4) during higher-intensity interval exercise compared to 3 (95% CI, 2–3, P < 0.001) in moderate-intensity continuous exercise, with no differences between groups (P = 0.50).
Cytokine Concentrations at Baseline and in Response to Exercise
Plasma IL-6, IL-10, and TNF-α concentrations during each experimental visit (baseline and after different protocols) are reported in Tables 2, 3, and findings are summarized below.
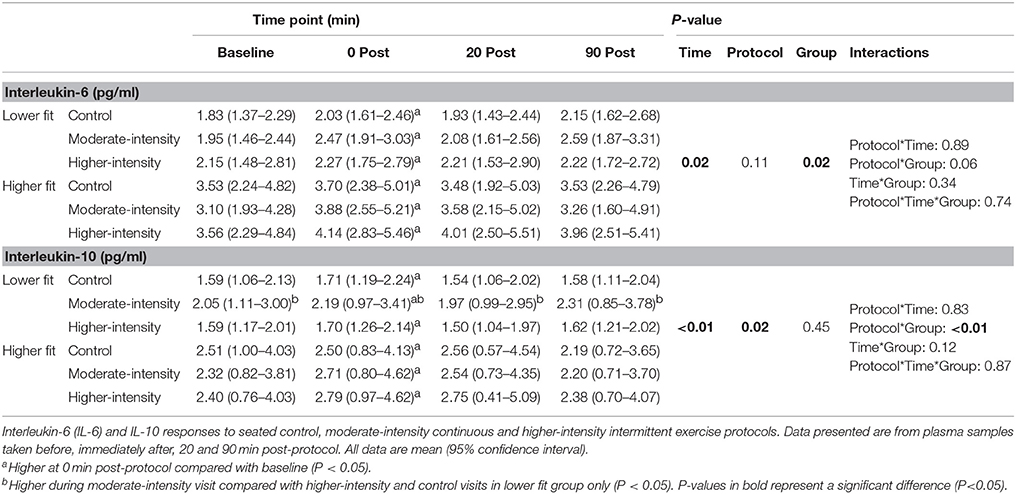
Table 2. Plasma IL-6 and IL-10 concentrations before and after control, moderate- and higher-intensity exercise in higher and lower fit groups.
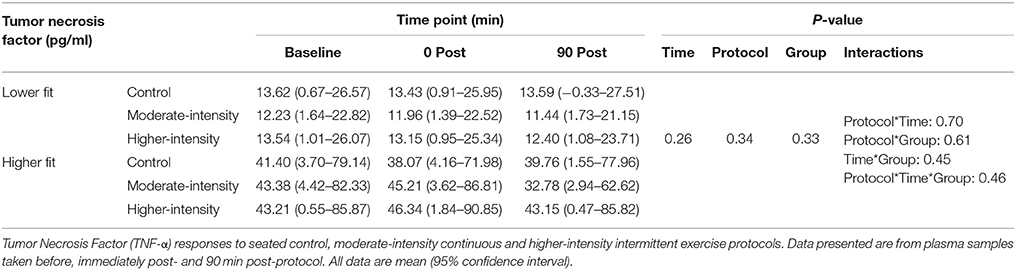
Table 3. Plasma TNF-α concentrations before and after control, moderate- and higher-intensity exercise in higher and lower fit groups.
Cytokine Concentrations at Baseline
Mean plasma IL-6 concentration at baseline across all experimental visits was 1.4 pg/ml higher in the higher-fit group compared to the lower-fit group (95% CI, 0.2–2.6, P = 0.02). There was a trend toward higher mean TNF-α concentrations in the higher-fit group compared to the lower-fit group (P = 0.16), and no group differences in mean IL-10 concentrations at baseline. There was no difference in cytokine concentrations at baseline between visits (P > 0.05).
Cytokine Concentrations in Response to Exercise
There was a time effect where mean IL-6 concentration in participants from both fitness groups increased by 0.4 pg/ml (95% CI, 0.1–0.7, P = 0.006) immediately post-protocol before returning to baseline values. Similarly, mean IL-10 concentration in participants from both fitness groups increased by 0.2 pg/ml (95% CI, 0.0–0.4, P = 0.002) immediately post-protocol before returning to baseline values. However, there were no group*time or protocol*time interactions for IL-6 or IL-10, indicating that the IL-6 and IL-10 responses were consistent between fitness groups and were not different between exercise and non-exercise protocols (Table 2). To account for the small baseline differences in IL-6 concentrations, analyses were repeated using delta (change from baseline) responses; however, this analysis did not change the findings (group*time*protocol interaction, P = 0.861). No differences in TNF-α concentrations were found over time, between groups or between protocols (Table 3).
Relationships Between Physical Activity, Fitness, and Cytokine Concentrations
Despite a significant difference in IL-6 concentration at baseline between lower and higher-fit groups, there was no correlation between IL-6 at baseline and VO2peak (r = 0.29, P = 0.12) in all participants. Higher IL-10 and TNF-α concentrations at baseline were associated with increased VO2peak (IL-10: r = 0.43, P = 0.02; TNF-α: r = 0.38, P = 0.04). No correlations were found between self-reported physical activity levels and IL-6, IL-10, or TNF-α concentrations at baseline. In addition, there were no correlations between self-reported physical activity levels or VO2peak, and changes in cytokine concentrations after moderate-intensity continuous exercise or higher-intensity interval exercise.
Discussion
This is the first study to compare the effects of lower and higher cardiorespiratory fitness on the circulating cytokine (IL-6, IL-10, and TNF-α) responses to short-term exercise in healthy older individuals. Our findings suggest that a single bout of moderate-intensity continuous or higher-intensity interval cycling exercise does not elicit a measurable cytokine response in healthy older adults, and these responses were similar in older adults with higher or lower levels of cardiorespiratory fitness.
Effect of Cardiorespiratory Fitness on Basal Cytokine Levels
We observed significantly higher mean IL-6 at baseline in the higher-fit group (3.4 pg/ml) compared to the lower-fit group (2.0 pg/ml, P = 0.02), with no group differences in IL-10 or TNF-α concentrations. Contrary to our findings, higher levels of cardiorespiratory fitness have previously been associated with lower circulating cytokine levels in young (Kullo et al., 2007; Gaeini et al., 2009; Lin et al., 2010), older (Valentine et al., 2009) and diseased populations, such as those with cancer (Jones et al., 2008). Conversely, recreationally active vs. endurance trained younger individuals show similar IL-6 or TNF-α concentrations at baseline, despite large differences in cardiorespiratory fitness (Scott et al., 2013; Landers-Ramos et al., 2014).
In an attempt to explain the higher IL-6 concentration observed at baseline in the higher-fit compared to the lower-fit group in this study, we considered whether the higher physical activity levels reported by the higher-fit group in the preceding 7 days before testing may have impacted on the observed cytokine concentrations. The circulating IL-6 response to exercise is transient, typically reaching a peak upon the cessation of exercise and returning to basal levels within 24 h (Leggate et al., 2010; Mendham et al., 2011; Perandini et al., 2015). The cytokine concentrations observed in the current study are unlikely to have been altered by a prior exercise bout, as the participants were asked to refrain from exercise or strenuous physical activity for at least 24 h prior to each experimental visit. While high physical activity levels such as those seen in the higher-fit group are typically considered to be beneficial for immune function (Estrela et al., 2017; Minuzzi et al., 2017), a U-shaped relationship between physical activity levels and infection (and subsequent inflammation) risk has also previously been proposed (Walsh et al., 2011; Gleeson and Walsh, 2012; Turner, 2016). Specifically, circulating inflammatory markers, such as IL-6, may be elevated in those undertaking heavy training loads. Despite this, we found no relationship between basal IL-6, IL-10, or TNF-α concentrations and self-reported physical activity levels in the preceding 7 days, suggesting that higher physical activity levels did not contribute to high circulating cytokines at baseline in our higher-fit group. After careful consideration, the cause of the higher basal IL-6 concentrations observed in the higher-fit group compared to the lower-fit group in this study is unclear. It is also unclear whether the higher basal circulating concentrations of IL-6 observed in the higher-fit group are indicative of a pro- or anti-inflammatory response. Further research of this complex cytokine incorporating comprehensive measurements of immune function is warranted to assess the effect of cardiorespiratory fitness and physical activity on basal inflammation in older adults.
Cytokine Responses to Exercise in Older Adults
Unlike many previous studies assessing cytokine responses to exercise, the experimental design of this study included a non-exercise control condition. Interestingly, we observed an increase in IL-6 and IL-10 concentrations immediately after all experimental protocols, with no difference in the cytokine response between the exercise and non-exercise protocols. These findings should be considered when interpreting previous studies, and highlight the importance of including a non-exercise protocol in the experimental design of future studies to prevent the reporting of false positive cytokine responses to exercise.
The exercise protocol intensity and duration used in this study is consistent with the current exercise prescription guidelines for health in older adults. Despite this, we observed no exercise-induced cytokine responses to exercise. These findings support the suggestion that acute cytokine responses to exercise may be blunted in older adults (Hamada et al., 2005; Reihmane et al., 2016) compared to previous observations in younger adults (Reihmane et al., 2013). Further studies comparing acute cytokine responses to short-term exercise in older and younger participant groups would help further clarify the effect of age on the inflammatory response to exercise.
Effect of Cardiorespiratory Fitness on Cytokine Responses to Exercise in Older Adults
There was no difference in the acute cytokine responses to a bout of exercise between older adults with lower and higher levels of cardiorespiratory fitness. These, findings concur with previous cross-sectional observations in young adults (Scott et al., 2013; Landers-Ramos et al., 2014) and middle-aged adults (Minuzzi et al., 2017). Studies assessing acute cytokine responses to exercise in younger adults have only included individuals with above-average levels of cardiorespiratory fitness, relative to population normative values (Scott et al., 2013; Landers-Ramos et al., 2014), which did not rule out the possibility that low levels of fitness might impact the cytokine response to exercise. The current study is the first to compare acute cytokine responses to exercise in participants with above average (37.4 ± 5.9 mL.kg−1.min−1) and below average (22.6 ± 2.8 mL.kg−1.min−1) cardiorespiratory fitness levels, relative to age-specific normative data (Stensvold et al., 2017). Our findings indicate that the blunted cytokine response to exercise previously reported among older adults (Hamada et al., 2005; Reihmane et al., 2016) is not likely to be due to low fitness levels.
In addition to having higher levels of cardiorespiratory fitness, the higher-fit group in this study were more physically active than the lower-fit group. However, we observed no relationships between self-reported physical activity levels and IL-6, IL-10, or TNF-α changes in response to exercise. These findings support previous observations in older individuals (Estrela et al., 2017). Estrela et al. (2017) found no difference in the IL-6 or TNF-α response to two successive bouts of maximal exercise between marathon runners with higher (~480 min/week) and lower training volumes (~240 min/week) (Estrela et al., 2017). Taken together, the findings of the current study and those reported previously suggest that neither cardiorespiratory fitness nor physical activity levels alter the acute cytokine responses to exercise in healthy older adults, and these findings appear to be consistent following both maximal and submaximal exercise.
Cytokine responses to exercise in all populations are known to be positively associated with both exercise intensity and duration (Fischer, 2006). Therefore, we adopted work- and time-matched exercise protocols to investigate the effect of exercise intensity on cytokine responses to exercise. We observed no differences in cytokine responses to moderate-intensity continuous exercise when compared to higher-intensity interval exercise of the same duration and total external workload. These findings contrast with previous observations of augmented IL-6 release in younger adults after interval exercise when compared to work- and time-matched continuous exercise (Leggate et al., 2010), albeit after longer duration exercise (58 min compared to 24 min in the current study). Further, we previously found that the IL-6 response was lower after interval exercise compared to continuous exercise (Windsor et al., 2017), although that study only included individuals with relatively low levels of cardiorespiratory fitness. Exercise duration has been reported to be the single most important factor determining the amplitude of the IL-6 response to exercise, accounting for more than 50% of the variation in plasma IL-6 (Fischer, 2006). Thus the contrasting findings between younger adults (Leggate et al., 2010) and older adults in the current study may be due to differences in the duration (and volume) of exercise. Taken together, the effect of exercise intensity on cytokine responses to exercise in older adults is still unclear, and the effect of exercise intensity in older adults after longer duration exercise is still to be determined.
When interpreting the findings of this study, it should be considered that we did not control for exercise induced changes in plasma volume. However, exercise induced fluid shifts in response to <30 min of submaximal exercise are typically small (Zouhal et al., 2001), and previous studies using a similar duration exercise protocol have found no changes in haematocrit or hemoglobin concentrations (Landers-Ramos et al., 2014). In addition, the non-exercise control visit in this study allowed us to highlight any non-exercise related variance in plasma cytokine concentrations such as those caused by postural-induced changes in plasma volume.
Conclusion
Shorts bouts of moderate-intensity continuous and higher-intensity interval cycling exercise did not influence circulating cytokine concentrations in healthy older adults, and this response was similar in older adults with higher or lower levels of cardiorespiratory fitness. The lack of an exercise-induced cytokine response in either group may suggest that the acute inflammatory response to short-term exercise is blunted in older adults, irrespective of cardiorespiratory fitness level. Further studies comparing cytokine responses to short-term exercise in older and younger adults would help clarify the effect of age on the inflammatory response to exercise.
Author Contributions
MW, TB, MP, JG, FR, and CA were responsible for the study conception and design. MW, TB, MP, LM, FR, and CA were responsible for the acquisition of data. MW, TB, LM, FR, and CA were responsible for the analysis and interpretation of data. MW, TB, and CA were responsible for drafting the manuscript. MW, TB, MP, LM, JG, FR, and CA were responsible for critical revision of the manuscript. All authors approved the final version of the manuscript for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was funded by grants from the National Health and Medical Research Council (1000967, 1022752, 1079369), The Townsville Hospital and the Inflammation and Healing Cluster at the University of the Sunshine Coast. JG work is supported by fellowships from the National Health and Medical Research Council (1117061) and the Queensland Government (Senior Clinical Research Fellowship).
References
Borg, G. A. (1982). Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14, 377–381. doi: 10.1249/00005768-198205000-00012
Castellano, V., Patel, D. I., and White, L. J. (2008). Cytokine responses to acute and chronic exercise in multiple sclerosis. J. Appl. Physiol. 104, 1697–1702. doi: 10.1152/japplphysiol.00954.2007
Das, A., Ambale-Venkatesh, B., Lima, J. A. C., Freedman, J. E., Spahillari, A., et al. (2017). Cardiometabolic disease in South Asians: a global health concern in an expanding population. Nutr. Metab. Cardiovasc. Dis. 27, 32–40. doi: 10.1016/j.numecd.2016.08.001
Estrela, A. L., Zaparte, A., da Silva, J. D. Jr., Moreira, J. C., and Bauer, M. E. (2017). Volume Exercise in older athletes influences inflammatory and redox responses to acute exercise. J. Aging Phys. Act. 25, 559–569. doi: 10.1123/japa.2016-0219
Fischer, C. P. (2006). Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 12, 6–33.
Gaeini, A. A., Fallahi, A. A., Kazemi, A., and Kordi, R. (2009). Association between cardiovascular fitness and inflammatory markers in boys aged 11-14 years. Iran. J. Pediatr. 19, 262–270.
Gibala, M. J., Little, J. P., Macdonald, M. J., and Hawley, J. A. (2012). Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 590, 1077–1084. doi: 10.1113/jphysiol.2011.224725
Gillen, J. B., Martin, B. J., MacInnis, M. J., Skelly, L. E., Tarnopolsky, M. A., and Gibala, M. J. (2016). Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS ONE 11:e0154075. doi: 10.1371/journal.pone.0154075
Gleeson, M., Bishop, N. C., Stensel, D. J., Lindley, M. R., Mastana, S. S., and Nimmo, M. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–610. doi: 10.1038/nri3041
Gleeson, M., and Walsh, N. P. (2012). The BASES expert statement on exercise, immunity, and infection. J. Sports Sci. 30, 321–324. doi: 10.1080/02640414.2011.627371
Goldhammer, E., Tanchilevitch, A., Maor, I., Beniamini, Y., Rosenschein, U., and Sagiv, M. (2005). Exercise training modulates cytokines activity in coronary heart disease patients. Int. J. Cardiol. 100, 93–99. doi: 10.1016/j.ijcard.2004.08.073
Hamada, K., Vannier, E., Sacheck, J. M., Witsell, A. L., and Roubenoff, R. (2005). Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J. 19, 264–266. doi: 10.1096/fj.03-1286fje
Heyward, V., and Gibson, A. (2014). Advanced Fitness Assessment and Exercise Prescription, 7th Edn. Champaign, IL: Human Kinetics.
Himmerich, H., Fulda, S., Linseisen, J., Seiler, H., Wolfram, G., Himmerich, S., et al. (2006). TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur. Cytokine Netw. 17, 196–201. doi: 10.1684/ecn.2006.0037
Hurtig-Wennlöf, A., Hagströmer, M., and Olsson, L. A. (2010). The international physical activity questionnaire modified for the elderly: aspects of validity and feasibility. Public Health Nutr. 13, 1847–1854. doi: 10.1017/S1368980010000157
Hwang, C.-L., Yoo, J. K., Kim, H. K., Hwang, H. K., Handberg, E. M., and Christou, D. D. (2016). Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp. Gerontol. 82(Suppl. C), 112–119. doi: 10.1016/j.exger.2016.06.009
Jones, L. W., Eves, N. D., Mackey, J. R., Peddle, C. J., Haykowsky, M., and Joy, A. A. (2008). Systemic inflammation, cardiorespiratory fitness, and quality of life in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 3, 194–195. doi: 10.1097/JTO.0b013e318160f36b
Kinugawa, T., Kato, M., Ogino, K., Osaki, S., Tomikura, Y., Igawa, O., et al. (2003). Interleukin-6 and tumor necrosis factor-alpha levels increase in response to maximal exercise in patients with chronic heart failure. Int. J. Cardiol. 87, 83–90. doi: 10.1016/S0167-5273(02)00200-0
Kleiven, O., Bjorkavoll-Bergseth, M., Melberg, T., Skadberg, O., Bergseth, R., Selvag, J., et al. (2017). High physical fitness is associated with reduction in basal- and exercise-induced inflammation. Scand. J. Med. Sci. Sports 28, 172–179. doi: 10.1111/sms.12878
Kohut, M. L., McCann, D. A., Russell, D. W., Konopka, D. N., Cunnick, J. E., and Vanderah, E. (2006). Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 20, 201–209. doi: 10.1016/j.bbi.2005.12.002
Kullo, I. J., Khaleghi, M., and Hensrud, D. D. (2007). Markers of inflammation are inversely associated withVo(2max) in asymptomatic men. J. Appl. Physiol. 102, 1374–1379. doi: 10.1152/japplphysiol.01028.2006
Landers-Ramos, R. Q., Jenkins, N. T., Spangenburg, E. E., Hagberg, J. M., and Prior, S. J. (2014). Circulating angiogenic and inflammatory cytokine responses to acute aerobic exercise in trained and sedentary young men. Eur. J. Appl. Physiol. 114, 1377–1384. doi: 10.1007/s00421-014-2861-6
Leggate, M., Nowell, M. A., Jones, S. A., and Nimmo, M. A. (2010). The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperones 15, 827–833. doi: 10.1007/s12192-010-0192-z
Lin, C. Y., Chen, P. C., Kuo, H. K., Lin, L. Y., Lin, J. W., and Hwang, J. J. (2010). Effects of obesity, physical activity, and cardiorespiratory fitness on blood pressure, inflammation, and insulin resistance in the National Health and Nutrition Survey 1999-2002. Nutr. Metab. Cardiovasc. Dis. 20, 713–719. doi: 10.1016/j.numecd.2009.06.005
Lira, F. S., Rosa, J. C., Yamashita, A. S., Koyama, C. H., Batista, M. L., et al. (2009). Endurance training induces depot-specific changes in IL-10/TNF-alpha ratio in rat adipose tissue. Cytokine 45, 80–85. doi: 10.1016/j.cyto.2008.10.018
Mendham, A. E., Donges, C. E., Liberts, E. A., and Duffield, R. (2011). Effects of mode and intensity on the acute exercise-induced IL-6 and CRP responses in a sedentary, overweight population. Eur. J. Appl. Physiol. 111, 1035–1045. doi: 10.1007/s00421-010-1724-z
Mendham, A. E., Duffield, R., Marino, F., and Coutts, A. J. (2015). Differences in the acute inflammatory and glucose regulatory responses between small-sided games and cycling in sedentary, middle-aged men. J. Sci. Med. Sport 18, 714–719. doi: 10.1016/j.jsams.2014.09.008
Minuzzi, L. G., Rama, L., Bishop, N. C., Rosado, F., Martinho, A., and Paiva, A. (2017). Lifelong training improves anti-inflammatory environment and maintains the number of regulatory T cells in masters athletes. Eur. J. Appl. Physiol. 117, 1131–1140. doi: 10.1007/s00421-017-3600-6
Monzillo, L. U., Hamdy, O., Horton, E. S., Ledbury, S., Mullooly, C., and Jarema, C. (2003). Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes. Res. 11, 1048–1054. doi: 10.1038/oby.2003.144
Perandini, L. A., Sales-de-Oliveira, D., Mello, S., Camara, N. O., Benatti, F. B., and Lima, F. R. (2015). Inflammatory cytokine kinetics to single bouts of acute moderate and intense aerobic exercise in women with active and inactive systemic lupus erythematosus. Exerc. Immunol. Rev. 21, 174–185.
Perneger, T. V. (1998). What's wrong with Bonferroni adjustments. Br. Med. J. 316, 1236–1238. doi: 10.1136/bmj.316.7139.1236
Petersen, A. M., and Pedersen, B. K. (2005). The anti-inflammatory effect of exercise. J. Appl. Physiol. 98, 1154–1162. doi: 10.1152/japplphysiol.00164.2004
Petersen, A. M., and Pedersen, B. K. (2006). The role of IL-6 in mediating the anti-inflammatory effects of exercise. J. Physiol. Pharmacol. 57(Suppl 10), 43–51.
Reihmane, D., and Dela, F. (2014). Interleukin-6: possible biological roles during exercise. Eur. J. Sport Sci. 14, 242–250. doi: 10.1080/17461391.2013.776640
Reihmane, D., Gram, M., Vigelsø, A., Helge, J. W., and Dela, F. (2016). Exercise promotes IL-6 release from legs in older men with minor response to unilateral immobilization. Eur. J. Sport Sci. 16, 1039–1046. doi: 10.1080/17461391.2015.1111939
Reihmane, D., Hansen, A. V., Gram, M., Kuhlman, A. B., Norregaard, J., and Dela, F. (2013). Immobilization increases interleukin-6, but not tumour necrosis factor-, release from the leg during exercise in humans. Exp. Physiol. 98, 778–783. doi: 10.1113/expphysiol.2012.069211
Rothman, K. J. (1990). No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46. doi: 10.1097/00001648-199001000-00010
Santos, R. V., Viana, V. A., Boscolo, R. A., Marques, V. G., Santana, M. G., and Lira, F. S. (2012). Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine 60, 731–735. doi: 10.1016/j.cyto.2012.07.028
Scott, J. P., Sale, C., Greeves, J. P., Casey, A., Dutton, J., and Fraser, W. D. (2013). Cytokine response to acute running in recreationally-active and endurance-trained men. Eur. J. Appl. Physiol. 113, 1871–1882. doi: 10.1007/s00421-013-2615-x
Starkie, R., Ostrowski, S. R., Jauffred, S., Febbraio, M., and Pedersen, B. K. (2003). Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 17, 884–886. doi: 10.1096/fj.02-0670fje
Steensberg, A., Fischer, C. P., Keller, C., Møller, K., and Pedersen, B. K. (2003). IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 285, E433–E437. doi: 10.1152/ajpendo.00074.2003
Stensvold, D., Sandbakk, S. B., Viken, H., Zisko, N., Reitlo, L. S., and Wisloff, U. (2017). Cardiorespiratory reference data in older adults: the generation 100 study. Med. Sci. Sports Exerc. 49, 2206–2215. doi: 10.1249/MSS.0000000000001343
Turner, J. E. (2016). Is immunosenescence influenced by our lifetime “dose” of exercise? Biogerontology 17, 581–602. doi: 10.1007/s10522-016-9642-z
Valentine, R. J., Vieira, V. J., Woods, J. A., and Evans, E. M. (2009). Stronger relationship between central adiposity and C-reactive protein in older women than men. Menopause 16, 84–89. doi: 10.1097/gme.0b013e31817fcb8f
Walsh, N. P., Gleeson, M., Shephard, R. J., Gleeson, M., Woods, J. A., and Bishop, N. C. (2011). Position statement part one: Immune function and exercise. Exerc. Immunol. Rev. 17, 6–63.
Windsor, M. T., Bailey, T. G., Perissiou, M., Greaves, K., Jha, P., and Leicht, A. S. (2017). Acute inflammatory responses to exercise in patients with abdominal aortic aneurysm. Med. Sci. Sports Exerc. doi: 10.1249/MSS.0000000000001501. [Epub ahead of print].
Yazdanyar, A., and Newman, A. B. (2009). The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin. Geriatr. Med. 25, 563–577, vii. doi: 10.1016/j.cger.2009.07.007
Keywords: inflammation, interleukin-6, interleukin-10, tumor necrosis factor-α, aging
Citation: Windsor MT, Bailey TG, Perissiou M, Meital L, Golledge J, Russell FD and Askew CD (2018) Cytokine Responses to Acute Exercise in Healthy Older Adults: The Effect of Cardiorespiratory Fitness. Front. Physiol. 9:203. doi: 10.3389/fphys.2018.00203
Received: 08 September 2017; Accepted: 23 February 2018;
Published: 15 March 2018.
Edited by:
Igor B. Mekjavic, Jožef Stefan Institute (IJS), SloveniaReviewed by:
Michail E. Keramidas, Royal Institute of Technology, SwedenHenning Bay Nielsen, Sanos Clinic and University of Copenhagen, Denmark
Diane Cooper, Athlone Institute of Technology, Ireland
Copyright © 2018 Windsor, Bailey, Perissiou, Meital, Golledge, Russell and Askew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher D. Askew, Y2Fza2V3QHVzYy5lZHUuYXU=
 Mark T. Windsor1
Mark T. Windsor1 Lara Meital
Lara Meital Christopher D. Askew
Christopher D. Askew